Abstract
Introduction
Middle meningeal artery embolization (MMAe) is increasingly utilized as a primary or secondary treatment for chronic subdural hematoma (cSDH) and is usually performed with liquid embolics or particles. Outcomes after MMAe with coiling as a standalone treatment, or an adjunct to other agents, have not been reviewed.
Methods
A systematic review of the literature was performed to identify all original research that included patients who underwent standalone or adjunctive coiling for MMAe. The primary outcome was the need for rescue treatment defined as any unplanned reintervention for recurrent or residual cSDH.
Results
A total of 10 studies comprising 346 patients (mean age 73 years, 39% female) who underwent MMAe with coils were included. The majority of embolizations were with coils and particles (n = 176), followed by standalone coiling (137) and coiling with liquid embolics (120). The pooled rate of rescue treatment after embolization was 9.4% (95% CI 6.4–13.6, I2 = 0). The pooled complication rate was 2.6% (95% CI 1.3–5.1, I2 = 0). In the subgroup analysis of four studies reporting results after standalone coiling, the pooled rescue treatment rate was 8.2% (95% CI 4.0–15.9, I2 = 0) and there were no complications.
Conclusion
MMAe with coils is safe and potentially effective, but additional studies evaluating long-term clinical and radiographic results after standalone coiling are needed.
Keywords: Middle meningeal artery, chronic subdural hematoma, embolization, coils
Introduction
Chronic subdural hematoma (cSDH) is a common neurosurgical condition predominantly affecting the elderly. Traditionally treatment has involved surgery, such as burr hole drainage or craniotomy. These are effective but associated with high recurrence rates, ranging from 10% to 30%.1–5 Recently, middle meningeal artery embolization (MMAe) has emerged as a promising alternative or adjunct treatment option aimed at reducing recurrence rates by targeting the dural neovasculature that sustains the hematoma. MMAe's efficacy is believed to be related to the devascularization of the fragile neovascular membranes associated with cSDH, which are thought to contribute to the slow accumulation of blood within the subdural space.6,7 By embolizing the middle meningeal artery (MMA), the blood supply to these membranes is reduced, thereby disrupting the cycle of hematoma expansion and promoting spontaneous resorption and resolution.8,9 Several agents have been described for MMAe including liquid embolic agents, particles, and coils.2,3,10–13 The ideal embolization material remains a topic of debate.
Most MMAe studies have described embolization with liquid embolics such as Onyx, n-butyl cyanoacrylate (n-BCA), or particles, with the goal of penetrating the distal capillaries of the MMA.10,14–17 However, these carry the risk of non-target embolization to collaterals to the ophthalmic artery or the petrosal MMA branch resulting in vision loss or facial palsy, respectively. An alternative is coil embolization, which can be used as a standalone treatment or an adjunct to liquid embolics and particles. Some studies have demonstrated that coiling, while not penetrating as deeply as liquid agents, is associated with low rates of hematoma recurrence.2,6,13 Coil embolization reduces the risk of distal embolization into dangerous anastomoses, making it a potentially safer alternative. 18
The goal of this study was to systematically review the literature on standalone and adjunctive coiling for MMAe to determine the efficacy and safety of this technique.
Methods
This study was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). 19
Search strategy and inclusion criteria
We performed a comprehensive search of the literature as of September 11, 2024, using PubMed, Scopus, and EMBASE. The search strategies for each database are provided in Supplemental Table 1. Studies published in English from the inception of the literature to the search date were included if the authors reported original data with at least five patients undergoing MMAe for cSDH with coils either as a primary or adjunct technique (i.e., in addition to a liquid embolic agent or particles). Studies that included patients who underwent MMAe without coils were included if the primary outcome was reported for the subgroup who underwent coiling. Case series and case-control studies were included. The rate of rescue treatment also had to be reported for inclusion. MMAe could be performed as a primary treatment for cSDH or as an adjunct to surgical evacuation.
Data extraction
A standardized form was used to extract data from the included studies in duplicate (JJS and HH). Discrepancies were resolved by the senior author (NG). Data was collected regarding age, sex, preoperative modified Rankin Scale (mRS), embolic agents used in addition to coils, coil types, and surgical intervention.
Critical appraisal
The JBI checklist for case series 20 was used to critically appraise the included studies. This is a 10-item checklist that assesses the risk of bias, completeness of reporting, and adequacy of statistical analysis for case series.
Outcomes
The primary outcome was rescue treatment after MMAe with coils, defined as any unplanned surgical or endovascular procedure to treat residual or recurrent cSDH. We also considered the procedural complication rate. All complications related to the procedure including but not limited to access complication, ischemia, vessel perforation or dissection, and non-target embolization were counted.
Statistical analysis
Pooled estimates for each outcome were calculated using random effects models and represented with forest plots. Logit-transformed proportions were calculated for each outcome. Heterogeneity was evaluated with the I-square (I2) statistic. Publication bias was evaluated using a funnel plot and Egger's test. Subgroup analyses of patients who underwent standalone coiling (no additional liquid embolic or particles) and patients who received particle and adjunctive coil embolization were performed. R version 4.0.2 with the “metafor” package was used.
Results
Description of studies
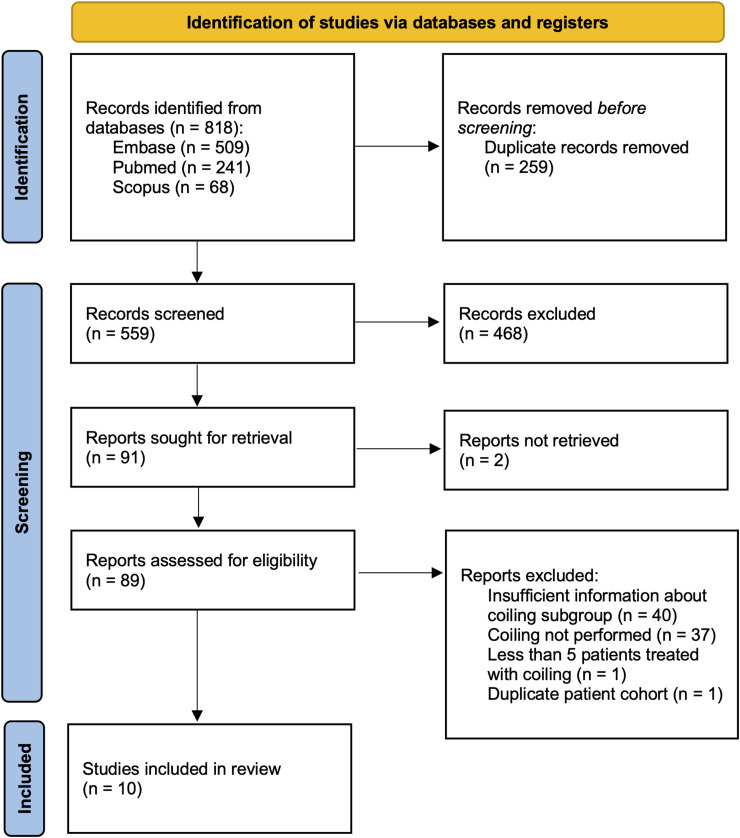
After screening 559 records, a total of 10 studies6,12,13,21,22–26 comprising 346 patients who underwent 445 embolizations met inclusion criteria (Figure 1). The results of the critical appraisal are shown in Table 1. Reporting criteria (participant demographics and clinic/site demographics) were the most commonly missed items from the JBI checklist. Only one study included patients from multiple institutions, seven were case series, and three were case-control studies.
Figure 1.
PRISMA flow diagram for study inclusion.
Table 1.
JBI checklist for case series.
| Study | Were there clear criteria for inclusion in the case series? | Was the condition measured in a standard, reliable way for all participants included in the case series? | Were valid methods used for the identification of the condition for all participants included in the case series? | Did the case series have consecutive inclusion of participants? | Did the case series have complete inclusion of participants? | Was there clear reporting of the demographics of the participants in the study? | Was there clear reporting of clinical information of the participants? | Were the outcomes or follow up results of cases clearly reported? | Was there clear reporting of the presenting site(s)/clinic(s) demographic information? | Was statistical analysis appropriate? |
|---|---|---|---|---|---|---|---|---|---|---|
| Campos et al. (2024) | Y | Y | Y | Y | U | N | Y | Y | N | NA |
| Perng et al. (2024) | Y | Y | Y | Y | N | N | Y | Y | N | Y |
| O'Gorman et al. (2024) | Y | Y | Y | Y | Y | N | Y | Y | N | NA |
| Iyer et al. (2023) | Y | Y | Y | Y | N | N | Y | Y | N | NA |
| Wali et al. (2023) | Y | Y | Y | Y | Y | N | N | Y | N | NA |
| Khorasanizadeh et al. (2022) | Y | Y | Y | Y | N | N | Y | Y | N | NA |
| Enriquez-Marulanda et al. (2021) | Y | Y | Y | Y | N | N | Y | Y | N | Y |
| Wei et al. (2021) | Y | Y | Y | Y | Y | N | Y | N | N | NA |
| Gomez-Paz et al. (2021) | Y | Y | Y | Y | Y | N | Y | Y | N | NA |
| Mureb et al. (2020) | Y | Y | Y | Y | N | N | Y | Y | N | NA |
Y: yes; N: no; U: unclear; NA: not applicable
The weighted mean age was 73 years (range: 61–80.5), and 39% of patients were female. The most common embolisate used was a combination of coils and particles (n = 176), followed by standalone coiling (137) and then coiling with liquid embolics (120). Coil embolization was limited to the main MMA trunk in five studies, including the main trunk and frontal/parietal branches in three studies, and selectively embolized the frontal/parietal branches in 1 study. One study did not report the locations of coil placement. Surgery was performed prior to embolization in 103 (29.8%) patients. Additional patient and procedural data are provided in Table 2.
Table 2.
Select study data.
| Study | No. patients | Mean age (years) | No. female (%) | Baseline mRS 0–2 (%) | No. Surgery prior to embolization (%) | Standalone coiling a | Coils + particles a | Coils + liquid embolic a | Coiling location (%) a | Coil type | Particle size (microns) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Campos et al. (2024) | 89 | 74.5 | 46 (51.7) | NR | 38 (42.7) | 17 (12.4) | 0 | 120 (87.6) b | Main trunk only (25.5) | NR | NA |
| Main trunk and either frontal or parietal branches (20.4) | |||||||||||
| Main trunk and both frontal and parietal branches (54) | |||||||||||
| Perng et al. (2024) | 27 | 78 | 5 (18.5) | 22 (81.5) | 0 | 27 (100) | 0 | 0 | Main trunk (100) | Interlock, Nester | NA |
| O'Gorman et al. (2024) | 22 | 61 | 11 (50) | NR | 0 | 13 (38.2) | 8 (23.5) | 0 | Frontal and parietal branches selectively (100) | Penumbra Smartcoil | 100–300 |
| Iyer et al. (2023) | 45 | 71 | 11 (24.4) | NR | 22 (48.9) | 45 (100) | 0 | 0 | Main trunk and either frontal or parietal branch (100) | Nester | NA |
| Wali et al. (2023) | 8 | 80.5 | 2 (25) | NR | 1 (12.5) | 0 | 8 (100) | 0 | Main trunk (100) | NR | 150–250 |
| Khorasanizadeh et al. (2022) | 78 | 72 | 28 (35.9) | 63 (80.7) | 22 (28.2) | 12 (12.8) | 82 (87.2) | 0 | Main trunk (58.3) | NR | 100–300 |
| Main trunk and either frontal or parietal branches (25) | |||||||||||
| Frontal or parietal branch selectively (16.7) c | |||||||||||
| Enriquez-Marulanda et al. (2021) | 36 | 76 | 17 (47.2) | 32 (88.9) | 10 (27.8) | 2 (4.4) | 43 (95.6) | 0 | Main trunk (100) | NR | 150–250 |
| Wei et al. (2021) | 10 | 63.1 | 0 | 10 (100) | 10 (100) | 20 (100) | 0 | 0 | Main trunk (100) | Target, Microplex, Axium | NA |
| Gomez-Paz et al. (2021) | 23 | 74 | 13 (56.5) | NR | 0 | 0 | 27 (100) | 0 | Main trunk (100) | NR | 150–250 |
| Mureb et al. (2020) | 8 | 75.4 | 1 (12.5) | 7 (87.5) | 0 | 0 | 8 (100) | 0 | NR | NR | 45–150 |
NR: not reported; NA: not applicable.
Absolute values and percentage are based on number of embolizations.
Onyx in 33 and n-BCA in 87 embolizations.
Only reported for standalone coiling subgroup.
Outcomes
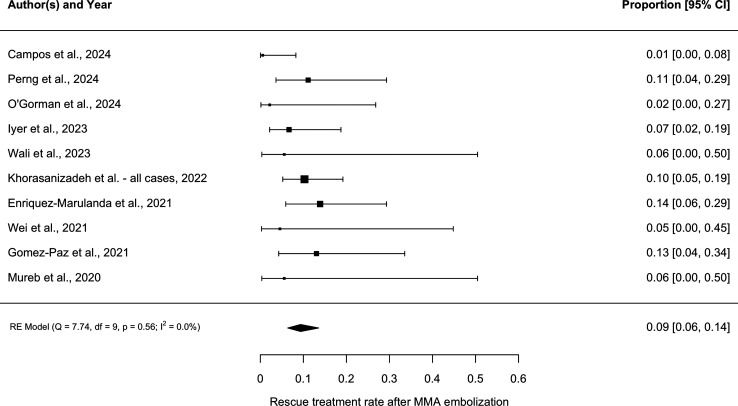
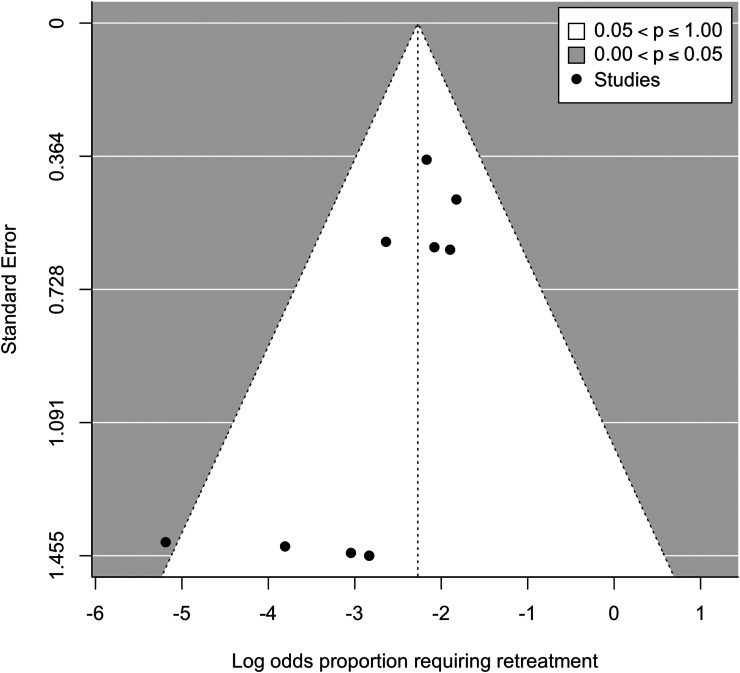
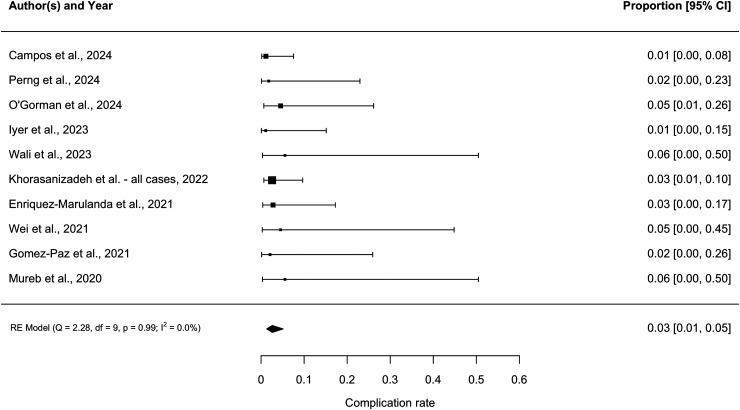
The weighted mean follow-up was 137 days (range: 72–182). As shown in Figure 2, the pooled rescue treatment rate was 9.4% (95% CI 6.4–13.6, I2 = 0). The funnel plot showed slight asymmetry, which was confirmed with Egger's test (p = .04). This suggested the presence of publication bias (Figure 3). As shown in Figure 4, the pooled complication rate was 2.6% (95% CI 1.3–5.1, I2 = 0). The complications included two non-target embolizations resulting in facial palsy (n = 1) and vision loss (1). These occurred in patients undergoing embolization with liquid embolic and particles, respectively. Additional complications included symptomatic M2 middle cerebral artery occlusion requiring thrombectomy (1), asymptomatic stroke (1), and femoral access site pseudoaneurysm (1).
Figure 2.
Forest plot demonstrating pooled rescue treatment rate among all 10 studies.
Figure 3.
Funnel plot demonstrating publication bias.
Figure 4.
Forest plot demonstrating pooled complication rate among all 10 studies.
Subgroup analysis
There were four studies2,6,13,24 consisting of 94 patients with a weighted mean age of 73 years that reported outcomes for patients who underwent standalone coiling. The pooled rescue treatment rate was 8.2% (95% CI 4.0–15.9, I2 = 0 [Supplemental Figure 1]). There were no complications.
There were four studies2,22,25,26 that reported outcomes for 121 patients with a weighted mean age of 73 years who underwent particle embolization with adjunctive coiling. The pooled rescue treatment rate was 9.2% (95% CI 5.2–15.8, I2 = 0 [Supplemental Figure 2]), and the pooled complication rate was 3.0% (95% CI 1.1–8.3, I2 = 0 [Supplemental Figure 3]). Additional data about the subgroups is shown in Supplemental Table 2.
Discussion
MMAe is a new treatment for cSDH that decreases recurrence rates compared to surgery alone. In a recent meta-analysis, MMAe was associated with lower rates of treatment failure and surgical rescue as well as higher rates of complete hematoma resolution. 27 None of the studies used standalone coiling as a primary treatment modality but instead reserved it for cases in which there were dangerous collaterals to the ophthalmic artery. 27 Standalone coiling has also been used when there is excessive MMA tortuosity precluding distal microcatheter position. There is limited data evaluating the efficacy of standalone and adjunctive coiling, and no reviews have been performed.
Traditionally, the goal of MMAe has been to obtain as distal penetration as safely possible to devascularize the neomembranes responsible for hematoma recurrence. With liquid embolic agents, this is achieved by obtaining a distal microcatheter position, using a balloon microcatheter, or creating a more dilute mixture of nBCA. The selection of smaller particles also facilitates distal penetration but increases the risk of reaching dangerous collaterals. One study did not identify any difference in treatment failure or complete hematoma resolution when comparing proximal and distal embolization. 28 A large multi-center cohort of 530 patients found that selective catheterization of MMA branches was associated with treatment failure compared to nonselective catheterization of the main MMA trunk. 29 Conversely, another study found that distal penetration of liquid embolics or particles was associated with more rapid hematoma resolution. 30 Therefore, there is conflicting evidence regarding the utility of distal penetration during MMAe.
The rescue treatment rate for all 10 studies and the subgroups in this review compares favorably to surgical cohorts. cSDH recurrence rates after the use of the subdural evacuating port system are as high as 33%. 31 A meta-analysis of over 15,000 patients undergoing burr hole evacuation found a recurrence rate of approximately 13%. 32 Risk factors for recurrence after surgery include mixed density hematoma, greater midline shift, larger hematoma size, and bilateral hematomas. 33
The rescue treatment rate for coils is also similar to retreatment rates associated with liquid embolics. In a comparison of Onyx to particles, unplanned rescue surgery occurred in 10% of patients in the Onyx group. 17 One cohort of patients with cSDH embolized with n-BCA reported a retreatment rate of only 5%. 34 There were high rates of distal penetration in this study with subdural membrane penetration at 84% and n-BCA cast reaching the midline at 55%. 34 Caution is needed when comparing the results in this review to other cohorts given possible differences in clinical characteristics, radiographic features, and selection for MMAe. Direct comparisons with adjustments for confounders are needed.
MMAe with coiling has several potential advantages over liquid embolics. Placement of coils is painless, unlike embolization with Onyx, which facilitates treatment with conscious sedation. A non-motion-degraded roadmap to navigate into distal MMA branches is not needed with coiling, which also obviates the need for general anesthesia. Avoiding general anesthesia is particularly important for the elderly population, which is the patient population predominantly affected by cSDH. Also, the coiling procedure is likely shorter, but direct comparisons are lacking. Coil embolization also avoids the risk of non-target embolization to the orbit or the facial nerve. In this review, neither of the two non-target embolizations occurred in patients undergoing standalone coiling.
MMAe with proximal coiling has disadvantages too. There is no penetration of the fragile neovascularity believed to be responsible for cSDH recurrence. The hematoma's microvascular bed is not affected due to the extensive network of dural collaterals that the MMA forms. Furthermore, ipsilateral repeat endovascular treatment is not possible when the MMA trunk is occluded, and repeat embolization is limited to embolization from the other side with liquid embolics and contralateral penetration.
Adjunctive proximal coiling of the MMA has been proposed as a method for reducing recanalization, 12 which may occur because of the network of dural collaterals that the MMA forms. The 9.2% rescue treatment rate for particle embolization with adjunctive coiling compares favorably to surgical cohorts and endovascular cohorts treated with liquid embolics, but direct comparisons are needed. Given dangerous skull base collaterals are not affected by coiling, this step likely adds minimal risk.
This review is limited by the small number of studies included. Several studies that included patients who underwent coiling but did not report their outcomes separately were excluded, potentially introducing bias into our results. There was not a standardized indication for rescue treatment used by the included studies. Patients could have been selected for standalone coiling who were expected to have a lower chance of recurrence, which would have been another source of bias. Finally, baseline and postoperative radiographic data were inconsistently reported among the studies, which precluded us from including outcomes regarding the rate and extent of hematoma resolution.
Additional large cohorts of patients undergoing MMAe with standalone coiling are needed to more accurately characterize clinical and radiographic outcomes of this relatively underexplored approach. The optimal technique will also need to be defined, as we found considerable variability in the type, number, and location of placed coils.
Conclusion
The results of this meta-analysis suggest that MMAe with coiling, both as a standalone and adjunctive treatment, is associated with low rates of rescue treatment and complications. Given the possibility of selection bias, small sample size, and the low quality of the included studies, caution is needed when extrapolating these results. Studies directly comparing standalone and adjunctive coiling with other embolic agents are needed.
Supplemental Material
Supplemental material, sj-docx-1-ine-10.1177_15910199241304852 for Middle meningeal artery embolization with standalone or adjunctive coiling for treatment of chronic subdural hematoma: Systematic review and meta-analysis by Haydn Hoffman, Jason J. Sims, Christopher Nickele, Violiza Inoa, Lucas Elijovich and Nitin Goyal in Interventional Neuroradiology
Acknowledgments
None.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval: Ethical approval was not required for this study because it was a review of previously published work Study data is available upon reasonable request.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Haydn Hoffman https://orcid.org/0000-0002-2967-6528
Jason J. Sims https://orcid.org/0009-0005-3393-0356
Violiza Inoa https://orcid.org/0000-0003-0399-3346
Supplemental material: Supplemental material for this article is available online.
References
- 1.Kolias AG, Chari A, Santarius Tet al. et al. Chronic subdural haematoma: modern management and emerging therapies. Nat Rev Neurol 2014; 10: 570–578. [DOI] [PubMed] [Google Scholar]
- 2.Khorasanizadeh M, Shutran M, Garcia A, et al. Middle meningeal artery embolization with isolated use of coils for treatment of chronic subdural hematomas: a case series. World Neurosurg 2022; 165: e581–e587. [DOI] [PubMed] [Google Scholar]
- 3.Ma L, Hoz SS, Doheim MF, et al. Impact of embolisate penetration, type, and technique on results after standalone middle meningeal artery embolization for chronic subdural hematoma. Neurosurgery 2024: 10–1227. [DOI] [PubMed] [Google Scholar]
- 4.Gelabert-González M, Iglesias-Pais M, García-Allut A, et al. Chronic subdural haematoma: surgical treatment and outcome in 1000 cases. Clin Neurol Neurosurg 2005; 107: 223–229. [DOI] [PubMed] [Google Scholar]
- 5.Nakaguchi H, Tanishima T, Yoshimasu N. Factors in the natural history of chronic subdural hematomas that influence their postoperative recurrence. J Neurosurg 2001; 95: 256–262. [DOI] [PubMed] [Google Scholar]
- 6.Perng PS, Chuang MT, Wong CE, et al. Simple coiling of middle meningeal artery embolization for chronic subdural hematoma: an inverse probability of treatment weighting matched cohort study. Intervent Neuroradiol 2024: 15910199241234407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang W, Huang J. Chronic subdural hematoma: epidemiology and natural history. Neurosurg Clin N Am 2017; 28: 205–210. [DOI] [PubMed] [Google Scholar]
- 8.Hashimoto T, Ohashi T, Watanabe D, et al. Usefulness of embolization of the middle meningeal artery for refractory chronic subdural hematomas. Surg Neurol Int 2013; 4: 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Edlmann E, Giorgi-Coll S, Whitfield PC, et al. Pathophysiology of chronic subdural haematoma: inflammation, angiogenesis and implications for pharmacotherapy. J Neuroinflammation 2017; 14: 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Al-Mufti F, Kaur G, Amuluru K, et al. Middle meningeal artery embolization using combined particle embolization and n-BCA with the dextrose 5% in water push technique for chronic subdural hematomas: a prospective safety and feasibility study. AJNR Am J Neuroradiol 2021; 42: 916–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tudor T, Capone S, Vivanco-Suarez J, et al. Middle meningeal artery embolization for chronic subdural hematoma: a review of established and emerging embolic agents. Stroke: Vasc Int Neurol 2023; 4: e000906. [Google Scholar]
- 12.Campos JK, Meyer BM, Zarrin DA, et al. Immediate procedural safety of adjunctive proximal coil occlusion in middle meningeal artery embolization for chronic subdural hematomas: experience in 137 cases. Intervent Neuroradiol 2024: 15910199231224003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iyer AM, Venkataraman SS, Kittel CA, et al. Coil embolization alone appears sufficient for middle meningeal artery embolization. Intervent Neuroradiol 2023: 15910199231217144. [DOI] [PubMed] [Google Scholar]
- 14.Scoville JP, Joyce E, Tonetti DA, et al. Radiographic and clinical outcomes with particle or liquid embolic agents for middle meningeal artery embolization of nonacute subdural hematomas. Interv Neuroradiol 2023; 29: 683–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Loh Y, Duckwiler G. A prospective, multicenter, randomized trial of the Onyx liquid embolic system and N-butyl cyanoacrylate embolization of cerebral arteriovenous malformations: clinical article. J Neurosurg 2010; 113: 733–741. [DOI] [PubMed] [Google Scholar]
- 16.Takeuchi Y, Morishita H, Sato Y, et al. Guidelines for the use of NBCA in vascular embolization devised by the committee of practice guidelines of the Japanese society of interventional radiology (CGJSIR), 2012 edition. Jpn J Radiol 2014; 32: 500–517. [DOI] [PubMed] [Google Scholar]
- 17.Shehabeldin M, Amllay A, Jabre R, et al. Onyx versus particles for middle meningeal artery embolization in chronic subdural hematoma. Neurosurgery 2023; 92: 979–985. [DOI] [PubMed] [Google Scholar]
- 18.Rinaldo L, Brinjikji W. Dangerous extracranial-intracranial anastomoses: what the interventionalist must know. Semin Intervent Radiol 2020; 37: 140–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moher D, Liberati A, Tetzlaff Jet al. et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol 2009; 62: 1006–1012. [DOI] [PubMed] [Google Scholar]
- 20.Munn Z, Barker TH, Moola S, et al. Methodological quality of case series studies: an introduction to the JBI critical appraisal tool. JBI Evid Synth 2020; 18: 2127–2133. [DOI] [PubMed] [Google Scholar]
- 21.O'Gorman J, Geevarghese R, Bodard S, et al. Embolization of middle meningeal arteries for symptomatic subacute subdural hematoma in patients with cancer. Acad Radiol 2024; 31: 4196–4200. [DOI] [PubMed] [Google Scholar]
- 22.Wali AR, Himstead A, Bravo J, et al. Helical coils augment embolization of the middle meningeal artery for treatment of chronic subdural hematoma: a technical note. J Cerebrovasc Endovasc Neurosurg 2023; 25: 214–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Enriquez-Marulanda A, Gomez-Paz S, Salem MM, et al. Middle meningeal artery embolization versus conventional treatment of chronic subdural hematomas. Neurosurgery 2021; 89: 486–495. [DOI] [PubMed] [Google Scholar]
- 24.Wei Q, Fan G, Li Z, et al. Middle meningeal artery embolization for the treatment of bilateral chronic subdural hematoma. Front Neurol 2021; 12: 651362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gomez-Paz S, Akamatsu Y, Salem MM, et al. Upfront middle meningeal artery embolization for treatment of chronic subdural hematomas in patients with or without midline shift. Interv Neuroradiol 2020; 27: 571–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mureb MC, Kondziolka D, Shapiro M, et al. DynaCT enhancement of subdural membranes after middle meningeal artery embolization: insights into pathophysiology. World Neurosurg 2020; 139: e265–e270. [DOI] [PubMed] [Google Scholar]
- 27.Sattari SA, Yang W, Shahbandi A, et al. Middle meningeal artery embolization versus conventional management for patients with chronic subdural hematoma: a systematic review and meta-analysis. Neurosurgery 2023; 92: 1142–1154. [DOI] [PubMed] [Google Scholar]
- 28.Catapano JS, Ducruet AF, Nguyen CL, et al. Middle meningeal artery embolization for chronic subdural hematoma: an institutional technical analysis. J Neurointerv Surg 2021; 13: 657–660. [DOI] [PubMed] [Google Scholar]
- 29.Salem MM, Kuybu O, Nguyen Hoang A, et al. Middle meningeal artery embolization for chronic subdural hematoma: predictors of clinical and radiographic failure from 636 embolizations. Radiology 2023; 307: e222045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Catapano JS, Ducruet AF, Srinivasan VM, et al. Radiographic clearance of chronic subdural hematomas after middle meningeal artery embolization. J Neurointerv Surg 2022; 14: 1279–1283. [DOI] [PubMed] [Google Scholar]
- 31.Golub D, Ashayeri K, Dogra S, et al. Benefits of the subdural evacuating port system (SEPS) procedure over traditional craniotomy for subdural hematoma evacuation. Neurohospitalist 2020; 10: 257–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lodewijkx R, Foppen M, Slot K, et al. Recurrent chronic subdural hematoma after burr-hole surgery and postoperative drainage: a systematic review and meta-analysis. Oper Neurosurg 2023; 25: 216–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maroufi SF, Farahbakhsh F, Macdonald RL, et al. Risk factors for recurrence of chronic subdural hematoma after surgical evacuation: a systematic review and meta-analysis. Neurosurg Rev 2023; 46: 270. [DOI] [PubMed] [Google Scholar]
- 34.Majidi S, Matsoukas S, Leacy D, , et al. Middle meningeal artery embolization for chronic subdural hematoma using N-butyl cyanoacrylate with D5 W push technique. Neurosurgery 2022; 90: 533–537. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-ine-10.1177_15910199241304852 for Middle meningeal artery embolization with standalone or adjunctive coiling for treatment of chronic subdural hematoma: Systematic review and meta-analysis by Haydn Hoffman, Jason J. Sims, Christopher Nickele, Violiza Inoa, Lucas Elijovich and Nitin Goyal in Interventional Neuroradiology