Abstract
This study aimed to isolate and characterize Seselopsis Tianschanica Schischk Polysaccharide (STSP), a natural functional ingredient, and to develop a compound fermented beverage of nutritional and health combining STSP with highland barley. Firstly, the STSP was isolated and characterized with ultrasound-assisted enzymatic method and chromatography, and analyzed the structural features of polysaccharide STSP-1. Then, a compounded fermented beverage integrating Tibet STSP and highland barley was created, with technology and flavor substances studied. Five kinds of organic acids, 18 kinds of amino acids, and 57 kinds of volatile flavor compounds were determined by GC–MS and HPLC, and quantified significantly enhancing the overall flavor profile of the compound drink-fermented. Moreover, it exhibited higher hydroxyl radical scavenging capacity (IC50 value was 42.68 μL) compared to conventional highland barley drink. This research is expected to provide a theoretical foundation for the utilization of STSP in the functional food industry and other industries.
Keywords: Seselopsis tianschanica schischk polysaccharide, Separation, Structural charactezation, Antioxygen, Fermented beverage
Highlights
-
•
A polysaccharide was isolated from STS, a medical-food homologus from Lhasa, Tibet.
-
•
Polysaccharide STSP-1 shows antioxidant activity significant medicinal potential.
-
•
A fermented beverage integrating Tibet STSP and highland barley was crafted.
-
•
This fermented beverages boasts a harmonious blend of flavor and functional benefits.
1. Introduction
At present, the medicine food homologous plant polysaccharide is mainly used in the development of functional food and beverage in the food field. Zhou (Zhou, 2021) used longan polysaccharide, jujube polysaccharide and lycium barbarum polysaccharide to prepare nutritious dietary powder with balanced nutrition, good preparability and good stability. The simulated fermentation experiment in vitro showed that it had the effect of regulating intestinal flora. Zhang (Zhang, 2011) using purslane polysaccharide, a sports functional beverage with certain anti sports fatigue and improving sports endurance was made. Under this trend, Tibet Seselopsis tianschanica schischk (STS) as a commonly used “medicinal and food homologous” plant, due to the geographical limitation, its development and utilization are less.
STS is the most distinctive dual-use plant for pharmaceutical/food purposes among the Tibetan community and grows at altitudes between 1500 and 2500 m, has not been cultivated by artificial introduction. (Fig. 1). Numerous scholars have carried out a series of explorations on the active substances in STS from Tibet. (Zhang et al., 2021). Table 1 provides a list of the main active ingredients and their effects of Tibet STS reported. Studies indicate that the polysaccharide from STS in Tibet (STSP) enhances superoxide dismutase (SOD) and glutathione peroxidase (GSH Px) activity and reduces acetylcholinesterase (AChE) in mice. It also reduces propylene glycol and nitric oxide, slows free radical oxidation damage, and exhibits an anti-hypoxia effect. (He et al., 2021). The STSP also has the function of regulating immune function. (Liu, Ding, et al., 2020). Furthermore,the anti-fatigue effect of STSP significantly increase the oxidative stress along with metabolic capacities observed in exhausted swimming mice (Duan et al., 2017). At present, the research of STSP in Tibet is focused on biological activity, but the subsequent structural characteristics and physical and chemical properties of further research are less (Huang & Huang, 2020). Therefore, this study has carried out further exploration and application research.
Fig. 1.
Seselopsis tianschanica schischk plant. a, physical picture, b, hand drawing. ①-Root, ②-Stem, ③-Leaf, ④-Flowers, ⑤-Cross section of meristematic fruit (Figure source: @PPBC.cn/9261212). c, root of seselopsis tianschanica schischk.
Table 1.
Main active ingredients in Tibet STS and their effects.
| Active ingredient | Efficacy | Reference |
|---|---|---|
| Ethanol extract | Regulate the body's immune function | (Li et al., 2017; Zhou, 2021) |
| Ethanol extract | Anti-aging effect | (Dong et al., 2020) |
| Ethanol extract | Obvious analgesic effect on primary dysmenorrhea in mice | (Zhou et al., 2016) |
| Ethanol extract | improve learning and memory | (Dong et al., 2016) |
| Ethanol extract | Anti-inflammatory effect | (Tian et al., 2018) |
| Polysaccharide | Antifatigue effect | (Duan et al., 2017) |
| Polysaccharide | Regulate the body's immune function | (Liu, Ding, et al., 2020) |
| Polysaccharide | Protective against sleep deprivation | (Ma et al., 2020) |
| Polysaccharide | Anti-oxidative stress effect | (He et al., 2021) |
| Essential oil | Anti-dysmenorrhea effect | (Qiu et al., 2019) |
Under the national health wave, incorporating “medicine food homology” plant into miscellaneous grains has become both healthy and trendy in terms of diet choices. Dong and Zhang (2016) combined the water soluble components of STS and Dendrobium purpureum juice to prepare a refreshing and delectable compound beverage with obvious aroma of STS. Highland barley possesses abundant nutrients and functions properties that cater to consumers' demand for diverse grains consumption trends (“miscellaneous grain fever”). In addition, highland barley is rich in amino acids, including many γ-Aminobutyric acid (GABA) (Hong and Kim, 2019). GABA serves as an essential inhibitory neurotransmitter within the human central nervous system by suppressing sympathetic nerve excitation while inducing hypnosis, sedation, and anxiety relief (Gu et al., 2020). The fermentation process combining highland barley and STSP yields a high-end compound beverage with both flavor and health functions. This can satisfy the demand for a healthy diet and open up a new path for the homology of medicine and food.
This research aims to develop a novel and superior highland barley fermented beverage product with antioxidant capabilities. The key ingredient of this beverage is STSP, an extract of Seselopsis tianschanica schischk, which laboratory testing conducted in preliminary stages, in conjunction with reports from the scientific literature, suggests antioxidant activity. Our goal is to enhance the nutritive quality and health-promoting effects of the traditional highland barley fermented beverage, while maintaining its inherent palatability. Furthermore, this study could offer innovative strategies and methodologies for the development and utilization of STSP and highland barley resources in Tibet, and establish a theoretical foundation for the industrialization of STSP antioxidant functional food in Tibet.
2. Materials and methods
2.1. Experimental materials
The Tibet STS was purchased from Lhasa Branch of Tibet Changdu Sapphire Medicine Co., Ltd. and identified as the root of Tibet STS by Tibet Tibetan Medical University; Highland barley, purchased in the local market of Lhasa; Papain (30,000 USP-U/mg, From Papaya lotion), Cellulase (50,000 USP-U/mg, From Aspergillus niger), phenol, sodium chloride, and other chemicals are analytically pure and purchased from Sinopharm Chemical Reagents Co., Ltd.; DEAE cellulose 52 and Sephadex G-100 were purchased from Beijing Solabo Technology Co., Ltd.; Series of dextran standard products were purchased from American Polymer Standard Company; Monosaccharide standard was purchased from Sigma Aldrich Company in the United States; Methanol, 2-octanol, acetonitrile, isobutanol, organic acid, amino acid and other standard products are chromatographically pure and purchased from Shanghai Amphora Experimental Technology Co., Ltd. The strains used in this article are those preserved in the laboratory, of which the yeast starter is purchased from Angel Yeast Co., Ltd., and the details are shown in Table 2.
Table 2.
Information of strains.
| Strain | Names of strains | Strain source | pure culture |
|---|---|---|---|
| Lactobacillus plantarum | BF-ZR | Sauerkraut | MRS |
| Lactobacillus casei | MYT | MeiYiTian of YILI fermented yogurt | |
| Lactobacillus fermentum | GM | Brightdairy fermented yogurt | |
| Pediococcus acidilactici | WM | Malic-Lactic acid baking powder of Lamandine | |
| Lactobacillus plantarum | L1901 | Fruit and vegetable enzyme powder of Bisour | |
| Yeast | H21 | Angel Yeast Co. LTD | YPD |
| Yeast | HL | ||
| Yeast | BH | ||
| Yeast | YL | ||
| Yeast | GH | ||
| Yeast | BTH |
2.2. Extraction process of crude polysaccharide
Select disease-free and mildew free STS from Tibet were selected and cleaned, put it in an oven at 50 °C, dried and crushed it, passed it through a 60 mesh sieve, and put it into a desiccator for storage. After that, the effects of the material liquid ratio (1:10, 1:20, 1:30, 1:40, 1:50 g·mL−1), the addition amount of complex enzyme (cellulase and papain, 4:6) (0 %, 1 %, 2 %, 3 %, 4 %), ultrasonic temperature (35, 45, 55, 65, 75 °C), ultrasonic time (35, 45, 55, 65, 75 min), and ultrasonic power (250, 300, 350, 400, 450 W) on the yield of crude polysaccharide were investigated respectively. After the end of ultrasonic, the enzyme was extinguished in water bath at 95 °C for 10 min, cooled, centrifuged at 4500 r·min−1 for 15 min, obtained the polysaccharide extract.
The phenol sulfuric acid method is used to determine the total sugar content of each tube of eluate. The linear regression equation is y = 0.213×-0.030, the correlation coefficient is R2 = 0.9971, where y (mg·mL−1) was the glucose concentration and x was the absorption value at OD490. The determination of reducing sugar is based on DNS method (Gramazio et al., 2020), The linear regression equation was y = 1.330×-0.015, and the correlation coefficient was R2 = 0.9944, where y (mg·mL−1) was the glucose concentration and x was the absorbance value at OD540. Polysaccharide content was calculated as total sugar minus reducing sugar.
On the basis of the single factor test, four important factors were selected from the above five factors, and the orthogonal design assistant II software was used to design the four factor three level test. The test table is shown in Table 3. The polysaccharide yield was determined, the calculation as formula (1).
| (1) |
Table 3.
Orthogonal optimization of factors and levels for the extraction of STSP.
| Level | Factors |
|||
|---|---|---|---|---|
| A (g·mL−1) material liquid ratio | B (°C) Ultrasonic temperature | C (min) Ultrasonic time | D (W) ultrasonic power | |
| 1 | 1:20 | 55 | 45 | 250 |
| 2 | 1:30 | 65 | 55 | 300 |
| 3 | 1:40 | 75 | 65 | 350 |
2.3. Isolation, purification and structure identification of STSP
The experimental scheme to purify the Tibet STSP was refered to Liu et al. (2014) with modify it appropriately. Ethanol was added to the solution to a final concentration of 80 %. The solution was stored at 4 °C for 24 h and centrifuged at 4500 r·min−1 for 15 min. The precipitate was dissolved in deionized water, rotary evaporated at 55 °C to obtain a pre-frozen solution and lyophilized for 72 h. DEAE-52 cellulose chromatography was used to purify a polysaccharide with varying charge loading at 10 mg·mL−1, 5 mL volume. NaCl elution was performed at 0, 0.1, 0.3, 0.5 mol·L−1 at 1 mL·min−1, collecting 10-min fractions. Sephadex G-100 (GE17–0060-01, Sigma-Aldrich, America) was used to further purify the polysaccharide from STS. Total sugar content was quantified using the phenol‑sulfuric acid method.
The relative molecular weight (Mw) of polysaccharides was determined by high performance gel permeation chromatography, which refered the methods of Ding et al. (2014). The monosaccharide composition of STS polysaccharide was determined by ion chromatography, with the ultraviolet spectrophotometer detecting nucleic acids and proteins at 260 and 280 nm. 5 mg of freeze-dried STS polysaccharide was analyzed by infrared spectrometry at 4000 cm−1–500 cm−1. The triple helix structure of polysaccharide was analyzed refer Xu et al. (2016).
2.4. Optimization of fermentation process of Tibet STS compound highland barley fermented beverage
Yeast activation: Active dry yeasts were selected for fermentation in a fixed flask volume of 150 mL (total capacity 250 mL), inoculated with 0.2 % yeast, and fermented for 24 h at 30 °C at 160 r·min−1. Lactobacillus activation: Under the condition of 150 mL bottle volume (total capacity 250 mL) and fermentation temperature 37 °C, 3 % active dry Lactobacillus was inoculated and fermented for 30 h in a constant temperature incubator. All consumables were sterilized at 121 °C for 15 min before inoculation.
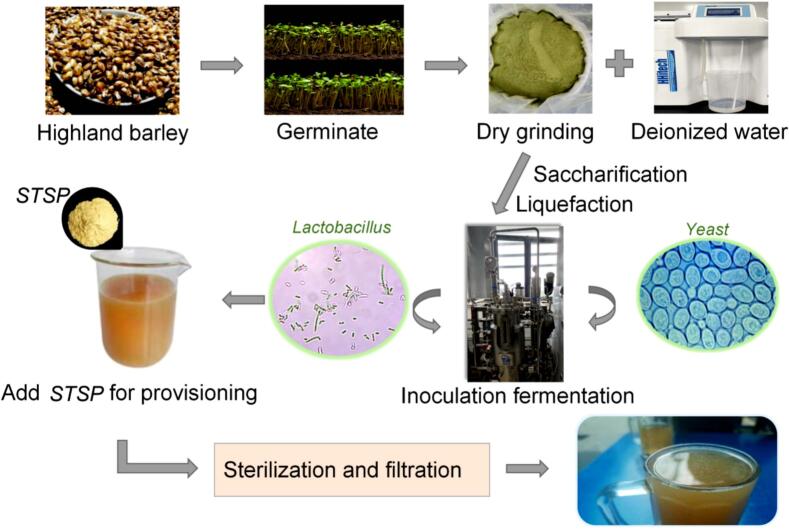
According to the technological process, the material liquid ratio, yeast strain, Lactobacillu strain and mixed bacteria fermentation process were optimized. When the material-to-liquid ratio was 1:5 or 1:10, the fermented highland barley beverage had strong flavor and deep color. However, it had issues such as thick taste and bitter taste, leading to poor sensory perception. When the ratio was adjusted to 1:15, the acidity of the fermented beverage became appropriate while maintaining a strong taste of highland barley rice with good overall flavor. This study uses yeast and Lactobacillu to ferment the beverage in order to impart their respective flavors into the beverage, and determines the inoculation sequence of both (Fig. 2). The inoculation fermentation method was conducted using total acid, ethanol content, pH value, and sensory evaluation as indicators; A) Simultaneous co-fermentation of Lactobacillus and Yeast for 34 h; B) Yeast is first inoculated, followed by the inoculation of Lactobacillus for continued fermentation for 18 h after 16 h; C) Lactobacillus is first inoculated for fermentation, followed by the inoculation of Yeast for further fermentation for 16 h after 18 h.
Fig. 2.
The process flow diagram of beverage made by STSP compound highland barley.
Add Tibetan concave celery polysaccharide to germinated highland barley juice in a certain proportion and mix well. The prepared Tibetan concave celery highland barley compound fermented beverage was sterilized at 85 °C for 20 min to obtain the compound fermented beverage.”
2.5. Determination of physical and chemical indexes and flavor substances during fermentation
The total acid content of compound fermented beverage during fermentation must conform to the National Standard GB/T 12456–2021-Determination of Total Acid in Food to carry out measurement (China, 2021); pH is measured by Mettler pH meter; The specific determination methods for free amino acid and organic acid content should be referenced from relevan literature(China Food Fermentation Industry Research Institute, 2018; Liu, 2015). Use M-100 biosensor analyzer to determine the ethanol content. For the analysis of flavor substances in compound drink-fermented, headspace solid-phase microextraction coupled with gas chromatography–mass spectrometry (HS-SPME-GC–MS) is employed (Luo, Fan and Xu, 2008), with specific determination methods outlined in GB/T 13662–2018.
2.6. Determination of antioxidant capacity of compound drink-fermented
The antioxidant capacity of compound drink-fermented (STSP was added after the fermentation of highland barley beverage), ordinary highland barley fermented beverage (No added STSP after the fermentation of highland barley beverage) and Vc were compared.
Refer to Xie, etc. for DPPH free radical scavenging capacity determination (Xie et al., 2012). The positive control was ascorbic acid (0.1 mg·mL−1), the calculation as formula (2)
| (2) |
Note: A1 is the absorbance of the sample solution; A2 is the absorbance of absolute ethanol instead of DPPH-absolute ethanol solution; A0 is the absorbance of deionized water instead of the sample solution.
The determination of ABTS+ radical scavenging capacity refer to Zhao et al. (2021). The calculation formula is as shown in (3)
| (3) |
Note: A1 is the absorbance of the sample solution; A2 is the absorbance of deionized water instead of ABTS solution; A0 is the absorbance of deionized water instead of the sample solution.
The determination of hydroxyl radical scavenging capacity refer to Chen and Huang (2019). The calculation formula is as shown in (4):
| (4) |
Note: A1 is the absorbance of the sample solution; A2 is the absorbance of deionized water instead of H2O2 solution; A0 is the absorbance of deionized water instead of the sample solution.
The determination of iron ion reduction capacity method refer to Shang et al. (2018). Ascorbic acid is used as a positive control, and deionized water is used as a blank control. The calculation formula is as shown in (5):
| (5) |
Note: A1 is the absorbance value of the sample solution; A2 is the absorbance value of deionized water instead of ferric chloride solution.
2.7. Sensory evaluation of compound drink-fermented
Got scores according to the requirements of the national standard Refer to GB/T 7101–2022 National Standard for Food Safety drink (Chinese, 2022). Invited 10 professionally trained sensory evaluators and set up a sensory evaluation team to conduct sensory evaluation of the product from four aspects of color, smell, taste and organizational state. The comprehensive score is 100 points, and finally take the average of the sensory scores of 10 people. All panelists provided their informed consent prior to each sensory evaluation session, and the study was approved by the Jiangnan University Medical Ethics Committee with the reference number JNU202312IRB13.
2.8. Data processing and analysis methods
In this study, three groups of experiments were set in parallel, ANOVA in Origin 2018 was used for statistical analysis, and validation in SPSS. All data were expressed as mean ± standard deviation. The difference between multiple comparisons was analyzed by one-way anova and Fisher's LSD test. Different letters indicated that there was a significant difference between the data, and p < 0.05 was considered significant.
3. Results and discussion
3.1. Extraction of polysaccharide from Tibet STS
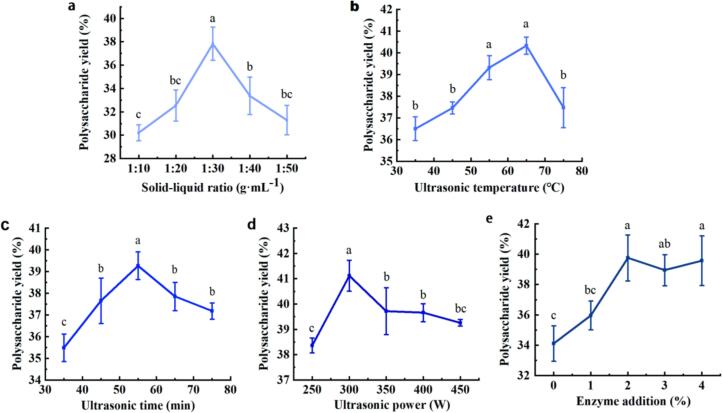
The single factor results are shown in Fig. 3. Under the conditions of fixed temperature 65 °C, power 250 W, time 55 min, and enzyme addition of 3 %, the effect of material-liquid ratio on the extraction yield of STSP was investigated. The experimental results (Fig. 3a) found that the increase of solvent ratio caused the osmotic pressure of the cells to increase. The higher the solvent ratio, the easier it was to accelerate the efflux of intracellular substances, and the increase of solvent ratio could enhance the ultrasonic action area, thereby increasing the ultrasonic efficiency (Li et al., 2023). This trend continued until the solid-liquid ratio reached 30 g·mL−1, whereupon the polysaccharide yield reached its maximum value of 37.84 % ± 1.42 %. After this point, the yield of polysaccharide gradually decreased, potentially due to an excess of solid-liquid ratio potentially reducing the intermolecular interaction, causing a significant reduction in the ultrasonic penetration rate (Zheng et al., 2016).
Fig. 3.
Optimization of polysaccharide rough bill of lading factors. a, material liquid ratio; b, ultrasonic temperature; c, ultrasonic time; d, ultrasonic power; e, compound enzyme addition amount. (Values with superstip letters a, b, c, and d are significantly different across columns (P < 0.05)).
When the ultrasonic temperature increased from 35 °C to 65 °C, the yield of STSP also increased rapidly (Fig. 3b). When the ultrasonic temperature exceeded 65 °C, the extraction yield decreased. This may be due to the combination of ultrasonic cavitation effect and cell wall diffusion effect, and ultrasonic temperature significantly enhances these two effects. The yield was directly proportional to the increasing temperature, yet excessive temperatures resulted in structural degradation due to the strong ultrasonic effect, leading to a decline in the yield. It was therefore concluded that the optimal ultrasonic temperature range lies between 55 °C and 75 °C.
The yield of STSP increased rapidly with the increase of extraction time and reached an extreme value at 55 min (Fig. 3c). The extraction yield began to decrease as the ultrasonic extraction time was increased. One plausible explanation is that the increase in ultrasonic time results in a superposition of thermal effects, thereby accelerating the outflow of the polysaccharide from the raw materials and increasing the yield of polysaccharide. Additionally, the accumulation of ultrasonic time causes the degradation of the polysaccharides in the solution, thus decreasing the production of polysaccharides. For this reason, the optimal ultrasonic time was determined to be between 45 and 65 min under these conditions.
When the ultrasonic power was increased from 250 W to 300 W, the yield of Tibetan foveal polysaccharide increased sharply, reaching 41.12 % ± 0.61 %, after which the extraction yield of polysaccharide began to decrease slowly (Fig. 3d). This may be due to the larger amplitude ultrasound causing more cavitation bubbles in the liquid medium and stronger pressure, which was able to destroy the cell wall and accelerate the diffusion effect(Wang et al., 2014). At the same time, strong shock waves and high-speed jets were also generated, enhancing the penetration of the solvent into the cell tissue(Wang et al., 2018).
When the amount of the complex enzyme increased from 0 % to 2 %, the yield of Tibetan concave celery polysaccharide increased sharply and then leveled off (Fig. 3e). The results suggest that elevating the amount of complex enzyme within a particular range can enhance the efficacy of the enzyme in hydrolyzing the cell wall, thereby promoting the dissolution of endolysin, thereby enhancing the extraction efficiency of the polysaccharide. However, if the amount of complex enzyme added is excessively high, the efficiency of enzymatic hydrolysis may be compromised by competitive inhibition, leading to a slight reduction in the yield of polysaccharide. To avoid reagent waste and cost expenditure in the subsequent process, a 2 % enzyme amount was determined as the optimal extraction enzyme amount for the polysaccharide extraction of STS.
On this basis, L9 (34) was used for orthogonal design. The orthogonal optimization of the extraction results and visual analysis of STSP are shown in Table 4. The R value has a positive correlation with the polysaccharide yield. The degree of influence of various factors on the polysaccharide yield is in the following order: material liquid ratio (MLR) > ultrasonic power (UP) > ultrasonic time (UTI) > ultrasonic temperature (UTE). The results of the orthogonal test were analyzed by variance, as shown in Table 5. The F value and P value can be used to determine whether this factor has a significant impact on the STSP yield. The ratio of material to liquid has a very significant impact on the polysaccharide yield, and the other three factors have no significant impact on the yield of STSP. Finalizing the experimental results showed that the optimal technological parameters for the extraction of STSP were as follows: the ratio of material to liquid was 1:30 (g·mL−1), the amount of compound enzyme was 2 %, the ultrasonic temperature was 55 °C, the ultrasonic time was 55 min, and the ultrasonic power was 350 W. Under this extraction condition, the yield of polysaccharide from STS was 41.37 % ± 1.40 %, the relative standard deviation (RSD) was 2.32 %, and the precision was good.
Table 4.
Orthogonal optimization results and visual analysis.
| Test number | Factors |
3 Replicates | STSP yield (%) | |||||
|---|---|---|---|---|---|---|---|---|
| A-MLR (g·mL−1) | B-UTE (°C) | C-UTI (min) | D-UP (W) | |||||
| 1 | 1 | 1 | 1 | 1 | 32.55 | 32.92 | 31.08 | 32.18 |
| 2 | 1 | 2 | 2 | 2 | 34.66 | 33.94 | 36.60 | 35.07 |
| 3 | 1 | 3 | 3 | 3 | 34.71 | 32.43 | 32.71 | 33.28 |
| 4 | 2 | 1 | 2 | 3 | 40.14 | 42.32 | 41.44 | 41.30 |
| 5 | 2 | 2 | 3 | 1 | 38.52 | 37.44 | 39.98 | 38.65 |
| 6 | 2 | 3 | 1 | 2 | 36.46 | 36.67 | 38.90 | 37.34 |
| 7 | 3 | 1 | 3 | 2 | 37.15 | 35.80 | 37.26 | 36.74 |
| 8 | 3 | 2 | 1 | 3 | 37.20 | 34.95 | 36.52 | 36.22 |
| 9 | 3 | 3 | 2 | 1 | 35.20 | 34.04 | 34.72 | 34.65 |
| K1 | 100.533 | 110.220 | 105.750 | 105.483 | ||||
| K2 | 117.290 | 109.937 | 111.020 | 109.147 | ||||
| K3 | 107.613 | 105.280 | 108.667 | 110.807 | ||||
| k1 | 33.511 | 36.740 | 35.250 | 35.161 | ||||
| k2 | 39.097 | 36.646 | 37.007 | 36.382 | ||||
| k3 | 35.871 | 35.093 | 36.222 | 36.936 | ||||
| Range | 5.586 | 1.647 | 1.757 | 1.774 | ||||
| Factors | A > D > C > B | |||||||
| Optimal scheme | A2B1C2D3 | |||||||
Table 5.
ANOVA of orthogonal optimization results.
| Source of variance | Deviation sum of squares | Degree of freedom | Mean square | F value | P value | Significance |
|---|---|---|---|---|---|---|
| A-MLR (g·mL−1) | 141.517 | 2 | 70.758 | 24.882 | 0.004 | ** |
| B-UTE (°C) | 15.389 | 2 | 7.695 | 2.706 | 0.127 | |
| C-UTI (min) | 13.939 | 2 | 6.970 | 2.451 | 0.148 | |
| D-UP (W) | 14.838 | 2 | 7.419 | 2.609 | 0.134 | |
| Error value | 22.750 | 8 | 2.844 |
Note: “* *” means that it has extremely significant effect on polysaccharide content (P < 0.01).
3.2. Isolation, purification and identification of polysaccharide from Tibet STS
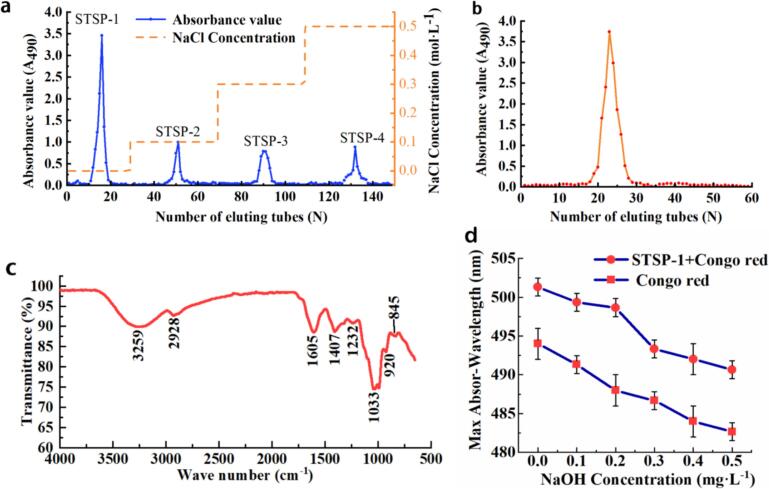
To characterize the polysaccharide components of the Tibet STS, a series of chromatographic techniques were employed to effectively separate and identify the key compounds. As shown in Fig. 4a, the polysaccharide from STS was eluted by 0, 0.1, 0.3, and 0.5 mol·L−1 of NaCl solution on DEAE-52 cellulose chromatography column, and four peaks were obtained. The peak shape was single and symmetrical, of which the first isolated STSP-1 was neutral polysaccharide without charge, and STSP-2, STSP-3, and STSP-4 were acid polysaccharides. The contents of the four polysaccharides were 79.80 ± 1.68 %, 10.82 ± 0.53 %, 5.85 ± 0.66 % and 3.53 ± 0.26 % respectively. Considering the collection time and desalination cost, STSP-1 was selected for subsequent separation and purification. As shown in Fig. 4b, after purification by Sephadex G-100 gel column, a single symmetrical single peak component is obtained, which proved that the obtained Tibet STS polysaccharide STSP-1 is a relatively homogeneous polysaccharide. The component solution is collected, dialyzed, and dried to obtain a purified polysaccharide sample with a content of 86.91 % ± 1.83 %. The total sugar content before purification was 70.29 % ± 2.90 %, and the content after purification was 86.91 % ± 1.83 %. The protein content before purification was 10.81 % ± 2.03 %, and its existence was not detected after purification. The physicochemical properties of the purified Tibetan STSP-1 were analyzed. The results show that it is easily soluble in water, insoluble in organic reagents such as chloroform and n-butanol; after the reaction of Molisch, there are obvious purple rings, indicating that the polysaccharide contains sugars; after the reaction of Feilin reagent, no brick red precipitation in the polysaccharide solution is formed, indicating that the polysaccharide does not contain soluble reducing sugars; after the reaction of iodine and potassium iodide, the color of the polysaccharide solution is yellow, not purple, indicating that there are no phenols in the polysaccharide.
Fig. 4.
Isolation and identification of polysaccharide from Tibet STS. a, preliminary separation of STSP from DEAE cellulose; b, STSP-1 was separated and purified by sephadex G-100; c, infrared spectrum analysis of STSP-1; d, results of Congo red with STSP-1. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Weight average molecular weight (Mw) and number average molecular weight (Mn) are key parameters used to evaluate the physicochemical properties of polysaccharides (Liu, Liu, et al., 2020). Therefore, the molecular weight of STSP-1 was determined using high performance gel permeation chromatography, and the results are shown in Table 6. The Mw and Mn of STSP-1 are 96.81 kDa and 56.28 kDa, respectively. The polydispersion coefficient of polysaccharides is used to measure the distribution of molecular weight heterogeneity of polysaccharides. The closer the coefficient is to 1, the smaller the molecular weight distribution and the more concentrated the molecular weight. The polydispersion coefficient (Mw/Mn) of STSP-1 is 1.72, indicating that STSP-1 is a relatively homogeneous polysaccharide. In this study, the monosaccharide composition of Tibetan STSP-1 was analyzed by high performance liquid chromatography. As shown in Table 6, the molar percentages of various monosaccharides were determined according to the peak area and peak time. STSP-1 consisted of arabinose, galactose, glucose, xylose and mannose, with a molar ratio of 5.63: 2.79: 86.36: 3.02: 1.59. Since the Polysaccharide and Monosaccharide composition of Tibetan STS has not been reported, a comparison was made with the results of studies on Angelica sinensis, which belongs to the same Apiaceae family as STS. It was reported that the polysaccharides of Angelica sinensis consist of glucose, galactose, arabinose, and xylose in a molar ratio of 8.4:2.7:1.8:1.0 (Chen et al., 2010). This composition is similar to the types and proportions of monosaccharides isolated and purified in this study, with glucose being the predominant component.
Table 6.
Molecular weight and monosaccharide composition of VTP-1.
| Indicators | VTP-1 |
|---|---|
| Weight average molecular weight (Mw,kDa) | 96.81 56.28 1.72 / 5.63 2.79 86.36 3.02 1.59 |
| Number average molecular weight (Mn,kDa) | |
| Polydispersion coefficient(Mw/Mn) | |
| Monosaccharide Content(%) | |
| Arabinose | |
| Galactose | |
| Glucose | |
| Xylose | |
| Mannose |
In order to further analyze the structure of STSP-1, FT-IR was used for analysis. As shown in Fig. 4c, STSP-1 has a broad and strong absorption peak at 3259 cm−1, indicating the existence of -OH in its intermolecular and intramolecular structure; The absorption peak at 2928 cm−1 is the stretching vibration of C—H, indicating that STSP-1 has typical polysaccharide structure characteristics (Xian et al., 2022); The absorption peak appeared at 1605 cm−1, which may be caused by the asymmetric stretching vibration of C O on the carboxylic acid group in the uronic acid or the amide I band in the protein (Zhao et al., 2021). There is no obvious absorption peak near 1540 cm−1, indicating that STSP-1 does not contain N—H groups, which is consistent with the protein content results of UV spectrum analysis (Xian et al., 2022). The absorption peak at 1407 cm−1 is caused by C C stretching vibration on the sugar chain. The absorption peak at 1232 cm−1 is caused by C—H stretching vibration. The absorption peak at 1033 cm−1 is caused by the stretching vibration of C—O and C-O-C in the pyran ring (Feng, 2022). The weak absorption peak at 920 cm−1isβ-Caused by the tensile vibration of glucopyranoside, at 845 cm−1: α-Characteristic absorption peak of type I glycoside bond (Guo et al., 2022). These results indicate that the polysaccharide STSP-1 contains α−/β-type glycoside bond. When the polysaccharide with triple helix structure combines with Congo red solution, the maximum absorption wavelength of the polysaccharide and Congo red complex will shift to red (Mutailifu et al., 2022). Therefore, Congo red assay was used to determine the triple helix structure of STSP-1. As shown in Fig. 4d, the maximum absorption wavelength of STSP-1 decreases with the increase of NaOH concentration, indicating that STSP-1 does not have a triple helix structure.
Studies have shown that the antioxidant activity of polysaccharides is closely related to its structural properties (molecular weight, monosaccharide composition, glycoside bond type and chain conformation, etc.) (Cui et al., 2023). The antioxidant activity of Tibetan STSP-1 is affected by a variety of factors, rather than a single factor. The STSP-1 extracted in this paper has a high molecular weight (Mw = 96.81 kDa) and exhibits strong antioxidant activity. The presence of rhamnose, arabinose, xylose, mannose, glucose, galactose, etc. in monosaccharides may have an impact on antioxidant activity (Li et al., 2024; Wang et al., 2022). Therefore, the rich monosaccharide composition of Tibetan concave celery polysaccharide may be the reason for its good antioxidant activity. In addition, alpha and beta-type glycosidic bonds in polysaccharides also affect the antioxidant activity of polysaccharides (Zhang & Zhu, 2024). STSP-1 contains both alpha and beta-type glycosidic bonds, which may also be the reason for its good antioxidant activity. In this study, the preliminary structure of polysaccharide was analyzed. Due to the complex antioxidant mechanism of polysaccharide, the structure of polysaccharide was not fully resolved. Therefore, the specific correlation between the antioxidant capacity and structural characteristics of polysaccharide needs to be further studied.
3.3. Fermentation technology and flavor of Tibet STS highland barley compound fermented beverage
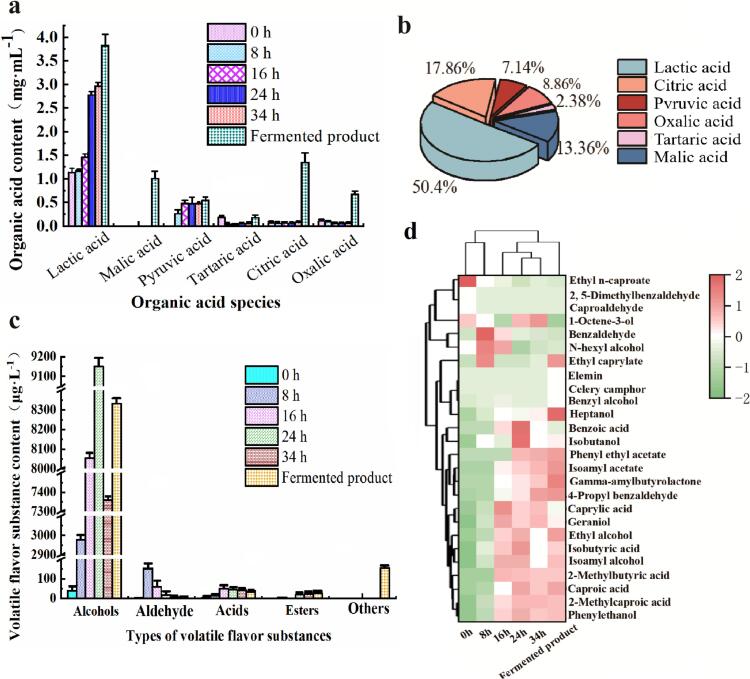
Highland barley is the most distinctive plateau crop. In this study, which was used as a carbon source for fermentation and combined with SPST to prepare a unique functional beverage. As depicted in Fig. 5a, the acid production initially decreases and subsequently stabilizes with an increase in the feed water ratio. Lactobacillus effectively utilizes nutrients from highland barley to produce acidd, but higher acid production leads to lower pH levels, the more restricted the growth of Lactobacillu. Additionally, as indicated in Fig. 5b, Tibet STSP was added to the fermented beverage post-fermentation, resulting in a significant enhancement of organic acid content including malic acid (13.36 %). The content of lactic acid, citric acid and oxalic acid also increased significantly. Citric acid imparts a refreshing sour taste while offering benefits such as reducing lipid peroxidation and inflammation, alleviating fatigue, and preventing diabetes. These findings demonstrate that the addition of STSP can enrich the flavor profile of compound drink-fermented.
Fig. 5.
Changes of acids and flavor substances in compound drink-fermented at different fermentation times. a, changes in the content of various organic acids; b, proportion of various organic acids in finished products; c, changes of volatile flavor compounds during fermentation; d, cluster analysis of composite fermented beverage samples at different fermentation times (green: low content; red: high content). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
According to the composition of the main volatile components in drink-fermented and sensory evaluation Table 7, it can be found that beverage B exhibits the highest sensory evaluation, which could be attributed to its elevated levels and diverse range of alcohols, acids, esters and aldehydes. Beverage A demonstrates the lowest sensory acceptability with a bitter taste profile and pronounced intensity. Similarly, beverage C also receives poor sensory evaluation due to an excessively sour taste. Considering factors such as total acid content, ethanol concentration, volatile flavor substances, and sensory evaluation results; the latter emerges as the most crucial indicator. Consequently, optimal outcomes are achieved by subjecting yeast fermentation for 16 h followed by lactobacillus fermentation for 18 h. When polysaccharide content reaches 20 %, the resulting beverage showcases a delightful blend of sweetness and acidity while maintaining good fluidity without any precipitation issues. As a result of these attributes combined together harmoniously, it attains the highest score in terms of sensory perception.
Table 7.
Content of main volatile components of compound drink-fermented and sensory evaluation.
| Category | Name of volatile component | Content(μg·L−1) |
Fragrance description | ||
|---|---|---|---|---|---|
| A | B | C | |||
| alcohols | Phenylethanol | 3387.16 ± 83.92 | 5996.87 ± 122.47 | 4954.48 ± 691.4 | Floral fragrance |
| Isoamyl alcohol | 2171.35 ± 8.35 | 2469.66 ± 72.31 | 2560.79 ± 287.78 | Banana and apple fragrance | |
| Ethanol | 342.09 ± 20.92 | 486.44 ± 26.86 | 331.85 ± 28.3 | – | |
| Isobutanol | 13.47 ± 1.76 | 13.21 ± 2.38 | 13.44 ± 6.57 | – | |
| N-hexanol | 1.52 ± 0.34 | 1.28 ± 0.17 | 1.82 ± 0.31 | Fruity aroma | |
| 1-Octen-3-ol | 1.21 ± 0.15 | 1.89 ± 0.46 | 1.29 ± 0.09 | Floral fragrance | |
| Heptanol | – | 0.97 ± 0.08 | 1.19 ± 0.18 | aromatic | |
| Geraniol | 0.24 ± 0.03 | 0.54 ± 0.12 | 0.84 ± 0.17 | Floral fragrance | |
| Benzyl alcohol | 0.81 ± 0.09 | 0.71 ± 0.07 | 0.73 ± 0.08 | Fruity aroma | |
| Acids | Caprylic acid | 31.64 ± 8.91 | 18.41 ± 0.47 | 23.54 ± 1.27 | Fruity aroma |
| Caproic acid | 2.88 ± 0.33 | 3.53 ± 0.37 | 2.92 ± 0.39 | Coconut oil flavor | |
| Methylbutyric acid | – | – | 2.50 ± 0.13 | Goat milk cheese flavor | |
| Isobutyric acid | 0.13 ± 0.08 | 0.37 ± 0.16 | 0.64 ± 0.14 | Irritating odor | |
| Butyrate | 0.27 ± 0.01 | 19.90 ± 2.12 | 0.57 ± 0.12 | Irritating odor | |
| Esters | Phenylethyl acetate | 59.52 ± 2.12 | – | – | Rose and honey fragrance |
| γ-Amyl butyrolactone | 0.49 ± 0.09 | 1.05 ± 0.02 | 0.70 ± 0.05 | – | |
| Ethyl acetate | 1.27 ± 0.08 | 1.70 ± 0.16 | 0.61 ± 0.07 | Floral and fruity fragrance | |
| Isoamyl acetate | 1.25 ± 0.02 | 0.15 ± 0.03 | 0.51 ± 0.01 | Banana scent | |
| Ethyl hexanoate | 0.14 ± 0.02 | 0.16 ± 0.01 | 0.15 ± 0.01 | Fruity aroma | |
| Aldehydes, phenols and others | Benzaldehyde | 18.46 ± 2.5 | 1.69 ± 0.14 | 2.23 ± 0.49 | Bitter almond flavor |
| Nonanal | 0.22 ± 0.11 | 0.38 ± 0.03 | 0.05 ± 0 | citrus | |
| 4-Ethylphenol | – | – | 2.38 ± 0.24 | – | |
| Tibet STS brain | 142.87 ± 11.08 | 142.08 ± 13.71 | 120.47 ± 13.49 | – | |
| Elemene | 8.93 ± 0.39 | 10.73 ± 1.27 | 8.90 ± 0.68 | – | |
| Description of sensory evaluation | Aroma coordination;The taste is sour and sweet, lactic acid is irritant, and bitter and astringent | Harmonious aroma, sweet and sour taste, obvious lactic acid taste, soft sour taste, good liquidity | Taste a little bitter, too sour, too sweet | ||
| Sensory score | 64.20 ± 7.05 | 80.10 ± 4.79 | 72.70 ± 8.50 | ||
A total of 57 volatile flavor compounds were identified in the compound fermented beverage during fermentation, comprising 18 alcohols, 6 aldehydes, 11 acids, 10 esters and 12 other flavor substances. As shown in Fig. 5c, throughout the fermentation process, the cumulative content of alcohols, aldehydes, and acids initially increased and then decreased with time while the cumulative content of esters exhibited an initial increase followed by stabilization. Among these compounds, alcohols and aldehydes emerged as the predominant volatile flavor substances throughout the entire fermentation process; moreover, the change trend in the cumulative content of alcohols was more pronounced compared to that of aldehydes.
Alcohols represent the predominant volatile flavor compounds in the fermentation stage of compound drink-fermented, compound fermented beverage exhibiting a wide range of concentrations in the fermentation process, with levels increasing from 38.32 ± 15.81 μg·L−1 to 7360.75 ± 267.98 μg·L−1. Phenylethanol possesses a delicate rose fragrance and demonstrates antibacterial properties, thereby incorporating into drink for sensory and functional enhancement. In addition, isoamyl alcohol is known for imparting a fruity flavor profile, which softens the overall taste of the beverage. Furthermore, numerous beneficial alcohols are synthesized during fermentation, contributing to the complex and multifaceted flavor profile of the beverage. There are many ways of alcohol synthesis, including yeast fermentation, glucose metabolism, amino acid decarboxylation and dehydrogenation (Zeng et al., 2022) etc. Among them, the alcohols with higher content are phenylethanol, isoamyl alcohol and ethanol, which reach the peak value at 24 h of fermentation, 6039.87 ± 1768.05 μg·L−1, 2713.68 ± 287.78 μg·L−1, 351.10 ± 93.41 μg·L−1 for ethanol respectively, whereas other alcohol contents remain unchanged throughout the entire fermentation period.
Aldehydes could promote the release of aroma in drink and coordinate various aroma components. The content increased from 3.18 ± 0.19 μg·L−1 to 5.47 ± 0.24 μg·L−1. Benzaldehyde was identified as the primary aldehyde compound during fermentation process, imparting a distinct bitter almond flavor that negatively affects the beverage's taste profile. However, its content significantly decreases throughout fermentation, reaching its peak at 8 h with a rapid drop from 150.36 ± 8.88 μg·L−1 to 3.96 ± 0.49 μg·L−1. The levels of other aldehydes remain relatively stable during fermentation process. Despite their low concentrations, acids, esters, and other volatile compounds play an irreplaceable role in contributing to the overall aroma profile of drink. Additionally, the addition of Tibet STSP extract into the finished product leads to a significant increase in volatile flavor components' content within the beverage matrix. The levels of isoamyl alcohol, ethanol, ethyl caproate show notable increments while two new volatile flavor substances-parsley brain and elementin are introduced. Additionally, the content of desirable volatile compounds also experiences an increase. All these findings collectively demonstrate that incorporating STSP enriches the variety and concentration of volatile flavor compounds in compound drink-fermented,resulting in a more harmonious and delicate sensory experience.
Furthermore, in order to visually and explicitly depict the fluctuation of volatile compound content in drink during fermentation, based on volatile compound content (>1 μg·L−1), a heatmap clustering methodology has been utilized. It can be observed from Fig. 5d that the significant volatile flavor substances in the five fermentation samples are categorized into two groups: one comprising 0 h ∼ 8 h, and the other consisting of 16 h and 24 h ∼ 34 h. The content of volatile flavor substances in the former category (0 h ∼ 8 h) is relatively lower compared to that in the latter category (16 h). Volatile flavor compounds present in drink are intricate, with their contents undergoing variations throughout fermentation. Notably, there is an increase in phenylethyl acetate content during later stages of fermentation, imparting a fruity aroma to the beverage while enhancing its overall taste profile. Phenylethanol, isoamyl alcohol (apple brandy), ethanol, and isobutanol (malt) represent four primary higher alcohols found within this beverage. These alcohols contribute a rich aroma to the compound fermented beverage while also facilitating dissolution of other aromatic (Chen, 2022). The thermogram illustrates color changes associated with various volatile compounds, thereby indicating significant fluctuations occurring during fermentation. Additionally, incorporation of STSP significantly boosts volatile compound content.
3.4. Study on antioxidant function of Tibet STS highland barley compound fermented beverage
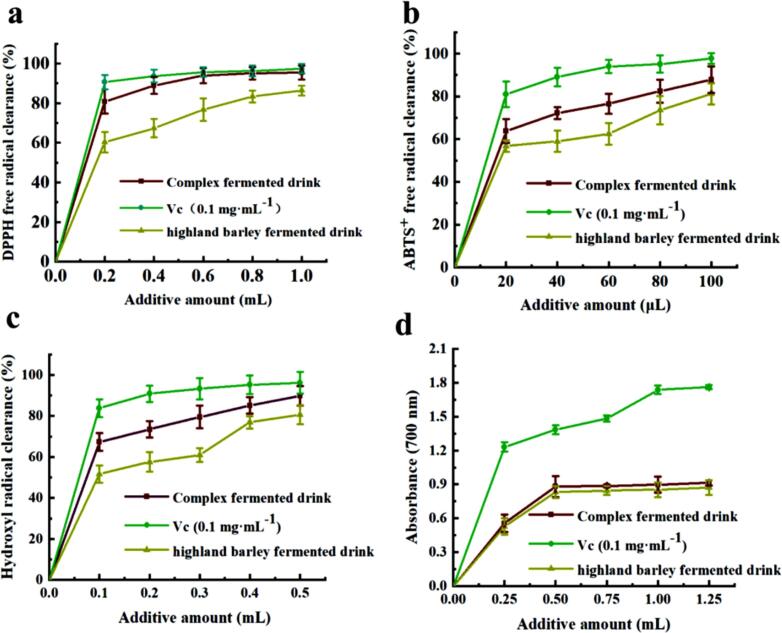
The in vitro antioxidant test was carried out on the compound beverage. The results depicted in Fig. 6a showed a gradual increase in the DPPH free radical scavenging rate of Tibet STSP highland barley compound fermented beverage with an increase in the added amount. The clearance rate of the compound fermented beverage was 95.41 % ± 3.49 %, slightly lower than that of Vc (97.37 % ± 2.37 %), and significantly higher than that of common highland barley beverage (86.39 % ± 2.55 %). Additionally, the IC50 value of the compound fermented beverage is 52.10 μL whereas for common highland barley beverage is 134.10 μL. This indicates that the addition of Tibet STSP can improve the DPPH scavenging capacity of highland barley drink. As illustrated in Fig. 6b, Tibet STSP highland barley compound fermented beverage exhibits a superior ability to remove ABTS+ compared to ordinary highland barley beverage. When the added amount is 100 μL, the clearance rate of ABTS+ in the compound fermented beverage added with the STSP was 87.77 ± 6.21 %. In addition, the IC50 value for the compound fermented beverage is 10.06 μL while for common highland barley beverage is 16.55 μL. These findings indicate that adding Tibet STS polysaccharide can improve both DPPH and hydroxyl radical scavenging capacitiesofhighl andbarleydrink. Fig. 6c demonstrates a gradual increase in the hydroxyl radical scavenging rate in Tibet STSP highland barley compound fermented beverage with the increase of the added amount. The clearance rate of the compound fermented beverage was 89.78 ± 4.71 %, lower than 96.16 ± 5.29 % of Vc, and higher than that of common highland barley beverage. When the added amount is 0.3 mL, the DPPH clearance rate of compound highland barley beverage is 79.50 ± 5.48 %, while when the added amount is 0.5 mL, the clearance rate of common highland barley beverage is 80.59 ± 4.66 %. In addition, the IC50 value of compound fermented beverage is 42.68 μL. The IC50 value of the common highland barley beverage is 110.20 μL. This indicates that the addition of Tibet STSP can improve the hydroxyl radical scavenging capacity of highland barley beverage, so the compound highland barley beverage has strong antioxidant capacity. It could be seen from Fig. 6d that the higher the amount of beverage added, the stronger the reduction ability to iron ions, and its absorbance value is always higher than that of ordinary highland barley beverage, so the reduction ability is significantly higher than that of ordinary highland barley beverage. All the above indicated that the addition of Tibet STSP can enhance the reduction ability of highland barley beverage, so the compound fermented beverage has strong antioxidant capacity.
Fig. 6.
Antioxidant function of Tibet STS highland barley compound fermented beveragea. a, DPPH free radical scavenging capacity; b, ability to scavenge ABTS+ free radicals; c, hydroxyl radical scavenging ability; d, Iron ion reduction ability.
4. Conclusions
The study evaluated the antioxidant capacities of STSP isolated and identified from Lhasa, Tibet, offering insights into their potential medicinal properties. A fermented beverage containing STSP and highland barley was evaluated, with a preliminary evaluation of its flavor properties. The findings highlight the significant medicinal potential of STSP and investigated the biological activities of foods and specific plants at high altitudes with the goal of enhancing human health and fostering economic growth. The results provide a scientific foundation for the utilization of traditional flora and pave the way for sustainable development in plateau regions.
CRediT authorship contribution statement
Tiantian Liu: Writing – review & editing, Validation, Investigation, Conceptualization. Mujia Nan: Supervision, Project administration. Suyi Zhang: Methodology, Formal analysis, Data curation. Hui Qin: Methodology, Formal analysis, Data curation. Zesu Zhao: Writing – original draft. Shuangping Liu: Investigation, Funding acquisition. Jian Mao: Supervision, Project administration.
Declaration of competing interest
We declare that the work described has not been published previously (except in the form of an abstract, a published lecture or academic thesis, see ‘Multiple, redundant or concurrent publication’ for more information), that it is not under consideration for publication elsewhere, that its publication is approved by all authors and tacitly or explicitly by the responsible authorities where the work was carried out, and that, if accepted, it will not be published elsewhere in the same form, in English or in any other language, including electronically without the written consent of the copyrightholder. To verify compliance, your article may be checked by Crossref Similarity Check and other originality or duplicate checking software.
Acknowledgements
This work was supported by the first phase of the connotation construction of the 14th Five-Year Plan of Tibetan medicine (2021ZYYGH008), the Fundamental Research Funds for the Central Universities (JUSRP221031), the Science and Technology Plan Project of Shaoxing City (2022B43001), and the Fundamental Research Funds for the Central Universities (JUSRP202401019).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2024.101988.
Contributor Information
Mujia Nan, Email: nmj6402@163.com.
Jian Mao, Email: maojian@jiangnan.edu.cn.
Appendix A. Supplementary data
Supplementary material shows the optimization of material-water ratio of compound fermented beverage, and antioxidant activities of STSP-1 in vitro.
Data availability
The data that support the findings of this study are available from the corresponding authors upon reasonable request.
References
- Chen F., Huang G.L. Extraction, derivatization and antioxidant activity of bitter gourd polysaccharide. International Journal of Biological Macromolecules. 2019;141:14–20. doi: 10.1016/j.ijbiomac.2019.08.239. [DOI] [PubMed] [Google Scholar]
- Chen X., Cao W., Sun Y., Mei Q.B. Physicochemical properties and anti-tumor activities in vitro of Angelica sinensis polysaccharide APS-bII. Science Technology and Engineering. 2010;10(8):1839–1843. https://en.cnki.com.cn/Article_en/CJFDTOTAL-KXJS201008003.htm. [Google Scholar]
- Chen X.Q. Guizhou University; Guiyang: 2022. Study on fermented glutinous rice beverage of Dendrobium Xiaoqu, master dissertation. [DOI] [Google Scholar]
- China Food Fermentation Industry Research Institute . Peking: Standards Press of China; 2018. Huangjiu. In Z. L. I. R. China food fermentation industry research institute. (Ed.), GB/T 13662-2018) Z. L. I. R. [Google Scholar]
- China M.O.H. GB12456–2021. Standards Press of China; Peking: 2021. National standards for food safety-determination of total acid in food. [Google Scholar]
- Chinese M.O.H. In: GB-7101-2022. Chinese M.O.H., editor. Standards Press of China; Peking: 2022. Food safety national standard-beverage. [Google Scholar]
- Cui Y.L., Chen Y.J., Wang S., Wang S.X., Yang J., Ismael M.…Lu X. Purification, structural characterization and antioxidant activities of two neutral polysaccharides from persimmon peel. International Journal of Biological Macromolecules. 2023;225:241–254. doi: 10.1016/j.ijbiomac.2022.10.257. [DOI] [PubMed] [Google Scholar]
- Ding H.H., Cui S.W., Goff H.D., Wang Q., Chen J., Han N.F. Soluble polysaccharides from flaxseed kernel as a new source of dietary fibres: Extraction and physicochemical characterization. Food Research International. 2014;56:166–173. doi: 10.1016/j.foodres.2013.12.005. [DOI] [Google Scholar]
- Dong S., Chai L., Zhang X., Hu Y., Yang H. Anti-aging effect of Vicatia thibetica de Boission aged mice induced by D-galactose. Journal of Traditional Chinese Veterinary Medicine. 2020;39(03):66–68. [Google Scholar]
- Dong S., Wang C., Zhang X., Zhu G., Du Y. Effects of Xigui on learning and memory function in mice. Chinese Journal of Pharmacovigilance. 2016;13(01):5–8. doi: 10.19803/j.1672-8629.2016.01.002. [DOI] [Google Scholar]
- Dong S., Zhang X. Development of compound beverage of seselopsis and dendrobium devonianum. Beverage Industry. 2016;19(06):42–45. doi:CNKI:SUN:BXJG.0.2018-01-010. [Google Scholar]
- Duan J.C., Luo S.D., Cao Z.G., Guo M.X., Shen L., Du Y.M., Liu X.P. The anti-exercise fatigue effect of crude polysaccharide of Vicatia thibertica de Boiss on exhausted swimming mice. Medicine, Chinese Traditional Patent. 2017;40(03):681–684. doi: 10.3969/j.issn.1001-1528.2018.03.035. [DOI] [Google Scholar]
- Feng X. Jiangnan University; Wuxi: 2022. The analysis of the crucial components in semi-dry Shaoxing Huangjiu on the alleviation of constipation, master dissertation. [Google Scholar]
- Gramazio P., Takayama M., Ezura H. Challenges and prospects of new plant breeding techniques for GABA improvement in crops : Tomato as an example. Frontiers in Plant Science. 2020;11 doi: 10.3389/fpls.2020.577980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu J.Y., Zhang H.H., Zhang J.X., Wen C.T., Ma H.L., Duan Y.Q., He Y.Q. Preparation, characterization and bioactivity of polysaccharide fractions from Sagittaria sagittifolia L. Carbohydrate Polymers. 2020;229(1) doi: 10.1016/j.carbpol.2019.115355. [DOI] [PubMed] [Google Scholar]
- Guo L.A., Kong N., Zhang X.Y., Ma H.L. Multimode ultrasonic extraction of polysaccharides from Maca (Lepidium meyenii): Optimization, purification, and in vitro immunoregulatory activity. Ultrasonics Sonochemistry. 2022;88 doi: 10.1016/j.ultsonch.2022.106062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He S., Zhang Y., Huang H., Xiao J., Yang X. Study on anti-oxidative stress effect of Xigui crude polysaccharide for hypoxic mice. Journal of Dali University. 2021;7(02):10–15. http://journal15.magtechjournal.com/Jwk_dlxyzk/ CN/abstract/abstract11528.shtml. [Google Scholar]
- Hong J., Kim K.J. Crystal structure of γ-aminobutyrate aminotransferase in complex with a PLP-GABA adduct from Corynebacterium glutamicum. Biochemical and Biophysical Research Communications. 2019;514(3):601–606. doi: 10.1016/j.bbrc.2019.04.194. [DOI] [PubMed] [Google Scholar]
- Huang H., Huang G. Extraction, separation, modification, structural characterization, and antioxidant activity of plant polysaccharides. Chemical Biology & Drug Design. 2020;96(5):1209–1222. doi: 10.1111/cbdd.13794. http://journal15.magtechjournal.com/ Jwk_dlxyzk/CN/Y2022/V7/I2/10. [DOI] [PubMed] [Google Scholar]
- Li D., Chen M., Meng X., Sun Y., Liu R., Sun T. Extraction, purification, structural characteristics, bioactivity and potential applications of polysaccharides from Avena sativa L.: A review. International Journal of Biological Macromolecules. 2024;265 doi: 10.1016/j.ijbiomac.2024.130891. [DOI] [PubMed] [Google Scholar]
- Li, J. C., Chen, Z. X., Shi, H. M., Yu, J., Huang, G. L., & Huang, H. L. (2023). Ultrasound-assisted extraction and properties of polysaccharide from Ginkgo biloba leaves. Ultrasonics Sonochemistry, 93, 106295. Doi: 10.1016/j.ultsonch.2023.106295. [DOI] [PMC free article] [PubMed]
- Li L., Guo M.X., Yang J., Yang Q.L., He Y.P., Shen L., Liu X.B. Effects of Xigui extract on immunosuppressive mice induced by cyclophosphamide. Chinese Journal of Hospital Pharmacy. 2017;37(03):244–247. doi: 10.13286/j.cnki.chinhosppharmacyj.2017.03.10. [DOI] [Google Scholar]
- Liu F., Chen G., Hu Q., Zhao S., Zhao L. Separation, purification and structure characteristics of Zn-binding polysaccharides from Flammulina velutipe. Food Science. 2014;35(05):1–7. [Google Scholar]
- Liu X.Q., Ding J.C., Li J.X., Meng D., Shen L., Guo M.X., Liu X.B. Immunomodulation of polysaccharides from Xigui in exercise-induced fatigue mice. Science and Technology of Food Industry. 2020;42(05):317–321. doi: 10.13386/j.issn1002-0306.2020040200. [DOI] [Google Scholar]
- Liu X.Y., Liu D., Chen Y.H., Zhong R.T., Gao L.Y., Yang C.F.…Zhao C. Physicochemical characterization of a polysaccharide from Agrocybe aegirita and its anti-ageing activity. Carbohydrate Polymers. 2020;236 doi: 10.1016/j.carbpol.2020.116056. [DOI] [PubMed] [Google Scholar]
- Liu Y. Jiangnan University; Wuxi: 2015. Characterization of microflora and their functions on flavor compounds in Shaoxing rice wine, master dissertation. [Google Scholar]
- Luo T., Fan W.L., Xu Y. Characterization of volatile and semi-volatile compounds in Chinese rice wines by headspace solid phase microextraction followed by gas chromatography-mass spectrometry. Journal of the Institute of Brewing. 2008;114(2):172–179. doi: 10.1002/j.2050-0416.2008.tb00323.x. [DOI] [Google Scholar]
- Ma J.J., Cao Z.G., Guo M.X., Shen L., Chen R.X., Liu X.B. Protective effects of Xigui polysaccharide on sleep deprivation injury in mice. Journal of Dali University. 2020;6(02):29–33. http://journal15.magtechjournal.com /Jwk_dlxyzk/CN/Y2021/V6/I2/29. [Google Scholar]
- Mutailifu P., Nuerxiati R., Lu C.F., Huojiaaihemaiti H., Abuduwaili A., Yili A. Extraction, purification, and characterization of polysaccharides from Alhagi pseudoalhagi with antioxidant and hypoglycemic activities. Process Biochemistry. 2022;121:339–348. doi: 10.1016/j.procbio.2022.06.026. [DOI] [Google Scholar]
- Qiu Y., Chen L., Liu J.X., Guo M.X., Liu X.B. Anti-dysmenorrhea effects of Xigui essential oil. Journal of Jinggangshan University (Natural Science) 2019;40(04):96–102. doi: 10.3969/j.issn.1674-8085.2019.04.019. [DOI] [Google Scholar]
- Shang H.M., Zhou H.Z., Duan M.Y., Li R., Wu H.X., Lou Y.J. Extraction condition optimization and effects of drying methods on physicochemical properties and antioxidant activities of polysaccharides from comfrey (Symphytum officinale L.) root. International Journal of Biological Macromolecules. 2018;112:889–899. doi: 10.1016/j.ijbiomac.2018.01.198. doi:1016/j.ijbiomac.2018.01.198. [DOI] [PubMed] [Google Scholar]
- Tian J.L., Guo M.X., Liu G.M., Shen L., Liu X.B. Effects of Xigui drug serum on inflammatory factors in lipopolysaccharide-stimulated murine macrophages RAW264.7. Journal of Pharmaceutical Research. 2018;37(09):479–499. doi: 10.13506/j.cnki.jpr.2018.09.001. [DOI] [Google Scholar]
- Wang F.F., Ye S.H., Ding Y., Ma Z.Y., Zhao Q.K., Zang M., Li Y. Research on structure and antioxidant activity of polysaccharides from Ginkgo biloba leaves. Journal of Molecular Structure. 2022;1252 doi: 10.1016/j.molstruc.2021.132185. [DOI] [Google Scholar]
- Wang L.B., Cheng L., Liu F.C., Li T.F., Yu Z.Y., Xu Y.Q., Yang Y. Optimization of ultrasound-assisted extraction and structural characterization of the polysaccharide from pumpkin (Cucurbita moschata) seeds. Molecules. 2018;23(5):1207. doi: 10.3390/molecules23051207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y.P., Liu Y., Hu Y.H. Optimization of polysaccharides extraction from Trametes robiniophila and its antioxidant activities. Carbohydrate Polymers. 2014;111:324–332. doi: 10.1016/j.carbpol.2014.03.083. [DOI] [PubMed] [Google Scholar]
- Xian R.H., Pu D.W., Fan Z.L., Yu C.Y., Liu Y.X., Ji Z.Q., Liu R. Lsolation, purification, structure characterisation and hypoglycemic activity analysis of polysaccharides from Heraclenm dissectum. Food and Fermentation Industries. 2022;49(18):113–118. doi: 10.13995/j.cnki.11-1802/ts.032678. [DOI] [Google Scholar]
- Xie J.H., Shen M.Y., Xie M.Y., Nie S.P., Chen Y., Li C.…Wang Y.X. Ultrasonic-assisted extraction, antimicrobial and antioxidant activities of Cyclocarya paliurus (Batal.) Iljinskaja polysaccharides. Carbohydrate Polymers. 2012;89(1):177–184. doi: 10.1016/j.carbpol.2012.02.068. [DOI] [PubMed] [Google Scholar]
- Xu Z., Wang H.D., Wang B.L., Fu L., Yuan M., Liu J.…Ding C.B. Characterization and antioxidant activities of polysaccharides from the leaves of Lilium lancifolium Thunb. International Journal of Biological Macromolecules. 2016;92(1):148–155. doi: 10.1016/j.ijbiomac.2016.07.028. [DOI] [PubMed] [Google Scholar]
- Zeng H., Wang Y.D., Han H.Y., Cao Y.P., Wang B. Changes in key aroma compounds and esterase activity of Monascus-fermented cheese across a 30-day ripening period. Foods. 2022;11(24):4026. doi: 10.3390/foods11244026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Zhu W.B. Ultrasound-assisted ethanol/K2HPO4 aqueous two-phase extraction of polysaccharides from Plantago asiatica L. seeds: Process optimization, physicochemical properties, and antioxidant activity. Phytochemical Analysis. 2024;35(3):586–598. doi: 10.1002/pca.3315. [DOI] [PubMed] [Google Scholar]
- Zhang L. Research on effects and mechanism about sport functional beverage of Portulaca oleracea L. polysaccharide on anti-exercise-induced fatigue and raise exercise tolerance. Journal of Anhui Agricultural Sciences. 2011;39(07):3949–3954. doi: 10.3969/j.issn.0517-6611.2011.07.067. [DOI] [Google Scholar]
- Zhang M.R., Zhao J.L., Sun F.Y. Research progress on chemical constituents and pharmacological action of Vicatia thibetica de Boiss. Chinese Journal of Pharmacology and Toxicology. 2021;35(10):804. [Google Scholar]
- Zhao W.J., Zhang W.Y., Liu L., Cheng Y.L., Guo Y.H., Yao W.R., Qian H. Fractionation, characterization and anti-fatigue activity of polysaccharides from Brassica rapa L. Process Biochemistry. 2021;106(1):163–175. [Google Scholar]
- Zheng Q., Ren D.Y., Yang N.N., Yang X.B. Optimization for ultrasound-assisted extraction of polysaccharides with chemical composition and antioxidant activity from the Artemisia sphaerocephala Krasch seeds. International Journal of Biological Macromolecules. 2016;91:856–866. doi: 10.1016/j.ijbiomac.2016.06.042. [DOI] [PubMed] [Google Scholar]
- Zhou Q.W., Guo M.X., Yang J.J., Lu M., Jin X.J., Shen L., Liu X.B. Study on the mechanism of seselopsis on primary dysmenorrhea lnduced by oxytocin in mice. Journal of Dali University. 2016;2(04):24–27. http://journal15.magtechjournal.com/Jwk_dlxyzk/CN/Y2017/V2/I4/24. [Google Scholar]
- Zhou W. Wuhan University of Light Industry; Wuhan: 2021. Probiotic activity of polysaccharides from Longan, goji and jujube and development of nutritional meal powder, master dissertation. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material shows the optimization of material-water ratio of compound fermented beverage, and antioxidant activities of STSP-1 in vitro.
Data Availability Statement
The data that support the findings of this study are available from the corresponding authors upon reasonable request.