Abstract
The reovirus attachment protein, ς1, is responsible for strain-specific patterns of viral tropism in the murine central nervous system and receptor binding on cultured cells. The ς1 protein consists of a fibrous tail domain proximal to the virion surface and a virion-distal globular head domain. To better understand mechanisms of reovirus attachment to cells, we conducted studies to identify the region of ς1 that binds cell surface carbohydrate. Chimeric and truncated ς1 proteins derived from prototype reovirus strains type 1 Lang (T1L) and type 3 Dearing (T3D) were expressed in insect cells by using a baculovirus vector. Assessment of expressed protein susceptibility to proteolytic cleavage, binding to anti-ς1 antibodies, and oligomerization indicates that the chimeric and truncated ς1 proteins are properly folded. To assess carbohydrate binding, recombinant ς1 proteins were tested for the capacity to agglutinate mammalian erythrocytes and to bind sialic acid presented on glycophorin, the cell surface molecule bound by type 3 reovirus on human erythrocytes. Using a panel of two wild-type and ten chimeric and truncated ς1 proteins, the sialic acid-binding domain of type 3 ς1 was mapped to a region of sequence proposed to form the more amino terminal of two predicted β-sheet structures in the tail. This unit corresponds to morphologic region T(iii) observed in computer-processed electron micrographs of ς1 protein purified from virions. In contrast, the homologous region of T1L ς1 sequence was not implicated in carbohydrate binding; rather, sequences in the distal portion of the tail known as the neck were required. Results of these studies demonstrate that a functional receptor-binding domain, which uses sialic acid as its ligand, is contained within morphologic region T(iii) of the type 3 ς1 tail. Furthermore, our findings indicate that T1L and T3D ς1 proteins contain different arrangements of receptor-binding domains.
Mammalian reoviruses display broad cell and tissue tropism in vivo (reviewed in reference 43) and infect numerous types of cultured cells (reviewed in reference 43). Viral attachment is mediated by outer-capsid protein ς1 (23, 26, 46), which is encoded by the S1 gene segment (27, 28, 49). Some strain-dependent patterns of reovirus spread and tropism in newborn mice segregate with the S1 gene (20, 44, 47, 48), indicating that ς1 plays a key role in the pathogenesis of reovirus-induced disease. Within the murine central nervous system, type 1 reovirus infects ependymal cells, whereas type 3 reovirus infects neurons (47). This difference in cell tropism is determined by receptor specificities of type 1 and type 3 ς1 proteins (13, 41). The ς1 protein also is responsible for strain-dependent differences in the attachment of virions to mammalian erythrocytes (9, 12, 49) and murine erythroleukemia (MEL) cells (9, 38). Sialic acid serves as a receptor for type 3 reovirus on MEL cells (9, 38) and also can function as a receptor on murine L929 (L) cells (11, 18, 30, 32, 33, 37). Type 3 ς1 protein also binds another receptor in addition to sialic acid (6, 8, 29, 30), but the identity of this receptor has not been determined.
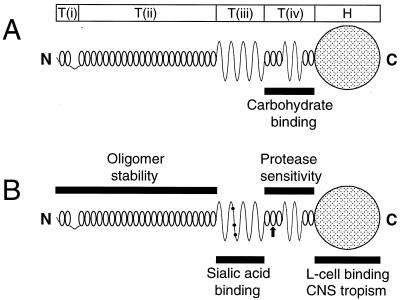
The ς1 protein is a homo-oligomer located at the vertices of the virion icosahedron (3, 16, 17, 24, 40). Electron microscopic analyses of virion-associated ς1 (17), ς1 isolated from virions (16, 17), and expressed ς1 (1) reveal that ς1 protein is a fibrous molecule consisting of an elongated tail domain and a virion-distal globular head domain. Five distinct, tandemly arranged morphologic regions of ς1, designated T(i), T(ii), T(iii), T(iv), and H, have been discerned using digitized image enhancements of ς1 electron micrographs (16). These morphologic regions correlate well with predictions of ς1 secondary structure (31). Sequences represented by morphologic regions in the tail are proposed to form an amino-terminal short (∼25-residue) α-helical coiled-coil and turn/loop [T(i)], a long (∼150-residue) α-helical coiled-coil [T(ii)], an eight-stranded cross β-sheet [T(iii), ∼65 residues], and two short regions of α-helical coiled-coil (two to three heptad repeats each) that flank a four-stranded cross β-sheet [T(iv), ∼75 residues]. Amino acid sequences carboxy terminal to morphologic region T(iv) (∼145 residues) are predicted to assume a more complex arrangement of secondary structures corresponding to the globular head domain (H) of ς1.
Previous studies of type 3 ς1 protein indicate that sequences in the tail bind sialic acid. Treatment of virions of reovirus strain type 3 Dearing (T3D) with protease to generate infectious subvirion particles (ISVPs), which are intermediates in reovirus disassembly, results in cleavage of ς1 and loss of the head and part of the T(iv) domain (8, 30). ISVPs retain the capacity for hemagglutination, demonstrating that sequences amino terminal to the cleavage site, which has been identified as residue 245 (8), are sufficient to bind sialic acid. Concordantly, sequence polymorphism within the fourth predicted β-strand of morphologic region T(iii), amino acid residues 198 to 204, determine the capacity of type 3 reoviruses to mediate hemagglutination and bind and infect MEL cells (9, 12). However, it is not known whether sequences within T(iii) constitute part of a sialic acid-binding domain or control this function at another site. Hemagglutination mediated by type 1 reovirus virions also is dependent on carbohydrate binding (25), although the specific carbohydrate has not been identified. Available evidence indicates that type 1 reovirus does not bind sialic acid (9, 12, 32, 38). Moreover, nothing is known about sequences in type 1 ς1 that mediate receptor binding.
To identify sequences in ς1 protein that bind carbohydrate, we generated chimeric and truncated ς1 proteins using the ς1 sequences of reovirus strains type 1 Lang (T1L) and T3D. Expressed ς1 proteins were tested in assays of carbohydrate binding, and morphologic region T(iii) was identified as the minimum structural domain in type 3 ς1 required to bind sialic acid. In contrast, sequences in the T(iv) region of type 1 ς1 protein were found to be required for hemagglutination, indicating that the minimal carbohydrate-binding domain in type 1 ς1 resides in the carboxy-terminal one-half of the molecule. Results from this study confirm the presence of discrete receptor-binding domains in the head and tail domains of type 3 ς1 and indicate that the topology of receptor-binding domains differs between type 1 and type 3 ς1 proteins.
MATERIALS AND METHODS
Cells and viruses.
Spinner-adapted L cells were grown in either suspension or monolayer cultures in Joklik's modified Eagle's minimal essential medium (Irvine Scientific, Santa Ana, Calif.) that was supplemented to contain 5% fetal bovine serum (Intergen, Purchase, N.Y.), 2 mM l-glutamine, 100 U of penicillin per ml, 100 μg of streptomycin per ml, and 0.25 μg of amphotericin (Irvine) per ml. Spodoptera frugiperda Sf21 and Trichoplusia ni Tn High Five insect cells were grown in either suspension or monolayer cultures using Grace's medium (Gibco, Grand Island, N.Y.) supplemented to contain 10% fetal bovine serum and 100 U of penicillin, 100 μg of streptomycin, and 0.25 μg of amphotericin per ml. Reovirus strains T1L and T3D are laboratory stocks. Purified virion preparations of reovirus were made using second- and third-passage L-cell lysate stocks of twice-plaque-purified reovirus as previously described (17). To obtain purified virions containing 35S-labeled proteins, Easy Tag Express-[35S] protein labeling mix (NEN, Boston, Mass.) was added to cell suspensions (∼12.5 μCi per ml) at the initiation of infection. Baculovirus vector strains were derived from Autographa californica nuclear polyhedrosis virus (Clontech Laboratories, Palo Alto, Calif.). Recombinant baculoviruses containing wild-type (wt) and mutant S1 gene cDNAs were generated and propagated as previously described (8).
Construction of recombinant S1 gene cDNAs.
Using primers specific for noncoding regions of the S1 gene, ς1-encoding S1 gene cDNAs were generated by reverse transcription-PCR (22) and cloned into the pCR2.1 vector (Invitrogen, San Diego, Calif.). Cloned T1L and T3D S1 gene cDNAs were used as template to generate chimeric and truncated S1 genes. Cloned chimeric S1 gene cDNAs were used as template to generate two additional chimeric constructs, 1-1-3-3-1 and 1-1-3-1-1.
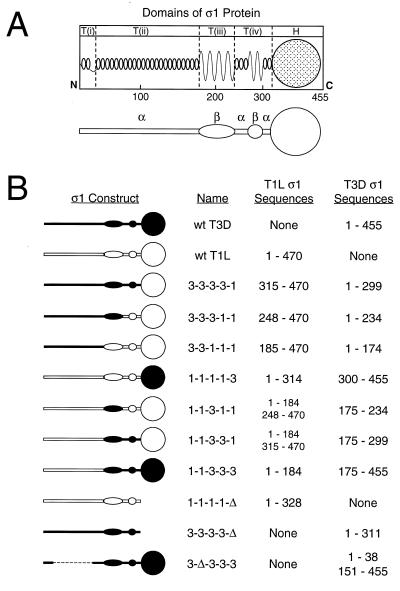
Chimeric S1 genes were produced using the splice-overlap-extension PCR technique (19) as previously described (8). Primers were designed to facilitate fusion of T1L and T3D S1 sequences and maintain the proper reading frame of sequences 3′ to the exchange locus. The same strategy was used to generate the mutant T3D S1 construct 3-Δ-3-3-3, which contains an internal in-frame deletion of sequences in the T(ii) region of the tail. Truncated S1 genes lacking 3′ sequences corresponding to the head domain were generated in PCRs using primers that inserted a stop codon at the desired position. S1 gene PCR products were cloned into the pCR2.1 vector and then transferred into baculovirus transfer vectors. Nucleotide sequences were determined for all cDNAs by automated analysis using an ABI model 377 (PE-Applied Biosystems, Norwalk, Conn.) and by manual analysis using phage T7 DNA polymerase (U.S. Biochemical, Cleveland, Ohio) and [35S]ATP. Chimeric and truncated S1 gene constructs used in this study are shown in Fig. 1.
FIG. 1.
Chimeric and truncated ς1 proteins used for studies of carbohydrate binding. (A) Model of ς1 structure depicting predicted secondary structures and correlating primary amino acid sequence with morphologic regions of ς1 (T(i), T(ii), T(iii), T(iv), and H) seen in computer-processed electron micrographic images of ς1 protein isolated from virions (16, 31). In the simplified version of this model shown below, α-helical regions of the tail domain are indicated by horizontal bars and regions of β-strand/β-turn are symbolized by ovoid shapes. The globular head domain (H) is depicted as a circle. (B) Sequence features of chimeric and truncated ς1 constructs. White symbols represent sequences derived from T1L ς1, and black symbols represent sequences derived from T3D ς1. Constructs are named according to the parental origin of ς1 morphologic regions as previously described (16, 31): 1, sequences derived from T1L ς1; and 3, sequences derived from T3D ς1. Sequences corresponding to morphologic regions T(i), T(ii), T(iii), T(iv), and H are represented by the first, second, third, fourth, and fifth characters, respectively, of construct names. Δ, deleted sequences. The sequences of T1L and T3D ς1 comprising each construct are denoted by numbers corresponding to ς1 amino acid residues reported by Nibert et al. (31) (T1L) and Bassel-Duby et al. (2) (T3D).
Expression of and purification of recombinant ς1 proteins.
Second- or third-passage stocks of recombinant baculovirus were used to infect insect cell monolayers (2.4 × 107 cells) at a multiplicity of infection of ≥5 PFU per cell. After 72 h of incubation, cells were harvested and resuspended in 2 ml of phosphate-buffered saline (PBS; 140 mM NaCl, 2.7 mM KCl, 8.3 mM Na2HPO4, 1.5 mM KH2PO4) containing 5 mM phenylmethylsulfonyl fluoride and Complete, Mini, EDTA-free protease inhibitor cocktail (Boehringer Mannheim, Indianapolis, Ind.). Cells were lysed by sonication, and supernatants were cleared of debris by centrifugation. To produce 35S-labeled ς1 proteins, culture medium was replaced after 24 h incubation with methionine-free Grace's medium (Gibco) supplemented to contain 10% fetal bovine serum, 100 U of penicillin, 100 μg of streptomycin, and 0.25 μg of amphotericin per ml, and 10 μCi of Easy Tag Express-[35S] protein labeling mix per ml. Cells were harvested after 48 h of incubation in 35S-containing medium. Expressed ς1 proteins containing an intact head domain were recovered from cell lysates using T1L ς1-specific monoclonal antibody (MAb) 5C6 (45) or T3D ς1-specific MAb 9BG5 (6) conjugated to cyanogen bromide-activated Sepharose (Pharmacia, Uppsala, Sweden). Beads containing adsorbed ς1 protein were washed five times with buffer consisting of 50 mM Tris (pH 8), 1.2 M NaCl, 0.4% sodium dodecyl sulfate (SDS), 0.2% Triton X-100, and 5 mM EGTA, followed by three washes with a solution of 50 mM triethanolamine (pH 11.6), 0.5 M NaCl, and 0.1% Triton X-100. Beads then were washed three times with virion storage buffer (150 mM NaCl, 10 mM MgCl2, 10 mM Tris [pH 7.5]). Truncation mutants of ς1 lacking the head domain were purified from cell lysates by precipitation with rabbit antiserum raised to virions of strain T1L or T3D. Precipitates were recovered using protein A-Sepharose (Pharmacia) and washed as described above.
Protease treatment of expressed ς1 proteins.
Aliquots of ς1-containing Sepharose beads in virion storage buffer were incubated at 15°C with 0, 0.1, 1.0, or 10 μg of Nα-p-tosyl-l-sulfonyl phenylalanyl chloromethyl ketone-treated bovine trypsin (Sigma Chemical Co., St. Louis, Mo.) per ml for 75 min. Reaction mixtures were mixed 1:1 with 2× protein sample buffer (3) and incubated at 100°C for 10 min. Reaction products were resolved by polyacrylamide gel electrophoresis (PAGE) in an SDS–10% polyacrylamide gel and visualized by autoradiography.
Assessment of ς1 multimerization status during SDS-PAGE.
35S-labeled purified reovirus virions (5 × 1010 particles) or expressed ς1 proteins adsorbed to MAb-conjugated Sepharose were suspended in protein sample buffer (62.5 mM Tris, 2% SDS, 5% 2-mercaptoethanol, 10% glycerol, 0.01% bromophenol blue [3]) adjusted to pH 6.8 or 8.3 and incubated at 100°C (pH 6.8) or 60°C (pH 8.3) for 10 min. Proteins were resolved in SDS–10% polyacrylamide gels and visualized by autoradiography.
Hemagglutination assay.
Clarified insect cell lysates containing expressed ς1 proteins were prepared as described above. Lysates were aliquoted into 96-well U-bottom microtiter plates (Costar, Cambridge, Mass.) and serially diluted twofold in 0.05 ml of PBS. Human type O+ erythrocytes or calf erythrocytes (Colorado Serum Co., Denver, Colo.) were washed twice in PBS and resuspended at a concentration of 1% (vol/vol). Erythrocytes (0.05 ml) were added to wells containing expressed protein and incubated at 4°C for at least 2 h. A partial or complete shield of erythrocytes on the well bottom was interpreted as a positive hemagglutination result; a smooth, round button of erythrocytes was interpreted as negative. The highest dilution of lysate sufficient to produce hemagglutination was designated to equal 1 hemagglutination unit (HA unit).
Glycophorin-binding assay.
Human type MN glycophorin or asialoglycophorin (Sigma) was biotinylated using ENZOTIN reagent (Enzo Diagnostics, Farmingdale, N.Y.) according to the supplier's instructions. Expressed ς1 proteins containing intact head domains were purified from insect cell lysates using MAb-conjugated Sepharose as described above, and 0.004 ml of the ς1 preparation was diluted in a total volume of 0.1 ml of PBS supplemented to contain 0.05% Tween 20 (J. T. Baker, Phillipsburg, N.J.) (PBS-T) and 0.034 μg of biotinylated glycophorin or biotinylated asialoglycophorin per ml. Beads were incubated at room temperature (RT) for 15 min, rinsed with three washes in 0.5 ml of PBS-T, then incubated at RT in 0.1 ml of PBS-T with 0.2 U of streptavidin-peroxidase conjugate (Boehringer) per ml for 15 min, and rinsed again with three washes in 0.5 ml of PBS-T. Finally, beads were incubated at RT in 0.1 ml of solution of chromogenic substrate [0.04% (wt/vol) 2,2′-azinobis(3-ethylbenzthiazolinesulfonic acid) (ABTS; Sigma) in 0.05 M phosphate-citrate buffer (pH 5.0) containing 0.0075% H2O2] for 15 min. Absorbance at 405 nm was determined using a Thermomax microplate reader (Molecular Devices, Crawley, United Kingdom). Nonspecific binding of biotinylated glycophorin and biotinylated asialoglycophorin was determined exactly as above, using MAb-conjugated Sepharose without ς1 protein.
RESULTS
Expression and folding of chimeric and truncated ς1 proteins.
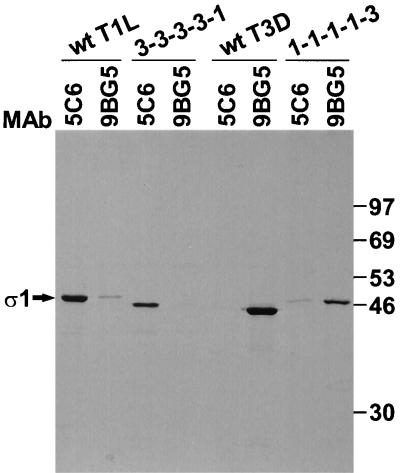
To identify structural domains in ς1 that bind carbohydrate, the ς1 proteins of reovirus strains T1L and T3D, seven T1L-T3D chimeric ς1 proteins, and three ς1 truncation mutants shown in Fig. 1 were expressed in insect cells using baculovirus vectors. Sequences exchanged among the ς1 chimeras correspond to morphologic regions of ς1 identified in electron micrographs (16). The specific exchange loci were at conserved positions between sequence regions distinguished by unique patterns of repeating apolar amino acids (31). MAbs specific for T1L and T3D ς1 proteins, 5C6 (45) and 9BG5 (6), respectively, were used to purify expressed ς1 proteins from cell lysates. The reactivity of MAbs 5C6 and 9BG5 with chimeric ς1 proteins demonstrates that these antibodies recognize epitopes in the ς1 head (Fig. 2). MAb 5C6 bound wt T1L ς1 and chimera 3-3-3-3-1, which has head-forming sequences derived from only T1L ς1. In contrast, MAb 9BG5 bound wt T3D ς1 and chimera 1-1-1-1-3, which has head-forming sequences from only T3D ς1. Consistent with this pattern, chimera 3-3-3-3-1 was not bound by MAb 9BG5, and chimera 1-1-1-1-3 was not bound by MAb 5C6. Either MAb 5C6 or MAb 9BG5 was capable of recovering ς1 proteins containing a head domain (Fig. 3 to 5), thus confirming expression of our panel of recombinant S1 gene constructs. Additionally, these antibodies bind conformationally sensitive epitopes in ς1 (4, 35, 45), which suggests that expressed ς1 proteins are properly folded.
FIG. 2.
Binding of anti-ς1 MAbs to expressed ς1 proteins. 35S-labeled wt and chimeric ς1 proteins were expressed in insect cells, and cell lysates were incubated with T1L ς1-specific MAb 5C6 and T3D ς1-specific MAb 9BG5 conjugated to Sepharose. Sepharose was washed under stringent conditions of salt, detergent, and pH to remove nonspecifically associated proteins, followed by incubation in protein sample buffer at 100°C to release antibody-bound ς1 protein. Samples were resolved in an SDS–10% polyacrylamide gel and visualized by autoradiography. Positions of molecular weight standards (in kilodaltons) are shown.
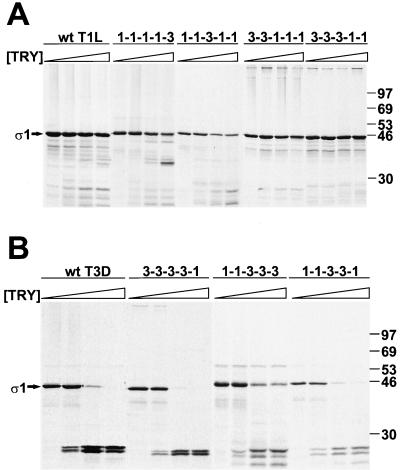
FIG. 3.
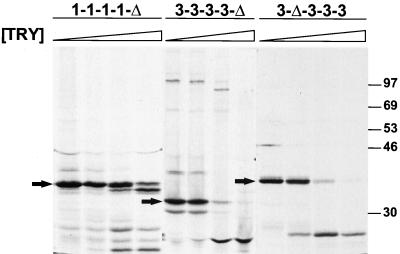
Effect of trypsin treatment on wt and chimeric ς1 proteins. 35S-labeled ς1 proteins were purified from insect cell lysates using anti-ς1 MAb 5C6 or 9BG5 conjugated to Sepharose followed by treatment at 15°C with 0, 0.1, 1.0, or 10 μg of bovine trypsin per ml for 75 min. Reaction mixtures were heated at 100°C in protein sample buffer; digestion products were resolved in an SDS–10% polyacrylamide gel and visualized by autoradiography. Sequences corresponding to the T(iv) region of proteins shown in panel A are derived from T1L ς1, whereas sequences corresponding to the T(iv) region of proteins shown in panel B are derived from T3D ς1.  , 0 to 10 μg of trypsin [TRY] per ml. Positions of molecular weight standards (in kilodaltons) are shown.
, 0 to 10 μg of trypsin [TRY] per ml. Positions of molecular weight standards (in kilodaltons) are shown.
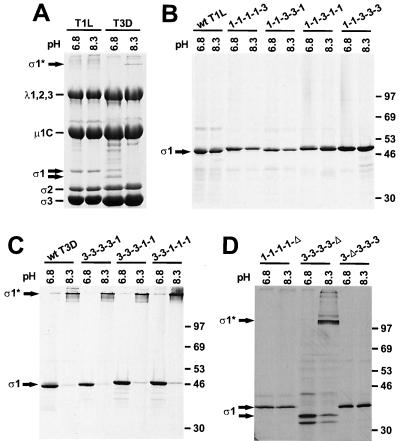
FIG. 5.
Oligomer stability of chimeric and truncated ς1 proteins. (A) 35S-labeled purified T1L and T3D virions were incubated at 100°C in pH 6.8 protein sample buffer or 60°C in pH 8.3 protein sample buffer for 10 min. Reaction products were resolved in an SDS–10% polyacrylamide gel and visualized by autoradiography. Viral structural proteins are labeled. (B and C) 35S-labeled full-length wt and chimeric ς1 proteins were purified from insect cell lysates using anti-ς1 MAb 5C6 or 9BG5 conjugated to Sepharose and treated as for panel A. Positions of molecular weight standards (in kilodaltons) are shown. (D) 35S-labeled truncated ς1 proteins were purified from insect cell lysates using MAb 9BG5 conjugated to Sepharose or antireovirus serum plus protein A-Sepharose and treated as for panel A. Positions of molecular weight standards (in kilodaltons) are shown. ς1*, bands corresponding to ς1 oligomers.
Identification of a protease-sensitive domain in morphologic region T(iv) of T3D ς1.
To identify protease-sensitive domains in T3D ς1, and to confirm that sequences in chimeric ς1 proteins fold into their native conformations, each protein was purified from cell lysates and treated with trypsin. Trypsin cleaves expressed (15) or virion-associated (8) T3D ς1 at Arg245 within morphologic region T(iv). T1L ς1 is resistant to cleavage by trypsin (5, 8, 14, 15, 30). Likewise, wt T1L ς1 and chimeric proteins containing the T(iv) region of T1L ς1 were resistant to cleavage (Fig. 3A). T1L ς1 truncation mutant 1-1-1-1-Δ, which contains tail-forming sequences only, was resistant to trypsin cleavage at lower enzyme concentrations but exhibited partial cleavage susceptibility at higher concentrations (∼10 μg of trypsin per ml) (Fig. 4); the predominant cleavage product migrated slightly faster than untreated protein. The wt T3D ς1 protein was cleaved by trypsin, as was each ς1 construct containing the T(iv) region of T3D ς1 (Fig. 3B and 4). Trypsin treatment of this group of expressed ς1 proteins resulted in the generation of stable cleavage products of approximately 25 kDa, which is characteristic of trypsin-treated wt T3D ς1 (8, 14, 15, 24, 30, 52). Thus, the pattern of susceptibility of chimeric ς1 proteins to cleavage by trypsin confirms the location of a protease-sensitive region in T3D ς1, T(iv), and is consistent with native folding of these molecules.
FIG. 4.
Effect of trypsin treatment on truncated ς1 proteins. 35S-labeled ς1 proteins were purified from insect cell lysates using antireovirus serum plus protein A-Sepharose, followed by treatment at 15°C with 0, 0.1, 1.0, or 10 μg of bovine trypsin per ml for 75 min. Reaction mixtures were heated at 100°C in protein sample buffer; digestion products were resolved in an SDS–10% polyacrylamide gel and visualized by autoradiography.  , 0 to 10 μg of trypsin [TRY] per ml. Positions of untreated ς1 deletion mutants are indicated by arrows. Positions of molecular weight standards (in kilodaltons) are shown.
, 0 to 10 μg of trypsin [TRY] per ml. Positions of untreated ς1 deletion mutants are indicated by arrows. Positions of molecular weight standards (in kilodaltons) are shown.
Identification of a domain in ς1 important for multimer stability.
As an additional test of protein folding, expressed ς1 proteins were examined for the capacity to maintain oligomeric structure during SDS-PAGE. In previous studies, it was shown that virion-associated (3, 51) and expressed (3, 24, 40) T3D ς1 protein migrates as an oligomer in SDS-polyacrylamide gels after solubilization in protein sample buffer under specific conditions of temperature and pH. When virions of T1L and T3D were disrupted at 60°C in pH 8.3 sample buffer, the ς1 protein of T1L migrated as a monomer, whereas T3D ς1 migrated as an oligomer (Fig. 5A). Incubation of virions at 100°C in pH 6.8 sample buffer resulted in the appearance of ς1 monomers only. This pattern was replicated by wt T1L and T3D ς1 proteins expressed in insect cells (Fig. 5B and C). Chimeric and truncated ς1 proteins containing T1L T(i) and T(ii) sequences migrated as monomers under these conditions, and ς1 proteins with T(i) and T(ii) sequences derived from T3D migrated as oligomers. Thus, results obtained using chimeric and truncated ς1 proteins show that sequences constituting morphologic regions T(i) and T(ii), which are predicted to form almost exclusively α-helical coiled coil, determine the difference in stability of T1L and T3D ς1 oligomers in SDS-polyacrylamide gels. Additionally, these results indicate that native tertiary and quaternary structures are maintained in the amino-terminal aspect of the tail domain of expressed ς1 proteins used for our studies.
Hemagglutination activity of chimeric ς1 proteins.
To identify sequences in ς1 that bind carbohydrate, chimeric and truncated proteins derived from T1L and T3D ς1 were expressed in insect cells, and ς1 proteins contained in cell lysates were tested for the capacity to mediate hemagglutination. In previous studies, it was shown that virions of T1L agglutinate human but not bovine erythrocytes, whereas virions of T3D agglutinate bovine erythrocytes more efficiently than human erythrocytes (9, 12). In accordance with this profile, wt T1L ς1 agglutinated human but not bovine erythrocytes, whereas wt T3D ς1 exhibited the reverse pattern (Fig. 6).
FIG. 6.
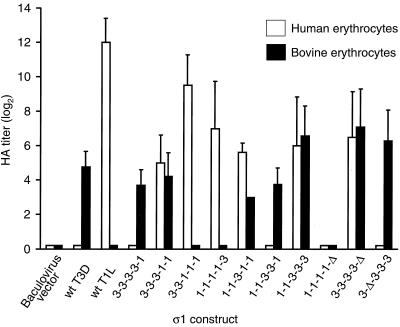
Hemagglutination activity of chimeric and truncated ς1 proteins. Insect cells (1.2 × 107) were inoculated with ς1-expressing recombinant baculoviruses, and cultures were harvested after 4 days of incubation. Cells were resuspended in 1 ml of PBS supplemented with protease inhibitors and disrupted by sonication, followed by centrifugation to clarify lysates. Supernatants were diluted twofold serially, and either human O+ or calf erythrocytes were added to each well. Hemagglutination reactions were scored after incubation at 4°C for at least 2 h. The hemagglutination pattern of each expressed ς1 protein was determined in 8 to 20 independent experiments, and a positive result was defined as ≥8 HA units of activity per 100 μl of lysate. For inclusion in the analysis, any given construct was required to display hemagglutination in two or more experiments; otherwise, the construct was deemed hemagglutination negative and assigned an HA titer of zero. Results are expressed as the mean log2 HA titer. Error bars indicate standard deviations of the mean.
Replacement of the T1L or T3D ς1 head domain with corresponding sequences from the heterologous protein (chimeras 1-1-1-1-3 and 3-3-3-3-1) did not alter the hemagglutination profile, demonstrating that sequences contained within the tail determine type-specific patterns of hemagglutination. Like wt T1L and 1-1-1-1-3 ς1 proteins, ς1 chimeras 3-3-1-1-1, 3-3-3-1-1, and 1-1-3-1-1 were capable of agglutinating human erythrocytes, which indicates that each contains the type 1 hemagglutination domain. The only T1L sequences common to all of these proteins correspond to morphologic region T(iv). These results support the conclusion that the T(iv) region of type 1 ς1 mediates hemagglutination, and therefore carbohydrate binding, by this protein.
In contrast to type 1 ς1 protein, the type 3 pattern of hemagglutination was linked to the presence of morphologic region T(iii). Chimeric proteins that agglutinated bovine erythrocytes, 3-3-3-3-1, 1-1-3-3-3, 3-3-3-1-1, 1-1-3-3-1, and 1-1-3-1-1, share sequences corresponding to the T3D T(iii) region, and no other sequences in T3D ς1 are held in common by these proteins. Furthermore, the only T3D sequences in chimera 1-1-3-1-1 are derived from the T(iii) region, which demonstrates that T(iii) sequences can mediate the type 3 reovirus pattern of hemagglutination independently of other sequences in type 3 ς1. These results provide strong evidence that a sialic acid-binding domain is located within the T(iii) region of type 3 ς1. Moreover, the finding that ς1 chimeras 3-3-3-1-1 and 1-1-3-1-1 agglutinated both human and bovine erythrocytes further supports a model of topologically distinct carbohydrate-binding domains in type 1 and type 3 ς1 proteins.
To determine whether a full-length ς1 molecule is necessary for functionality of the hemagglutination domain, three ς1 deletion mutants, 1-1-1-1-Δ, 3-3-3-3-Δ, and 3-Δ-3-3-3 (Fig. 4 and 5D), were tested for their hemagglutination capacity. Both the 3-3-3-3-Δ and 3-Δ-3-3-3 deletion mutants agglutinated bovine erythrocytes, demonstrating that sequences forming the type 3 ς1 globular head domain and long, fibrous α-helical segment of the tail are dispensable for hemagglutination. These results agree with those from experiments using ς1 chimeras in which hemagglutination activity of type 3 ς1 segregates with morphologic region T(iii). When tested in hemagglutination assays with human and bovine erythrocytes, truncation mutant 1-1-1-1-Δ failed to agglutinate cells from either species. This result contrasts with the capacity of morphologic region T(iv) of T1L ς1 to mediate hemagglutination of human erythrocytes by chimeric ς1 proteins and suggests that head-forming sequences (of either T1L or T3D ς1) must be present to facilitate the type 1 pattern of hemagglutination mediated by T(iv).
Deletion mutant 3-3-3-3-Δ efficiently agglutinated human erythrocytes in addition to bovine erythrocytes (Fig. 6). Thus, sequences in the tail of T3D ς1 are sufficient to mediate hemagglutination of human erythrocytes. Chimeric ς1 protein 1-1-3-3-3 also agglutinated both types of erythrocytes efficiently, which indicates either species-independent hemagglutination mediated by T3D sequences or the presence of both type 1 and type 3 hemagglutination domains within this construct. The former mechanism is more consistent with our other results, which do not localize hemagglutination activity of type 1 ς1 to morphologic regions T(i) or T(ii) and which show that sequences in the T3D tail can mediate hemagglutination of human erythrocytes (e.g., 3-3-3-3-Δ).
Binding of expressed ς1 proteins to sialic acid.
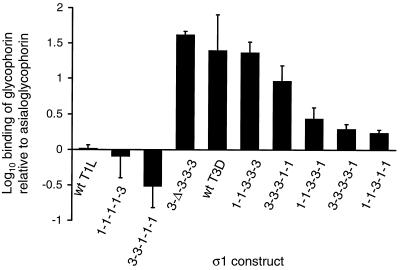
To confirm that sequences in morphologic region T(iii) of type 3 ς1 protein bind sialic acid, we performed a quantitative assay to assess sialic acid-dependent binding of expressed ς1 proteins to glycophorin. Glycophorin is a highly sialylated glycoprotein on the erythrocyte surface (10), and sialic acid residues of human erythrocyte glycophorin are bound by type 3 reovirus (34). Expressed ς1 proteins were captured on Sepharose beads conjugated to MAb 5C6 or 9BG5 and treated with biotinylated human glycophorin or asialoglycophorin. Expressed ς1 proteins segregated into two groups according to their capacity to bind glycophorin relative to asialoglycophorin (Fig. 7). The first group, which includes wt T1L ς1 and chimeric ς1 proteins 1-1-1-1-3 and 3-3-1-1-1, did not exhibit appreciable binding to glycophorin over background. The second group, which includes wt T3D ς1 and recombinant ς1 proteins 3-Δ-3-3-3, 1-1-3-3-3, 3-3-3-1-1, 1-1-3-3-1, 3-3-3-3-1, and 1-1-3-1-1, bound glycophorin 2- to 40-fold more efficiently than asialoglycophorin. Thus, the latter group of recombinant ς1 proteins exhibited sialic acid-dependent glycophorin binding. The only type 3 ς1 sequences common to all members of this group correspond to morphologic region T(iii), which indicates that T(iii) sequences are sufficient to mediate binding of sialic acid by type 3 ς1 protein. Accordingly, expressed proteins that exhibited only background binding to glycophorin (wt T1L, 1-1-1-1-3, and 3-3-1-1-1) contain T(iii) sequences derived from type 1 ς1. It was not possible to test truncation mutants 1-1-1-1-Δ and 3-3-3-3-Δ in the glycophorin-binding assay since these experiments would require domain-specific antibodies that bind the ς1 tail, and such antibodies currently are unavailable. Results of the glycophorin-binding and hemagglutination assays demonstrate that sialic acid is bound by sequences within morphologic region T(iii) of the type 3 ς1 tail domain, amino acid residues 175 to 234.
FIG. 7.
Binding of expressed ς1 proteins to glycophorin and asialoglycophorin. Expressed wt and chimeric ς1 proteins were purified from insect cell lysates using MAb-conjugated Sepharose, followed by treatment of equivalent amounts of ς1 with biotinylated glycophorin or biotinylated asialoglycophorin. Sepharose beads then were incubated with streptavidin-peroxidase conjugate, followed by the addition of chromogenic substrate. The reaction was allowed to develop for 15 min, and supernatant absorbance at 405 nm was determined using a microplate reader. Nonspecific binding of glycophorin and asialoglycophorin was determined using MAb-conjugated Sepharose without ς1 protein, and raw absorbance data were corrected accordingly to derive values of specific absorbance. The results are presented as the mean log10 ratio of specific glycophorin binding to specific asialoglycophorin binding by ς1. Error bars represent the standard deviation of three independent experiments.
DISCUSSION
This study was designed to identify the minimal domain in reovirus attachment protein ς1 capable of binding carbohydrate. Using a panel of expressed chimeric and truncated ς1 proteins derived from strains T1L and T3D, we found that sequences predicted to form an eight-stranded β-sheet in the T3D ς1 tail are sufficient to mediate binding of sialic acid by T3D ς1; the head domain, which contains sequences that bind an unidentified receptor on L cells (8, 14, 29, 30, 42, 50, 52), is dispensable for sialic acid binding. Carbohydrate binding by type 1 ς1 protein also is mediated by sequences in the tail predicted to form a β-sheet structure; however, these sequences are contained in a morphologic region of the tail, T(iv), that is distinct from that involved in binding sialic acid by type 3 ς1. The organization of receptor-binding domains in ς1 protein has important implications concerning mechanisms used by reovirus to achieve a stable virus-receptor complex that facilitates viral entry into cells.
T3D ς1 protein expressed in prokaryotic or eukaryotic cells retains the capacity to bind sialic acid (29, 34) and mediate hemagglutination (1, 26). Our approach was to exploit differences in carbohydrate specificity of type 1 and type 3 ς1 and unique hemagglutination patterns mediated by these proteins to identify sequences in ς1 that bind carbohydrate. We took advantage of the unique domain organization of ς1 protein (8, 16, 30, 31) to design chimeric molecules and truncation mutants that were likely to isolate independent functional units of sequence. Exchange loci in chimeric proteins were created at positions of conserved residues located between sequence units corresponding to morphologic regions seen in electron micrographic images of ς1 protein (16) (Fig. 1). Assays to test proper folding of ς1 sequences, including oligomer stability, susceptibility to protease cleavage, and binding to conformationally sensitive MAbs, indicate that natural ς1 conformation was preserved in the amino-terminal portion of the tail domain, carboxy-terminal portion of the tail domain, and head domain, respectively, of baculovirus-expressed ς1 proteins (Fig. 2 to 5). These results support a model of modular ς1 structure (31) and validate the use of chimeric and truncated ς1 proteins to define structure-function relationships.
MAbs 5C6 and 9BG5, specific for type 1 and type 3 ς1 proteins, respectively (6, 45), were used to test fidelity of ς1 folding and for purification of ς1 from insect cell lysates. MAb 9BG5 binds sequences in the T3D ς1 head domain (4, 14, 29, 42, 52). Consistent with the epitope specificity of 9BG5, wt T3D ς1 and chimeric and truncated ς1 proteins containing the T3D head were bound efficiently and specifically by this MAb (Fig. 2 to 5). Prior to this study, the domain in T1L ς1 bound by MAb 5C6 was not known. MAb 5C6 specifically bound expressed ς1 proteins containing the T1L ς1 head domain, and sequences in the head but not the tail were sufficient for 5C6 binding (Fig. 2). Therefore, these results indicate that MAb 5C6 binds the head domain of T1L ς1.
In a previous study, we found that a sequence polymorphism in the T(iv) region of type 3 ς1 protein, isoleucine or threonine at amino acid position 249, is a determinant of T3D ς1 cleavage susceptibility at Arg245 during treatment of virions with trypsin to generate ISVPs (8). In T3D ς1, position 249 is occupied by a threonine residue, which interrupts a heptad repeat sequence predicted to form α-helical coiled coil (31). Although the specific amino acid at position 249 regulates cleavage susceptibility of T3D ς1 protein, it is possible that sequences outside the T(iv) region are also required. The cleavage profiles of chimeric and truncated ς1 proteins demonstrate that all sequences necessary for cleavage of expressed T3D at Arg245 by trypsin are contained within morphologic region T(iv) (Fig. 3 and 4). These results support the hypothesis that the isoleucine-threonine polymorphism at position 249 is the minimal determinant of T3D ς1 susceptibility to protease.
Previous studies of T3D ς1 oligomerization demonstrated that oligomer stability under conditions of SDS-PAGE is mediated by sequences corresponding to the amino-terminal half of T3D ς1 (24, 40). In agreement with these findings, baculovirus-expressed T3D ς1 tail-forming sequences representing morphologic regions T(i) through T(iv) migrated as oligomers in SDS-polyacrylamide gels (Fig. 5C and D). Interestingly, the T1L ς1 oligomer is labile under the same conditions. This difference in oligomer stability was mapped to sequences corresponding to the T(i) and T(ii) regions of T1L and T3D ς1 proteins, which is consistent with the finding that the amino-terminal 161 amino acids of T3D ς1 protein form a stable oligomer (24) and that destabilizing mutations in T3D ς1 selected during persistent reovirus infection of cultured L cells occur in the T(ii) region (51). Thus, differences in T1L and T3D ς1 oligomer stability are determined by strain-specific properties of the extended region of predicted α-helical coiled coil in the tail domain. Accordingly, T3D ς1-derived deletion mutant 3-Δ-3-3-3, in which about 75% of region T(ii) sequences are missing, migrated as a monomer under these conditions of SDS-PAGE. Since the entirety of region T(i) sequences are intact in this deletion mutant, these results suggest that region T(ii) sequences alone determine oligomer stability.
The hemagglutination characteristics of chimeric and truncated ς1 proteins used for these studies is the strongest evidence to date that morphologic region T(iii) contains the complete hemagglutination domain of type 3 ς1 protein (Fig. 6). These results agree with our previous findings indicating that the hemagglutination domain of type 3 ς1 protein is located amino terminal to Arg245 in the tail (8, 30) and that sequence polymorphism within T(iii) is a determinant of hemagglutination capacity (9, 12) and sialic acid-dependent viral infectivity (9). An intriguing finding from the domain mapping experiments was that the T1L hemagglutination pattern segregated with sequences in morphologic region T(iv). Prior to this study, no functions had been ascribed to any specific region of type 1 ς1 protein, but these results indicate that a carbohydrate-binding domain resides in the carboxy-terminal aspect of the tail.
Because a construct corresponding to sequences found only in the T1L ς1 tail was unable to mediate hemagglutination in our experimental system, it is possible that the head domain is required for proper folding of T(iv) sequences into a conformation functional for carbohydrate binding. Irregular folding of the 1-1-1-1-Δ T(iv) region is suggested by results of trypsin treatment of this construct; at higher concentrations of trypsin, a cleavage product that migrated slightly faster than untreated 1-1-1-1-Δ was produced, which is indicative of subterminal cleavage, possibly within morphologic region T(iv) (Fig. 4). The loss of T1L ς1 hemagglutination activity by truncation of head-forming sequences contrasts with the behavior of construct 3-3-3-3-Δ, in which hemagglutination activity remains fully intact (and expanded to include human erythrocytes) in the absence of a head domain. Perhaps this differential effect of the head domain on hemagglutination by type 1 and type 3 ς1 proteins reflects the relative proximity of the hemagglutination domain to the head and the potential differences in steric interactions that would follow.
Using wt and chimeric ς1 proteins in a quantitative assay of sialic acid binding, we were able to show that specific binding to sialic acid by type 3 ς1 also segregates with morphologic region T(iii) (Fig. 7). This finding confirms and extends the results of hemagglutination assays as a biologically relevant assessment of ς1 function. Sialic acid is the minimal determinant of type 3 reovirus attachment (33), and results obtained using expressed ς1, glycophorin, and asialoglycophorin indicate that virus binding to sialic acid occurs through direct interactions between this carbohydrate and sequences in morphologic region T(iii). In previous studies (9, 12), we identified three amino acid residues within a single predicted β-strand of T(iii)—Asn198, Arg202, and Pro204—that determine the capacity of reovirus to bind sialic acid and carry out sialic acid-dependent infection of cultured cells. Findings made in the present study indicate that the effect of these residues on receptor binding is a local one, confined to region T(iii), and provide support for a model in which Asn198, Arg202, and Pro204 comprise part of the ς1 sialic acid-binding domain.
Among ς1 constructs containing the T3D T(iii) region, only truncation mutant 3-3-3-3-Δ and chimeric ς1 protein 1-1-3-3-3 agglutinated human erythrocytes in addition to bovine erythrocytes (Fig. 6). Despite this observation, all constructs containing morphologic region T(iii) from T3D ς1 were able to bind sialic acid presented on human glycophorin (Fig. 7), which is the sialylated glycoprotein bound by type 3 reovirus on human erythrocytes (34). The glycophorin-binding assay was designed to test the capacity of expressed ς1 proteins to specifically bind sialic acid, and the inability of most ς1 proteins to agglutinate human erythrocytes may reflect the more complex ς1-receptor interactions that occur during hemagglutination when virion-associated ς1 molecules cross-link cell surface glycophorin, and perhaps other sialoglycoconjugates, on adjacent cells.
The capacity of constructs 3-3-3-3-Δ and 1-1-3-3-3 to agglutinate both bovine and human erythrocytes suggests that sequences in the head domain of T3D ς1 influence hemagglutination capacity of sequences in the tail and that certain combinations of sequences from T1L and T3D ς1 proteins enhance agglutination of human erythrocytes by T3D ς1-derived sequences. Alternatively, because T3D ς1 protein has a lower avidity for human than for bovine erythrocytes (9, 12), relative amounts of chimeric and truncated ς1 proteins in insect cell lysates may influence the capacity of T3D ς1-derived sequences to mediate agglutination of human erythrocytes.
We observed that sialic acid-dependent binding of glycophorin by expressed ς1 proteins spanned a range of 20-fold. This variability may be due to the heterogeneous sequence contexts of ς1 proteins containing morphologic region T(iii) of type 3 ς1, leading to differences in individual affinities for sialic acid. Although the amount of expressed ς1 protein used for the glycophorin-binding assays was not quantitated, the results of sialoglycophorin binding were normalized to asialoglycophorin binding for each construct, which should have minimized the effects of ς1 concentration differences on the binding results.
Our findings permit discrete topographical assignment of receptor-binding activities of type 3 ς1 protein to morphologic region T(iii) of the tail (this study) and the head domain (8, 14, 29, 30, 42, 50, 52) (Fig. 8). These results explain our previous findings that ISVPs of T3D containing a cleaved ς1 protein bind sialylated cellular receptors (8, 30) and that sequences in morphologic region T(iii) determine viral capacity for sialic acid binding (9). Although the two receptor-binding domains are clearly distinct in images of isolated ς1 protein (16), the steric relationship of the head and T(iii) on the virion surface is not known. The ς1 protein exhibits considerable flexibility (1, 7, 16, 17), and it is possible that sequences in these two regions of the molecule approximate one another when ς1 rests in its virion-associated conformation. If so, the relative proximity of receptor-binding domains in the head and tail may influence viral receptor specificity, stability of viral attachment, or subsequent events, such as endocytic uptake of virus and intravesicular viral disassembly. The mechanism of receptor engagement employed by type 3 ς1 protein may not be shared by type 1 ς1 since the carbohydrate-binding domain of the latter was mapped to more head-proximal sequences, morphologic region T(iv). Thus, diversity in viral attachment strategies may account for some serotype-dependent patterns of reovirus biology that segregate with ς1 protein (9, 13, 36, 38, 41, 44, 48). As higher-resolution models of ς1 structure are developed, the individual and corporate activities of ς1 receptor-binding domains will become clearer. This information will have particular import in the area of reovirus pathogenesis, where viral cell tropism at the level of receptor recognition determines the pathologic outcome of infection (13, 41, 48).
FIG. 8.
Functional domains of the reovirus attachment protein. Morphologic regions of ς1 [T(i), T(ii), T(iii), T(iv), and H] seen in computer-processed electron micrographic images of ς1 protein isolated from virions (16) are correlated with ς1 predicted secondary structure (31). (A) Type 1 ς1 protein. Tail-forming sequences adjacent to the head, morphologic region T(iv), are required for binding to carbohydrate, the nature of which has not been identified for type 1 ς1. (B) Type 3 ς1 protein. All sequences necessary to bind sialic acid are contained in a predicted region of β-sheet in the tail constituting morphologic region T(iii). The sialic acid-binding domain is discrete from other sequences located in the head that bind an unidentified receptor on L cells (8, 14, 29, 30, 42, 50, 52) and determine viral tropism in the murine central nervous system (CNS) (4, 21, 39). These two receptor-binding domains are bridged by a region of sequence, T(iv), that confers susceptibility of strain T3D ς1 to cleavage by trypsin. Filled circles denote amino acid residues Asn198, Arg202, and Pro204, which determine viral capacity for sialic acid-dependent binding and infectivity (9). An arrow denotes the predicted location of amino acid residue 249, which is the minimal determinant of ς1 susceptibility to cleavage by intestinal proteases (8). Stability of T3D ς1 oligomers in SDS-polyacrylamide gels was mapped to sequences corresponding to morphologic regions T(i) plus T(ii).
ACKNOWLEDGMENTS
This work was supported by Public Health Service Award AI38296 (J.D.C. and T.S.D.) from the National Institute of Allergy and Infectious Diseases and the Elizabeth B. Lamb Center for Pediatric Research.
We acknowledge the National Cell Culture Center for purification of monoclonal antibodies.
REFERENCES
- 1.Banerjea A C, Brechling K A, Ray C A, Erikson H, Pickup D J, Joklik W K. High-level synthesis of biologically active reovirus protein ς1 in a mammalian expression vector system. Virology. 1988;167:601–612. [PubMed] [Google Scholar]
- 2.Bassel-Duby R, Jayasuriya A, Chatterjee D, Sonenberg N, Maizel J V, Jr, Fields B N. Sequence of reovirus haemagglutinin predicts a coiled-coil structure. Nature. 1985;315:421–423. doi: 10.1038/315421a0. [DOI] [PubMed] [Google Scholar]
- 3.Bassel-Duby R, Nibert M, Homcy C, Fields B, Sawutz D. Evidence that the sigma 1 protein of reovirus serotype 3 is a multimer. J Virol. 1987;61:1834–1841. doi: 10.1128/jvi.61.6.1834-1841.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bassel-Duby R, Spriggs D R, Tyler K L, Fields B N. Identification of attenuating mutations on the reovirus type 3 S1 double-stranded RNA segment with a rapid sequencing technique. J Virol. 1986;60:64–67. doi: 10.1128/jvi.60.1.64-67.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bodkin D K, Nibert M L, Fields B N. Proteolytic digestion of reovirus in the intestinal lumens of neonatal mice. J Virol. 1989;63:4676–4681. doi: 10.1128/jvi.63.11.4676-4681.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burstin S J, Spriggs D R, Fields B N. Evidence for functional domains on the reovirus type 3 hemagglutinin. Virology. 1982;117:146–155. doi: 10.1016/0042-6822(82)90514-1. [DOI] [PubMed] [Google Scholar]
- 7.Centonze V E, Chen Y, Severson T F, Borisy G G, Nibert M L. Visualization of individual reovirus particles by low-temperature, high-resolution scanning electron microscopy. J Struct Biol. 1995;115:215–225. doi: 10.1006/jsbi.1995.1046. [DOI] [PubMed] [Google Scholar]
- 8.Chappell J D, Barton E S, Smith T H, Baer G S, Duong D T, Nibert M L, Dermody T S. Cleavage susceptibility of reovirus attachment protein ς1 during proteolytic disassembly of virions is determined by a sequence polymorphism in the ς1 neck. J Virol. 1998;72:8205–8213. doi: 10.1128/jvi.72.10.8205-8213.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chappell J D, Gunn V L, Wetzel J D, Baer G S, Dermody T S. Mutations in type 3 reovirus that determine binding to sialic acid are contained in the fibrous tail domain of viral attachment protein ς1. J Virol. 1997;71:1834–1841. doi: 10.1128/jvi.71.3.1834-1841.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chasis J A, Mohandas N. Red blood cell glycophorins. Blood. 1992;80:1869–1879. [PubMed] [Google Scholar]
- 11.Choi A H C, Paul R W, Lee P W K. Reovirus binds to multiple plasma membrane proteins of mouse L fibroblasts. Virology. 1990;178:316–320. doi: 10.1016/0042-6822(90)90412-k. [DOI] [PubMed] [Google Scholar]
- 12.Dermody T S, Nibert M L, Bassel-Duby R, Fields B N. A ς1 region important for hemagglutination by type 3 reovirus strains. J Virol. 1990;64:5173–5176. doi: 10.1128/jvi.64.10.5173-5176.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dichter M A, Weiner H L. Infection of neuronal cell cultures with reovirus mimics in vitro patterns of neurotropism. Ann Neurol. 1984;16:603–610. doi: 10.1002/ana.410160512. [DOI] [PubMed] [Google Scholar]
- 14.Duncan R, Horne D, Strong J E, Leone G, Pon R T, Yeung M C, Lee P W K. Conformational and functional analysis of the C-terminal globular head of the reovirus cell attachment protein. Virology. 1991;182:810–819. doi: 10.1016/0042-6822(91)90622-i. [DOI] [PubMed] [Google Scholar]
- 15.Duncan R, Lee P W K. Localization of two protease-sensitive regions separating distinct domains in the reovirus cell-attachment protein ς1. Virology. 1994;203:149–152. doi: 10.1006/viro.1994.1465. [DOI] [PubMed] [Google Scholar]
- 16.Fraser R D, Furlong D B, Trus B L, Nibert M L, Fields B N, Steven A C. Molecular structure of the cell-attachment protein of reovirus: correlation of computer-processed electron micrographs with sequence-based predictions. J Virol. 1990;64:2990–3000. doi: 10.1128/jvi.64.6.2990-3000.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Furlong D B, Nibert M L, Fields B N. Sigma 1 protein of mammalian reoviruses extends from the surfaces of viral particles. J Virol. 1988;62:246–256. doi: 10.1128/jvi.62.1.246-256.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gentsch J R, Pacitti A F. Effect of neuraminidase treatment of cells and effect of soluble glycoproteins on type 3 reovirus attachment to murine L cells. J Virol. 1985;56:356–364. doi: 10.1128/jvi.56.2.356-364.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Horton R M, Hunt H D, Ho S N, Pullen J K, Pease L R. Engineering hybrid genes without the use of restriction enzymes: gene splicing by overlap extension. Gene. 1989;77:61–68. doi: 10.1016/0378-1119(89)90359-4. [DOI] [PubMed] [Google Scholar]
- 20.Kauffman R S, Wolf J L, Finberg R, Trier J S, Fields B N. The ς1 protein determines the extent of spread of reovirus from the gastrointestinal tract of mice. Virology. 1983;124:403–410. doi: 10.1016/0042-6822(83)90356-2. [DOI] [PubMed] [Google Scholar]
- 21.Kaye K M, Spriggs D R, Bassel-Duby R, Fields B N, Tyler K L. Genetic basis for altered pathogenesis of an immune-selected antigenic variant of reovirus type 3 Dearing. J Virol. 1986;59:90–97. doi: 10.1128/jvi.59.1.90-97.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kowalik T F, Yang Y-Y, Li J K-K. Molecular cloning and comparative sequence analyses of bluetongue virus S1 segments by selective synthesis of specific full-length DNA copies of dsRNA genes. Virology. 1990;177:820–823. doi: 10.1016/0042-6822(90)90557-8. [DOI] [PubMed] [Google Scholar]
- 23.Lee P W K, Hayes E C, Joklik W K. Protein ς1 is the reovirus cell attachment protein. Virology. 1981;108:156–163. doi: 10.1016/0042-6822(81)90535-3. [DOI] [PubMed] [Google Scholar]
- 24.Leone G, Duncan R, Mah D C, Price A, Cashdollar L W, Lee P W K. The amino-terminal heptad repeat region of reovirus cell attachment protein ς1 is responsible for ς1 oligomer stability and possesses intrinsic oligomerization function. Virology. 1991;182:336–345. doi: 10.1016/0042-6822(91)90677-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lerner A M, Cherry J D, Finland M. Haemagglutination with reoviruses. Virology. 1963;19:58–65. doi: 10.1016/0042-6822(63)90024-2. [DOI] [PubMed] [Google Scholar]
- 26.Masri S A, Nagata L, Mah D C, Lee P W K. Functional expression in Escherichia coli of cloned reovirus S1 gene encoding the viral cell attachment protein ς1. Virology. 1986;149:83–90. doi: 10.1016/0042-6822(86)90089-9. [DOI] [PubMed] [Google Scholar]
- 27.McCrae M A, Joklik W K. The nature of the polypeptide encoded by each of the ten double-stranded RNA segments of reovirus type 3. Virology. 1978;89:578–593. doi: 10.1016/0042-6822(78)90199-x. [DOI] [PubMed] [Google Scholar]
- 28.Mustoe T A, Ramig R F, Sharpe A H, Fields B N. Genetics of reovirus: identification of the dsRNA segments encoding the polypeptides of the μ and ς size classes. Virology. 1978;89:594–604. doi: 10.1016/0042-6822(78)90200-3. [DOI] [PubMed] [Google Scholar]
- 29.Nagata L, Masri S A, Pon R T, Lee P W K. Analysis of functional domains on reovirus cell attachment protein ς1 using cloned S1 gene deletion mutants. Virology. 1987;160:162–168. doi: 10.1016/0042-6822(87)90056-0. [DOI] [PubMed] [Google Scholar]
- 30.Nibert M L, Chappell J D, Dermody T S. Infectious subvirion particles of reovirus type 3 Dearing exhibit a loss in infectivity and contain a cleaved ς1 protein. J Virol. 1995;69:5057–5067. doi: 10.1128/jvi.69.8.5057-5067.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nibert M L, Dermody T S, Fields B N. Structure of the reovirus cell-attachment protein: a model for the domain organization of ς1. J Virol. 1990;64:2976–2989. doi: 10.1128/jvi.64.6.2976-2989.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pacitti A, Gentsch J R. Inhibition of reovirus type 3 binding to host cells by sialylated glycoproteins is mediated through the viral attachment protein. J Virol. 1987;61:1407–1415. doi: 10.1128/jvi.61.5.1407-1415.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paul R W, Choi A H, Lee P W K. The α-anomeric form of sialic acid is the minimal receptor determinant recognized by reovirus. Virology. 1989;172:382–385. doi: 10.1016/0042-6822(89)90146-3. [DOI] [PubMed] [Google Scholar]
- 34.Paul R W, Lee P W K. Glycophorin is the reovirus receptor on human erythrocytes. Virology. 1987;159:94–101. doi: 10.1016/0042-6822(87)90351-5. [DOI] [PubMed] [Google Scholar]
- 35.Pelletier J, Nicholson R, Bassel-Duby R, Fields B N, Sonenberg N. Expression of reovirus type 3 Dearing ς1 and ςs polypeptides in Escherichia coli. J Gen Virol. 1987;68:135–145. doi: 10.1099/0022-1317-68-1-135. [DOI] [PubMed] [Google Scholar]
- 36.Rodgers S E, Barton E S, Oberhaus S M, Pike B, Gibson C A, Tyler K L, Dermody T S. Reovirus-induced apoptosis of MDCK cells is not linked to viral yield and is blocked by Bcl-2. J Virol. 1997;71:2540–2546. doi: 10.1128/jvi.71.3.2540-2546.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rubin D H, Weiner D B, Dworkin C, Greene M I, Maul G G, Williams W V. Receptor utilization by reovirus type 3: distinct binding sites on thymoma and fibroblast cell lines result in differential compartmentalization of virions. Microb Pathog. 1992;12:351–365. doi: 10.1016/0882-4010(92)90098-9. [DOI] [PubMed] [Google Scholar]
- 38.Rubin D H, Wetzel J D, Dworkin C, Williams W V, Cohen J A, Dermody T S. Binding of type 3 reovirus by a domain of the ς1 protein important for hemagglutination leads to infection of murine erythroleukemia cells. J Clin Investig. 1992;90:2536–2542. doi: 10.1172/JCI116147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spriggs D R, Bronson R T, Fields B N. Hemagglutinin variants of reovirus type 3 have altered central nervous system tropism. Science. 1983;220:505–507. doi: 10.1126/science.6301010. [DOI] [PubMed] [Google Scholar]
- 40.Strong J E, Leone G, Duncan R, Sharma R K, Lee P W K. Biochemical and biophysical characterization of the reovirus cell attachment protein ς1: evidence that it is a homotrimer. Virology. 1991;184:23–32. doi: 10.1016/0042-6822(91)90818-V. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tardieu M, Weiner H L. Viral receptors on isolated murine and human ependymal cells. Science. 1982;215:419–421. doi: 10.1126/science.6276976. [DOI] [PubMed] [Google Scholar]
- 42.Turner D L, Duncan R, Lee P W K. Site-directed mutagenesis of the C-terminal portion of reovirus protein ς1: evidence for a conformation-dependent receptor binding domain. Virology. 1992;186:219–227. doi: 10.1016/0042-6822(92)90076-2. [DOI] [PubMed] [Google Scholar]
- 43.Tyler K L, Fields B N. Reoviruses. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 1597–1623. [Google Scholar]
- 44.Tyler K L, McPhee D A, Fields B N. Distinct pathways of viral spread in the host determined by reovirus S1 gene segment. Science. 1986;233:770–774. doi: 10.1126/science.3016895. [DOI] [PubMed] [Google Scholar]
- 45.Virgin H W, IV, Mann M A, Fields B N, Tyler K L. Monoclonal antibodies to reovirus reveal structure/function relationships between capsid proteins and genetics of susceptibility to antibody action. J Virol. 1991;65:6772–6781. doi: 10.1128/jvi.65.12.6772-6781.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weiner H L, Ault K A, Fields B N. Interaction of reovirus with cell surface receptors. I. Murine and human lymphocytes have a receptor for the hemagglutinin of reovirus type 3. J Immunol. 1980;124:2143–2148. [PubMed] [Google Scholar]
- 47.Weiner H L, Drayna D, Averill D R, Jr, Fields B N. Molecular basis of reovirus virulence: role of the S1 gene. Proc Natl Acad Sci USA. 1977;74:5744–5748. doi: 10.1073/pnas.74.12.5744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weiner H L, Powers M L, Fields B N. Absolute linkage of virulence and central nervous system tropism of reoviruses to viral hemagglutinin. J Infect Dis. 1980;141:609–616. doi: 10.1093/infdis/141.5.609. [DOI] [PubMed] [Google Scholar]
- 49.Weiner H L, Ramig R F, Mustoe T A, Fields B N. Identification of the gene coding for the hemagglutinin of reovirus. Virology. 1978;86:581–584. doi: 10.1016/0042-6822(78)90099-5. [DOI] [PubMed] [Google Scholar]
- 50.Williams W V, Guy H R, Rubin D H, Robey F, Myers J N, Kieber-Emmons T, Weiner D B, Greene M I. Sequences of the cell-attachment sites of reovirus type 3 and its antiidiotypic/antireceptor antibody: modeling of their three-dimensional structures. Proc Natl Acad Sci USA. 1988;85:6488–6492. doi: 10.1073/pnas.85.17.6488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wilson G J, Wetzel J D, Puryear W, Bassel-Duby R, Dermody T S. Persistent reovirus infections of L cells select mutations in viral attachment protein ς1 that alter oligomer stability. J Virol. 1996;70:6598–6606. doi: 10.1128/jvi.70.10.6598-6606.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yeung M C, Lim D, Duncan R, Shahrabadi M S, Cashdollar L W, Lee P W K. The cell attachment proteins of type 1 and type 3 reovirus are differentially susceptible to trypsin and chymotrypsin. Virology. 1989;170:62–70. doi: 10.1016/0042-6822(89)90352-8. [DOI] [PubMed] [Google Scholar]