Abstract
Gene expression analysis is a fundamental technique to elucidate the regulatory mechanisms of genes of interest or to reveal the patterns of plant response to environmental stimuli. Traditionally, gene expression analyses have required RNA extraction, followed by cDNA synthesis and qPCR analyses. However, this conventional method is costly and time-consuming, limiting the amount of data collected. The protocol outlined in this study, which utilizes a chemiluminescence system, offers a cost-effective and rapid method for assessing the expression of Arabidopsis (Arabidopsis thaliana) genes, exemplified by analyzing the nitrate-inducible expression of a major nitrate transporter gene, nitrate transporter 2.1 (NRT2.1). A reporter construct, containing the NRT2.1 promoter fused to the firefly luciferase gene, was introduced into wild-type and mutant Arabidopsis plants. Seeds obtained from the transgenic lines were grown for 3 days in 96-well microplates containing a nitrate-free nutrient solution. After 3 days, the nutrient solution was replaced with a fresh batch, which was supplemented with luciferin potassium. One hour later, nitrate was added at various concentrations, and the temporal expression pattern of NRT2.1 was analyzed by monitoring the chemiluminescence signals. This method allowed for the cost-effective, quantitative, and high-throughput analysis of NRT2.1 expression over time under the effects of various nutrient conditions and genetic backgrounds.
Key features
• Small-scale and immediate assessment of NRT2.1 promoter activity using 3-day-old Arabidopsis seedlings expressing the firefly luciferase gene under the control of the Arabidopsis NRT2.1 promoter.
• Comparison of various Arabidopsis genotypes and nutrient conditions using 96-well microplates.
• Quantitative assessment of the temporal changes in gene expression levels.
Graphical overview
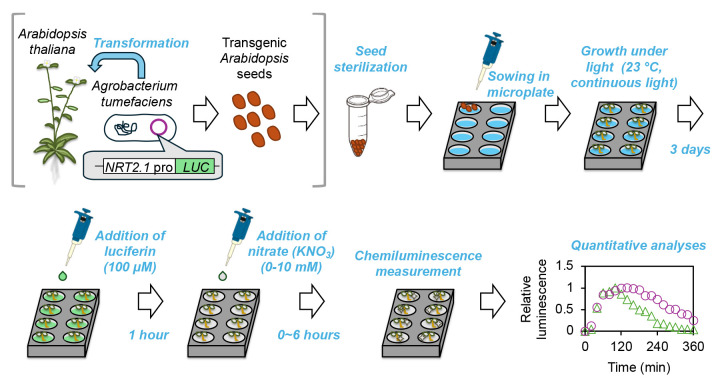
Graphical summary of the microplate-based NRT2.1 expression monitoring system in planta.
Note: The steps within gray square brackets are part of a general protocol and are not included in this manuscript.
Keywords: Arabidopsis thaliana, Nitrate, Gene expression, Luciferase, Microplate, Seedling, Temporal expression pattern
Background
Gene expression analysis is a fundamental approach used to understand the plant response to environmental stimuli and examine the effects of gene manipulation. The current method of gene expression analysis typically involves RNA extraction, cDNA synthesis, and qPCR, which often limits the number of samples that can be handled at one time. In addition, RNA extraction and purification per se typically cost >$3 per sample, potentially hindering the analysis of a large number of samples. Consequently, many laboratories perform gene expression analyses only at a few time points, which may obscure the investigation of important genes that exhibit time-dependent expression patterns owing to the effects of multiple transcriptional regulators. In such cases, analysis of gene expression at several time points is crucial for elucidating the mechanisms employed by plants to fine-tune gene expression and for understanding the role of each transcriptional regulator in this process.
Arabidopsis nitrate transporter 2.1 gene (NRT2.1), which encodes a major high-affinity nitrate transporter required for nitrate uptake by the root [1], is an example of a gene regulated by multiple transcriptional regulators. NRT2.1 expression is under the control of multiple transcription factors with antagonistic functions, including NIN-like protein (NLP) transcription factors, which act as nitrate-activated transcriptional enhancers, and nitrate-inducible GARP-type transcriptional repressor1 (NIGT1) proteins, which act as NLP-inducible transcriptional repressors [2] (Figure 1A). NRT2.1 shows a bell-shaped expression pattern upon nitrate supply [2,3], owing to its initial NLP-induced upregulation [4], followed by NIGT1-mediated repression. To understand the contribution of different transcription factors to NRT2.1 expression, it is crucial to analyze the temporal expression pattern of NRT2.1 in different genetic backgrounds, which necessitates the analysis of NRT2.1 expression in numerous samples.
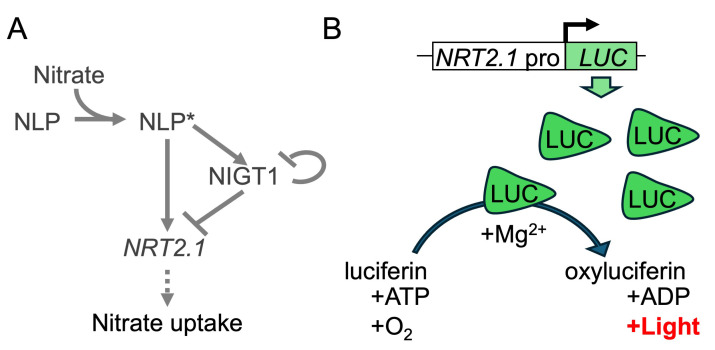
Figure 1. Regulatory mechanism of Arabidopsis NRT2.1 and the principle of luciferase (LUC) assay.
(A) The known regulatory mechanism of NRT2.1 in Arabidopsis. Nitrate directly binds to and activates NLP. Activated NLP (NLP*) directly binds to the promoters of NRT2.1 and NIGT1, enhancing their expression. Subsequently, NIGT1 suppresses the expression of not only NRT2.1 but also NIGT1 genes. (B) Mode of action of LUC. The level of LUC protein increases upon the activation of NRT2.1 promoter (NRT2.1pro). LUC protein catalyzes the conversion of luciferin to an excited state (oxyluciferin) with the aid of Mg2+. Subsequently, light is emitted during the transition of oxyluciferin to the ground state.
Reporter proteins, such as green fluorescent protein (GFP) and luciferase (LUC), are versatile biological tools that facilitate the analysis of protein localization or promoter activity without laborious procedures. Firefly-derived LUC gene is often used as a reporter gene to determine promoter activity in plant and animal cells because of its high sensitivity and linear range. To analyze the effects of transcription factors on target promoters, LUC is often expressed under the control of a given promoter in the analysis of plant genes in a protoplast-based assay system [5]. LUC has also been used in planta to analyze the temporal expression patterns of genes, such as those related to the circadian rhythm [6,7]. The abundance of LUC protein increases upon activation of the promoter regulating LUC expression. LUC protein converts its substrate, luciferin, to oxyluciferin with the help of a magnesium ion, which emits light. As long as the substrates (luciferin, ATP, and oxygen) are not depleted, the intensity of emitted light is assumed to be proportional to the amount of LUC protein, which enables the assessment of promoter activity (Figure 1B).
This protocol describes a detailed, step-by-step method optimized for a microplate-based LUC reporter assay system, which was used to evaluate the temporal activity of the NRT2.1 promoter. This rapid, cost-effective, and high-throughput method holds potential for analyzing the expression of various genes.
Materials and reagents
Biological materials
Transgenic Arabidopsis thaliana seeds harboring the LUC gene under the control of NRT2.1 promoter [2]
Reagents
Sodium hypochlorite (FUJIFILM Wako Pure Chemical, catalog number: 197-02206)
D-luciferin potassium salt (FUJIFILM Wako Pure Chemical, catalog number: 120-05114)
Potassium nitrate (FUJIFILM Wako Pure Chemical, catalog number: 160-04035)
Ammonium succinate (Kanto Chemical, catalog number: 01319-30)
Murashige and Skoog salts without nitrogen, phosphorus, and iron (FUJIFILM Wako Pure Chemical, special order)
2-Morpholinoethanesulfonic acid (MES) (Dojindo, catalog number: 343-01626)
Potassium hydroxide (FUJIFILM Wako Pure Chemical, catalog number: 168-21815)
Potassium dihydrogen phosphate (FUJIFILM Wako Pure Chemical, catalog number: 169-02425)
Iron (II) sulfate heptahydrate (FUJIFILM Wako Pure Chemical, catalog number: 094-01082)
Sucrose (FUJIFILM Wako Pure Chemical, catalog number: 196-00015)
Solutions
MES buffer (see Recipes)
10 mM iron (II) sulfate solution (see Recipes)
1 M potassium dihydrogen phosphate solution (see Recipes)
100 mM ammonium succinate solution (see Recipes)
1× Murashige and Skoog solution (N-, P-, Fe-free) (see Recipes)
0.1× N-free MS medium (see Recipes)
1 M potassium nitrate stock solution (see Recipes)
10 mM D-luciferin potassium stock solution (see Recipes)
Recipes
-
170 mM MES buffer
Note: Adjust the pH of 170 mM MES buffer to 5.7 using potassium hydroxide solution. Filter-sterilize the buffer and store at room temperature.
Reagent Final concentration Quantity or Volume 2-Morpholinoethanesulfonic acid 170 mM 3.6 g Potassium hydroxide n/a n/a Ultrapure water (Milli-Q) n/a Up to 100 mL Total n/a 100 mL -
10 mM iron (II) sulfate stock solution (10 mL)
Note: Filter the solution, aliquot into 1.5 mL tubes, and store at -20 °C to prevent precipitation.
Reagent Final concentration Quantity or Volume Iron (II) sulfate heptahydrate 10 mM 27.8 mg Ultrapure water (Milli-Q) n/a Up to 10 mL Total n/a 10 mL -
1 M potassium dihydrogen phosphate stock solution (10 mL)
Note: Autoclave or filter-sterilize the solution and store at room temperature.
Reagent Final concentration Quantity or Volume Potassium dihydrogen phosphate 1 M 1.36 g Ultrapure water (Milli-Q) n/a Up to 10 mL Total n/a 10 mL -
100 mM ammonium succinate stock solution (10 mL)
Note: Autoclave or filter-sterilize the solution and store at room temperature.
Reagent Final concentration Quantity or Volume Ammonium succinate 100 mM 0.15 g Ultrapure water (Milli-Q) n/a Up to 10 mL Total n/a 10 mL -
1× Murashige and Skoog medium (without N, P, and Fe) (1 L)
Note: To conduct studies involving nutrient-deficiency treatments, make a specialized Murashige and Skoog plant salt mixture lacking KNO3, NH4NO3, KH2PO4, and FeSO4, and store the solution at 4 °C.
Reagent Final concentration Quantity or Volume Murashige and Skoog plant salt mixture (N-, P-, and Fe-free) 1× 1 bag Ultrapure water (Milli-Q) n/a Up to 1 L Total n/a 1 L -
0.1× N-free MS medium + 500 µM ammonium succinate (100 mL)
Note: Mix all components, except iron (II) sulfate solution, and autoclave. After autoclaving, add the iron (II) sulfate solution and mix thoroughly. Store the solution at 4 °C.
Reagent Final concentration Quantity or Volume 1× Murashige and Skoog medium (without N, P, and Fe) 0.1× 10 mL Sucrose 0.5% (w/v) 0.5 g 100 mM ammonium succinate stock solution 500 µM 0.5 mL 1 M potassium dihydrogen phosphate stock solution 125 µM 12.5 µL 170 mM MES stock solution 3.5 mM 2.1 mL 10 mM iron (II) sulfate stock solution (add after autoclave) 10 µM 100 µL Ultrapure water (Milli-Q) n/a Up to 100 mL Total n/a 100 mL -
1 M potassium nitrate stock solution (10 mL)
Note: Autoclave or filter-sterilize the solution and store at room temperature.
Reagent Final concentration Quantity or Volume Potassium nitrate 1 M 1.0 g Ultrapure water (Milli-Q) n/a Up to 10 mL Total n/a 10 mL -
10 mM luciferin potassium stock solution (10 mL)
Note: Aliquot the solution into 1.5 mL tubes and store at -80 °C.
Reagent Final concentration Quantity or Volume Luciferin potassium 10 mM 31.8 mg Ultrapure water (Milli-Q) n/a Up to 10 mL Total n/a 10 mL
Laboratory supplies
Black 96-well microplates (Greiner, F-bottom, catalog number: 655077)
Transparent lids for 96-well plates (e.g., TPP, catalog number: 92696)
1.5 mL tubes (e.g., Watson, catalog number: 131-415C)
200 µL pipette tips (e.g., Watson, catalog number: 110-705C)
Surgical tape (e.g., 3M Company, catalog number: 1530-0)
Beaker
Equipment
Microplate reader (Tecan, model: Infinite M1000)
Electronic balance
Autoclave
Tabletop centrifuge
pH meter
Magnetic stirrer
Clean bench
Growth chamber (equipped with LED lights)
Pipettes (200 µL, single- and 8-channel)
Software and datasets
Magellan software (Tecan)
Microsoft Excel (Microsoft)
Procedure
-
Seed preparation
Place transgenic Arabidopsis seeds in a 1.5 mL tube.
-
Sterilize the seeds with 0.7% sodium hypochlorite solution for 5 min at room temperature.
Mix 860 µL of sterile deionized water and 140 µL of 5% sodium hypochlorite in a 1.5 mL tube.
Vigorously mix the contents of the tube using a vortex mixer.
Centrifugate briefly (~5 s) at 2,000× g and remove the supernatant with a pipette.
Rinse the seeds with 1 mL of sterile deionized water, vortex, and remove the supernatant after a brief centrifugation (~5 s at 2,000× g).
Repeat step A2d 4–5 times.
Stratify the seeds by placing them in a refrigerator (4 °C), along with a small amount of water, enough to cover the seeds, for 2–3 days.
Note: The water and pipette tips used above must be autoclaved or purchased sterile to ensure that plants stay free from infection during growth.
-
Seed sowing and plant growth
-
Add 200 µL of nutrient solution (0.1× N-free MS medium + 500 µM ammonium succinate) to each well of a 96-well microplate.
Note: Black microplates are recommended as white microplates may produce higher background signals.
Fill the gap between wells with sterile deionized water to prevent evaporation of the nutrient solution.
-
Place four seeds in each well using a pipette tip.
Note: To ensure that plants stay free from infection during growth, the above steps must be performed on a clean bench.
Cover the microplate with a transparent lid and seal the lid with surgical tape.
-
Incubate the plate under continuous light (60 µE; around 30 cm from the light source in our case) in a growth chamber maintained at 23 °C for 3 days.
To apply the phosphorus deficiency treatment, remove the nutrient solution from the wells 15 h before measurement of LUC activity; wash the seedlings with 200 µL of 0.1× N- and P-free MS medium (+500 µM ammonium succinate) once; and add 200 µL of fresh 0.1× N-free MS medium (+500 µM ammonium succinate) containing a reduced amount of phosphorus (0 or 5 µM compared with 125 µM in the control solution).
Note: Three-day-old plants were found to be optimal, 2-day-old plants produced relatively lower signal intensity, and 4-day-old plants were too tall and sometimes exceeded the well depth.
-
-
Treatment
-
Uncover the plate 1 h before treatment onset and remove the solution using an 8-channel pipette.
Note: Ensure that the seedlings are not mechanically damaged by the pipette tip.
-
Add a new nutrient solution (200 µL) containing 100 µM of luciferin potassium to each well using a single-channel or an 8-channel pipette.
In the phosphorus deficiency treatment, the phosphorus concentration in the treatment solution should remain as low as in the growth solution (0 or 5 µM compared with 125 µM in the control solution).
Cover the plate with a lid and incubate under light for 1 h in the same growth chamber set at 23 °C with continuous illumination.
Add potassium nitrate at a final concentration of 0–10 mM to each well and mix the contents of each well by pipetting with an 8-channel pipette (at least 5 times). We typically add 2 µL of potassium nitrate solution concentrated 100-fold (e.g., 2 µL of 500 mM potassium nitrate for a final concentration of 5 mM).
Note: The inclusion of appropriate controls, such as non-transgenic plants or nitrate-free medium, is recommended to account for background signals.
-
-
Measurement
Immediately after adding potassium nitrate, transfer the plate to the microplate reader.
-
Record the chemiluminescence as described below:
Shake the plate for 2 s with the linear and 3 mm settings of the instrument.
Read the chemiluminescence of each well for 1.5 s.
Notes:
i. The reading duration can be adjusted according to signal strength.
ii. No wavelength filter was applied during the luminescence measurement.
-
After recording the signal from all wells, transfer the plate back to the incubator set at 23 °C with continuous illumination.
Note: This step is necessary to maintain signal intensity over time, likely because the emission of chemiluminescence by LUC requires ATP as a substrate (Figure 1B).
Perform steps D2 and D3 at regular intervals (e.g., at 20-min intervals for a total of 360 min).
Data analysis
Export the raw values (fluorescence units) to Microsoft Excel and open the data.
Note: We typically prepare eight wells (n = 8) for each sample group and calculate the mean value. Plot the mean fluorescence units against time (Figure 2). If the values are expressed on a relative scale (e.g., 0–1 scale), consider 0 and 1 as the lowest and highest values, respectively, with other values as intermediate.
Figure 2. Summary of data analysis.
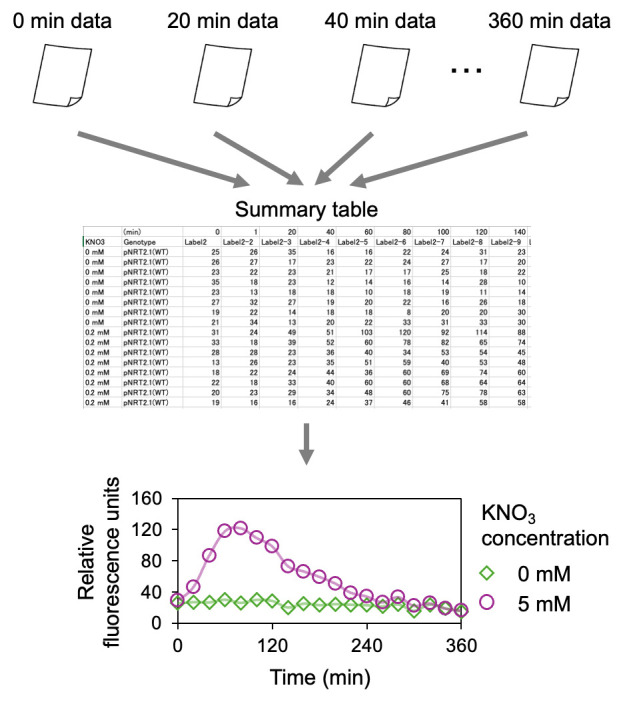
Raw data files containing luminescence signal values collected at different time points are merged, and a summary table is prepared. A mean value is calculated for each time point and nitrate condition. A graph is subsequently constructed based on the data.
Validation of protocol
This protocol, or parts of it, has been used and validated in the following research article:
General notes and troubleshooting
General notes
-
Effects of different genetic backgrounds and nutrient conditions
This method can be used to analyze the effect of nutrient signaling-related genes and nutrient conditions on gene expression. For example, in our previous study, the NRT2.1pro-LUC transgenic line was crossed with knockout mutants (such as nlp6nlp7, phr1phl1, and nigt1.1/1.2/1.3/1.4) defective in nutrient signaling. Then, plants homozygous for all intended loci were selected and analyzed [3]. The temporal expression pattern of NRT2.1 was compared between the wild-type and mutants, enabling a quantitative evaluation of the contribution of each transcription factor. Additionally, we performed a time-course analysis of the effect of phosphorus deficiency on NRT2.1 expression [3].
-
Applications in semi-quantitative imaging analysis
The promoter-LUC transgenic lines can also be used for semi-quantitative imaging analysis. Briefly, plants grown on agar plates or in liquid culture under different nutrient conditions are supplied with luciferin potassium, and chemiluminescence is detected with a CCD imager. This analysis has been adopted in our previous studies [2,8].
-
Potential delay in chemiluminescence detection
We note that the measurement of LUC activity does not account for the abundance of mRNA per se. Since the synthesis of LUC protein and decay of LUC activity do not necessarily coincide with those of NRT2.1 mRNA, there could be a slight discrepancy between LUC activity and NRT2.1 expression. In our previous mathematical modeling study, we explicitly incorporated the process of LUC activity decay (half-life 15.3 min [9]) in the formula, which resulted in a slight difference between the timing of peak LUC activity and that of maximum NRT2.1 expression [3]. Nevertheless, the correlation coefficient calculated using LUC signal intensity and experimentally determined NRT2.1 expression level was >0.95, indicating that the LUC signal could be used as a first-order approximation of NRT2.1 promoter activity under different environmental conditions or at different time points.
-
Applications in other organisms
A similar microplate-based quantitative LUC assay could likely be established for other plant species, provided the following conditions are met: (1) seeds and seedlings of the plant species fit easily within the microplate wells; and (2) growth conditions, including culture duration and the number of seeds per well, are optimized for the species of interest.
Troubleshooting
Problem 1: Signal intensities are too low.
Possible cause: Weak promoter activity or small plant size.
Solution: Try using a different promoter or larger plants and/or increase the chemiluminescence reading time.
Acknowledgments
This study was partly supported by Japan Society for the Promotion of Science (KAKENHI, grant no. 22H04977). This protocol is based on our recent publication [3].
Competing interests
The authors declare that they have no competing interests.
References
- 1. Okamoto M., Vidmar J. J. and Glass A. D. M.(2003). Regulation of NRT1 and NRT2 Gene Families of Arabidopsis thaliana: Responses to Nitrate Provision. Plant Cell Physiol. 44(3): 304-317. [DOI] [PubMed] [Google Scholar]
- 2. Maeda Y., Konishi M., Kiba T., Sakuraba Y., Sawaki N., Kurai T., Ueda Y., Sakakibara H. and Yanagisawa S.(2018). A NIGT1-centred transcriptional cascade regulates nitrate signalling and incorporates phosphorus starvation signals in Arabidopsis . Nat Commun. 9(1): 1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ueda Y. and Yanagisawa S.(2023). Transcription factor module NLP–NIGT1 fine-tunes NITRATE TRANSPORTER2.1 expression. Plant Physiol. 193(4): 2865-2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Liu K. H., Liu M., Lin Z., Wang Z. F., Chen B., Liu C., Guo A., Konishi M., Yanagisawa S., Wagner G., et al.(2022). NIN-like protein 7 transcription factor is a plant nitrate sensor. Science. 377(6613): 1419-1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yoo S. D., Cho Y. H. and Sheen J.(2007). Arabidopsis mesophyll protoplasts: a versatile cell system for transient gene expression analysis. Nat Protoc. 2(7): 1565-1572. [DOI] [PubMed] [Google Scholar]
- 6. Urquiza-García U. and Millar A. J.(2019). Expanding the bioluminescent reporter toolkit for plant science with NanoLUC. Plant Methods. 15(1): 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dixon L. E., Hodge S. K., Ooijen G., Troein C., Akman O. E. and Millar A. J.(2014). Light and circadian regulation of clock components aids flexible responses to environmental signals. New Phytol. 203(2): 568-577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ueda Y., Kiba T. and Yanagisawa S.(2020). Nitrate‐inducible NIGT1 proteins modulate phosphate uptake and starvation signalling via transcriptional regulation of SPX genes. Plant J. 102(3): 448-466. [DOI] [PubMed] [Google Scholar]
- 9. Van Leeuwen, W., Hagendoorn, M. J. M., Ruttink, T., Van Poecke, R., Van Der Plas, L. H. W. and Van Der Krol, A. R.(2000). The use of the luciferase reporter system for in planta gene expression studies. Plant Mol Biol Rep. 18(2): 143-144. [Google Scholar]