Abstract
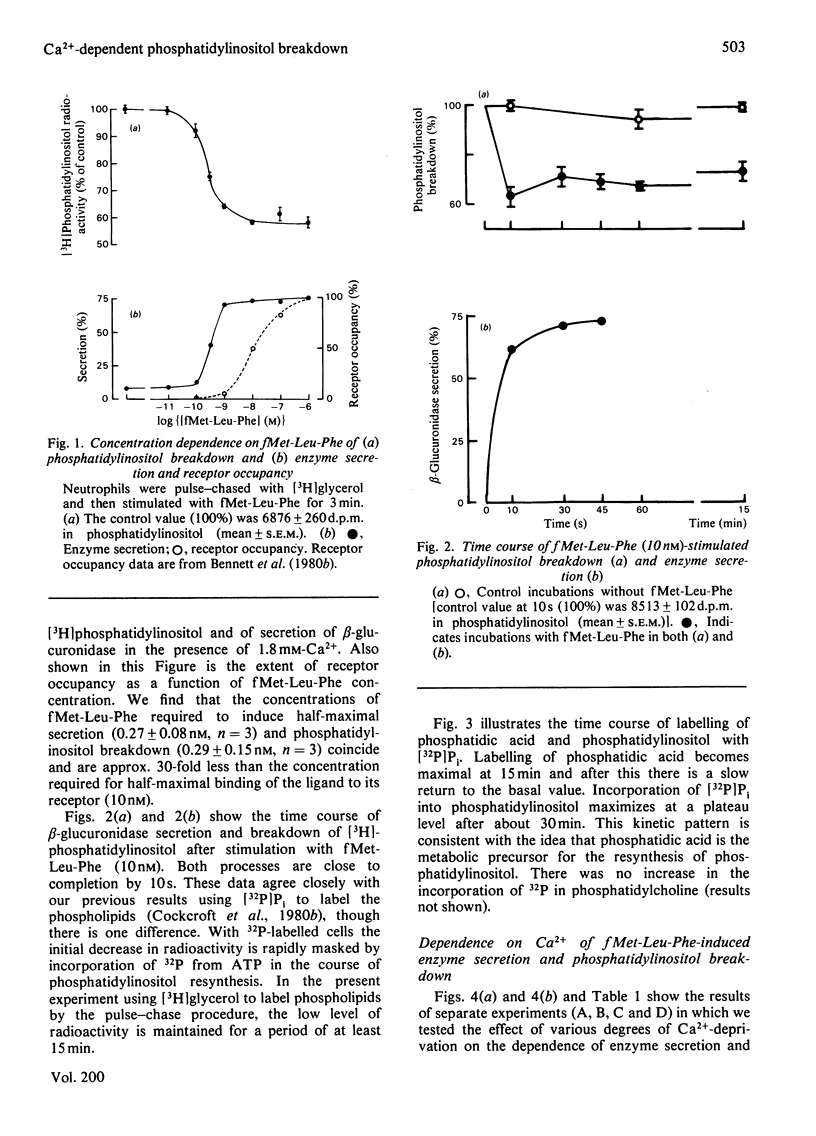
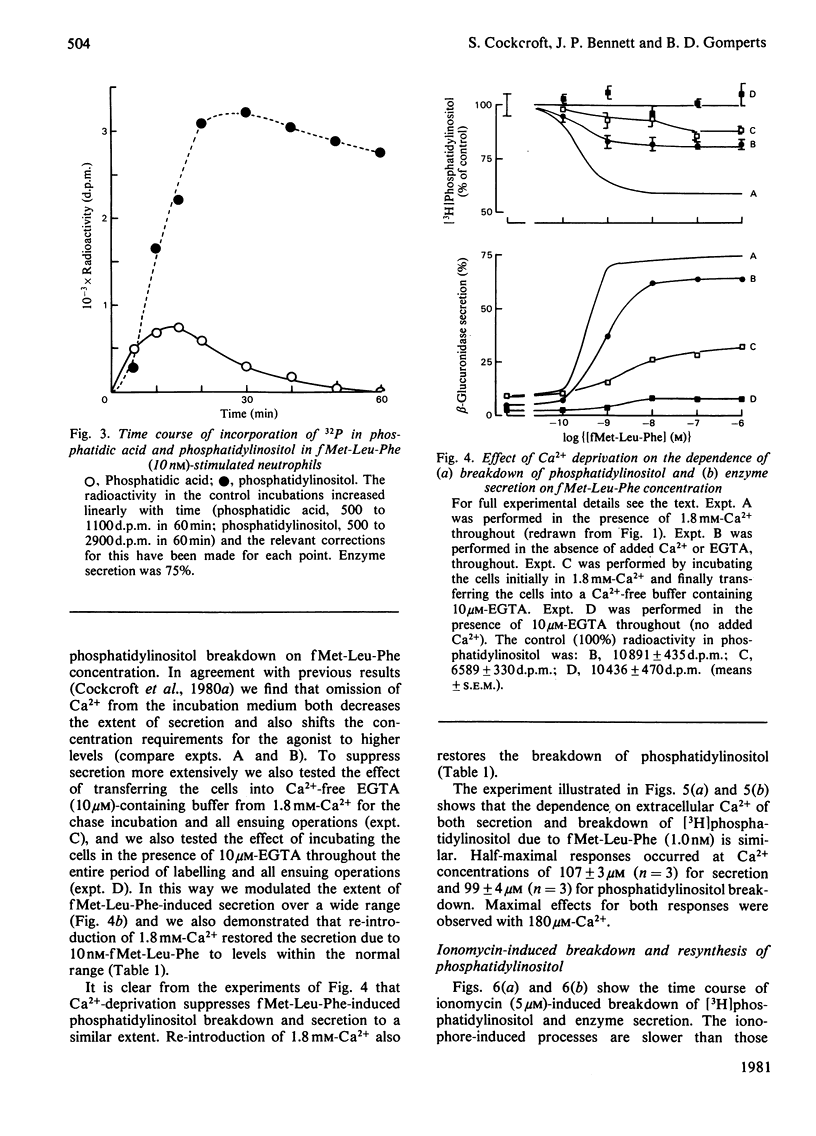
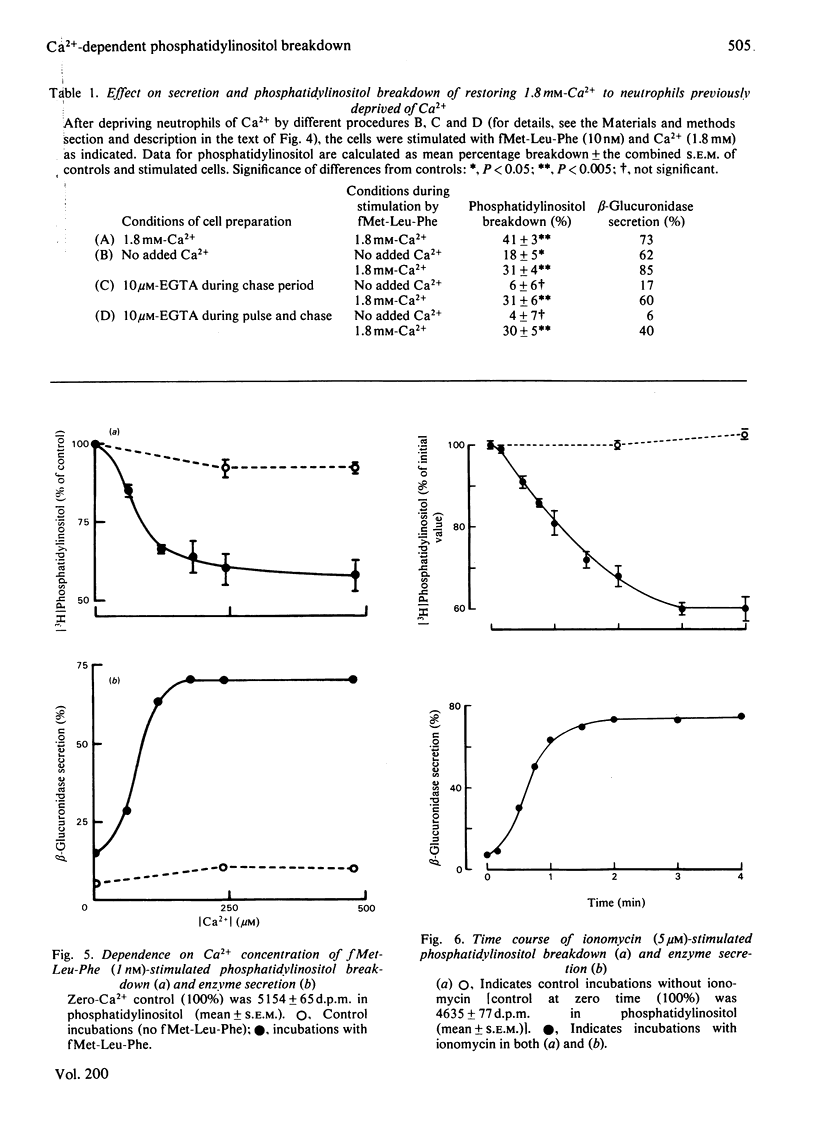
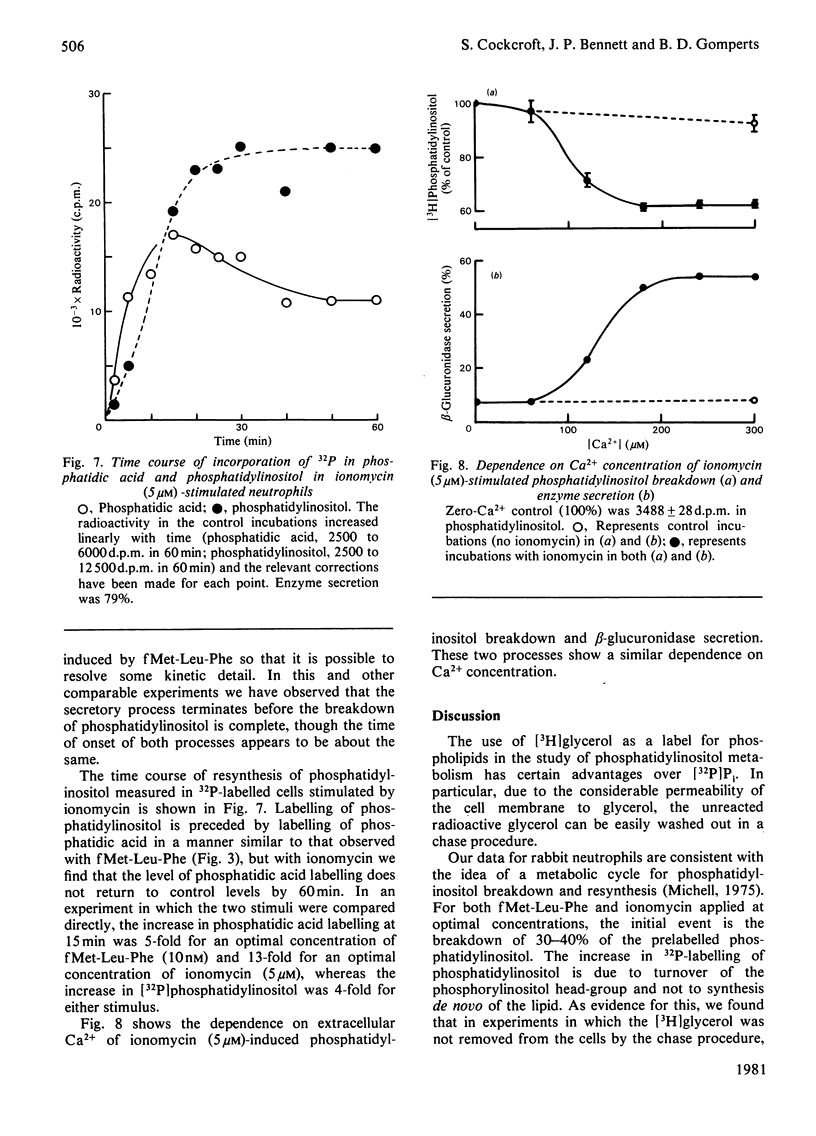
1. We have measured the breakdown of [3H]phosphatidylinositol in rabbit neutrophils prelabelled with [3H]glycerol by a pulse-chase procedure. With a view to defining a possible causal relationship between phosphatidylinositol breakdown and enzyme secretion in these cells, we have compared the characteristics of both these processes induced by either the receptor-directed agonist formylmethionyl-leucylphenylalanine (fMet-Leu-Phe) or the Ca2+-ionophore ionomycin. 2. The dependence on fMet-Leu-Phe concentration of phosphatidylinositol breakdown and secretion is identical (half-maximal at 0.3 nM). This is 30-fold less than that required for half-maximal occupation of receptors. 3. Both secretion and breakdown of phosphatidylinositol due to fMet-Leu-Phe are modulated by extracellular Ca2+. The sensitivity to Ca2+ of both processes is enhanced by pretreatment to deplete cell Ca2+. The concentration of Ca2+ required to cause half-maximal effects of both processes in Ca2+-depleted cells on stimulation with 1nM-fMet-Leu-Phe is 100 microM. Ionomycin-stimulated secretion and breakdown of phosphatidylinositol are completely dependent on extracellular Ca2+ over similar concentration ranges. 4. Both secretion and phosphatidylinositol breakdown due to fMet-Leu-Phe approach completion by 10s. With ionomycin these processes are slower, terminating by 2 min. 5. In the presence of [32P]Pi, labelling of [32P]phosphatidic acid reaches a maximum 15 min after stimulation with either fMet-Leu-Phe or ionomycin. This precedes the labelling of [32P]phosphatidylinositol and shows the expected precursor-product relationship. 6. We conclude from these results that in rabbit neutrophils a rise in cytosol [Ca2+] is both sufficient and necessary to cause secretion and phosphatidylinositol breakdown. In cells depleted of Ca2+, the occupation of receptors by fMet-Leu-Phe is without effect on these two processes.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allan D., Michell R. H. A comparison of the effects of phytohaemagglutinin and of calcium ionophore A23187 on the metabolism of glycerolipids in small lymphocytes. Biochem J. 1977 May 15;164(2):389–397. doi: 10.1042/bj1640389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker E. L., Showell H. J. The ability of chemotactic factors to induce lysosomal enzyme release. II. The mechanism of release. J Immunol. 1974 Jun;112(6):2055–2062. [PubMed] [Google Scholar]
- Bennett J. P., Cockcroft S., Gomperts B. D. Ionomycin stimulates mast cell histamine secretion by forming a lipid-soluble calcium complex. Nature. 1979 Dec 20;282(5741):851–853. doi: 10.1038/282851a0. [DOI] [PubMed] [Google Scholar]
- Bennett J. P., Cockcroft S., Gomperts B. D. Use of cytochalasin B to distinguish between early and late events in neutrophil activation. Biochim Biophys Acta. 1980 Oct 2;601(3):584–591. doi: 10.1016/0005-2736(80)90560-x. [DOI] [PubMed] [Google Scholar]
- Bennett J. P., Hirth K. P., Fuchs E., Sarvas M., Warren G. B. The bacterial factors which stimulate neutrophils may be derived from procaryote signal peptides. FEBS Lett. 1980 Jul 11;116(1):57–61. doi: 10.1016/0014-5793(80)80528-x. [DOI] [PubMed] [Google Scholar]
- Cockcroft S., Bennett J. P., Gomperts B. D. Stimulus-secretion coupling in rabbit neutrophils is not mediated by phosphatidylinositol breakdown. Nature. 1980 Nov 20;288(5788):275–277. doi: 10.1038/288275a0. [DOI] [PubMed] [Google Scholar]
- Cockcroft S., Bennett J. P., Gomperts B. D. f-MetLeuPhe-induced phosphatidylinositol turnover in rabbit neutrophils is dependent on extracellular calcium. FEBS Lett. 1980 Jan 28;110(1):115–118. doi: 10.1016/0014-5793(80)80036-6. [DOI] [PubMed] [Google Scholar]
- Cockcroft S., Gomperts B. D. Evidence for a role of phosphatidylinositol turnover in stimulus-secretion coupling. Studies with rat peritoneal mast cells. Biochem J. 1979 Mar 15;178(3):681–687. doi: 10.1042/bj1780681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein I., Hoffstein S., Gallin J., Weissmann G. Mechanisms of lysosomal enzyme release from human leukocytes: microtubule assembly and membrane fusion induced by a component of complement. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2916–2920. doi: 10.1073/pnas.70.10.2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michell R. H. Inositol phospholipids and cell surface receptor function. Biochim Biophys Acta. 1975 Mar 25;415(1):81–47. doi: 10.1016/0304-4157(75)90017-9. [DOI] [PubMed] [Google Scholar]
- Michell R. H., Jafferji S. S., Jones L. M. Receptor occupancy dose--response curve suggests that phosphatidyl-inositol breakdown may be intrinsic to the mechanism of the muscarinic cholinergic receptor. FEBS Lett. 1976 Oct 15;69(1):1–5. doi: 10.1016/0014-5793(76)80640-0. [DOI] [PubMed] [Google Scholar]
- Michell R. H., Jafferji S. S., Jones L. M. The possible involvement of phosphatidylinositol breakdown in the mechanism of stimulus-response coupling at receptors which control cell-surface calcium gates. Adv Exp Med Biol. 1977;83:447–464. doi: 10.1007/978-1-4684-3276-3_41. [DOI] [PubMed] [Google Scholar]
- Naccache P. H., Showell H. J., Becker E. L., Sha'afi R. I. Transport of sodium, potassium, and calcium across rabbit polymorphonuclear leukocyte membranes. Effect of chemotactic factor. J Cell Biol. 1977 May;73(2):428–444. doi: 10.1083/jcb.73.2.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedel J. E., Kahane I., Cuatrecasas P. Receptor-mediated internalization of fluorescent chemotactic peptide by human neutrophils. Science. 1979 Sep 28;205(4413):1412–1414. doi: 10.1126/science.472759. [DOI] [PubMed] [Google Scholar]
- O'Flaherty J. T., Kreutzer D. L., Showell H. S., Becker E. L., Ward P. A. Desensitization of the neutrophil aggregation response to chemotactic factors. Am J Pathol. 1978 Dec;93(3):693–706. [PMC free article] [PubMed] [Google Scholar]
- Putney J. W., Jr, Weiss S. J., Van De Walle C. M., Haddas R. A. Is phosphatidic acid a calcium ionophore under neurohumoral control? Nature. 1980 Mar 27;284(5754):345–347. doi: 10.1038/284345a0. [DOI] [PubMed] [Google Scholar]
- Salmon D. M., Honeyman T. W. Proposed mechanism of cholinergic action in smooth muscle. Nature. 1980 Mar 27;284(5754):344–345. doi: 10.1038/284344a0. [DOI] [PubMed] [Google Scholar]
- Showell H. J., Freer R. J., Zigmond S. H., Schiffmann E., Aswanikumar S., Corcoran B., Becker E. L. The structure-activity relations of synthetic peptides as chemotactic factors and inducers of lysosomal secretion for neutrophils. J Exp Med. 1976 May 1;143(5):1154–1169. doi: 10.1084/jem.143.5.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skipski V. P., Peterson R. F., Barclay M. Quantitative analysis of phospholipids by thin-layer chromatography. Biochem J. 1964 Feb;90(2):374–378. doi: 10.1042/bj0900374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. J., Ignarro L. J. Bioregulation of lysosomal enzyme secretion from human neutrophils: roles of guanosine 3':5'-monophosphate and calcium in stimulus-secretion coupling. Proc Natl Acad Sci U S A. 1975 Jan;72(1):108–112. doi: 10.1073/pnas.72.1.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOODIN A. M., WIENEKE A. A. The accumulation of calcium by the polymorphonuclear leucocyte treated with staphylococcal leucocidin and its significance in the extrusion of protein. Biochem J. 1963 Jun;87:487–495. doi: 10.1042/bj0870487. [DOI] [PMC free article] [PubMed] [Google Scholar]


