Abstract
Background
Psoriasis was long regarded as an inflammatory disease limited to the skin. Data from dermatologic, rheumatologic and cardiologic research now show it to be a systemic disease, for which the term psoriatic disease is used.
Methods
This paper is based on a selective literature search with special attention to the findings of clinical trials and other current publications, as well as the recommendations of international guidelines.
Results
Immunologically mediated inflammation of the skin, arteries, bones, and joints is a central feature of psoriatic disease. Other diseases that are known to be associated with psoriatic disease include hypertension, metabolic syndrome, and depression. The main risk factor for the development of psoriatic disease is obesity, which also increases the likelihood of psoriatic arthritis. The main known trigger factors are stress, infection, and, less commonly, medication. Psoriatic disease is characterized by complex genetics and by a characteristic pattern of inflammation that involves elements of both innate and acquired immunity and, in particular, the cytokines interleukin 17 and 23. The inflammatory processes underlying psoriatic disease can now be targeted with modern biologic and other therapies.
Conclusion
In view of the complexity of psoriatic disease, structured management is now recommended so that physicians and patients can work together to determine the optimal treatment strategy.
CME plus+
This article has been certified by the North Rhine Academy for Continuing Medical Education.
The questions on this article may be found at http://daebl.de/RY95. The closing date for entries is 11 July 2025.
Participation is possible at cme.aerztebatt.de
Psoriasis is a common, chronic, and incurable disease, affecting 2 out of every 100 people in Germany (1). It is characterized by sharply defined, red, raised plaques that are usually covered with scales. Frequently affected sites are the hairy scalp (79.9%), the extensor surfaces of the upper and lower limbs, the lumbosacral region, the genital area including the anal cleft (30.7%), and the nails (45.5%) (e1). In principle, however, any area of the skin can be affected. Itching of the lesions is common and very distressing (2).
Psoriasis is still widely thought of as being merely a skin disease. Yet people with psoriasis have long been known to suffer disproportionately from hypertension, dyslipidemia, metabolic syndrome/diabetes mellitus, and cardiovascular disease (e2). Large-scale studies have identified further comorbidities including Crohn‘s disease, depression, and metabolic fatty liver disease. One in five persons with psoriasis suffers from psoriatic arthritis (PsA) with involvement of musculoskeletal structures such as the entheses, the joints themselves, or the spine; in most such cases, rheumatoid factors or antibodies against cyclic citrullinated peptides cannot be detected. PsA was also previously classified as a comorbidity of psoriasis, rather than a component of psoriatic disease. The links between psoriasis and obesity and tobacco smoking were also recognized long ago.
These and many other findings have led to a redefinition of psoriasis. It is no longer regarded as a disease of the skin, but rather as a systemic inflammatory disease, and is therefore called psoriatic disease.
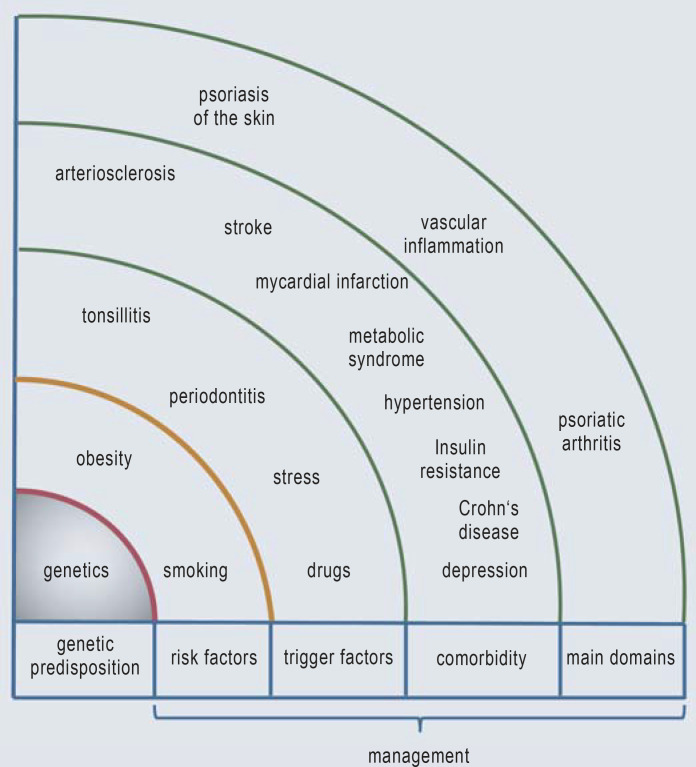
The currently known elements of psoriatic disease are summarized in Figure 1. Psoriatic disease involves inflammation in at least three organ systems (domains): the skin, the blood vessels, and the bones and joints (3).
Figure 1.
Psoriatic dsease (modified from: [3])
Common inflammatory signature
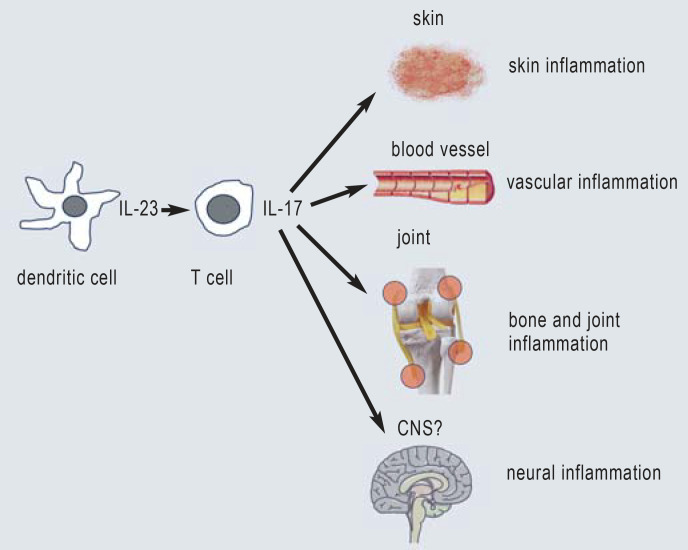
A particular signature of inflammation is common to all three domains of psoriatic disease (Figure 2). The factors leading to the development of psoriasis are still unclear but are thought to include autoantigens and signals that particularly target the epidermal keratinocytes. Central to this process is the activation of antigen-presenting dendritic cells to produce the cytokine interleukin (IL)-23 (4). IL-23 can activate T cells (CD4– and CD8-positive), which, in turn, release cytokines of the IL-17 family, the most important of which are IL-17A and IL-17F (5). Since Th17 immunity is physiologically required for the control of bacterial and mycotic infections, especially with Candida, stimulation with IL-17 cytokines represents a danger signal for keratinocytes. This leads to increased proliferation, impaired differentiation and the release of antimicrobial proteins (including β-defensin 2, LL-37) and chemokines (e.g., CXCL8, CCL20) in the epidermis. As a result, neutrophilic granulocytes are recruited; these form the typical Munro‘s microabscesses in the stratum corneum. Studies have shown that special T cell populations can form during immunological activation that are still present in the epidermis and dermis even after the skin lesions regress completely. These tissue-resident memory cells (Trm) (6) are thought to be at least partly responsible for new disease relapses (7). A current topic of study is whether a reduction in Trms through early, effective treatment can alter the natural, chronic course of the disease and the development of comorbidities. Regulatory T cells (Treg) play an important role in the inflammatory response in the skin and presumably elsewhere as well (8). Any decline in their number and/or activity promotes the inflammatory reaction.
Figure 2.
Key cell types, cytokines, and target tissues in psoriatic disease
The genetics underlying psoriatic disease is complex, and the presence of the currently known susceptibility genes does not adequately account for the development of the disease (9). A familial clustering of psoriatic disease and the findings of twin studies suggest an important genetic component. New concepts define psoriasis as an “MHC-1-opathy” (10) because of its association with HLA-C*06:02, similarly to ankylosing spondylitis (HLA-B*27) and Behçet‘s disease (HLA-B*51).
An almost identical inflammatory signature to that seen in the skin is also seen in the two other domains, i.e., the bones and joints and the blood vessels. These central cytokines are sensitive target structures for modern therapeutic agents that can be used with a highly beneficial effect (Figure 2).
Vascular inflammation in psoriatic disease
Cardiological research, in particular, has shown that psoriatic disease causes arterial inflammation, especially in the coronary arteries, along with marked inflammation of the pericoronary adipose tissue, promoting the formation of so-called non-calcifying plaques on the endothelial cells (11). This vascular inflammation arises even in younger people with psoriasis and markedly elevates the risk of both myocardial infarction (3-fold) and stroke (1.6-fold) and lowers the life expectancy (12, e3, e4). There are common genetic features (13), but these play no more than a small role in vascular inflammation (e5). Cytokines of the IL-17 family, IL-6, and TNF are especially involved in the development of the initial non-calcifying arteriosclerotic plaques. Of particular interest is an open, experimental study that showed reduced inflammation of the pericoronary adipose tissue after treatment with IL-17 inhibitors, as well as with tumor necrosis factor (TNF) or IL-12/23 inhibitors (14). In a controlled trial, treatment with an IL-17A inhibitor resulted in improved endothelial function at one year (15). Registry data also indicate a lowering of mortality by suitable systemic therapy (16).
Inflammation of musculoskeletal structures in psoriatic disease
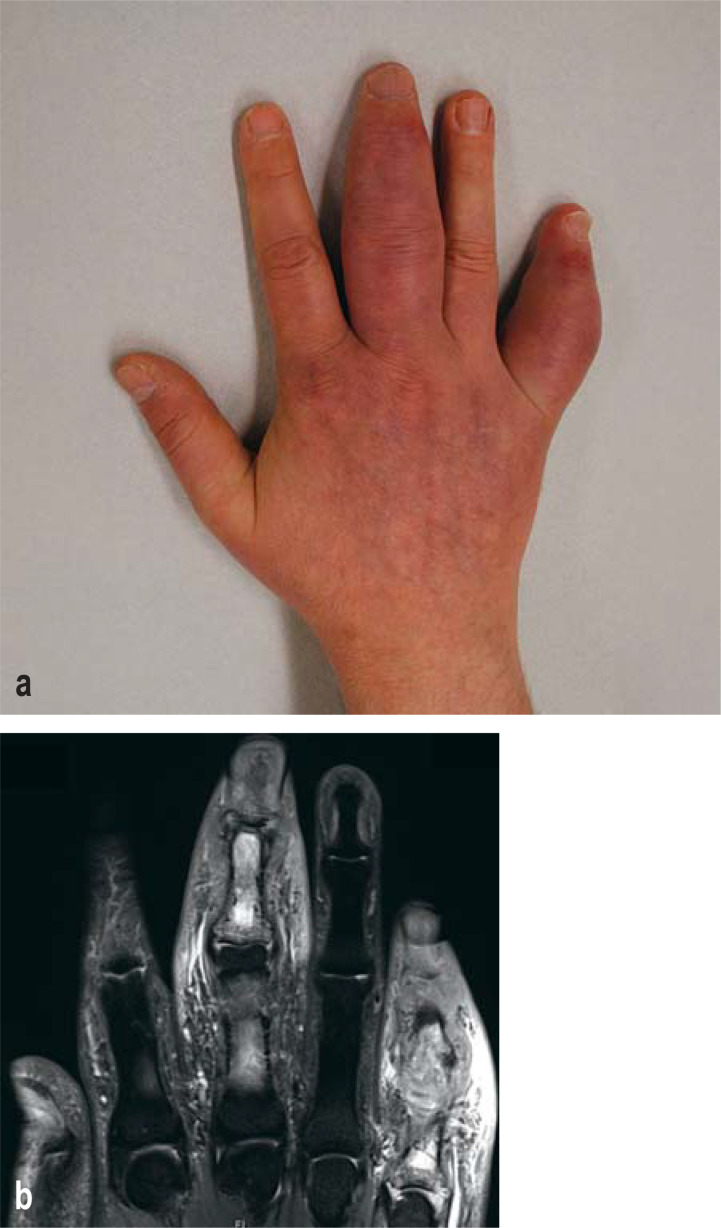
Modern imaging, especially MRI and high-resolution ultrasonography with power Doppler, has shown that people with cutaneous psoriasis and no joint symptoms already have inflammation at the points of attachment of tendons and ligaments to the bones (entheses), which can be detected regardless of the severity of the psoriatic disease (MRI [e6]; ultrasonography [e7]). Beyond the highly typical inflammation of the Achilles tendon insertion, which is sometimes misidentified as a “heel spur,” any of the well over a thousand tendon insertions on the limbs and the spine can be affected. Enthesitis apparently arises because of initial microtraumata due to mechanical stress reactions, in turn favoring the invasion of the above-mentioned cell populations along with inflammatory cytokines (17). In contrast to synovitis, which arises relatively late in the disease course, enthesitis can be a very early sign of musculoskeletal involvement. If peripheral arthritis is present, it is usually an asymmetric oligoarthritis (2–5 joints), unlike in rheumatoid arthritis, where a symmetric polyarthritis of the small joints of the fingers and in the feet is typical (e8). In dactylitis, there is marked tenosynovitis with inflammation of the subcutaneous fat, although synovitis is not necessarily present. Moreover, periosteitis is typical of, and pathognomonic for, dactylitis. This is well seen on both MRI and ultrasonography, which impressively show the massive inflammation (Illustration) (18). 25–70% of cases also show axial involvement i.e., involvement of the spine (e9). Along with inflammation of the vertebral bodies themselves (spondylitis), enthesitis is typically seen, e.g., at the insertions of the erector spinae muscles (19).
Illustration:
Psoriatic arthritis with dactylitis: a) clinical photograph; b) magnetic resonance imaging (MRI).
Comorbidity in psoriatic disease
As early as 1992, a large-scale association study showed that hypertension, type 2 diabetes mellitus, dyslipidemia, and cardiovascular diseases are important accompanying diseases of psoriasis (20). Obesity, which used to be considered a comorbidity, is now held to be an independent risk factor for psoriatic disease (e10). Other comorbidities are chronic inflammatory bowel diseases, especially Crohn‘s disease (21), metabolic fatty liver disease (22), and uveitis (e11).
Depression and anxiety disorders were once thought psychological responses to the stigmatizing skin disease but are now known to have a neuroinflammatory component (especially for depression) (3). All conditions associated with psoriasis can already occur in children (23).
The relationship between these conditions and psoriatic disease follows a similar inflammatory pattern. This is also true of inflammatory changes in the central nervous system, perhaps explaining why, in one study, 21.1% of women with psoriatic disease suffered from chronic depression (compared to 14.2% in the general population) (e12). Yet hypertension can also activate IL-17 cytokines and, in turn, be exacerbated by them (24). In Crohn‘s disease, a central role is played by IL-23, and there are genetic associations as well. An imbalance of adipokines is known to be present in psoriasis (e13).
The authors of this review assume that the comorbidities of psoriatic disease are associated with its particular inflammatory pattern and should therefore be alleviated by treatments directed against elements of the inflammatory response. Targeted therapies with inhibitors of TNF, IL-17, or IL-23 alleviate depression as well when they alleviate psoriasis (e14).
The evidence from clinical studies suggests that the alleviation of severe depression comes about not only as a secondary effect of the alleviation of the skin condition, but also through an anti-inflammatory effect in the central nervous system (25).
Triggers of psoriatic disease
Many patients with psoriatic disease state that certain situations make it worse or were the initial trigger for it. Streptococcal tonsillitis is a known trigger factor In childhood and adolescence. Infections that can be triggers in later life include periodontitis (usually associated with smoking) and HIV infection.
In adulthood, stress, particularly at work, is the most important trigger factor; it is cited by more than 90% of people with psoriasis surveyed (26). Hormonal factors may also play a role in the onset and progression of psoriasis (e15). Although drugs are no longer held to be major trigger factors (26), drug-induced psoriasis certainly does exist. Some of the drugs that can cause it are beta-blockers, ACE inhibitors, hydroxychloroquine, lithium salts, and immune checkpoint inhibitors.
Risk factors for psoriatic disease
The most important risk factor for psoriatic disease is obesity. This rule applies not only to the three major domains (skin, blood vessels, bones/joints), but also to the comorbidities. Studies on large populations have shown that obesity is still a risk factor even after all confounders have been accounted for; in other words, it is an independent risk factor for psoriatic disease. Large cohort studies of bariatric surgery from Denmark and Sweden have shown that a veer high body mass index (BMI) (above 30 and above 40, respectively) nearly doubles the risk of developing psoriasis (27, 28). Vascular inflammation is also exacerbated by obesity. Obesity has long been recognized as an important risk factor for PsA (29). All of the diseases that are associated with psoriasis are also worsened by obesity.
The pathophysiological link between obesity and psoriatic disease has been well known for many years (30). When body weight increases due to uncontrolled calorie intake, a marked inflammatory reaction occurs in the adipose tissue, mainly (but not only) in the abdomen, which alters the adipokine profile toward a metabolic phenotype (with an increase in leptin, and resistin, among others, and a decrease in adiponectin). There is also a greater release of IL-6 and TNF, in turn contributing to systemic inflammation. Aside from the influence of obesity on cytokines, cellular changes have recently been shown as well, particularly in the important Treg system, which has anti-inflammatory and regulatory properties. A diet rich in calories and long-chain fatty acids lowers number of Tregs and stimulates the formation of IL-17-producing gamma/delta T cells (31).
Beyond the well-documented effect of weight loss on all components of psoriatic disease and comorbidity, an initial report of a single case has shown that the new glucagon-like-peptide-1 (GLP-1) agonist semaglutide can alleviate cutaneous and vascular inflammation in -addition to counteracting diabetes and promoting body weight loss (32).
Psychosocial aspects of psoriatic disease
The health-related quality of life and well-being, as defined by the World Health Organization, are impaired in psoriatic disease of any severity to an extent resembling that seen in other serious diseases, including cancer (e16, e17). Stigmatization and self-stigmatization contribute to marked impairment in patients who are already suffering from depression (33). The cumulative burden of psoriatic disease can have a lasting effect, which has been called cumulative life course impairment (34). Physicians caring for patients with chronic psoriatic disease must be aware of these major psychosocial effects.
The treatment of psoriatic disease
The complexity of psoriatic disease requires an individually tailored approach, with, as a rule, long-term drug treatment to achieve as much improvement as possible in all three major domains, as well as in the comorbidities (35). Unfortunately, in Germany at present, the drugs that can bring about an asymptomatic state, or nearly so, and that can exert a broad anti-inflammatory effect are prescribed only reluctantly to most patients with psoriatic disease, and often only after the failure of so-called conventional treatment.
There is no longer any justification for the old strategy of saving the best drugs for last. According to the current guideline, biologic agents can be used for first-line therapy, particularly for severely affected patients, if conventional drugs are not expected to yield adequate relief (36). It was recently shown that patients who have had psoriasis for less than two years can be effectively treated with the IL-23p19 inhibitor guselkumab, so that they experience a longer-lasting remission (37). The current “hit hard, hit early” strategy is also based on analogous beneficial results in rheumatology and gastroenterology. Early and highly effective treatment for rheumatoid arthritis prevents irreversible joint destruction more effectively than step treatment, which begins with the mildest form of treatment and is then escalated as necessary. Especially for PsA, rapid, disease-arresting treatment is needed regardless of the severity of skin involvement. The ability to arrest the course of PsA has been shown for modern biologic agents (e18; e19), but not for conventional agents such as methotrexate or leflunomide. Moreover, methotrexate is ineffective in some PsA subtypes, such as axial or nail involvement, but continues to be prescribed for these conditions despite the contrary recommendation in the rheumatology guidelines (38). Early evidence suggests that the probability of developing PsA over the course of psoriatic disease can be lessened with biologic agents (39).
Optimal treatment adherence can only be achieved when the appropriate treatment has been chosen in a shared decision-making process (40). This involves evaluating the psoriatic disease in all of its individually relevant dimensions and identifying the patient’s goals for treatment. As a wide variety of approved treatments are available, there is room for the patient’s preferences to be taken into account. There is also an increasing amount of room for adjusting the dosage or dose intervals of biologic agents within the parameters of their approval, on the basis of the patient’s body weight, thereby improving the treatment of overweight patients and others. The overriding goal of treatment is to restore these patients’ well-being and quality of life to normal levels as far as possible. The treatment of comorbidities requires close interdisciplinary cooperation among dermatologists, primary care physicians, rheumatologists, and psychologists. Awareness of the dimensions of psoriatic disease beyond the skin lesions is essential so that physicians of multiple specialties can cooperate optimally to help their patients.
eTable. Randomized and controlled trials of relevant systemic therapies for psoriasis vulgaris*1.
| First author | Year | ClinicalTrials.gov | PASI90 response | Time of observation | Undesired events (UE) | |
| Conventional drugs and small molecules | ||||||
| Dimethy lfumarat | Mrowietz et al. (e20) | 2017 | NCT01726933 | 18.4% | 16 weeks | gatrointestional complaints, flush, lymphopenia |
| MTX s.c. | Warren et al. (e21) | 2017 | NCT02902861 | 18% | 16 weeks | myelosuppression, hepatic and renal toxicity, nausea |
| Apremilast | Papp et al. (e22) | 2015 | NCT01194219 | 9.8% | 16 weeks | gastrointestinal complaints, depression |
| Paul et al. (e23) | 2015 | NCT01232283 | 8.8% | 16 weeks | ||
| Deucravacitinib | Armstrong et al. (e24) | 2023 | NCT03624127 | 35.5% | 16 weeks | upper respiratory infections |
| Strober et al. (e25) | 2023 | NCT03611751 | 27% | 16 weeks | ||
| Anti-TNF biologic agents | ||||||
| Adalimumab | Menter et al. (e26) | 2018 | NCT00237887 | 37% | 12 weeks | elevated risk of infection, reactivation of latent tuberculosis, infusion reactions (only infliximab) |
| Certolizumab | Gottlieb et al. (e27) | 2018 |
NCT02326298 NCT02326272 |
45.9% | 16 weeks | |
| Etanercept | Papp et al. (e28) | 2005 | Nicht angegeben | 21% | 12 weeks | |
| Infliximab | Reich et al. (e29) | 2005 | NCT00106834 | 57% | 10 weeks | |
| Anti-IL17 biologic agents | ||||||
| Bimekizumab | Gordon et al. (e30) | 2021 | NCT03410992 | 91% | 16 weeks | elevated risk of infection, particularly for Candida; induction of chronic inflammatory bowel disease, rarely neutropenia |
| Brodalumab | Papp et al. (e31) | 2016 | NCT01708590 | 70.3% | 12 weeks | |
| Ixekizumab | Gordon et al. (e32) | 2016 | NCT01474512 | 70.9% | 12 weeks | |
| Secukinumab | Langley et al. (e33) | 2014 |
NCT01365455 NCT01358578 |
54.2–59.2% | 12 weeks | |
| Anti-IL12/23 biologic agents | ||||||
| Ustekinumab | Leonardi et al. (e34) | 2008 | NCT00267969 | 36.7–41.6% | 12 weeks | low risk of infection |
| Papp et al. (e35) | 2008 | NCT00307437 | 42,.%–50.9% | 12 weeks | ||
| Anti-IL23 biologic agents | ||||||
| Guselkumab | Blauvelt et al. (e36) | 2017 | NCT02207231 | 73.3% | 16 weeks | low risk of infection |
| Reich et al. (e37) | 2017 | NCT02207244 | 70.0% | 16 weeks | ||
| Risankizumab | Gordon et al. (e38) | 2018 |
NCT02684370 NCT02684357 |
74.8–75.3% | 16 weeks | |
| Tildrakizumab*2 | Reich et al. (e39) | 2017 |
NCT01722331 NCT01729754 |
35.0–39.0% | 12 weeks | |
*1 The key approval studies in adult patients with moderate to severe psoriasis vulgaris are listed. The clinical endpoint is the percentage achievement of a 90% reduction in the Psoriasis Area and Severity Index (PASI) after the induction phase. A PASI90 response corresponds to lesion-free or almost lesion-free skin.
*2 Marked discrepancy in the PASI90 response between the RCT and real-world data (50% PASI90 after 16 weeks, e.g. in the Austrian Psoriasis Registry (e40)).
s.c.: subkutan
Questions on the article.
Psoriasis as a Systemic Disease
The submission deadline is 11 July 2025. Only one answer is possible per question. Please select the answer that is most appropriate.
Question 1
Approximately what percentage of the German population suffers from psoriasis?
0.1%
2%
10%
20%
35%
Question 2
As described in this article, what areas of the skin are commonly affected by the typical red, raised plaques of psoriasis?
the hairy scalp, natal cleft, and nails
the upper back, scalp, and palms
the dorsal surfaces of the feet, the chest, and the abdomen
the face, chest, and soles
the nails, soles, and upper back
Question 3
As described in this article, what are the three main domains for inflammation in psoriatic disease?
the skin, lungs, and eyes
the mucous membranes, blood vessels, and glands
the joints, mucous membranes, and glands
the skin, blood vessels, and joints
the skin, lungs, and glands
Question 4
What interleukins play a central role in the inflammatory reaction that is characteristic of psoriatic disease?
IL-18 and IL-11
IL-17 and IL-23
IL-13 and IL-27
IL-9 and IL-7
IL-3 and IL-13
Question 5
What does the abbreviation Trm in the article stand for?
t-cell relayed memory
therapy-resistant memory cells
tissue-resident memory cells
t-cell regulated memory
tissue-relayed memory
Question 6
People with psoriatic disease have a higher risk of developing certain other diseases. Which of the following elevated risks are mentioned in the article?
gastric ulcer (double the risk)
cystic fibrosis (risk three times as high)
polycystic ovarian syndrome (risk five times as high)
myocardial infarction (risk as high)
blindness (risk five times as high)
Question 7
According to the article, what disease of children is a frequent trigger for the onset or worsening of psoriasis?
varicella-zoster infection (chickenpox)
influenza infection
infectious mononucleosis
three-day fever (HHV 6 infection)
streptococcal tonsillitis
Question 8
What do 90% of adult patients with psoriatic disease say is the main trigger factor for their disease?
stress (mainly at work)
sleep deprivation
high ambient temperatures (sweating)
a high-protein diet
contact with chlorinated water, e.g., in swimming pools
Question 9
According to the text, which of the following is an independent risk factor for psoriatic disease?
hypertension
depression
obesity
migraine
anxiety disorder
Question 10
What treatment is recommended in this article for patients suffering from severe psoriasis, and especially psoriatic arthritis, in accordance with a hit-hard-and-early strategy, in case conventional treatments are not expected to yield adequate relief?
step treatment (mildest treatment at first, then escalation if necessary)
first-line treatment with biologic agents
first-line treatment with methotrexate
first-line treatment with leflunomide
first-line treatment with corticosteroids
Acknowledgments
Translated from the original German by Ethan Taub, M.D.
Footnotes
Conflict of interest statement
SG has received research funding from Almirall-Hermal and Amgen. He has served as a paid consultant for AbbVie, Almirall-Hermal, Amgen, Bristol-Myers Squibb, Boehringer-Ingelheim, Celgene, Eli Lilly, Janssen-Cilag, Klinge Pharma, Leo Pharma, Neubourg Skin Care GmbH, Novartis, Pfizer, and UCB. He has received lecture honoraria from AbbVie, Almirall-Hermal, Amgen, Biogen Idec, Bristol-Myers Squibb, Boehringer-Ingelheim, Celgene, Eli Lilly, Hexal AG, Janssen-Cilag, Leo Pharma, Medac, Neuborg Skin Care GmbH, Novartis, Pfizer, Sandoz Biopharmaceuticals, and UCB-Pharma, and reimbursement of travel expenses and meeting participation fees from AbbVie, Almirall-Hermal, Bristol-Myers Squibb, Boehringer-Ingelheim, Celgene, Eli Lilly, Janssen-Cilag, Leo Pharma, Novartis, Pfizer, and UCB Pharma. He serves on the advisory boards of AbbVie, Almirall-Hermal, Amgen, Bristol-Myers Squibb, Boehringer-Ingelheim, Celgene, Eli Lilly, Janssen-Cilag, Klinge Pharma, Leo Pharma, Neuborg Skin Care GmbH, Novartis, Pfizer, and UCB.
FL has received financial support from Novartis, Galapagos, MorphoSys, Almirall, Bristol-Myers-Squibb, AbbVie, Lilly, Janssen, and Amgen. He has received honoraria for lectures, presentations, manuscript preparation, and continuing medical education events from AbbVie, Novartis, LEO Pharma, Lilly, Almirall, Janssen, Amgen, UCB, Bristol-Myers-Squibb, and Biogen. He has received reimbursement of travel expenses and meeting participation fees from Janssen and UCB. He serves on the advisory boards of AbbVie, Novartis, LEO Pharma, Lilly, Almirall, Janssen, Amgen, UCB, Bristol-Myers-Squibb, and Union Therapeutics.
UM is a paid consultant for AbbVie, Almirall, Amgen, Biogen, Boehringer-Ingelheim, Bristol-Myers Squibb, Eli Lilly, Formycin, Janssen-Cilag, LEO Pharma, Merck, Sharp & Dohme, Novartis, and UCB Pharma. He has received honoraria for continuing medical education events from AbbVie, Almirall, Amgen, Boehringer-Ingelheim, Biogen, Eli Lilly, Janssen-Cilag, Merck, Sharp & Dohme, Novartis, and UCB Pharma. He has received reimbursement of travel expenses and meeting participation fees from AbbVie, Almirall, Amgen, Biogen, Boehringer-Ingelheim, Eli Lilly, Janssen-Cilag, Novartis, and UCB Pharma. He serves on the advisory boards of Almirall, Amgen, Eli Lilly, Formycon, LEO Pharma, Novartis, UCB Pharma, and UNION Therapeutics.
PS is a paid consultant for AXIOM Health, AMGEN, AbbVie, Astra Zeneca, Biogen, Bristol-Myers Squibb, Boehringer Ingelheim, Celgene, Celltrion, Chugai Pharma Marketing Ltd, Deutscher Psoriasis-Bund, Fresenius Kabi, Gilead Sciences, Galapagos Pharma, Hexal Pharma, Janssen-Cilag, Johnson & Johnson, Lilly, medi-login, Medac, Mediri GmbH, Novartis Pharma, Onkowissen GmbH, Pfizer, Roche Pharma, Rheumazentrum Rhein-Ruhr, Sanofi-Genzyme, Swedish Orphan Biovitrum, and UCB Pharma. He serves on the advisory board of AMGEN, AbbVie, Biogen, Bristol-Myers Squibb, Gilead Sciences, Hexal Pharma, Janssen-Cilag, Johnson & Johnson, Lilly, Mediri GmbH, Novartis Pharma, Onkowissen GmbH, Pfizer, Roche Pharma, Sanofi-Genzyme, and UCB Pharma.
WS is a paid consultant for AbbVie, Almirall, Amgen, Bristol-Myers Squibb, Boehringer-Ingelheim, Janssen, LEO Pharma, Lilly, Novartis, Pfizer, Sanofi Genzyme, and UCB. She has received honoraria for continuing medical education events from AbbVie, Almirall, Amgen, Bristol-Myers Squibb, Boehringer-Ingelheim, Janssen, LEO Pharma, Lilly, Novartis, Pfizer, Sanofi Genzyme, and UCB. She has received reimbursement of travel expenses from AbbVie, Almirall, Amgen, Bristol-Myers Squibb, Boehringer-Ingelheim, Janssen, LEO Pharma, Lilly, Novartis, Pfizer, Sanofi Genzyme, and UCB. She serves on the advisory boards of AbbVie, Almirall, Amgen, Bristol-Myers Squibb, Boehringer-Ingelheim, Janssen, LEO Pharma, Lilly, Novartis, Pfizer, Sanofi Genzyme, and UCB. She receives writing support from Almirall, Boehringer-Ingelheim, LEO Pharma, and medi GmbH Bayreuth.
References
- 1.Hagenstrom K, Muller K, Garbe C, Augustin M. Prevalence of psoriasis and psoriatic arthritis in Germany—analysis of claims data. J Dtsch Dermatol Ges. 2024;22:45–54. doi: 10.1111/ddg.15269. [DOI] [PubMed] [Google Scholar]
- 2.van de Kerkhof PC, Reich K, Kavanaugh A, et al. Physician perspectives in the management of psoriasis and psoriatic arthritis: results from the population-based Multinational Assessment of Psoriasis and Psoriatic Arthritis survey. J Eur Acad Dermatol Venereol. 2015;29:2002–2010. doi: 10.1111/jdv.13150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mrowietz U, Sümbül M, Gerdes S. Depression, a major comorbidity of psoriatic disease, is caused by metabolic inflammation. J Eur Acad Dermatol Venereol. 2023;37:1731–1738. doi: 10.1111/jdv.19192. [DOI] [PubMed] [Google Scholar]
- 4.Griffiths CEM, Armstrong AW, Gudjonsson JE, Barker J. Psoriasis. Lancet. 2021;397:1301–1315. doi: 10.1016/S0140-6736(20)32549-6. [DOI] [PubMed] [Google Scholar]
- 5.Prinz I, Sandrock I, Mrowietz U. Interleukin-17 cytokines: Effectors and targets in psoriasis—a breakthrough in understanding and treatment. J Exp Med. 2020:217. doi: 10.1084/jem.20191397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheuk S, Wikén M, Blomqvist L, et al. Epidermal Th22 and Tc17 cells form a localized disease memory in clinically healed psoriasis. J Immunol. 2014;192:3111–3120. doi: 10.4049/jimmunol.1302313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Puig L, Costanzo A, Muñoz-Elías EJ, et al. The biological basis of disease recurrence in psoriasis: a historical perspective and current models. Br J Dermatol. 2022;186:773–781. doi: 10.1111/bjd.20963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim J, Moreno A, Krueger JG. The imbalance between Type 17 T-cells and regulatory immune cell subsets in psoriasis vulgaris. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.1005115. 1005115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kocaaga A, Kocaaga M. Psoriasis: An immunogenetic perspective. Glob Med Genet. 2022;9:82–89. doi: 10.1055/s-0042-1743259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuiper JJ, Prinz JC, Stratikos E, et al. EULAR study group on ‘MHC-I-opathy‘: identifying disease-overarching mechanisms across disciplines and borders. Ann Rheum Dis. 2023;82:887–896. doi: 10.1136/ard-2022-222852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou W, Abdelrahman KM, Dey AK, et al. Association among noncalcified coronary burden, fractional flow reserve, and myocardial injury in psoriasis. J Am Heart Assoc. 2020;9 doi: 10.1161/JAHA.119.017417. e017417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gelfand JM, Neimann AL, Shin DB, Wang X, Margolis DJ, Troxel AB. Risk of myocardial infarction in patients with psoriasis. Jama. 2006;296:1735–1741. doi: 10.1001/jama.296.14.1735. [DOI] [PubMed] [Google Scholar]
- 13.Patrick MT, Li Q, Wasikowski R, et al. Shared genetic risk factors and causal association between psoriasis and coronary artery disease. Nat Commun. 2022;13:6565. doi: 10.1038/s41467-022-34323-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elnabawi YA, Oikonomou EK, Dey AK, et al. Association of biologic therapy with coronary inflammation in patients with psoriasis as assessed by perivascular fat attenuation index. JAMA Cardiol. 2019;4:885–891. doi: 10.1001/jamacardio.2019.2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.von Stebut E, Reich K, Thaçi D, et al. Impact of secukinumab on endothelial dysfunction and other cardiovascular disease parameters in psoriasis patients over 52 weeks. J Invest Dermatol. 2019;139:1054–1062. doi: 10.1016/j.jid.2018.10.042. [DOI] [PubMed] [Google Scholar]
- 16.Langley RG, Poulin Y, Srivastava B, et al. Reduced risk of mortality associated with systemic psoriasis treatment in the Psoriasis Longitudinal Assessment and Registry (PSOLAR): a nested case-control analysis. J Am Acad Dermatol. 2021;84:60–69. doi: 10.1016/j.jaad.2020.08.032. [DOI] [PubMed] [Google Scholar]
- 17.Schett G, Rahman P, Ritchlin C, McInnes IB, Elewaut D, Scher JU. Psoriatic arthritis from a mechanistic perspective. Nat Rev Rheumatol. 2022;18:311–325. doi: 10.1038/s41584-022-00776-6. [DOI] [PubMed] [Google Scholar]
- 18.Girolimetto N, Giovannini I, Crepaldi G, et al. Psoriatic dactylitis: current perspectives and new insights in ultrasonography and magnetic resonance imaging. J Clin Med. 2021;10:2604. doi: 10.3390/jcm10122604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gottlieb AB, Merola JF. Axial psoriatic arthritis: An update for dermatologists. J Am Acad Dermatol. 2021;84:92–101. doi: 10.1016/j.jaad.2020.05.089. [DOI] [PubMed] [Google Scholar]
- 20.Henseler T, Christophers E. Disease concomitance in psoriasis. J Am Acad Dermatol. 1995;32:982–986. doi: 10.1016/0190-9622(95)91336-x. [DOI] [PubMed] [Google Scholar]
- 21.Najarian DJ, Gottlieb AB. Connections between psoriasis and Crohn‘s disease. J Am Acad Dermatol. 2003;48:805–821. doi: 10.1067/mjd.2003.540. quiz 22-4. [DOI] [PubMed] [Google Scholar]
- 22.Bellinato F, Gisondi P, Mantovani A, Girolomoni G, Targher G. Risk of non-alcoholic fatty liver disease in patients with chronic plaque psoriasis: an updated systematic review and meta-analysis of observational studies. J Endocrinol Invest. 2022;45:1277–1288. doi: 10.1007/s40618-022-01755-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Augustin M, Glaeske G, Radtke MA, Christophers E, Reich K, Schäfer I. Epidemiology and comorbidity of psoriasis in children. Br J Dermatol. 2010;162:633–636. doi: 10.1111/j.1365-2133.2009.09593.x. [DOI] [PubMed] [Google Scholar]
- 24.Davis GK, Fehrenbach DJ, Madhur MS. Interleukin 17A: key player in the pathogenesis of hypertension and a potential therapeutic target. Curr Hypertens Rep. 2021;23:13. doi: 10.1007/s11906-021-01128-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wittenberg GM, Stylianou A, Zhang Y, et al. Effects of immunomodulatory drugs on depressive symptoms: a mega-analysis of randomized, placebo-controlled clinical trials in inflammatory disorders. Mol Psychiatry. 2020;25:1275–1285. doi: 10.1038/s41380-019-0471-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mrowietz U, Dieckmann T, Gerdes S, Szymczak S, von Spreckelsen R, Körber A. ActiPso: definition of activity types for psoriatic disease: a novel marker for an advanced disease classification. J Eur Acad Dermatol Venereol. 2021;35:2027–2033. doi: 10.1111/jdv.17434. [DOI] [PubMed] [Google Scholar]
- 27.Maglio C, Peltonen M, Rudin A, Carlsson LMS. Bariatric surgery and the incidence of psoriasis and psoriatic arthritis in the swedish obese subjects study. Obesity (Silver Spring) 2017;25:2068–2073. doi: 10.1002/oby.21955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Egeberg A, Sørensen JA, Gislason GH, Knop FK, Skov L. Incidence and prognosis of psoriasis and psoriatic arthritis in patients undergoing bariatric surgery. JAMA Surg. 2017;152:344–349. doi: 10.1001/jamasurg.2016.4610. [DOI] [PubMed] [Google Scholar]
- 29.Zabotti A, De Marco G, Gossec L, et al. EULAR points to consider for the definition of clinical and imaging features suspicious for progression from psoriasis to psoriatic arthritis. Ann Rheum Dis. 2023;82:1162–1170. doi: 10.1136/ard-2023-224148. [DOI] [PubMed] [Google Scholar]
- 30.Vata D, Tarcau BM, Popescu IA, et al. Update on obesity in psoriasis patients. Life (Basel) 2023;13:1947. doi: 10.3390/life13101947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sivasami P, Elkins C, Diaz-Saldana PP, et al. Obesity-induced dysregulation of skin-resident PPARγ(+) Treg cells promotes IL-17A-mediated psoriatic inflammation. Immunity. 2023;56:1844–1861. doi: 10.1016/j.immuni.2023.06.021. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Malavazos AE, Meregalli C, Sorrentino F, et al. Semaglutide therapy decreases epicardial fat inflammation and improves psoriasis severity in patients affected by abdominal obesity and type-2 diabetes. Endocrinol Diabetes Metab Case Rep. 2023;2023 doi: 10.1530/EDM-23-0017. 23–0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sommer R, Topp J, Mrowietz U, Zander N, Augustin M. Perception and determinants of stigmatization of people with psoriasis in the German population. J Eur Acad Dermatol Venereol. 2020;34:2846–2855. doi: 10.1111/jdv.16436. [DOI] [PubMed] [Google Scholar]
- 34.Kimball AB, Gieler U, Linder D, Sampogna F, Warren RB, Augustin M. Psoriasis: is the impairment to a patient‘s life cumulative? J Eur Acad Dermatol Venereol. 2010;24:989–1004. doi: 10.1111/j.1468-3083.2010.03705.x. [DOI] [PubMed] [Google Scholar]
- 35.Mrowietz U, Steinz K, Gerdes S. Psoriasis: to treat or to manage? Exp Dermatol. 2014;23:705–709. doi: 10.1111/exd.12437. [DOI] [PubMed] [Google Scholar]
- 36.Nast A, Altenburg A, Augustin M, et al. Deutsche S3-Leitlinie zur Therapie der Psoriasis vulgaris, adaptiert von EuroGuiDerm—Teil 1: Therapieziele und Therapieempfehlungen. J Dtsch Dermatol Ges. 2021;19:934–951. doi: 10.1111/ddg.14508_g. [DOI] [PubMed] [Google Scholar]
- 37.Schäkel K, Reich K, Asadullah K, et al. Early disease intervention with guselkumab in psoriasis leads to a higher rate of stable complete skin clearance (‚clinical super response‘): Week 28 results from the ongoing phase IIIb randomized, double-blind, parallel-group, GUIDE study. J Eur Acad Dermatol Venereol. 2023;37:2016–2027. doi: 10.1111/jdv.19236. [DOI] [PubMed] [Google Scholar]
- 38.Coates LC, Soriano ER, Corp N, et al. Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA): updated treatment recommendations for psoriatic arthritis 2021. Nat Rev Rheumatol. 2022;18:465–479. doi: 10.1038/s41584-022-00798-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Acosta Felquer ML, LoGiudice L, Galimberti ML, Rosa J, Mazzuoccolo L, Soriano ER. Treating the skin with biologics in patients with psoriasis decreases the incidence of psoriatic arthritis. Ann Rheum Dis. 2022;81:74–79. doi: 10.1136/annrheumdis-2021-220865. [DOI] [PubMed] [Google Scholar]
- 40.Yee D, Kingston P, Lee K, et al. Shared decision-making and satisfaction with care in patients with psoriasis: A population-based study in the United States. J Am Acad Dermatol. 2023;89:920–926. doi: 10.1016/j.jaad.2023.03.039. [DOI] [PubMed] [Google Scholar]
- E1.Piaserico S, Riedl E, Pavlovsky L, et al. Comparative effectiveness of biologics for patients with moderate-to-severe psoriasis and special area involvement: week 12 results from the observational Psoriasis Study of Health Outcomes (PSoHO) Front Med (Lausanne) 2023;10 doi: 10.3389/fmed.2023.1185523. 1185523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E2.Henseler T, Christophers E. Disease concomitance in psoriasis. J Am Acad Dermatol. 1995;32:982–986. doi: 10.1016/0190-9622(95)91336-x. [DOI] [PubMed] [Google Scholar]
- E3.Samarasekera EJ, Neilson JM, Warren RB, Parnham J, Smith CH. Incidence of cardiovascular disease in individuals with psoriasis: a systematic review and meta-analysis. J Invest Dermatol. 2013;133:2340–2346. doi: 10.1038/jid.2013.149. [DOI] [PubMed] [Google Scholar]
- E4.Svedbom A, Stahle M. The psoriasis area and severity index is an independent risk factor for cardiovascular events: a prospective register study. J Eur Acad Dermatol Venereol. 2023;37:1841–1847. doi: 10.1111/jdv.19168. [DOI] [PubMed] [Google Scholar]
- E5.Gao N, Kong M, Li X, et al. The association between psoriasis and risk of cardiovascular disease: a mendelian randomization analysis. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.918224. 918224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E6.Faustini F, Simon D, Oliveira I, et al. Subclinical joint inflammation in patients with psoriasis without concomitant psoriatic arthritis: a cross-sectional and longitudinal analysis. Ann Rheum Dis. 2016;75:2068–2074. doi: 10.1136/annrheumdis-2015-208821. [DOI] [PubMed] [Google Scholar]
- E7.Chen ZT, Chen RF, Li XL, et al. The role of ultrasound in screening subclinical psoriatic arthritis in patients with moderate to severe psoriasis. Eur Radiol. 2023;33:3943–3953. doi: 10.1007/s00330-023-09493-4. [DOI] [PubMed] [Google Scholar]
- E8.Braun J, Wassenberg S. [Outcome parameters for use in psoriatic arthritis] Z Rheumatol. 2006;65:110. doi: 10.1007/s00393-006-0046-3. 2-8, 20-3. [DOI] [PubMed] [Google Scholar]
- E9.Gladman DD. Axial disease in psoriatic arthritis. Curr Rheumatol Rep. 2007;9:455–460. doi: 10.1007/s11926-007-0074-2. [DOI] [PubMed] [Google Scholar]
- E10.Wolk K, Mallbris L, Larsson P, Rosenblad A, Vingård E, Stahle M. Excessive body weight and smoking associates with a high risk of onset of plaque psoriasis. Acta Derm Venereol. 2009;89:492–497. doi: 10.2340/00015555-0711. [DOI] [PubMed] [Google Scholar]
- E11.Kim BR, Choi SW, Choi CW, et al. Risk of uveitis in patients with psoriasis in Korea: a nationwide population-based cohort study. J Eur Acad Dermatol Venereol. 2023;37:1336–1343. doi: 10.1111/jdv.19060. [DOI] [PubMed] [Google Scholar]
- E12.Duvetorp A, Mrowietz U, Nilsson M, Seifert O. Sex and age influence the associated risk of depression in patients with psoriasis: a retrospective population study based on diagnosis and drug-use. Dermatology. 2021;237:595–602. doi: 10.1159/000509732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E13.Gerdes S, Osadtschy S, Rostami-Yazdi M, Buhles N, Weichenthal M, Mrowietz U. Leptin, adiponectin, visfatin and retinol-binding protein-4—mediators of comorbidities in patients with psoriasis? Exp Dermatol. 2012;21:43–47. doi: 10.1111/j.1600-0625.2011.01402.x. [DOI] [PubMed] [Google Scholar]
- E14.Timis TL, Beni L, Mocan T, Florian IA, Orasan RI. Biologic therapies decrease disease severity and improve depression and anxiety symptoms in psoriasis patients. Life (Basel) 2023;13:1219. doi: 10.3390/life13051219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E15.Ceovic R, Mance M, Bukvic Mokos Z, et al. Psoriasis: female skin changes in various hormonal stages throughout life—puberty, pregnancy, and menopause. Biomed Res Int. 2013;2013 doi: 10.1155/2013/571912. 571912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E16.Rapp SR, Feldman SR, Exum ML, Fleischer AB, Jr, Reboussin DM. Psoriasis causes as much disability as other major medical diseases. J Am Acad Dermatol. 1999;41:401–407. doi: 10.1016/s0190-9622(99)70112-x. [DOI] [PubMed] [Google Scholar]
- E17.Sommer R, Westphal L, Mrowietz U, Gerdes S, Augustin M. Measuring well-being in psoriasis: psychometric properties of the WHO-5 questionnaire. J Eur Acad Dermatol Venereol. 2022;36:e986–e987. doi: 10.1111/jdv.18396. [DOI] [PubMed] [Google Scholar]
- E18.Deodhar A, Helliwell PS, Boehncke WH, et al. Guselkumab in patients with active psoriatic arthritis who were biologic-naive or had previously received TNFalpha inhibitor treatment (DISCOVER-1): a double-blind, randomised, placebo-controlled phase 3 trial. Lancet. 2020;395:1115–1125. doi: 10.1016/S0140-6736(20)30265-8. [DOI] [PubMed] [Google Scholar]
- E19.McInnes IB, Asahina A, Coates LC, et al. Bimekizumab in patients with psoriatic arthritis, naive to biologic treatment: a randomised, double-blind, placebo-controlled, phase 3 trial (BE OPTIMAL) Lancet. 2023;401:25–37. doi: 10.1016/S0140-6736(22)02302-9. [DOI] [PubMed] [Google Scholar]
- E20.Mrowietz U, Szepietowski JC, Loewe R, et al. Efficacy and safety of LAS41008 (dimethyl fumarate) in adults with moderate-to-severe chronic plaque psoriasis: a randomized, double-blind, Fumaderm(®)—and placebo-controlled trial (BRIDGE) Br J Dermatol. 2017;176:615–623. doi: 10.1111/bjd.14947. [DOI] [PubMed] [Google Scholar]
- E21.Warren RB, Mrowietz U, von Kiedrowski R, et al. An intensified dosing schedule of subcutaneous methotrexate in patients with moderate to severe plaque-type psoriasis (METOP): a 52 week, multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389:528–537. doi: 10.1016/S0140-6736(16)32127-4. [DOI] [PubMed] [Google Scholar]
- E22.Papp K, Reich K, Leonardi CL, et al. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: Results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM] 1) J Am Acad Dermatol. 2015;73:37–49. doi: 10.1016/j.jaad.2015.03.049. [DOI] [PubMed] [Google Scholar]
- E23.Paul C, Cather J, Gooderham M, et al. Efficacy and safety of apremilast, an oral phosphodiesterase 4 inhibitor, in patients with moderate-to-severe plaque psoriasis over 52 weeks: a phase III, randomized controlled trial (ESTEEM 2) Br J Dermatol. 2015;173:1387–1399. doi: 10.1111/bjd.14164. [DOI] [PubMed] [Google Scholar]
- E24.Armstrong AW, Gooderham M, Warren RB, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, placebo-controlled phase 3 POETYK PSO-1 trial. J Am Acad Dermatol. 2023;88:29–39. doi: 10.1016/j.jaad.2022.07.002. [DOI] [PubMed] [Google Scholar]
- E25.Strober B, Thaçi D, Sofen H, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: Efficacy and safety results from the 52-week, randomized, double-blinded, phase 3 Program fOr Evaluation of TYK2 inhibitor psoriasis second trial. J Am Acad Dermatol. 2023;88:40–51. doi: 10.1016/j.jaad.2022.08.061. [DOI] [PubMed] [Google Scholar]
- E26.Menter A, Tyring SK, Gordon K, et al. Adalimumab therapy for moderate to severe psoriasis: a randomized, controlled phase III trial. J Am Acad Dermatol. 2008;58:106–115. doi: 10.1016/j.jaad.2007.09.010. [DOI] [PubMed] [Google Scholar]
- E27.Gottlieb AB, Blauvelt A, Thaçi D, et al. Certolizumab pegol for the treatment of chronic plaque psoriasis: results through 48 weeks from 2 phase 3, multicenter, randomized, double-blinded, placebo-controlled studies (CIMPASI-1 and CIMPASI-2) J Am Acad Dermatol. 2018;79:302–314. doi: 10.1016/j.jaad.2018.04.012. e6. [DOI] [PubMed] [Google Scholar]
- E28.Papp KA, Tyring S, Lahfa M, et al. A global phase III randomized controlled trial of etanercept in psoriasis: safety, efficacy, and effect of dose reduction. Br J Dermatol. 2005;152:1304–1312. doi: 10.1111/j.1365-2133.2005.06688.x. [DOI] [PubMed] [Google Scholar]
- E29.Reich K, Nestle FO, Papp K, et al. Infliximab induction and maintenance therapy for moderate-to-severe psoriasis: a phase III, multicentre, double-blind trial. Lancet. 2005;366:1367–1374. doi: 10.1016/S0140-6736(05)67566-6. [DOI] [PubMed] [Google Scholar]
- E30.Gordon KB, Foley P, Krueger JG, et al. Bimekizumab efficacy and safety in moderate to severe plaque psoriasis (BE READY): a multicentre, double-blind, placebo-controlled, randomised withdrawal phase 3 trial. Lancet. 2021;397:475–486. doi: 10.1016/S0140-6736(21)00126-4. [DOI] [PubMed] [Google Scholar]
- E31.Papp KA, Reich K, Paul C, et al. A prospective phase III, randomized, double-blind, placebo-controlled study of brodalumab in patients with moderate-to-severe plaque psoriasis. Br J Dermatol. 2016;175:273–286. doi: 10.1111/bjd.14493. [DOI] [PubMed] [Google Scholar]
- E32.Gordon KB, Blauvelt A, Papp KA, et al. Phase 3 trials of Ixekizumab in moderate-to-severe plaque psoriasis. N Engl J Med. 2016;375:345–356. doi: 10.1056/NEJMoa1512711. [DOI] [PubMed] [Google Scholar]
- E33.Langley RG, Elewski BE, Lebwohl M, et al. Secukinumab in plaque psoriasis–results of two phase 3 trials. N Engl J Med. 2014;371:326–338. doi: 10.1056/NEJMoa1314258. [DOI] [PubMed] [Google Scholar]
- E34.Leonardi CL, Kimball AB, Papp KA, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 1) Lancet. 2008;371:1665–1674. doi: 10.1016/S0140-6736(08)60725-4. [DOI] [PubMed] [Google Scholar]
- E35.Papp KA, Langley RG, Lebwohl M, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 52-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 2) Lancet. 2008;371:1675–1684. doi: 10.1016/S0140-6736(08)60726-6. [DOI] [PubMed] [Google Scholar]
- E36.Blauvelt A, Papp KA, Griffiths CE, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the continuous treatment of patients with moderate to severe psoriasis: results from the phase III, double-blinded, placebo- and active comparator-controlled VOYAGE 1 trial. J Am Acad Dermatol. 2017;76:405–417. doi: 10.1016/j.jaad.2016.11.041. [DOI] [PubMed] [Google Scholar]
- E37.Reich K, Armstrong AW, Foley P, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the treatment of patients with moderate to severe psoriasis with randomized withdrawal and retreatment: results from the phase III, double-blind, placebo- and active comparator-controlled VOYAGE 2 trial. J Am Acad Dermatol. 2017;76:418–431. doi: 10.1016/j.jaad.2016.11.042. [DOI] [PubMed] [Google Scholar]
- E38.Gordon KB, Strober B, Lebwohl M, et al. Efficacy and safety of risankizumab in moderate-to-severe plaque psoriasis (UltIMMa-1 and UltIMMa-2): results from two double-blind, randomised, placebo-controlled and ustekinumab-controlled phase 3 trials. Lancet. 2018;392:650–661. doi: 10.1016/S0140-6736(18)31713-6. [DOI] [PubMed] [Google Scholar]
- E39.Reich K, Papp KA, Blauvelt A, et al. Tildrakizumab versus placebo or etanercept for chronic plaque psoriasis (reSURFACE 1 and reSURFACE 2): results from two randomised controlled, phase 3 trials. Lancet. 2017;390:276–288. doi: 10.1016/S0140-6736(17)31279-5. [DOI] [PubMed] [Google Scholar]
- E40.Graier T, Weger W, Jonak C, et al. Real-world effectiveness of anti-interleukin-23 antibodies in chronic plaque-type psoriasis of patients from the Austrian Psoriasis Registry (PsoRA) Sci Rep. 2022;12 doi: 10.1038/s41598-022-18790-9. 15078. [DOI] [PMC free article] [PubMed] [Google Scholar]