Abstract
Interaction of erythropoietin (Epo) with its cell surface receptor activates signal transduction pathways which result in the proliferation and differentiation of erythroid cells. Infection of erythroid cells with the Friend spleen focus-forming virus (SFFV) leads to the interaction of the viral envelope glycoprotein with the Epo receptor and renders these cells Epo independent. We previously reported that SFFV induces Epo independence by constitutively activating components of several Epo signal transduction pathways, including the Jak-Stat and the Raf-1/mitogen-activated protein kinase (MAPK) pathways. To further evaluate the mechanism by which SFFV activates the Raf-1/MAPK pathway, we investigated the effects of SFFV on upstream components of this pathway, and our results indicate that SFFV activates Shc and Grb2 and that this leads to Ras activation. While studies with a dominant-negative Ras indicated that Ras was required for Epo-induced proliferation of normal erythroid cells, the Epo-independent growth of SFFV-infected cells can still occur in the absence of Ras, although at reduced levels. In contrast, protein kinase C (PKC) was shown to be required for the Epo-independent proliferation of SFFV-infected cells. Further studies indicated that PKC, which is thought to be involved in the activation of both Raf-1 and MAPK, was required only for the activation of MAPK, not Raf-1, in SFFV-infected cells. Our results indicate that Ras and PKC define two distinct signals converging on MAPK in both Epo-stimulated and SFFV-infected erythroid cells and that activation of only PKC is sufficient for the Epo-independent proliferation of SFFV-infected cells.
Infection of erythroid cells with the Friend spleen focus-forming virus (SFFV) induces a rapidly occurring erythroleukemia in susceptible adult mice due to expression of its unique envelope gene (for a review, see reference 56). The SFFV envelope glycoprotein associates with the Epo receptor at the cell surface (8, 23) and is thought to be responsible for the proliferation of SFFV-infected erythroid cells in the absence of its normal regulator, erythropoietin (Epo). Interactions between the SFFV envelope glycoprotein and the Epo receptor may alter cell growth by activating signal transduction pathways that are normally regulated by Epo.
Previous studies have shown that growth factor stimulation of the Epo receptor activates several signal transduction pathways including the Jak-Stat pathway (24, 44, 51, 53, 69), the Raf-1/mitogen-activated protein kinase (MAPK) pathway (6, 15, 45, 65, 66), and components of the phosphatidylinositol 3-kinase (PI 3-kinase) pathway (14, 27, 28, 31, 32, 41, 43). Using the Epo-dependent HCD-57 erythroleukemia cell line, we have previously demonstrated that infection with SFFV, which abrogates the Epo dependence of HCD-57 cells (55), activates Stat DNA-binding activity in the absence of Epo (51) and constitutively activates components of the serine/threonine kinase cascade in the Raf-1/MAPK pathway, specifically, Raf-1, MAPK kinase (MEK), and MAPK (48).
Activation of the Raf-1/MAPK pathway by cytokines such as Epo requires activation of receptor tyrosine kinases which phosphorylate the cytokine receptor and provide a binding site for adapter molecules that localize Raf-1 at the cell membrane. Adapter molecules that have been identified as components of the activated Epo-receptor complex include the SH2 domain-containing adapter proteins Grb2 and Shc (13, 15, 28). Grb2, which is constitutively associated with the guanine nucleotide-releasing factor Sos1 (54), binds the tyrosine-phosphorylated Epo receptor either directly through its SH2 domain or indirectly through binding to EpoR-associated, tyrosine-phosphorylated Shc (15). Binding of Grb2 to the receptor is thought to translocate Sos1 to the membrane, where it activates the exchange of GDP for GTP on Ras guanine nucleotide-binding proteins (20, 35, 57). Ras-GTP has been shown to activate the Raf-1/MAPK cascade by binding Raf-1 and anchoring it at the cell membrane where it is phosphorylated and activated by other kinases (36, 46, 62). Activated Raf-1 then phosphorylates and activates the dually specific kinase MEK, which phosphorylates and activates MAPK (12, 16, 34). Although the specific kinases involved in Raf-1 activation have not been completely identified, members of the protein kinase C (PKC) family of serine kinases have been implicated as potential activators of Raf-1. It has been demonstrated that PKC-mediated serine phosphorylation directly activates Raf-1 in hematopoietic cells stimulated with interleukin-3 (7) and in other cell types stimulated with the phorbol ester 12-O-tetradecanoylphorbol-13-acetate (4, 33, 37, 39). Stimulation of erythroid cells with Epo induces phosphorylation and activation of calcium-dependent isoforms of the PKC family of serine/threonine kinases (59). In addition, PKC is required for Epo-induced activation of MAPK in normal erythroid progenitor cells (18). However, studies using a variety of mitogen- and growth factor-activated cells have demonstrated the existence of both PKC-dependent and PKC-independent modes of Raf-1 activation, and the requirement for PKC in Raf-1 activation varies with the specific growth factor receptor being stimulated (1, 29).
To evaluate the mechanism of SFFV-induced activation of the Raf-1/MAPK cascade, we investigated the effect of SFFV infection on upstream components in the Raf-1/MAPK pathway using HCD-57 erythroleukemia cells infected with SFFV. Our results indicate that upstream events required for Epo-induced activation of the Raf-1/MAPK pathway, specifically tyrosine phosphorylation of Shc, formation of the Shc-Grb2 complex, and Ras-GTP binding, are constitutively activated in erythroid cells infected with SFFV but are not essential for the Epo-independent proliferation of these cells. In contrast, PKC, which is also constitutively activated in SFFV-infected cells, is absolutely required for the Epo-independent activation of MAPK by SFFV and for the proliferation of SFFV-infected cells in the absence of Epo.
MATERIALS AND METHODS
Cell lines.
HCD-57 cells, an Epo-dependent erythroleukemia cell line (55), were maintained in Iscove's modified Dulbecco minimal essential medium supplemented with 30% fetal calf serum (FCS), 5 × 10−5 M 2-mercaptoethanol, and 0.3 U of Epo/ml. HCD-57 cells in which Epo dependence had been abrogated by infection with either SFFVP or SFFVA, two different variants of SFFV (55), were maintained in the same medium without Epo.
Western blot analysis.
Cells grown to a density of 106 cells/ml were washed once in medium without serum and incubated in medium plus 1.5% FCS at 37°C overnight. The next day the cells were centrifuged and resuspended in fresh starvation medium for an additional 2 h. Epo (0.3 U/ml) was added to Epo-stimulated cells for 15 min. Cells were then centrifuged, and the pellet was washed twice with ice-cold phosphate-buffered saline (PBS) containing 1 mM Na3VO4. Cells were resuspended in 1 ml of Triton-X lysis buffer (20 mM Tris [pH 7.4], 150 mM NaCl, 10% glycerol, 1% Triton X-100, 2 mM EDTA, 2 mM NaPPi, 2 mM NaF, 0.5 mM phenylmethylsulfonyl fluoride, 1 μg each of aprotinin and leupeptin per ml, 1 mM Na3VO4). Insoluble material was removed by centrifugation, and protein concentrations were determined using a Bio-Rad Laboratories (Hercules, Calif.) protein assay kit. For immunoprecipitations, 1 mg of protein was incubated for 2 h with anti-Shc (C20) or anti-Grb2 (C23) antibodies (Santa Cruz Biotechnology, Santa Cruz, Calif.) and the antigen-antibody complexes were collected with protein A-agarose beads (Gibco-BRL, Gaithersburg, Md.). The beads were washed three times with TBST (50 mM Tris-HCL [pH 7.3], 150 mM NaCl, 0.05% Tween 20), resuspended in protein loading buffer, and boiled for 5 min at 95°C. Proteins were resolved by electrophoresis on a sodium dodecyl sulfate (SDS)-polyacrylamide gel and blotted onto nitrocellulose. Blots were blocked with 5% nonfat milk in TBST plus 0.2% Tween 20 and incubated with one of the above antibodies at room temperature for 1 h. Blots were washed three times with TBST plus 0.2% Tween 20 and incubated at room temperature for 30 min with anti-rabbit or anti-mouse immunoglobulin G antibodies conjugated to horseradish peroxidase. Blots were washed three times with TBST plus 0.2% Tween 20, and protein bands were detected by enhanced chemiluminescence (Amersham Corp., Arlington Heights, Ill. Tyrosine phosphorylation of Shc was detected using the 4G10 antiphosphotyrosine monoclonal antibody purchased from United Biotechnologies, Inc. (UBI) (Lake Placid, N.Y.).
Proliferation assay.
Cells were starved in 96-well microtiter plates at a concentration of 2 × 104 cells/well in Iscove's medium plus 1.5% FCS and various dilutions of H7-dihydrochloride (H7), staurosporine (both from Sigma Biosciences, St. Louis, Mo.), bisindolylmaleimide Boehringer Mannheim Corp., Indianapolis, Ind.), or HA1004 (Sigma Biosciences). Cells were incubated for 18 h at 37°C. The microtiter plate was then centrifuged, and medium was discarded and replaced with Iscove's Dulbecco minimal essential medium plus 20% FCS containing the inhibitors. Epo (0.3 U/ml) was added to HCD-57 cells. Cells were grown for 8 h at 37°C and then labeled by adding 10 μM bromodeoxyuridine (BrdU) to each well. Cells were grown for an additional 18 h in labeling medium. Labeling medium was removed, and cell proliferation was determined using a colorimetric cell proliferation enzyme-linked immunosorbent assay kit purchased from Boehringer Mannheim.
Kinase assays.
For kinase assays, cells were preincubated with staurosporine (40 nM), bisindolylmaleimide (20 μM), or HA1004 (40 μM) overnight and then washed twice with PBS, resuspended in medium containing 1.5% FCS and fresh inhibitors at various concentrations, and incubated for 2 h at 37°C. After 2 h, cells were either left unstimulated or were stimulated with 0.6 U of Epo/ml for 15 min. Cells were then centrifuged, and lysates were prepared as described above. For PKC kinase assays, PKC was purified from 1 mg of protein using an anti-PKC (MC5) antibody (Santa Cruz Biotechnology) which recognizes the α, β, and γ isoforms of PKC. Immunoprecipitates were washed twice with lysis buffer and twice with assay dilution buffer (20 mM MOPS [morpholinepropanesulfonic acid; pH 7.2], 25 mM β-glycerol phosphate, 1 mM sodium orthovanadate, 1 mM dithiothreitol, 1 mM CaCl2), and the cell pellet was then resuspended in 25 μl of assay dilution buffer. For each reaction, 10 μl of purified PKC was incubated for 10 min at 30°C in a 60-μl reaction mixture containing 10 μl of substrate cocktail, 10 μl of inhibitor cocktail, 10 μl of lipid activator, 10 μl of [γ-32P]ATP, and 10 μl of assay dilution buffer included in the PKC assay kit purchased from UBI. The reaction was stopped by spotting 25 μl of reaction mixture onto p81 phosphocellulose paper. The filters were washed twice with 0.75% phosphoric acid and once with acetone. Incorporation of 32P into the substrate peptide was measured by scintillation counting. Protein loading was determined by Western blot analysis using 10 μl of purified PKC.
For Raf-1 and MAPK immune complex kinase assays, protein was purified from 1 mg of lysate using anti-Raf-1 or Erk-2 polyclonal (C14) antibodies from Santa Cruz Biotechnology. Immunoprecipitates were washed twice in lysis buffer and twice in kinase buffer (20 mM Tris-HCL [pH 7.4], 20 mM NaCl, 10 mM MgCl2, 1 mM dithiothreitol). For Raf-1 kinase assays, immunoprecipitates were resuspended in 35 μl of kinase buffer containing 10 μM ATP, 20 μCi of [γ-32P]ATP, and 2 μg of glutathione S-transferase (GST)-kinase-inactive MEK (K97A) (UBI). MEK activity was assayed in 35 μl of kinase buffer containing 10 μM ATP, 20 μCi of [γ-32P]ATP, 1 μg of leupeptin/ml, 1 μM okadaic acid, and 4 μg of myelin basic protein (UBI). Reaction mixtures were incubated for 30 min at 30°C. Protein loading buffer was added, and reaction mixtures were boiled at 95°C for 10 min. Proteins were resolved on SDS-polyacrylamide gels and transferred to nitrocellulose. Phosphorylated substrate bands were visualized by autoradiography. Protein loading was determined by Western blotting of the filter.
Activated Ras interaction assay.
Ras activation was evaluated using an activated-Ras interaction assay (64) as described by de Rooij and Bos (17). The pGEX-RBD plasmid encoding the Ras-binding domain (amino acids 1 to 149) of Raf-1 fused to GST was obtained from D. Shalloway (Cornell University, Ithaca, N.Y.). Protein expression was induced in Escherichia coli by incubation with 1 mM IPTG (isopropyl-β-d-thiogalactopyranoside) for 1 to 2 h at 37°C. Cells were lysed by sonication (two times for 15 s, maximum output) and centrifuged at 28,000 × g for 20 min. Supernatants were collected, and 10% NP-40 was added to obtain a final concentration of 0.5% NP-40. Glutathione-Sepharose beads (Gibco-BRL) were washed once with cold 25 mM HEPES (pH 7.5)–150 mM NaCl and resuspended in 10 ml of HEPES lysis buffer (20 mM HEPES [pH 7.5], 120 mM NaCl, 10% glycerol, 2 mM EDTA, 10 μg of leupeptin/ml, 10 μg of aprotinin/ml). GST–Ras-binding domain (RBD) was bound to glutathione-Sepharose beads by adding 300 to 500 μl of packed beads to the supernatant and rocking for 20 to 30 min in the cold room. Beads were collected and washed six to eight times with 0.6 ml of HEPES lysis buffer plus 0.5% NP-40. For affinity purification of Ras-GTP, HCD-57 cell lines starved overnight in 1.5% FCS and either left unstimulated or stimulated with Epo for 15 min at 37°C were lysed in 1 ml of Mg-lysis buffer (25 mM HEPES [pH 7.5], 150 mM NaCl, 1% NP-40, 0.25% sodium deoxycholate, 10% glycerol, 25 mM NaF, 10 mM MgCl2, 1 mM EDTA, 1 mM Na3VO4, 10 μg of leupeptin/ml, 10 μg of aprotinin/ml). One milligram of whole-cell lysate was incubated with 15 to 30 μg of GST-RBD bound to glutathione-Sepharose (10 to 15 μl of packed beads) for 30 min at 4°C with rocking. Bound proteins were eluted with SDS-polyacrylamide gel electrophoresis sample buffer, resolved on a 10% acrylamide gel, and analyzed by Western blotting. Blots were probed with anti-pan Ras (Santa Cruz Biotechnology) antibodies.
DNA transfection and colony assays.
The pUSE plasmid expressing the N-17 dominant-negative Ras mutant (22) was obtained from UBI. The pEGFP-N2 N-terminal protein fusion vector, which expresses the green fluorescent protein (GFP) and carries the neomycin resistance gene, was obtained from Clontech (Palo Alto, Calif.). HCD-57 cells were cotransfected by electroporation (Bio-Rad Gene Pulser electroporator) with pEGFP-N2 (8 μg) and either pUSE H-rasN17 (80 μg) or the empty vector (80 μg). For electroporation, cells were resuspended in 50% medium plus 1.5% FCS and 50% PBS and 2 × 106 to 4 × 106 cells were electroporated using 0.24 kV and three consecutive pulses at 960, 500, and 250 μF. Transfected cells were identified and sorted for expression of GFP by fluorescence-activated cell sorting (FACS). Cells expressing GFP were either plated in soft-agar colony assays or lysed in Triton-X lysis buffer as previously described for Western blotting. A modification of the method of Stanley et al. (61) was used for the colony assays. Immediately following cell sorting, 104 GFP+ cells were plated in 60-mm-diameter, plates in 5 ml of medium containing 0.35% SeaPlaque agarose. Plates were incubated in a fully humidified incubator at 37°C and 5% CO2 and scored for colony formation on day 28. For some studies, stable cell lines expressing the N17 H-ras mutant were established by selecting the cells on G418 (700 μg/ml).
RESULTS
Ras-GTP-binding activity is constitutive in SFFV-infected cells.
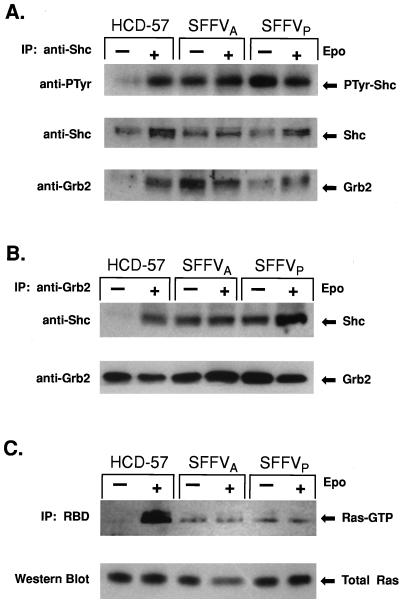
In cells stimulated with Epo, growth factor-induced tyrosine phosphorylation of Shc leads to the formation of a Grb2-Shc complex which translocates Sos1 to the membrane and activates Ras-GTP binding activity (15). We evaluated the effect of SFFV infection on tyrosine phosphorylation of Shc and formation of Grb2-Shc complexes in HCD-57 cells by Western blot analysis (Fig. 1A and B). Uninfected HCD-57 cells and HCD-57 cells infected with SFFVA or SFFVP were growth factor starved overnight and then stimulated in complete medium with or without Epo for 15 min. Shc was purified from the cells by immunoprecipitation using an anti-Shc antibody. Western blot analysis using antiphosphotyrosine antibodies (Fig. 1A) indicated that the low level of tyrosine-phosphorylated Shc expressed in uninfected HCD-57 cells before Epo stimulation was greatly increased after exposure to Epo. In contrast, HCD-57 cells infected with SFFV showed high levels of tyrosine-phosphorylated Shc both before and after Epo stimulation. The blot was stripped and reprobed with anti-Shc antibodies to verify that similar levels of Shc protein were loaded for all samples. The same blot was subsequently probed with anti-Grb2 antibodies to determine if tyrosine-phosphorylated Shc was associated with Grb2. High levels of Grb2 were detected in Shc immunoprecipitates from uninfected HCD-57 cells stimulated with Epo and in SFFV-infected HCD-57 cells in the presence and absence of Epo. As shown in Fig. 1B, the presence of a Grb2-Shc complex was confirmed by Western analysis using anti-Shc antibodies to detect Shc protein in Grb2 immunoprecipitates. Anti-Shc antibodies detected Shc in the Grb2 immunoprecipitates from uninfected HCD-57 cells stimulated with Epo and in both stimulated and unstimulated SFFV-infected HCD-57 cells. In contrast, the Grb2-Shc complex was not detected in uninfected HCD-57 cells in the absence of Epo. The presence of Sos in the Grb2-Shc complex was verified by reprobing the blot with anti-Sos antibodies. As expected, Sos, which constitutively associates with Grb2 (54), was detected in anti-Grb2 immunoprecipitates in all of the samples (data not shown).
FIG. 1.
Shc, Grb2, and Ras are constitutively activated in SFFV-infected erythroid cells. Uninfected or SFFV-infected HCD-57 cells were left unstimulated or were stimulated with Epo for 15 min. (A) Extracts were immune precipitated (IP) with anti-Shc antiserum and then immunoblotted with antiserum to phosphotyrosine (PTyr), Shc, or Grb2. (B) Extracts were immune precipitated with anti-Grb2 antiserum and then immunoblotted with antiserum to Shc or Grb2. (C) Ras-GTP was affinity purified from 1 mg of whole-cell lysate using the minimal RBD fused to GST. Ras-GTP-bound proteins were identified by blotting with anti-Ras antiserum. Ras protein levels were determined by immunoblotting 100 μg of total-cell lystate with anti-Ras antiserum.
The presence of the Grb2-Shc complex in SFFV-infected HCD-57 cells in the absence of Epo strongly suggested that Ras-GTP-binding activity might also be constitutively activated in these cells. To determine if Ras was constitutively activated in SFFV-infected cells, we used the minimal RBD of Raf-1 as a probe for detecting Ras-GTP (Fig. 1C). The RBD of Raf-1 specifically interacts with Ras-GTP and has little or no affinity for Ras-GDP, the inactive form of Ras (11, 17, 64). Uninfected HCD-57 cells and HCD-57 cells infected with SFFVP or SFFVA were starved for growth factor and then stimulated in complete medium with or without Epo for 15 min. Ras-GTP was immunoprecipitated from 1 mg of total-cell lysate using a GST-RBD fusion protein. Bound proteins were eluted and evaluated by Western blot analysis using anti-Ras antibodies (Fig. 1C). Ras-GTP was detected in HCD-57 cells stimulated with Epo but was not detected in unstimulated HCD-57 cells. In contrast, Ras-GTP was present in SFFV-infected HCD-57 cells in the absence of Epo and stimulation with Epo had little or no effect on Ras-GTP levels. Analysis of 100 μg of total-cell lysate indicated that equal amounts of Ras protein were present in the samples. However, a significantly lower level of Ras-GTP was detected in the SFFV-infected cells, even in the presence of Epo, than in HCD-57 cells stimulated with Epo.
Activated Ras is not required for the Epo-independent proliferation of SFFV-infected cells.
To determine if Ras-GTP is required for the Epo-independent proliferation of SFFV-infected cells, we evaluated the effect of a dominant-negative GTP-binding mutant of Ras, H-rasN17, on colony formation of uninfected and SFFV-infected HCD-57 cells (Table 1). Cells were transfected by electroporation with the pUSE vector expressing H-rasN17 or a control vector together with pEGFP-N2 as a selection marker. Cells expressing GFP were purified by FACS and plated in soft-agar colony assays. The effect of the H-rasN17 mutant on cell growth was evaluated by determining the extent of colony formation on day 28. H-rasN17 inhibited colony formation of HCD-57 cells stimulated with Epo by greater than 83% in comparison to colony formation of cells expressing the vector alone (Table 1). However, H-rasN17 only partially inhibited (25 to 31%) colony formation of SFFV-infected HCD-57 cells (Table 1). Furthermore, HCD-57 cells infected with SFFV were still growing in the presence of the dominant-negative Ras mutant after 3 months in culture, whereas uninfected HCD-57 cells expressing dominant-negative Ras failed to grow.
TABLE 1.
Effect of a dominant-negative Ras mutant on colony formation of uninfected and SFFV-infected HCD-57 cells
| Cells | Mean no. of colonies/104 cellsa ± SD with:
|
% Inhibitionb | |
|---|---|---|---|
| pUSE vector | pUSE H-rasN17 | ||
| HCD-57 | 83 ± 1.1 | 13 ± 0.9 | 83 |
| SFFVA | 342 ± 2.1 | 235 ± 3.0 | 31 |
| SFFVP | 291 ± 5.3 | 217 ± 2.6 | 25 |
HCD-57 cells were plated in 60-mm-diameter plates containing 0.35% SeaPlaque agarose and 5 ml of medium plus 0.6 U of Epo/ml. SFFV-infected HCD-57 cells were plated in the same medium without Epo. Colonies were counted on day 28. Values are based on results from three plates.
Percent inhibition was determined relative to the vector control.
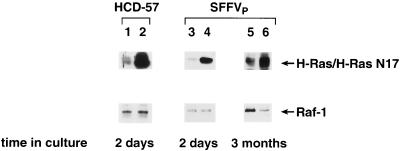
Expression of the H-rasN17 mutant was verified by Western blot analysis of protein lysates prepared from transfected cells (Fig. 2). The protein expressed by H-rasN17 is the same size as endogenous p21 Ras, but it is expressed at significantly higher levels. Expression of the dominant-negative Ras mutant in uninfected and SFFV-infected HCD-57 cells was confirmed by the high level of Ras protein expression detected in cells transfected with H-rasN17 in comparison to that in cells transfected with the vector alone (Fig. 2). Taken together, these results indicate that, unlike normal erythroid cells, which require Ras-GTP for Epo-induced proliferation, SFFV-infected erythroid cells can proliferate in the absence of activated Ras.
FIG. 2.
Expression of dominant-negative Ras in uninfected and SFFV-infected HCD-57 cells transfected with H-rasN17. Cell lysates were prepared for Western blot analysis from uninfected and SFFVP-infected HCD-57 cells cotransfected with a vector expressing the dominant-negative H-rasN17 mutant (lanes 2, 4, and 6) or a control vector (lanes 1, 3, and 5) plus the pEGFP-N2 N-terminal fusion vector expressing GFP and the neomycin resistance gene. Cells expressing GFP were purified by FACS and evaluated for H-rasN17 expression 2 days after transfection (lanes 1 to 4) or 3 months after G418 selection (lanes 5 and 6) using an anti-Ras antibody that detects both endogenous and mutant forms of the Ras protein. Protein loading was determined by immunoblotting with an anti-Raf-1 antibody.
PKC is constitutively activated in HCD-57 cells infected with SFFV.
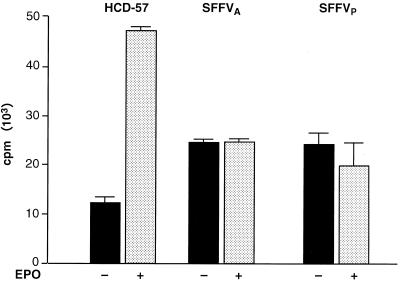
Ras-GTP activates the Raf-1/MAPK pathway by localizing Raf-1 at the cell membrane, where it is phosphorylated by other kinases. PKC has been identified as a Raf-1 kinase that can directly phosphorylate and activate Raf-1 in response to mitogen or growth factor stimulation (4, 7, 33). Therefore, we analyzed the role of PKC in activation of the Raf-1/MAPK pathway in SFFV-infected cells. We first used a PKC kinase assay to evaluate the phosphotransferase activity of PKC purified from HCD-57 cells infected with SFFV. Uninfected and SFFV-infected HCD-57 cells were starved overnight and then analyzed for PKC activity after stimulation for 15 min in complete medium with or without Epo (Fig. 3). In the absence of Epo, uninfected HCD-57 cells expressed a low level of PKC activity, and the level was greatly increased (almost fourfold) after Epo stimulation. In contrast, SFFV-infected HCD-57 cells grown in the absence of Epo expressed significantly more PKC than uninfected HCD-57 cells and this level did not increase after Epo stimulation. Interestingly, the level of activation of PKC in SFFV-infected cells grown in the presence or absence of Epo was only about 50% of that in uninfected HCD-57 cells grown in the presence of Epo. Thus, Epo induction of PKC appears to be inhibited in SFFV-infected cells.
FIG. 3.
PKC is constitutively activated in SFFV-infected erythroid cells. Uninfected and SFFV-infected HCD-57 cells were left unstimulated or were stimulated with Epo for 15 min. PKC was then purified from freshly prepared cell lysates using a pan-PKC antibody that recognizes the α, β, and γ isoforms of PKC. PKC kinase activity was determined using a PKC kinase assay that measures incorporation of 32P into a substrate peptide. Results are the means ± standard deviations of triplicate samples.
PKC inhibitors block proliferation of both uninfected and SFFV-infected HCD-57 cells.
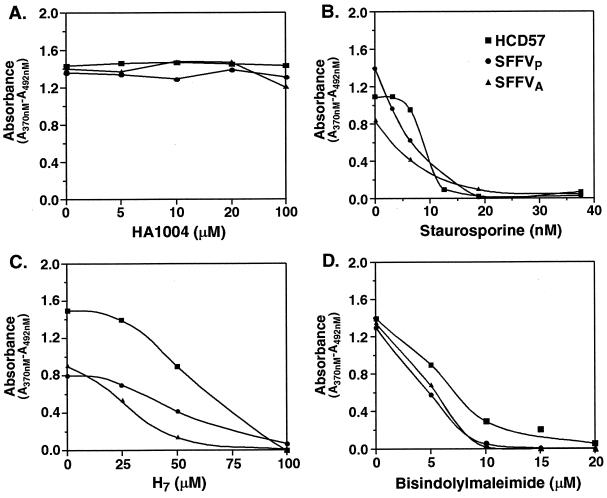
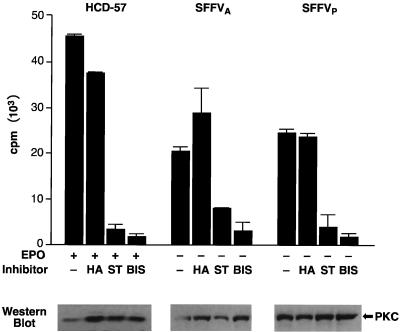
Previous experiments have demonstrated that PKC is required for Epo-induced proliferation of erythroid cells (7, 60). We evaluated the requirement for PKC in the growth factor-independent proliferation of HCD-57 cells infected with SFFV using PKC inhibitors. Cells were incubated overnight with one of three PKC inhibitors, staurosporine, H7, or bisindolylmaleimide, at various concentrations or with protein kinase A (PKA) inhibitor HA1004, which has no effect on PKC activity (49). Staurosporine and H7 are broad-spectrum protein kinase inhibitors that are PKC specific at low concentrations (30, 63), while bisindolylmaleimide is a specific inhibitor of the calcium-dependent PKC isoforms PKCα, -β1, -βII, and -γ (67). The effect of the inhibitors on cell growth was evaluated using a 3-day BrdU incorporation assay. As shown in Fig. 4, all three PKC inhibitors blocked proliferation (≥90% inhibition) of both uninfected HCD-57 cells and HCD-57 cells infected with SFFVP or SFFVA at doses compatible with a PKC-specific effect (30, 63, 67). Staurosporine and H7 almost completely blocked proliferation of both uninfected and SFFV-infected HCD-57 cells at 20 nM and 100 μM, respectively (Fig. 4B and C), while 10 or 20 μM bisindolylmaleimide was required to completely block the proliferation of SFFV-infected or uninfected HCD-57 cells, respectively (Fig. 4D). In contrast, the PKA inhibitor HA1004 had no effect on cell proliferation at concentrations as high as 100 μM (Fig. 4A). Cell viabilities of both uninfected and SFFV-infected HCD-57 cells as assessed by trypan blue exclusion counting were ≥80% for cells starved overnight in medium without inhibitors and ≥72% for cells starved overnight in medium with inhibitors.
FIG. 4.
PKC inhibitors block proliferation of SFFV-infected erythroid cells. Uninfected (■) and SFFV-infected (● and ▴) HCD-57 cells were left untreated or were treated with various concentrations of PKC inhibitors (B to D) or a PKA inhibitor (A). The effects of the inhibitors on cell proliferation were determined using a 3-day BrdU incorporation assay.
To determine if the PKC inhibitors used in this assay were specifically inhibiting PKC activity, immunoprecipitates of PKC from uninfected and SFFV-infected HCD-57 cells grown overnight in the presence or absence of staurosporine (40 nM), bisindolylmaleimide (20 μM), or HA1004 (40 μM) were evaluated in a PKC kinase assay (Fig. 5). Consistent with the results of the proliferation assay, PKC kinase activity was significantly inhibited (80 to 90%) in both uninfected and SFFV-infected HCD-57 cells treated with staurosporine or bisindolylmaleimide, while the PKA inhibitor HA1004 had no significant effect on the level of PKC activation. Western analysis of PKC purified by immunoprecipitation from untreated or inhibitor-treated cells verified that the level of PKC expression was not affected by the inhibitors (Fig. 5, lower panel).
FIG. 5.
PKC inhibitors block both Epo-dependent and Epo-independent PKC activity. Uninfected and SFFV-infected HCD-57 cells were treated with the PKC inhibitors staurosporine (ST) (40 nM) or bisindolylmaleimide (BIS) (20 μM) or with the PKA inhibitor HA1004 (HA) (40 μM) or were left untreated. PKC was purified from the cells using a pan-PKC antibody, and the effects of the inhibitors on PKC kinase activity was determined using a PKC kinase assay that measures incorporation of 32P into a substrate peptide. Results shown are the means ± standard deviations of triplicate samples. The lower panel indicates by Western blotting using a pan-PKC antibody that equivalent amounts of PKC protein were present in each sample.
PKC is required for activation of MAPK but is not required for activation of Raf-1 or MEK-1 in either uninfected or SFFV-infected HCD-57 cells.
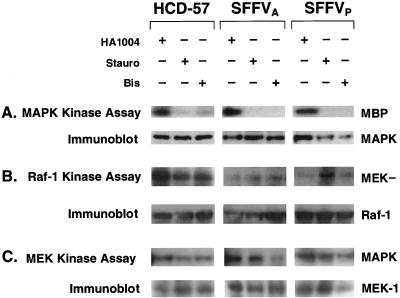
Since PKC has been shown to mediate both cell proliferation and activation of the MAPK signal transduction pathway (58, 68), we next determined if PKC was required for activation of MAPK in Epo-stimulated HCD-57 cells or HCD-57 cells infected with SFFV (Fig. 6A). HCD-57 cells stimulated with Epo and unstimulated HCD-57 cells infected with SFFV were incubated overnight in the presence of the PKC inhibitor staurosporine (40 nM) or bisindolylmaleimide (20 μM) or in the presence of PKA inhibitor HA1004 (40 μM). MAPK was purified from cell lysates by immunoprecipitation and analyzed in an immune complex kinase assay using myelin basic protein as a substrate. As shown in Fig. 6A, MAPK activity was detected in the presence of the PKA inhibitor HA1004 in HCD-57 cells stimulated with Epo and in SFFV-infected HCD-57 cells grown in the absence of Epo. In contrast, MAPK activity was significantly inhibited in both uninfected and SFFV-infected HCD-57 cells treated with the PKC inhibitor staurosporine or bisindolylmaleimide (Fig. 6A). The low level of MAPK activity remaining in uninfected HCD-57 cells treated with bisindolylmaleimide most likely reflects a requirement for higher concentrations of the inhibitor to completely block PKC activity in Epo-stimulated cells (Fig. 4D).
FIG. 6.
PKC is required for activation of MAPK but not Raf-1 or MEK in uninfected and SFFV-infected HCD-57 cells. Uninfected and SFFV-infected HCD-57 cells were treated with the PKC inhibitor staurosporine (Stauro) (40 nM) or bisindolylmaleimide (Bis) (20 μM) or the PKA inhibitor HA1004 (40 μM). Uninfected HCD-57 cells were then stimulated with Epo for 15 min, while SFFV-infected HCD-57 cells were left unstimulated. Cell lysates were assayed in immune complex assays for MAP kinase activity (A), Raf-1 kinase activity (B), and MEK kinase activity (C). Protein loading was determined by immunoblotting with the appropriate antibody. MEK−, GST-kinase-inactive MEK.
It has been previously demonstrated that Raf-1, MEK, and MAPK are constitutively activated in SFFV-infected HCD-57 cells (48). Therefore, to determine if PKC was responsible for the constitutive activation of MAPK through phosphorylation and activation of Raf-1, we evaluated the effect of the PKC inhibitors on Raf-1 kinase activity in an immune complex kinase assay (Fig. 6B). We found that Raf-1 kinase was still active in Epo-stimulated HCD-57 cells treated with staurosporine or bisindolylmaleimide, as well as in control cells treated with HA1004. Although Raf-1 kinase activity appeared to be partially inhibited in Epo-stimulated HCD-57 cells treated with the PKC inhibitors shown in Fig. 6B, this inhibition was not reproducible. Similarly, Raf-1 kinase activity could still be detected in SFFV-infected HCD-57 cells treated with the PKC inhibitors (Fig. 6B).
Finally, we evaluated the effect of the PKC inhibitors on the kinase activity of the Raf-1 substrate MEK-1, a dually specific kinase which directly phosphorylates and activates MAPK in the Raf-1/MAPK pathway. Consistent with the results of the Raf-1 kinase assay, MEK kinase activity was also unaffected by the PKC inhibitors (Fig. 6C). These results demonstrate that PKC is required for activation of MAPK but is not required for activation of Raf-1 or MEK in either HCD-57 cells stimulated with Epo or unstimulated HCD-57 cells infected with SFFV.
DISCUSSION
Previous studies from our laboratory determined that infection of the Epo-dependent erythroid cell line HCD-57 with SFFV induces constitutive activation of the Raf-1/MAPK signal transduction pathway and that activation of this pathway is required for maximum proliferation of SFFV-infected cells in the absence of Epo (48). In this study we have evaluated the upstream events involved in activation of Raf-1 and the requirement for PKC in SFFV-induced activation of the Raf-1/MAPK pathway. We have determined that both Ras and PKC are constitutively activated in HCD-57 erythroid cells infected with SFFV. We further show that, while activated Ras is required for Epo-induced proliferation of uninfected HCD-57 cells, the Epo-independent growth of SFFV-infected cells utilizes both Ras-dependent and Ras-independent mechanisms. In contrast, both uninfected and SFFV-infected HCD-57 cells required PKC for proliferation. Although PKC was not required for activation of Raf-1, it was required for activation of MAPK in both uninfected and SFFV-infected cells.
The role of Ras in activation of the Raf-1/MAPK kinase cascade in response to growth factor stimulation of tyrosine kinase receptors is well known. The first step in Ras-mediated activation of Raf-1 requires conversion of Ras-GDP to Ras-GTP, the activated form of Ras, which then binds to Raf-1 and localizes it at the cell membrane. A complex containing tyrosine-phosphorylated Shc, Grb2, and the guanine nucleotide-releasing factor Sos, which is required for activation of Ras-GTP-binding activity, was detected in both uninfected HCD-57 cells stimulated with Epo and unstimulated SFFV-infected cells. The presence of this complex correlated with expression of Ras-GTP in both the uninfected and virus-infected cells. These results indicate that Ras is activated by Epo in uninfected HCD-57 cells and that it is constitutively activated in HCD-57 cells infected with SFFV. Constitutive activation of Ras in SFFV-infected cells is consistent with our previous results demonstrating that Raf-1, a major effector of Ras function in protein tyrosine kinase-activated signaling pathways, is also constitutively activated by SFFV (48).
The requirement for activated Raf-1 in Epo-induced proliferation of erythroid cells has been previously established (6, 47). In this study, we demonstrate using the dominant-negative Ras mutant H-rasN17 that Ras-GTP is also required for the mitogenic response induced by Epo. However, the Epo-independent proliferation of SFFV-infected HCD-57 cells is only partially blocked by expression of the H-rasN17 mutant, indicating that Ras-independent mechanisms supporting cell growth have also been activated by the virus. This is consistent with results from our previous experiments in which inhibition of Raf-1 using antisense oligonucleotides almost completely inhibited proliferation of uninfected HCD-57 cells but only partially inhibited the Epo-independent proliferation of SFFV-infected HCD-57 cells (48). Both Ras-independent pathways (3, 5, 10, 25, 26, 32, 68) and Ras activation of effectors other than Raf-1 (38, 40, 42, 52) have been identified in other experimental systems. Growth of SFFV-infected cells in the absence of either Ras-GTP binding or Raf-1 kinase activity (48) suggests that other kinases or alternate signal transduction pathways are used by SFFV to promote proliferation of erythroid cells in the absence of Epo.
Our studies also show that PKC, which is activated in normal erythroid cells in response to Epo, is constitutively activated in SFFV-infected erythroid cells. While PKC inhibitors blocked both cell proliferation and activation of MAPK in uninfected and SFFV-infected HCD-57 cells, they did not inhibit activation of Raf-1 or MEK. Various isotypes of PKC have been identified as activators of Raf-1 both in vitro and in vivo (4, 7, 33), and a requirement for Ras and/or Raf-1 in PKC-mediated activation of MAPK has been demonstrated for some (4, 9, 37, 39, 58) but not all growth factors and mitogens (3, 5, 10, 26, 58, 68). Our studies indicate that while PKC is not required for either Epo- or SFFV-induced activation of Raf-1 or MEK-1 in HCD-57 cells, it is required for activation of MAPK.
The PKC inhibitors blocked MAPK activation even in the presence of activated upstream components of the Raf-1/MAPK pathway including MEK, the kinase that directly phosphorylates and activates MAPK. This result suggests that more than one signal is required for both Epo- and SFFV-induced activation of MAPK in HCD-57 cells. MEK-dependent and MEK-independent signaling pathways converging on MAPK in a cell-type specific manner have also been previously identified (19, 21, 25, 32). Alternatively, PKC may affect MAPK activity in uninfected and SFFV-infected HCD-57 cells by inhibiting a negative regulator of MAPK, as suggested by a recent study of events contributing to prolonged activation of MAPK (25).
Our results demonstrate that activation of at least two signaling pathways is required for Epo-induced proliferation of HCD-57 cells: the Ras/Raf signaling pathway and a PKC-dependent pathway converging on MAPK. Although the Epo-independent proliferation of SFFV-infected cells required activation of a PKC-dependent pathway, activation of Ras-GTP or Raf-1 was not sufficient to promote cell growth. Interestingly, infection of HCD-57 cells with SFFV seems to inhibit the Epo-induced activation of Ras-GTP and PKC in these cells compared with uninfected HCD-57 cells. It is curious that a subpopulation of SFFV-infected HCD-57 cells still require activation of the Ras/Raf-1 pathway for proliferation. Perhaps SFFV employs two distinct mechanisms for activation of Epo-independent growth, only one of which requires activation of Ras or Raf. Alternatively, this subpopulation may not express high enough levels of activated substrates in alternate growth pathways to sustain proliferation in the absence of Ras/Raf-1 activity. Finally, since the SFFV-infected HCD-57 cells used for these studies were selected on the basis of Epo independence and not virus expression, it is possible that some of the cells do not express the SFFV envelope protein and are proliferating in the absence of Epo due to nonviral activation of a Ras-dependent signal transduction pathway. Bao et al. (2) recently reported the isolation of an Epo-independent, apoptosis-resistant subclone of HCD-57 cells (HCD57-SREI) in the absence of SFFV infection. Interestingly, unlike SFFV-infected HCD-57 cells, HCD57-SREI cells do not show constitutive activation of Shc or MAPK.
Several groups have reported involvement of PI-3 kinase in activation of MAPK by growth factors, including Epo (32). PI-3 kinase is a downstream effector of Ras which has been shown to contribute to activation of Raf-1 and MAPK by a pathway that does not require PKC (21). Alternatively, it has been shown that Epo-induced recruitment of PI-3 kinase to the Epo receptor can activate MAPK by a Ras-independent, PKC-mediated mechanism (32), suggesting that PI-3 kinase may mediate the Ras-independent, PKC-dependent proliferation of SFFV-infected cells. Recent studies from our laboratory (50) indicate that PI 3-kinase is constitutively activated in SFFV-infected HCD-57 cells and that activation of this pathway is required for the Epo-independent proliferation of SFFV-infected cells. Thus, PI 3-kinase may be the primary activator of PKC in SFFV-infected cells and this may result in activation of MAPK. We have also shown that the insulin receptor substrate-related adapter molecules Gab1 and Gab2 are constitutively phosphorylated in SFFV-infected cells and associate with SHP-2, Shc, and SHIP. Formation of such multimolecular complexes may be responsible for the constitutive activation of Ras and Raf seen in SFFV-infected cells. Studies are in progress to determine if inhibitors of PI 3-kinase inhibit the activation of PKC in SFFV-infected cells.
ACKNOWLEDGMENTS
We thank D. Shalloway for the gift of the pGEX-RBD plasmid and Karen Cannon for helpful assistance in the preparation of the manuscript.
REFERENCES
- 1.App H, Hazan R, Zilberstein A, Ullrich A, Schlessinger J, Rapp U. Epidermal growth factor (EGF) stimulates association and kinase activity of Raf-1 with the EGF receptor. Mol Cell Biol. 1991;11:913–919. doi: 10.1128/mcb.11.2.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bao H, Jacobs-Helber S M, Lawson A E, Penta K, Wickrema A, Sawyer S T. Protein kinase B (c-akt), phosphatidylinositol 3-kinase, and Stat5 are activated by erythropoietin (EPO) in HCD57 erythroid cells but are constitutively active in an EPO-independent, apoptosis-resistant subclone (HCD57-SREI cells) Blood. 1999;93:3757–3773. [PubMed] [Google Scholar]
- 3.Büscher D, Hipskind R A, Krautwald S, Reimann T, Baccarini M. Ras-dependent and -independent pathways target the mitogen-activated protein kinase network in macrophages. Mol Cell Biol. 1995;15:466–475. doi: 10.1128/mcb.15.1.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cai H, Smola U, Wixler V, Eisenmann-Tappe I, Diaz-Meco M T, Moscat J, Rapp U, Cooper G M. Role of diacylglycerol-regulated protein kinase C isotypes in growth factor activation of the Raf-1 protein kinase. Mol Cell Biol. 1997;17:732–741. doi: 10.1128/mcb.17.2.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carel K, Kummer J L, Schubert C, Leitner W, Heidenreich K A, Draznin B. Insulin stimulates mitogen-activated protein kinase by a Ras-independent pathway in 3T3-L1 adipocytes. J Biol Chem. 1996;271:30625–30630. doi: 10.1074/jbc.271.48.30625. [DOI] [PubMed] [Google Scholar]
- 6.Carroll M P, Spivak J L, McMahon M, Weich N, Rapp U R, May W S. Erythropoietin induces Raf-1 activation and Raf-1 is required for erythropoietin-mediated proliferation. J Biol Chem. 1991;266:14964–14969. [PubMed] [Google Scholar]
- 7.Carroll M P, May W S. Protein kinase C-mediated serine phosphorylation directly activates Raf-1 in murine hematopoietic cells. J Biol Chem. 1994;269:1249–1256. [PubMed] [Google Scholar]
- 8.Casadevall N, Lacombe C, Muller O, Gisselbrecht S, Mayeux P. Multimeric structure of the membrane erythropoietin receptor of murine erythroleukemia cells (Friend cells) J Biol Chem. 1991;266:16015–16020. [PubMed] [Google Scholar]
- 9.Chao T S, Foster D A, Rapp U R, Rosner M R. Differential Raf requirement for activation of mitogen-activated protein kinase by growth factors, phorbol esters, and calcium. J Biol Chem. 1994;269:7337–7341. [PubMed] [Google Scholar]
- 10.Chen Q, Lin T H, Der C J, Juliano R L. Integrin-mediated activation of MEK and mitogen-activated protein kinase is independent of Ras. J Biol Chem. 1996;271:18122–18127. doi: 10.1074/jbc.271.30.18122. [DOI] [PubMed] [Google Scholar]
- 11.Chuang E, Barnard D, Hettich L, Zhang X F, Avruch J, Marshall M S. Critical binding and regulatory interactions between Ras and Raf occur through a small, stable N-terminal domain of Raf and specific Ras effector residues. Mol Cell Biol. 1994;14:5318–5325. doi: 10.1128/mcb.14.8.5318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crews C M, Erikson R L. Purification of a murine protein-tyrosine/threonine kinase that phosphorylates and activates the Erk-1 gene product: relationship to the fission yeast byr1 gene product. Proc Natl Acad Sci USA. 1992;89:8205–8209. doi: 10.1073/pnas.89.17.8205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cutler R L, Liu L, Damen J E, Krystal G. Multiple cytokines induce the tyrosine phosphorylation of Shc and its association with Grb2 in hematopoietic cells. J Biol Chem. 1993;268:21463–21465. [PubMed] [Google Scholar]
- 14.Damen J E, Mui A L-F, Puil L, Pawson T, Krystal G. Phosphatidylinositol 3-kinase associates, via its Src homology 2 domains, with the activated erythropoietin receptor. Blood. 1993;81:3204–3210. [PubMed] [Google Scholar]
- 15.Damen, J. E., L. Liu, R. L. Cutler, and G. Krystal. Erythropoietin stimulates the tyrosine phosphorylation of Shc and its association with Grb2 and a 145-Kd tyrosine phosphorylated protein. Blood 82:2296–2303. [PubMed]
- 16.Dent P, Haser W, Haystead T A, Vincent L A, Roberts T M, Sturgill T W. Activation of mitogen-activated protein kinase kinase by v-Raf in NIH3T3 cells and in vitro. Science. 1992;257:1404–1407. doi: 10.1126/science.1326789. [DOI] [PubMed] [Google Scholar]
- 17.de Rooij J, Bos J L. Minimal Ras-binding domain of Raf1 can be used as an activation-specific probe for Ras. Oncogene. 1997;14:623–625. doi: 10.1038/sj.onc.1201005. [DOI] [PubMed] [Google Scholar]
- 18.Devemy E, Billat C, Haye B. Activation of Raf-1 and mitogen-activated protein kinases by erythropoietin and inositolphosphate-glycan in normal erythroid progenitor cells: involvement of protein kinase C. Cell Signal. 1997;9:41–46. doi: 10.1016/s0898-6568(96)00095-2. [DOI] [PubMed] [Google Scholar]
- 19.Dhanasekaran N, Reddy E P. Signaling by dual specificity kinases. Oncogene. 1998;17:1447–1455. doi: 10.1038/sj.onc.1202251. [DOI] [PubMed] [Google Scholar]
- 20.Downward J. The GRB2/Sem-5 adaptor protein. FEBS Lett. 1994;338:113–117. doi: 10.1016/0014-5793(94)80346-3. [DOI] [PubMed] [Google Scholar]
- 21.Duckworth B C, Cantley L C. Conditional inhibition of the mitogen-activated protein kinase cascade by wortmannin. J Biol Chem. 1997;272:27665–27670. doi: 10.1074/jbc.272.44.27665. [DOI] [PubMed] [Google Scholar]
- 22.Feig L A, Cooper G M. Inhibition of NIH3T3 cell proliferation by a mutant ras protein with preferential affinity for GDP. Mol Cell Biol. 1988;268:3235–3243. doi: 10.1128/mcb.8.8.3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferro F E, Kozak S L, Hoatlin M E, Kabat D. Cell surface site for mitogenic interaction of erythropoietin receptors with the membrane glycoprotein encoded by Friend erythroleukemia virus. J Biol Chem. 1993;268:5741–5747. [PubMed] [Google Scholar]
- 24.Gouilleux F, Pallard C, Dusanter-Fourt I, Wakao H, Haldosen L-A, Norstedt G, Levy D, Groner B. Prolactin, growth hormone, erythropoietin and granulocyte-macrophage colony stimulation factor induce MGF-Stat5 DNA binding activity. EMBO J. 1995;14:2005–2013. doi: 10.1002/j.1460-2075.1995.tb07192.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grammar T C, Blenis J. Evidence for MEK-independent pathways regulating the prolonged activation of the ERK-MAP kinases. Oncogene. 1997;14:1635–1642. doi: 10.1038/sj.onc.1201000. [DOI] [PubMed] [Google Scholar]
- 26.Hawes B E, van Biesen T, Koch W J, Luttrell L M, Lefkowitz R J. Distinct pathways of Gi- and Gq-mediated mitogen-activated protein kinase activation. J Biol Chem. 1995;270:17148–17153. doi: 10.1074/jbc.270.29.17148. [DOI] [PubMed] [Google Scholar]
- 27.He T C, Zhuang H, Jiang N, Waterfield M D, Wojchowski D M. Association of the p85 regulatory subunit of phosphatidylinositol 3-kinase with an essential erythropoietin receptor subdomain. Blood. 1993;82:3530. [PubMed] [Google Scholar]
- 28.He T-C, Jiang N, Zhuang H, Wojchowski D M. Erythropoietin-induced recruitment of Shc via a receptor phosphotyrosine-independent, Jak2-associated pathway. J Biol Chem. 1995;270:11055–11061. doi: 10.1074/jbc.270.19.11055. [DOI] [PubMed] [Google Scholar]
- 29.Heidecker G, Kolch W, Morrison D K, Rapp U R. The role of Raf-1 phosphorylation in signal transduction. Adv Cancer Res. 1992;58:53–73. doi: 10.1016/s0065-230x(08)60290-0. [DOI] [PubMed] [Google Scholar]
- 30.Hidaka H, Inagaki M, Kawamoto S, Sasaki Y. Isoquinolinesulfonamides, novel and potent inhibitors of cyclic nucleotide dependent protein kinase and protein kinase C+ Biochemistry. 1984;23:5036. doi: 10.1021/bi00316a032. [DOI] [PubMed] [Google Scholar]
- 31.Jaster R, Bittorf T, Brock J. Involvement of phosphatidylinositol 3-kinase in the mediation of erythropoietin-induced activation of p70S6k. Cell Signal. 1997;9:175–179. doi: 10.1016/s0898-6568(96)00138-6. [DOI] [PubMed] [Google Scholar]
- 32.Klingmüller U, Wu H, Hsiao J G, Toker A, Duckworth B C, Cantley L C, Lodish H F. Identification of a novel pathway important for proliferation and differentiation of primary erythroid progenitors. Proc Natl Acad Sci USA. 1997;94:3016–3021. doi: 10.1073/pnas.94.7.3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kolch W, Heidecker G, Kochs G, Hummel R, Vahidi H, Mischak H, Finkenzeller G, Marme D, Rapp U R. Protein kinase C alpha activates RAF 1 by direct phosphorylation. Nature. 1993;364:249–252. doi: 10.1038/364249a0. [DOI] [PubMed] [Google Scholar]
- 34.Kyriakis J M, App H, Zhang X F, Banerjee P, Brautigan D L, Rapp U R, Avruch J. Raf-1 activates MAP kinase-kinase. Nature. 1992;358:417–421. doi: 10.1038/358417a0. [DOI] [PubMed] [Google Scholar]
- 35.Li N, Batzer A, Daly R, Yajnik V, Skolnik E, Chardin P, Bar-Sagi D, Margolis B, Schlessinger J. Guanine-nucleotide-releasing factor hSos1 binds to Grb2 and links receptor tyrosine kinases to Ras signalling. Nature. 1993;363:85–87. doi: 10.1038/363085a0. [DOI] [PubMed] [Google Scholar]
- 36.Marais R, Light Y, Paterson H F, Marshall C J. Ras recruits Raf-1 to the plasma membrane for activation by tyrosine phosphorylation. EMBO J. 1995;14:3136–3145. doi: 10.1002/j.1460-2075.1995.tb07316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marais R, Light Y, Mason C, Paterson H, Olson M F, Marshall C J. Requirement of Ras-GTP-Raf complexes for activation of Raf-1 by protein kinase C. Science. 1998;280:109–112. doi: 10.1126/science.280.5360.109. [DOI] [PubMed] [Google Scholar]
- 38.Marcus S, Polverino A, Chang E, Robbins D, Cobb M H, Wigler M H. Shk1, a homolog of the Saccharomyces cerevisiae Ste20 and mammalian p65PAK protein kinases, is a component of a Ras/Cdc42 signaling module in the fission yeast Schizosaccharomyces pombe. Proc Natl Acad Sci USA. 1995;92:6180–6184. doi: 10.1073/pnas.92.13.6180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marquardt B, Frith D, Stabel S. Signaling from TPA to MAP kinase requires protein kinase C, Raf and MEK; reconstitution of the signaling pathway in vitro. Oncogene. 1994;9:3213–3218. [PubMed] [Google Scholar]
- 40.Marshall M S. Ras target proteins in eukaryotic cells. FASEB J. 1995;9:1311–1318. doi: 10.1096/fasebj.9.13.7557021. [DOI] [PubMed] [Google Scholar]
- 41.Mayeux P, Dusanter-Fourt I, Muller O, Mauduit P, Sabbah M, Druker B, Vainchenker W, Fischer S, Lacombe C, Gisselbrecht S. Erythropoietin induces the association of phosphatidylinositol 3′-kinase with a tyrosine-phosphorylated protein complex containing the erythropoietin receptor. Eur J Biochem. 1993;216:821–828. doi: 10.1111/j.1432-1033.1993.tb18203.x. [DOI] [PubMed] [Google Scholar]
- 42.Minden A, Lin A, McMahon M, Lange-Carter C, Derijard B, Davis R J, Johnson G L, Karin M. Differential activation of ERK and JNK mitogen-activated protein kinases by Raf-1 and MEKK. Science. 1994;266:1719–1723. doi: 10.1126/science.7992057. [DOI] [PubMed] [Google Scholar]
- 43.Miura O, Nakamura N, Ihle J N, Aoki N. Erythropoietin-dependent association of phosphatidylinositol 3-kinase with tyrosine-phosphorylated erythropoietin receptor. J Biol Chem. 1994;269:614–620. [PubMed] [Google Scholar]
- 44.Miura O, Nakamura N, Quelle F W, Witthuhn B A, Ihle J N, Aoki N. Erythropoietin induces association of the JAK2 protein tyrosine kinase with the erythropoietin receptor in vivo. Blood. 1994;84:1501–1507. [PubMed] [Google Scholar]
- 45.Miura Y, Miura O, Ihle J N, Aoki N. Activation of the mitogen-activated protein kinase pathway by the erythropoietin receptor. J Biol Chem. 1994;269:29962–29969. [PubMed] [Google Scholar]
- 46.Morrison D K, Cutler R E. The complexity of Raf-1 regulation. Curr Opin Cell Biol. 1997;9:174–179. doi: 10.1016/s0955-0674(97)80060-9. [DOI] [PubMed] [Google Scholar]
- 47.Muszynski K W, Ruscetti F W, Heidecker G, Rapp U, Troppmair J, Gooya J M, Keller J R. Raf-1 protein is required for growth factor-induced proliferation of hematopoietic cells. J Exp Med. 1995;181:2189–2199. doi: 10.1084/jem.181.6.2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Muszynski K W, Ohashi T, Hanson C, Ruscetti S K. Both the polycythemia- and anemia-inducing strains of Friend spleen focus-forming virus induce constitutive activation of the Raf-1/mitogen-activated protein kinase signal transduction pathway. J Virol. 1998;72:919–925. doi: 10.1128/jvi.72.2.919-925.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Neveu I, Jehan F, Houlgatte R, Wion D, Brachet P. Activation of nerve growth factor synthesis in primary glial cells by phorbol 12-myristate 13-acetate: role of protein kinase C. Brain Res. 1992;570:316–322. doi: 10.1016/0006-8993(92)90596-2. [DOI] [PubMed] [Google Scholar]
- 50.Nishigaki K, Hanson C, Ohashi T, Thompson D, Muszynski K, Ruscetti S. Erythroid cells rendered Epo-independent by infection with Friend spleen focus-forming virus show constitutive activation of phosphatidylinositol 3-kinase and Akt kinase: involvement of insulin receptor substrate-related adapter proteins. J Virol. 2000;74:3037–3045. doi: 10.1128/jvi.74.7.3037-3045.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ohashi T, Masuda M, Ruscetti S K. Induction of sequence-specific DNA-binding factors by erythropoietin and the spleen focus-forming virus. Blood. 1995;85:1454–1462. [PubMed] [Google Scholar]
- 52.Olson M F, Ashworth A, Hall A. An essential role for Rho, Rac, and Cdc42 GTPases in cell cycle progression through G1. Science. 1995;269:1270–1272. doi: 10.1126/science.7652575. [DOI] [PubMed] [Google Scholar]
- 53.Penta K, Sawyer S T. Erythropoietin induces the tyrosine phosphorylation, nuclear translocation, and DNA binding of STAT1 and STAT5 in erythroid cells. J Biol Chem. 1995;270:31282–31287. doi: 10.1074/jbc.270.52.31282. [DOI] [PubMed] [Google Scholar]
- 54.Ravichandran K S, Lorenz U, Shoelson S E, Burakoff S J. Interaction of Shc with Grb2 regulates association of Grb2 with mSOS. Mol Cell Biol. 1995;15:593–600. doi: 10.1128/mcb.15.2.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ruscetti S K, Janesch N J, Chakraborti A, Sawyer S T, Hankins W D. Friend spleen focus-forming virus induces factor independence in an erythropoietin-dependent erythroleukemia cell line. J Virol. 1990;64:1057–1062. doi: 10.1128/jvi.64.3.1057-1062.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ruscetti S K. Deregulation of erythropoiesis by the Friend spleen focus-forming virus. Int J Biochem Cell Biol. 1999;31:1089–1109. doi: 10.1016/s1357-2725(99)00074-6. [DOI] [PubMed] [Google Scholar]
- 57.Schlessinger J. SH2/SH3 signaling proteins. Curr Opin Genet Dev. 1994;4:25–30. doi: 10.1016/0959-437x(94)90087-6. [DOI] [PubMed] [Google Scholar]
- 58.Schönwasser D C, Marais R M, Marshall C J, Parker P J. Activation of the mitogen-activated protein kinase/extracellular signal-regulated kinase pathway by conventional, novel, and atypical protein kinase C isotypes. Mol Cell Biol. 1998;18:790–798. doi: 10.1128/mcb.18.2.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Spangler R, Bailey S C, Sytkowski A J. Erythropoietin increases c-myc mRNA by a protein kinase C-dependent pathway. J Biol Chem. 1991;266:681–684. [PubMed] [Google Scholar]
- 60.Spivak J L, Fisher J, Isaacs M A, Hankins W D. Protein kinases and phosphatases are involved in erythropoietin-mediated signal transduction. Exp Hematol. 1992;20:500–504. [PubMed] [Google Scholar]
- 61.Stanley E R, Bartocci A, Patinkin D, Rosendaal M, Bradley T R. Regulation of very primitive, multipotent, hematopoietic cells by hematopoietin-1. Cell. 1986;45:667–674. doi: 10.1016/0092-8674(86)90781-6. [DOI] [PubMed] [Google Scholar]
- 62.Stokoe D, Macdonald S G, Cadwallader K, Symons M, Hancock J F. Activation of Raf as a result of recruitment to the plasma membrane. Science. 1994;264:1463–1467. doi: 10.1126/science.7811320. [DOI] [PubMed] [Google Scholar]
- 63.Tamaoki T, Nomoto H, Takahashi I, Kato Y, Morimoto M, Tomita F. Staurosporine, a potent inhibitor of phospholipid/Ca++ dependent protein kinase. Biochem Biophys Res Commun. 1986;135:397. doi: 10.1016/0006-291x(86)90008-2. [DOI] [PubMed] [Google Scholar]
- 64.Taylor S J, Shalloway D. Cell cycle-dependent activation of Ras. Curr Biol. 1996;6:1621–1627. doi: 10.1016/s0960-9822(02)70785-9. [DOI] [PubMed] [Google Scholar]
- 65.Todokoro K, Sugiyama M, Nishida E, Nakaya K. Activation of mitogen-activated protein kinase cascade through erythropoietin receptor. Biochem Biophys Res Commun. 1994;203:1912–1919. doi: 10.1006/bbrc.1994.2411. [DOI] [PubMed] [Google Scholar]
- 66.Torti M, Marti K B, Altschuler D, Yamamoto K, Lapetina E G. Erythropoietin induces p21ras activation and p120GAP tyrosine phosphorylation in human erythroleukemia cells. J Biol Chem. 1992;267:8293–8298. [PubMed] [Google Scholar]
- 67.Toullec D, Pianetti P, Coste H, Bellevergue P, Grand-Perret T, Ajakane M, Baudet V, Boissin P, Boursier E, Loriolle F, et al. The bisindolylmaleimide GF 109203X is a potent and selective inhibitor of protein kinase C. J Biol Chem. 1991;266:15771–15781. [PubMed] [Google Scholar]
- 68.Ueda Y, Hirai S, Osada S, Suzuki A, Mizuno K, Ohno S. Protein kinase C delta activates the MEK-ERK pathway in a manner independent of Ras and dependent on Raf. J Biol Chem. 1996;271:23512–23519. doi: 10.1074/jbc.271.38.23512. [DOI] [PubMed] [Google Scholar]
- 69.Wakao H, Harada N, Kitamura T, Mui A L-F, Miyajima A. Interleukin 2 and erythropoietin activate STAT5/MGF via distinct pathways. EMBO J. 1995;14:2527–2535. doi: 10.1002/j.1460-2075.1995.tb07250.x. [DOI] [PMC free article] [PubMed] [Google Scholar]