Abstract
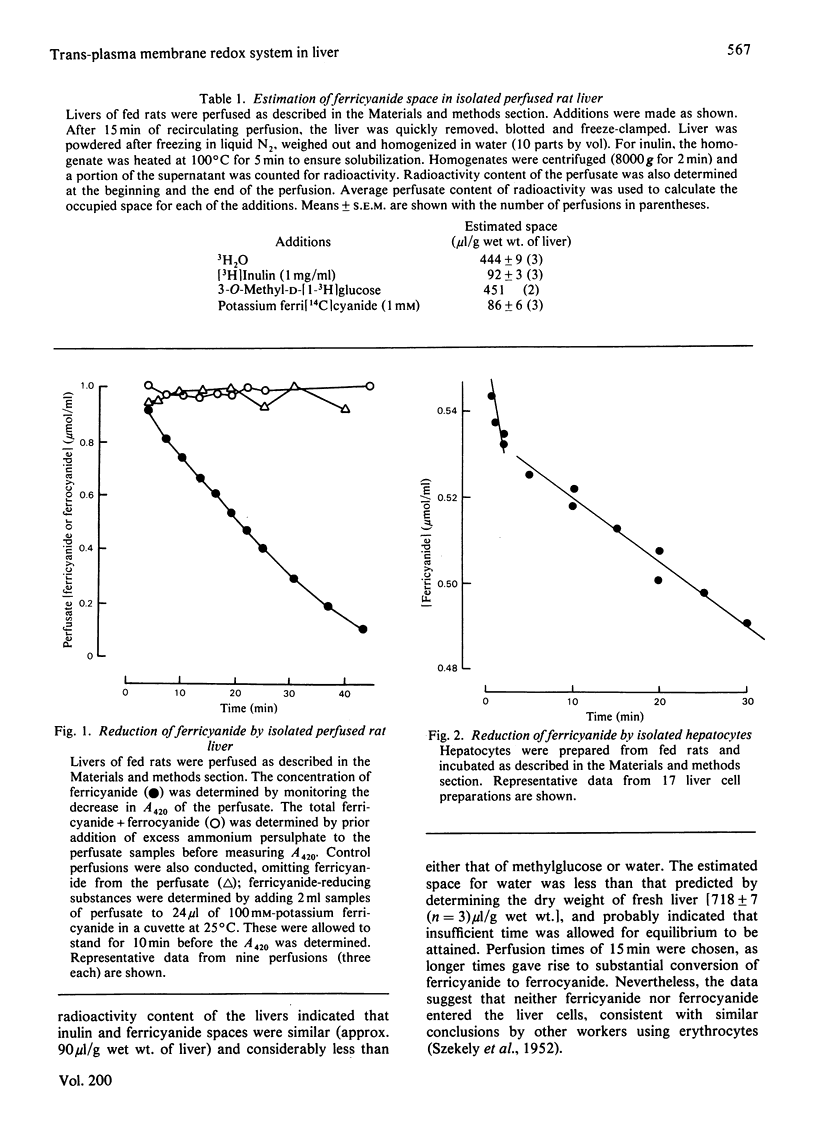
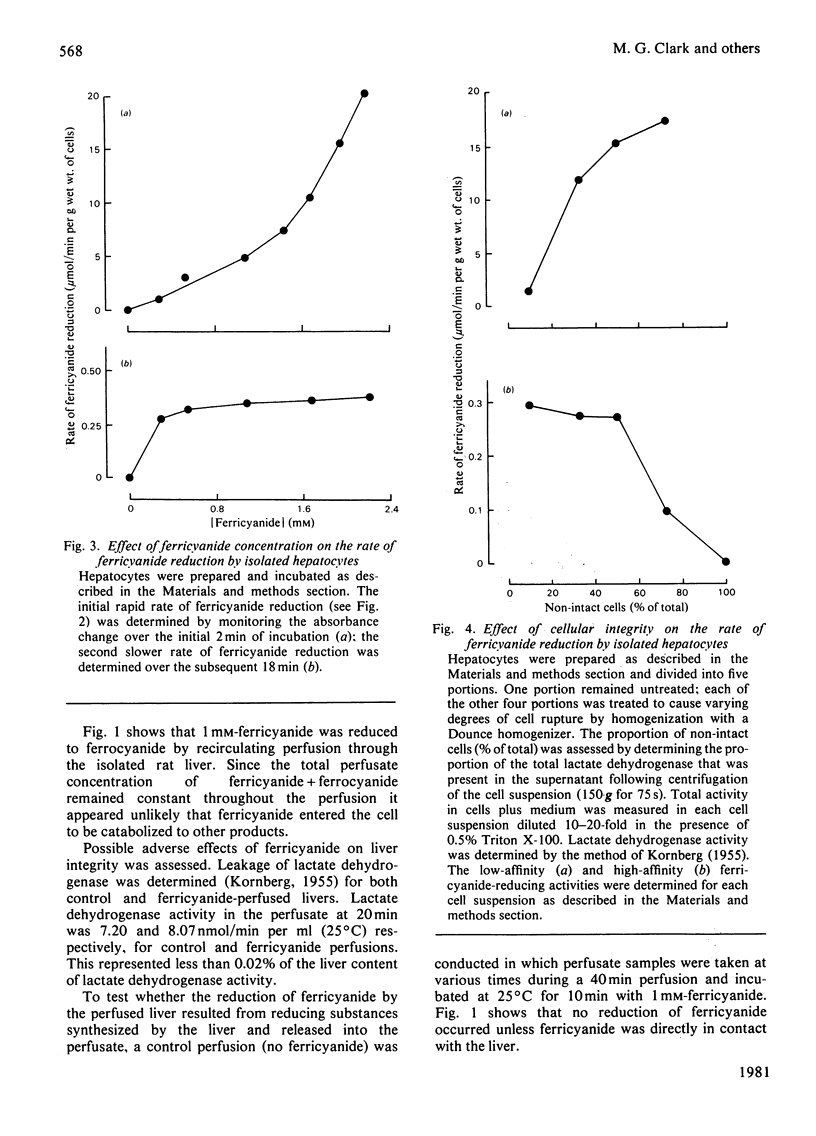
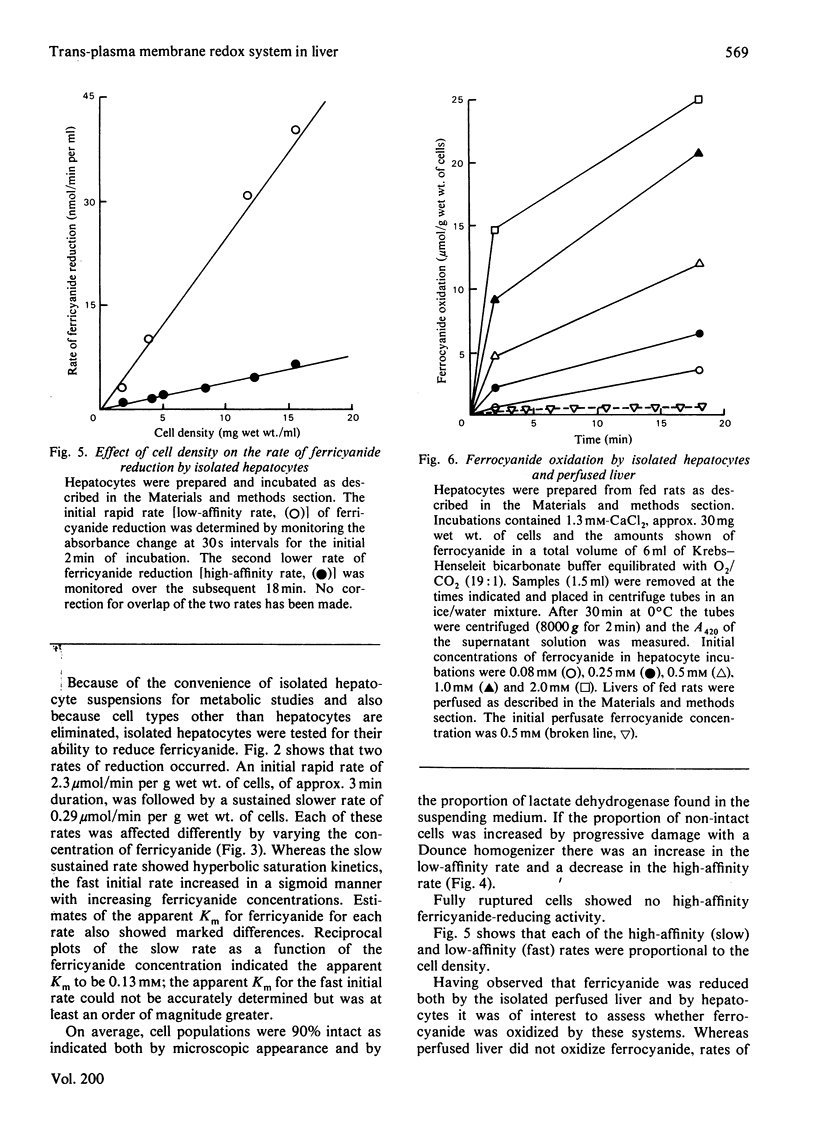
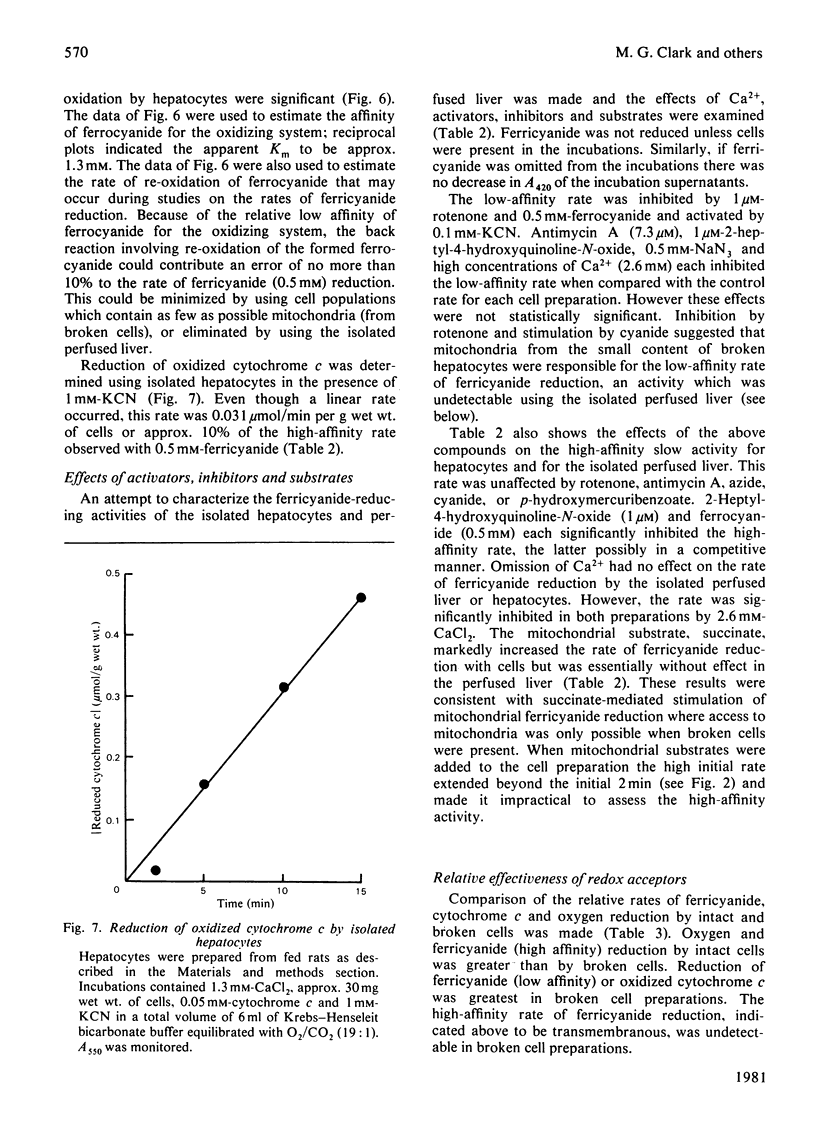
1. Reduction of ferricyanide by the isolated perfused rat liver and by isolated rat hepatocytes was studied. 2. Ferricyanide was reduced to ferrocyanide by the perfused liver at a linear rate of 0.22μmol/min per g of liver. Ferricyanide was not taken up by the liver and the perfusate concentration of ferricyanide+ferrocyanide remained constant throughout the perfusion. Perfusate samples from livers perfused without ferricyanide did not reduce ferricyanide. 3. Isolated hepatocytes reduced ferricyanide in a biphasic manner. The initial rate of 2.3μmol/min per g of cells proceeded for approx. 3min and derived from low-affinity sites (apparent Km>1.3mm). The secondary rate of 0.29μmol/min per g of cells was maintained for the remainder of the incubation and derived from higher affinity sites (apparent Km0.13mm). Disruption of the cells resulted in an increase in the low-affinity rate and a decrease in the high-affinity rate. 4. Ferrocyanide was oxidized by isolated hepatocytes but not by perfused liver. The apparent Km for ferrocyanide oxidation by hepatocytes was 1.3mm. 5. Oxidized cytochrome c was reduced by isolated hepatocytes in the presence of 1mm-KCN but at a rate less than that of the reduction of ferricyanide. 6. Properties of the ferricyanide-reducing activities of intact hepatocytes and the perfused liver were examined. The low-affinity rate, present only in cell and broken cell preparations, was inhibited by 1μm-rotenone and 0.5mm-ferrocyanide, and stimulated by 0.1mm-KCN. The mitochondrial substrate, succinate, also stimulated this rate. The perfused liver showed only a high-affinity activity for ferricyanide reduction. This activity was also present in liver cells and was unaffected by rotenone, antimycin A, KCN, NaN3, or p-hydroxymercuribenzoate but was inhibited by 2.6mm-CaCl2, 2-heptyl-4-hydroxyquinoline-N-oxide and ferrocyanide. Overall, these results are consistent with the occurrence of a trans-plasma membrane redox system of liver that reduces extracellular ferricyanide to ferrocyanide. The reduction process shows properties which are similar to that of the NADH:ferricyanide oxidoreductase found in isolated liver plasma membranes but different from that of mitochondria.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AVRON M., SHAVIT N. A SENSITIVE AND SIMPLE METHOD FOR DETERMINATION OF FERROCYANIDE. Anal Biochem. 1963 Dec;6:549–554. doi: 10.1016/0003-2697(63)90149-0. [DOI] [PubMed] [Google Scholar]
- Berry M. N., Friend D. S. High-yield preparation of isolated rat liver parenchymal cells: a biochemical and fine structural study. J Cell Biol. 1969 Dec;43(3):506–520. doi: 10.1083/jcb.43.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane F. L., Goldenberg H., Morré D. J., Löw H. Dehydrogenases of the plasma membrane. Subcell Biochem. 1979;6:345–399. doi: 10.1007/978-1-4615-7945-8_6. [DOI] [PubMed] [Google Scholar]
- DePierre J. W., Ernster L. Enzyme topology of intracellular membranes. Annu Rev Biochem. 1977;46:201–262. doi: 10.1146/annurev.bi.46.070177.001221. [DOI] [PubMed] [Google Scholar]
- Dormandy T. L., Zarday Z. The mechanism of insulin action: the immediate electrochemical effects of insulin on red-cell systems. J Physiol. 1965 Oct;180(4):684–707. doi: 10.1113/jphysiol.1965.sp007724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarasch E. D., Kartenbeck J., Bruder G., Fink A., Morré D. J., Franke W. W. B-type cytochromes in plasma membranes isolated from rat liver, in comparison with those of endomembranes. J Cell Biol. 1979 Jan;80(1):37–52. doi: 10.1083/jcb.80.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kant J. A., Steck T. L. Specificity in the association of glyceraldehyde 3-phosphate dehydrogenase with isolated human erythrocyte membranes. J Biol Chem. 1973 Dec 25;248(24):8457–8464. [PubMed] [Google Scholar]
- Kilberg M. S., Christensen H. N. Electron-transferring enzymes in the plasma membrane of the Ehrlich ascites tumor cell. Biochemistry. 1979 Apr 17;18(8):1525–1530. doi: 10.1021/bi00575a021. [DOI] [PubMed] [Google Scholar]
- Löw H., Crane F. L. Redox function in plasma membranes. Biochim Biophys Acta. 1978 Jul 31;515(2):141–161. doi: 10.1016/0304-4157(78)90002-3. [DOI] [PubMed] [Google Scholar]
- MANYAI S., SZEKELY M. Die Wirkung von Natriumfluorid und Monojodessigsäure auf die Glykolyse von menschlichen roten Blutkörperchen. Acta Physiol Acad Sci Hung. 1954;5(1-2):7–18. [PubMed] [Google Scholar]
- Orringer E. P., Roer M. E. An ascorbate-mediated transmembrane-reducing system of the human erythrocyte. J Clin Invest. 1979 Jan;63(1):53–58. doi: 10.1172/JCI109277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramasarma T., Mackellar W., Crane F. L. Nature of NADH: acceptor oxidoreductase in plasma membranes of mouse liver. Indian J Biochem Biophys. 1980 Jun;17(3):163–167. [PubMed] [Google Scholar]
- STRITTMATTER P., VELICK S. F. A microsomal cytochrome reductase specific for diphosphopyridine nucleotide. J Biol Chem. 1956 Jul;221(1):277–286. [PubMed] [Google Scholar]
- SZEKELY M., MANYAI S., STRAUB F. B. Uber den mechanismus der osmotischen hämolyse. Acta Physiol Acad Sci Hung. 1952;3(3-4):571–584. [PubMed] [Google Scholar]
- Wang C. S., Alaupovic P. Isolation and partial characterization of human erythrocyte membrane NADH: (acceptor) oxidoreductase. J Supramol Struct. 1978;9(1):1–14. doi: 10.1002/jss.400090102. [DOI] [PubMed] [Google Scholar]
- Zamudio I., Cellino M., Canessa-Fischer M. The relation between membrane structure and NADH: (acceptor) oxidoreductase activity of erythrocyte ghosts. Arch Biochem Biophys. 1969 Jan;129(1):336–345. doi: 10.1016/0003-9861(69)90184-2. [DOI] [PubMed] [Google Scholar]


