Abstract
Discordance in perception of disease activity between adolescent patients with lupus and their providers may influence disease outcomes. We found that patients endorsed higher perceptions of disease activity than providers. Discordance was present at all levels of disease activity, particularly in patients with high activity, nephritis, and/or taking corticosteroids or mycophenolate mofetil.
Differences in perception of disease activity can negatively impact medication adherence and clinical outcomes in adults with systemic lupus erythematosus (aSLE). Up to 20% of patients with SLE are diagnosed in childhood (pediatric patients with SLE [pSLE]) and can experience more significant morbidity than aSLE.1 Although broad immunosuppression remains the standard of treatment, these medications do not address or treat the noninflammatory symptoms associated with pSLE. The relationship between patients with SLE and their providers continues to be important in formulating and achieving treatment goals and positive clinical outcomes.2
Providers and patients prioritize different aspects of disease when characterizing disease activity, severity, and impact on a patient’s quality of life. aSLE have reported more frequent pain, fatigue, and negative physical and psychosocial functioning when assessing disease activity, whereas providers characterize disease activity and severity based on clinical signs of disease and accrual of end-organ damage.3 This disconnect in understanding treatment goals between providers and aSLEs can result in discordance in perceptions of disease and has been shown to affect treatment adherence.4 A similar relationship between discordance and medication adherence has not been studied in pSLE.
Patient-reported outcome (PRO) measures have been utilized to quantify patients’ perceptions of disease activity, damage, and impact on health-related quality of life (HRQoL) in aSLE and those with other rheumatic diseases.5-7 This finding contrasts with validated aSLE disease activity measures, which focus on disease severity and accrual of organ damage. The combination of patient-reported disease activity, HRQoL, and accumulated damage has been shown to be crucial when measuring treatment response and adherence in aSLE.4 Data are scarce investigating the associations between HRQoL, disease activity outcome measures, and their impact on pSLE treatment and outcomes. We investigate the level of discordance between providers and pSLEs of varying disease severity to better understand perceptions of disease activity, its association with disease severity, and medication use.
Methods
Patient Sample and Procedures
We conducted a prospective study within two tertiary pediatric rheumatology centers in New York. All patients between 12 and 21 years old who fulfilled either 1997 American College of Rheumatology or 2012 Systemic Lupus Erythematosus International Collaborating Clinics groups criteria were screened and, if consenting, were enrolled from September 2019 to August 2022.8,9 This study was approved by the Northwell Health (#20-1108) and Montefiore Medical Center IRBs (#2019-10021). Informed consent and assent were obtained per protocol from participants and caregivers.
Clinical data were collected at baseline and prospectively every 3 months for 24 months. Data from the baseline assessment are presented in this study, including demographics, duration of disease, medication regimens, and pSLE disease manifestations.
Patients and providers completed global assessment (GA) scores at baseline and prospectively every 3 months. GA were based on numerical ratings of perceived disease activity on a 10-cm visual analog scale. A score of 0 signifies perception of no disease activity and 10 perception of very high disease activity. Providers were blinded to the patients’ GA, and completed current medication regimens and Systemic Lupus Erythematosus Disease Activity Index scores at every study visit.10 We used the STROBE cohort checklist when writing our report.11
Statistical Analysis
Descriptive statistics were used to characterize demographics, disease manifestations, and medications, to examine general data distributions, and to identify any outliers in the study sample. SLEDAI scores were reported as medians and IQR. Absolute discordance was measured by subtracting the provider GA score from the patient GA score; those with differences in GA scores were deemed discordant. Continuous variables were defined for patient- and provider-reported levels of disease activity and discordance. Mann-Whitney U tests were calculated to compare and analyze discordance scores of prevalent clinical disease manifestations, and medication regimens, including those on hydroxychloroquine, oral and/or intravenous (IV) corticosteroids, rituximab, or mycophenolate mofetil (MMF). A P value of <.05 was considered statistically significant.
Patients were stratified based on disease activity into 3 groups: clinically inactive (SLEDAI ≤4, indicated by presence of serologies or hypocomplementemia), moderate activity (SLEDAI 5-8), and high activity (SLEDAI >8, with involvement of ≥1 organ systems). Patient and provider GA scores and discordance were reported as medians and IQR within each disease activity group and analyzed with a Kruskal-Wallis test. Any missing survey data were excluded from final analyses.
Results
Demographics and Clinical Features
We enrolled 98 pSLEs (79% female) with a mean age of 17 years (range, 12-21 years) and mean disease duration of 3.4 years (range, <1-12 years). Forty-three percent of patients self-reported as Hispanic, 31% Black, 22% Asian American, 7% White, and 4% Alaskan Native/American Indian; 13% were multiracial.
At enrollment, 93% of patients had serologies positive for antinuclear antibodies, 45% positive antidouble-stranded DNA antibodies, and 40% had hypocomplementemia. The most common clinical manifestations in the last 10 days were nephritis (39%), rash (20%), Raynaud’s symptomatology (17%), arthritis (15%), and neuropsychiatric lupus (8%). Eighty-five percent of patients were on hydroxychloroquine, 72% on MMF, 81% on oral or IV corticosteroids, 17% had prior use of cyclophosphamide and 8% were on rituximab.
Discordance
There was no statistical significance noted between the two centers on discordance, t(85) =.83; P = .40; hence, the data were combined for analyses. A t-test found marginal differences on discordance by gender, with higher levels for males, male, mean 3.54 ± 2.70; female, mean, 2.32 ± 2.07; t(83) = 1.86; P = .07. Spearman’s correlations between discordance and income and education yielded no significant results. Spearman’s correlation between discordance and mean disease duration yielded a significant negative correlation (r = −0.23; P = .03), indicating that the longer the duration of disease, the less discordance was reported. Mann-Whitney U tests on race and ethnicity categories yielded only significant differences for those that selected White race (median, 5; IQR, 1.75-7.25) vs did not select (median, 2 (IQR, 1-4; U = 121.5; P = .04), indicating greater discordance in the white participants. Perceptions of disease activity were measured and reported as patient and provider GA, scored from 0 to 10. The mean patient GA score was 3.20 ± 2.74 and provider GA score was 1.33 ± 1.60, yielding a mean patient-provider absolute discordance of 2.48 ± 2.18 (Figure). The median GA score was 2.50 (IQR, 1.00-5.25) and provider GA score was 1 (IQR, 0-2), yielding a median patient-provider absolute discordance of 2 (IQR, 1-4).
Figure.
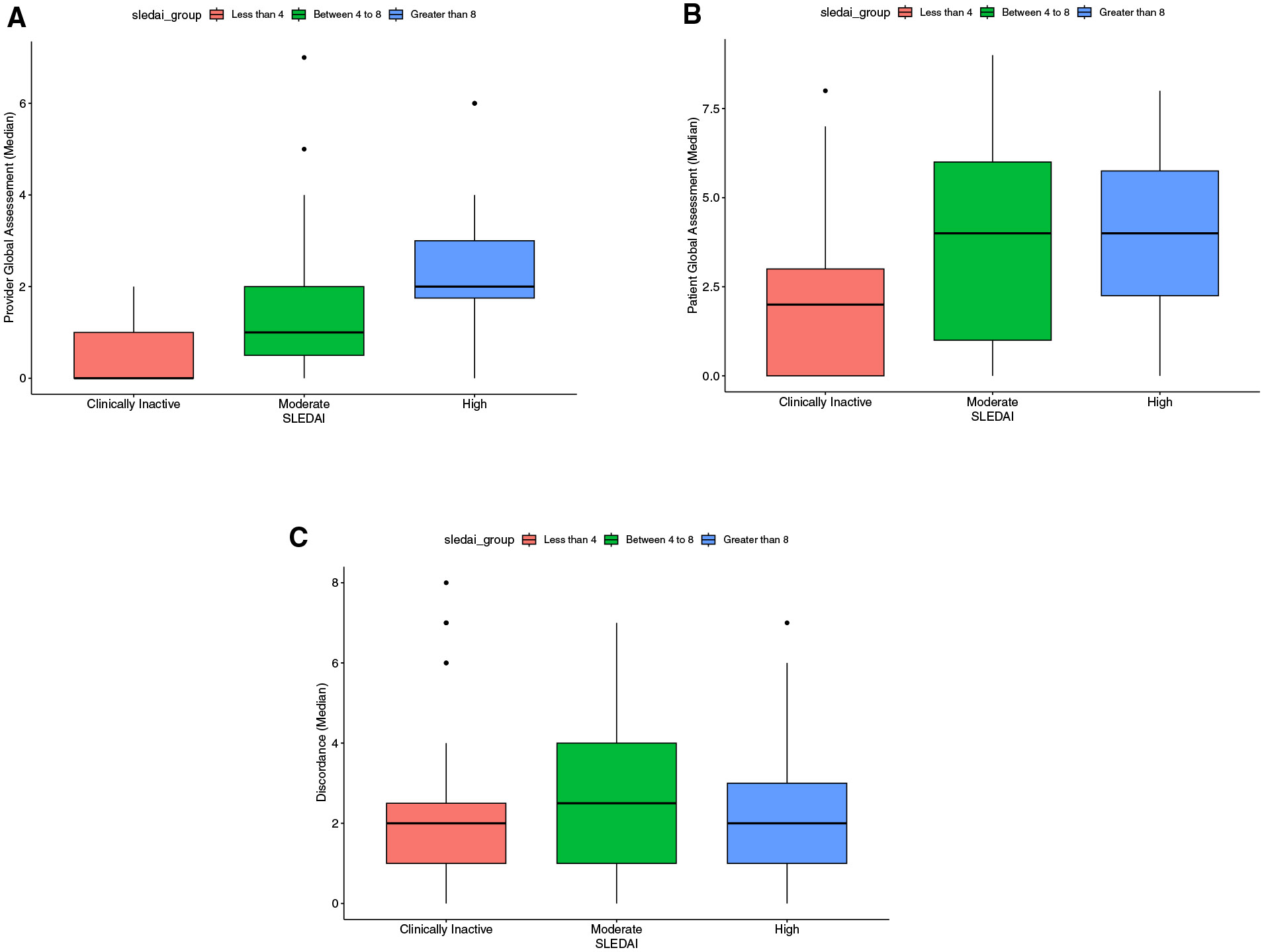
A, Provider GA stratified by SLEDAI scores. B, Patient GA stratified by SLEDAI scores. C, Absolute discordance in perception of disease activity between adolescents with lupus and their providers, stratified by SLEDAI scores.
Owing to the low prevalence in some categories, Mann-Whitney U testing on clinical manifestations (yes/no) and discordance were run for variables with at least 8 in a group (Table). Marginal differences in greater discordance were found with neuropsychiatric lupus. Significantly higher discordance was found with nephritis compared with those without (P = .03).
Table.
Associations between clinical manifestations, medication use, and absolute discordance in perception of disease activity between adolescents with lupus and their providers
| Characteristics | Yes | No | U-value |
|---|---|---|---|
| Clinical manifestation | |||
| Lupus nephritis | 2 (1.0-5.5)/53 | 2 (0-3)/34 | U = 656.00; P = .03* |
| Rash | 2 (1-5)/19 | 2 (1.00-3.75)/68 | U = 546.50; P = .30 |
| Raynaud’s | 2 (1.0-5.5)/17 | 2 (1-3)/68 | U = 497.00; P = .37 |
| Arthritis | 2 (1-4)/13 | 2 (1-4)/74 | U = 426.00; P = .51 |
| Neuropsychiatric | 3 (2.00-5.75)/8 | 2 (1-4)/79 | U = 197.00; P = .08 |
| Medications | |||
| Hydroxychloroquine | 2 (1-4)/77 | 1 (0-2)/7 | U = 167.50; P = .09 |
| Corticosteroids (oral or IV pulse) | 2 (1.0-4.5)/53 | 1.50 (0-2)/18 | U =.321.50; P = .04* |
| Rituximab | 3.50 (2-6)/8 | 2 (1-4)/78 | U = 190.00; P = .07 |
| MMF | 2 (1-4)/67 | 1 (0-2)/20 | U = 374.50; P = .002* |
| Past cyclophosphamide | 1 (0-2)/17 | 2 (1-4)/65 | U = 368.00, P = .03* |
Values are median discordance scores (IQR)/number.
Discordance was calculated as the absolute value of patient-provider ratings of disease activity.
P < .05.
Mann-Whitney U tests on specific current medication use and discordance revealed a marginal finding of greater discordance while on treatment with hydroxychloroquine or rituximab. Patients on oral or IV corticosteroids and/or MMF had significantly greater discordance than patients not taking these medications (P = .04 and P = .002, respectively). There were no patients currently being treated with IV cyclophosphamide at study enrollment; however, there was significantly greater discordance in patients who had received cyclophosphamide in the past (P = .03).
The mean SLEDAI score was 6 (range, 0-38). When pSLEs were stratified by disease activity, 55 (56.1%) had currently inactive disease activity, 19 (19.4%) moderate disease activity, and 19 (19.4%) high disease activity. A series of Kruskal-Wallis tests found no significant differences in patient GA scores on clinically inactive, moderate, or high disease activity, H(2) 4.63; P = .1, or discordance, H(2) =.005; P = .998. However, a significant difference in provider GA scores between the 3 SLEDAI groups was found, H(2) = 18.15, P < .001. The high disease activity group had the highest levels of difference in provider GA scores compared with moderate and clinically inactive groups. A series of follow-up Mann-Whitney U tests (with a Bonferroni correction to .02) yielded significant differences only between the high vs clinically inactive groups (U = 154; P < .001) and marginal differences between the high vs moderate groups (U = 92; P = .045).
Discussion
We found discordance in disease perception between pSLEs and their providers, with greater absolute discordance in patients with nephritis and patients, currently taking oral or IV corticosteroids, past cyclophosphamide, and/or current MMF. Patient-provider discordance may impact the patient-provider relationship negatively by impacting patient understanding of disease damage, long-term morbidity, and the importance of treatment adherence. Thus, understanding discordance and its determinants is critical to improving patient care and clinical outcomes in pSLEs.
Although there is no standard definition or measurement of patient-provider discordance, our results agree with our prior findings using the Childhood Arthritis and Rheumatology Research Alliance Registry, in which we found significant discordance between patient and provider GA and worse perceptions of disease in pSLE compared with their providers.12 Importantly, our results here expand on this finding by demonstrating that discordance does not depend on disease activity as measured by SLEDAI scores. Patients reported higher GA scores across all SLEDAI groups, possibly owing to contributions of subjective, noninflammatory factors of pSLE, such as pain and fatigue, to their perception of disease.7,13,14 This discrepancy implies that subjective measures of disease activity and HRQoL may hold greater influence on PROs, irrespective of objective measures of disease activity. Discordance may reflect providers’ perceptions of subjective patient concerns and their impact on HRQoL.
Our findings of discordance across all categories of lupus disease activity suggest a difference in evaluation of disease between patients and providers and a potential limitation in communication, as has been reported previously in aSLEs.7 Our study demonstrated the benefit of PROs (ie, GA score) in routine clinical practice to help address factors contributing to patient perception of disease activity. Although the specific factors and determinants for discordance need to be explored further, using PROs may allow for more open discussions between providers and patients when provider-based objective measurement tools are not enough to capture these subjective measures.
Discordance between patients and providers on perceptions of disease activity was higher in those with nephritis when compared with other clinical manifestations. Lupus nephritis occurs in ≤80% of pSLE diagnoses, compared with 20%-40% of aSLEs.2 Therefore, early diagnosis and treatment is imperative in pSLE to decrease renal failure, end-stage renal disease, and mortality. Golder et al demonstrated that patients and providers report renal disease as a high-level concern when assessing SLE disease severity, but the degree of concern is skewed toward providers rating renal disease as more significant for disease severity when compared with patients’ concerns.4 This may be due to evidence of active disease using objective measures rather than the effect of renal disease on HRQoL and daily functioning.
We assessed specific medication use with discordance in perception of disease severity between patients and providers, noting greater discordance in patients taking hydroxychloroquine or rituximab, and significantly greater discordance in patients on corticosteroids or MMF, and patients who had received cyclophosphamide in the past. Because B-cell-depleting agents (eg, rituximab) and immunosuppressives (eg, cyclophosphamide, MMF) are reserved typically for significant and/or refractory SLE manifestations, it can be inferred that providers assessed patients requiring these medications as having higher disease severity than those not requiring these medications. Corticosteroids are commonly used as initial or adjunctive immunosuppressive therapy when treating acute pSLE flares. However, patients with long-term corticosteroid use may experience adverse effects, which may become a barrier to medication adherence. Sloan et al demonstrated that adult patients with rheumatic diagnoses exhibited discordance regarding treatment adherence and need for escalation in therapy based on perception of disease activity, particularly with discrepancies in patients’ concerns for adverse effects and daily functioning compared with providers’ objective measures to assess disease severity and changes to medications.15 There may be potential in using HRQoL measures and PROs when assessing disease activity to improve the discordance gap in pSLE.
Limitations of our study include the relatively small sample size and recruitment from only 2 centers in the same general geographic region. However, because pSLEs from both centers were enrolled with different races and ethnicities, disease durations of <1-12 years, widely variable disease severities and manifestations, and on different medication regimens, our data may be generalizable to a larger cohort. Another limitation is that provider race, ethnicity, years in clinical practice, and additional demographic factors were not analyzed as a subset with GA and SLEDAI. This factor may have impacted some of our findings.
The results of our study suggest evidence of discordance among providers and pSLEs. We conclude that adolescent patients with SLE endorsed higher perceptions of disease activity than their providers. Discordance between patients and providers on perceptions of disease activity was present at various levels of disease severity, but particularly in patients with nephritis and patients taking MMF and/or corticosteroids. Our data suggest that further investigation into noninflammatory and difficult-to-treat symptomatology is warranted. Using and incorporating these findings with PRO measures may augment provider assessments of pSLE and lead to improved clinical outcomes.
Declaration of Competing Interest
K.K.R., K.R., and S.B. are the recipients of grant funding (#2019-10021) from the National Institute on Disability, Independent Living and Rehabilitation Research (NIDILRR).
T.B.R. is supported by the Childhood Arthritis and Rheumatology Research Alliance/Arthritis Foundation Career Development Award and the National Institute of Arthritis and Musculoskeletal Skin Diseases (NIAMS) (K23AR080803).
The authors declare no conflicts of interest.
The authors thank the patients for their participation. We are grateful to Andrea La Bella for her expertise in patient enrollment and database management. We thank Drs Beth Gottlieb, Katherine Steigerwald, and Lydia Thomas for their clinical care and contributing their patients.
Glossary
- aSLE
Adults with systemic lupus erythematosus
- GA
Global assessment
- HRQoL
Health-related quality of life
- IV
Intravenous
- MMF
Mycophenolate mofetil
- PRO
Patient-reported outcome
- pSLE
Pediatric patients with SLE systemic lupus erythematosus
- SLE
Systemic lupus erythematosus
- SLEDAI
Systemic Lupus Erythematosus Disease Activity Index
Footnotes
CRediT authorship contribution statement
Zanab Mian: Writing – review & editing, Writing – original draft, Visualization, Validation, Investigation. Terrence Calistro: Writing – review & editing, Software, Resources, Formal analysis. Kimberly Rapoza: Visualization, Validation, Software, Resources, Methodology, Funding acquisition, Formal analysis, Conceptualization. Shari Salzhauer Berkowitz: Writing – review & editing, Visualization, Methodology, Investigation, Funding acquisition, Conceptualization. Tamar B. Rubinstein: Writing – review & editing, Visualization, Methodology, Investigation, Formal analysis, Conceptualization. Heather M. Walters: Writing – review & editing, Visualization, Resources, Investigation. B. Anne Eberhard: Writing – review & editing, Visualization, Resources, Investigation. Kathleen Kenney-Riley: Writing – review & editing, Methodology, Funding acquisition, Conceptualization. Joyce S. Hui-Yuen: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Conceptualization.
Data Statement
Data sharing statement available at www.jpeds.com.
References
- 1.Brunner HI, Higgins GC, Wiers K, Lapidus SK, Olson JC, Onel K, et al. Prospective validation of the provisional criteria for the evaluation of response to therapy in childhood-onset systemic lupus erythematosus. Arthritis Care Res 2010;62:335–44. 10.1002/acr.20103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yen JC, Abrahamowicz M, Dobkin PL, Clarke AE, Battista RN, Fortin PR. Determinants of discordance between patients and physicians in their assessment of lupus disease activity. J Rheum 2003;30:1967–76. [PubMed] [Google Scholar]
- 3.Devilliers H, Amoura Z, Basencenot JF, Bonnotte B, Pasquali JL, Wahl D, et al. Responsiveness of the 36-item short form health survey and the Lupus quality of life questionnaire in SLE. Rheumatology 2015;54:940–9. 10.1093/rheumatology/keu410 [DOI] [PubMed] [Google Scholar]
- 4.Golder V, Ooi JJY, Antony AS, Ko T, Morton S, Kandane-Rathnayake R, et al. Discordance of patient and physician health status concerns in systemic lupus erythematosus. Lupus 2018;27:501–6. 10.1177/0961203317722412 [DOI] [PubMed] [Google Scholar]
- 5.Giancane G, Campone C, Gicchino MF, Alongi A, Bava C, Rosina S, et al. Determinants of discordance between criteria for inactive disease and low disease activity in juvenile idiopathic arthritis. Arthritis Care Res (Hoboken) 2021;73:1722–9. 10.1002/acr.24415 [DOI] [PubMed] [Google Scholar]
- 6.Tory H, Zurakowski D, Kim S for the CARRA Juvenile Dermatomyositis Quality Measures Workgroup for the CARRA Registry Investigators. Patient and Physician Discordance of Global disease Assessment in Juvenile Dermatomyositis: findings from the Childhood Arthritis & Rheumatology Research Alliance Legacy Registry. Pediatric Rheumatology 2020;18:1–8. 10.1186/s12969-020-0402-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rogers JL, Eudy AM, Pisetsky D, Criscione-Schreiber LG, Sun K, Doss J, et al. Using clinical characteristics and patient-reported outcome measures to categorize systemic lupus erythematosus subtypes. Arthritis Care Res 2021;73:386–93. 10.1002/acr.24135 [DOI] [PubMed] [Google Scholar]
- 8.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1997;40:1725. 10.1002/art.1780400928 [DOI] [PubMed] [Google Scholar]
- 9.Petri M, Orbai AM, Alarcon G, Gordon C, Merrill JT, Fortin PR, et al. Derivation and validation of the systemic lupus international collaborating clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum 2012;64:2677–86. 10.1002/art.34473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bombardier C, Gladman DD, Urowitz MB, Caron D, Chang CH. Derivation of the SLEDAI. A Disease Activity Index for Lupus Patients. The committee on prognosis studies in SLE. Arthritis Rheum 1992;35:630–40. 10.1002/art.1780350606 [DOI] [PubMed] [Google Scholar]
- 11.von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. J Clin Epidemiol 2008;61:344–9. 10.1016/j.jclinepi.2007.11.008 [DOI] [PubMed] [Google Scholar]
- 12.Kenney-Riley K, Berkowitz SS, Rapoza K. Understanding Patient-Provider Discordance in Adolescents with Lupus: The Role of Pain and Antidepressant Medication Use. Health Psychology Open 2020;7:1–7. 10.1177/20551029209777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yen JC, Neville C, Fortin PR. Discordance between patients and their physicians in the assessment of lupus disease activity: relevance for clinical trials. Lupus 1999;8(8):660–70. 10.1191/096120399680411362 [DOI] [PubMed] [Google Scholar]
- 14.Neville C, Clarke AE, Joseph L, Belisle P, Ferland D, Fortin PR. Learning from discordance in patient and physician global assessments of systemic lupus erythematosus disease activity. J Rheumatol 2000;27(3):675–9. [PubMed] [Google Scholar]
- 15.Sloan M, Lever E, Gordon C, Harwood R, Georgopoulou S, Naughton F, et al. Medication decision-making and adherence in lupus: patient–physician discordance and the impact of previous ‘adverse medical experiences’. Rheumatology 2022;61:1417–29. 10.1093/rheumatology/keab534 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing statement available at www.jpeds.com.


