Abstract
To report a novel variation in the TRIM8 gene in a Chinese patient who developed focal segmental glomerulosclerosis (FSGS) and neurogenic bladder. Retrospective analysis of the clinical manifestations, laboratory results, renal biopsy results, and genetic data of the patient with FSGS complicated with neurogenic bladder. The patient was a 6-year and 8-month-old Chinese Zang ethnic boy with low-set ears, widely-spaced eyes (inner canthal distance exceeds the 95th percentile of normal inner canthal distance), a small jaw, and a short neck. He could not walk and speak complete sentences until age of 2 years. At age of 4 years, the boy was noticed to have daytime urinary incontinence, hesitancy, and urgency. Combined with urodynamic examination and magnetic resonance imaging examination results, the patient was diagnosed with a neurogenic bladder. Proteinuria was also found. The levels of uric acid, serum creatinine, and blood urea nitrogen were increased. Vitamin D deficiency, hypokalemia, hypocalcium, and hypophosphorus were detected. Urinary ultrasound showed shrinkage of both kidneys. After hospital admission, he was diagnosed with FSGS and stage 3b chronic kidney disease (CKD). Eight months after the first diagnosis, the disease progressed to stage 5 CKD. Gene analysis using whole-exome capture and sequencing revealed a de novo heterozygous pathogenic variation in the TRIM8 gene [NM_030912.2.2:c.1484G>A (p.RP495 *)]. This pathogenic TRIM8 variation and the combined clinical manifestations of neurogenic bladder and FSGS have not been previously reported in the literature. We report a rare Chinese case of FSGS and neurogenic bladder associated with a novel de novo heterozygous variation in the TRIM8 gene. The findings expanded the clinical spectrum of TRIM8 pathogenic variations.
Keywords: Focal segmental glomerulosclerosis, neurogenic bladder, TRIM8, pathogenic variation, mutation
Introduction
TRIM8 protein, a member of the TRIM (tripartite motif-containing) protein family, has many important biological functions such as exerting anti-viral or anti-bacterial functions, potentiating protective responses to genotoxic stress, promoting cell survival and genomic stability in normal cells, yet exerting oncogenic functions in p53-null or p53-mutated cells.1–3 TRIM8 also regulates the self-renewal or differentiation of stem cells. 1 It has been recently found that TRIM8 is involved in the regulation of neural development and synaptic function. 4 TRIM8 is widely expressed in murine and human tissues, among which the highest TRIM8 expression are central nervous tissues and kidneys. 5 Compatibly, TRIM8 dysfunction or mutations have been associated with rare genetic forms of neuro-renal syndrome, manifested as early onset of intractable epileptic seizures, intellectual disability, developmental delay, and refractory nephropathy. 6
Focal segmental glomerulosclerosis (FSGS) is one of the common causes of steroid-resistant nephrotic syndrome or refractory nephropathy that eventually results in end-stage renal disease (ESRD) in children.7,8 Mutations/pathogenic variations of the TRIM8 gene have been recently recognized as the genetic basis for childhood FSGS that, in most cases, was accompanied by neurological symptoms.6,9–15 To date, 23 cases of neuro-renal syndrome with de novo pathogenic variations of the TRIM8 gene have been reported worldwide, and a total of 17 nucleotide variations have been found. However, few cases of neuro-renal syndrome with TRIM8 variations have been reported from China. 15 It appeared that the cases of neuro-renal syndrome with TRIM8 variations were particularly rare, which might be related to the low incidence of the genetic form of the disease and also the incomplete understanding or reporting of the disease. This paper describes a case of FSGS with neurogenic bladder in a Chinese child carrying a novel de novo pathogenic variation in the TRIM8 gene [NM_030912.2.2:c.1484G>A (P.RP495*)]. The clinical characteristics, diagnosis, and treatment are discussed in combination with an updated systematic literature review of the disease.
Case presentation
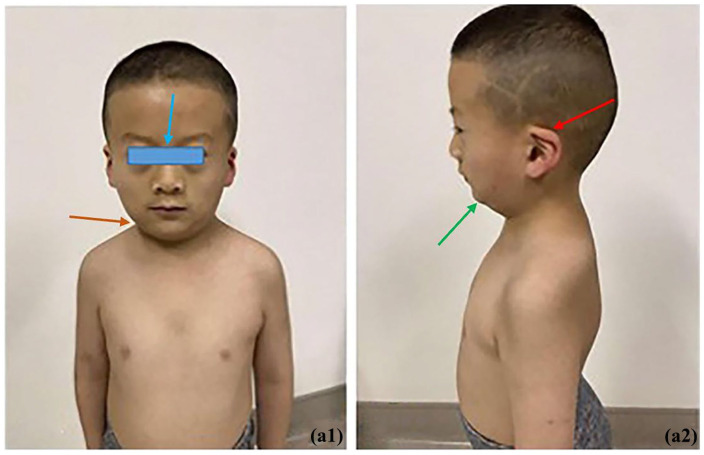
The patient was a 6-year and 8-month-old boy from the Zang ethnic minority group, in Sichuan Province, China. The grandmothers of the child’s parents were biological sisters. Both parents were healthy and had no family history of genetic diseases. The boy was the only child of the parents. He was born at full term and delivered by cesarean section with no birth asphyxia. His birth weight was 3500 g and body length unknown. The boy was fed artificial milk with occasional supplementary foods after birth. His mother did not use nor was exposed to alcohol, tobacco, or drugs during pregnancy. The boy could not walk and speak complete sentences until he was 2 years old, but his family did not pay attention to those matters. When he was about 4 years old, his family noticed that the child tended to have urinary hesitancy, had daytime urinary incontinence, accompanied by urgent urination; yet he had smooth urination with an apparently normal urine volume, and without urine dripping, dysuria, pain during urination or nocturnal enuresis. He was admitted to our hospital (West China Second Hospital of Sichuan University) because of acute bronchitis when he was 6 years and 8 months old (in April 2022). Then he was also found to have 3+ urinary protein, and pathocast was detected; routine blood sample test revealed elevated urea (11.3 mmol/L, normal range: 3.2–8.2 mmol/L), elevated creatinine (117 µmol/L, normal range: 17.3–54.6 µmol/L), and decreased blood vitamin D (8.7 ng/mL, normal range: 30–100ng/mL). Therefore, he was hospitalized because of acute upper respiratory tract infection, and vitamin D deficiency, with additional manifestations of urinary incontinence, proteinuria, and the hypothesis of neurogenic bladder. Meanwhile, the child presented unique facial features: low-set ears, widely-spaced eyes, a small jaw, and a short neck, as shown in Figure 1. The patient’s Wechsler intelligence test score was 94, 16 weight 23 kg (age-specific weight Z-score: +1), height 117 cm (age-specific height Z-score: −1), BMI 18.3 kg/m² (age-specific BMI Z-score: +1). The urodynamic examination after hospitalization revealed a bladder capacity of about 211 mL, decreased bladder fullness sensation, decreased bladder compliance, no inhibition of detrusor contraction during storage phase, and no urine leakage when severe coughing, hypo-contraction of detrusor during voiding phase, and a post-void residual urine volume of 40 mL. Magnetic resonance imaging indicated an arachnoid cyst or a large occipital cistern. Those examination results were consistent with the diagnosis of neurogenic bladder.
Figure 1.
Unique facial features of the children: Red arrow: low-set ears; Blue arrow: widely-spaced eyes; Green arrow: small jaw; Orange arrow: short neck. Left figure is the front view, and right figure left-side view.
Urinary ultrasound examination showed that the size of the right kidney was 6.4 × 2.7 × 3.0 cm, and the size of the left kidney was 6.7 × 2.8 × 2.5 cm. The lengths of both kidneys were shorter than the normal value for the patient’s age (reference value: 8.33 cm), suggesting shrinkage of his kidneys. Enhanced parenchymal echoes of both kidneys and separation of the left kidney assembly were detected. Micturition urography revealed separation of both renal assemblies, slightly enhanced parenchymal echoes of both kidneys and no obvious vesicoureteral reflux. The 24-h urine protein excretion quantity was 4.67 g. Urine protein electrophoresis detected larger and smaller molecular-weight protein components on both sides of the albumin band, suggesting mixed proteinuria. (99mTc-Dimercaptosuccinic Acid renal imaging) examination revealed reduced perfusion of both kidneys and severe functional impairment, accompanied by poor drainage of the bilateral upper urinary tract. During the in-patient period, the patient’s creatinine clearance rate was 50.74 mL/min according to Schwartz’s calculation, and multiple electrolyte disorders (hypokalemia, hypocalcium, hypophosphorus) and secondary hyperparathyroidism were detected.
To further identify the cause of renal injury in the patient and to further develop follow-up treatment plans, the patient underwent a B-ultrasound-guided percutaneous renal aspiration biopsy with informed consent. The results showed FSGS, accompanied by multiple sclerosis (sclerosis was detected in 6 of the 8 visible glomeruli in the specimen), as shown in Figure 2. Cystic dilatation of renal tubules was also detected. In addition, diffused fusion of the foot process was observed by using electron microscopy.
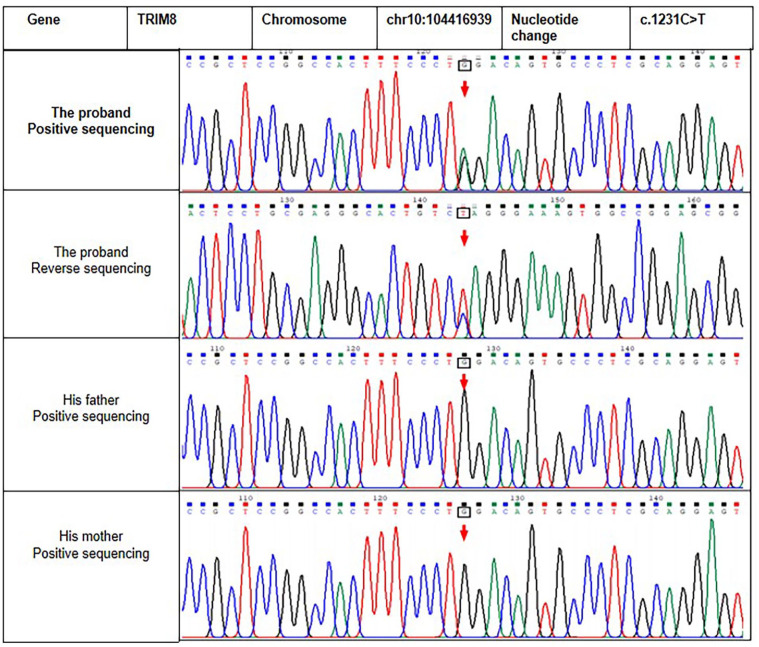
Figure 2.
Sanger sequencing results of the proband and his parents. The arrows in upper two panels indicate the position of the de novo base substitution in a heterozygous state in the proband.
Considering the consanguineous connection of the grandmothers of the child’s parents and the clinical manifestations of the patient, we generated a hypothesis of genetically related neuro-renal disease. The whole-exome capture and sequencing of the genomic DNA of the child was conducted under the premise of informed consent. The results showed a heterozygous variation in exon 6 of the TRIM8 gene [NM_030912.2.2: c.1484G>A, (p.TP495*)], which was a nonsense mutation in the coding region of the TRIM8 gene and could theoretically lead to premature termination of the translation of encoded protein. This variation has not been reported in the literature and is not in the large-scale population frequency database. The variation was not detected in the peripheral blood samples of the patient’s parents, suggesting that the variation was a de novo variation in the patient, as shown in Figure 3. According to the guidelines from the American College of Medical Genetics and Genomics, 17 the novel de novo variation was identified as pathogenic and is a rare variation.
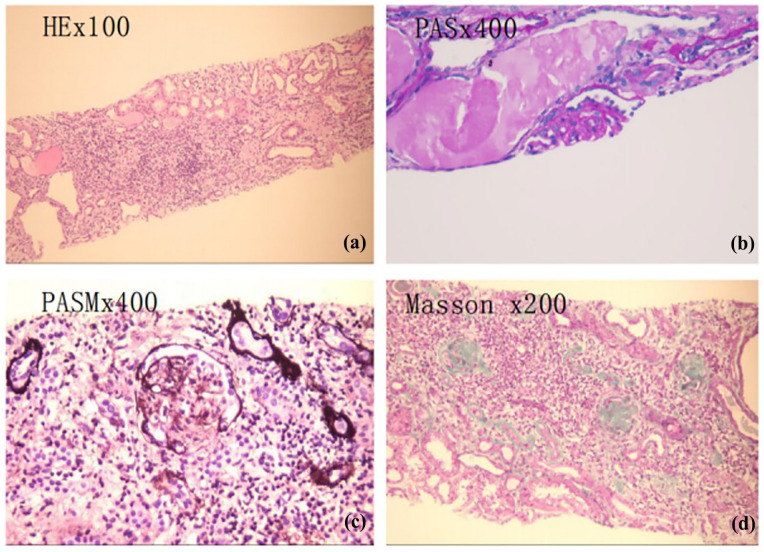
Figure 3.
Renal pathological presentation of the proband. (a) HE staining, ×100 magnification. (b) PAS, staining, ×400 magnification. (c) PASM staining, ×400 magnification. (d) Masson staining, ×200 magnification. Eight glomeruli could be seen deeply, among which six glomeruli had bulbous sclerosis, and one incomplete glomerulus could be seen. Segmental sclerosis was suspected. PASM staining results indicated six glomeruli with bulbous sclerosis.
HE: hematoxylin-eosin; PAS: periodic acid Schiff; PASM: periodic acid-silver methenamine.
Combined with the clinical manifestations, laboratory tests, genetic analysis, and renal biopsy results, the primary diagnosis of the patient was a genetic form of FSGS, stage 3b chronic kidney disease (CKD), and neurogenic bladder, accompanied by global developmental delay.
During hospitalization, the patient was given a full course of ceftazidime to fight infection, captopril to relieve proteinuria, solinaxin succinate to regulate abnormal bladder activity, piperazine ferulate to protect kidneys, compound α-ketoacid tablets to regulate metabolism, calcium supplement and vitamin D supplement and other symptomatic treatments. After the observation of effective symptom control, the patient was discharged. After discharge, the patient and his family were instructed to observe the intelligence, executive function, and language skills of the patient, urine volume, and urinary incontinence, to avoid infection, to continue taking the aforementioned oral drugs, excessive sodium intake should be avoided in children with CKD, and a low sodium intake of 1.5–2.4 g/d is recommended for children who require sodium restriction, protein intake for children with CKD stage 4–5 is 100%–120% of the recommended daily intake, 18 and to visit our physicians for outpatient follow-up with biofeedback therapy.
During the follow-up period up to the date of this report writing, the patient did not develop epilepsy or convulsion, but his renal function deteriorated progressively, urine protein fluctuated between 3+ and 4+, blood creatinine and urea nitrogen continued to rise, and the symptoms of urinary urgency and daytime urinary incontinence improved. When he was 7 years and 4 months old (8 months after the first diagnosis), the child was diagnosed with stage 5 CKD and received regular hemodialysis. In July 2024, the child received a kidney transplant at West China Hospital of Sichuan University, and now his renal function is slowly recovering.
Discussion
We reported the first patient with a novel de novo pathogenic variation in the TRIM8 gene who developed neurogenic bladder and FSGS. To date, 23 patients with neuro-renal syndrome (not including our patient) have been reported to harbor de novo pathogenic variations in the TRIM8 gene (Table 1). Epilepsy, as a severe neurological disorder, was manifested in 20 out of the 23 patients. Seventeen out of 23 patients had developmental delay or intellectual instability. Although our patient did not develop epilepsy, we conjecture that neurogenic bladder, which significantly impacts the quality of life, 19 is another neurological phenotype associated with a pathogenic variation in the TRIM8 gene. This TRIM8 variation-associated neural manifestation has not been reported before.
Table 1.
Reported TRIM8 variations in patients with neuro-renal syndrome and clinical data (n = 23 cases).
| Nucleotide change | Amino-acid change | Gender | Ethnic origin | Ab onset | ESRD onset (Y) | Renal disease | Neuro disease | Facial features | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| c.1099_1100 insG | p.C367fs | Male | Japanese | 2M | NA | NA | Epilepsy, GDD | Small, upward-slanting palpebral fissures, long arched eyebrows | 10 |
| c.1117_1117delG | p.Ala374Argfs*16 | Male | NA | 5M | NA | NA | Epilepsy, hypospadias, hypotonia, intellectual disability, and SB | Mild facial features, long philtrum | 11 |
| c.1163delT | p.Phe388 Serfs* | Female | European | 2.2Y | 3 | FSGS, SRNS | Epilepsy, GDD, mild cerebral atrophy atrophy | NA | 6 |
| c.1198_1220del | p.Tyr400ArgfsTer2 | Male | British | 2Y | 5 | FSGS | Well-controlled epilepsy, MDD | NA | 12 |
| c.1201_1202delGGInsTA | p.Gly401* | Female | European | 3Y | 5 | SRNS, DMS | Epilepsy, GDD, Mild cerebral atrophy atrophy | NA | 6 |
| c.1231C>T | p.Gln411* | Male | Italian | 4.5Y | 4.8 | FSGS, SRNS | Epilepsy, GDD, cerebral atrophy | NA | 6 |
| c.1240C>T | p.Gln414* | Female | German | Birth | 1.1 | SRNS, DMS | Epilepsy, GDD, cerebral atrophy, hypotonia | NA | 6 |
| c.1267 C>T | p.Gln423* | Male | African American | 11Y | 12 | FSGS, NS | Epilepsy, GDD | NA | 6 |
| c.1267 C>T | p.Gln423* | Male | NA | 4M | NA | NA | Epilepsy, DD | Straight eyebrows, synophrys, sunken eyes, flat, long philtrum | 11 |
| c.1331 C>A | p.Ser444* | Female | NA | 1.8Y | NA | NRP | Epilepsy, DD | None | 11 |
| c.1333C>T | p.Gln445* | Female | Italian | 13.7Y | 19.7 | FSGS, SRNS | Epilepsy, cerebral atrophy, spastic dystonic quadriplegia | NA | 6 |
| c.1338 T>A | p.Tyr446* | Female | NA | 2Y | NA | Mesangial glomerulonephritis with non-specific IgM deposits | Epilepsy, generalized hypotonia, ataxia, dysmetria, stereotypic movements and abnormal ocular movement | Gingival overgrowth, synophrys, long philtrum | 11 |
| c.1375 C>T | p.Gln459* | Male | Korean | 4Y | 5 | FSGS, SRNS | Epilepsy, GDD, autism | NA | 6 |
| c.1375 C>T | p.Gln459* | Male | German | 7.9Y | 9.8 | FSGS, NRP | Epilepsy, GDD | Secondary microcephaly | 6 |
| c.1375 C>T | p.Gln459* | Male | European | 3Y | 5 | FSGS, NS | Epilepsy, GDD, mesial temporal sclerosis | NA | 6 |
| c.1375 C>T | p.Gln459* | Female | Ashkenazi Jewish | 5M | NA | Spontaneously resolved proteinuria | Epilepsy during sleep, hypotonia, hyporeflexia, and GDD | left simian crease, fine hairs | 11 |
| C1380T>A | p.Tyr460* | Male | Hispanic | 2.4Y | >4 | FSGS, SRNS | Epilepsy, intellectual disability, DD | NA | 6, 13 |
| c.1380T>G | p.Tyr460* | Female | Middle- eastern | 6Y | 8 | FSGS, NRP | Epilepsy, GDD, cerebral atrophy, hypotonia | NA | 6 |
| c.1461C>A | p.Tyr487* | Female | Japanese | 3Y | 9 | FSGS | None | NA | 14 |
| c.1461C>A | p.Tyr487* | Female | Chinese | 18M | 6 | FSGS | MDD | NA | 15 |
| c.1461C>G (a pair of monozygotic twins) | p.Tyr487* | Male | Irish/Hispanic | 6Y | 14 | FSGS, SRNS | Mild GDD, Tourette’s syndrome-like symptoms, autism spectrum | NA | 6 |
| c.1453C>T | p.Gln485* | Female | Chinese | 3.3Y | NA | FSGS | None | NA | 15 |
Note. Ab: abnormality; DD: developmental delay; DMS: diffuse mesangial sclerosis; ESRD: end-stage renal disease; FSGS: focal segmental glomerulosclerosis; M: month; MDD: mild developmental delay; NA: not available; NS: nephrotic syndrome; NRP: nephrotic-range proteinuria; Ref: reference; SB: stereotypic behavior; SRNS: steroid-resistant nephrotic syndrome; Y: year.
Primary FSGS, as one of the common pathological causes of refractory nephropathy in children, has a poor prognosis. 7 About 50% of the patients with general forms of FSGS gradually develop ESRD.7,20 Among the 23 previously reported patients with neural-renal syndrome and TRIM8 variations, 15 patients had FSGS, of whom 14 (~ 93%) progressed to ESRD (Table 1). Although the number of reported patients with TRIM8-associated FSGS is still small, it appears that TRIM8-associated FSGS has an even worse prognosis than general forms of FSGS.
The child reported in this paper started to present developmental abnormalities at age of 2 years and neurogenic bladder symptoms at the age of 4 years. At age of 6 years and 8 months when his was admitted to our hospital because of acute bronchitis, he was found to have FSGS with stage 3b CKD, which quickly progressed to ESRD 8 months after the first diagnosis, requiring regular hemodialysis treatment.
The childhood onset of FSGS accompanied by neurological diseases and developmental delay may indicate a general genetic cause of these diseases. Whole-exome capture and sequencing of the genomic DNA of the subjects can be conducted to analyze genetic changes including point variations, small fragment insertions, deletion variations, and large fragment copy number variations based on the second-generation sequencing data. We benefited from this technique in finding the novel de novo heterozygous nonsense variation in the TRIM8 gene.
TRIM8 normally encodes a 551 amino-acid protein, a member of a large family of TRIM proteins, which are characterized by the succession of a RING domain, 1 or 2 B-box domains, a coiled-coil domain, and a variable C-terminal region. The RING domain endows TRIM proteins with E3 ubiquitin ligase activity. TRIM8 homodimerizes via its coiled-coil domain, and its C-terminus is required for correct nuclear localization.1–3 TRIM8 is involved in multiple important biological processes with a wide range of cellular and molecular functions, including proliferation, differentiation, immune response, and signal transduction.1–3 Central nervous tissues and kidneys normally have the highest expression of TRIM8 protein. 5 Those functions and characteristics of the TRIM8 protein are compatible with the association between TRIM8 pathogenic variations and nephrotic syndrome, encephalopathy, and other neurological diseases.
Specifically, it is known that TRIM8 induces p53-dependent cell cycle arrest in normal cells.1,2 Podocytes in normal glomeruli are terminally differentiated and barely have the capacity for proliferation. The maintenance of these features of podocytes may involve normal TRIM8-p53 signaling. In contrast, the cellular lesion of FSGS is characterized by aberrant cell proliferation14,21; and kidney specimens from the patients with TRIM8 pathogenic variations demonstrated significantly reduced nuclear expression of TRIM8 in podocytes and parietal epithelial cells in the glomeruli.13,14 Thus, one of the hypotheses on the mechanism underlying TRIM8 pathogenic variation-associated FSGS is that the reduced nuclear expression or mis-localization of TRIM8 leads to aberrant proliferation and loss of differentiation of podocytes, which may result in the development of FSGS. In addition, it is known that TRIM8 inhibits SOCS1 (suppressor of cytokine signaling 1) protein in normal cells.1–3 Indeed, in podocytes and parietal epithelial cells in the glomeruli from normal controls, the strong nuclear expression of TRIM8 is accompanied by weak cytoplasmic expression of SOCS1 protein. However, the SOCS1 protein is over-expressed in kidney specimens from patients with TRIM8 pathogenic variations compared with normal controls. The consequence of the SOCS1 over-expression in patients is the inhibition of cytokine signaling such as the typical STAT (signal transducer and activator of transcription) signaling. Yet STAT signaling is actually activated in the kidneys of patients with FSGS,22,23 thus challenging the mechanistic role of the TRIM8-SOCS1 pathway.
In the neural system, TRIM8 plays an important role in regulating synaptic function and neural development. 4 The underlying mechanism for the association between pathogenic TRIM8 variations and a unique subgroup of neurological diseases is an interesting topic to be investigated.
In our patient reported here, the novel heterozygous de novo nonsense mutation in exon 6 of the TRIM8 gene (c.1484G>A, p.TP495*) is in line with previous findings of truncating variations located in exon 6 of the C-terminal of the gene (Table 1). Of note, consistent with a previous observation, 13 the pathogenic variations/mutations in all the reported patients so far, including the one in our patient, were found to be de novo and heterozygous. This suggests a dominant negative effect of those heterozygous de novo variations, which could theoretically result in premature termination of translation, thus encoding a truncated protein. It is believed that a truncated TRIM8 protein may be detrimental to the stability and the proper nuclear localization of TRIM8 protein and thereby may lead to the loss of its normal functions or gain of detrimental functions,6,14,15 eventually resulting in a variety of clinical manifestations of neuro-renal syndrome.
Conclusion
In summary, we reported a Chinese child with FSGS who had a novel de novo heterozygous variation in the TRIM8 gene and initially presented with global developmental delay and neurogenic bladder that has never been reported before. The reported results expanded the clinical phenotypic spectrum of TRIM8 variants. Abnormal TRIM8 gene often affects the nervous system and kidneys, and its pathological manifestations can be nephrotic syndrome which in most cases is accompanied by neurological diseases such as epilepsy or neurogenic bladder, and developmental delays. Clinicians in clinical practice need to be vigilant for the genetic basis of those patients with childhood-onset neurological and renal manifestations to prevent misdiagnosis and missed diagnosis. Genetic testing is important for early diagnosis, thus initiating early treatment, and improving knowledge of patients and families. If patients are diagnosed early, when hematuria and proteinuria occur, drugs such as Angiotensin-converting enzyme inhibitor and Angiotonin Ⅱ Receptor Blocker can be used to reduce these symptoms, thus slowing down the process of chronic progressive fibrosis of the kidneys. In China, some traditional Chinese medicines can be used to delay the effects of chronic progressive fibrosis, such as Cordyceps sinensis extract and total astragalus glycosides, which have a protective effect on the kidneys. Clinicians can regulate the patient’s diet at an early stage to control the total protein and high-purine food, focusing on high-quality protein, and avoiding food and drugs that have toxic effects on the kidneys.
Acknowledgments
We are grateful to the patient and his family who participated in this study. We also express our gratitude to all of the pediatricians, who provided the patient’s clinical data. We thank Dr. Wen Deng, from The University of Hong Kong, for editing the English text of a draft of this article.
Footnotes
Author contributions: Y.-N.G. conceptualization; D.-Y.L. and Y.L. methodology; L.-L.L. and X.-Y.C. validation; L.-L.L. and X.-Y.C. formal analysis; Y.-N.G. investigation; Y.-N.G. resources; D.-Y.L. and Y.L. data curation; D.-Y.L. and Y.L. writing—original draft preparation; Y.-N.G. writing—review and editing; Y.-N.G. visualization; Y.-N.G. supervision; Y.-N.G. project administration; Y.-N.G. funding acquisition. All authors have read and agreed to the published version of the manuscript.
Data availability statement: The datasets for this article are not publicly available due to concerns regarding participant/patient anonymity. Requests to access the datasets should be directed to the corresponding author.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethics approval: Ethical approval to report this case was obtained from the Ethics Committee of the West China Second University Hospital. The ethics approval number is (2022) Clinical ethics approval No.5.
Informed consent: Written informed consent was obtained from the individual(s), and minor(s)’ legal guardian/next of kin, for the publication of any potentially identififiable images or data included in this article.
ORCID iD: Yan-Nan Guo  https://orcid.org/0000-0002-0024-2806
https://orcid.org/0000-0002-0024-2806
References
- 1. Caratozzolo MF, Marzano F, Mastropasqua F, et al. TRIM8: making the right decision between the oncogene and tumour suppressor role. Genes (Basel) 2017; 8(12): 354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bhaduri U, Merla G. Rise of TRIM8: a molecule of duality. Mol Ther Nucleic Acids 2020; 22: 434–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Marzano F, Guerrini L, Pesole G, et al. Emerging roles of TRIM8 in health and disease. Cells 2021; 10(3): 561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ding C, Zhang C, Kopp R, et al. Transcription factor POU3F2 regulates TRIM8 expression contributing to cellular functions implicated in schizophrenia. Mol Psychiatry 2021; 26(7): 3444–3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Reymond A, Meroni G, Fantozzi A, et al. The tripartite motif family identifies cell compartments. EMBO J 2001; 20(9): 2140–2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Weng PL, Majmundar AJ, Khan K, et al. De novo TRIM8 variants impair its protein localization to nuclear bodies and cause developmental delay, epilepsy, and focal segmental glomerulosclerosis. Am J Hum Genet 2021; 108(2): 357–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. D’Agati VD, Kaskel FJ, Falk RJ. Focal segmental glomerulosclerosis. N Engl J Med 2011; 365(25): 2398–2411. [DOI] [PubMed] [Google Scholar]
- 8. De Vriese AS, Sethi S, Nath KA, et al. Differentiating primary, genetic, and secondary FSGS in adults: a clinicopathologic approach. J Am Soc Nephrol 2018; 29(3): 759–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Epi4K and EPGP Investigators. De novo mutations in epileptic encephalopathies. Nature 2013; 501(7466): 217–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sakai Y, Fukai R, Matsushita Y, et al. De novo truncating mutation of TRIM8 causes early-onset epileptic encephalopathy. Ann Hum Genet 2016; 80(4): 235–240. [DOI] [PubMed] [Google Scholar]
- 11. Assoum M, Lines MA, Elpeleg O, et al. Further delineation of the clinical spectrum of de novo TRIM8 truncating mutations. Am J Med Genet A 2018; 176(11): 2470–2478. [DOI] [PubMed] [Google Scholar]
- 12. McClatchey MA, du Toit ZD, Vaughan R, et al. Focal segmental glomerulosclerosis and mild intellectual disability in a patient with a novel de novo truncating TRIM8 mutation. Eur J Med Genet 2020; 63(9): 103972. [DOI] [PubMed] [Google Scholar]
- 13. Warren M, Takeda M, Partikian A, et al. Association of a de novo nonsense mutation of the TRIM8 gene with childhood-onset focal segmental glomerulosclerosis. Pediatr Nephrol 2020; 35(6): 1129–1132. [DOI] [PubMed] [Google Scholar]
- 14. Shirai Y, Miura K, Kaneko N, et al. A novel de novo truncating TRIM8 variant associated with childhood-onset focal segmental glomerulosclerosis without epileptic encephalopathy: a case report. BMC Nephrol 2021; 22(1): 417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Li X, Wei Y, Wang M, et al. Two children with steroid-resistant significant proteinuria due to nonsense mutations of the TRIM8 gene: a case report and literature review. Front Pediatr 2022; 10: 918373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Watkins MW, Canivez GL, Dombrowski SC, et al. Long-term stability of Wechsler Intelligence Scale for Children-fifth edition scores in a clinical sample. Appl Neuropsychol Child 2022; 11(3): 422–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Drackley A, Brew C, Wlodaver A, et al. Utility and outcomes of the 2019 American College of Medical Genetics and Genomics-Clinical Genome Resource Guidelines for Interpretation of Copy Number Variants with Borderline Classifications at an Academic Clinical Diagnostic Laboratory. J Mol Diagn 2022; 24(10): 1100–1111. [DOI] [PubMed] [Google Scholar]
- 18. KDOQI. Clinical practice guideline for nutrition in children with CKD: 2008 update. Am J Kidney Dis 2009; 53(3): S11–S104. [DOI] [PubMed] [Google Scholar]
- 19. Panicker JN. Neurogenic bladder: epidemiology, diagnosis, and management. Semin Neurol 2020; 40(5): 569–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gipson DS, Trachtman H, Kaskel FJ, et al. Clinical trial of focal segmental glomerulosclerosis in children and young adults. Kidney Int 2011; 80(8): 868–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang S, Kim JH, Moon KC, et al. Cell-cycle mechanisms involved in podocyte proliferation in cellular lesion of focal segmental glomerulosclerosis. Am J Kidney Dis 2004; 43: 19–27. [DOI] [PubMed] [Google Scholar]
- 22. Tao J, Mariani L, Eddy S, et al. JAK-STAT signaling is activated in the kidney and peripheral blood cells of patients with focal segmental glomerulosclerosis. Kidney Int 2018; 94(4): 795–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pekkucuksen NT, Liu LP, Aly R, et al. Extracellular vesicles from focal segmental glomerulosclerosis pediatric patients induce STAT3 activation and mesangial cell proliferation. PLoS One 2022; 17(11): e0274598. [DOI] [PMC free article] [PubMed] [Google Scholar]