Abstract
This study reports the demulsification activity of a newly developed nonionic demulsifier (NID) via the condensation of glycolic acid ethoxylate lauryl ether with amine. The demulsification performance of the developed NID was assessed under room and moderate temperatures (25 and 60 °C), while the concentrations of NID were varied from 100 to 700 ppm at both temperatures in order to observe their oil–water separation efficiency. The demulsification mechanism was expatiated by determining the viscosity and elastic modulus of emulsion in the presence and absence of the NID. Adsorption at the oil and water interface was analyzed through a series of interfacial tension measurements. Accordingly, under the aforementioned temperatures, the optimal demulsification efficiency of the NID was 95% (25 °C) and 99% (60 °C) at 500 ppm. Viscosity determination at both temperatures revealed a drastic reduction in emulsion viscosity in the presence of NID, and the viscosity drop was of high magnitude at moderate temperatures (60 °C). Likewise, the elastic modulus measurements in bulk rheology revealed that the presence of NID in the emulsion weakened the elastic strength. Again, the interfacial modulus test exhibited the percolation of NID at the oil–water interface and the displacement of asphaltenes. Interfacial tension (IFT) measurements of the oil–water system at different NID concentrations showed that the particles were adsorbed at the oil–water interface. The IFT values of the oil–water system in the presence of NID ranged from 1.84 to 3.02 mN/m as compared to that of the NID-free oil–water system recorded as 16.11 mN/m. It is envisaged that this new nonionic demulsifier would be very useful in oilfields and petrochemical industries.
1. Introduction
Crude oil emulsions are inevitably a serious challenge in the petroleum industry, especially during hydrocarbon exploitation and processing. Owing to the presence of solid particles, resins, asphaltenes, and other natural substances in crude oil, the formation of oil emulsions becomes feasible in the course of mining, processing, and transportation.1 However, the corrosive implications of emulsions on equipment, pipeline damage, and increased operational cost necessitate the need to separate water at upstream prior to the conveyance of crude oil to the refining plant for further processing.2
Demulsification in the petroleum industry refers to the means or technique applied to effectively separate water and crude oil from the produced oil emulsion, such that the crude oil transported through pipelines to the refinery contains a minimal amount of water. The notable applied demulsification techniques are primarily physical, biological, and chemical.3−5 Physical demulsification techniques include microwave irradiation,6,7 gravitational settling,8 membrane separation,9−11 filtration,12 and ultrasonic method.13−15 Biological demulsification involves the use of bacteria to demulsify oil emulsions aided by bacteria cells or extracellular compounds.16 Chemical demulsification is one of the techniques employed in the petroleum and petrochemical industries to break crude emulsions and emulsified oil–water mixtures, and this method is known to be efficient for lowering the emulsion stability and enhancing the film-thinning by using chemicals called demulsifiers. These chemical demulsifiers are amphiphilic and neutralize the emulsion stability by absorbing at the oil–water interface and modifying the interfacial properties.17 Chemical demulsification is composed of two mechanisms: the demulsifier demonstrates a high interfacial activity and migrates to the oil–water interface, and the demulsifier displaces the interfacial films and promotes film drainage by lowering the tension gradient.17 Eventually, oil–water separation takes place via coalescence or flocculation.
Numerous research activities have been conducted by many researchers to develop varieties of chemical demulsifiers.18−24 Among the notable chemical demulsifiers in the petroleum and petrochemical industries are ionic liquids.25−35 In a demulsification study by Qu et al.,2 two unique ionic liquids (PPBD and PPBH) with four ionic centers and four hydrophobic branches were synthesized for oil–water emulsion separation at a low demulsification temperature (40 °C). The outcome of their work showed that the addition of 250 mg/L of PPBH and PPBD in separate emulsions resulted in 96.34 and 95.23% demulsification efficiency (DE), respectively, at 40 °C. In another investigation by Lei and his colleagues,36 a Gemini ionic liquid (PGE-TFA) with interfacial activity was synthesized for breaking crude oil emulsions. The 500 mg/L PGE-TGA achieved about 95.53% oil–water separation within 90 min at 40 °C. At elevated demulsification temperature (70 °C), they reported that 500 mg/L PGE-TGA could only achieve a slight increase in demulsification efficiency (97.72%). Also, ethylene oxide,22,23,32,37,38 propylene oxide,22,23,30,37,39−41 and their derivatives are very prominent among other ionic liquid demulsifiers. Several literature studies have assessed the demulsification performances of these ionic liquids in the presence of different water types,42−44 at various temperatures,2,22,23,45 in the presence of salts,45−48 and the impact of acidic and alkaline conditions23,36,45,49,50 on the activity of these demulsifiers. However, one of the key salient issues with some, if not many, ionic liquids is their poor demulsification activity at room temperature (e.g., 20–25 °C) and temperatures within 60 °C.22 Besides, the cost implication and toxicity concerns of ionic liquids are becoming a high priority in oilfields coupled with their tedious and hazardous preparation steps. Owing to these alarming factors, researchers are intensifying tremendous efforts to design new and efficient chemical demulsifiers that would be cost-effective, eco-friendly, and efficiently separate oil and water at low and moderate temperatures.
In this study, a nonionic demulsifier (NID) was developed by condensation of glycolic acid ethoxylate lauryl ether with amine. The use of such nonionic demulsifiers has not been extensively studied in the demulsification of crude oil emulsions. In demulsification research that was carried out by Zhang et al.,1 an oxygen-enriched nonionic demulsifier was developed, and the authors claimed this nonionic demulsifier achieved 100% dehydration in less than 25 min. Thus, the present study reports the demulsification performance of an in-house developed nonionic chemical demulsifier. The synthetic route and chemical structure characterization of this demulsifier have already been reported in our preceding report.51 This demulsifier has not been reported in the literature for demulsification application, and this is the first report on the use of this chemical as a demulsifier of crude oil emulsion. In addition, this laboratory-grown nonionic demulsifier is eco-friendly, less toxic, and inexpensive, and its oil–water efficiencies are comparable to many other chemical demulsifiers.22,24,49,50,52,53 In this work, stable emulsions were produced from crude oil and laboratory-synthesized seawater, and the demulsification performance of this new chemical demulsifier was tested at various concentrations and at room and moderate temperatures (25 and 60 °C). The demulsification mechanism of this nonionic demulsifier was studied via rheological means under the aforementioned temperatures by measuring the rheological properties of the produced emulsions in the presence and absence of the synthesized demulsifier. By doing so, the rheological measurements shed light on how this new demulsifier was able to diffuse and displace the emulsifiers at the interface of oil and water. Likewise, the interfacial tension (IFT) measurements were performed at various concentrations of this nonionic demulsifier (NID) in order to assess its migration and aggregation at the oil–water interface. It is envisaged that this NID would be very useful in the treatment of emulsions in the petroleum and petrochemical industries.
2. Experimental Section
2.1. Materials
At 15 °C, the oil possesses an API gravity of 32.5 and viscosity of 10.9 mPa.s. The oil contains saturates (36.2%), asphaltenes (2.8%), resins (11%), and aromatics (50%). This crude oil is obtained from one of the oilfields in Saudi Arabia. The laboratory-produced seawater was prepared from the following salts: Na2SO4, NaCl, MgCl2, NaHCO3, and CaCl2, with a total of 57,700 mg/L salinity (Table 1). This particular seawater concentration was used because oilfields have high salinity during crude oil processing. So, this concentration was used to mimic the oilfields scenario. The precursor chemicals and reagents used in the synthesis of the nonionic demulsifier (NID) were supplied by Sigma-Aldrich. The precursor chemicals and reagents used in the synthesis of the nonionic demulsifier (NID) such as glycolic acid ethoxylate lauryl ether (CAS 220622-96-8) were supplied by Sigma-Aldrich.
Table 1. Ions in the Laboratory-Synthesized Seawater.
| ions | concentration (mg/L) |
|---|---|
| Na+ | 18,300 |
| Ca2+ | 700 |
| Mg2+ | 2100 |
| SO2–4 | 4300 |
| Cl– | 32,200 |
| HCO–3 | 100 |
| total | 57,700 |
2.2. Emulsification and Demulsification Methods
To produce a stable crude oil emulsion, seawater was added systematically inside a beaker filled with a determined amount of crude oil. A 30 mL portion of water was added gradually into 20 mL of crude oil and agitated under a constant speed of 1100 ppm. Prior to mixing, formation water in crude oil was removed using the centrifuge. The mixing proceeded until a single phase was detected after 1 h. The produced emulsion was examined under ambient conditions for 6 weeks to ensure that there was no phase separation. After 6 weeks of preparation, there was no noticeable oil/water separation. A bottle test procedure was utilized to investigate the demulsification activity of the synthesized NID. Then, six g (6 g) of NID was dissolved in a 50 mL bottle containing 14 g (14 g) of 2-propanol, making the bulk demulsifier solution 30 wt %. Afterward, various concentrations of the NID ranging from 100, 300, 500, and 700 ppm were formulated and injected into vials containing the produced emulsions and tightly covered. The demulsification performance was thereafter studied at room temperature (25 °C) and inside the oven at moderate temperature (60 °C). Besides, a blank emulsion sample without the NID was prepared as a control sample. All samples were hand shaken for 10 s, and oil/water separation was monitored periodically. The demulsification efficiency of NID at various concentrations was evaluated using the formula54 shown in eq 1. The demulsification rate describing the extent of water removal from emulsion with respect to time was determined by eq 2.
| 1 |
| 2 |
where V is the volume of separated water, Vo is the volume of initial water in the produced emulsion, and t is the periodic settling time (min) of the separated water.
2.3. Synthesis of Nonionic Demulsifier (NID)
A round-bottom flask fitted with a condenser was placed on a magnetic stirrer and filled with a 1:1.5:0.1 ratio of glycolic acid ethoxylate lauryl ether, amine, and sodium fluoride, respectively. The reaction progressed for 12 h at 160 °C with a continuous flow of argon. Eventually, the extra amine was evaporated by reduced pressure, and sodium fluoride was removed by filtration to obtain the nonionic demulsifier (NID), as shown in Scheme 1. The Fourier transform infrared (FTIR) spectrum (Figure 1a) of NID revealed an adsorption band at 3412 cm–1 that corresponds to the N–H stretching vibration. The resonating peak that appeared at 1658 cm–1 was mainly due to the stretching vibration of the amide group. Moreover, the CH2 bending vibration was observed at 1460 cm–1. Two distinguished signals were noticed at 2921 and 2855 cm–1 and assigned to the asymmetric and symmetric stretching vibrations of the C–H bond in the aliphatic tail. The prominent sharp peak at 1097 cm–1 is connected to the ether (C–O–C) group. Represented in Figure 1b is the thermal analysis of NID, as determined by the thermogravimetric analysis (TGA). The initial weight losses at temperatures less than 100 °C are invariably due to the evaporation of residual moisture, while the sharp weight loss at 298 °C above exhibited the temperature effect on the structural degradation of NID. Since the demulsification activities in several oilfields are carried out at 50–100 °C, NID would withstand these temperatures without quick degradation. Regarding the 1H NMR of NID (Figure 1c), the signals at δ 0.88 ppm are the single methyl group at one end, and the peak at δ 2.33 ppm is the two methyl groups at the second end attached to tertiary nitrogen. The signals of multiple –CH2– groups of the lipophilic tail were detected at δ 1.25 ppm. The prominent large peak at δ 3.67 ppm was connected to the ethoxy (–C–C–O–) groups, and the proton of the amide group was observed at δ 7.56 ppm. Represented in Figure 1d is the 13C NMR of NID in which the peak at δ 14.0 ppm is the methyl group at one end, and the peak at δ 45.3 ppm is the two methyl groups at the second end attached to tertiary nitrogen. The repeating –CH2– units were resonated at δ 29.4 ppm, and the repeating ethoxy groups were observed at δ 70.4 ppm. The carbonyl carbon of amide groups was detected at δ 169.8 ppm. According to the electrospray ionization mass spectrometry (ESI-MS) data of NID (Figure 1e), the base peak at m/z 813.6 seems to agree with the chief component being NID where a = 11 and b = 11. The following peaks before and after the base peak were the distribution of the ethoxy groups having a mass difference of (m/z 44), which is the mass of one ethoxy group.
Scheme 1. Synthetic Route of the Developed Nonionic Demulsifier (NID).
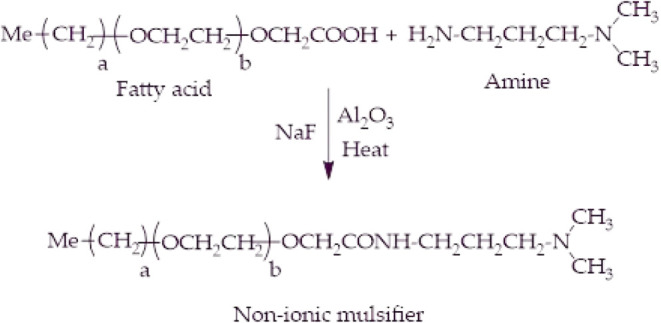
Figure 1.
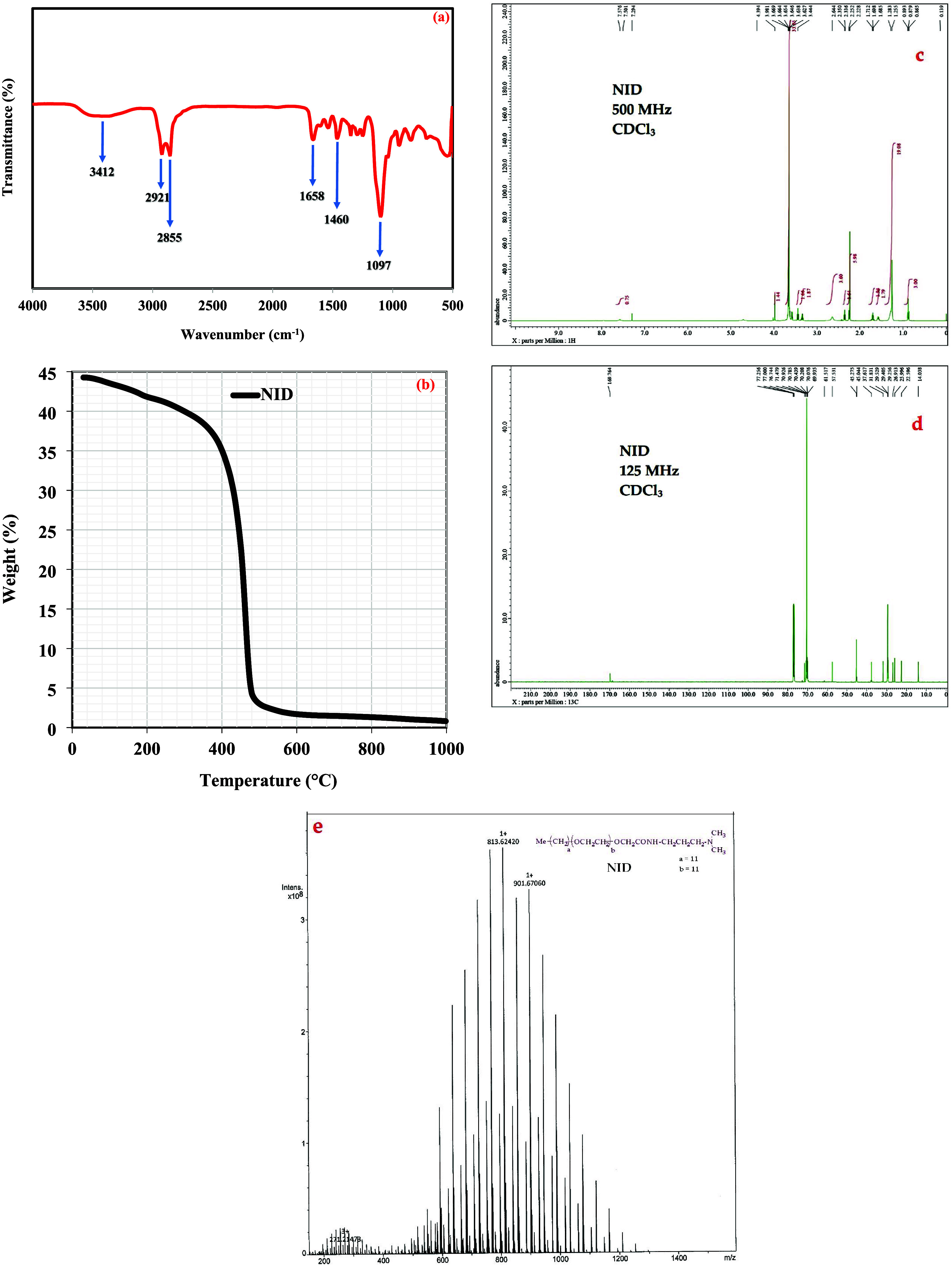
(a) FTIR spectrum, (b) TGA curve, (c) 1H NMR spectra, (d) 13C NMR spectra, and (e) ESI-MS analysis of the NID developed.
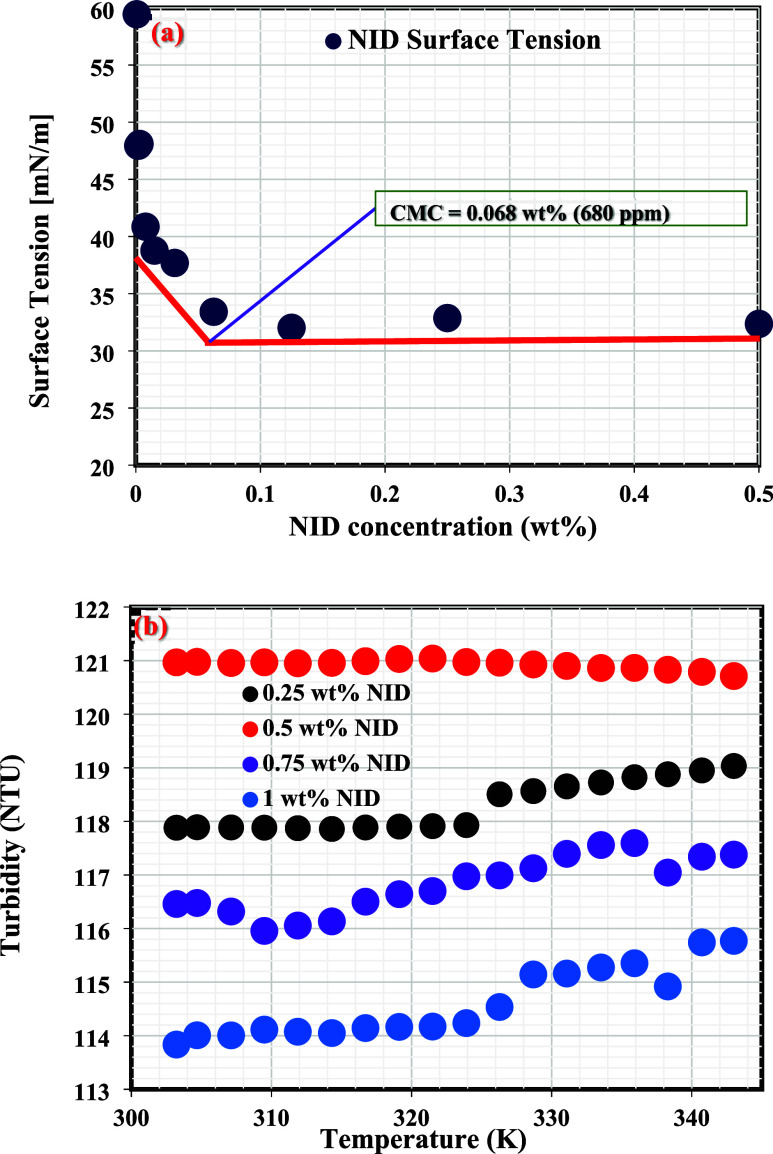
Figure 2a shows the surface tension of the NID aqueous solution measured at various concentrations. The data plot revealed a reduction in surface tension with the increasing concentration of NID until the critical micelle concentration (cmc) is reached at 0.068 wt % (680 ppm). Following the cmc point, the air/water interface would be surrounded by NID molecules, and a further increase in the NID concentration would therefore not have any effect on the surface tension. Figure 2b presents the turbidity of the NID aqueous solution at varying concentrations under different temperatures. The NID turbidity level at 0.25 wt % was nearly constant until 324 K, and a fair increment in turbidity was then experienced up to 342 K. At 0.5 wt % NID, the turbidity was almost constant throughout the entire temperature; however, the turbidity level of NID at 0.5 wt % was higher than that of 0.25 wt %, suggesting enhanced solubility of NID at 0.5 wt % and the formation of micelles.1 However, a reduction in the turbidity set in as the NID concentration was increased to 0.75 wt %, and this reduction intensified at 1 wt % NID across all of the temperatures. This observation suggests a decrease in the solubility of NID molecules in water since 0.75 wt % and above already surpassed the cmc55 of the developed NID in aqueous solution.
Figure 2.
(a) Surface tension of NID aqueous solution measured at room temperature (25 °C). (b) Turbidity curves of NID aqueous solution at different concentrations and temperatures.
2.4. Characterizations
The investigation of emulsion morphology was performed using a Leica DM2000 microscope to ensure accurate analysis. The chemical composition of the NID was elucidated through Fourier transform infrared (FTIR) spectroscopy. The thermal resilience of the chemical demulsifier was determined by employing a thermogravimetric analyzer (TGA) from TA Instruments.
Bulk rheology of produced emulsions and oil–water interfacial modulus measurements were carried out using a TA rheometer (DHR-3). For emulsion viscosity determinations, a fixed shear rate of 0.01 s–1 was applied, while storage modulus measurements were conducted at a 5% strain, depicting the region of linear viscoelasticity. The concentric cylinder geometry was used during bulk rheology tests, while double wall ring (DWR) interface geometry was employed for oil–water interfacial modulus measurements.
To evaluate the NID adsorption at the interface of oil and water, the surface activity described as the surface tension and interfacial tension (IFT) was performed at room temperature (25 °C) by using the Biolin Scientific machine. The interfacial tension (IFT) values were also determined for the oil–water system without a NID (blank sample). The turbidity measurement of NID aqueous solution at various concentrations was determined using the Data Physics Multi Scan 20 stability analysis system. A vial filled with NID aqueous solution was placed in the scan tower of the system. The turbidity was calculated from the obtained transmission and backscattering intensity at the specified temperatures (300 to 343 K). The unit of turbidity was recorded as the nephelometric turbidity unit (NTU).
3. Results and Discussion
3.2. Characterization of the Produced Emulsion
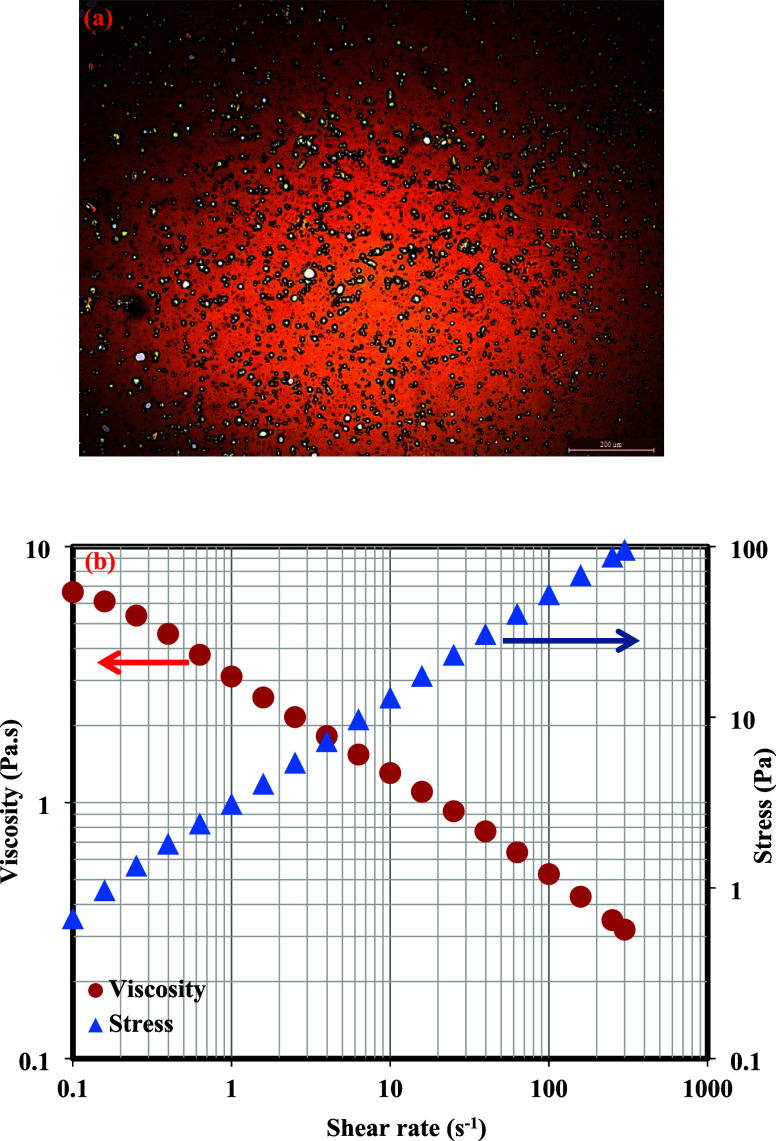
Emulsions reported in this study were produced by the rigorous agitation of crude oil from an oilfield and laboratory-formulated seawater. After proper preparation, the stability of the formed emulsion was monitored for 8 weeks without any visual separation of the phases. Figure 3a shows the optical microscopic image of the formed emulsion in which the droplets analyzed under the microscope possessed average sizes in the range of 0.1–17 μm. By observation, water droplets were dispersed in the crude oil, indicating that the produced emulsion was water-in-oil (W/O), and this was also confirmed by checking the emulsion conductivity, which is close to being zero. Figure 3b relates the viscosity and stress behavior of this emulsion as a function of the shear rate from 0.1 to 1000 s–1. The emulsion viscosity was observed to be declining as the shear rate increased. This is expected due to the aggregation of molecules in the emulsion sample.42 The stress behavior of this emulsion was observed to have increased as shear rate values increased, indicating shear thinning characteristics.42
Figure 3.
(a) Optical image of the produced crude oil emulsion. (b) Viscosity behavior and stress of the produced emulsion.
3.3. Demulsification Performance of NID
This section reports the demulsification performance of NID at room and moderate temperatures (25 and 60 °C). Varying NID concentrations from 100, 300, 500, and 700 ppm were tested to assess their potential to break the produced crude oil emulsions and effectively separate crude oil and water into clear phases under the aforementioned demulsification temperatures. Figure 4a reflects the demulsification efficiencies at various NID concentrations (100–700 ppm) under room temperature (25 °C). As can be seen in this figure, the blank emulsion remained unchanged (no separation) for 12 h of monitoring; however, oil/water separation was noticed to have increased with the increasing NID concentration for 12 h demulsification duration. After 12 h of demulsification activity at room temperature, the demulsification efficiencies of NID at 100, 300, 500, and 700 ppm were evaluated to be 45, 68, 95, and 93%, respectively. Following this, the demulsification activity of NID was tested inside the oven at a moderate temperature (60 °C) because many chemical demulsifiers could work better for demulsification at elevated temperatures, hence among the key reasons for conducting the current demulsification study at room and moderate temperatures. Shown in Figure 4b is the demulsification activity of various concentrations of NID at 60 °C. Just like the demulsification condition of the blank emulsion under room temperature, the blank sample again did not reveal any observable oil/water separation during the entire 120 min (2 h) demulsification duration at 60 °C. However, under this demulsification temperature (60 °C), oil/water separation was observed to be rapid at different NID concentrations. The demulsification efficiency of NID at 100, 300, 500, and 700 ppm after 2 h of demulsification activity was estimated to be 75, 88, 99, and 96%, respectively. It is necessary to note that the demulsification activity and the demulsification duration were shorter at moderate temperature (60 °C) compared with the demulsification activity under room temperature (25 °C) conditions. This scenario is expected because increasing the demulsification temperature would generally contribute to the instability of emulsion, and this would favor a swift oil/water separation.42 The demulsification efficiency (DE) of NID at 25 and 60 °C is obviously considerable, and its performance is compared with some notable chemical demulsifiers, as shown in Table 2. As can be seen from this table, NID would be a promising demulsifier that can compete favorably with the existing chemical demulsifiers employed for the separation of oil–water mixtures in the petroleum industry. Further, Figure 5a,b relates the demulsification rate at both temperatures (25 and 60 °C), describing the extent to which every tested concentration of NID was able to dispel water under a specified period during the demulsification activity. Generally, the demulsification activity of the NID proceeded at a faster rate under moderate temperature as compared to the demulsification activity experienced under room temperature.
Figure 4.
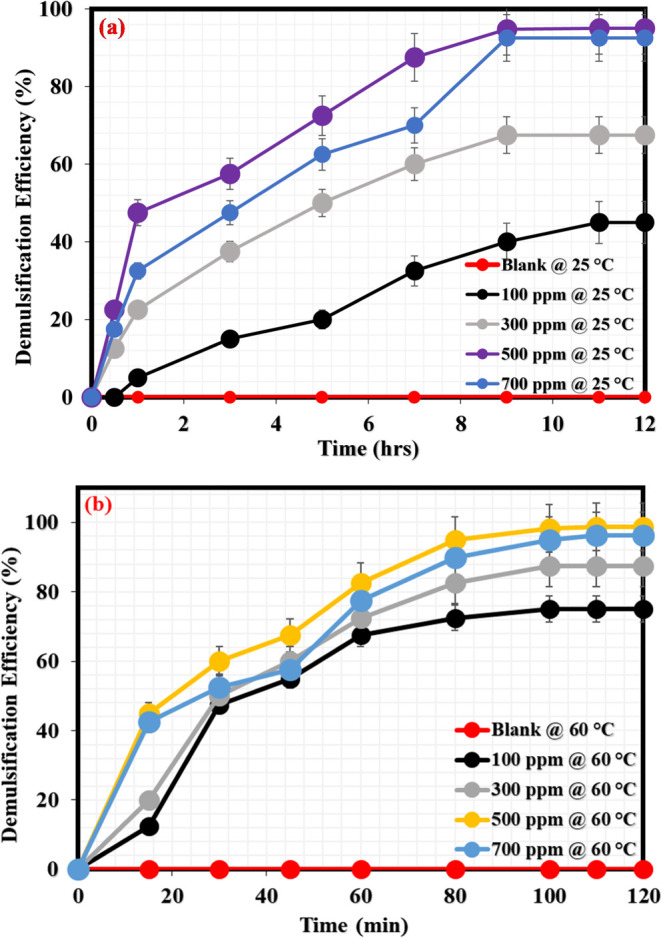
(a) Demulsification efficiency at different NID concentrations (25 °C); (b) demulsification efficiency at different NID concentrations (60 °C).
Table 2. Demulsification Efficiency (DE) of NID in Relation to Some Existing Chemical Demulsifiers.
| chemical demulsifier | concentration (ppm) | temperature (°C) | DE (%) | reference |
|---|---|---|---|---|
| poly(amido amine) demulsifier | 50 | ambient | 91.5 | Kuang et al.56 |
| gemini ionic liquid demulsifier (Y12) | 400 | 50 | 100 | Ding et al.23 |
| low-temperature ionic liquid demulsifier (EBTA) | 250 | 40 | 95.36 | Tang et al.21 |
| ionic liquid PPBH | 250 | 40 | 96.34 | Qu et al.2 |
| ionic liquid PPBD | 250 | 40 | 95.23 | Qu et al.2 |
| nonionic demulsifier | 500 | 25 | 95 | this work |
| nonionic demulsifier | 500 | 60 | 99 | this work |
Figure 5.
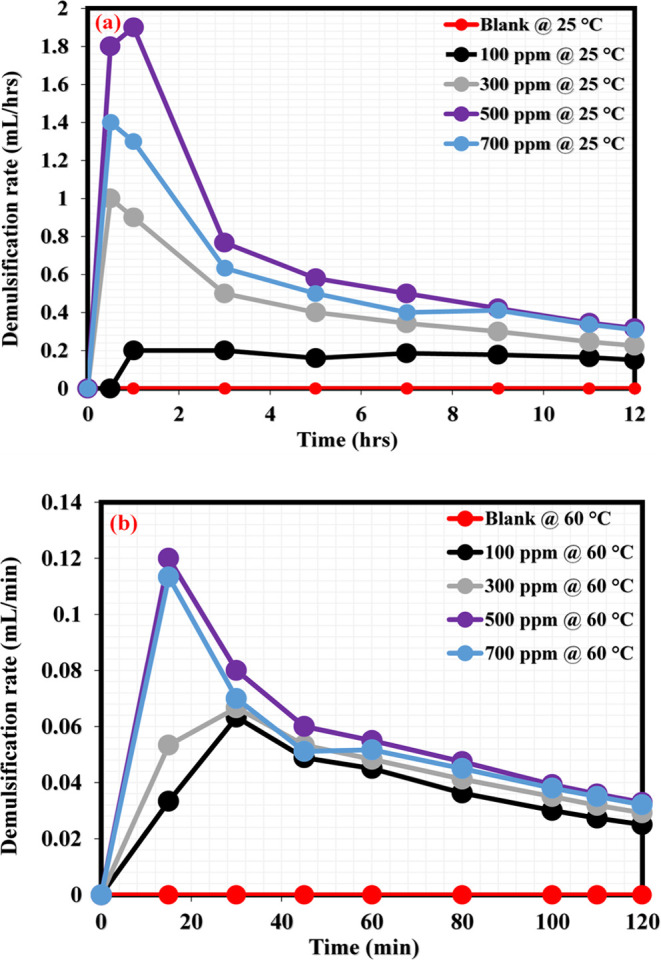
(a) Demulsification rate of various NID concentrations at room temperature (25 °C); (b) demulsification rate of various NID concentrations at moderate temperature (60 °C).
3.4. Viscosity and Elastic Modulus Measurements
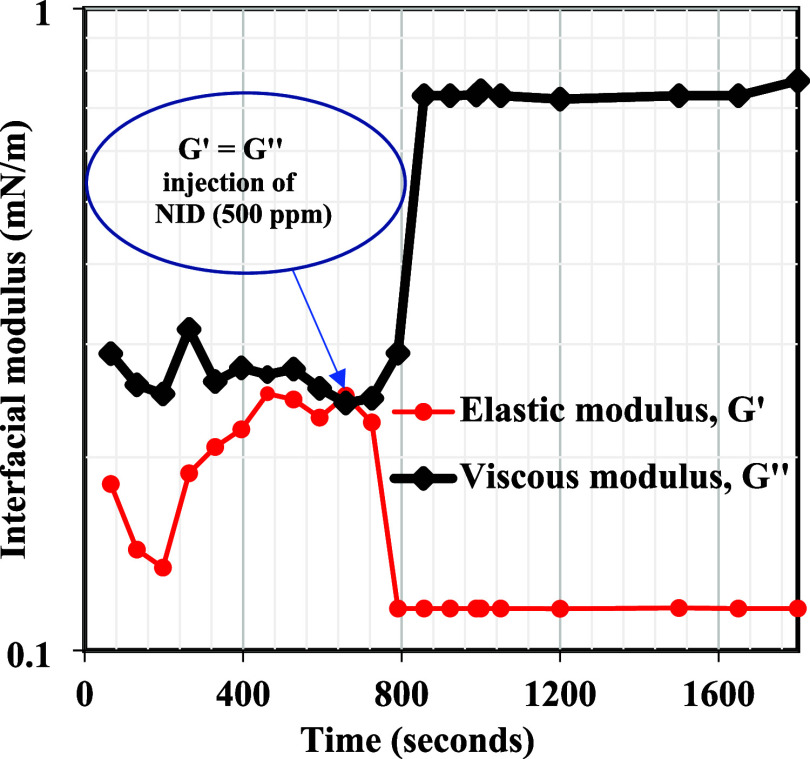
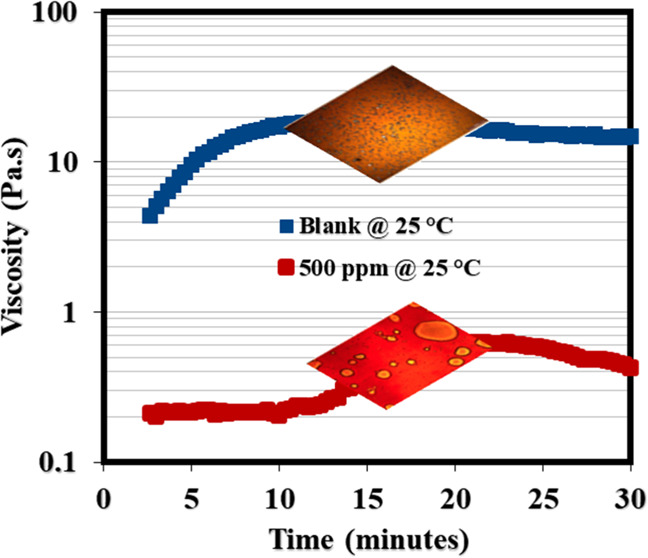
This section presents the application of rheology to shed more light on the demulsification mechanism of the synthesized NID. The demulsification of emulsions via the bottle test is a simple physical technique that is practically not adequate to establish the working mechanism of a typical chemical demulsifier. Rheology is another reliable method among other methods that could be applied to assess the workability of a chemical demulsifier upon its injection into the produced emulsion. The bulk rheology test of produced crude oil emulsion before and after the addition of NID is among the reliable means of establishing the efficacy of an actively working demulsifier.37,,58 This technique is reasonable and well adopted by the researchers because the crude oil exploration comes out of oil wells to the subsurface in the form of real emulsion before it is conveyed to the separator tank for separation. It is expected that the viscosity of the emulsion should differ before and after demulsifier injection. Usually, the emulsion viscosity drops in the presence of an active demulsifier. This means that an excellent demulsifier would penetrate and adsorb at the oil/water interface and subsequently rupture the interfacial films22,44,49,59 that are bonding the oil–water molecules. Likewise, the interfacial films of emulsion possess a sort of mechanical strength22,60,61 enabling the oil–water interface to remain intact, and as far as the current study is concerned, the NID developed was not added to the crude oil directly; rather, it was incorporated into the formed emulsion in order to examine the weakening of oil–water bonding molecules at the interface, which would definitely lead to emulsion viscosity reduction. Represented in Figure 6a,b is the viscosity profile of the produced emulsion before and after the injection of 500 ppm of NID at 25 and 60 °C. As can be noticed at both temperatures, the viscosity of the emulsion infused with 500 ppm of NID dropped considerably as compared to that of the blank sample. The viscosity reduction at both temperatures is an indication of the adsorption of NID at the oil/water interface and the systematic rupture of emulsifiers binding the oil/water molecules together at the interface. Hence, a working and active chemical demulsifier incorporated inside the emulsion would diminish the emulsion viscosity, and the viscosity decrease becomes intense as temperature advances. Likewise, the interfacial films at the oil–water interface exhibit a sort of elastic strength owing to the buildup and influx of emulsifiers at the oil/water interface. Ideally, a working and active chemical demulsifier would destabilize the emulsifying substances at the interface, enabling the stability of the emulsion. Hence, elastic modulus measurement can therefore be used to reveal the elastic strength of the produced emulsion prior to and after the injection of a working demulsifier. Displayed in Figure 7a,b are the elastic modulus curves of blank emulsion and emulsion injected with 500 ppm of NID. It was obvious in these plots that the elastic modulus of emulsion incorporated with the NID retarded across the tested frequencies as compared to that of the blank sample. The decrease in the elastic modulus of emulsion injected with the NID was, however, more glaring under low frequencies in the range of 0.1–10 rad/s. Generally, the elastic modulus of the emulsion filled with the NID diminished across the entire frequency as compared to the blank sample because the presence of the NID weakened the elastic strength at the oil–water interface. Furthermore, the oil–water interfacial modulus measurement shown in Figure 8 gave another insight into the activity of the NID demulsifier. The asphaltene aggregates were building up at the oil–water interface until the elastic modulus (G′) intercepted the viscous modulus (G″) at about 750 s during measurement, indicating asphaltene accumulation at the oil–water interface was acting like rigid elastic films that were holding oil–water molecules intact. At this juncture of similar viscoelasticity (G′ = G″) behavior, 500 ppm of NID was carefully injected, and this led to a sharp reduction in G′ and an increase in G″ at about 800 s during the test. The sharp reduction in G′ and sudden increase in G″ signified the rupture of asphaltenes molecules at the oil–water interface, and G′ and G″ remained unchanged until 1800 s, which probably indicated complete dissociation of emulsifying materials at the oil–water interface.
Figure 6.
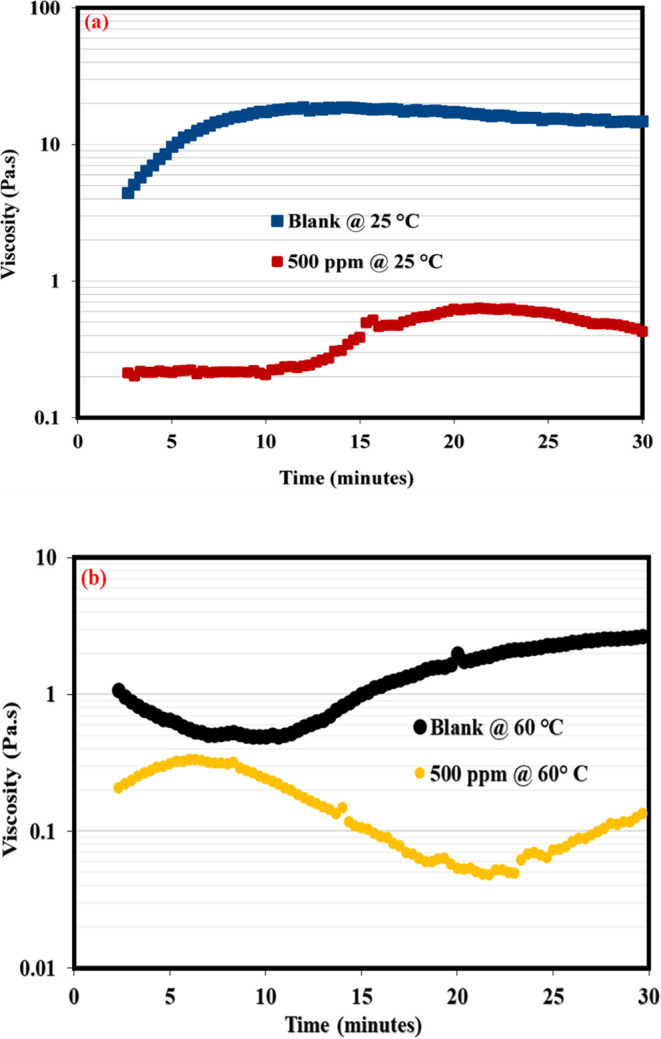
Viscosity profile of the produced emulsion with and without NID at ambient temperature: (a) 25 and (b) 60 °C.
Figure 7.
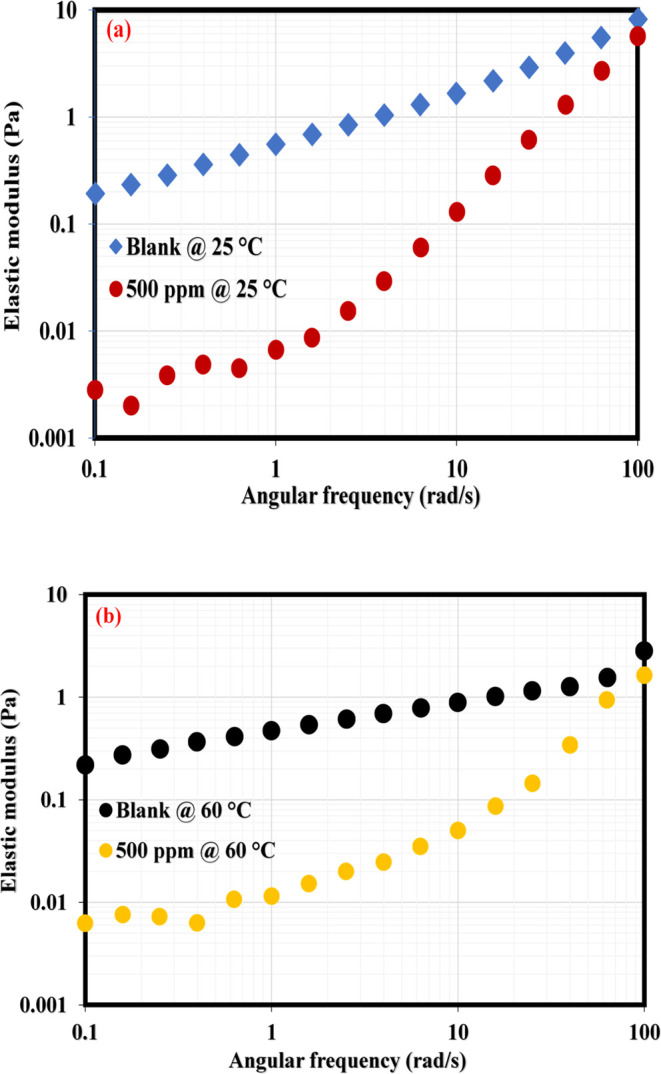
Elastic modulus measurements of the produced emulsion with and without NID: (a) 25 and (b) 60 °C.
Figure 8.
Oil–water interfacial modulus measurements in the presence of an NID.
3.5. Interfacial Tension Measurements
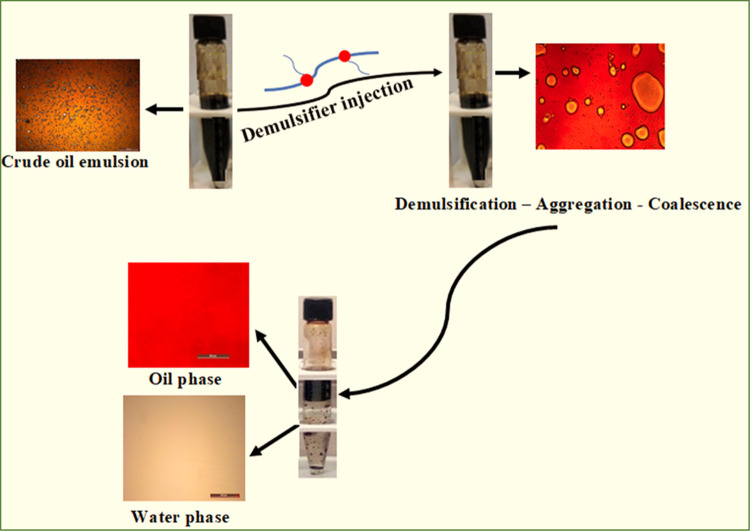
Interfacial tension (IFT) measurement is very key in describing the stability of emulsion and the penetration of demulsifier at the interface of oil/water as well as the adsorption mechanism of the interfacial films. Figure 9 illustrates the IFT measurements revealing the adsorption of different NID dosages behavior at the oil/water interface and the oil–water interfacial behavior in the absence of NID. As can be seen in these plots, oil–water IFT values at 100, 300, 500, and 700 ppm of NID concentrations after 500 s of dynamic measurements were found to be 3.02, 1.84, 2.41, and 1.84 mN/m, respectively, while the IFT value of the oil–water system without the NID was recorded to be 16.11 mN/m under the same duration. It should be noted that the higher IFT values recorded at 500 ppm of NID could be attributed to the uneven and inconsistent migration of NID to the oil–water interface, and similar results have been reported.2,62 The inference from these IFT results is that the produced NID was able to migrate to the oil–water interface, and its gradual adsorption of the interfacial films was manifested by a sharp decrease in the IFT in the presence of its various concentrations as compared to the IFT value of the blank sample. The outcomes of the IFT measurements are in good agreement with past studies reported in the literature.2,23 A simple demulsification mechanism elucidating how the demulsifier displaces the oil–water interfacial film is displayed in Figure 10, elucidating the breaking of the produced emulsion by NID, followed by the aggregation of water droplets, resulting in coalescence and gradual oil/water separation.
Figure 9.
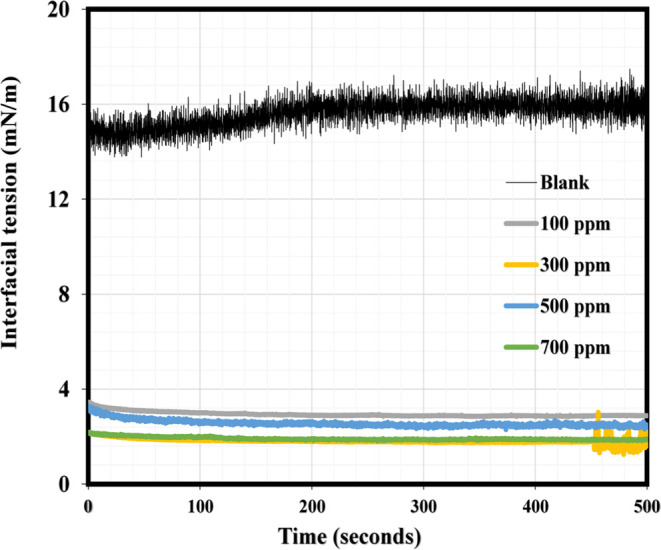
Oil–water dynamic IFT measurements at various concentrations of NID.
Figure 10.
Proposed demulsification mechanism for the NID demulsifier.
4. Conclusions
The current study investigates the demulsification activity of a newly developed nonionic demulsifier (NID). Emulsions produced from crude oil and seawater were destabilized by the NID of varying concentrations at room and moderate temperatures (25 and 60 °C). The outcomes of demulsification tests showed that about 95 and 99% water removal was achieved at 25 and 60 °C, respectively. The rigid interfacial films triggering the stability of emulsion at the interface of oil and water were broken by subjecting the produced emulsion to viscosity and elastic modulus tests at both temperatures in the presence of the NID. The viscosity behavior and elastic modulus of the produced emulsion were observed to have dropped owing to the displacement of the natural emulsifiers resting at the oil/water interface. Besides, the interfacial tension (IFT) measurements established the penetration and adsorption of the NID at the oil/water interface. Accordingly, the IFT values of oil–water at varying NID concentrations were evaluated to be within 1.84–3.02 mN/m, and that of the NID-free oil–water system was estimated to be 16.11 mN/m.
Acknowledgments
The authors are grateful to the Center for Integrative Petroleum Research at King Fahd University of Petroleum & Minerals for providing the necessary support and research facilities.
The authors declare no competing financial interest.
References
- Zhang X.; He C.; Zhou J.; Tian Y.; He L.; Sui H.; Li X. Demulsification of Water-in-Heavy Oil Emulsions by Oxygen-Enriched Non-Ionic Demulsifier: Synthesis, Characterization and Mechanisms. Fuel 2023, 338, 127274 10.1016/j.fuel.2022.127274. [DOI] [Google Scholar]
- Qu Q.; Li H.; Li S.; Hu Z.; Zhu M.; Chen J.; Sun X.; Tang Y.; Zhang Z.; Mi Y.; Yu W. Synthesis and Demulsification Mechanism of an Ionic Liquid with Four Hydrophobic Branches and Four Ionic Centers. Chemosphere 2023, 340, 139802 10.1016/j.chemosphere.2023.139802. [DOI] [PubMed] [Google Scholar]
- Kwon W.-T.; Park K.; Han S. D.; Yoon S. M.; Kim J. Y.; Bae W.; Rhee Y. W. Investigation of Water Separation from Water-in-Oil Emulsion Using Electric Field. J. Ind. Eng. Chem. 2010, 16 (5), 684–687. 10.1016/j.jiec.2010.07.018. [DOI] [Google Scholar]
- Kang W.; Li M.; Yang H.; Kang X.; Wang F.; Jiang H.; Zhang M.; Zhu T.; Sarsenbekuly B. Coalescence Behavior of Aqueous Drops in Water-in-Oil Emulsions under High-Frequency Pulsed AC Fields. J. Ind. Eng. Chem. 2021, 93, 415–422. 10.1016/j.jiec.2020.10.020. [DOI] [Google Scholar]
- Xiang Z.; Zhao X.; Wang G.; Qi C.; Zhou S.; Li J.; Gao Y. Diblock Copolymer Worms Stabilized PH-Responsive Pickering Emulsions: An Efficient and Recyclable Platform for Claisen-Schmidt Condensation Reaction. J. Ind. Eng. Chem. 2023, 117, 538–545. 10.1016/j.jiec.2022.10.044. [DOI] [Google Scholar]
- Santos D.; da Rocha E. C. L.; Santos R. L. M.; Cancelas A. J.; Franceschi E.; Santos A. F.; Fortuny M.; Dariva C. Demulsification of Water-in-Crude Oil Emulsions Using Single Mode and Multimode Microwave Irradiation. Sep. Purif. Technol. 2017, 189, 347–356. 10.1016/j.seppur.2017.08.028. [DOI] [Google Scholar]
- Lv X.; Song Z.; Yu J.; Su Y.; Zhao X.; Sun J.; Mao Y.; Wang W. Study on the Demulsification of Refinery Oily Sludge Enhanced by Microwave Irradiation. Fuel 2020, 279, 118417 10.1016/j.fuel.2020.118417. [DOI] [Google Scholar]
- Mohayeji M.; Farsi M.; Rahimpour M. R.; Shariati A. Modeling and Operability Analysis of Water Separation from Crude Oil in an Industrial Gravitational Coalescer. J. Taiwan Inst. Chem. Eng. 2016, 60, 76–82. 10.1016/j.jtice.2015.10.025. [DOI] [Google Scholar]
- Huang X.; Zhang J.; Peng K.; Na Y.; Xiong Y.; Liu W.; Liu J.; Lu L.; Li S. Functional Magnetic Nanoparticles for Enhancing Ultrafiltration of Waste Cutting Emulsions by Significantly Increasing Flux and Reducing Membrane Fouling. J. Membr. Sci. 2019, 573, 73–84. 10.1016/j.memsci.2018.11.074. [DOI] [Google Scholar]
- Ahmad F.; Lau K. K.; Shariff A. M.; Murshid G. Process Simulation and Optimal Design of Membrane Separation System for CO2 Capture from Natural Gas. Comput. Chem. Eng. 2012, 36, 119–128. 10.1016/j.compchemeng.2011.08.002. [DOI] [Google Scholar]
- Wang Q.; Yu Z.; Zhu X.; Xiang Q.; Chen H.; Pang Y. ZIF-67 Modified MXene/Sepiolite Composite Membrane for Oil–Water Separation and Heavy Metal Removal. J. Ind. Eng. Chem. 2022, 115, 314–328. 10.1016/j.jiec.2022.08.014. [DOI] [Google Scholar]
- Del Colle R.; Longo E.; Fontes S. R. Demulsification of Water/Sunflower Oil Emulsions by a Tangential Filtration Process Using Chemically Impregnated Ceramic Tubes. J. Membr. Sci. 2007, 289 (1–2), 58–66. 10.1016/j.memsci.2006.11.048. [DOI] [Google Scholar]
- Wang Z.; Gu S.; Zhou L. Research on the Static Experiment of Super Heavy Crude Oil Demulsification and Dehydration Using Ultrasonic Wave and Audible Sound Wave at High Temperatures. Ultrason. Sonochem. 2018, 40, 1014–1020. 10.1016/j.ultsonch.2017.08.037. [DOI] [PubMed] [Google Scholar]
- Xu X.; Cao D.; Liu J.; Gao J.; Wang X. Research on Ultrasound-Assisted Demulsification/Dehydration for Crude Oil. Ultrason. Sonochem. 2019, 57, 185–192. 10.1016/j.ultsonch.2019.05.024. [DOI] [PubMed] [Google Scholar]
- Ye F.; Zhang Z.; Mi Y.; Huang Z.; Yuan H.; Zhang Z.; Luo Y. Carbon Nanotubes Grafted with β-Cyclodextrin by an Ultrasonication Method and Its Demulsification Performance in Oily Wastewater. Colloids Surf., A 2020, 600, 124939. 10.1016/j.colsurfa.2020.124939. [DOI] [Google Scholar]
- Wen Y.; Cheng H.; Lu L. J.; Liu J.; Feng Y.; Guan W.; Zhou Q.; Huang X. F. Analysis of Biological Demulsification Process of Water-in-Oil Emulsion by Alcaligenes Sp. S-XJ-1. Bioresour. Technol. 2010, 101 (21), 8315–8322. 10.1016/j.biortech.2010.05.088. [DOI] [PubMed] [Google Scholar]
- Shehzad F.; Hussein I. A.; Kamal M. S.; Ahmad W.; Sultan A. S.; Nasser M. S. Polymeric Surfactants and Emerging Alternatives Used in the Demulsification of Produced Water: A Review. Polym. Rev. 2017, 1–39. 10.1080/15583724.2017.1340308. [DOI] [Google Scholar]
- Tahami S.; Movagharnejad K. Demulsification of Water in Crude Oil Emulsions via Phosphonium-Based Ionic Liquids: Statistical Modeling and Optimization. J. Mol. Liq. 2024, 411, 125748 10.1016/j.molliq.2024.125748. [DOI] [Google Scholar]
- Ding Y.; Li H.; Jia J.; Ouyang S.; Jiang W.; Chen L.; Zhao Y.; Zhang Z.; Mi Y. Synthesis of a Demulsifier for Treating Crude Oil-in-Water Emulsion through a Straightforward Low-Temperature Process. J. Mol. Liq. 2024, 410, 125635 10.1016/j.molliq.2024.125635. [DOI] [Google Scholar]
- Abdullah M. M. S.; Al-Lohedan H. A.; Faqihi N. A.; Al-Maswari B. M. Efficient Demulsification of Crude Oil Emulsion Using Novel Sugar-Based Surfactant. ACS Omega 2024, 9, 32144–32152. 10.1021/acsomega.4c04299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y.; Zhu M.; Chen J.; Sun X.; Li H.; Hu Z.; Li S.; Qu Q.; Zhang Z.; Mi Y. A Low-Temperature Ionic Liquid Demulsifier Derived from Recycled PET Waste Plastics. J. Mol. Liq. 2024, 394, 123737 10.1016/j.molliq.2023.123737. [DOI] [Google Scholar]
- Shen L.; Liu T.; Li H.; Li S.; Hu Z.; Jiang X.; Liu H.; Zhang Z.; Mi Y.; Yu W. Quadruple-Branched Jellyfish-like Demulsifier Used for Completely Demulsifying Water-in-Oil Emulsion at Low Temperature and Its Demulsification Mechanism. Fuel 2024, 358, 130153 10.1016/j.fuel.2023.130153. [DOI] [Google Scholar]
- Ding Y.; Ai G.; Du J.; Yu G.; Lai L.; Yan X.; Yu W.; Mi Y. Synthesis and Demulsification Performance of a Gemini Ionic Liquid with Dual Cationic Active Centers. Sep. Purif. Technol. 2024, 330, 125242 10.1016/j.seppur.2023.125242. [DOI] [Google Scholar]
- Hasiri M.; Kantzas A. Efficiency of Oil Separation and Demulsification Following Sonication Gel Degradation: Influence of Cr(III) Ions, NaCl Concentrations, and Sodium-Based Retarders. Fuel 2024, 357, 129940 10.1016/j.fuel.2023.129940. [DOI] [Google Scholar]
- Jia Z.; Niu Z.; Yang Z.; Li X.; Wang J.; He X.; Sui H.; He L. Interfacial Behaviors of Ionic Liquid Cations and Asphaltenes at Oil–Water Interface: Dynamic Diffusion and Interfacially Competitive Adsorption. Energy Fuels 2020, 34, 1259–1267. 10.1021/acs.energyfuels.9b02679. [DOI] [Google Scholar]
- Ezzat A. O.; Al-Lohedan H. A.. Dehydration of Heavy Crude Oil Emulsions Using Novel Imidazolium-Based Poly Ionic Liquids. J. Mol. Liq. 2021, 326. 115284. 10.1016/j.molliq.2021.115284. [DOI] [Google Scholar]
- Atta A. M.; Al-Lohedan H. A.; Ezzat A. O. Synthesis and Application of Geminal Dicationic Ionic Liquids and Poly (Ionic Liquids) Combined Imidazolium and Pyridinium Cations as Demulsifiers for Petroleum Crude Oil Saline Water Emulsions. J. Mol. Liq. 2021, 325, 115264 10.1016/j.molliq.2020.115264. [DOI] [Google Scholar]
- Hassanshahi N.; Hu G.; Li J. Application of Ionic Liquids for Chemical Demulsification: A Review. Molecules 2020, 23, 4915 10.3390/molecules25214915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R.; Feng Y.; Zhong Y.; Zou Y.; Yang M.; Liu Y.; Zhou Y. Enhancing Demulsification Performance for Oil-Water Separation through Encapsulating Ionic Liquids in the Pore of MIL-100(Fe). Langmuir 2021, 37 (27), 8232–8239. 10.1021/acs.langmuir.1c00945. [DOI] [PubMed] [Google Scholar]
- Abdullah M. M. S.; Al-Lohedan H. A. Novel Bio-Based Amphiphilic Ionic Liquids for the Efficient Demulsification of Heavy Crude Oil Emulsions. Molecules 2021, 26 (20), 6119. 10.3390/molecules26206119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adewunmi A. A.; Kamal M. S.; Olatunji S. O. Demulsification of Crude Oil Emulsions Using Ionic Liquids: A Computational Intelligence Approach. J. Pet. Sci. Eng. 2022, 208, 109279 10.1016/j.petrol.2021.109279. [DOI] [Google Scholar]
- Ezzat A. O.; Tawfeek A. M.; Al-Lohedan H. A. Synthesis and Application of Novel Gemini Pyridinium Ionic Liquids as Demulsifiers for Arabian Heavy Crude Oil Emulsions. Colloids Surf., A 2022, 634, 127961 10.1016/j.colsurfa.2021.127961. [DOI] [Google Scholar]
- Abdullah M. M. S.; Al-Lohedan H. A. Alginate-Based Poly Ionic Liquids for the Efficient Demulsification of Water in Heavy Crude Oil Emulsions. Fuel 2022, 320, 123949 10.1016/j.fuel.2022.123949. [DOI] [Google Scholar]
- Masri A. N.; Sulaimon A. A.; Zakaria M. Z.; Abidemi A. S. Imidazolium-Based Ionic Liquids as Demulsifier for Water-Crude Oil Emulsion. Mater. Today Proc. 2022, 64, 1597. 10.1016/j.matpr.2022.03.098. [DOI] [Google Scholar]
- Atta A. M.; Al-Lohedan H. A.; Abdullah M. M. S.; ElSaeed S. M. Application of New Amphiphilic Ionic Liquid Based on Ethoxylated Octadecylammonium Tosylate as Demulsifier and Petroleum Crude Oil Spill Dispersant. J. Ind. Eng. Chem. 2016, 33, 122–130. 10.1016/j.jiec.2015.09.028. [DOI] [Google Scholar]
- Lei M.; Huang H.; Liu J.; Peng F. A Gemini Ionic Liquid and Its Low-Temperature Demulsification Performance in Water-in-Crude Oil Emulsions. Colloids Surf., A 2023, 671, 131696 10.1016/j.colsurfa.2023.131696. [DOI] [Google Scholar]
- Al-Sabagh A. M.; Nasser N. M.; Abd El-Hamid T. M. Investigation of Kinetic and Rheological Properties for the Demulsification Process. Egypt. J. Pet. 2013, 22 (1), 117–127. 10.1016/j.ejpe.2012.11.013. [DOI] [Google Scholar]
- Le Follotec A.; Pezron I.; Noik C.; Dalmazzone C.; Metlas-Komunjer L. Triblock Copolymers as Destabilizers of Water-in-Crude Oil Emulsions. Colloids Surf., A 2010, 365 (1–3), 162–170. 10.1016/j.colsurfa.2010.02.025. [DOI] [Google Scholar]
- Abdullah M. M. S.; Al-Lohedan H. A.; Faqihi N. A. Efficacy of Curcumin-Based Amphiphilic Ionic Liquids towards the Demulsification of Water-in-Heavy Crude Oil Emulsions. Colloids Surf., A 2021, 628, 127320 10.1016/j.colsurfa.2021.127320. [DOI] [Google Scholar]
- Abdullah M. M. S.; Al-Lohedan H. A. Demulsification of Water in Heavy Crude Oil Emulsion Using a New Amphiphilic Ionic Liquid Based on the Glycolysis of Polyethylene Terephthalate Waste. J. Mol. Liq. 2020, 307, 112928 10.1016/j.molliq.2020.112928. [DOI] [Google Scholar]
- Ezzat A. O.; Atta A. M.; Al-Lohedan H. A. Demulsification of Stable Seawater/Arabian Heavy Crude Oil Emulsions Using Star-like Tricationic Pyridinium Ionic Liquids. Fuel 2021, 304, 121436 10.1016/j.fuel.2021.121436. [DOI] [Google Scholar]
- Adewunmi A. A.; Kamal M. S. Demulsification of Water-in-Oil Emulsions Using Ionic Liquids: Effects of Counterion and Water Type. J. Mol. Liq. 2019, 279, 411–419. 10.1016/j.molliq.2019.02.008. [DOI] [Google Scholar]
- Faisal W.; Almomani F. A Critical Review of the Development and Demulsification Processes Applied for Oil Recovery from Oil in Water Emulsions. Chemosphere 2022, 291, 133099 10.1016/j.chemosphere.2021.133099. [DOI] [PubMed] [Google Scholar]
- Yonguep E.; Chowdhury M. Optimization of the Demulsification of Crude Oil-in-Water Emulsions Using Response Surface Methodology. S. Afr. J. Chem. Eng. 2021, 36, 105–117. 10.1016/j.sajce.2021.02.002. [DOI] [Google Scholar]
- Adewunmi A. A.; Shahzad Kamal M.; Amao A. O.; Ivan Solling T. Extracted Quartz as Efficient Natural Demulsifier for Crude Oil-Water Emulsions: Effect of Monovalent/Divalent Salts, PH and Modeling Study. J. Pet. Sci. Eng. 2021, 206, 109069 10.1016/j.petrol.2021.109069. [DOI] [Google Scholar]
- Doğan M.; Göksel Saraç M.; Aslan Türker D. Effect of Salt on the Inter-Relationship between the Morphological, Emulsifying and Interfacial Rheological Properties of O/W Emulsions at Oil/Water Interface. J. Food Eng. 2020, 275, 109871 10.1016/j.jfoodeng.2019.109871. [DOI] [Google Scholar]
- Ríos G.; Pazos C.; Coca J. Destabilization of Cutting Oil Emulsions Using Inorganic Salts as Coagulants. Colloids Surf., A 1998, 138, 383–389. 10.1016/S0927-7757(97)00083-6. [DOI] [Google Scholar]
- Xia L. X.; Lu S. W.; Cao G. Y. Salt-Assisted Microwave Demulsification. Chem. Eng. Commun. 2004, 191 (8), 1053–1063. 10.1080/00986440490276380. [DOI] [Google Scholar]
- Deng J.; Zhu M.; Wang Z.; Liu Y. Novel Iron-Modified Chitosan for Effective Demulsification and Oil Recovery of Emulsified Oily Wastewater. Mater. Chem. Phys. 2023, 305, 127848 10.1016/j.matchemphys.2023.127848. [DOI] [Google Scholar]
- Ye F.; Zhang X.; Jiang X.; Liu H.; Tang Y.; Qu Q.; Shen L.; Zhang Z.; Mi Y.; Yan X. Demulsification of Crude Oil Emulsions Using a Three-Branched Betaine Type Ionic Liquid and Its Demulsification Mechanism. Geoenergy Sci. Eng. 2023, 230, 212265 10.1016/j.geoen.2023.212265. [DOI] [Google Scholar]
- Shakil Hussain S. M.; Kamal M. S.; Murtaza M. Synthesis of Novel Ethoxylated Quaternary Ammonium Gemini Surfactants for Enhanced Oil Recovery Application. Energies 2019, 12 (9), 11–13. 10.3390/en12091731. [DOI] [Google Scholar]
- Jia X.; Wei L.; Fu M.; Liu C.; Gu Y.; Qin W.; Zhang L.; Geng X.; Guo H. Journal, A. One-Pot Preparation of Environmentally Friendly, High Demulsification Rate and Novel Functional Magnetic Demulsifier: Used for Oil and Water Separation in Crude Oil Emulsion. Arabian J. Chem. 2023, 16, 105134 10.1016/j.arabjc.2023.105134. [DOI] [Google Scholar]
- Sun H.; Li X.; Li X. Magnetically Recyclable Polydopamine-Polyquaternium Modified Fe3O4 Nanoparticles for Demulsification of Asphaltene-Rich ASP Flooding Produced Water. J. Water Process Eng. 2023, 56, 104460 10.1016/j.jwpe.2023.104460. [DOI] [Google Scholar]
- Adewunmi A. A.; Kamal M. S.; Solling T. I.; Salami B. A. Palm Oil Fuel Ash (POFA) as a Demulsifier for Crude Oil Emulsions: Performance and Mechanism. J. Pet. Sci. Eng. 2019, 183, 106430 10.1016/j.petrol.2019.106430. [DOI] [Google Scholar]
- Li M.; Meng L.; Yuanyi Y.; Li Z.; Xiaoyang G. Mechanical Properties of Oil Well Cement Stone Reinforced with Hybrid Fiber of Calcium Carbonate Whisker and Carbon Fiber. Pet. Explor. Dev. 2015, 42, 104–111. 10.1016/S1876-3804(15)60012-X. [DOI] [Google Scholar]
- Kuang J.; Mi Y.; Zhang Z.; Ye F.; Yuan H.; Liu W.; Jiang X.; Luo Y. A Hyperbranched Poly(Amido Amine) Demulsifier with Trimethyl Citrate as Initial Cores and Its Demulsification Performance at Ambient Temperature. J. Water Process Eng. 2020, 38, 101542 10.1016/j.jwpe.2020.101542. [DOI] [Google Scholar]
- Hippmann S.; Ahmed S. S.; Fröhlich P.; Bertau M. Demulsification of Water/Crude Oil Emulsion Using Natural Rock Alginite. Colloids Surf., A 2018, 553, 71–79. 10.1016/j.colsurfa.2018.05.031. [DOI] [Google Scholar]
- Husain A.; Adewunmi A. A.; Kamal M. S.; Mahmoud M.; Al-Harthi M. A. Demulsification of Heavy Petroleum Emulsion Using Pyridinium Ionic Liquids with Distinct Anion Branching. Energy Fuels 2021, 35 (20), 16527–16533. 10.1021/acs.energyfuels.1c02286. [DOI] [Google Scholar]
- Wang D.; Yang D.; Huang C.; Huang Y.; Yang D.; Zhang H.; Liu Q.; Tang T.; Gamal El-Din M.; Kemppi T.; Perdicakis B.; Zeng H. Stabilization Mechanism and Chemical Demulsification of Water-in-Oil and Oil-in-Water Emulsions in Petroleum Industry: A Review. Fuel 2021, 15, 119390 10.1016/j.fuel.2020.119390. [DOI] [Google Scholar]
- Shu G.; Bu K.; Zhao B.; Zheng S. Separation of SAGD Produced Emulsions through a Combined Pre-Dewatering and Demulsification Process. J. Pet. Sci. Eng. 2021, 201, 920–4105. 10.1016/j.petrol.2021.108493. [DOI] [Google Scholar]
- Elmobarak W. F.; Almomani F. Evaluation of the Efficiency of Ionic Liquids in the Demulsification of Oil-in-Water Emulsions. Environ. Technol. Innov. 2021, 24, 102003 10.1016/j.eti.2021.102003. [DOI] [Google Scholar]
- Hazrati N.; Miran Beigi A. A.; Abdouss M. Demulsification of Water in Crude Oil Emulsion Using Long Chain Imidazolium Ionic Liquids and Optimization of Parameters. Fuel 2018, 229, 126–134. 10.1016/j.fuel.2018.05.010. [DOI] [Google Scholar]