Abstract
The production of secreted proteins that bind cytokines and block their activity has been well characterized as an immune evasion strategy of the orthopoxviruses vaccinia virus (VV) and cowpox virus (CPV). However, very limited information is available on the expression of similar cytokine inhibitors by ectromelia virus (EV), a virulent natural mouse pathogen that causes mousepox. We have characterized the expression and binding properties of three major secreted immunomodulatory activities in 12 EV strains and isolates. Eleven of the 12 EVs expressed a soluble, secreted 35-kDa viral chemokine binding protein with properties similar to those of homologous proteins from VV and CPV. All of the EVs expressed soluble, secreted receptors that bound to mouse, human, and rat tumor necrosis factor alpha. We also detected the expression of a soluble, secreted interleukin-1β (IL-1β) receptor (vIL-1βR) by all of the EVs. EV differed from VV and CPV in that binding of human 125I-IL-1β to the EV vIL-1βR could not be detected. Nevertheless, the EV vIL-1βR prevented the interaction of human and mouse IL-1β with cellular receptors. There are significant differences in amino acid sequence between the EV vIL-1βR and its VV and CPV homologs which may account for the results of the binding studies. The conservation of these activities in EV suggests evolutionary pressure to maintain them in a natural poxvirus infection. Mousepox represents a useful model for the study of poxvirus pathogenesis and immune evasion. These findings will facilitate future study of the role of EV immunomodulatory factors in the pathogenesis of mousepox.
Mousepox is a devastating disease of laboratory mice that continues to cause major disruption to biomedical research via outbreaks in animal facilities (14; N. S. Lipman, H. Nguyen, and S. Perkins, Letter, Science 284:1123, 1999). This infection is caused by Ectromelia virus (EV), an orthopoxvirus (OPV) that is closely related to Vaccinia virus (VV), Cowpox virus (CPV), and Variola virus (smallpox virus) (31). Like variola virus, EV has a restricted host range, causes a severe disease with a high mortality rate, and produces skin lesions in the later stages of a natural infection (18). These analogies with smallpox led to mousepox being extensively studied as an experimental model of OPV pathogenesis before the eradication of smallpox (17). However, in comparison with VV and CPV, EV has been poorly characterized at the molecular level.
The OPVs are complex cytoplasmic viruses with large, double-stranded DNA genomes that can encode more than 100 gene products. In recent years, it has been recognized that several of these gene products inhibit host cytokine responses in a number of different ways (23, 33, 42, 44). Among these immunomodulatory factors are several soluble, secreted proteins which downregulate inflammatory responses by sequestering cytokines and preventing their interaction with cellular receptors. OPV receptors and binding proteins for interleukin-1β (IL-1β) (vIL-1βR), tumor necrosis factor (TNF) (vTNFR), alpha/beta interferon (IFN-α/β) (vIFN-α/βR), IFN-γ, IL-18, and CC chemokines (vCKBP) are expressed by VV and CPV. Analysis of genomic sequence information suggests that several of these are also expressed by variola virus (37, 39).
Since EV may well be a natural mouse pathogen and the pathogenesis of mousepox has been extensively characterized in the past, it is clearly a preferred model for studying the role of OPV immunomodulatory factors, such as soluble cytokine receptors, in viral pathogenesis. However, to date, information regarding the expression and in vitro or in vivo characterization of such gene products for EV is scarce. In vitro characterizations of EV receptors and binding proteins for IFN-γ (32), TNF (25), and IL-18 (10, 43) have been published, and secreted vIFN-α/βR has been detected in EV-infected cell supernatants (13); however, there is no information concerning EV homologs of vIL-1βR or vCKBP. In addition, there has been no study of the differential expression of such gene products by EVs isolated from geographically and temporally distinct outbreaks in laboratory mouse colonies. Such studies have proved useful in the identification of novel VV gene products not expressed by the most commonly used strains (1, 5). Additionally, since soluble cytokine receptors have important effects on the pathogenesis of VV infection in mice (2, 45, 46), knowledge of their expression by EV is relevant to the choice of strain to be used in in vivo studies with this virus. Finally, it is possible that EV immunomodulatory genes have undergone recent evolutionary adaptations which influence the specific disease phenotype produced by this highly virulent pathogen.
We have characterized the expression, binding properties and, where necessary, gene sequences of EV homologs of vCKBP, vTNFR, and vIL-1βR in 12 distinct strains and isolates of EV. Our main findings were (i) that functional forms of vCKBP, vIL-1βR, and vTNFR are expressed by EV; (ii) that there is very little variability between different isolates with regard to their expression of these (glyco)proteins; and (iii) that the EV vIL-1βR has undergone significant adaptation to function in the mouse system, distinguishing it clearly from the homologous proteins of VV and CPV.
MATERIALS AND METHODS
Cells and viruses.
BSC-1 (African green monkey kidney) cells were cultured in Glasgow minimum essential medium supplemented with 10% fetal calf serum (FCS). Human U937 cells and mouse EL4 6.1 C10 cells, which express a large number of IL-1 binding sites (26), were cultured in RPMI 1640 medium supplemented with 10% FCS. Spodoptera frugiperda (Sf-21) insect cells and Autographa californica nuclear polyhedrosis virus were cultured in TC-100 medium (Sigma) containing 10% FCS.
VV strains Lister and Western Reserve (WR), recombinant VVs WR vΔB15R (4) and Lister Δ35K (36), CPV strain Brighton Red (BR), and all EV isolates were grown in BSC-1 cells. VV strains Lister and WR and CPV strain BR were obtained from G. L. Smith (Sir William Dunn School of Pathology, University of Oxford). VV Lister Δ35K was provided by A. H. Patel (Institute of Virology, Glasgow, United Kingdom). EV isolates were obtained from the following sources: Hampstead, Moscow, and Mill Hill (original stocks from K. Dumbell) from J. Williamson (St. Mary's Hospital, Imperial College School of Medicine, London, United Kingdom); Ishibashi I-111 (Ishibashi) from Y. Ichihashi (Faculty of Medicine, Niigata University, Niigata, Japan); Naval Medical Research Institute (Naval) and plaque-purified Moscow (Mos-3-P2) from R. M. L. Buller (School of Medicine, Saint Louis University); MP-1, MP-2, MP-3, MP-4, and MP-5 from H. Meyer (Institute of Microbiology, Federal Armed Forces Medical Academy, Munich, Germany); Weill Medical College of Cornell University (Cornell) from H. Meyer and N. Lipmann (Weill Medical College of Cornell University, New York, N.Y.); and Hampstead Egg from A. Mullbacher (John Curtin School of Medical Research, Australian National University, Canberra, Australia). The viral species of all OPVs were confirmed by diagnostic PCR amplification of the gene encoding the A-type inclusion body protein followed by restriction enzyme analysis (30).
Reagents.
Recombinant human 125I-macrophage inflammatory protein 1α (MIP-1α), 125I-RANTES (regulated upon activation, normal T-cell expressed, and secreted), and 125I-IL-8 (all at 2,000 Ci/mmol) were obtained from Amersham (Little Chalfont, United Kingdom). Recombinant human 125I-TNF alpha (TNF-α) (58.3 μCi/μg), 125I-fractalkine (soluble form, 2,200 Ci/mmol), and 125I-IL-1β (107 μCi/μg) were obtained from DuPont-New England Nuclear. Recombinant mouse IL-1β was obtained from R & D systems and radioiodinated to a specific activity of 2.2 × 108 cpm/μg using the Iodogen method (28). The following cytokines used in competition experiments were all obtained from Peprotech EC: recombinant human RANTES, MIP-1α, IL-8, growth-related oncogene alpha (GRO-α), lymphotactin, fractalkine (soluble form), TNF-α, TNF-β (lymphotoxin alpha [LT-α]), and IL-1β; recombinant mouse RANTES, MIP-1α, KC (also known as N51 or the mouse homolog of GRO-α), and IL-1α; and recombinant rat TNF-α.
Preparation of medium for binding assays.
FCS-free medium from OPV-infected BSC-1 or baculovirus-infected Sf-21 cell cultures was collected at 2 (OPV) or 3 (baculovirus) days postinfection. BSC-1 cell supernatants were adjusted to 20 mM HEPES (pH 7.4), and any infectious VV, EV, or CPV present in them was inactivated with 4,5′,8-trimethypsoralen and exposure to UV light (47). The binding medium was RPMI 1640 containing 20 mM HEPES (pH 7.4) and 0.1% (wt/vol) bovine serum albumin. In some instances, supernatants were concentrated and dialyzed against phosphate-buffered saline as described previously (4, 5).
Binding assays.
In cross-linking experiments with 125I-chemokine and 125I-IL-1β, supernatants from 104 OPV-infected BSC-1 cells or baculovirus-infected Sf-21 cells were incubated with radioiodinated cytokine or chemokine in a volume of 25 μl for 2 h at room temperature. Viral proteins were cross-linked to labeled cytokines or chemokines with 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide (EDC; 40 mM) or ethylene glycol-bis-succinamidyl succinate (EGS; 1 mg/ml) (5). Samples were then analyzed by denaturing sodium dodecyl sulfate (SDS)–12.5% polyacrylamide gel electrophoresis (PAGE) and autoradiography. In competition assays with U937 cells, supernatants were incubated in a final volume of 100 μl with 67 pM 125I-chemokine, 400 pM mouse 125I-IL-1β, or 190 pM human 125I-IL-1β for 1 h at 4°C. Subsequently, 2.5 × 106 U937 or EL4 6.1 C10 cells were added in 50 μl of binding medium before 2 h of incubation at 4°C. The amount of bound 125I-chemokine was determined by phthalate oil centrifugation as previously described (4, 5). Binding assays with human 125I-TNF-α and human and mouse 125I-IL-1β were performed by precipitation of receptor-ligand complexes with polyethylene glycol and filtration as described previously (1, 4).
Extraction of viral DNA and sequencing of viral genes.
Viral DNA was prepared from BSC-1 cells infected with EV by extraction from viral cores (15). The vCKBP genes from EV strains Mill Hill and Hampstead were PCR amplified with Taq DNA polymerase using the upstream oligonucleotide EVCKBP-3 (5′-TTATAGTAAGTTTTTTACCC-3′), based on the sequence in EV strain Moscow of the neighboring TNFR II homolog pseudogene (accession no. U86380), and the downstream oligonucleotide EVCKBP-4 (5′-TTTGTGAATGTAGTTAAGAAC-3′), based on the sequence of the 35-kDa vCKBP gene in VV strain Lister (36). The EV Hampstead homolog of the OPV vIL-1βR gene was amplified by PCR with Taq DNA polymerase using two oligonucleotides based on the sequence of VV WR (41). The upstream oligonucleotide was B15R-8 (5′-GAGTTGTACATCTTGAC-3′), and the downstream oligonucleotide, based on the sequence of the neighboring B16L gene, was EVB16L-1 (5′-CAATATAGAATTAGTTAGGGC-3′). DNA sequencing was carried out by the DNA sequencing service of the Department of Biochemistry, University of Cambridge, and the sequence data were analyzed using the GCG software package.
Expression in the baculovirus system.
The EV Hampstead vIL-1βR gene was produced as a recombinant protein fused to a C-terminal His6 tag in the baculovirus system. The EV vIL-1βR was amplified by PCR with Pfu DNA polymerase using viral DNA as a template. The two oligonucleotides used, EVB15RH (5′-CCGAAGCTTATGAGTGCATTATTGACTATTC-3′) and EVB15RN (5′-CGCGCGGCCGCCTTTATGCTAATAGTAAGTG-3′), provided HindIII and NotI restriction enzyme sites at the 5′ and 3′ ends of the open reading frame (ORF), respectively. The DNA fragment was cloned into HindIII- and NotI-digested pBAC-1 to produce pVS1. The DNA sequence of the insert was confirmed not to contain mutations by sequencing. The recombinant baculovirus AcEVIL1R (AcVS1) was produced as described previously (5). The recombinant baculoviruses AcB15R, AcA53R, and Ac35K have been described before (1, 4, 5).
Nucleotide sequence accession numbers.
The GenBank accession numbers for the sequences reported in this paper are AJ277111, AJ277112, and AJ277110.
RESULTS
EV isolates.
We wanted to assess the degree of variation in the expression of immunomodulatory gene products by different EVs, whether occurring naturally or induced by passage in the laboratory. Therefore, we assembled a collection of EV isolates from temporally and geographically diverse laboratory mousepox outbreaks as well as several strains derived from them. These included EV Hampstead (1930), the first EV to be isolated (27); the highly virulent EV Moscow isolate (1947) (7); several isolates from German and Austrian outbreaks (EVs MP-1, MP-2, MP-3, MP-4, and MP-5) (34); and two recent isolates from the United States, EVs Naval (1995) and Cornell (1998) (14; Lipman et al., Letter). We also obtained two plaque-purified viruses, EV Mos-3-P2 (12), which is derived from EV Moscow, and EV Ishibashi I-111 (22), as well as two egg-passaged derivatives of the Hampstead isolate, EVs Hampstead Egg (16) and Mill Hill. Both EV Hampstead Egg and EV Ishibashi I-111 have been reported to show substantially lower virulence than other isolates after footpad inoculation of mice (18, 19). The results obtained here were identical for EV Mos-3-P2 strain and its parental Moscow isolate; therefore, only those for the latter are shown.
Expression and binding specificity of the EV homolog of vCKBP.
While it is known that several strains of VV (including VV Lister) and CPV (including CPV BR) express a secreted vCKBP with specificity for CC chemokines (5, 20, 40), no data are currently available concerning the expression, activity, or gene sequence of any EV homolog of this protein. Supernatants from BSC-1 cells that were uninfected (mock) or infected with VV, WR, VV Lister, VV Lister Δ35K, CPV BR, or one of the EVs described above were screened for the presence of secreted vCKBP by cross-linking to human 125I-MIP-1α (Fig. 1a, upper panel). As reported previously (5), a 125I-MIP-1α–vCKBP complex was observed with VV Lister and CPV BR but not with mock, VV WR, or VV Lister Δ35K. VV WR expresses a truncated 7.5-kDa protein from the vCKBP gene that has no chemokine binding activity, and VV Lister Δ35K is an insertion mutant with an inactivated vCKBP gene (36, 48). Inclusion of an excess of unlabeled MIP-1α inhibited the formation of a cross-linked 125I-chemokine–vCKBP complex in the VV Lister supernatant, demonstrating the specificity of chemokine binding. Complexes between the labeled chemokine and secreted vCKBP were formed in all of the EV supernatants except that of EV Mill Hill. The EV complexes were the same size as the chemokine-vCKBP complex from CPV BR (35 kDa) and slightly smaller than the VV Lister complex. A similar experiment was performed with human 125I- RANTES (Fig. 1a, lower panel), and a 125I-RANTES–vCKBP complex was observed with all 10 of the EV isolates tested (EVs Cornell and Mill Hill were not included).
FIG. 1.
Expression and binding specificity of the EV vCKBP. (a) Media from BSC-1 cell cultures uninfected (mock) or infected with the indicated viruses were incubated with 400 pM human 125I-MIP-1α (upper panel) or human 125I-RANTES (lower panel) and treated with the cross-linker EDC before SDS-PAGE and autoradiography. Positions of the 125I-chemokine (upper panel only) and 125I-chemokine–vCKBP complexes and molecular size markers are indicated. Hamp., Hampstead. VVL, VV Lister; Cold CK, 1,000-fold molar excess of unlabeled homologous chemokine was included. (b) Cross-linking of 125I-RANTES to media from EV- and VV-infected BSC-1 cell cultures in the absence (NC) or presence of a 50-, 250-, 1,000-, or 2,000-fold molar excess of the indicated human (h) or mouse (m) chemokine; Lptn, lymphotactin. (c) Cross-linking of human 125I-RANTES to media from BSC-1 cell cultures infected with the indicated viruses in the absence or presence of a 50-, 250-, 1,000-, 2,000-, or 5,000-fold molar excess of soluble human (h) fractalkine.
The chemokine binding specificity of the vCKBP observed in EV supernatants was determined by performing a series of competition experiments with human 125I-RANTES and several different unlabeled chemokine competitors. The results obtained were essentially identical for EV Hampstead and VV Lister supernatants (Fig. 1b). The four CC chemokines tested, human and mouse RANTES and MIP-1α, competed effectively with 125I-RANTES for binding to the EV or VV vCKBP, while neither human IL-8, human GRO-α, and mouse KC (CXC chemokines) nor human lymphotactin (C chemokine) could inhibit the formation of a 125I-RANTES–vCKBP complex at any of the doses tested. The binding specificity of the vCKBP was similar in nine other EV isolates (EV Cornell was not tested), as demonstrated in competition experiments in which a 2,000-fold molar excess of either human or mouse RANTES, human IL-8, mouse KC, or human lymphotactin was used (data not shown).
Although it is clear that the vCKBPs of VV, CPV, and EV bind CC chemokines with much higher affinities than those of the CXC or C class, there have been no published investigations of their binding of the fourth class of chemokine, the CX3C class, as represented by the membrane-bound chemokine fractalkine (neurotactin) (9). Here, a soluble form of human fractalkine was used as an unlabeled competitor in a cross-linking assay with human 125I-RANTES and VV Lister, CPV BR, and EV Hampstead supernatants (Fig. 1c). With each virus, fractalkine failed to inhibit the formation of a 125I-RANTES–vCKBP complex, even when present at a 5,000-fold molar excess over the labeled ligand. In addition, experiments with human 125I-fractalkine and the cross-linkers EDC and EGS failed to produce a complex with vCKBP in any of the above-mentioned VV, CPV, and EV supernatants, excluding those of EVs Cornell and Mill Hill, which were not tested (data not shown).
The vCKBP of EV blocks the binding of CC chemokines to cellular receptors.
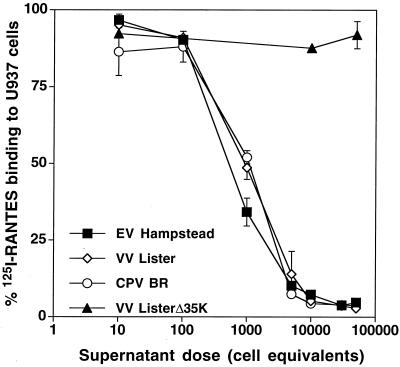
We confirmed that vCKBP from EV Hampstead can prevent the specific binding of 125I-RANTES to chemokine receptors on human U937 cells (Fig. 2) and is therefore likely to be functional as a chemokine inhibitor in vivo. Preincubation of the 125I-chemokine with supernatants from as few as 10,000 EV Hampstead-, VV Lister-, or CPV BR-infected BSC-1 cells reduced its binding to U937 cells to background levels. The dose dependence of this effect was similar for each of the three viruses. Supernatants from VV Lister Δ35K, a vCKBP insertion mutant, did not significantly reduce the binding of 125I-RANTES at any of the doses tested.
FIG. 2.
Competitive inhibition of chemokine-receptor binding by the EV vCKBP. U937 cells were incubated with human 125I-RANTES that had been preincubated with various doses of medium equivalent to the indicated numbers of BSC-1 cells infected with different viruses. Cells were then separated from soluble radioactivity by phthalate oil centrifugation, and cell-bound radioactivity was measured with a gamma counter. Data are expressed as the mean and standard deviation of duplicate determinations. In the absence of viral supernatant, total radioactivity bound to the cells was 5,598 ± 341 cpm (n = 8), while in the presence of a 1,000-fold excess of unlabeled human RANTES, it was 159 ± 3 cpm (n = 2), or 2.8% of the total binding.
Sequence of the EV vCKBP gene.
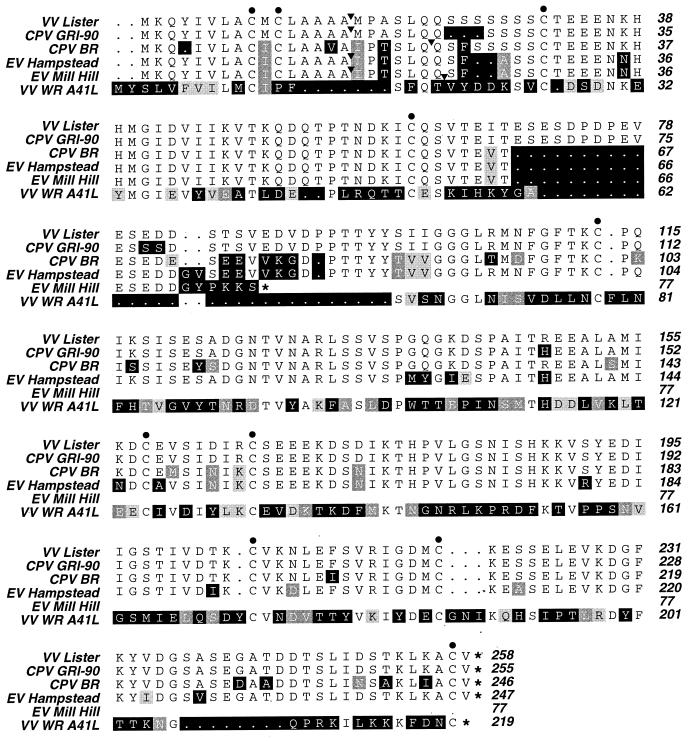
We sequenced a segment of the genome of EV Hampstead that contained a predicted vCKBP ORF encoding 247 amino acids (Fig. 3). The amino acid sequence of the EV vCKBP shared between 85.8 and 87.3% identity with predicted vCKBP sequences from VV Lister (36), CPV BR (21), and CPV GRI-90 (38). The EV Hampstead sequence contained a deletion of 10 amino acids corresponding to residues 69 to 78 of the VV Lister sequence. These amino acids were also absent in the CPV BR sequence (21) but were present in the sequence of another CPV strain, GRI-90 (38). This deletion and a stretch of 21 adjacent, variable amino acids in the C-terminal portion were found to align with a 29-amino-acid deletion in the amino acid sequence of the related VV protein encoded by the A41L ORF (41).
FIG. 3.
Comparison of the predicted amino acid sequences of OPV vCKBPs. The top five amino acid sequences are from the OPV vCKBP genes (accession numbers D00612, Y15035, J02066, AJ277111, and AJ277112), whereas the bottom sequence is from the related A41L (SalF3L) gene from VV WR (accession number D11079). In this alignment, residues which differ from the corresponding position in the VV Lister sequence are highlighted. Identical amino acids are black on white, nonconservative substitutions are white on black, semiconservative substitutions are white on dark grey, and conservative substitutions are black on pale grey. The predicted sites of N-terminal secretory signal peptide cleavage are indicated by arrowheads. Filled circles indicate the positions of invariant cysteine residues. Dots indicate amino acid deletions, and asterisks indicate stop codons.
Sequencing of the vCKBP gene in EV Mill Hill provided an explanation for the failure to observe vCKBP activity in medium from cells infected with this virus. The predicted vCKBP ORF in this EV was truncated by a stop codon after the sequence encoding amino acid 77; this stop codon was introduced by a frameshift mutation in which the first base of codon 73 was deleted. Otherwise, the sequence determined for EV Mill Hill was identical to that determined for EV Hampstead.
Expression of soluble TNFRs by EVs.
The Moscow strain of EV expresses a secreted vTNFR (CrmD) that is also present in CPV BR (25) but does not express functional homologs of the other two known OPV vTNFRs, CrmB and CrmC. A full-length, presumably functional CrmD gene has also been sequenced in EVs MP-3, MP-4, and Munich-SF (25). We used a filter-based binding assay (1) to screen EV culture supernatants for the presence of secreted 125I-TNF binding proteins and examined their binding specificity by including unlabeled cytokine competitors in the assay. We detected specific binding of human 125I-TNF-α in all EV supernatants tested (Fig. 4a). In most instances, the signal was of an intensity similar to that obtained with CPV BR supernatants but was lower than that detected with VV Lister supernatants. Expressed as a proportion of the 125I-TNF-α binding signal observed with VV Lister, the EV Cornell binding signal was slightly more intense than those of the other EVs. Experiments in which molar excesses of unlabeled human, mouse, or rat TNF-α were included in binding assays revealed that they bound to vTNFRs from EV Hampstead, but no significant binding of human LT-α to soluble receptors could be demonstrated with these assays (Fig. 4b). This EV TNF binding specificity was similar to that found for VV Lister and CPV in such binding assays (Fig. 4b) (1).
FIG. 4.
Expression and binding specificity of secreted EV vTNFRs. (a) Secretion of vTNFRs by 12 different EVs. Supernatants from 5.4 × 104 BSC-1 cells infected with the indicated viruses were incubated with 300 pM human 125I-TNF-α at room temperature for 2 h. Receptor-bound 125I-TNF was separated from free 125I-TNF by filtration through Whatman GF/C glass fiber filters after polyethylene glycol precipitation. Specific 125I-TNF binding was determined by subtraction of background with medium from values obtained with mock-infected cells. Data are expressed as the mean and standard deviation of four separate determinations. Hamp., Hampstead. VVL, VV Lister; Cold hTNFα, 500-fold molar excess of unlabeled human TNF-α was included. (b) Binding specificity of vTNFRs from EV Hampstead, VV Lister, and CPV BR. Binding assays with human 125I-TNF-α and the indicated supernatants were performed as for panel a in the absence or presence of the indicated molar excesses of unlabeled human, mouse, or rat TNF-α or human LT-α. Data represent the percentage of binding in the absence of a competitor and are expressed as the mean and standard deviation of duplicate (VV Lister and CPV BR) or triplicate (EV Hampstead) determinations after subtraction of background 125I-TNF binding.
Several strains of VV, including VV Lister, have been reported to express a membrane-associated TNF binding activity at the surface of infected cells (1). We screened for the presence of this activity in EV by determining the binding of human 125I-TNF-α to BSC-1 cells either uninfected or infected with EV Moscow, Hampstead, or Naval. However, under conditions where the activity was clearly detected with VV Lister, no such activity could be observed with the EV isolates (data not shown).
Analysis of the secreted vIL-1βR expressed by EV.
Using a filter-based assay of IL-1 binding in solution similar to that used for TNF, we screened EV supernatants for the presence of a soluble, secreted vIL-1βR that bound to human 125I-IL-1β (Fig. 5a). As previously described (4), specific binding of this cytokine was observed with medium from BSC-1 cells infected with VV WR but not from cultures infected with a VV which lacks VV vIL-1βR gene B15R (vΔB15R). However, no binding of human 125I-IL-1β could be detected in any of the EV supernatants. To examine the possibility that this assay specifically failed to detect an EV vIL-1βR–125I-IL-1β complex which was nevertheless formed, we developed another assay in which the vIL-1βR was cross-linked to 125I-IL-1β by use of EGS. Ligand-receptor complexes were then detected by SDS-PAGE and autoradiography. Using this assay, we failed to detect any EV vIL-1βR–125I-IL-1β complexes under conditions in which specific binding of human 125I-IL-1β to the vIL-1βR from VV WR (58-kDa complex) or CPV BR (52-kDa complex) was easily detectable (Fig. 5c).
FIG. 5.
Expression of vIL-1βRs by EV. (a and b) Detection of the EV vIL-1βR with a filter-based binding assay. Supernatants from 5.4 × 104 BSC-1 or 2 × 104 Sf-21 cells infected with the indicated viruses were incubated with 100 pM human 125I-IL-1β (a) or 200 pM mouse 125I-IL-1β (b), and the formation of ligand-receptor complexes was measured as described in the legend to Fig. 4. Data are expressed as the mean and standard deviation of four separate determinations. Hamp., Hampstead. (c and d), Cross-linking assay for vIL-1βRs. Supernatants from 104 BSC-1 or Sf-21 cells infected with the indicated viruses were incubated with either 1.15 nM human 125I-IL-1β (c) or 1.27 nM mouse 125IL-1β (d). Complexes between vIL-1βRs and the labeled cytokine were cross-linked by use of EGS, and the products were analyzed by SDS-PAGE and autoradiography. The positions of 125I-IL-1β, 125I-IL-1β–vIL-1βR complexes, and molecular size markers are indicated. Cold competition of binding to the labeled cytokine was achieved by inclusion of a 500-fold molar excess of homologous human (h) or mouse (m) unlabeled IL-1β.
Since EV may be a natural pathogen of mice, we repeated the above experiments using mouse 125I-IL-1β as the radiolabeled ligand. Surprisingly, we could detect binding of this cytokine in all EV supernatants using the filter-based binding assay (Fig. 5b). The levels of specifically bound cytokine were significantly lower in media from EV infections than in VV WR or CPV BR supernatants. Cross-linking experiments (Fig. 5d) demonstrated that a 66-kDa 125I-ligand–receptor complex was formed in all EV supernatants. This complex comigrated with the 125I-IL-1β–vIL-1βR complex seen with VV WR and was larger than the 57-kDa CPV BR complex.
Further investigation of the binding specificity of the EV vIL-1βR using the filter-based binding assay revealed that unlabeled human IL-1β and mouse IL-1β could compete with mouse 125I-IL-1β for binding to the EV vIL-1βR, whereas mouse IL-1α could not (Fig. 6a). Similar results were obtained with the cross-linking assay (data not shown). These results indicated that the binding of human 125I-IL-1β to the EV vIL-1βR does take place, but at levels which are below the limits of detection of the filter-based or cross-linking assays. To determine whether the binding of the two different types of IL-1β was of sufficiently high affinity to prevent their interaction with cellular receptors, we performed cell binding experiments in which iodinated human or mouse IL-1β was incubated with viral supernatants before binding to EL4 6.1 C10 cells was assessed. These cells express large amounts of membrane IL-1R (26). The experiments (Fig. 6b) revealed that the EV vIL-1βR could, like its VV WR homolog, inhibit the binding of both human and mouse 125I-IL-1β to the IL-1R-expressing cells. Higher doses of both EV Hampstead and VV WR supernatants were required to block human 125I-IL-1β binding than to block that of mouse 125I-IL-1β, although the concentration of the human cytokine used was twofold lower than that of the mouse cytokine. Control uninfected or VV vΔB15R-infected BSC-1 cell supernatants produced no significant decrease in either human or mouse 125I-IL-1β binding.
FIG. 6.
Binding specificity of the EV vIL-1βR and its sequestration of IL-1β in vitro. (a) Filter-based assay of binding to 200 pM mouse 125I-IL-1β by vIL-1βR in supernatants from 5.4 × 104 BSC-1 cells infected with VV WR or EV Hampstead. Binding was determined in the absence (NC) or presence of the indicated molar excesses of unlabeled human (h) and mouse (m) cytokines. Results are the mean and standard deviation of duplicate determinations. (b) Inhibition of cellular IL-1R binding by media from EV-infected cells. Binding of 400 pM mouse or 190 pM human 125I-IL-1β to 2.5 × 106 EL4 6.1 C10 cells was determined by phthalate oil centrifugation after preincubation of the cytokine with various doses of medium equivalent to the indicated numbers of BSC-1 cells either uninfected or infected with different viruses. Specific binding of 125I-IL-1β was determined by measuring binding in the presence or absence of a 500-fold molar excess of human IL-1β and subtracting the former value from the latter value. Values for human and mouse cytokines were 1,125 and 1354 cpm, respectively. Data, expressed as the percentage of specific binding, are the mean and standard deviation of duplicate (mouse) and triplicate (human) determinations.
Sequence of the EV vIL-1βR and its expression in the baculovirus system.
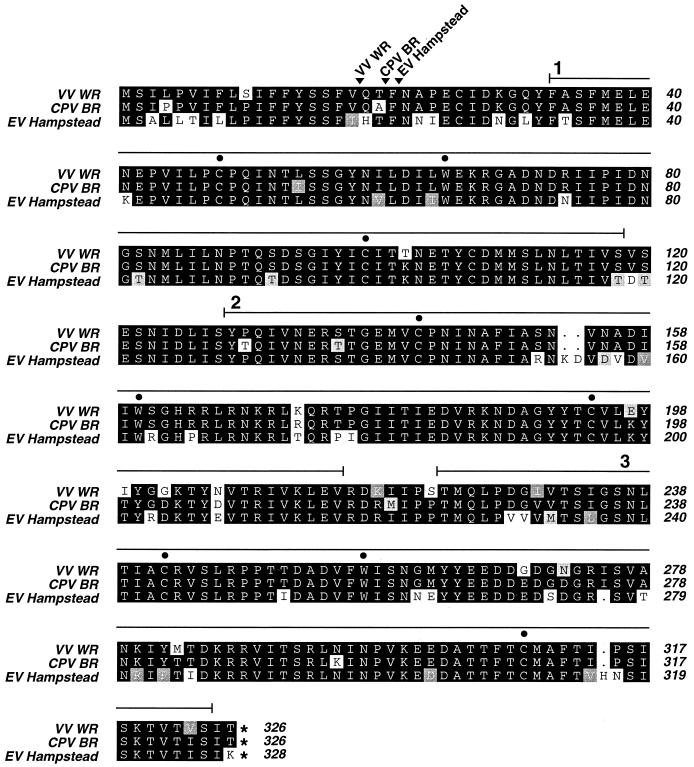
Sequencing of the EV homolog of the VV vIL-1βR gene revealed that the EV gene encodes a 328-amino-acid polypeptide whose sequence is distinct from those of its VV and CPV homologs. The EV sequence differs from the vIL-1βR consensus sequence at 51 amino acid residues, compared to only 14 and 9 residues for the VV and CPV sequences, respectively (Fig. 7). The EV vIL-1βR amino acid sequence is 81.8% identical to that of VV WR and 83.4% identical to that of CPV BR, while the VV WR and CPV BR sequences share 93.4% identical amino acids. Thirty of the 51 mutated EV amino acid residues are concentrated in three discrete regions which together constitute only 27% of the total EV vIL-1βR sequence. These regions are amino acids 3 to 32, before the first immunoglobulin (Ig) domain (10 mutations); amino acids 152 to 178, in the second Ig domain (11 mutations); and amino acids 253 to 285, in the third Ig domain (9 mutations).
FIG. 7.
Amino acid sequence comparison of OPV vIL-1βRs. Identical residues are white on black, conservative substitutions are white on dark grey, semiconservative substitutions are black on pale grey, and nonconservative substitutions are black on white. The Ig domains (1, 2, and 3) are indicated by an unbroken line above the sequences, and their characteristic cysteine and tryptophan residues are indicated by filled circles. Predicted sites of N-terminal signal peptide cleavage in the three vIL-1βRs are marked by labeled arrowheads. Dots indicate amino acid deletions, and asterisks indicate stop codons. The accession numbers of the sequences shown are as follows: VV WR, D11079; CPV BR, M95202; and EV Hampstead, AJ277110.
Comparison of the EV vIL-1βR gene with a conceptual ORF constructed from the nonfunctional vIL-1βR gene of variola virus revealed that, of the 51 EV amino acid positions that differ from the VV and CPV sequences, only 1 is identical in EV and variola virus. The variola virus sequence matches the VV and/or CPV sequences at 42 of these positions, suggesting that the EV gene has also diverged from the fully functional, ancestral vIL-1βR gene which underwent mutational inactivation in variola virus (data not shown).
To demonstrate that the mouse IL-1β binding factor detected in EV supernatants is encoded by this gene, we constructed a recombinant baculovirus, AcEVIL1R, which expresses the secreted protein encoded by this EV ORF fused to a C-terminal His6 tag. Supernatants from Sf-21 cells infected with this virus were tested for the presence of factors binding to human and mouse 125I-IL-1β. In both cross-linking and filter-based binding assays, recombinant EV vIL-1βR bound to mouse but not human 125I-IL-1β (Fig. 5). In the same experiments, recombinant VV WR vIL-1βR expressed by the baculovirus AcB15R bound both the human and the mouse cytokines, as reported previously (4). No IL-1β binding was detected with supernatants from either a wild-type baculovirus (A. californica nuclear polyhedrosis virus) or a control recombinant baculovirus expressing the VV Lister 35-kDa vCKBP (Ac35K) (Fig. 5). In cross-linking assays with mouse 125I-IL-1β, the two different types of recombinant vIL-1βR formed cytokine-receptor complexes that comigrated at a molecular mass of 52 kDa (Fig. 5d, right panel). In both instances, larger aggregates (above 100 kDa) were detected, but these were not observed in analogous experiments with human 125I-IL-1β (Fig. 5c, right panel).
DISCUSSION
The immunomodulatory factors expressed by OPVs have a major influence on the pathogenesis of the acute infections produced by these complex viruses. For example, deletion of the vIL-1βR and vIFN-α/βR genes from VV has marked effects on the infections produced by this virus in mice (2, 4, 45, 46). However, to date, there have been few in vivo studies of the function of the products of such genes in the presence of the immune system encountered by an OPV in its natural host (10). Such studies can be performed using the mousepox model, since all available evidence suggests that EV is likely to be a natural mouse pathogen (18). Here we have taken steps toward exploiting the full potential of this model by characterizing the expression and properties of three major EV immunomodulatory activities.
Several general observations about EV cytokine inhibitors can be made on the basis of data presented here and elsewhere (13, 25, 26, 32, 43). First, EV expresses soluble cytokine inhibitors belonging to all of the classes which have thus far been identified in OPVs, including proteins that bind to and inhibit IL-1β, TNF, CC chemokines, IFN-γ, IFN-α/β, and IL-18 (10, 13, 25, 32, 43). Second, the expression of all of these receptors is now known to be uniformly conserved in EVs isolated from laboratory outbreaks that occurred at different times and in widespread geographic locations (43) (V. Smith and A. Alcami, unpublished data). Finally, whereas particular cytokine receptors expressed by different OPVs tend to be virtually identical in terms of their amino acid sequences and binding specificities, there is an example of an EV receptor, the vIFN-γR (32), which has an amino acid sequence and binding properties distinct from those of other OPV receptors. These differences may have resulted from a prolonged period of viral coevolution with the mouse immune system. Here we present evidence that the sequence of the EV vIL-1βR has also diverged from those of its OPV homologs. This sequence divergence may have resulted in quantitative, if not qualitative, differences in its inhibition of IL-1β activity.
EV is a further example of an OPV which produces a comprehensive repertoire of inhibitors of inflammatory cytokines. Biochemical, biological, or sequence data suggest that certain CPV strains produce an even broader range of soluble cytokine receptors than EV, including a panel of three distinct soluble vTNFRs. Various strains of VV express subsets of all of the above types of cytokine receptors (6, 42) as well as a novel membrane-bound TNF binding factor (1), while sequencing data suggest that variola virus expresses functional forms of all but the vIL-1βR (37, 39). Retention of most of these immunomodulators by OPVs may therefore be essential for a productive infection to occur. The loss of one or two activities may in fact be compensated for by redundancy in the anti-inflammatory effects of the remaining gene products. The study of mutant EVs from which multiple immunomodulatory genes have been deleted would be informative in this regard.
The retention of functional vTNFRs, vIL-1βRs, and vCKBPs by all of the EV isolates studied here is interesting, since similar studies with multiple VV strains have revealed considerable interstrain heterogeneity in the expression of these immunomodulators (1, 4, 5). The most obvious explanation for this observation is that all of the EVs were originally isolated from laboratory mousepox outbreaks that were presumably caused by virus transmitted from a natural host reservoir (14, 18; Lipman et al., Letter). In contrast, the natural host of VV is unknown, and this virus has been derived from a range of different hosts and conditions of laboratory passage (3). If different mousepox outbreaks were caused by transmission from discrete natural reservoirs of the virus, then there must be considerable pressure to conserve functional immunomodulatory factors in the wild. It is also interesting to note that the Hampstead Egg strain, which was extensively passaged on chick chorioallantoic membranes to induce attenuation (16), has retained functional copies of all three of the genes studied here, despite the fact that it shows reduced virulence in experimental infections. In contrast, laboratory passage of EV Mill Hill appears to have resulted in the loss of the functional vCKBP expressed by the parental Hampstead isolate.
We report for the first time that EV encodes a vCKBP that effectively sequesters CC chemokines, presumably preventing their induction of multiple proinflammatory effects, such as an increase in the influx of leukocytes into sites of infection (8). The in vitro properties of the EV protein appear to be very similar to those described for its VV and CPV homologs (5, 20, 24, 40) and are likely, on the basis of sequence data, to closely resemble those of the predicted variola virus protein (29). The sequence of the EV protein contains a short deletion of 10 amino acids which are also absent from the sequence of the CPV BR protein (21). This deletion may be responsible for the slightly smaller size of the EV and CPV vCKBP–125I-chemokine complexes than of complexes seen with VV Lister in our cross-linking experiments. Interestingly, sequence information shows that this deletion event has not occurred in CPV GRI-90 (38), raising the possibility that it may have occurred independently in both CPV BR and EV. According to the recently reported X-ray crystallographic structure of the vCKBP from CPV BR (11), this region of the protein lies within an extended loop that forms contacts between the two molecules present in the vCKBP homodimer. However, this dimer is unlikely to be formed in solution. In the monomeric form of vCKBP, the loop would be exposed on the surface of the molecule. Nevertheless, the variability of this region between otherwise fully functional vCKBPs suggests that it does not have a major role in chemokine binding. The absence of the domain from the related protein encoded by the VV A41L gene is further evidence that it is not involved in the overall folding or function of this class of molecules.
Until recently, the production of vCKBPs was thought to be a mechanism of immune evasion restricted exclusively to poxviruses (23). However, the identification of an unrelated soluble, secreted vCKBP produced by the murine gamma herpesvirus MHV-68 (35) has extended this strategy to a second major family of large DNA viruses. Here we have demonstrated that vCKBPs from VV, CPV, and EV do not bind the CX3C chemokine fractalkine and therefore are absolutely specific for CC chemokines. In contrast, the MHV-68 vCKBP, encoded by the M3 gene, can bind to chemokines from all four classes. This difference in binding specificity may reflect the different roles of chemokines in the control of herpesvirus and poxvirus infections, and the study of its structural basis will be highly relevant for the design of therapeutic chemokine inhibitors.
All of the EVs which we tested produced secreted TNF binding activity. This result extends the previous report that EV Moscow and three other EV isolates express functional forms of the vTNFR CrmD, which is also expressed by CPV. Sequence analysis of the EV homologs of the vTNFRs CrmB and CrmC has shown their genes to be truncated (25). Thus far, sequence data therefore provide no evidence for the presence of any vTNFR other than CrmD in this virus. If this is the case, it seems that VV (CrmC), EV (CrmD), CPV (CrmB, CrmC, and CrmD), and variola virus (CrmB) all produce different subsets of the OPV vTNFR family. These differences in interactions with the host TNF system may contribute to the different disease phenotypes produced by these viruses. Competition experiments revealed that EV supernatants contained a factor(s) capable of binding human, mouse, and rat TNF-α but not human LT-α. This observation is inconsistent with results from previous studies (25) which detected LT-α binding in supernatants from EV-infected cells. This discrepancy may be due to the use of different assay methods in the two studies.
We report for the first time that EV, like VV and CPV (2, 4, 45), expresses a vIL-1βR that is capable of binding to both mouse IL-1β and human IL-1β and preventing their interaction with mouse cellular IL-1R. Like other known OPV vIL-1βRs, the EV protein does not bind mouse IL-1α (2). There is a discussion of unpublished data (18) which states that, in contrast to our results, mouse IL-1β binding activity was undetectable in medium from EV-infected cells under conditions in which binding of the VV vIL-1βR was observed. This contradiction with our findings is explicable because in our assays, the IL-1β binding signals measured in infected-cell supernatants were lower for EV than for VV or CPV. It is also interesting to note that although we demonstrated, using cold competition experiments, that the EV vIL-1βR binds human IL-1β, it was not possible to directly detect binding of the viral protein to human 125I-IL-1β using two different methods of assay. In the same experiments, the binding of VV and CPV vIL-1βRs to human 125I-IL-1β was readily detected.
There are two possible explanations for these unusual binding data. One is that the vIL-1βR is secreted at much lower levels by EV than by VV or CPV, with a corresponding reduction in the amounts of labeled cytokine bound in assays. However, natural and recombinant vIL-1βRs from both VV and EV are secreted in sufficient quantities to detect the binding of murine 125I-IL-1β in cross-linking and filter-based binding assays. The second explanation is that the difference in binding of human 125I-IL-1β and mouse 125I-IL-1β by the EV vIL-1βR results from amino acid sequence changes that have modified its absolute and relative affinities for the two cytokines, albeit not to the extent that its ability to sequester them has been lost. These possibilities are not mutually exclusive, and examination of the sequence of the EV vIL-1βR gene provides circumstantial evidence for both.
The amino acid sequence of the EV vIL-1βR has diverged from those of its functional CPV and VV homologs and from that of the presumed product of the functional ancestor of the variola virus vIL-1βR gene. Three regions of the protein carry an especially high proportion of unique amino acid residues. The predicted 21-amino-acid N-terminal signal sequence of the EV vIL-1βR differs from those of its VV and CPV homologs at six positions. Such mutations may affect the efficiency of secretion of the EV vIL-1βR from infected cells. Two other regions that carry a high proportion of mutated residues are in the center of the second and third Ig domains. It is possible that such mutations have an influence on the interaction of the protein with IL-1β either via direct interactions with the ligand or via influence on the overall folding of the protein. Unfortunately, there is no available evidence concerning the contribution of different domains or residues of the vIL-1βR to cytokine binding. Interestingly, alignment of the EV vIL-1βR amino acid sequence with that of a soluble, extracellular form of the human type I IL-1R which has been structurally characterized in a complex with human IL-1β (49) reveals that several of the unique EV amino acid residues, most notably five between amino acids 152 and 158, align with regions of the human receptor shown to contact IL-1β in the receptor-ligand complex (data not shown).
Previous studies of the effects of the vIL-1βR on VV pathogenesis have revealed that this receptor is responsible for the suppression of febrile responses and attenuation of the virus after intranasal inoculation of mice (2, 4). It has been suggested that the expression of this activity is associated with VV virulence in humans, since certain highly reactogenic VV vaccine strains, such as VV Copenhagen and VV Tashkent, are vIL-1βR negative, whereas the safer Lister strain is vIL-1βR positive. In addition, variola virus, which causes a systemic infection associated with high fever, does not express a functional vIL-1βR, while CPV, which produces a milder infection, is vIL-1βR positive. EV does not fit with this hypothesis because, although it produces a severe systemic infection in mice, it expresses a functional vIL-1βR that is present in all of the isolates which we tested. In light of this finding, it would be interesting to assess the contribution of the EV vIL-1βR to mortality and morbidity in the mousepox model.
In conclusion, we have demonstrated that EV, a species-specific, highly virulent OPV, expresses three major types of immunomodulatory cytokine receptors. The expression of these molecules is highly conserved among different virus isolates, suggesting that they have a positive influence on the persistence of this virus in the wild. We have also found evidence that the EV vIL-1βR gene has undergone some evolutionary changes which are presumably the result of an extended period of adaptation in a restricted range of natural hosts. The study of the function of these molecules using the laboratory mousepox model should provide further insights into their roles in vivo.
ACKNOWLEDGMENTS
We thank Neil Bryant for confirmation of the identity of EV strains and isolates using diagnostic PCR. We are grateful to Mark Buller, Yasuo Ichihashi, Hermann Meyer, Arno Mullbacher, John Williamson, and Neil Lipman for providing the EV isolates and strains.
This work was funded by the Wellcome Trust (grant 051087/Z/97/Z). A.A. is a Wellcome Trust senior research fellow.
REFERENCES
- 1.Alcami A, Khanna A, Paul N L, Smith G L. Vaccinia virus strains Lister, USSR and Evans express soluble and cell-surface tumour necrosis factor receptors. J Gen Virol. 1999;80:949–959. doi: 10.1099/0022-1317-80-4-949. [DOI] [PubMed] [Google Scholar]
- 2.Alcami A, Smith G L. A mechanism for the inhibition of fever by a virus. Proc Natl Acad Sci USA. 1996;93:11029–11034. doi: 10.1073/pnas.93.20.11029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alcami A, Smith G L. Receptors for gamma-interferon encoded by poxviruses: implications for the unknown origin of vaccinia virus. Trends Microbiol. 1996;4:321–326. doi: 10.1016/0966-842x(96)10051-2. [DOI] [PubMed] [Google Scholar]
- 4.Alcami A, Smith G L. A soluble receptor for interleukin-1β encoded by vaccinia virus: a novel mechanism of virus modulation of the host response to infection. Cell. 1992;71:153–167. doi: 10.1016/0092-8674(92)90274-g. [DOI] [PubMed] [Google Scholar]
- 5.Alcami A, Symons J A, Collins P D, Williams T J, Smith G L. Blockade of chemokine activity by a soluble chemokine binding protein from vaccinia virus. J Immunol. 1998;160:624–633. [PubMed] [Google Scholar]
- 6.Alcami A, Symons J A, Khanna A, Smith G L. Poxviruses: capturing cytokines and chemokines. Semin Virol. 1998;5:419–427. [Google Scholar]
- 7.Andrewes C H, Elford W J. Infectious ectromelia: experiments on interference and immunisation. Br J Exp Pathol. 1947;28:278–285. [PMC free article] [PubMed] [Google Scholar]
- 8.Baggiolini M. Chemokines and leukocyte traffic. Nature. 1998;392:565–568. doi: 10.1038/33340. [DOI] [PubMed] [Google Scholar]
- 9.Bazan J F, Bacon K B, Hardiman G, Wang W, Soo K, Rossi D, Greaves D R, Zlotnik A, Schall T J. A new class of membrane-bound chemokine with a CX3C motif. Nature. 1997;385:640–644. doi: 10.1038/385640a0. [DOI] [PubMed] [Google Scholar]
- 10.Born T L, Morrison L A, Esteban D J, VandenBos T, Thebeau L G, Chen N, Spriggs M K, Sims J E, Buller R M. A poxvirus protein that binds to and inactivates IL-18, and inhibits NK cell response. J Immunol. 2000;164:3246–3254. doi: 10.4049/jimmunol.164.6.3246. [DOI] [PubMed] [Google Scholar]
- 11.Carfi A, Smith C A, Smolak P J, McGrew J, Wiley D C. Structure of a soluble secreted chemokine inhibitor vCCI (p35) from cowpox virus. Proc Natl Acad Sci USA. 1999;96:12379–12383. doi: 10.1073/pnas.96.22.12379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen W, Drillien R, Spehner D, Buller R M. Restricted replication of ectromelia virus in cell culture correlates with mutations in virus-encoded host range gene. Virology. 1992;187:433–442. doi: 10.1016/0042-6822(92)90445-u. [DOI] [PubMed] [Google Scholar]
- 13.Colamonici O R, Domanski P, Sweitzer S M, Larner A, Buller R M. Vaccinia virus B18R gene encodes a type I interferon-binding protein that blocks interferon-α transmembrane signaling. J Biol Chem. 1995;270:15974–15978. doi: 10.1074/jbc.270.27.15974. [DOI] [PubMed] [Google Scholar]
- 14.Dick E J, Jr, Kittell C L, Meyer H, Farrar P L, Ropp S L, Esposito J J, Buller R M, Neubauer H, Kang Y H, McKee A E. Mousepox outbreak in a laboratory mouse colony. Lab Anim Sci. 1996;46:602–611. [PubMed] [Google Scholar]
- 15.Esposito J, Condit R, Obijeski J. The preparation of orthopoxvirus DNA. J Virol Methods. 1981;2:175–179. doi: 10.1016/0166-0934(81)90036-7. [DOI] [PubMed] [Google Scholar]
- 16.Fenner F. Studies in mousepox (infectious ectromelia of mice). VI. A comparison of the virulence and infectivity of three strains of ectromelia virus. Aust J Exp Biol Med Sci. 1949;27:31–43. doi: 10.1038/icb.1949.3. [DOI] [PubMed] [Google Scholar]
- 17.Fenner F, Anderson D A, Arita I, Jezek Z, Ladnyi I D. Smallpox and its eradication. Geneva, Switzerland: World Health Organization; 1988. [Google Scholar]
- 18.Fenner F, Buller R M L. Mousepox. In: Nathanson N, editor. Viral pathogenesis. Philadelphia, Pa: Lippincott-Raven; 1997. pp. 535–553. [Google Scholar]
- 19.Fenner F, Wittek R, Dumbell K R. The orthopoxviruses. London, United Kingdom: Academic Press, Inc.; 1989. [Google Scholar]
- 20.Graham K A, Lalani A S, Macen J L, Ness T L, Barry M, Liu L Y, Lucas A, Clark-Lewis I, Moyer R W, McFadden G. The T1/35kDa family of poxvirus-secreted proteins bind chemokines and modulate leukocyte influx into virus-infected tissues. Virology. 1997;229:12–24. doi: 10.1006/viro.1996.8423. [DOI] [PubMed] [Google Scholar]
- 21.Hu F Q, Smith C A, Pickup D J. Cowpox virus contains two copies of an early gene encoding a soluble secreted form of the type II TNF receptor. Virology. 1994;204:343–356. doi: 10.1006/viro.1994.1539. [DOI] [PubMed] [Google Scholar]
- 22.Ichihashi Y, Matsumoto S. Studies on the nature of Marchal bodies (A-type inclusion) during ectromelia virus infection. Virology. 1966;29:264–275. doi: 10.1016/0042-6822(66)90033-x. [DOI] [PubMed] [Google Scholar]
- 23.Lalani A S, Barrett J W, McFadden G. Modulating chemokines: more lessons from viruses. Immunol Today. 2000;21:100–106. doi: 10.1016/s0167-5699(99)01556-x. [DOI] [PubMed] [Google Scholar]
- 24.Lalani A S, Ness T L, Singh R, Harrison J K, Seet B T, Kelvin D J, McFadden G, Moyer R W. Functional comparisons among members of the poxvirus T1/35kDa family of soluble CC-chemokine inhibitor glycoproteins. Virology. 1998;250:173–184. doi: 10.1006/viro.1998.9340. [DOI] [PubMed] [Google Scholar]
- 25.Loparev V N, Parsons J M, Knight J C, Panus J F, Ray C A, Buller R M, Pickup D J, Esposito J J. A third distinct tumor necrosis factor receptor of orthopoxviruses. Proc Natl Acad Sci USA. 1998;95:3786–3791. doi: 10.1073/pnas.95.7.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.MacDonald H R, Lees R K, Bron C. Cell surface glycoproteins involved in the stimulation of interleukin 1-dependent interleukin 2 production by a subline of EL4 thymoma cells. I. Functional characterization by monoclonal antibodies. J Immunol. 1985;135:3944–3950. [PubMed] [Google Scholar]
- 27.Marchal J. Infectious ectromelia. A hitherto undescribed virus disease of mice. J Pathol Bacteriol. 1930;33:713–728. [Google Scholar]
- 28.Markwell M A, Fox C F. Surface-specific iodination of membrane proteins of viruses and eucaryotic cells using 1,3,4,6-tetrachloro-3α,6α-diphenylglycouril. Biochemistry. 1978;17:4807–4817. doi: 10.1021/bi00615a031. [DOI] [PubMed] [Google Scholar]
- 29.Massung R F, Esposito J J, Liu L I, Qi J, Utterback T R, Knight J C, Aubin L, Yuran T E, Parsons J M, Loparev V N, Selivanov N A, Cavallaro K F, Kerlavage A R, Mahy B W J, Venter J C. Potential virulence determinants in terminal regions of variola smallpox virus genome. Nature. 1993;366:748–751. doi: 10.1038/366748a0. [DOI] [PubMed] [Google Scholar]
- 30.Meyer H, Ropp S L, Esposito J J. Gene for A-type inclusion body protein is useful for a polymerase chain reaction assay to differentiate orthopoxviruses. J Virol Methods. 1997;64:217–221. doi: 10.1016/S0166-0934(96)02155-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moss B. Poxviridae: the viruses and their replication. In: Fields B N, Knipe D M, Howley P M, Chanock R M, Melnick J, Monath T P, Roizman B, Straus S E, editors. Virology. 3rd ed. Vol. 2. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 2637–2671. [Google Scholar]
- 32.Mossman K, Upton C, Buller R M, McFadden G. Species specificity of ectromelia virus and vaccinia virus interferon-γ binding proteins. Virology. 1995;208:762–769. doi: 10.1006/viro.1995.1208. [DOI] [PubMed] [Google Scholar]
- 33.Nash P, Barrett J, Cao J X, Hota-Mitchell S, Lalani A S, Everett H, Xu X M, Robichaud J, Hnatiuk S, Ainslie C, Seet B T, McFadden G. Immunomodulation by viruses: the myxoma virus story. Immunol Rev. 1999;168:103–120. doi: 10.1111/j.1600-065x.1999.tb01286.x. [DOI] [PubMed] [Google Scholar]
- 34.Osterrieder N, Meyer H, Pfeffer M. Characterization of the gene encoding the A-type inclusion body protein of mousepox virus. Virus Genes. 1994;8:125–135. doi: 10.1007/BF01703611. [DOI] [PubMed] [Google Scholar]
- 35.Parry C M, Simas J P, Smith V P, Stewart C A, Minson A C, Efstathiou S, Alcami A. A broad spectrum secreted chemokine binding protein encoded by a herpesvirus. J Exp Med. 2000;191:573–578. doi: 10.1084/jem.191.3.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Patel A H, Gaffney D F, Subak-Sharpe J H, Stow N D. DNA sequence of the gene encoding a major secreted protein of vaccinia virus, strain Lister. J Gen Virol. 1990;71:2013–2021. doi: 10.1099/0022-1317-71-9-2013. [DOI] [PubMed] [Google Scholar]
- 37.Shchelkunov S N, Massung R F, Esposito J J. Comparison of the genome DNA sequences of Bangladesh-1975 and India-1967 variola viruses. Virus Res. 1995;36:107–118. doi: 10.1016/0168-1702(94)00113-q. [DOI] [PubMed] [Google Scholar]
- 38.Shchelkunov S N, Safronov P F, Totmenin A V, Petrov N A, Ryazankina O I, Gutorov V V, Kotwal G J. The genomic sequence analysis of the left and right species-specific terminal region of a cowpox virus strain reveals unique sequences and a cluster of intact ORFs for immunomodulatory and host range proteins. Virology. 1998;243:432–460. doi: 10.1006/viro.1998.9039. [DOI] [PubMed] [Google Scholar]
- 39.Shchelkunov S N, Totmenin A V, Loparev V N, Safronov P F, Gutorov V V, Chizhikov V E, Knight J C, Parsons J M, Massung R F, Esposito J J. Alastrim smallpox variola minor virus genome DNA sequences. Virology. 2000;266:361–386. doi: 10.1006/viro.1999.0086. [DOI] [PubMed] [Google Scholar]
- 40.Smith C A, Smith T D, Smolak P J, Friend D, Hagen H, Gerhart M, Park L, Pickup D J, Torrance D, Mohler K, Schooley K, Goodwin R G. Poxvirus genomes encode a secreted, soluble protein that preferentially inhibits β chemokine activity yet lacks sequence homology to known chemokine receptors. Virology. 1997;236:316–327. doi: 10.1006/viro.1997.8730. [DOI] [PubMed] [Google Scholar]
- 41.Smith G L, Chan Y S, Howard S T. Nucleotide sequence of 42 kbp of vaccinia virus strain WR from near the right inverted terminal repeat. J Gen Virol. 1991;72:1349–1376. doi: 10.1099/0022-1317-72-6-1349. [DOI] [PubMed] [Google Scholar]
- 42.Smith G L, Symons J A, Khanna A, Vanderplasschen A, Alcami A. Vaccinia virus immune evasion. Immunol Rev. 1997;159:137–154. doi: 10.1111/j.1600-065x.1997.tb01012.x. [DOI] [PubMed] [Google Scholar]
- 43.Smith V P, Bryant N A, Alcami A. Ectromelia, vaccinia and cowpox viruses encode secreted interleukin-18 binding proteins. J Gen Virol. 2000;81:1223–1230. doi: 10.1099/0022-1317-81-5-1223. [DOI] [PubMed] [Google Scholar]
- 44.Spriggs M K. One step ahead of the game: viral immunomodulatory molecules. Annu Rev Immunol. 1996;14:101–130. doi: 10.1146/annurev.immunol.14.1.101. [DOI] [PubMed] [Google Scholar]
- 45.Spriggs M K, Hruby D E, Maliszewski C R, Pickup D J, Sims J E, Buller R M, VanSlyke J. Vaccinia and cowpox viruses encode a novel secreted interleukin-1-binding protein. Cell. 1992;71:145–152. doi: 10.1016/0092-8674(92)90273-f. [DOI] [PubMed] [Google Scholar]
- 46.Symons J A, Alcami A, Smith G L. Vaccinia virus encodes a soluble type I interferon receptor of novel structure and broad species specificity. Cell. 1995;81:551–560. doi: 10.1016/0092-8674(95)90076-4. [DOI] [PubMed] [Google Scholar]
- 47.Tsung K, Yim J H, Marti W, Buller R M, Norton J A. Gene expression and cytopathic effect of vaccinia virus inactivated by psoralen and long-wave UV light. J Virol. 1996;70:165–171. doi: 10.1128/jvi.70.1.165-171.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Venkatesan S, Gershowitz A, Moss B. Complete nucleotide sequences of two adjacent early vaccinia virus genes located within the inverted terminal repetition. J Virol. 1982;44:637–646. doi: 10.1128/jvi.44.2.637-646.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vigers G P, Anderson L J, Caffes P, Brandhuber B J. Crystal structure of the type-I interleukin-1 receptor complexed with interleukin-1β. Nature. 1997;386:190–194. doi: 10.1038/386190a0. [DOI] [PubMed] [Google Scholar]