Abstract
Background
Initially conceptualized as a bridge to heart transplantation, the left ventricular assist device (LVAD) has become an important option for improving survival in patients with severe heart failure and poor prognosis.
Case summary
We report the case of a patient suffering from severe chronic heart failure, complicated by ST-elevation myocardial infarction due to left main coronary artery stenosis (NYHA IV, INTERMACS profile 1). Despite support with veno-arterial extracorporeal membrane oxygenation, inotropes, and catecholamine therapy, the patient’s cardiac function did not recover sufficiently. Consequently, the decision was made to proceed with LVAD implantation as destination therapy. The initial LVAD implantation was uneventful, and the patient received anticoagulant therapy according to standard operating procedure. However, pump thrombosis occurred on the first post-operative day, necessitating an LVAD exchange. Following an extended stay in the cardiac surgery intensive care unit, the patient was eventually discharged. Approximately 15 months later, the patient developed a driveline infection, involving most of the intrapericardial components of the LVAD. A second LVAD exchange was required, and the patient received a third LVAD. To mitigate the risk of recurrent infection, suppressive antibiotic therapy with ampicillin/sulbactam was initiated.
Discussion
This is the first reported case of a patient surviving three LVAD implantations and highlights an instance of off-label use of lifelong antibiotic therapy following a driveline infection.
Keywords: Veno-arterial extracorporeal membrane oxygenation, Left ventricular assist device, Pump exchange, Thrombosis, Case report
Learning points.
A lifelong anticoagulation with phenprocoumon and aspirin or clopidogrel (international normalized ratio between 2.0 and 2.5) is needed for patients with a left ventricular assist device (LVAD).
Antiseptic precautions of the driveline and exit site are necessary to minimize the occurrence of infections in patients with left ventricular assist device.
Driveline infections of LVAD are one of the most common complications after LVAD implantation.
Pump thrombosis incidence is up to 13% within the first-year post-implant.
Introduction
The left ventricular assist device (LVAD) is a medical technology born in the early 90s as a bridge to heart transplantation for patients with advanced heart failure, who carry a particularly elevated mortality risk.1 In 2001, the REMATCH clinical trial demonstrated that patients with advanced heart failure receiving LVAD, who were not candidates for cardiac transplantation, survived longer than patients at the same pre-operative stage treated with guideline-directed medical therapy (GDMT) alone (reduction of 48% in the risk of death from any cause in the LVAD group).2 The change from pulsatile devices to continuous flow devices (CF-LVAD) further increased survival, also decreasing the onset of infections, pump failure, and neurological dysfunction.3 Today, third-generation LVADs, such as the HeartMate 3, are compact and can be implanted at the apex of the left ventricle using minimally invasive techniques.4,5 Advances in biocompatible surface materials, fully internalized systems (including implantable batteries with wireless energy transfer), and synchronization of blood flow with a co-pumping aortic transvalvular stream are expected to enhance patient compliance and improve the overall efficiency of LVAD technology.6 Left ventricular assist device implantation is generally indicated for patients with chronic heart failure, persistent NYHA classes III and IV who, despite GDMT, have a poor prognosis. The Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) has subdivided NYHA III–IV patients into seven classes depending on ejection fraction.7 The best outcome after LVAD implantation was observed in INTERMACS classes 2 and 3.8 After post-operative bridging with heparin, long-term therapy with phenprocoumon [international normalized ratio (INR) 2.0–3.0] and aspirin (100 mg) is necessary.9,10
The most frequent complications in the post-operative period are stroke, right ventricular failure, gastrointestinal bleeding (due to arteriovenous malformation and angiodysplasia related to continuous flow of CF-LVAD), and driveline infections. The incidence of pump thrombosis (major complication) is low, between 0.014 and 0.05 events per year and can be attributed to device-related and non-device-related factors.11,12 Infection in LVAD patients is another major complication, with an incidence of 9%–11.2% per patient-year for driveline infections13 and a mortality up to 44%.14 The main goal in improving patient outcome is to reduce the incidence of these complications.7
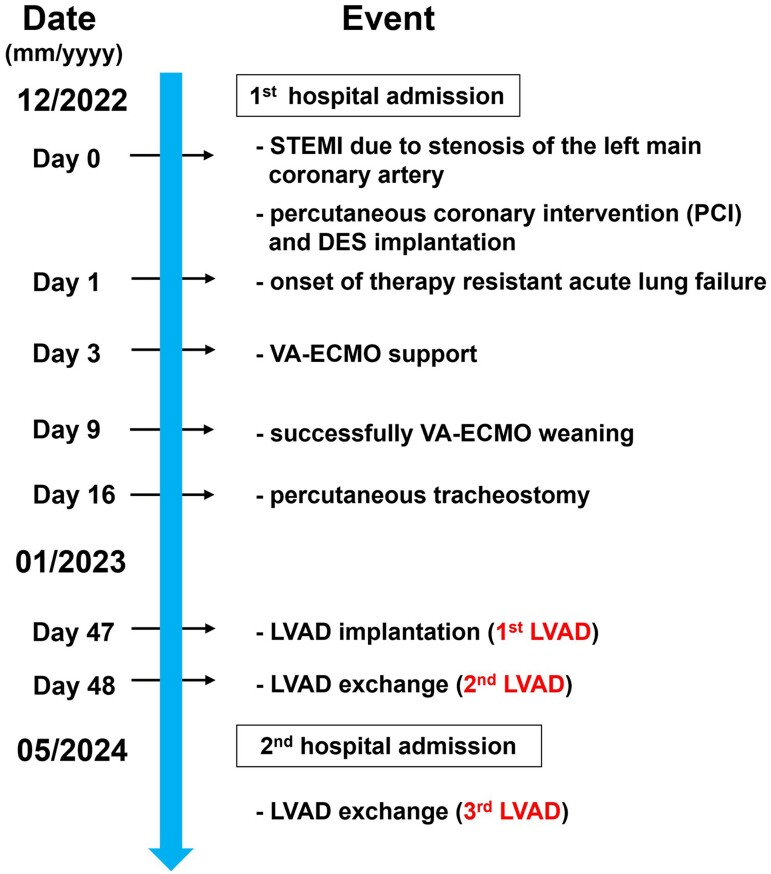
Summary figure
Timeline of the clinical history of the patient from STEMI myocardial infarction up to the third LVAD.
Case presentation
A 70-year-old male patient was admitted to the hospital presenting with typical angina and electrocardiographic signs of ST-elevation myocardial infarction (STEMI). An urgent coronary angiography revealed a high-grade stenosis of the left main coronary artery (LMCA). The occluded artery was recanalized using a drug-eluting stent via percutaneous coronary intervention. After the intervention, tachycardic arrhythmias occurred in the intensive care unit (ICU), which were treated with electrolytes and antiarrhythmics.
Chest X-ray on the first post-interventional day revealed bilateral pleural effusions and pneumonic infiltrates resulting in therapy resistant respiratory failure. On the fourth post-interventional day, the decision was made to proceed to veno-arterial extracorporeal membrane oxygenation (VA-ECMO). With targeted antibiotic therapy, it was possible to resolve the pulmonary infection. On Day 10, the patient was successfully weaned off VA-ECMO after two trials of levosimendan. Weaning proved to be difficult, and a percutaneous tracheostomy was performed subsequently. Despite ongoing inotropic and catecholamine support, it was not possible to re-establish satisfactory cardiac function. The patient was classified as NYHA IV, INTERMACS 2 which sliding rapidly to INTERMACS I. Heart team evaluation found the patient qualified for LVAD implantation as destination therapy.
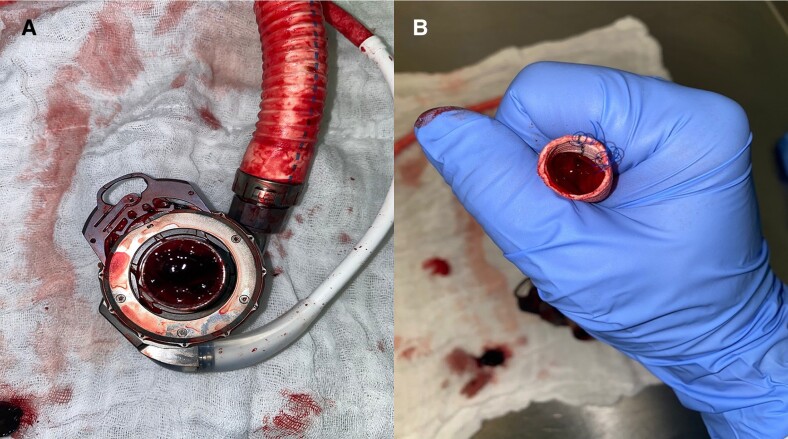
After induction of general anaesthesia and airway-management via the tracheostomy, a median sternotomy was performed. The pericardium was opened and after heparinization, the ascending aorta and the superior vena cava were cannulated in standard fashion for commencing cardiopulmonary bypass. The implantation of the first LVAD was performed without complications in Beating Heart fashion. The patient was transferred to the cardiac surgery ICU post-operatively on medium-dose inotropic and catecholamine support. On the first post-operative day, there was a sudden onset of persistent LVAD low flow (below 1 L/min), Puls Index 1.3 with a severe drop in blood pressure. Volume administration, inotropes, and catecholamines showed no effect. Laboratory values were consistent with an uneventful post-operative course. Without prior computed tomography (CT), the patient underwent emergency re-sternotomy. Inspection of the LVAD confirmed the diagnosis of thrombosis of the inflow cannula (Figure 1A) and outflow cannula (Figure 1B).
Figure 1.
Pump thrombosis of the first LVAD on the first post-operative day. The figure shows the thrombosis in the inflow cannula (A) and outflow cannula (B).
The LVAD was replaced, and the patient was transferred to ICU in stable condition. After several weeks, the patient was discharged from the hospital. Anticoagulant and antiplatelet medication consisted of phenprocoumon titrated to an INR of 2.0–3.0 and aspirin (100 mg). In the following months, regular check-ups were performed. The LVAD was functioning normally, and the patient was feeling well.
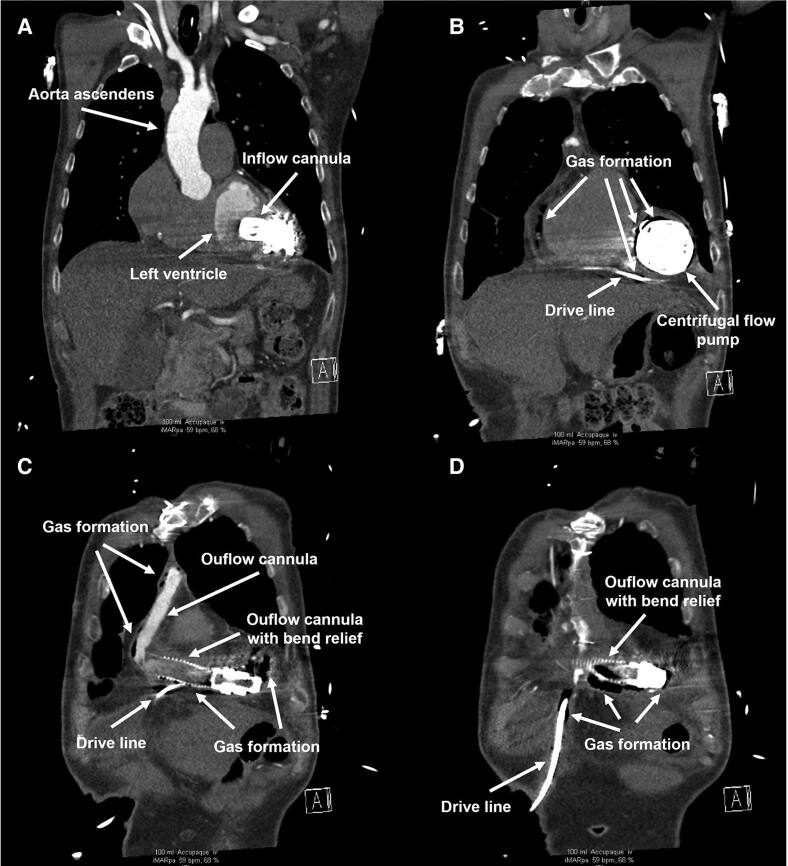
Approximately 15 months after LVAD implantation, the patient was hospitalized with a quick Sequential Organ Failure Assessment ≥ 2, suggestive of sepsis. Procalcitonin value was 0.35 ng/mL, and white blood cell count was 14.14 × 109/L. Thoracic and abdominal CT images revealed gas formation around the centrifugal flow pump, driveline, and outflow cannula (Figure 2). Furthermore, thrombotic formations were discovered in the outflow cannula.
Figure 2.
Computed tomography frontal view images after 16 months. The images show the development of gas around the components of the second LVAD (A–D): driveline, pump, outflow cannula.
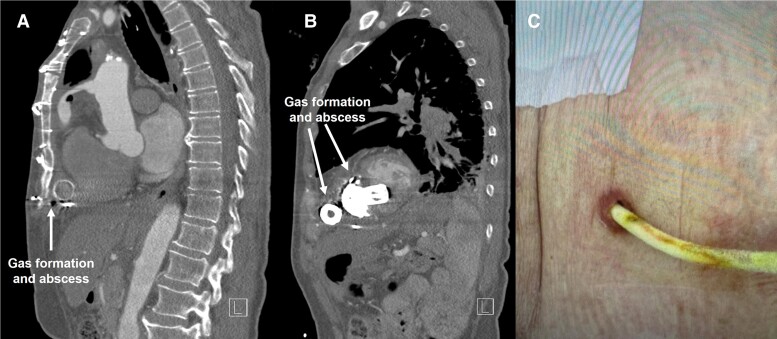
Emergent surgical exploration showed abscess formation around the pump, outflow cannula, and along the driveline to the driveline exit site (Figure 3). With exception of the inflow-sewing ring, the components of the system were replaced for the second time and the patient received the third LVAD. With irrigations vacuum, microbiological evaluation of the samples of the abscess revealed Finegoldia magna. The post-operative course in the cardiac surgery ICU was characterized by a progressive improvement in the patient’s status. The patient was gradually weaned off inotropic and catecholamine support. Antibiotic therapy with vancomycin and meropenem was sustained for six weeks. To prevent further infection of LVAD components, an off-label suppressive therapy with ampicillin/sulbactam was prescribed. The next follow-up is scheduled after 120 days.
Figure 3.
Computed tomography sagittal view images after 16 months. Gas formation and abscess around the outflow cannula (A) and centrifugal flow pump (B). Infection of the driveline exit site (C).
Discussion
The HeartMate III is a third-generation CF-LVAD that has been used successfully in Europe and Middle East with a two-year overall survival rate of 83.4%.15
We present a unique case of prolonged survival in a patient who underwent three successive LVAD implantations. The pre-existing chronic heart failure was complicated by a STEMI due to a stenosis of LMCA. Despite VA-ECMO support, it was impossible to achieve an adequate cardiac function without inotropes and catecholamines. The pre-operative status of the patient was NYHA IV and INTERMACS 2. Although heart transplantation was a viable option, the patient declined to be placed on the transplant waiting list, leading to the decision for LVAD implantation as destination therapy. The cause of pump thrombosis in the first LVAD remains unclear, as post-operative anticoagulation was managed within standard guidelines. Activated partial thromboplastin time was consistently maintained between 60 and 80 s using unfractionated heparin and repeated heparin-induced thrombocytopaenia test was negative. While pump malfunction could be a possible cause, it was not definitively proved. Driveline exit site infection is a well-documented risk in LVAD patients. In this case, suppressive antibiotic therapy with ampicillin/sulbactam was chosen based on the sensitivity profile of F. magna, as recommended by the clinical microbiology team. The off-label use of lifelong antibiotic therapy for driveline infections has been described in the literature.16 We hypothesize that the patient’s survival can be attributed to several factors: high patient compliance, normal function of the right ventricle prior to LVAD implantation (tricuspid annular plane systolic excursion was 16 mm), and partially preserved function of the left ventricular posterior wall and septum after LVAD implantation. While there are reports of patients with LVADs surviving for more than 10 years,17 to our knowledge, no case has been published regarding survival following the implantation of three LVADs.
A limitation in the management of this case was the absence of advanced coagulation testing, including assessment of coagulation factors and antibodies that might predispose to thrombotic events, which should have been performed after the initial pump thrombosis.
Lead author biography

Dr Luca Scarsella is an attending anaesthesiologist and emergency medicine physician at Helios University Hospital in Wuppertal, Germany. Originally from Italy, he completed his medical degree there before moving to Germany to pursue his residency in anaesthesiology. Dr Scarsella’s research interests centre on advanced heart failure management and infection-related outcomes in critically ill patients in the ICU, under the mentorship of Prof. Dr Serge C. Thal.
Acknowledgements
We would like to thank the clinical staff and the nurses who provided care for the patient.
Consent: The patient has provided written informed consent, confirming compliance with COPE guidelines.
Funding: This work was supported by department funding.
Contributor Information
Luca Scarsella, Department of Anesthesiology, Center for Clinical and Translational Research, Helios University Hospital Wuppertal, Heusnerstraße 40, 42283 Wuppertal, Germany.
Alexander Bentley, Department of Anesthesiology, Center for Clinical and Translational Research, Helios University Hospital Wuppertal, Heusnerstraße 40, 42283 Wuppertal, Germany.
Mohamed Ishaq Amer, Department of Cardiac Surgery, Helios University Hospital Wuppertal, Arrenberger Str.20, 42117 Wuppertal, Germany.
Serge C Thal, Department of Anesthesiology, Center for Clinical and Translational Research, Helios University Hospital Wuppertal, Heusnerstraße 40, 42283 Wuppertal, Germany.
Data availability
The data underlying this article are available in the article.
References
- 1. Berardi C, Bravo CA, Li S, Khorsandi M, Keenan JE, Auld J, et al. The history of durable left ventricular assist devices and comparison of outcomes: HeartWare, HeartMate II, HeartMate 3, and the future of mechanical circulatory support. J Clin Med 2022;11:2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rose EA, Gelijns AC, Moskowitz AJ, Heitjan DF, Stevenson LW, Dembitsky W, et al. Long-term use of a left ventricular assist device for end-stage heart failure. N Engl J Med 2001;345:1435–1443. [DOI] [PubMed] [Google Scholar]
- 3. Long JW, Kfoury AG, Slaughter MS, Silver M, Milano C, Rogers J, et al. Long-term destination therapy with the HeartMate XVE left ventricular assist device: improved outcomes since the REMATCH study. Congest Heart Fail 2005;11:133–138. [DOI] [PubMed] [Google Scholar]
- 4. Schmitto JD, Krabatsch T, Damme L, Netuka I. Less invasive HeartMate 3 left ventricular assist device implantation. J Thorac Dis 2018;10:S1692–S1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Carrozzini M, Bejko J, Gerosa G, Bottio T. Bilateral mini-thoracotomy approach for minimally invasive implantation of HeartMate 3. Artif Organs 2019;43:593–595. [DOI] [PubMed] [Google Scholar]
- 6. Varshney AS, DeFilippis EM, Cowger JA, Netuka I, Pinney SP, Givertz MM. Trends and outcomes of left ventricular assist device therapy: JACC focus seminar. J Am Coll Cardiol 2022;79:1092–1107. [DOI] [PubMed] [Google Scholar]
- 7. Kormos RL, Cowger J, Pagani FD, Teuteberg JJ, Goldstein DJ, Jacobs JP, et al. The society of thoracic surgeons Intermacs database annual report: evolving indications, outcomes, and scientific partnerships. Ann Thorac Surg 2019;107:341–353. [DOI] [PubMed] [Google Scholar]
- 8. Melendo-Viu M, Dobarro D, Raposeiras Roubin S, Llamas Pernas C, Moliz Cordón C, Vazquez Lamas M, et al. Left ventricular assist device as a destination therapy: current situation and the importance of patient selection. Life (Basel) 2023;13:1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. HeartMate III . HeartMate III. Instructions for use. Abbott. 2019. https://manuals.sjm.com/.
- 10. Yin E. Pearls in anticoagulation management for patients with left ventricular assist devices. Tex Heart Inst J 2023;50:e238154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Starling RC, Moazami N, Silvestry SC, Ewald G, Rogers JG, Milano CA, et al. Unexpected abrupt increase in left ventricular assist device thrombosis. N Engl J Med 2014;370:33–40. [DOI] [PubMed] [Google Scholar]
- 12. Fatullayev J, Samak M, Sabashnikov A, Zeriouh M, Rahmanian PB, Choi YH, et al. Continuous-flow left ventricular assist device thrombosis: a danger foreseen is a danger avoided. Med Sci Monit Basic Res 2015;21:141–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Qu Y, Peleg AY, McGiffin D. Ventricular assist device-specific infections. J Clin Med 2021;10:453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Herrmann M, Weyand M, Greshake B, von Eiff C, Proctor RA, Scheld HH, et al. Left ventricular assist device infection is associated with increased mortality but is not a contraindication to transplantation. Circulation 1997;95:814–817. [DOI] [PubMed] [Google Scholar]
- 15. Zimpfer D, Gustafsson F, Potapov E, Pya Y, Schmitto J, Berchtold-Herz M, et al. Two-year outcome after implantation of a full magnetically levitated left ventricular assist device: results from the ELEVATE Registry. Eur Heart J 2020;41:3801–3809. [DOI] [PubMed] [Google Scholar]
- 16. Eckmann C, Sunderkötter C, Becker K, Grabein B, Hagel S, Hanses F, et al. Left ventricular assist device-associated driveline infections as a specific form of complicated skin and soft tissue infection/acute bacterial skin and skin structure infection—issues and therapeutic options. Curr Opin Infect Dis 2024;37:95–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhigalov K, Mashhour A, Szczechowicz M, Mkalaluh S, Baranov A, Easo J, et al. Long-term left ventricular assist device (LVAD): a rare case of 10 years’ support and follow-up. Am J Case Rep 2019;20:1035–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article are available in the article.