Abstract
In modern science, the exchange of scientific material between different institutions and collaborating working groups constitutes an indispensable endeavor. For this purpose, bacterial strains are frequently shipped to collaborators to advance joint research projects. Bacterial strains are usually safely shipped as cultures on solid medium, whereas the shipment of liquid cultures requires specific safety measures due to the risk of leakage. Cyanobacterial cultures are frequently maintained as liquid stock cultures, and this problem typically arises. This protocol describes a new method for the shipment of liquid cyanobacterial stock cultures by agarose gel embedding (SCAGE). More specifically, a cyanobacterial culture is mixed with low-melting agarose and cast into sterile plastic bags, resulting in a thin, solid cyanobacterial agarose gel (cyanogel) that can be easily shipped. After delivery, subsequent regeneration of the cyanogel material in liquid media results in full recovery of the examined bacterial strains. Thus, the packaging method devised in the present study comprises an innovative technique to facilitate the shipment of bacterial strains, whilst eliminating previously encountered issues like cell culture leakage.
Key features
• New packaging procedure to reduce culture leakage.
• Novel technique facilitating improved shipment conditions.
• Validated method leading to recovery of tested bacterial strains after 14 days.
Graphical overview
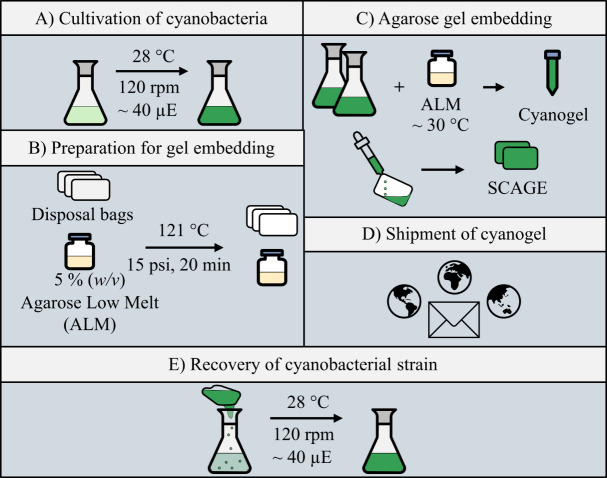
Schematic representation of steps for gel embedding and recovery of cyanobacteria
Keywords: Synechocystis sp. PCC6803 GT, Anabaena (Nostoc) sp. PCC7120, Agarose gel embedding, Shipment of cyanobacteria, Cyanobacterial culture collection
Background
Cyanobacteria are photoautotrophic microorganisms that constitute one of the biggest, most diverse genera of prokaryotes [1]. Among their many characteristics, their ability to produce oxygen and fixate carbon dioxide (CO2) has piqued the interest of scientists worldwide. In recent years, a critical need for a sustainable, CO2-neutral production platform of biofuels and chemicals has emerged and, hence, the investigation of cyanobacterial cell factories has exponentially gained significance [2–4]. Further research on these promising applications of cyanobacteria is necessary and will efficiently accelerate through collaborations between the scientific community.
The exchange of cyanobacterial strains between laboratories is a common procedure to advance joint research projects. Traditionally, bacterial strains are shipped on solid agar plates, in liquid cultures, or cryopreserved and shipped on dry ice. Solid agar plates are effective at preventing leakage but are prone to desiccation, particularly during prolonged shipment. Liquid cultures are suitable for short-term transport but require more complex packaging to prevent leakage. Prolonged shipment under the abovementioned conditions increases the risk of loss of viability of cyanobacterial cells. Cryopreservation is widely used for the long-term preservation of eukaryotic cell lines [5], bacteria, and cyanobacteria [6]; however, shipping cryopreserved samples requires specialized equipment and incurs high costs. Additionally, the shipment of samples on dry ice is prohibited in more than 50% of countries worldwide [7]. Moreover, depending on the cyanobacterial strain, the “reawakening” process can take up to 30 days [6].
Herein, we describe a shipment method for cyanobacteria that increases shipment safety and maintains cells for prolonged times in a viable state by embedding the bacterial strain in an agarose matrix (shipment of cyanobacteria by agarose gel embedding, SCAGE). This agarose gel (cyanogel) is inherently unlikely to display leakage due to the solid state of the material. Moreover, the risk of bacterial desiccation or damage to bacterial vials is greatly reduced due to the embedment in a sterile sealed bag. The casting of thin cyanogels allows for low-cost, efficient shipment in padded envelopes. Furthermore, cyanobacterial cells remain viable and can be successfully recovered after 14 days. A recent study also demonstrated the effective transportation of eukaryotic cell lines using agarose gel-based preservation [8]. This protocol highlights the casting of cyanogels and the subsequent recovery procedure for cyanobacteria.
Materials and reagents
Biological materials
Synechocystis sp. PCC6803, glucose-tolerant (GT) [9]
Anabaena (Nostoc) sp. PCC7120 [10]
Reagents
NaNO3 (Sigma-Aldrich, CAS: 7631-99-4)
K2HPO4 (water-free) (Sigma-Aldrich, CAS: 7758-11-4)
MgSO4·7H2O (AppliChem, CAS: 10034-99-8)
CaCl2·2H2O (Merck, CAS: 10035-04-8)
Na2-EDTA·2H2O (Merck, CAS: 6381-92-6)
Na2CO3 (Merck, CAS: 497-19-8)
Fe(III)-citrate (Sigma-Aldrich, CAS: 3522-50-7)
Citric acid (Roth, CAS: 77-92-9)
H3BO3 (Merck, CAS: 10043-35-3)
MnCl2·4H2O (Roth, CAS: 13446-34-9)
ZnSO4·7H2O (Fluka, CAS: 7758-99-8)
Na2MoO4·2H2O (Sigma-Aldrich, CAS: 10102-40-6)
CuSO4·5H2O (Fluka, CAS: 7758-99-8)
Co(NO3)2·6H2O (Sigma-Aldrich, CAS: 10026-22-9)
NaHCO3 (Fisher Chemica, CAS: 144-55-8)
Agarose low melt (Roth, CAS: 39346-81-1)
Solutions
BG11 medium (see Recipes)
5% (w/v) agarose low melting (ALM) solution (see Recipes)
Recipes
-
BG11 medium (modified after Rippka et al. [11]):
Prepare 200 mL of each stock solution (1–7) from Table 1, 100 mL of trace element solution (Table 2), and 50 mL of 1 M NaHCO3 solution (Table 3). Use Milli-Q water for each solution.
-
Stock solutions 1–7 and the trace element solution are sterilized by autoclaving (121 °C, 15 psi, 20 min), while the 1 M NaHCO3 solution is sterilized by filter sterilization (0.22 μm).
Note: Store solutions 1–6 at room temperature and solution 7 [Fe(III)-citrate & citric acid] protected from light at room temperature. Store trace solution at 4 °C and 1 M NaHCO3 at -20 °C.
-
To prepare 1 L of BG11 (1×), add 5 mL of each stock solution 1–7 (200×), 1 mL of the trace element solution (1,000×), and up to 995 mL of Milli-Q water. Sterilize by autoclaving (121 °C, 15 psi, 20 min). Add 5 mL of 1 M NaHCO3 solution before use.
Note: For the modified BG11 medium, conduct the following adjustments:
Na2CO3: 0.04 g/L (originally 0.02 g/L).
Ferric ammonium citrate was replaced by Fe(III)-citrate.
-
5% (w/v) agarose low melting (ALM) solution
To prepare 100 mL of 5% (w/v) ALM solution, add 5 g of ALM in 100 mL of BG11 medium. Sterilize by autoclaving (121 °C, 15 psi, 20 min). Store at room temperature.
Table 1. Stock solutions (200×) for BG11 medium .
| Solution | Chemical | Molar mass M (g/mol) | Molarity c (mM) in 200× stock solution | Mass concentration β (g/L) in 200× stock solution | Molarity c (mM) 1× medium | Mass concentration β (g/L) in 1× medium | Mass m (g) in 200 mL stock solution |
|---|---|---|---|---|---|---|---|
| 1 | NaNO3 | 84.99 | 35230 | 300 | 17.65 | 1.5 | 60 |
| 2 | K2HPO4 (water-free) | 174.18 | 35.9 | 6.25 | 0.18 | 0.03125 | 1.25 |
| 3 | MgSO4·7H2O | 246.48 | 60.9 | 14.8 | 0.3 | 0.075 | 3 |
| 4 | CaCl2 ·2H2O | 147.02 | 49.0 | 7.2 | 0.24 | 0.036 | 1.44 |
| 5 | Na2-EDTA·2H2O | 372.24 | 0.5 | 0.2 | 0.003 | 0.001 | 0.04 |
| 6 | Na2CO3 | 105.99 | 75.5 | 8 | 0.38 | 0.04 | 1.6 |
| 7 | Fe(III)-citrate | 244.94 | 4.9 | 1.2 | 0.024 | 0.006 | 0.24 |
| 8 | Citric acid | 192.13 | 6.2 | 1.2 | 0.031 | 0.006 | 0.24 |
Table 2. Trace element solution (1,000×) for BG11 medium.
| Chemical | Molar mass M (g/mol) | Molarity c (mM) in 1,000× stock solution | Mass concentration β (g/L) in 1,000× stock solution | Molarity c (μM) 1× medium | Mass m (mg) in 100 mL stock solution |
|---|---|---|---|---|---|
| H3BO3 | 61.83 | 46.26 | 2.86 | 46.26 | 286 |
| MnCl2·4H2O | 197.91 | 9.15 | 1.81 | 9.15 | 181 |
| ZnSO4·7H2O | 287.54 | 0.77 | 0.222 | 0.77 | 22.2 |
| Na2MoO4·2H2O | 241.95 | 1.61 | 0.39 | 1.61 | 39 |
| CuSO4·5H2O | 249.68 | 0.32 | 0.079 | 0.32 | 7.9 |
| Co(NO3)2·6H2O | 291.03 | 0.17 | 0.0494 | 0.17 | 4.9 |
Table 3. 1 M NaHCO3 stock solution for BG11 medium.
| Chemical | Molar mass M (g/mol) | Molarity c (mM) | Mass concentration β (g/L) in 1 M stock solution | Molarity c (mM) 1× medium | Mass concentration β (g/L) in 1× medium | Mass m (g) in 100 mL stock solution |
|---|---|---|---|---|---|---|
| NaHCO3 | 84.01 | 1,000 | 84 | 5 | 0.42 | 8.4 |
Laboratory supplies
Pipette tips with filter (Mettler Toledo, type RAININ)
Serological pipette, 5 mL (Sarstedt, catalog number: 86.1253.001)
Conical centrifuge tubes, 15 mL (Sarstedt, catalog number: 62.554.502)
Disposal bags (Roth, catalog number: E706.1)
Grease-proof paper Profissimo (DM-Drogerie Markt, catalog number: 470799)
Rubber band “Alco 758” 80 × 4 mm (Lyreco, catalog number: 5.112.077)
Steristopper (VWR, catalog number: HERE1013700)
Plastic cuvettes (Sarstedt, catalog number: 67.742)
Erlenmeyer wide-neck flask DIN ISO 24450, 100 mL (VWR, catalog number: 214-1131)
Laboratory bottle, 1 L (VWR, catalog number: 215-1517P)
Beaker, 800 mL (Roth, catalog number: X694.1)
Aluminum foil (Roth, catalog number: 1399.1)
Sterile indicator strip (Roth, catalog number: XC20.1)
Multimark overhead marker permanent (Faber-Castell, catalog number: 151304)
Equipment
Pipettes (Mettler Toledo, model: Pipet-Lite XLS series)
Pipetboy (VWR, catalog number: 613-4438)
Rotary shaker (GFL, catalog number: GFL-3017)
OSRAM LUMILUM DE LUX Daylight lamp (OSRAM, catalog number: L58W/954)
Biosafety cabinet (Bioquell, model: ABS1500CLS2-MK2)
Microwave (OK, model: OMW 330 D-M)
Centrifuge 5804 R (Eppendorf, catalog number: 5804 R)
Centrifuge rotor S-4-72 (Eppendorf, catalog number: S-4-72)
Photometer (Thermo Scientific, model: Spectronic Helios model δ)
Foil welding apparatus (BOSCH, catalog number: FG16-0717901002)
Procedure
Note: All steps should be carried out under sterile conditions.
-
Cultivation of cyanobacteria
Put the Steristopper (paper plug) on top of clean Erlenmeyer flasks and cover the Erlenmeyer flasks with grease-proof paper. Secure the grease-proof paper with a rubber band.
Sterilize the prepared Erlenmeyer flask by autoclaving (121 °C, 15 psi, 20 min) and let it cool to room temperature.
Open the Erlenmeyer flask under sterile conditions and inoculate Synechocystis sp. PCC6803 GT or Anabaena (Nostoc) sp. PCC7120 in 50 mL of BG11 medium. Close the Erlenmeyer flask after inoculation.
Grow Synechocystis sp. PCC6803 GT or Anabaena (Nostoc) sp. PCC7120 under continuous light illumination (~ 40 μmol m-2 s-1) at 120 rpm and 28 °C until the stationary phase (OD750 > 4) is reached.
-
Preparation for gel embedding of cyanobacteria
Sterilize disposable bags in a beaker covered with aluminum foil by autoclaving (121 °C, 15 psi, 20 min) and let them cool down to room temperature.
Prepare 5% (w/v) ALM solution (see Recipes).
-
Gel embedding
Collect 15 mL of liquid bacterial culture in a conical reaction tube and harvest cells by centrifugation at 4000× g for 10 min at room temperature. Discard the supernatant and resuspend cells in 2 mL of BG11 medium.
-
Simultaneously, heat the 5% (w/v) ALM suspension until a clear, homogenous solution is obtained.
Caution: The flask containing the ALM solution will be hot and even a boiling delay could be observed. Open the cap of the bottle slightly. Use appropriate safety measures to prevent burning yourself.
Let the solution cool down to approximately 30 °C under the sterile hood.
-
Once the appropriate temperature is reached, add 3 mL of the 5% (w/v) ALM solution to the resuspended cyanobacterial strain (obtained in step C1) and mix through careful pipetting, resulting in the cyanogel.
Note: Re-use the serological pipette to mix the components to save time before the cyanogel is solidified. The concentration of ALM in the resulting cyanogel will be 3% (w/v).
Open the disposable bag under sterile conditions and transfer the cyanogel into the bag by slow and careful pipetting.
Situate the disposable bag containing the gel on a flat surface and apply a slight amount of pressure with your hands on the upper side of the bag to evenly distribute the cyanogel within the bag. A smooth plane should be obtained (8 cm × 20 cm × ≤1 mm), which can then be sealed with the foil welding machine (section D).
-
Sealing the cyanogel
-
Place the bag between the contacts of a foil welding machine and close the machine for 3 s.
Caution: Be cautious when pressing the machine down since the contacts will get hot. Take measures to prevent burning yourself.
Open the apparatus again and wait for 5 s.
Note: Removing the cyanogel before letting it cool may result in deformation or breakage of the bag. Usually, 5 s is enough to let the sealed bag cool down.
Label the bag appropriately, e.g., with a permanent marker, for later identification of the cyanobacterial strain contained within.
-
-
Packaging of embedded cyanobacteria
-
Package the embedded cyanobacteria in an appropriate container (e.g., an envelope) and send it to the location of your choice.
Note: Depending on the destination of your cyanobacterial samples, different regulations regarding labeling may apply.
-
-
Recovering of cyanobacteria out of the cyanogel
Prepare 50 mL of BG11 medium in an Erlenmeyer flask under sterile conditions.
Open the received cyanogel and squeeze the gel out of the disposal bag into the BG11 medium.
-
Note: Use a sterile pair of scissors for cutting an edge of the cyanogel for easier and more precise gel squeezing.
Let the cyanobacteria grow under dimmed light conditions (flask wrapped with a paper towel) of ~20 µmol m2·s1 at 120 rpm for 24 h at 28 °C.
After 24 h of incubation, remove the paper towel and cultivate the cyanobacteria under standard cultivation conditions.
Validation of protocol
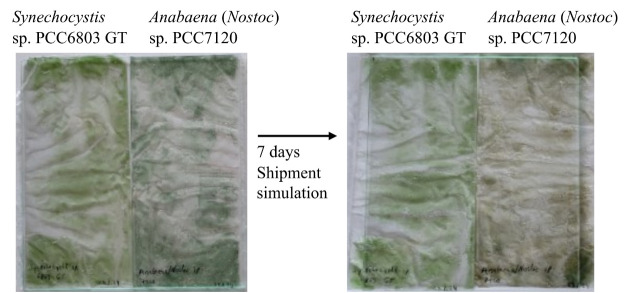
For validation of the protocol presented here, the procedure was performed with two different cyanobacterial strains [Synechocystis sp. PCC6803 and Anabaena (Nostoc) sp. PCC7120]. Synechocystis and Anabaena (Nostoc) were cultivated under standard conditions until an OD750 of 9.3 and 5.2 was reached, respectively. 25 mL of culture was centrifuged and resuspended in 8 mL of BG11 medium; 2 mL of resuspended cyanobacteria were mixed with 3 mL of BG11 medium and used as a control group. Triplicates of 2 mL culture were mixed with 3 mL of 5% (w/v) ALM and cast into disposable bags. Bags were sealed with a foil welding machine and were stored for one week in a non-transparent, padded envelope at room temperature in the dark to simulate packaging shipment (Figure 1).
Figure 1. Disposal bags filled with cyanobacteria.
Left: Synechocystis sp. PCC6803 GT; right: Anabaena (Nostoc) sp. PCC7120 embedded in 5% (w/v) ALM [cyanogel, end concentration 3% (w/v) ALM] before and after shipment simulation.
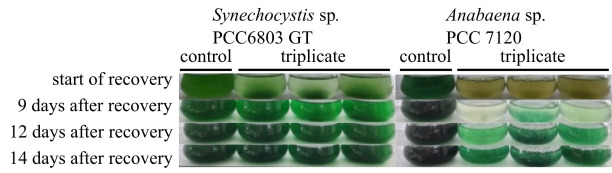
After one week of shipment simulation, regeneration of cyanobacterial strains was performed according to the described method. Full recovery of both strains was reached after 14 days of cultivation ( Figure 2 ).
Figure 2. Recovery of cyanobacteria over 14 days.
Left: Synechocystis sp. PCC6803 GT; right: Anabaena (Nostoc) sp. PCC7120.
Acknowledgments
This project was conducted with the support of the Korea Evaluation Institute of Industrial Technology (KEIT) grant funded by the Korean government (MOTIE) (No. RS-2022-00155902) and Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2021R1I1A3055799).
Competing interests
The authors have no conflicts of interest to declare.
References
- 1. Stanier R. Y. and Cohen-Bazire G.(1977). PHOTOTROPHIC PROKARYOTES: THE CYANOBACTERIA. Annu Rev Microbiol. 31(1): 225 274 274. 10.1146/annurev.mi.31.100177.001301 [DOI] [PubMed] [Google Scholar]
- 2. Clark J. H., Luque R. and Matharu A. S.(2012). Green Chemistry, Biofuels, and Biorefinery. Annu Rev Chem Biomol Eng. 3(1): 183 207 207. 10.1146/annurev-chembioeng-062011-081014 [DOI] [PubMed] [Google Scholar]
- 3. Gao X., Sun T., Pei G., Chen L. and Zhang W.(2016). Cyanobacterial chassis engineering for enhancing production of biofuels and chemicals. Appl Microbiol Biotechnol. 100(8): 3401 3413 3413. 10.1007/s00253-016-7374-2 [DOI] [PubMed] [Google Scholar]
- 4. Wirth T. E., Gray C. B. and Podesta J. D.(2003). The Future of Energy Policy. Foreign Affairs. 82. http://dx.doi.org/10.2307/20033654
- 5. Heydarzadeh S., Kheradmand Kia S., Boroomand S. and Hedayati M.(2022). Recent developments in cell shipping methods. Biotechnol Bioeng. 119(11): 2985 3006 3006. 10.1002/bit.28197 [DOI] [PubMed] [Google Scholar]
- 6. Esteves-Ferreira A. A., Corrêa D. M., Carneiro A. P. S., Rosa R. M., Loterio R. and Araújo W. L.(2012). Comparative evaluation of different preservation methods for cyanobacterial strains. J Appl Phycol. 25(4): 919 929 929. 10.1007/s10811-012-9927-9 [DOI] [Google Scholar]
- 7. FedEx(2024). Dangerous Goods- Countries and Territories Served. FedEx. Retrieved August 28, 2024, from https://www.fedex.com/en-us/service-guide/dangerous-goods/international-locations.html
- 8. Yang L., Li C., Chen L. and Li Z.(2009). An Agarose-Gel Based Method for Transporting Cell Lines. Curr Chem Genomics. 3: 50 53 53. 10.2174/1875397300903010050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kaneko T., Sato S., Kotani H., Tanaka A., Asamizu E., Nakamura Y., Miyajima N., Hirosawa M., Sugiura M., Sasamoto S., et al.(1996). Sequence Analysis of the Genome of the Unicellular Cyanobacterium Synechocystis sp. Strain PCC6803. II. Sequence Determination of the Entire Genome and Assignment of Potential Protein-coding Regions. DNA Res. 3(3): 109 136 136. 10.1093/dnares/3.3.109 [DOI] [PubMed] [Google Scholar]
- 10. Kaneko T.(2001). Complete Genomic Sequence of the Filamentous Nitrogen-fixing Cyanobacterium Anabaena sp. Strain PCC 7120. DNA Res. 8(5): 205 213 213. 10.1093/dnares/8.5.205 [DOI] [PubMed] [Google Scholar]
- 11. Rippka R., Stanier R. Y., Deruelles J., Herdman M. and Waterbury J. B.(1979). Generic Assignments, Strain Histories and Properties of Pure Cultures of Cyanobacteria. Microbiology(N Y). 111(1): 1 61 61. 10.1099/00221287-111-1-1 [DOI] [Google Scholar]