Abstract
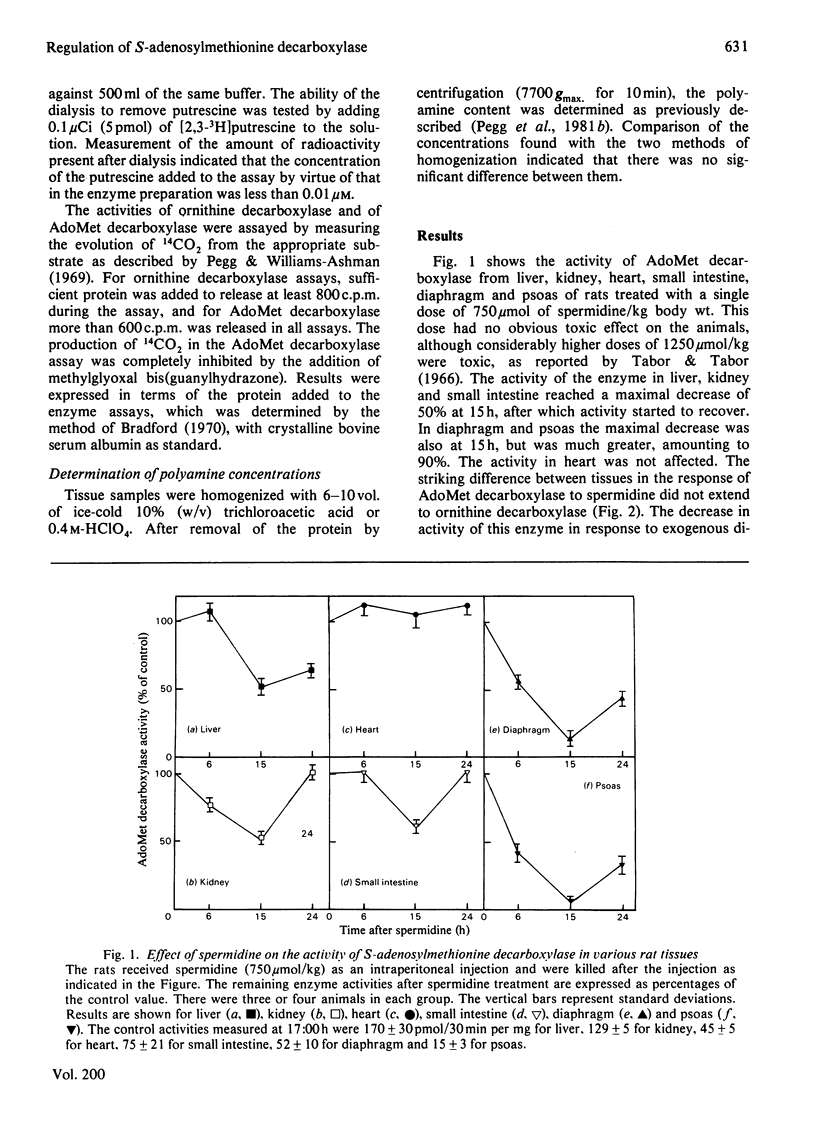
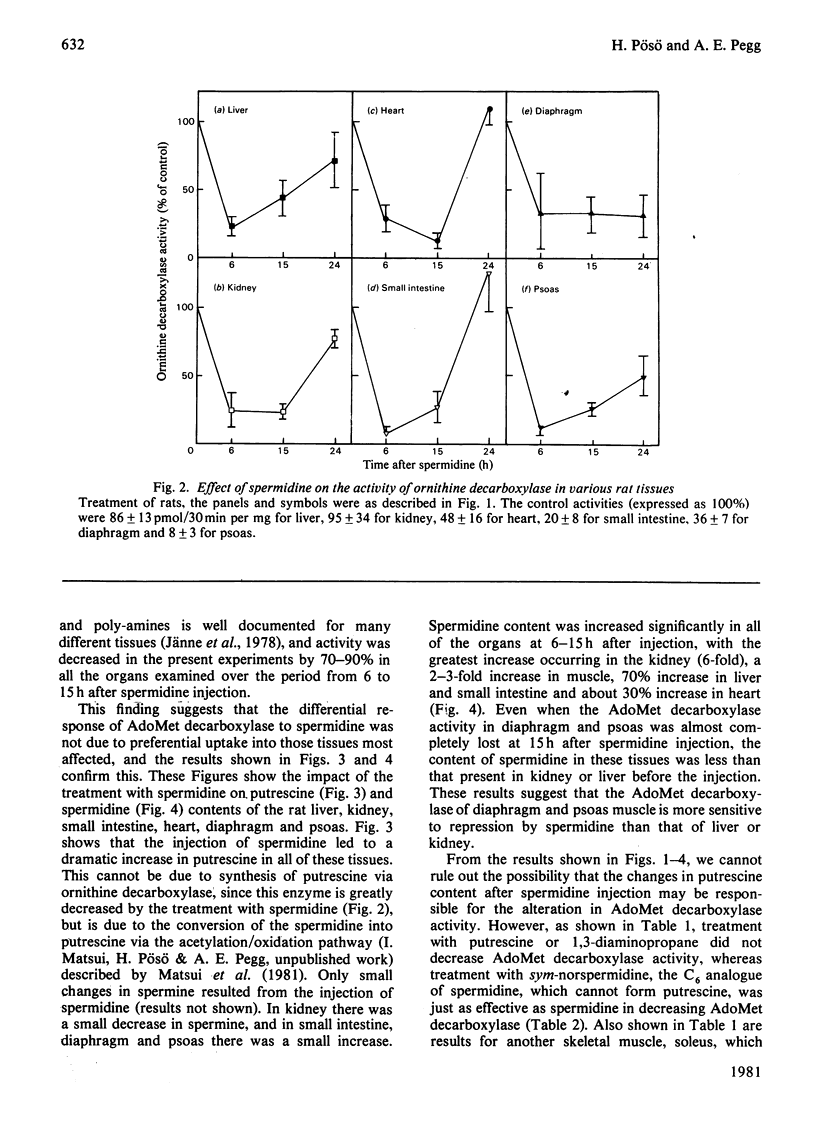
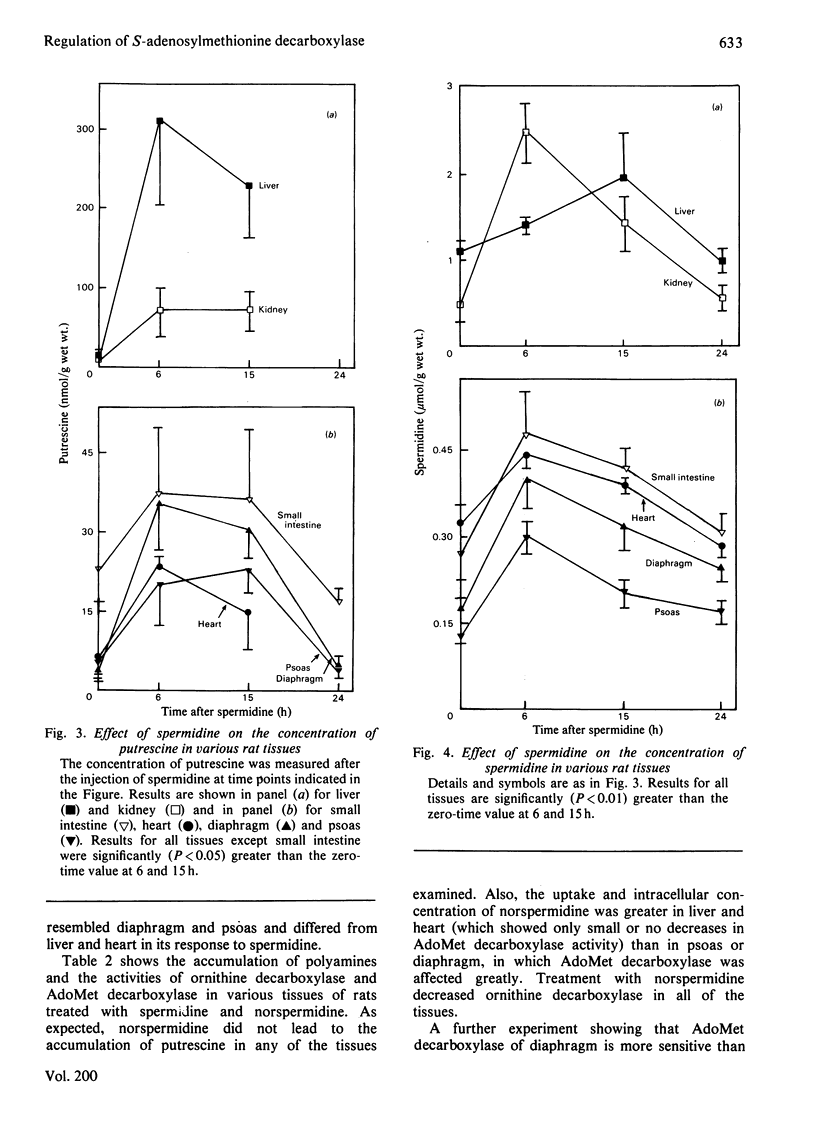
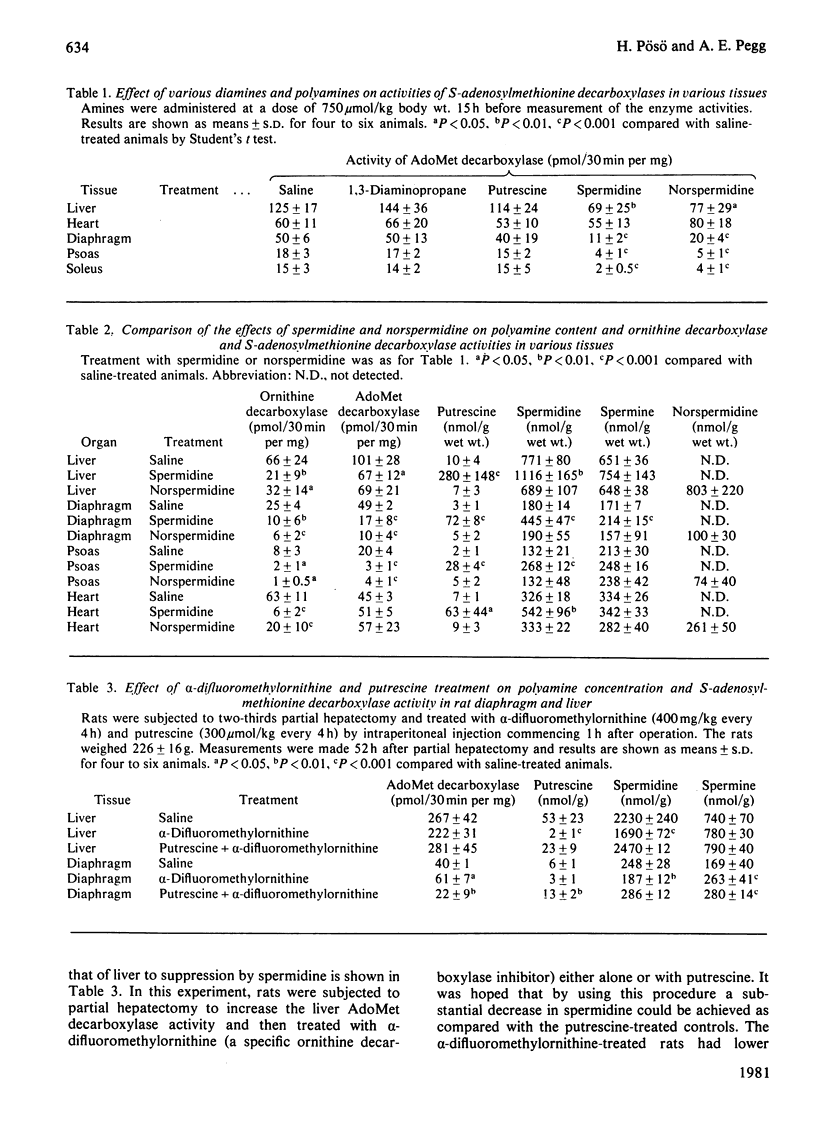
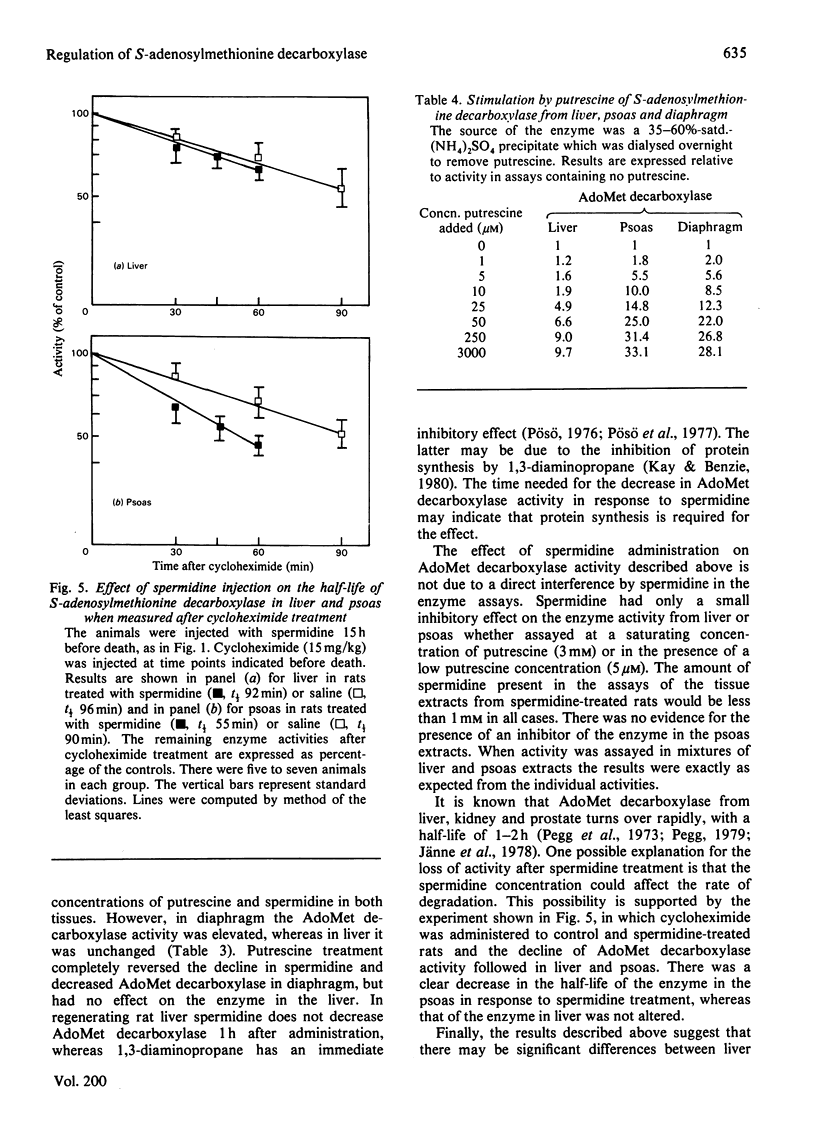
1. Administration of spermidine or sym-norspermidine decreased the activity of AdoMet (S-adenosylmethionine) decarboxylase in extracts prepared from rat liver, Kidney, psoas, diaphragm, soleus and small intestine, but not heart. The decline in psoas, diaphragm and soleus was much greater than that in liver and kidney. The difference in sensitivity to spermidine could not be explained by changes in the uptake and accumulation of the polyamine, because much higher contents were found in liver and kidney that in diaphragm and psoas. 2. Spermidine administration also led to a substantial increase in putrescine in all tissues examined. However, the rise in putrescine was not responsible for the decline in AdoMet decarboxylase activity, since norspermidine, which cannot form putrescine, also produced the decline. Also, administration of putrescine or 1,3-diaminopropane did not decrease AdoMet decarboxylase. 3. The decline in skeletal-muscle AdoMet decarboxylase activity in response to spermidine may be due to an increased rate of degradation of the enzyme protein. The t1/2 (half-time) for the decline in activity after inhibition of protein synthesis by cycloheximide was almost halved in the psoas of spermidine-treated rats. Spermidine treatment did not change the t1/2 in liver. 4. These results raise the possibility that there are at least two different forms of AdoMet decarboxylase and that the enzyme from psoas or diaphragm differs from that in liver. Additional support for this hypothesis was obtained by comparing the activation by putrescine of AdoMet decarboxylase from these tissues. The liver enzyme was stimulated 10-fold, but the muscle enzyme was stimulated 30-fold.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alhonen-Hongisto L. Regulation of S-adenosylmethionine decarboxylase by polyamines in Ehrlich ascites-carcinoma cells grown in culture. Biochem J. 1980 Sep 15;190(3):747–754. doi: 10.1042/bj1900747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Canellakis E. S., Viceps-Madore D., Kyriakidis D. A., Heller J. S. The regulation and function of ornithine decarboxylase and of the polyamines. Curr Top Cell Regul. 1979;15:155–202. [PubMed] [Google Scholar]
- Conover C. A., Rozovski S. J., Belur E. R., Aoki T. T., Ruderman N. B. Ornithine decarboxylase activity in insulin-deficient states. Biochem J. 1980 Nov 15;192(2):725–732. doi: 10.1042/bj1920725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das D. K., Steinberg H. The effect of fructose on purine metabolism in the lung. Life Sci. 1980 Jan 7;26(1):1–10. doi: 10.1016/0024-3205(79)90181-4. [DOI] [PubMed] [Google Scholar]
- Demetriou A. A., Cohn M. S., Tabor C. W., Tabor H. Identification of pyruvate in S-adenosylmethionine decarboxylase from rat liver. J Biol Chem. 1978 Mar 10;253(5):1684–1686. [PubMed] [Google Scholar]
- Eloranta T. O., Raina A. M. Formation of CO2 from the carboxyl group of S-adenosylmethionine by liver membrane-associated enzymes involves the demethylation-transsulphuration pathway. Biochem Biophys Res Commun. 1978 Sep 14;84(1):23–30. doi: 10.1016/0006-291x(78)90257-7. [DOI] [PubMed] [Google Scholar]
- Fillingame R. H., Morris D. R. S-adenosyl-L-methionine decarboxylase during lymphocyte transformation: decreased degradation in the presence of a specific inhibitor. Biochem Biophys Res Commun. 1973 Jun 8;52(3):1020–1025. doi: 10.1016/0006-291x(73)91039-5. [DOI] [PubMed] [Google Scholar]
- Hannonen P., Raina A., Jänne J. Polyamine synthesis in the regenerating rat liver: stimulation of S-adenosyl methionine decarboxylase, and spermidine and spermine synthases after partial hepatectomy. Biochim Biophys Acta. 1972 Jun 26;273(1):84–90. doi: 10.1016/0304-4165(72)90194-8. [DOI] [PubMed] [Google Scholar]
- Heller J. S., Fong W. F., Canellakis E. S. Induction of a protein inhibitor to ornithine decarboxylase by the end products of its reaction. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1858–1862. doi: 10.1073/pnas.73.6.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins D., Manchester K. L. Control of activity of S-adenosylmethionine decarboxylase in muscle by spermidine. FEBS Lett. 1980 Jan 14;109(2):299–302. doi: 10.1016/0014-5793(80)81109-4. [DOI] [PubMed] [Google Scholar]
- Hopkins D., Manchester K. L. The influence of nerve section on the metabolism of polyamines in rat diaphragm muscle. Biochem J. 1981 May 15;196(2):603–610. doi: 10.1042/bj1960603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins D., Manchester K. L. The influence of nerve section on the metabolism of polyamines in rat diaphragm muscle. Biochem J. 1981 May 15;196(2):603–610. doi: 10.1042/bj1960603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hölttä E., Hannonen P., Pispa J., Jänne J. Effect of methylglyoxal bis(guanylhydrazone) on polyamine metabolism in normal and regenerating rat liver and rat thymus. Biochem J. 1973 Nov;136(3):669–676. doi: 10.1042/bj1360669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jänne J., Pösö H., Raina A. Polyamines in rapid growth and cancer. Biochim Biophys Acta. 1978 Apr 6;473(3-4):241–293. doi: 10.1016/0304-419x(78)90015-x. [DOI] [PubMed] [Google Scholar]
- Kay J. E., Benzie C. R. Direct effects of 1,3-diaminopropane on reticulocyte lysate protein synthesis. FEBS Lett. 1980 Dec 1;121(2):309–312. doi: 10.1016/0014-5793(80)80370-x. [DOI] [PubMed] [Google Scholar]
- Lutaya G., Griffiths J. R. Rapid formation of spermine skeletal muscle during tetanic stimulation. FEBS Lett. 1981 Jan 26;123(2):186–188. doi: 10.1016/0014-5793(81)80283-9. [DOI] [PubMed] [Google Scholar]
- Mamont P. S., Duchesne M. C., Grove J., Tardif C. Initial characterization of a HTC cell variant partially resistant to the anti-proliferative effect of ornithine decarboxylase inhibitors. Exp Cell Res. 1978 Sep;115(2):387–393. doi: 10.1016/0014-4827(78)90292-6. [DOI] [PubMed] [Google Scholar]
- Matsui I., Wiegand L., Pegg A. E. Properties of spermidine N-acetyltransferase from livers of rats treated with carbon tetrachloride and its role in the conversion of spermidine into putrescine. J Biol Chem. 1981 Mar 10;256(5):2454–2459. [PubMed] [Google Scholar]
- McAnulty P. A., Williams J. P. Polyamines and their biosynthetic decarboxylases in various tissues of the young rat during recovery from undernutrition. Biochem J. 1977 Jan 15;162(1):109–121. doi: 10.1042/bj1620109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegg A. E., Corti A., Williams-Ashman H. G. Paradoxical enhancement of S-adenosylmethionine decarboxylase in rat tissues following administration of the specific inhibitor methyl glyoxal bis(guanylhydrazone). Biochem Biophys Res Commun. 1973 May 15;52(2):696–701. doi: 10.1016/0006-291x(73)90768-7. [DOI] [PubMed] [Google Scholar]
- Pegg A. E. Evidence for the presence of pyruvate in rat liver S-adenosylmethionine decarboxylase. FEBS Lett. 1977 Dec 1;84(1):33–36. doi: 10.1016/0014-5793(77)81051-x. [DOI] [PubMed] [Google Scholar]
- Pegg A. E., Hibasami H., Matsui I., Bethell D. R. Formation and interconversion of putrescine and spermidine in mammalian cells. Adv Enzyme Regul. 1980;19:427–451. doi: 10.1016/0065-2571(81)90027-3. [DOI] [PubMed] [Google Scholar]
- Pegg A. E., Hibasami H. Polyamine metabolism during cardiac hypertrophy. Am J Physiol. 1980 Nov;239(5):E372–E378. doi: 10.1152/ajpendo.1980.239.5.E372. [DOI] [PubMed] [Google Scholar]
- Pegg A. E. Inhibition of spermidine formation in rat liver and kidney by methylglyoxal bis(guanylhydrazone). Biochem J. 1973 Mar;132(3):537–540. doi: 10.1042/bj1320537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegg A. E. Investigation of the turnover of rat liver S-adenosylmethionine decarboxylase using a specific antibody. J Biol Chem. 1979 May 10;254(9):3249–3253. [PubMed] [Google Scholar]
- Pegg A. E. Purification of rat liver S-adenosyl-L-methionine decarboxylase. Biochem J. 1974 Aug;141(2):581–583. doi: 10.1042/bj1410581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegg A. E., Shuttleworth K., Hibasami H. Specificity of mammalian spermidine synthase and spermine synthase. Biochem J. 1981 Aug 1;197(2):315–320. doi: 10.1042/bj1970315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegg A. E., Williams-Ashman H. G. On the role of S-adenosyl-L-methionine in the biosynthesis of spermidine by rat prostate. J Biol Chem. 1969 Feb 25;244(4):682–693. [PubMed] [Google Scholar]
- Pösö H., Hannonen P., Himberg J. J., Jänne J. Adenosylmethionine decarboxylase from various organisms: relation of the putrescine activation of the enzyme to the ability of the organism to synthesize spermine. Biochem Biophys Res Commun. 1976 Jan 12;68(1):227–234. doi: 10.1016/0006-291x(76)90033-4. [DOI] [PubMed] [Google Scholar]
- Pösö H., Kallio A., Scalabrino G., Jänne J. Specific inhibition of the synthesis of putrescine and spermidine by 1,3-diaminopropane in rat liver in vivo. Biochim Biophys Acta. 1977 Mar 29;497(1):288–297. doi: 10.1016/0304-4165(77)90162-3. [DOI] [PubMed] [Google Scholar]
- Pösö H., Sinervirta R., Jänne J. S-adenosylmethionine decarboxylase from baker's yeast. Biochem J. 1975 Oct;151(1):67–73. doi: 10.1042/bj1510067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raina A., Jänne J. Physiology of the natural polyamines putrescine, spermidine and spermine. Med Biol. 1975 Jun;53(3):121–147. [PubMed] [Google Scholar]
- Raina A., Pajula R. L., Eloranta T. A rapid assay method for spermidine and spermine synthases. Distribution of polyamine-synthesizing enzymes and methionine adenosyltransferase in rat tissues. FEBS Lett. 1976 Sep 1;67(3):252–255. doi: 10.1016/0014-5793(76)80540-6. [DOI] [PubMed] [Google Scholar]
- Sakai T., Hori C., Kano K., Oka T. Purification and characterization of S-adenosyl-L-methionine decarboxylase from mouse mammary gland and liver. Biochemistry. 1979 Dec 11;18(25):5541–5548. doi: 10.1021/bi00592a003. [DOI] [PubMed] [Google Scholar]
- Sakai T., Perry J. W., Hori C., Oka T. Putrescine and the regulation of S-adenosyl-L-methionine decarboxylase in cultured mouse mammary gland. Biochim Biophys Acta. 1980 Aug 7;614(2):577–582. doi: 10.1016/0005-2744(80)90246-6. [DOI] [PubMed] [Google Scholar]
- Sturman J. A. Subcellular distribution of S-adenosylmethionine decarboxylase in rat liver. Evidence of decarboxylation of S-adenosylmethionine separate from synthesis of spermidine. Biochim Biophys Acta. 1976 Mar 25;428(1):56–69. doi: 10.1016/0304-4165(76)90108-2. [DOI] [PubMed] [Google Scholar]
- TABOR H., TABOR C. W. SPERMIDINE, SPERMINE, AND RELATED AMINES. Pharmacol Rev. 1964 Sep;16:245–300. [PubMed] [Google Scholar]
- Williams-Ashman H. G., Canellakis Z. N. Polyamines in mammalian biology and medicine. Perspect Biol Med. 1979 Spring;22(3):421–453. doi: 10.1353/pbm.1979.0013. [DOI] [PubMed] [Google Scholar]
- Wilson J., Corti A., Hawkins M., Williams-Ashman H. G., Pegg A. E. The decarboxylation of S-adenosylmethionine by detergent-treated extracts of rat liver. Biochem J. 1979 Jun 15;180(3):515–522. doi: 10.1042/bj1800515. [DOI] [PMC free article] [PubMed] [Google Scholar]


