Abstract
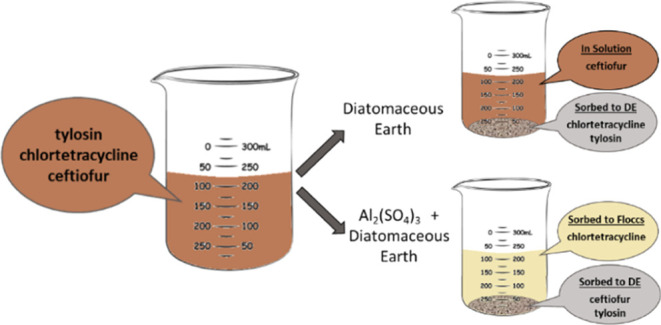
Use of antibiotics is common practice in agriculture; however, they can be released into the environment, potentially causing antimicrobial resistance. Naturally mined diatomaceous earth with bentonite was tested as a remediation material for tylosin, chlortetracycline, and ceftiofur in wastewater from a beef cattle feedlot. Langmuir binding affinity in 10 mM sodium phosphate buffer at pH 6.7 was established prior to testing wastewater to determine binding potential. Chlortetracycline was found to have a binding affinity of 15.2 mM–1 and a binding capacity of 123 mg per g of diatomaceous earth while ceftiofur showed a much lower binding affinity and loading at 7.8 mM–1 and 3 mg per g of diatomaceous earth, respectively. From spiked wastewater, tylosin (50 μg mL–1, pH 8) and chlortetracycline (300 μg mL–1, pH 6) were removed (100 and 80%, respectively) when treated with 20 mg of diatomaceous earth while ceftiofur (300 μg mL–1, pH 8) remained in solution. When the spiked wastewater was flocculated with aluminum sulfate, a change in pH from 8 to 4 was observed, and chlortetracycline was removed from the wastewater; tylosin and ceftiofur remained in solution. When subsequently treated with diatomaceous earth, ceftiofur and tylosin were completely removed by diatomaceous earth from the flocculated wastewater.
1. Introduction
Antibiotics (AB) are commonly used during animal production to ensure health and are projected to have increased use by the year 2030.1 These compounds and their degradation products pose a threat of accumulation in the environment and development of antimicrobial resistance. Orally administered AB can pass through livestock undigested2,3 and deposit in feedlot surfaces4 and wastewater ponds.5 Wastewater from these ponds is often released into the environment through irrigation systems, resulting in the environmental loading of AB and their metabolites to agricultural fields. There, they can leach from the soil after a rainfall,6 enter shallow groundwater,7 be taken up by plants,8−10 sorb to soils,11,12 and affect nontarget organisms.13 Despite being heavily researched for decades, there is a notable gap in the literature establishing methods to remediate AB in beef wastewater ponds; the objective of this work was to establish the feasibility of using readily available high-affinity binding agents to remove AB from beef wastewater.
Diatomaceous earth (DE) is the amorphous silica skeleton of algae that is deposited at the bottom of bodies of water around the world.14 Particles of DE vary in the shape, size, and purity of the deposits. It has found many commercial uses such as filtering, whitening, and use as an anticaking additive for animal feed. Most recent application research has focused on the use of DE as a support for new materials,15 remediation of aqueous bacteria,16 water desalination,17 hydrogen sorption,18 heavy metal sorption,19−23 dye sorption,24 and removal of ciprofloxacin from aqueous solutions.25
Previous studies showed the sorption of tylosin to different kinds of DE products and demonstrated an increased efficiency in binding when the organic matter covering food chemical codex grade DE was removed. A limitation of previous studies is that they were conducted using only buffered solutions26,27 which does not reflect the conditions of wastewater. To overcome these limitations, this work was performed to determine whether DE is a viable option for wastewater treatment. Diatomaceous earth was used alone and, in addition to a common wastewater treatment method, flocculation by aluminum sulfate (alum). Three antibiotics were considered: tylosin (TYL), chlortetracycline (CTC), and ceftiofur (CEF) (Figure 1). Tylosin is a macrolide antibiotic commonly used in agriculture that is structurally similar to the antibiotic erythromycin, which is commonly used in human medicine. Chlortetracycline is a broad-spectrum antibiotic in the tetracycline class that has been used to treat infections and promote animal growth while also being a prescribed antibiotic for human health. Ceftiofur is a third-generation cephalosporin antibiotic used in agriculture to treat respiratory diseases and foot rot. All three of these antibiotics have been shown to cause antimicrobial resistance in cattle pathogens.28 The objective of this work was to test the ability of DE to remove common livestock AB from beef wastewater as a standalone method and in tandem with flocculation by alum.
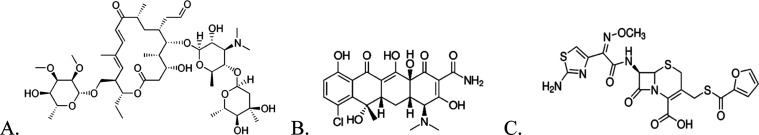
Figure 1.
(A) Structure of tylosin. (B) Structure of chlortetracycline. (C) Structure of ceftiofur.
2. Materials and Methods
2.1. Materials
Red Lake DE with calcium bentonite was purchased from Tractor Supply (Brentwood, TN). Tylosin Tartrate salt (CAS 74610–55–2) and chlortetracycline (CAS 64–72–2) were purchased from MP Biomedical (Santa Ana, CA). Ceftiofur sodium salt (CAS 104010–37–9) was purchased from Thermo Scientific. Hydrogen peroxide (30%, CAS 7722–84–1), sodium phosphate monobasic (CAS 10049–21–5), and sodium phosphate dibasic (CAS 7558–79–4) were purchased from Sigma-Aldrich (St. Louis MO). Alum powder was purchased from nuts.com
2.2. Digestion of Diatomaceous Earth Sample
Ten grams of DE was placed in a 2 L Erlenmeyer flask, 250 mL of fresh 3% hydrogen peroxide was added, briefly mixed, and then allowed to sit at room temperature for 72 h. After 72 h, 50 mL of freshly prepared 3% hydrogen peroxide was added to the flask, mixed, and observed for the evolution of small bubbles. If bubbles were observed, a fresh 450 mL of 3% hydrogen peroxide was added to the flask, mixed, and allowed to sit for another 24 h. When bubbles were no longer observed after addition of fresh hydrogen peroxide, the sample was transferred to an aluminum pan and placed in an oven at 105 °C for 4 h to dry. Once the DE was dry, the resulting cake was broken up into a fine powder using a mortar and pestle and transferred to a bottle for storage. Treated diatomaceous earth is termed DE hereafter.
2.3. Binding
Solutes were investigated by batch adsorption in a single component system in sodium phosphate buffer composed of NaH2PO4 and Na2HPO4. Increasing amounts of CTC (0.1 to 5 mg mL–1 in 10 mM sodium phosphate buffer (PB) pH 6.7) were added to tubes containing 5 mg of DE. Tubes were vortexed (30 s) and allowed to shake on a wrist action shaker for 2 h before centrifugation at 13,226g for 30 min.
Due to low solubility of CEF, increasing amounts of DE (5 to 40 mg) were placed into microcentrifuge tubes and CEF in 10 mM sodium phosphate buffer (PB) pH 6.7 was added (1 mg mL–1). Samples were vortexed, shaken, and centrifuged, as detailed in the previous paragraph.
The absorbances of AB were measured on a Thermo Scientific Nanodrop. Standard curves were established for each antibiotic using 367 nm for CTC and 292 nm for CEF, and a linear equation was applied to describe the relationship of absorbance vs concentration. The concentration of the free antibiotic in the supernatant was determined from the linear equation. Bound AB was determined from the difference between the total concentration of AB added to the sample and AB remaining in solution after binding. Langmuir isotherms were constructed using the following equation
where m is AB bound to DE (g g–1), p is the concentration of free AB (M), KL (M–1) is the Langmuir binding constant, and b (g g–1) is the maximum amount of AB sorbed per mass of adsorbent. Nonlinear isotherms were fit using the equation solver in Microsoft Excel.
2.4. Collection of Wastewater
Wastewater was collected from the primary holding pond of the beef feedlot at the US Meat Animal Research Center (Clay Center, NE) and stored in glass bottles at 4 °C until ready for use.
2.5. Wastewater Spike and Treatment
Wastewater from a beef confined animal feeding operation was measured into a glass bottle and spiked in batch adsorption, in a single component system with 50 μg mL–1 TYL, 300 μg mL–1 CTC, or 300 μg mL–1 CEF. For wastewater spiked only with chlortetracycline, the pH was adjusted to pH 5.6 using hydrochloric acid to improve solubility. Spiked wastewater was allowed to equilibrate for 5 days at 4 °C in the dark before treatment. The spiked wastewater (10 mL) was measured into a 15 mL falcon tube, and the dry DE (5, 10, or 20 for TYL and CTC; 20, 40, or 60 g for CEF) was added. Tubes were capped and placed on their sides in a wrist action shaker for 30 min at 175 rpm. Tubes were centrifuged at 512g for 5 min to pellet the diatomaceous earth. The supernatant, 700 μL, was filtered through a spin-x cellulose acetate centrifuge tube for 5 min at 13,226g to remove any suspended fine particles. For samples treated with alum, 6 mg/mL alum was added to the spiked wastewater and allowed to flocculate for 30 min. Samples were then centrifuged at 512g for 5 min and the supernatant decanted. The supernatant was then treated with diatomaceous earth as mentioned above.
2.6. UPLC Analysis
All wastewater samples were analyzed via UPLC-PDA to separate the target AB from other UV-absorbing compounds found in the wastewater. After filtration, the samples were placed into vials and injected onto a Waters Acquity H-class UPLC system. Tylosin (5 μL injected) and ceftiofur (10 μL injected) were analyzed in 80:20 methanol/water isocratic mobile phase on a Waters BEH C18 column (1.7 μm particle size, 2.1 mm × 50 mm) at 40 °C and a flow rate of 0.1 mL/min. Chlortetracycline (10 μL injected) was analyzed on a Waters BEH Shield RP18 column (1.7 μm particle size, 2.1 mm × 50 mm) at 40 °C in a 50:50 methanol/water isocratic mobile phase with a flow rate of 0.1 mL/min. Antibiotic elution peaks were measured at 289 nm for TYL, 367 nm for CTC, and 292 nm for CEF. Empower 3 software was used to integrate the area under antibiotic peaks; the percent AB removed was calculated by the difference in the area of peak before and after treatments.
3. Results and Discussion
3.1. Sorption of Chlortetracycline to DE
To assess the potential of DE as a sorption material for antibiotic removal, Langmuir binding isotherms were constructed in PB for each antibiotic. Previously published studies investigated interactions of TYL with diatomaceous earth and reported binding capacity (b) and binding affinity (KL) of TYL to DE at 88 mg g–1 and 23.5 mM–1, respectively, when assessed in 10 mM PB pH 6.7. Sorption of tylosin to diatomaceous earth is a physisorption process with charge–charge interactions being the mode of sorption at neutral pH.26 The zero point charge (pHzpc) of different types of DE was experimentally determined to be between 2.13 ± 0.03 and 1.89 ± 0.04 for all products tested.27 Because of this, the pH of the media plays an important role in the sorption of the solute to the DE surface.
Chlortetracycline showed excellent sorption to the surface of DE in aqueous PB at pH 6.7 after 2 h. The m (g g–1) and p (M) were calculated from the spectroscopic data and plotted as the y and x values, respectively (Figure 2). From this plot, the Langmuir isotherm was fit, and the Langmuir binding capacity (b) and binding affinity (KL) were calculated to be 15.2 mM–1 and 123 mg of CTC per g of DE, respectively. Chlortetracycline has three pKa values: 3.3 (−OH), 7.55 (ketodiol), and 9.3 (−NH+(CH3)2). Speciation plots of CTC as a function of pH have been previously published.29 At pH 6.7, chlortetracycline exists as a zwitterion carrying an overall net neutral charge, while DE has a negative charge. Charge–charge interactions were determined to be partially responsible for TYL binding to DE,26 so it is likely these same interactions are occurring between the positively charged dimethylammonium group of CTC and the negatively charged silanol groups on the surface of DE.
Figure 2.
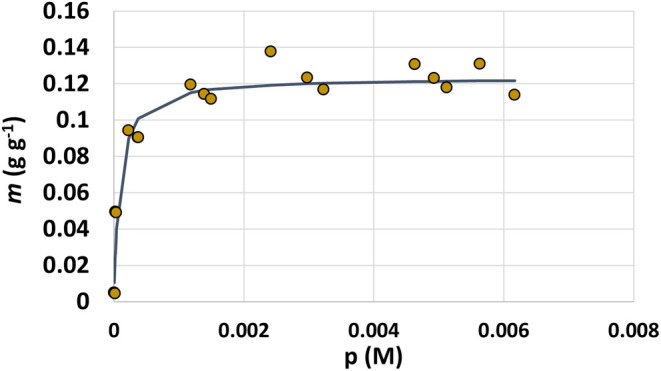
Langmuir isotherm for CTC in 10 mM PB pH 6.7 binding to DE. Chlortetracycline binding data are represented by circular data points. The isotherm is shown as the solid line fitted to the data.
3.2. Sorption of Ceftiofur to DE
Binding of CEF to DE in 10 mM PB at pH 6.7 was evaluated by Langmuir isotherm (Figure 3) by the same method as CTC and TYL. Binding capacity, m (g g–1), was calculated to be 3 mg g–1 DE with a binding affinity (KL) of 7.8 mM–1. Ceftiofur has a pKa of 2.68 corresponding to the carboxylic acid group.30 At pH 6.7, both CEF and DE carry a negative charge; charge–charge repulsions between CEF and DE most likely account for the very low binding capacity and affinity of CEF for DE when compared to those of CTC and TYL.
Figure 3.
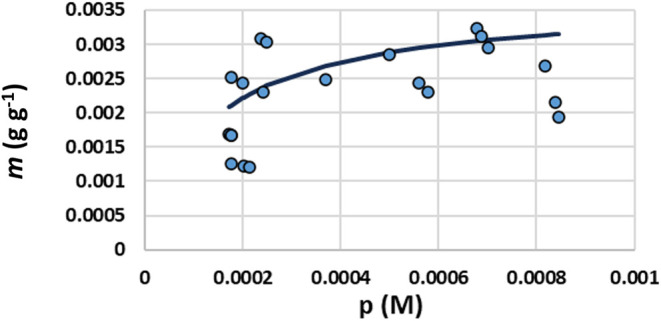
Langmuir binding isotherm for CEF binding to DE in 10 mM PB pH 6.7. Ceftiofur binding data are represented by circular data points. The isotherm is shown as the solid line fitted to the data.
3.3. Antibiotic Sorption to DE in the Absence and Presence of Alum
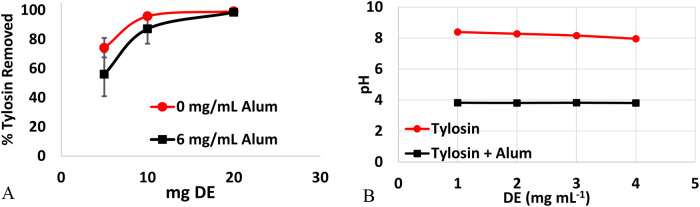
Increasing masses of DE were added to the wastewater samples spiked with TYL (300 μg mL–1), and the percent TYL removed was calculated. Tylosin removal from wastewater was considered when only DE was used and when DE treatment followed flocculation with alum (Figure 4A). At the lowest mass of DE (5 mg), 74 ± 7% of tylosin was removed. Increasing the mass of DE to 10 and 20 mg improved the removal of tylosin to 96 ± 2 and 99 ± 0.2%, respectively. In the presence of alum, a decrease in binding for 5 and 10 mg of DE was observed: 56 ± 15% and 87 ± 10%, respectively. However, at the highest mass of DE (20 mg), binding in the presence of alum remained nearly 100%. Previous research indicates the importance of charge–charge interactions between the TYL and DE.26 Upon flocculation with alum, wastewater samples were noted to have a lower pH (pH 3.8) compared to before treatment (pH 8.4 to 8.0) (Figure 4B). The pKa of TYL is 7.5, corresponding to the –NH+(CH3)2 group, which will be protonated at a pH less than 7.5 and deprotonated at a pH greater than 7.5.29 Previously published studies determined that charge–charge interactions were important in TYL sorption but not the sole interaction responsible for sorption. Therefore, binding in wastewater (pH 8.4) when TYL is neutral is most likely due to other noncovalent interactions. Addition of alum lowered the pH and created a more favorable scenario for charge–charge interactions where TYL was positively charged, while DE remained negatively charged. Because of this, it would be expected to observe an increased level of binding of TYL when the water is pretreated with alum. However, due to this not being the observed response, changes in binding efficiency are more likely due to increased competition for the sorption sites on DE and not because of the introduction of unfavorable binding conditions.
Figure 4.
(A) Tylosin removal from beef wastewater by DE as a function of increasing the mass of DE up to 99.2 ± 0.2%. When treated first with alum followed by DE (black, square), up to 98.5 ± 1.4% was removed. (B) Changes in pH of samples of tylosin wastewater after treatment with DE (red) and treatment with alum (black) prior to DE treatment.
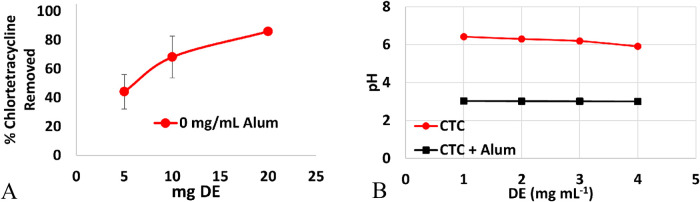
Chlortetracycline was spiked into wastewater at 300 μg mL–1; however, due to the limited solubility of CTC, the wastewater was adjusted to a pH of 6 to achieve a concentration high enough to be measured. A portion of CTC was removed from wastewater by DE when 5 mg (44 ± 12%) and 10 mg (68 ± 15%) of DE were added to wastewater. However, when 20 mg of DE was added, 86.0 ± 0.2% of CTC was removed (Figure 5A). Wastewater treated with alum prior to treatment with DE resulted in the removal of CTC in the flocculation process, and therefore, the performance of DE in the presence of alum could not be isolated. Chlortetracycline is structurally similar to tetracycline, which binds to aluminum hydrous oxides.31 The pH of samples as a function of DE was monitored to determine if binding efficiency effects were related to pH (Figure 5B). As increasing amounts of DE were added, only minor changes in pH were observed (pH 6.4–5.9). Chlortetracycline in wastewater (pH 6.4) exists as a zwitterion carrying a net neutral charge. Binding at this pH is attributed significantly to charge–charge interactions between the positively charged –NH+(CH3)2 group and the negative surface charge of DE. Addition of alum significantly decreased the pH of the samples (pH 3.0), as was observed in other AB-spiked wastewater treated with alum. Further addition of DE did not have any further effect on the pH of the samples.
Figure 5.
(A) Removal of CTC from wastewater by increasing the amount of DE up to 86.0 ± 0.2%. No data is collected for the removal of CTC by DE after treatment with alum due to CTC being removed by flocculation. (B) Change in pH as a function of DE for wastewater containing CTC and wastewater containing CTC after treatment with alum.
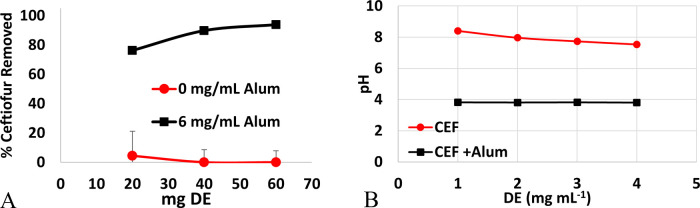
Wastewater spiked with 50 μg mL–1 CEF was used to determine the ability of DE to remove CEF from solution before and after treatment with alum. Wastewater treated with DE showed no removal of CEF from water (Figure 6A). Wastewater had a pH of 8.4, which decreased to 7.5 upon increased additions of DE (Figure 5B). At pH 7.5 and above, CEF carries a negative charge and most likely cannot bind the negatively charged surface of DE due to charge–charge repulsions. Wastewater that was treated with alum prior to addition of DE showed increased sorption of CEF to DE. This favorable binding was most likely caused by the change in pH resulting in more favorable charge–charge interactions. After flocculation with alum, wastewater had a pH of 3.8, which is near the pKa of the −COOH group on CEF and above the pHzpc of DE. When the carboxylic acid is protonated, there are no longer charge–charge repulsions between CEF and the negatively charged surface of DE, allowing for sorption through hydrogen bonds or other noncovalent interactions.
Figure 6.
(A) No CEF was removed when wastewater was treated with DE (red, circle). When wastewater was first treated with alum, followed by treatment of DE (black, square), up to 93.8 ± 0.3% of the CEF was removed. (B) Change in pH as a function of DE for wastewater containing CEF and wastewater containing CEF after treatment with alum.
4. Conclusions
Single component batch adsorption studies were conducted to determine if DE is a viable affinity binding material for the remediation of CTC and CEF from buffered aqueous solutions. It was determined that DE was capable of sorbing CTC at near-neutral pH but was much less efficient at sorbing CEF at the same pH. The success of CTC sorption by DE was most likely due to favorable charge–charge interactions between the positively charged functional group of CTC and the negatively charged surface of DE. The lower efficiency of sorption of CEF to DE was attributed to unfavorable binding between negatively charged CEF and negatively charged DE at pH 6.7. Sorption of TYL, CTC, and CEF in wastewater to DE was tested to determine whether the material could function as a viable remediation material for AB in agricultural wastewater. When wastewater was treated with DE, TYL and CTC were removed from the solution, while CEF remained in the wastewater. Sorption of TYL and CTC to DE was attributed to favorable charge–charge interactions between the negative surface of DE and the positively charged functional groups of TYL and CTC. When wastewater was flocculated with alum and decanted prior to treatment with DE, CTC was removed by the flocculation process while CEF and TYL remained in solution. Following addition of DE to flocculated wastewater, both TYL and CEF were removed from the solution, ultimately resulting in the removal of all three antibiotics in a 2-step process. Changes in the efficiency of AB sorption to DE are most likely due to the reduction in pH, from 8 to 4, which created more favorable binding conditions between the AB and DE. This work demonstrated the potential of DE to be used as a sorption medium for remediation of agricultural wastewater containing antibiotics from beef production practices.
Acknowledgments
The authors wish to thank Lisa Stone for secretarial support. Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the USDA. The USDA prohibits discrimination in all its programs and activities on the basis of race, color, national origin, age, disability, and, where applicable, sex, marital status, familial status, parental status, religion, sexual orientation, genetic information, political beliefs, reprisal, or because all or part of an individual’s income is derived from any public assistance program (not all prohibited bases apply to all programs). Persons with disabilities who require alternative means for communication of program information (Braille, large print, audiotape, etc.) should contact USDA’s TARGET Center at (202) 720-2600 (voice and TDD). To file a complaint of discrimination, write to USDA, Director, Office of Civil Rights, 1400 Independence Avenue, S.W., Washington, DC 20250-9410, or call (800) 795-3272 (voice) or (202) 720-6382 (TDD). USDA is an equal opportunity provider and employer.
Author Contributions
B.W. and B.S. conceptualized the project. B.S. designed and performed the experiments and performed the analysis. B.S., B.W., C.W., and M.S. wrote and reviewed the manuscript.
USDA appropriated funds. Project Plan 3040–63000–002–00D.
The authors declare no competing financial interest.
References
- Mulchandani R.; Wang Y.; Gilbert M.; Van Boeckel T. P. Global trends in antimicrobial use in food-producing animals: 2020 to 2030. PLOS Glob Public Health 2023, 3 (2), e0001305 10.1371/journal.pgph.0001305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinman S. E.; Matheson J. C.. Draft Environmental Impact Statement: Subtherapeutic Antibacterial Agents in Animal Feeds Food Drug Administration: Washington, DC., 1978. [Google Scholar]
- Kim K.-R.; Owens G.; Kwon S.; So S.; Lee D. B.; Ok Y. S. Occurrence and environmental fate of veterinary antibiotics in the terrestrial environment. Water, Air, Soil Pollut. 2011, 214, 163–174. 10.1007/s11270-010-0412-2. [DOI] [Google Scholar]
- Trejo B.; Russell M.; Bartelt-Hunt S.; Beni N. N.; Snow D. D.; Messer T. L. Occurrence and persistence of antibiotics administered to cattle in a newly established feedlot. J. Environ. Qual. 2023, 52, 119–1205. 10.1002/jeq2.20516. [DOI] [PubMed] [Google Scholar]
- Zhang Y.; Zhang C.; Parker D. B.; Snow D. D.; Zhou Z.; Li X. Occurrence of antimicrobials and antimicrobial resistance genes in beef cattle storage ponds and swine treatment lagoons. Sci. Total Environ. 2013, 463–464, 631–638. 10.1016/j.scitotenv.2013.06.016. [DOI] [PubMed] [Google Scholar]
- Barrios R. E.; Khuntia H. K.; Bartelt-Hunt S. L.; Gilley J. E.; Schmidt A. M.; Snow D. D.; Li X. Fate and transport of antibiotics and antibiotic resistance genes in runoff and soil as affected by the timing of swine manure slurry application. Sci. Total Environ. 2020, 712, 136505 10.1016/j.scitotenv.2020.136505. [DOI] [PubMed] [Google Scholar]
- Bartelt-Hunt S.; Snow D. D.; Damon-Powell T.; Miesbach D. Occurrence of steroid hormones and antibiotics in sallow groundwater impacted by livestock waste control facilities. J. Contam. Hydrol. 2011, 123, 94–103. 10.1016/j.jconhyd.2010.12.010. [DOI] [PubMed] [Google Scholar]
- Marques R. Z.; Wistuba N.; Brito J. C. M.; Bernardoni V.; Rocha D. C.; Gomes M. P. Crop irrigation (soybean, bean, and corn) with enrofloxacin-contaminated water leads to yield reductions and antibiotic accumulation. Ecotoxicol. Environ. Saf. 2021, 216, 112193 10.1016/j.ecoenv.2021.112193. [DOI] [PubMed] [Google Scholar]
- Tadić D.; Hernandez M. J. B.; Cerqueira F.; Matamoros V.; Piña B.; Bayona J. M. Occurrence and human health risk assessment of antibiotics and their metabolites in vegetables grown in field-scale agriculture systems. J. Hazard. Mater. 2021, 401 (5), 123424 10.1016/j.jhazmat.2020.123424. [DOI] [PubMed] [Google Scholar]
- Zhao F.; Yang L.; Chen L.; Li S.; Sun L. Bioaccumulation of antibiotics in crops under long-term manure application: Occurrence, biomass response and human exposure. Chemosphere 2019, 219, 882–895. 10.1016/j.chemosphere.2018.12.076. [DOI] [PubMed] [Google Scholar]
- Tadjine S.; Kies F. K. Insights into agricultural soil contamination by a verterinary antibiotic tylosin through continuous run-off conditions. Soil Sediment Contam. 2024, 33 (8), 1652–1673. 10.1080/15320383.2024.2323512. [DOI] [Google Scholar]
- Lin L.; Hu X.; Liang J.; Huang Z.; Yu G.; Chong Y. Adsorption of tylosin in wastewater by iron rich farmland soil and the effect of iron reduction and common cations. J. Water Reuse Desalin. 2021, 2, 248–256. 10.2166/wrd.2021.106. [DOI] [Google Scholar]
- Ribeiro A. R.; Sures B.; Schmidt T. C. Ecotoxicity of the two veterinarian antibiotics ceftiofur and cefapirin before and after photo-transformation. Sci. Total Environ. 2018, 619–620, 866–873. 10.1016/j.scitotenv.2017.11.109. [DOI] [PubMed] [Google Scholar]
- Dolley T. P.Diatomite. In U.S. Geological Survey Minerals Yearbook; USGS: Reston, VA, 2000; Vol. 1, pp 25.1–25.4. [Google Scholar]
- Przybek A. Assessment of Physico-Chemical Behavoir and soprtvity-Diatomaceous earth as support for paraffinic phase-change. Materials 2024, 17 (19), 4691 10.3390/ma17194691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson T.; Brineman R.; Schultze C.; Barkovskii A. L. Efficient removal of bacteria from aqueous media with kaolinite and diatomaceous earth products. J. Appl. Microbiol. 2020, 129 (3), 466–473. 10.1111/jam.14642. [DOI] [PubMed] [Google Scholar]
- Alsar Z.; Duskinova B.; Insepov Z. New Sorption Properties of Diatomaceous Earth for Water Desalination and Reducing Salt Stress of Plants. Eurasian Chem.-Technol. J. 2020, 22 (2), 89–97. 10.18321/ectj955. [DOI] [Google Scholar]
- Wang L.; Jin Z.; Liu R.; Huang X.; Su Y.; Li C.; Zhang Q. Hydrogen sorption capacity of diatomaceous earth for geological hydrogen storage. Int. J. Hydrogen Energy 2024, 62, 883–891. 10.1016/j.ijhydene.2024.02.376. [DOI] [Google Scholar]
- Dhanapal R.; Ravindran R.; Seethalakshmi N.; Selvakumar R. Surface functionalized diatomaceous earth for effective adsorption of strontium from aqueous solution. J. Radioanal. Nucl. Chem. 2019, 319, 1301–1306. 10.1007/s10967-018-06406-4. [DOI] [Google Scholar]
- Hamadneh I.; Al-Jundub N. W.; Al-Bshaish A. A.; Al-Dujiali A. H. Adsorption of lanthanum(III), samarium(III), europium(III) and gadolinium(III) on raw and modified diatomaceous earth: equilibrium, kinetic and thermodynamic study. Desalin. Water Treat. 2021, 215, 119–135. 10.5004/dwt.2021.26762. [DOI] [Google Scholar]
- Hamadneh I.; Alatawi A.; Zalloum R.; Albuqain R.; Alsotari S.; Khalili F. I.; Al-Dujaili A. H. Comparison of Jordanian and standard diatomaceous earth as an adsorbent for removal of Sm(III) and Nd(III) from aqueous solution. Environ. Sci. Pollut. Res. 2019, 26, 20969–20980. 10.1007/s11356-019-05294-9. [DOI] [PubMed] [Google Scholar]
- Sosa G. L.; Morantes C. F.; Flores F. M.; Sánchez R. M. T.; Zalts A.; Ramirez S. A. Characterization of diatomaceous earth modified by organic ligands for enhanced zinc adsorption. J. Environ. Chem. Eng. 2019, 7 (4), 103197 10.1016/j.jece.2019.103197. [DOI] [Google Scholar]
- Salih S. S.; Mahdi A.; Kadhom M.; Ghosh T. K. Competitive adsorption of As(III) and As(V) onto chitosan/diatomaceous earth adsorbent. J. Environ. Chem. Eng. 2019, 7 (5), 103407 10.1016/j.jece.2019.103407. [DOI] [Google Scholar]
- Semião M. A.; Haminiuk C. W. I.; Maciel G. M. M. Residual diatomaceous earth as a potential and cost effective biosorbent of the azo textile dye Reactive Blue. J. Environ. Chem. Eng. 2020, 8 (1), 103617 10.1016/j.jece.2019.103617. [DOI] [Google Scholar]
- García-Alonso J. A.; Sulbarán-Rangel B. C.; Bandala E. R.; del Real-Olvera J. Adsorption and kinetic studies of the removal of ciprofloxacin from aqueous solutions by diatomaceous earth. Desalin. Water Treat. 2019, 162, 331–340. 10.5004/dwt.2019.24313. [DOI] [Google Scholar]
- Stromer B. S.; Woodbury B.; Williams C. F. Tylosin sorption to diatomaceous earth described by Langmuir isotherm and Freundlich isotherm models. Chemosphere 2018, 193, 912–920. 10.1016/j.chemosphere.2017.11.083. [DOI] [PubMed] [Google Scholar]
- Stromer B. S.; Woodbury B.; Williams C. F. The Efficacy of Three Diatomaceous Earth Sources for Removing Tylosin from Aqueous Systems. J. Environ. Quality 2019, 48, 1863–1871. 10.2134/jeq2018.11.0409. [DOI] [Google Scholar]
- Cameron A.; McAllister T. A. Antimicrobial usage and resistance in beef production. J. Anim. Sci. Biotechnol. 2016, 7, 68 10.1186/s40104-016-0127-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiang Z.; Adams C. Potentiometric determination of acid dissociation constants (pKa) for human and veterinary antibiotics. Water Res. 2004, 38, 2874–2890. 10.1016/j.watres.2004.03.017. [DOI] [PubMed] [Google Scholar]
- Ribeiro A. R.; Schmidt T. C. Determination of acid dissociation constants (pKa) of cephalosporin antibiotics: Computational and experimental approaches. Chemosphere 2017, 169, 524–533. 10.1016/j.chemosphere.2016.11.097. [DOI] [PubMed] [Google Scholar]
- Gu C.; Karthikeyan K. G. Interaction of Tetracycline with Aluminum Iron Hydrous Oxides. Environ. Sci. Technol. 2005, 39 (8), 2660–2667. 10.1021/es048603o. [DOI] [PubMed] [Google Scholar]