ABSTRACT
Background
The associations between self-reported chronic kidney disease–associated pruritus (CKD-aP) and patient-reported outcomes (PROs) have been reported using various instruments to assess itch. Data collection via multiple CKD-aP instruments allows the evaluation of different domains and measurements of CKD-aP burden and may help tailor data capture for future research or clinical care.
Methods
An electronic PRO (ePRO) survey was distributed to European hemodialysis (HD) patients enrolled in the Dialysis Outcomes and Practice Patterns Study (DOPPS) in 2021–23. The DOPPS is an international cohort study that aims to investigate practice patterns and outcomes in HD patients. The ePRO survey included multiple CKD-aP instruments: Average Itch and Worst Itching Intensity Numerical Rating Scales (AI-NRS, WI-NRS) and a Kidney Disease Quality of Life (KDQOL)-36 single question. Linear and logistic regression were used to estimate adjusted associations between CKD-aP instruments and various PROs.
Results
This analysis included 769 patients who completed the WI-NRS from HD facilities in France, Germany, Italy, Spain, Sweden and the UK. The correlation between WI-NRS and the KDQOL-36 itch question was 0.88 overall and 0.46 among patients at least somewhat bothered by itch. Mean WI-NRS scores stratified by response to the KDQOL-36 itch question were 8.1, 6.4, 4.1 and 3.1 for extremely, very much, moderately and somewhat bothered, respectively. Patients with worse WI-NRS scores reported worse sleep quality, greater fatigue, more depressive symptoms, and lower mental and physical quality of life; these associations were similar to those observed for the KDQOL-36 itch question.
Conclusion
Correlation between CKD-aP instruments was high overall, but moderate among the subgroup of patients bothered by itch; differences can be partially attributed to the recall period for the KDQOL-36 (4 weeks) vs the AI- and WI-NRS (24 h). The consistent associations of these instruments with poor outcomes underscores the importance to identify and effectively treat HD patients suffering from pruritus.
Keywords: hemodialysis, patient-reported outcomes, pruritus
KEY LEARNING POINTS.
What was known:
Pruritus is a burdensome symptom commonly experienced by people with kidney failure.
Randomized controlled trials of interventions to treat pruritus often use patient-reported outcomes that are different than those usually implemented in observational studies.
Understanding the relationship between multiple measures of pruritus is important to contextualize findings between different studies.
This study adds:
We found that instruments commonly used in pruritus research [e.g. the single Kidney Disease Quality of Life (KDQOL)-36 pruritus question] are relatively well correlated with single measure numerical scales [e.g. Average Itch and Worst Itching Intensity Numerical Rating Scales (AI- and WI-NRS)] that were used to measure pruritus in clinical trials among kidney failure patients.
The mean WI-NRS scores corresponding to different levels of response to the KDQOL-36 pruritus question are also useful to help translate findings from different settings.
Potential impact:
Our findings provide support to better interpret pruritus measures in the dialysis population.
Future researchers can use our results to design new studies investigating risk factors or interventions for pruritus.
INTRODUCTION
Chronic kidney disease–associated pruritus (CKD-aP) affects up to two-thirds of patients receiving dialysis [1]. Patients with greater severity of CKD-aP more often report lower physical and mental quality of life, lower sleep quality, greater fatigue and more depression, and face higher risks of adverse clinical outcomes [2]. CKD-aP is frequently underreported by patients, underrecognized by nephrologists and undertreated in many dialysis centers [3].
Although many validated patient-reported outcomes (PROs) of CKD-aP are available, limitations often prevent their implementation in clinical practice [4]. Questionnaire burden has been a longstanding problem [5], but recent studies have shown that single items, such as the itchy skin question from the Kidney Disease Quality of Life (KDQOL)-36 or visual analog scale (VAS), correlate well with multidimensional patient-reported outcome measures (PROMs) assessing pruritus-related quality of life, such as the Skindex-10 score [6, 7].
Large observational studies of CKD-aP have commonly used the KDQOL-36 itch question. However, randomized controlled trials (RCTs) rarely use this question to assess the efficacy of pruritus treatments due to its longer recall period of 4 weeks and the restricted, discrete levels categorizing pruritus intensity [8]. Instead, low-burden validated single-item numerical rating scales (NRS), such as the Worst Itching Intensity NRS (WI-NRS) and the Average Itch NRS (AI-NRS) are more commonly used in RCTs [9]. It is difficult to compare findings between observational studies and RCTs because the correlations between some of these different CKD-aP instruments have not been estimated in the kidney failure population. Moreover, associations between numerical scales for itch and other PROs such as sleep disturbance, health-related quality of life (HRQOL) and depression have not been described in large, representative populations of patients with kidney failure. These limitations restrict the application and interpretation of numerical scales to assess pruritus in populations with kidney failure.
In this cross-sectional analysis of a longitudinal study using electronic-PROMs among in-center hemodialysis (HD) patients in six western European countries, we report the associations between WI-NRS, AI-NRS and other CKD-aP measures, including the single-item KDQOL-36 itch question and the 5-D Itch scale. We also describe the associations between pruritus numerical scales and low-burden measures of fatigue, depression and sleep quality that can be implemented in clinical practice.
MATERIALS AND METHODS
Study design and data sources
We analyzed cross-sectional baseline data from an electronic patient-reported outcome (ePRO) prospective ancillary study nested within the Dialysis Outcomes and Practice Patterns Study (DOPPS) phase 7 (2021–23), a multicenter, international study of adult chronic in-center HD patients.
Patients at European DOPPS sites in six countries (France, Germany, Italy, Spain, Sweden and the UK) were approached for their consent to participate in this ancillary study. Consented patients agreed to complete a brief ePRO questionnaire each quarter during an 18-month data collection period. Only baseline data were available at the time of this analysis.
The DOPPS data collected during this study period includes patient demographics, socioeconomic measures, comorbidities, laboratory measures, medication prescriptions and prospective follow-up for hospitalization and mortality. The data yielded by the ePRO questionnaires was merged into the overall DOPPS dataset.
Exposure assessment
The primary exposure of interest was the severity level of self-reported itch measured using the WI-NRS, a single-item instrument that has been developed for dermatologic conditions and validated among end-stage kidney disease (ESKD) patients with pruritus [9]. The WI-NRS measures the worst itching intensity experienced over the past 24 h on a numerical scale from 0 to 10, with higher scores indicating worse itch intensity. A ≥3-point difference in the WI-NRS has been identified as a minimally clinically important difference (MCID) [9]. For most analyses, we categorized WI-NRS scores into five levels: no pruritus (0), mild pruritus (1–3), moderate pruritus (4–6), severe pruritus (7, 8) and very severe pruritus (9, 10), following previous studies [10]. In secondary analyses, we substituted the AI-NRS, which captures the average itch in the past 24 h.
Additional CKD-aP measures were: (i) KDQOL-36 itch question, in which patients were asked to rate the extent to which they were bothered by itchy skin during the past 4 weeks (1, not at all; 2, somewhat; 3, moderately; 4, very much; 5, extremely) [11]; and (ii) 5-D Itch scale, assessing five dimensions of itch (degree, duration, direction, disability and distribution) during the past 2 weeks. Included CKD-aP instruments are summarized in Supplementary data, Table S1.
Outcome assessment
HRQOL was measured by the KDQOL-36 Physical Component Summary (PCS) and Mental Component Summary (MCS) scores. Low PCS and MCS have been associated with worse clinical outcomes among ESKD patients and are validated measures of HRQOL in this population [12]. The MCID for MCS and PCS is considered to be 5 points [13].
Fatigue was measured using the SONG-HD (Standardized Outcomes in Nephrology-Hemodialysis) Fatigue questionnaire. The SONG-HD Fatigue measure consists of three items to represent fatigue-related dimensions experienced during the prior week, that assess (i) the effect of fatigue on life participation, (ii) tiredness and (iii) level of energy. These dimensions are assessed on a 4-point Likert scale indicating increasing severity, and ranges from 0 (not at all) to 3 (severely). An overall fatigue score is calculated by summing the responses across the three questions, resulting in a scale ranging from 0 (no fatigue) to 9 (maximum fatigue) [14].
Sleep quality was assessed by the single-item sleep quality scale (SQS), a widely used, self-reported questionnaire designed to evaluate sleep quality in various populations [15]. The range for possible SQS scores is from 0 (terrible quality) to 10 (excellent quality). Depression was defined by the 10-item Center for Epidemiologic Studies Depression Scale (CESD-10), a self-reported questionnaire abbreviated from the original 20-item CES-D scale [16], designed to measure depressive symptoms in the general population [17]. The CESD-10 evaluates various aspects of depressive symptoms, including affective, cognitive and somatic manifestations [17]. The scale has demonstrated strong validity, reliability and internal consistency across diverse populations, including patients with chronic conditions such as ESKD [18]. CESD-10 scores range from 0 to 30. Scores of 10 or higher are indicative of depressive symptoms.
Statistical analysis
We performed descriptive analyses to summarize patient characteristics by CKD-aP severity using WI-NRS categories. The Spearman correlation coefficient was used to estimate the correlation between CKD-aP instruments, both overall and among a subgroup of patients who reported being at least somewhat bothered by itch on the KDQOL-36 itch question. To estimate mean differences [with 95% confidence intervals (CI)] in PROs measured on a continuous scale, we used linear regression models. Such models assume that observations are independent, that the relationship between the exposure and outcome is linear, and that the variance of errors terms is consistent across all levels of the independent variables. For binary outcomes, we used Poisson regression models to estimate prevalence ratios (with 95% CIs). All models were adjusted for potential confounders including age, sex, race and 12 summary comorbidities. Poisson models assume independence of observations and linearity between the log of the mean outcome and the independent variables.
Secondary analyses were conducted substituting the AI-NRS (measuring average itch) and KDQOL-36 itch question (4-week recall period) as the exposure variable, to evaluate the consistency of the associations between CKD-aP and PROs.
To reduce questionnaire burden, patients reporting being “not at all” bothered by itchy skin on the KDQOL-36 itch question were asked to skip all other CKD-aP assessments, including WI-NRS. A score of 0 for the WI-NRS and AI-NRS was imputed for patients who reported being “not bothered at all” by itchy skin on the KDQOL-36 itch question because these data were clearly not missing at random. These individuals not at all bothered by pruritus are important to include and served as the reference group for analyses of associations with PROs. We performed correlation analyses both with and without these “not at all bothered” patients to first assess how strongly the WI-NRS and the KDQOL-36 itch question were correlated across the spectrum of patients and second to assess how they distinguished between itch severity among those with established itch.
To assess how the correlation between KDQOL-36 and the WI-NRS scores varied by imputation assumption, acknowledging that a small but non-zero proportion of patients responding “not at all bothered” may have scored >0 on the WI-NRS if given the opportunity, we considered two scenarios as sensitivity analyses. The first considered that 80% of patients who reported not being bothered by itchy skin in the KDQOL-36 would have a WI-NRS of 0; the second scenario assumed assume 60% for the same proportion.
RESULTS
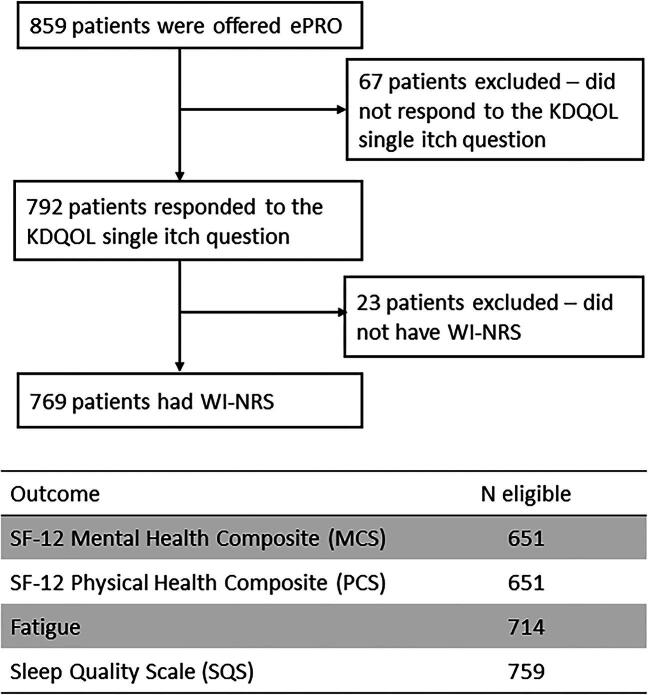
A total of 859 HD patients from France (N = 161), Germany (N = 230), Italy (N = 128), Spain (N = 165), Sweden (N = 83) and the UK (N = 92) completed the ePRO baseline questionnaire. After excluding 90 patients without data available on the primary CKD-aP exposure (WI-NRS), 769 were included in the analysis (Fig. 1). Patient characteristics were similar between the included and excluded cohort of patients (Supplementary data, Table S2).
Figure 1:
Flow-chart of sample inclusion and exclusion.
Patient characteristics by categories of pruritus defined by WI-NRS are described in Table 1. Among the 769 patients, 420 (55%) had no pruritus, 141 (18%) mild pruritus, 125 (16%) moderate pruritus, 57 (7%) severe pruritus, and 26 (3%) very severe pruritus. The mean age ranged from 63 years in the very severe pruritus group to 67 years in the mild pruritus group. The median duration of dialysis was longest in the severe pruritus group (4.2 years), followed by the group without pruritus (3.0 years). The proportion of female patients was highest in the severe pruritus group (44%) and lowest in the mild pruritus group (26%). The prevalence of cardiovascular comorbidities was slightly higher among patients with more severe pruritus, while the frequency of diabetes was similar across groups by pruritus severity. The prevalence of catheter use increased with pruritus severity, while single pool Kt/V values were similar across all groups. Serum albumin levels were lowest in the severe pruritus group (3.56 g/dL), and serum phosphorus levels were highest in the severe and very severe pruritus groups (5.25 and 5.21 mg/dL, respectively). C-reactive protein levels were generally higher with pruritus severity, with the highest median value observed in the severe pruritus group (10.0 mg/L). The distribution of patient characteristics by pruritus severity as measured by the AI-NRS was similar to WI-NRS (Supplementary data, Table S3).
Table 1:
Sample characteristics by pruritus severity as measured by WI-NRS.
| Characteristics | No pruritus (0) | Mild pruritus (1–3) | Moderate pruritus (4–6) | Severe pruritus (7–8) | Very severe pruritus (9–10) |
|---|---|---|---|---|---|
| Patients, N (%) | 420 (55) | 141 (18) | 125 (16) | 57 (7) | 26 (3) |
| Age, years | 65.8 (13.4) | 67.0 (14.6) | 65.9 (14.3) | 65.9 (14.8) | 63.0 (15.4) |
| Duration of dialysis, years | 3.0 (1.0, 7.1) | 2.8 (0.9, 5.4) | 2.9 (1.0, 6.2) | 4.2 (1.3, 7.6) | 4.0 (1.3, 9.0) |
| Female, % | 39 | 26 | 32 | 44 | 42 |
| Black race, % | 6 | 3 | 5 | 5 | 6 |
| Coronary artery disease, % | 30 | 20 | 33 | 38 | 31 |
| Cerebrovascular disease, % | 12 | 11 | 11 | 9 | 12 |
| Congestive heart failure, % | 18 | 15 | 20 | 23 | 31 |
| Other cardiovascular disease, % | 28 | 29 | 31 | 38 | 42 |
| Peripheral vascular disease, % | 29 | 15 | 21 | 36 | 23 |
| Hypertension, % | 90 | 89 | 91 | 88 | 85 |
| Diabetes, % | 40 | 34 | 38 | 47 | 38 |
| Cancer (non-skin) , % | 19 | 27 | 24 | 25 | 12 |
| Gastrointestinal bleeding, % | 5 | 4 | 5 | 2 | 8 |
| Lung disease, % | 11 | 12 | 14 | 12 | 35 |
| Neurologic disease, % | 11 | 9 | 9 | 13 | 4 |
| Any psychiatric disorder, % | 15 | 12 | 17 | 20 | 15 |
| Catheter use, % | 30 | 31 | 40 | 38 | 35 |
| Single pool Kt/V | 1.55 (0.37) | 1.56 (0.35) | 1.47 (0.28) | 1.59 (0.35) | 1.54 (0.30) |
| Serum albumin, g/dL | 3.76 (0.48) | 3.74 (0.52) | 3.73 (0.51) | 3.56 (0.52) | 3.72 (0.58) |
| Serum phosphorus, mg/dL | 4.84 (1.72) | 4.84 (1.74) | 4.70 (1.58) | 5.25 (1.71) | 5.21 (2.24) |
| C-reactive protein, mg/L | 6.0 (2.4, 20.0) | 8.0 (3.1, 19.3) | 8.1 (3.0, 20.0) | 10.0 (4.3, 24.0) | 9.5 (4.6, 21.0) |
Results are expressed as mean (standard deviation), median (interquartile range) or prevalence (%).
Among all patients, the correlation with WI-NRS was 0.97 for AI-NRS and 0.88 for the KDQOL-36 itch question. These results were consistent in multiple imputation scenarios (Supplementary data, Table S3). For patients with at least some degree of pruritus as measured by the KDQOL-36 itch question at baseline, the correlation between WI-NRS and the KDQOL-36 itch question was much lower (0.46) while AI-NRS and WI-NRS remained very highly correlated (0.90).
The correlation between the 5-D Itch and KDQOL-36 itch question was 0.54 while for both AI-NRS and WI-NRS it was 0.67. Among patients with at least some degree of pruritus, the 5-D Itch domain most highly correlated with the KDQOL-36 itch question was the degree domain (r = 0.60), while the least correlated was the direction domain (r = 0.28). The correlation between all 5-D Itch domains and the KDQOL-36 itch question is provided in Supplementary data, Table S4.
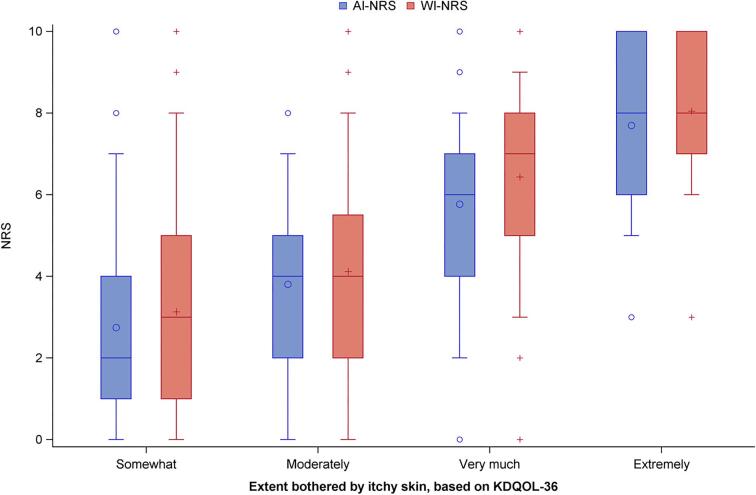
Figure 2 depicts mean NRS scores by the categories of the KDQOL-36 itch question. The mean WI-NRS scores stratified by response to the KDQOL-36 itch question were 8.1 for extremely bothered, 6.4 for very much bothered, 4.1 for moderately bothered and 3.1 for somewhat bothered. The AI-NRS and WI-NRS responses were consistent, with most patients having similar responses (Fig. 2).
Figure 2:
Distribution of WI-NRS and AI-NRS, by KDQOL-36 itchy skin question response.
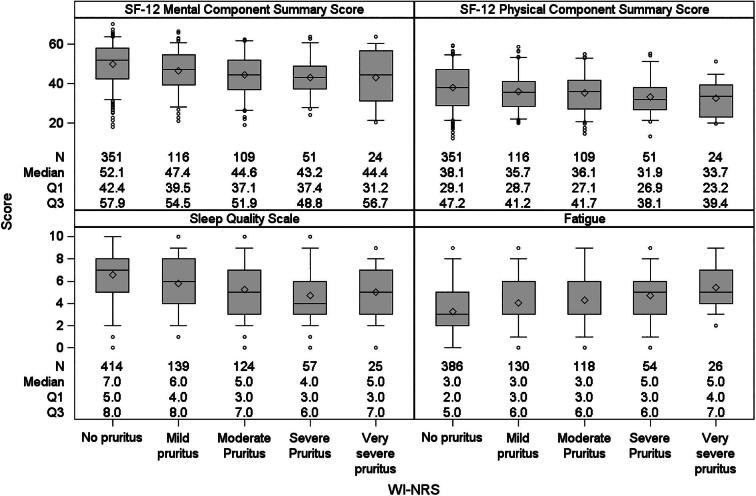
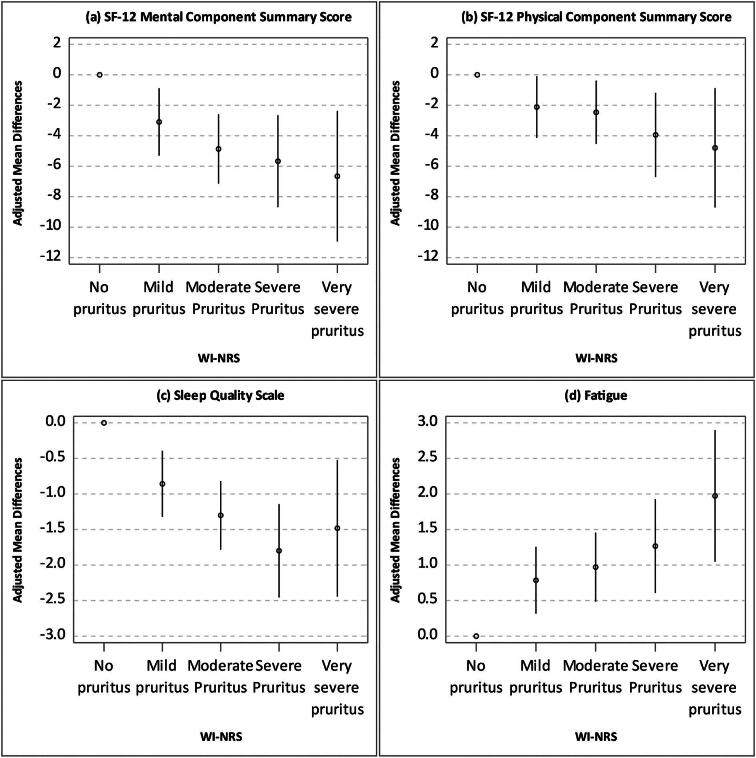
Patients with worse WI-NRS scores tended to have worse sleep quality, greater fatigue and lower MCS and PCS scores (Fig. 3). Consistent results by AI-NRS are shown in Supplementary data, Fig. S1. After adjusting for potential confounders, WI-NRS was inversely associated with MCS, PCS and fatigue (Fig. 4). Higher WI-NRS scores linearly associated with poorer KDQOL-36 scores, more so with MCS than PCS. Supplementary data, Fig. S2 summarizes similar results by AI-NRS.
Figure 3:
Distribution of general health-related quality of life, sleep quality and fatigue, stratified by WI-NRS.
Figure 4:
Adjusted mean differences between WI-WRS categories for general health-related quality of life, sleep quality and fatigue. *Adjusted for age at patient questionnaire, gender, Black race and 12 summary comorbidities.
Patients having WI-NRS scores consistent with being moderately to very severely bothered by itch had an approximately 2-fold higher adjusted prevalence of reporting depressive symptoms consistent with the diagnosis of depression (i.e. having a CES-D score ≥10) compared with those without itchy skin (Table 2). Associations were similar when substituting AI-NRS as the exposure variable. For comparison purposes, Supplementary data, Figs S3 and S4 display four of the main patient-reported outcomes in relationship to patient responses to the single KDQOL-36 itch question, with results being similar to those seen using WI-NRS.
Table 2:
Adjusted prevalence ratio of depression (CES-D ≥10) across WI-NRS and AI-NRS itch severity categories.
| Adjusteda prevalence ratio (95% CI) for WI-NRS categories | Adjusteda prevalence ratio (95% CI) for AI-NRS categories | |
|---|---|---|
| No pruritus (0) | ref | ref |
| Mild pruritus (1–3) | 1.15 (0.85,1.57) | 1.39 (1.06,1.82) |
| Moderate pruritus (4–6) | 2.04 (1.68,2.48) | 2.01 (1.61,2.50) |
| Severe pruritus (7–8) | 2.08 (1.64,2.63) | 2.00 (1.54,2.59) |
| Very severe pruritus (9–10) | 1.98 (1.42,2.78) | 2.22 (1.34,3.66) |
Adjusted for age, gender, Black race and 12 summary comorbidities.
DISCUSSION
In this cross-sectional analysis of a prospective international study using ePRO data collection among HD patients, we found that the correlation between CKD-aP instruments was very high overall, but moderate among the subgroup of patients bothered by itch. Higher WI-NRS scores were associated with worse mental and physical components of HRQOL and a higher prevalence of depression. Our findings confirm the extensive literature describing poorer quality of life among patients with CKD-aP [2] and show that low-burden, electronically captured PROMs can be deployed in real-world settings among ESKD patients.
Our results are consistent with earlier work by Reich et al. [10] and Phan et al. [19], based upon patients with itchy skin followed in dermatology clinics in Germany, Poland and Japan. A high test–retest reproducibility was described by these studies, with correlation coefficients >0.80 between the VAS, NRS, and either a 4- or 5-point verbal rating scale (VRS) in the assessment of pruritus. Although the 5-point VRS used by Reich et al. [10] consists of response categories very similar to the KDQOL single itch question used in our study, the time frame of the past 24 h typically used for the VRS is considerably shorter than the time frame of past 4 weeks used for the KDQOL-36 itch question. Despite this time-frame difference between the VRS and KDQOL-36 itch question, it is informative to see the relatively high correlation in patient responses in our study between the WI-NRS and KDQOL-36 itch question. Although it is not surprising to see some absolute differences in the severity of being bothered by itch in the past 24 h compared with the past 4 weeks, the general degree of being bothered by itch was quite similar across instruments and recall periods. Thus, a patient's response to the single KDQOL itch question pertaining to the past 4 weeks generally provides a good indication to the extent that patients have been bothered by itch during the past 24 h.
An important question is whether key patient-reported and clinical outcomes substantially differ in relationship to the extent a patient has been bothered by itch over the past 4 weeks vs the past 24 h. Our study demonstrates that the association between pruritus and PROs is consistent across different instruments with varying recall periods. Patients bothered by itchy skin both in the 4-week period and in the 24-h period tended to have worse sleep quality, more depressive symptoms and lower quality of life, both mental and physical, even after adjustment for multiple confounders. Therefore, our results suggest that instruments with different recall periods not only yield similar measures of pruritus severity but are also similarly associated with PROs.
Our results also confirm and expand prior studies on the associations between CKD-aP and patient characteristics and outcomes [20]. Similar to prior studies using different measures for CKD-aP [1], we found that the strength of the association between pruritus severity and mental or physical components of quality of life is clinically meaningful [12]. Furthermore, our research provides the first detailed analysis of the associations between the SONG-HD Fatigue instrument and the WI-NRS and AI-NRS among ESKD patients. By confirming the association between pruritus and fatigue measured by an instrument designed specifically for HD patients, we demonstrate that fatigue in ESKD is strongly and consistently linked to pruritus. It is noteworthy that the SONG-HD Fatigue instrument and the sleep quality scale did not appear to be any more strongly related to CKD-aP severity in the present study, as seen previously by Sukul et al. [1] when using the single question PROs of “feeling washed out or drained” and “poor sleep quality (≥3 vs <3 nights/week of restless sleep)” as surrogates for fatigue and sleep quality, respectively.
Our study has several limitations. Although specific cut-points to define pruritus severity based on the WI-NRS have not been clearly defined, our approach is consistent with prior studies in similar populations [10]. Moreover, this is a cross-sectional analysis, and we cannot rule out reverse causality in the described associations. Also, the severity of CKD-aP in our study is lower than previously reported, which may suggest that our sample is not completely representative of the general in-center HD population in Europe. In fact, although our results are consistent with prior DOPPS studies including several European countries [1], our analyses are restricted to Western European countries. Moreover, by study design, to limit questionnaire burden, patients who did not report pruritus in the KDQOL-36 itch question were not eligible to answer additional pruritus questions, leading to informative missingness for numerical scales and the 5-D Itch scale. We addressed this in our primary analysis by imputing a score of 0 for WI-NRS and AI-NRS when patients reported being not at all bothered by itchy skin on the KDQOL-36 question. Two reasons explain this decision. First, a complete case analysis, excluding patients with missing NRS, could lead to a strong bias towards the null because the missing data, consequent to the study design, are not random. Second, and relatedly, patients reporting being not bothered by pruritus in the last 30 days (i.e. KDQOL-36) most likely would report minimal or no pruritus within the last 24 h (i.e. WI-NRS or AI-NRS). Although imputing a score of 0 for WI-NRS and AI-NRS could overestimate the true correlation with the KDQOL-36 itch question, we believe the bias in the imputed case is smaller than in the complete case analysis. Because of this, the correlation between CKD-aP instruments was unsurprisingly sensitive to the imputation assumption, and we reported both approaches. However, our analyses with distinct imputation assumptions suggest that the correlation between CKD-aP measures remains high despite the informative missing in our study design.
Our study also has noteworthy strengths, demonstrating the feasibility of deploying an ePRO data collection process in real-world settings of HD patients in Europe. We studied the correspondence between diverse data instruments to measure pruritus intensity and in future analyses will be able to investigate the longitudinal changes of pruritus intensity and associations with outcomes including PROs, healthcare resource utilization and clinical outcomes via linking to the DOPPS core study. Our results will assist in the development of new prospective studies, including clinical trials, that aim to assess interventions or risk factors for CKD-aP outcomes in the HD population. Our results also aid in the interpretation of observational studies and clinical trials employing multiple CKD-aP measures. Since the correlation between KDQOL-36 and numerical scales (WI- and AI-NRS) is high, studies exploring interventions and risk factors for pruritus measured on different scales should be consistent in the HD population, despite expected differences. Finally, our correlation results can be used in the future for meta-analyses combining studies using multiple measures for CKD-aP. Further investigations are needed to generalize our findings to longitudinal measures and additional CKD-aP measures assessing multiple pruritus dimensions, such as the 5-D Itch scale.
In conclusion, this cross-sectional analysis of the DOPPS ePRO study shows that the KDQOL-36 itch question, as well as the AI-NRS and WI-NRS measures of pruritus severity are each similarly associated with worse PROs among HD patients. Instruments with varying recall periods are highly correlated and produce comparable results regarding pruritus severity and its associations with PROs. These findings help to inform clinical practice in trying to decide which instrument to choose for assessing CKD-aP in patients. Further analysis from the DOPPS ePRO study will enable interpretation of long-term changes in pruritus and associated risk factors among HD patients.
Supplementary Material
ACKNOWLEDGEMENTS
Jennifer McCready-Maynes and Shauna Leighton, employees of Arbor Research Collaborative for Health, provided editorial support for this paper.
Contributor Information
Murilo Guedes, Arbor Research Collaborative for Health, Ann Arbor, USA.
Charlotte Tu, Arbor Research Collaborative for Health, Ann Arbor, USA.
Nidhi Sukul, Division of Nephrology, Department of Internal Medicine, University of Michigan, Ann Arbor, USA; Division of Nephrology, Veterans Affairs Ann Arbor Health System, Ann Arbor, USA.
Elham Asgari, Department of Nephrology, Guy's, St Thomas Hospital, London, UK.
Fitsum Guebre-Egziabher, Service de Nephrologie, Hopital Edouard Herriot, Hospices Civils de Lyon, INSERM U1060 université Lyon-1, Lyon, France.
Despina Ruessmann, CSL-Vifor, Glattbrugg, Switzerland.
Thilo Schaufler, CSL-Vifor, Glattbrugg, Switzerland.
Hugh Rayner, Birmingham Heartlands Hospital, Birmingham, UK.
Michel Jadoul, Department of Nephrology, Cliniques universitaires Saint-Luc, Université catholique de Louvain, Brussels, Belgium.
Laura Labriola, Department of Nephrology, Cliniques universitaires Saint-Luc, Université catholique de Louvain, Brussels, Belgium.
Roberto Pecoits-Filho, Arbor Research Collaborative for Health, Ann Arbor, USA.
Ronald L Pisoni, Arbor Research Collaborative for Health, Ann Arbor, USA.
Angelo Karaboyas, Arbor Research Collaborative for Health, Ann Arbor, USA.
FUNDING
This manuscript was directly supported by Vifor. Global support for the ongoing DOPPS Programs is provided without restriction on publications by a variety of funders. For details see https://www.dopps.org/AboutUs/Support.aspx.
AUTHORS’ CONTRIBUTIONS
Substantial contributions to the conception or design of the work; or the acquisition, analysis or interpretation of data for the work: T.S., C.T., H.R., A.K., L.L., D.R., R.L.P., F.G.-E., M.G., R.P.-F. Drafting the work or revising it critically for important intellectual content: T.S., H.R., N.S., A.K., L.L., R.L.P., F.G.-E., M.J., E.A., M.G., R.P.-F. Final approval of the version to be published: T.S., N.S., A.K., L.L., R.L.P., F.G.-E., M.J., M.G., R.P.-F. Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved: T.S., N.S., A.K., R.L.P., F.G.-E., M.G., R.P.-F.
DATA AVAILABILITY STATEMENT
The data underlying this article will be shared on reasonable request to the corresponding author.
CONFLICT OF INTEREST STATEMENT
N.S. declares honoraria from Medscape for a pre-recorded lecture on CKD-aP. E.A. is the chair of the British Transplant Society Standards and Guidelines. F.G.-E. and H.R. have no conflicts of interest to declare. D.R. and T.S. are employees of CSL Vifor, and have stock/stock option from CSL Vifor. M.J. declares a research grant from AstraZeneca, consulting fee from AstraZeneca, Astellas, Boehringer-Ingelheim, CSL Vifor, Cardiorenal, GSK, Vertex; honoraria from AstraZeneca, Bayer, Boehringer-Ingelheim; payment for expert testimony from Astellas; support for attending meetings from AstraZeneca, Boehringer; and is the cochair (volunteer) of Kidney Disease: Improving Global Outcomes (KDIGO). L.L. declares honoraria from Amgen. M.G., C.T., R.P.-F., R.L.P. and A.K. are employees of Arbor Research Collaborative for Health which administers the DOPPS. Global support for the ongoing DOPPS Programs is provided without restriction on publications by a variety of funders. For details see https://www.dopps.org/AboutUs/Support.aspx. All funding is provided to Arbor Research Collaborative for Health, and not to the authors directly. R.P.-F. has received consulting fees from Bayer, Boehringer, AstraZeneca, GSK, Akebia, Fibrogen and Fresenuis Medical Care; and honoraria from Bayer, Boehringer, AstraZeneca, GSK, Akebia, Fibrogen and Fresenuis Medical Care; and has participated on a DMSB for Bayer, Boehringer, AstraZeneca, GSK, Akebia and Fresenuis Medical Care.
REFERENCES
- 1.Sukul N, Karaboyas A, Csomor PAet al. Self-reported pruritus and clinical, dialysis-related, and patient-reported outcomes in hemodialysis patients. Kidney Med 2020;3:42–53.e1. 10.1016/j.xkme.2020.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pisoni RL, Wikström B, Elder SJet al. Pruritus in haemodialysis patients: international results from the Dialysis Outcomes and Practice Patterns Study (DOPPS). Nephrol Dial Transplant 2006;21:3495–505. 10.1093/ndt/gfl461 [DOI] [PubMed] [Google Scholar]
- 3.Rayner HC, Larkina M, Wang Met al. International comparisons of prevalence, awareness, and treatment of pruritus in people on hemodialysis. Clin J Am Soc Nephrol 2017;12:2000–7. 10.2215/CJN.03280317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Combs SA, Teixeira JP, Germain MJ. Pruritus in kidney disease. Semin Nephrol 2015;35:383–91. 10.1016/j.semnephrol.2015.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Breckenridge K, Bekker HL, Gibbons Eet al. How to routinely collect data on patient-reported outcome and experience measures in renal registries in Europe: an expert consensus meeting. Nephrol Dial Transplant 2015;30:1605–14. 10.1093/ndt/gfv209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mathur VS, Lindberg J, Germain Met al. A longitudinal study of uremic pruritus in hemodialysis patients. Clin J Am Soc Nephrol 2010;5:1410–9. 10.2215/CJN.00100110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lopes MB, Karaboyas A, Sukul Net al. Utility of a single itch-related question and the skindex-10 questionnaire for assessing pruritus and predicting health-related quality of life in patients receiving hemodialysis. Kidney Med 2022;4:100476. 10.1016/j.xkme.2022.100476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simonsen E, Komenda P, Lerner Bet al. Treatment of uremic pruritus: a systematic review. Am J Kidney Dis 2017;70:638–55. 10.1053/j.ajkd.2017.05.018 [DOI] [PubMed] [Google Scholar]
- 9.Vernon MK, Swett LL, Speck RMet al. Psychometric validation and meaningful change thresholds of the worst Itching Intensity Numerical Rating Scale for assessing itch in patients with chronic kidney disease-associated pruritus. J Patient Rep Outcomes 2021;5:134. 10.1186/s41687-021-00404-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reich A, Heisig M, Phan NQet al. Visual analogue scale: evaluation of the instrument for the assessment of pruritus. Acta Derm Venereol 2012;92:497–501. 10.2340/00015555-1265 [DOI] [PubMed] [Google Scholar]
- 11.Hays RD, Kallich JD, Mapes DLet al. Development of the kidney disease quality of life (KDQOL) instrument. Qual Life Res 1994;3:329–38. 10.1007/BF00451725 [DOI] [PubMed] [Google Scholar]
- 12.Sinclair A, Cimon K, Loncar Met al. Dialysis Modalities for the Treatment of End-Stage Kidney Disease: a Health Technology Assessment [Internet]. (CADTH Optimal Use Report, No. 6.2b.) Appendix 10, Validity of Outcomes for Health-Related Quality of Life Instruments—Clinical Review. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health, 2017. Available from: https://www.ncbi.nlm.nih.gov/books/NBK532011/ (4 June 2024; date last accessed) [PubMed] [Google Scholar]
- 13.Pagels AA, Söderkvist BK, Medin Cet al. Health-related quality of life in different stages of chronic kidney disease and at initiation of dialysis treatment. Health Qual Life Outcomes 2012;10:71. 10.1186/1477-7525-10-71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ju A, Teixeira-Pinto A, Tong Aet al. Validation of a core patient-reported outcome measure for fatigue in patients receiving hemodialysis: the SONG-HD fatigue instrument. Clin J Am Soc Nephrol 2020;15:1614–21. 10.2215/CJN.05880420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Snyder E, Cai B, DeMuro Cet al. A new single-item sleep quality scale: results of psychometric evaluation in patients with chronic primary insomnia and depression. J Clin Sleep Med 2018;14:1849–57. 10.5664/jcsm.7478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas 1977;1385–401. 10.1177/014662167700100306 [DOI] [Google Scholar]
- 17.Björgvinsson T, Kertz SJ, Bigda-Peyton JSet al. Psychometric properties of the CES-D-10 in a psychiatric sample. Assessment 2013;20:429–36. 10.1177/1073191113481998 [DOI] [PubMed] [Google Scholar]
- 18.Lopes AA, Albert JM, Young EWet al. Screening for depression in hemodialysis patients: associations with diagnosis, treatment, and outcomes in the DOPPS. Kidney Int 2004;66:2047–53. 10.1111/j.1523-1755.2004.00977.x [DOI] [PubMed] [Google Scholar]
- 19.Phan NQ, Blome C, Fritz Fet al. Assessment of pruritus intensity: prospective study on validity and reliability of the visual analogue scale, numerical rating scale and verbal rating scale in 471 patients with chronic pruritus. Acta Derm Venereol 2012;92:502–7. 10.2340/00015555-1246 [DOI] [PubMed] [Google Scholar]
- 20.Aresté N, Sanchez-Alvarez JE, Prieto-Velasco Met al. Prevalence and severity of pruritus in Spanish patients with chronic kidney disease and impact on quality of life: a cross-sectional study. Clin Kidney J 2023;16:1035–7. 10.1093/ckj/sfac246 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.