Abstract
Objective
A gold standard surgical treatment for osteochondral lesions (OCLs) of the talus still needs to be established. Still, autologous matrix-induced chondrogenesis (AMIC) is a commonly applied 1-stage procedure that has achieved good short- and mid-term results. The present cohort study aimed to assess whether the long-term, 10-year results can confirm the previous findings.
Design
All patients underwent an open AMIC procedure using a collagen type I/III bilayer matrix for a talar OCL. General demographic data, preoperative magnetic resonance imaging findings, intraoperative details, and German version of the Foot Function Index (FFI-D) scores preoperatively and at 1, 5, and 10 years as well as European Foot and Ankle Society (EFAS) and American Orthopedic Foot & Ankle Society (AOFAS) scores at 10 years after surgery were analyzed. The primary outcome variable was the procedure’s longitudinal effect, and several variables’ influence on the outcome was tested.
Results
Of 47 consecutive patients, 18 (38%) were included. Of the 18 patients, 6 (33%) were female, and 12 (67%) were male, with a mean age of 39 ± 15 (range = 15-62) and an average body mass index (BMI) of 26 ± 5 (range = 20-38) kg/m². The mean defect size was 1.4 ± 0.9 (range = 0.2-4) cm². The FFI-D total score showed a significant decrease from preoperatively to 1 year postoperatively (56 ± 19 to 34 ± 27; P = 0.001) with a further nonsignificant decrease to the 5-year (34 ± 27 to 21 ± 20; P = 0.16) and 10-year follow-up (21 ± 20 to 15 ± 13; P = 1.00). All the single items decreased significantly from preoperatively to the 5- and 10-year mark. Although not significant, most items improved from 5 to 10 years postoperatively. Age positively correlated with the preoperative, 5-year, and 10-year follow-up FFI-D total score.
Conclusions
AMIC, as a single-step surgical intervention, is a viable long-term treatment option. Patient selection regarding symptoms and findings is vital to achieve satisfying results.
Keywords: AMIC, osteochondral defects, osteochondral lesion, talus, sports
Introduction
The talus’ osteochondral lesions (OCLs) are a heterogeneous group of pathologies commonly defined by a defect of the articular cartilage and the subchondral bone. 1 OCLs occur in about two-thirds of chronic ankle instability and acute ankle fractures, but also without a clear traumatic context.2-4 The interest in a reliable and sustainable treatment option is high, considering hyaline cartilage’s poor intrinsic repair mechanism based on its hypocellular, avascular, and alymphatic characteristics.5,6 This often causes a lasting defect or insufficient fibrotic scar tissue within the healing process, leading to ankle joint osteoarthritis.7,8 Regarding the symptoms, patients can be asymptomatic or profoundly limited in their daily and sporting activities. 9 Therefore, treatment remains challenging. Under conservative treatment, osteoarthritis progression takes place in about one-third of patients, and an even more significant number fail to restore their level of sports activity. 8 The central aspect of conservative management is adjusting activities with stress on the ankle joint. 10 Since most patients are young and carry an active lifestyle, surgical treatment is usually recommended.11,12 Surgical treatment options for OCLs can be divided into 3 groups: (1) reattachment of the osteochondral fragment; (2) bone marrow stimulation (BMS) by microfracture, nano-fracture, or microdrilling with or without a scaffold to achieve fibrocartilage proliferation; and (3) autologous or allogenic graft to restore the defect with hyaline cartilage. 13 Fixation of an osteochondral flake is only possible in an acute traumatic context with appropriate morphologic attributes regarding size and bone stock. A gold standard treatment for OCLs outside this category has yet to be realized. 7 Since its establishment in the 1980s, BMS has long been used to treat OCLs. 14 Its technique is relatively simple and affordable. Nevertheless, their use is limited to lesions of less than 1 cm².15,16 Autologous matrix-induced chondrogenesis (AMIC) combines bone marrow–derived mesenchymal progenitor cells and a collagen type I/III bilayer matrix. The surrounding avital tissue is debrided within this single-stage procedure to form stable edges. The subchondral sclerotic bone is penetrated using the BMS technique. The matrix is fitted to the defect and attached by fibrin glue. The released progenitor cells migrate toward the membrane, avoiding leakage into the joint space.17,18 The 5-year evaluation of patients treated with AMIC for OCLs of the talus showed promising results. Long-term results are still minimal. Therefore, this study aimed to assess the stability of these previous results in the long term.
Materials and Methods
Study Design
We performed a cohort study using 10-year follow-up data. All patients were treated at a single specialized orthopedic foot and ankle clinic and thoroughly informed about their treatment. They provided written consent to the treatment and the use of their clinical and imaging data for research purposes. The institutional review board approved the present study. The follow-up data focused on patient-reported outcome measures (PROMS) as several studies have shown a poor correlation of clinical outcome and imaging after cartilage reconstruction.19,20
Patients
Of 21 consecutive patients undergoing an open AMIC procedure for a talar OCL without malleolar osteotomy from June 2010 to December 2011 and taking part in the 5-year follow-up, 18 were included in this study. The dropouts were caused by missing follow-ups, changes of address, or lack of interest.
Surgical Technique and Postoperative Management
The 5-year follow-up study accurately depicted the operative procedure. 17 In summary, all patients were treated by an open ventral approach to the ankle joint. All unstable cartilage and necrotic bone were debrided. The subchondral sclerosis was perforated using a 1.2-mm Kirschner wire (=microdrilling) or microfracture. The bone defect was reconstructed to the level of the subchondral bone lamella using autologous cancellous bone. After assessing the correct size, the collagen matrix was attached using commercially available fibrin glue. The postoperative regimen consisted of partial weight-bearing with 10 kg for 6 weeks and a gradual increase in loading for another 6 weeks.
Data Collection
Following our standardized quality management policy, the patients were asked to complete a standardized foot and ankle questionnaire (Foot Function Index, German version [FFI-D]) 21 preoperatively, 1 and 5 years postoperatively. At the 10-year follow-up examination between June 2020 and November 2022, the patients were asked to complete the FFI-D again and additionally the EFAS (European Foot and Ankle Society) and AOFAS (American Orthopedic Foot & Ankle Society) score.22,23
Statistical Analysis
Statistical analysis was performed using JAPS 0.18.1 (University of Amsterdam). The data are presented as the mean and standard deviation. General descriptive statistical analyses were performed. Data were compared using the t-test and repeated measurement analysis of variance (post hoc Bonferroni test). Correlations were determined by calculating the Spearman coefficient for normally distributed values. The P values ≤0.05 were considered to indicate statistical significance.
Results
Demographic Data
Eighteen patients were included in this analysis. Of these patients, 6 (33%) were female, and 12 (67%) were male. The mean age at the time of surgery was 39 ± 15 (range = 15-62), and the average body mass index (BMI) was 26 ± 5 (range = 20-38) kg/m². The mean duration of symptoms was 52 ± 43 months (range = 5-132). Three of the 18 (17%) patients were smokers, and 10 (56%) had undergone an ipsilateral surgical treatment previously. Finally, 8 (44%) had not experienced any trauma before the beginning of the symptoms, 2 (11%) had experienced trauma within the previous year, and 8 (44%) had experienced trauma >12 months before surgery.
Location and Size of the Defect
All 18 patients (100%) underwent preoperative magnetic resonance imaging (MRI). Sagittal and coronal views were used to determine the defect size and location. The mean defect size was 1.4 ± 0.9 (range = 0.2-4) cm². The defect was located medial to the talus in 13 (67%), central in 2 (11%), and lateral in 3 (17%) patients.
Intraoperative Details
Surgery was performed from June 8, 2010, to December 6, 2011. For 17 patients (94%), additional procedures, such as cancellous bone graft, reconstruction of lateral ligaments, neurolysis of the peroneus superficial nerve, arthroscopy before arthrotomy, reconstruction of the peroneal tendon, hardware removal, lengthening of the Achilles tendon, and reconstruction of the syndesmosis, were required.
Subjective Outcome (FFI-D)
The FFI-D total score showed a significant decrease from preoperatively to 1 year postoperatively (56 ± 19 to 34 ± 27; P = 0.001) with a further nonsignificant decrease to the 5-year (34 ± 27 to 21 ± 20; P = 0.16) and 10-year follow-up (21 ± 20 to 15 ± 13; P = 1.00) ( Fig. 1 , Table 1).
Figure 1.
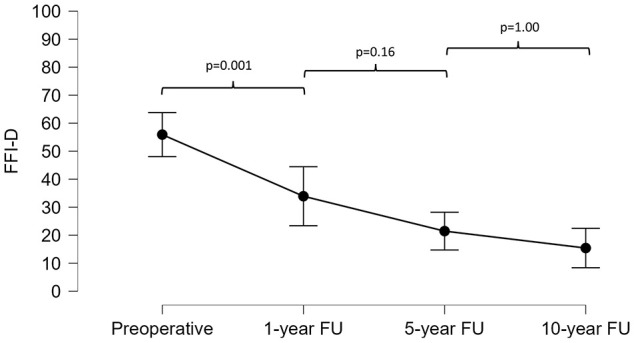
Progression of the German version of the Foot Function Index (FFI-D) total score at the preoperative and 1-, 5-, and 10-year follow-up assessments.
Table 1.
Comparison of the German Version of the Foot Function Index (FFI-D) Total Score at the Preoperative and 1-, 5- and 10-Year Follow-up Assessments.
| FFI-D | Mean Difference | P value | |
|---|---|---|---|
| Preoperative | 1-year FU | 22.00 | <0.01 |
| 5-year FU | 34.46 | <0.001 | |
| 10-year FU | 40.50 | <0.001 | |
| 1-year FU | 5-year FU | 12.46 | 0.16 |
| 10-year FU | 18.50 | <0.01 | |
| 5-year FU | 10-year FU | 6.05 | 1.00 |
P value adjusted for comparing a family of 6.
FFI-D = German version of the Foot Function Index; FU = follow-up.
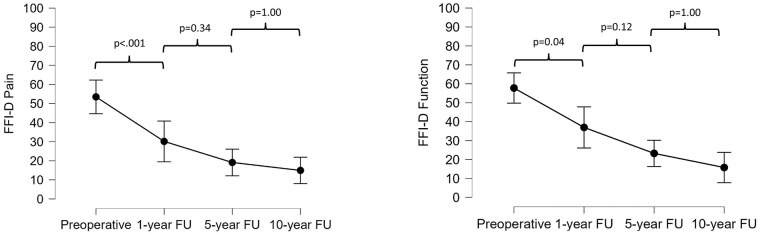
Comparable results were obtained for the FFI-D subscales of function and pain. These decreased significantly from preoperatively to 1 year postoperatively (58 ± 18 to 37 ± 29; P = 0.04 and 53 ± 22 to 30 ± 25; P < 0.001), and further, but non-significantly to the 5-year (37 ± 29 to 23 ± 20; P = 0.12 and 30 ± 25 to 20 ± 20; P = 0.34) and 10-year follow-up (23 ± 20 to 15 ± 15; P = 1.00 and 20 ± 20 to 15 ± 12; P = 1.00) ( Fig. 2 ).
Figure 2.
Progression of the German version of the Foot Function Index (FFI-D) pain and function scores at the preoperative and 1-, 5-, and 10-year follow-up assessments.
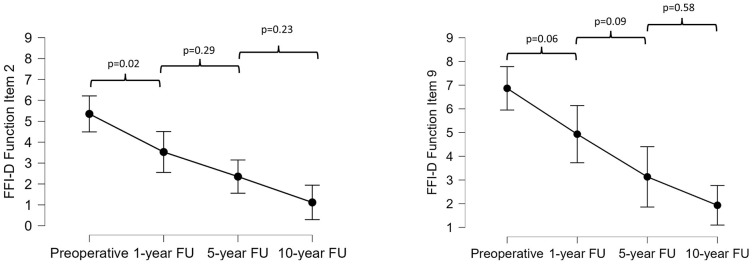
Furthermore, each item of the FFI-D at each follow-up examination (preoperatively and 1, 5, and 10 years postoperatively) was analyzed separately. Overall, significant differences (analysis of variance) could be found for all the items in the pain subscale. Items 1 (worst foot pain), 2 (morning foot pain), 6 (pain standing with shoes), 7 (foot pain at end of day), and 8 (pain at night) improved significantly from preoperatively to 5 and 10 years postoperatively, whereas items 3 to 5 (pain walking barefoot, pain standing barefoot, pain walking with shoes) improved considerably from 1 year to 10 years postoperatively (Suppl. Table S1). Similarly, significant differences were found for all the items in the function subscale. All items improved significantly from preoperatively to 5 and 10 years postoperatively. Except for items 5 (difficulty descending stairs) and 7 (difficulty getting up from chair), all items showed a significant decrease from 1 year to 10 years postoperatively (Suppl. Table S1). Items 2 (difficulty walking on uneven surfaces) and 9 (difficulty with sports/leisure activities) had the most linear improvement with P values of <0.001 from preoperatively as well as 1 year postoperatively to the 10-year follow-up ( Fig. 3 ).
Figure 3.
Progression of the German version of the Foot Function Index (FFI-D) function items 2 and 9 at the preoperative and 1-, 5-, and 10-year follow-up assessments.
Factors Affecting FFI-D Scores
Gender and previous surgical interventions did not significantly influence the FFI-D or subgroup scores. Age showed a moderate but significant positive correlation with the preoperative, 5-year, and 10-year follow-up FFI-D total score and subscale for function and the preoperative subscale for pain outcomes analyzed. The BMI and size of the lesion showed a similar positive correlation for the preoperative FFI-D and subscale for pain (Table 2). The smoking status correlated positively only at the 5-year mark for all 3 measurements. Etiology (trauma) and defect location showed a barely significant correlation for the subscale pain at the 5-year follow-up. At the same time, the duration of symptoms showed a mild, significant positive correlation between the FFI-D overall score and the subscale for function at the 10-year follow-up.
Table 2.
Correlation of Age, Body Mass Index, Lesion Size, and Symptom Duration with the German Version of the Foot Function Index (FFI-D) Scores (N = 18 Patients).
| Variable | FFI Score Preoperatively |
FFI Score at 1-Year FU |
FFI Score at 5-Year FU |
FFI Score at 10-Year FU |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pain | Function | Total | Pain | Function | Total | Pain | Function | Total | Pain | Function | Total | |
| Age | ||||||||||||
| Correlation coefficient | 0.63 | 0.54 | 0.59 | 0.40 | 0.30 | 0.37 | 0.42 | 0.53 | 0.50 | 0.34 | 0.61 | 0.54 |
| P value | <0.01 | 0.02 | 0.01 | 0.11 | 0.24 | 0.15 | 0.08 | 0.02 | 0.04 | 0.17 | <0.01 | 0.02 |
| BMI | ||||||||||||
| Correlation coefficient | 0.53 | 0.41 | 0.50 | 0.09 | 0.02 | 0.06 | 0.19 | 0.17 | 0.16 | 0.11 | 0.25 | 0.17 |
| P value | 0.02 | 0.09 | 0.04 | 0.73 | 0.94 | 0.83 | 0.45 | 0.49 | 0.52 | 0.68 | 0.31 | 0.49 |
| Lesion size | ||||||||||||
| Correlation coefficient | 0.56 | 0.48 | 0.56 | 0.28 | 0.27 | 0.28 | 0.36 | 0.44 | 0.37 | 0.45 | 0.45 | 0.47 |
| P value | 0.03 | 0.06 | 0.03 | 0.32 | 0.34 | 0.32 | 0.17 | 0.09 | 0.16 | 0.08 | 0.08 | 0.07 |
BMI = body mass index; FFI-D = German version of Foot Function Index; FU = follow-up.
Bold indicates P values < 0.05.
EFAS Scores
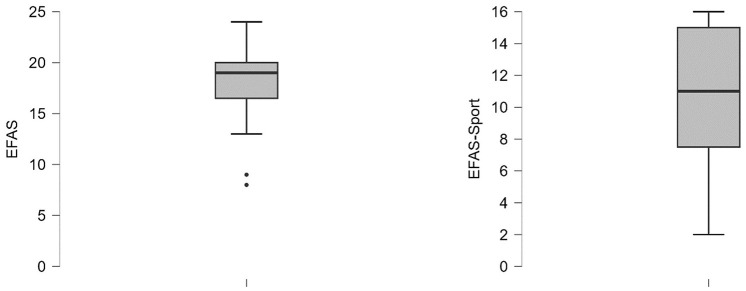
EFAS scores were evaluated at the 10-year follow-up. Total scores were 18 ± 4 and 11 ± 4 for EFAS Sport, respectively ( Fig. 4 ). Age showed a significant, negative correlation in the 2 score outcomes. The location correlated significantly with EFAS overall; both the etiology (trauma) and size of the defect negatively with EFAS overall, and the duration of symptoms negatively with EFAS sports scores.
Figure 4.
EFAS outcome at 10-year follow-up assessments.
Discussion
The 5-year follow-up from our clinic showed promising results for using the AMIC technique. 17 Due to later dropouts, that study included 3 more patients. The FFI-D total score decreased significantly from pre- to 1 year postoperative, with a further, nonsignificant decrease between the 1- and 5-year follow-ups. Similar results were observed for the FFI-D subscales function and pain. 17 Several studies matched these results with a follow-up period from 12 to 60 months. 24 In a meta-analysis, Walther et al. included a total of 323 patients. The studies used assessment tools such as AOFAS, the visual analogue scale for pain (VAS), the Foot Function Index (FFI), and the Tegner score. A statistically significant improvement in functional outcomes was seen throughout the studies. Only 1% of patients required revision surgery. Thus, the authors support using the AMIC procedure to treat OCLs based on these mid-term results. 24 The same applies to arthroscopically performed AMIC procedure.25,26
A more long-term evaluation of outcome variables is desirable since most patients treated for OCLs are young.27-29 This study showed that the function improved even more between 5 and 10 years postop, with a further decrease in the FFI-D overall score and the subscales, function, and pain. This indicates a satisfactory sustainability of the AMIC treatment of OCLs of the talus. However, we do not know of any other study that has such an extended follow-up to verify our results. Two studies with a mean 5-year follow-up included single cases with longer follow-up. Weigelt et al. 30 showed the example of an 18-year-old patient with an AOFAS = 100 and a VAS = 0.8 years after treating a large OCL of the medial talar dome. Wiewiorski et al. 28 also found a significant improvement in AOFAS from 43 to 76 and VAS from 6.9 to 2.3 with a follow-up to a maximum of 87 months (mean 46.9 ± 17.8) but did not distinguish between this range. Our mean AOFAS was 77 and therefore similar at 10 years but in accordance with other findings (Suppl. Fig. S1).
The results for items 2 (walking on uneven surfaces) and 9 (sports/leisure activities) of the FFI-D function subscale showed linear improvement up to the 10-year follow-up mark. Wiewiorski et al., 28 who focused on sports activities, did not show significant improvement between pre- and postoperatively. In contrast, this study did show significant improvement from preoperatively to the mid-term and long-term follow-up as well as from 1 year postoperatively to long term. Using different PROMS (Tegner score vs FFI-D), a direct comparison of the 2 studies is impossible. The same applies to Weigelt et al. (Tegner score), who found that 79% of patients acquired a total return to previous sports activities after 5 years and significantly improved score outcomes. 30 As well as in our earlier study, most demographic characteristics had no to mild effects on the score results. Naturally, age, BMI, size of the lesion, and duration of symptoms showed some correlation with the outcome but must be interpreted very carefully due to the small number of patients.
Considering other locations, good long-term clinical results were stated in 7- and 9-year follow-ups after the AMIC procedure of the knee joint.31-33 The same applies to an 8-year follow-up after treatment of acetabular chondral lesions of the hip with AMIC. 34 That study compared AMIC with microfracture alone and found a 0-conversion rate into hip arthroplasty versus 22% in the latter group. All the other long-term studies share that they do not compare AMIC to any other technique. The AMIC procedure performed significantly better for more extensive lesions at the mid-term follow-up of a randomized controlled trial (RCT). 35 Autologous chondrocyte implantation (ACI) has also been used as a treatment option. The apparent downsides are the donor-side morbidity, the 2-step procedure, and the necessity of laboratory processing. 36 Regardless of these factors, in a systematic review, the 2 techniques reach similar mid-term results in functional outcomes and complications. 37 The same authors recently published a meta-analysis on the outcome of allograft versus autograft for the treatment of OCLs of the talus. They showed significantly better results in AOFAS and MOCART (Magnetic Resonance Observation of Cartilage Repair Tissue) scores in the autograft group. 38 However, the same perioperative disadvantages apply to ACI. Thus, AMIC seems to be a promising operative treatment option.
The further improvement of the FFI-D scores within this study can give suggestions regarding patient selection and management. Patients with specific complaints preoperatively and high demands postoperatively are more likely to benefit from surgical treatment and positively impact PROMS. A progression in osteoarthritis is not regularly seen in OCL. 8 Thus, the risks might outweigh the benefits of a preventive surgical approach. Acknowledging the findings that a structured nonoperative treatment with activity reduction, bracing, physical therapy, and temporarily restricted weight-bearing can lead to satisfying results 8 in mild to moderate lesions and symptoms, a conservative attempt may be considered. Patients who fail conservative treatment or have severe symptoms and findings on MRI will most likely improve after the AMIC procedure, even in the long term, and can be guided accordingly pre- and postoperatively.
One of the limitations of our study is the small sample size, which decreased further compared with the 5-year follow-up due to dropouts. In addition, a control group is lacking in comparing different operative techniques. The limitations regarding FFI-D were outlined previously. 17 AOFAS and EFAS were only acquired at the 10-year follow-up. A longitudinal change cannot be evaluated. A comparison between different study results is limited because various scores were used.
Conclusions
Our study shows that PROMS results in function and pain are stable even between 5- and 10-year follow-ups. AMIC, as a single-step surgical intervention, is a viable long-term treatment option. Patient selection regarding symptoms and findings is vital to achieve satisfying results.
Supplemental Material
Supplemental material, sj-docx-1-car-10.1177_19476035241301896 for Long-Term Results after Autologous Matrix-Induced Chondrogenesis for Osteochondral Lesions of the Talus: A 10-Year Cohort Study by Lukas Deiss, Markus Walther, Kathrin Pfahl, Hubert Hörterer, Alexander Mehlhorn, Anke Röser and Oliver Gottschalk in CARTILAGE
Supplemental material, sj-jpg-2-car-10.1177_19476035241301896 for Long-Term Results after Autologous Matrix-Induced Chondrogenesis for Osteochondral Lesions of the Talus: A 10-Year Cohort Study by Lukas Deiss, Markus Walther, Kathrin Pfahl, Hubert Hörterer, Alexander Mehlhorn, Anke Röser and Oliver Gottschalk in CARTILAGE
Footnotes
Acknowledgments and Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
M. Walther and O. Gottschalk received reimbursements for travel expenses and speaker’s fees from Geistlich Pharma AG (Wolhusen, Switzerland). The other author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
ORCID iDs: Lukas Deiss  https://orcid.org/0009-0005-9722-0484
https://orcid.org/0009-0005-9722-0484
Markus Walther  https://orcid.org/0000-0003-1122-1303
https://orcid.org/0000-0003-1122-1303
Oliver Gottschalk  https://orcid.org/0000-0002-6512-6275
https://orcid.org/0000-0002-6512-6275
Supplementary material for this article is available on the Cartilage website at http://cart.sagepub.com/supplemental.
References
- 1. Körner D, Gueorguiev B, Niemeyer P, Bangert Y, Zinser W, Aurich M, et al. Parameters influencing complaints and joint function in patients with osteochondral lesions of the ankle-an investigation based on data from the German Cartilage Registry (KnorpelRegister DGOU). Arch Orthop Trauma Surg. 2017;137(3):367-73. [DOI] [PubMed] [Google Scholar]
- 2. Hintermann B, Boss A, Schäfer D. Arthroscopic findings in patients with chronic ankle instability. Am J Sports Med. 2002;30(3):402-9. [DOI] [PubMed] [Google Scholar]
- 3. Loren GJ, Ferkel RD. Arthroscopic assessment of occult intra-articular injury in acute ankle fractures. Arthroscopy. 2002;18(4):412-21. [DOI] [PubMed] [Google Scholar]
- 4. Kerkhoffs GMMJ, Karlsson J. Osteochondral lesions of the talus. Knee Surg Sports Traumatol Arthrosc. 2019;27(9):2719-20. [DOI] [PubMed] [Google Scholar]
- 5. Filardo G, Perdisa F, Roffi A, Marcacci M, Kon E. Stem cells in articular cartilage regeneration. J Orthop Surg Res. 2016;11:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mei-Dan O, Carmont MR, Laver L, Mann G, Maffulli N, Nyska M. Platelet-rich plasma or hyaluronate in the management of osteochondral lesions of the talus. Am J Sports Med. 2012;40(3):534-41. [DOI] [PubMed] [Google Scholar]
- 7. Migliorini F, Maffulli N, Bell A, Hildebrand F, Weber CD, Lichte P. Autologous matrix-induced chondrogenesis (AMIC) for osteochondral defects of the talus: a systematic review. Life (Basel). 2022;12(11):1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Weigelt L, Laux CJ, Urbanschitz L, Espinosa N, Klammer G, Götschi T, et al. Long-term prognosis after successful nonoperative treatment of osteochondral lesions of the talus: an observational 14-year follow-up study. Orthop J Sports Med. 2020;8(6):924183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. van Dijk CN, Reilingh ML, Zengerink M, van Bergen CJ. Osteochondral defects in the ankle: why painful. Knee Surg Sports Traumatol Arthrosc. 2010;18(5):570-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Walther M, Gottschalk O, Madry H, Müller PE, Steinwachs M, Niemeyer P, et al. Etiology, classification, diagnostics, and conservative management of osteochondral lesions of the talus. 2023 recommendations of the working group “clinical tissue regeneration” of the German society of orthopedics and traumatology. Cartilage. 2023;14(3):292-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Amendola A, Panarella L. Osteochondral lesions: medial versus lateral, persistent pain, cartilage restoration options and indications. Foot Ankle Clin. 2009;14(2):215-27. [DOI] [PubMed] [Google Scholar]
- 12. Zengerink M, Struijs PA, Tol JL, van Dijk CN. Treatment of osteochondral lesions of the talus: a systematic review. Knee Surg Sports Traumatol Arthrosc. 2010;18(2):238-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Loveday D, Clifton R, Robinson A. Interventions for treating osteochondral defects of the talus in adults. Cochrane Database Syst Rev. 2010;2010(8):CD008104. [DOI] [PubMed] [Google Scholar]
- 14. Steadman JR, Rodkey WG, Rodrigo JJ. Microfracture: surgical technique and rehabilitation to treat chondral defects. Clin Orthop Relat Res. 2001;391:S362-9. [DOI] [PubMed] [Google Scholar]
- 15. Chuckpaiwong B, Berkson EM, Theodore GH. Microfracture for osteochondral lesions of the ankle: outcome analysis and outcome predictors of 105 cases. Arthroscopy. 2008;24(1):106-12. [DOI] [PubMed] [Google Scholar]
- 16. Rothrauff BB, Murawski CD, Angthong C, Becher C, Nehrer S, Niemeyer P, et al. Scaffold-based therapies: proceedings of the international consensus meeting on cartilage repair of the ankle. Foot Ankle Int. 2018;39(1 suppl):41S-7. [DOI] [PubMed] [Google Scholar]
- 17. Gottschalk O, Altenberger S, Baumbach S, Kriegelstein S, Dreyer F, Mehlhorn A, et al. Functional medium-term results after autologous matrix-induced chondrogenesis for osteochondral lesions of the talus: a 5-year prospective cohort study. J Foot Ankle Surg. 2017;56(5):930-6. [DOI] [PubMed] [Google Scholar]
- 18. Götze C, Nieder C, Felder H, Migliorini F. AMIC for focal osteochondral defect of the talar shoulder. Life (Basel). 2020; 10(12):328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gottschalk O, Mazet J, Kerschl F, Schenk H, Suero EM, Hörterer H, et al. Correlation between EFAS- and MOCART score and clinical outcome after AMIC. Arch Orthop Trauma Surg. 2023;143(6):2895-900. [DOI] [PubMed] [Google Scholar]
- 20. Casari FA, Germann C, Weigelt L, Wirth S, Viehöfer A, Ackermann J. The role of magnetic resonance imaging in autologous matrix-induced chondrogenesis for osteochondral lesions of the talus: analyzing MOCART 1 and 2.0. Cartilage. 2021;13(1 suppl):639S-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Naal FD, Impellizzeri FM, Huber M, Rippstein PF. Cross-cultural adaptation and validation of the Foot Function Index for use in German-speaking patients with foot complaints. Foot Ankle Int. 2008;29(12):1222-8. [DOI] [PubMed] [Google Scholar]
- 22. Richter M, Agren PH, Besse JL, Cöster M, Kofoed H, Maffulli N, et al. EFAS score—multilingual development and validation of a patient-reported outcome measure (PROM) by the score committee of the European Foot and Ankle Society (EFAS). Foot Ankle Surg. 2018;24(3):185-204. [DOI] [PubMed] [Google Scholar]
- 23. Kitaoka HB, Alexander IJ, Adelaar RS, Nunley JA, Myerson MS, Sanders M. Clinical rating systems for the ankle-hindfoot, midfoot, hallux, and lesser toes. Foot Ankle Int. 1994;15(7):349-53. [DOI] [PubMed] [Google Scholar]
- 24. Walther M, Valderrabano V, Wiewiorski M, Usuelli FG, Richter M, Baumfeld TS, et al. Is there clinical evidence to support autologous matrix-induced chondrogenesis (AMIC) for chondral defects in the talus? A systematic review and meta-analysis. Foot Ankle Surg. 2021;27(3):236-45. [DOI] [PubMed] [Google Scholar]
- 25. Efrima B, Barbero A, Maccario C, Indino C, Nocera C, Albagli A, et al. Significant clinical improvement after arthroscopic autologous matrix-induced chondrogenesis for osteochondral lesions of the talus: a 5-year follow-up. Cartilage. Epub 2024 March 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Usuelli FG, de Girolamo L, Grassi M, D’Ambrosi R, Montrasio UA, Boga M. All-arthroscopic autologous matrix-induced chondrogenesis for the treatment of osteochondral lesions of the talus. Arthrosc Tech. 2015;4(3):e255-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. DʼAmbrosi R, Villafañe JH, Indino C, Liuni FM, Berjano P, Usuelli FG. Return to sport after arthroscopic autologous matrix-induced chondrogenesis for patients with osteochondral lesion of the talus. Clin J Sport Med. 2019;29(6):470-5. [DOI] [PubMed] [Google Scholar]
- 28. Wiewiorski M, Werner L, Paul J, Anderson AE, Barg A, Valderrabano V. Sports activity after reconstruction of osteochondral lesions of the talus with autologous spongiosa grafts and autologous matrix-induced chondrogenesis. Am J Sports Med. 2016;44(10):2651-8. [DOI] [PubMed] [Google Scholar]
- 29. Richter M, Zech S. Matrix-associated stem cell transplantation (MAST) in chondral lesions at the ankle as part of a complex surgical approach- 5-year-follow-up in 100 patients. Foot Ankle Surg. 2019;25(3):264-71. [DOI] [PubMed] [Google Scholar]
- 30. Weigelt L, Hartmann R, Pfirrmann C, Espinosa N, Wirth SH. Autologous matrix-induced chondrogenesis for osteochondral lesions of the talus: a clinical and radiological 2- to 8-year follow-up study. Am J Sports Med. 2019;47(7):1679-86. [DOI] [PubMed] [Google Scholar]
- 31. de Girolamo L, Schönhuber H, Viganò M, Bait C, Quaglia A, Thiebat G, et al. Autologous matrix-induced chondrogenesis (AMIC) and AMIC enhanced by autologous concentrated bone marrow aspirate (BMAC) allow for stable clinical and functional improvements at up to 9 years follow-up: results from a randomized controlled study. J Clin Med. 2019;8(3):392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Schiavone Panni A, Del Regno C, Mazzitelli G, D’Apolito R, Corona K, Vasso M. Good clinical results with autologous matrix-induced chondrogenesis (Amic) technique in large knee chondral defects. Knee Surg Sports Traumatol Arthrosc. 2018;26(4):1130-6. [DOI] [PubMed] [Google Scholar]
- 33. Kaiser N, Jakob RP, Pagenstert G, Tannast M, Petek D. Stable clinical long term results after AMIC in the aligned knee. Arch Orthop Trauma Surg. 2021;141(11):1845-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. de Girolamo L, Jannelli E, Fioruzzi A, Fontana A. Acetabular chondral lesions associated with femoroacetabular impingement treated by autologous matrix-induced chondrogenesis or microfracture: a comparative study at 8-year follow-up. Arthroscopy. 2018;34(11):3012-23. [DOI] [PubMed] [Google Scholar]
- 35. Volz M, Schaumburger J, Frick H, Grifka J, Anders S. A randomized controlled trial demonstrating sustained benefit of Autologous Matrix-Induced Chondrogenesis over microfracture at five years. Int Orthop. 2017;41(4):797-804. [DOI] [PubMed] [Google Scholar]
- 36. Giannini S, Buda R, Vannini F, Di Caprio F, Grigolo B. Arthroscopic autologous chondrocyte implantation in osteochondral lesions of the talus: surgical technique and results. Am J Sports Med. 2008;36(5):873-80. [DOI] [PubMed] [Google Scholar]
- 37. Migliorini F, Maffulli N, Baroncini A, Knobe M, Tingart M, Eschweiler J. Matrix-induced autologous chondrocyte implantation versus autologous matrix-induced chondrogenesis for chondral defects of the talus: a systematic review. Br Med Bull. 2021;138(1):144-54. [DOI] [PubMed] [Google Scholar]
- 38. Migliorini F, Maffulli N, Baroncini A, Eschweiler J, Knobe M, Tingart M, et al. Allograft versus autograft osteochondral transplant for chondral defects of the talus: systematic review and meta-analysis. Am J Sports Med. 2022;50(12):3447-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-car-10.1177_19476035241301896 for Long-Term Results after Autologous Matrix-Induced Chondrogenesis for Osteochondral Lesions of the Talus: A 10-Year Cohort Study by Lukas Deiss, Markus Walther, Kathrin Pfahl, Hubert Hörterer, Alexander Mehlhorn, Anke Röser and Oliver Gottschalk in CARTILAGE
Supplemental material, sj-jpg-2-car-10.1177_19476035241301896 for Long-Term Results after Autologous Matrix-Induced Chondrogenesis for Osteochondral Lesions of the Talus: A 10-Year Cohort Study by Lukas Deiss, Markus Walther, Kathrin Pfahl, Hubert Hörterer, Alexander Mehlhorn, Anke Röser and Oliver Gottschalk in CARTILAGE