Abstract
Retrovirus entry into cells is mediated by specific interactions between the retrovirally encoded Env envelope glycoprotein and a host cell surface receptor. Though a number of peptide motifs responsible for the structure as well as for the binding and fusion activities of Env have been identified, only a few quantitative data concerning the infection process are available. Using an inducible expression system, we have expressed various amounts of ecotropic and amphotropic Env at the surfaces of Moloney murine leukemia virus-derived vectors and assayed for the infectivity of viral particles. Contrary to the current view that numerous noncooperative Env-viral receptor interactions are required for cell infection, we report here that very small amounts of Env are sufficient for optimal infection. However, increasing Env density clearly accelerates the rate at which infectious attachment to cells occurs. Moreover, our data also show that a surprisingly small number of Env molecules are sufficient to drive infection, albeit at a reduced efficiency, and that, under conditions of low expression, Env molecules act cooperatively. These observations have important consequences for our understanding of natural retroviral infection as well as for the design of cell-targeted infection techniques involving retroviral vectors.
Retroviral env gene-encoded glycoproteins (Env) form knobbed spikes on the surfaces of virions and play a critical role in infection of target cells by attaching viral particles to specific host cell receptors and mediating the fusion of viral and host cell membranes (10, 22). However, Env binding to the receptor is, most probably, not the primary event of infection but rather is preceded by an Env-independent adsorption step (39, 43). Envs are synthesized as polyproteins targeted for translation to the endoplasmic reticulum via cleaved amino-terminal signal peptides. They are subsequently subjected to glycosylation, oligomerization, and further proteolytic processing by cellular proteases to give two subunits remaining associated via noncovalent (and sometimes covalent) bonds. These two subunits are SU, a hydrophilic extracellular glycoprotein responsible for binding to viral receptors, and TM, a transmembrane polypeptide tethering Env complexes to viral particles and playing the major part in the fusion of the viral envelope with the cellular membrane (10, 23, 28).
The structure and function of murine leukemia virus (MuLV) Env have been studied in detail. On the basis of their host range, MuLVs have been classified into six subgroups (ecotropic, amphotropic, polytropic, xenotropic, MDEV, and 10A1) (10, 22). The envelope motifs that bind to the various viral receptors and determine the infection specificity have been mapped within the N-terminal one-third of SU (gp70), which bears the most-variable regions of MuLV Envs (2–6, 13, 16, 21, 22, 34, 38, 41, 51). Thus, ecotropic MuLVs enter cells after binding to a cationic amino acid transporter (CAT-1) and amphotropic MuLVs enter cells after binding to a sodium-phosphate symporter (PIT-2) (22). In addition to an amino-terminal fusion peptide of TM [p15(E) in MuLVs] (16, 25, 56), various fusion-influencing determinants have been identified. At the level of SU, the latter include an N-terminal peptide (2) and a central proline-rich region (29, 50) mediating envelope conformational changes upon the binding of Env to its cognate receptor, the consequences of which are fusion activation through the unmasking of the TM N-terminal fusion peptide and the decreased stability of SU-TM heterodimers (29). At the level of TM, the last 16 amino acids (R peptide) exert a fusion-inhibitory effect in virus-producing cells (24, 44, 45, 54, 56) relieved at the time of budding or within virions through proteolytic processing of p15(E) by the viral protease. Motifs important for correct SU-TM interactions have also been identified. In addition to motifs responsible for noncovalent interactions between MuLV subunits, SU and TM are linked by a disulfide bond thought to be stable (40) owing to a CWLC motif at the beginning of the C-terminal domain of SU and a CX6CC sequence in TM (42). Moreover, one signal for N-linked glycosylation, located at the beginning of the C-terminal domain of SU, seems essential for both the folding of the C-terminal domain of SU and the stability of the interactions between SU and TM (33). In addition, a “leucine zipper-like” motif or a motif contained within it, downstream of the fusion peptide in the extracellular domain of p15(E), is essential for trimerization of SU-TM heterodimers through homomeric interactions of TM subunits (16, 32). However, there are also multiple other contacts in both SU and TM domains responsible for oligomerization (49) which allow functional interactions between SU-TM heterodimers within envelope protein complexes (46, 53). Finally, it has recently been found that, in addition to attaching MuLVs to their receptors, SU also sensitizes cells to infection (30). This last work also shows that, although they recognize different receptors for binding to the cell surface, the different classes of MuLVs use a common entry pathway, which is activated by a conserved feature of their envelope glycoprotein (30).
So far, the quantitative aspects of cell infection by retroviruses have been little documented. Analysis of the kinetics for the binding of both purified Env and purified virions to cells (9, 14, 17, 26, 27, 51) has led to the idea that the binding of MuLV is essentially due to multivalent noncooperative interactions whereby viral particles, each bearing multiple copies of oligomeric Env, bind to target cells, each bearing multiple copies of the receptor (51). As another approach to address the issue of how many Env-receptor interactions are necessary for infection, we have produced MuLV particles expressing various amounts of ecotropic and amphotropic Env. Unexpectedly, our data indicate that a small number of Env molecules are sufficient for mediating infection, albeit at a low efficiency. Surprisingly, they also show that the threshold of Env abundance required for efficient infection is very low and that Env can be incorporated in vast excess into viral particles with no further improvement of viral titers. The implications of our observation are discussed with respect to both wild-type infection and cell targeting by recombinant retroviral vectors.
MATERIALS AND METHODS
Plasmids and expression vectors.
The pRL-TK Renilla luciferase reporter plasmid was obtained from Clontech. pUHC-13-3 expresses the firefly luciferase gene under the control of the Tet operator, and pUHD-15-1 expresses the tetracycline-regulatable tTA transactivator (19). Ecotropic and amphotropic Env sequences were amplified by PCR from plasmids FBEMOSALF and FB4070ASALF (11), respectively. They were then cloned into the EcoRI and XbaI sites and SacII and XbaI sites located downstream of the Tet operator sequences of the plasmid pUHD-10-3 (19) to give the PM361 and PM377 tTA-responsive expression plasmids, respectively.
Cell lines.
TelCeb6 cells were derived from the TE671 human rhabdomyosarcoma (11). They produce Env glycoprotein-lacking retroviral particles carrying a nuclear localization signal–β-galactosidase reporter gene. Tel A and 3A2B6 are stable cell lines derived from TelCeb6 cells by transfection of amphotropic and ecotropic Env expression vectors, respectively. Mouse NIH 3T3 fibroblasts, A431 human keratinocytes, H9 human T cells, and mouse C2C12 myoblasts are available from the American Type Culture Collection. TE671-derived cells and NIH 3T3 cells were cultured in Dulbecco modified Eagle medium (DMEM) (Gibco/BRL), whereas A431 and H9 cells were grown in RPMI 1640 medium (Gibco/BRL) and C2C12 cells were grown in a 1:1 mixture of HAM-F12 (Eurobio) and DMEM. All culture media were supplemented with a mixture of 10% heat-inactivated fetal calf serum (Biomedia), 2 mM glutamine, 100 μg of streptomycin/ml, and 100 U of penicillin/ml.
Cell transfections.
Transient and stable transfections were carried out using the calcium phosphate precipitation procedure (48). The pRL-TK plasmid was used for normalization of transient-transfection experiments. In this case, 2 × 105 cells per well of six-well culture plates (Nunc) were transfected using 2 μg of the relevant plasmid plus 0.1 μg of pRL-TK. To obtain stable virus-producing cell clones expressing inducible ampho- and ecotropic Env, TelCeb6 cells were stably transfected with pUHD-15-1 and individual clones were tested in transient-transfection assays for their ability to express the firefly luciferase gene from pUHC-13-3 in a doxycycline (DOX)-dependent manner. The Tel179.9 clone used in this study was found to be the best responder. It was then stably transfected with eco- and amphotropic Env expression plasmids PM361 and PM377 to give CPM64 and CPM79 cells, respectively.
Luciferase and β-galactosidase assays and virus assay.
Renilla and firefly luciferase assays were carried out using the dual-luciferase reporter assay system from Promega according to the supplier's specifications. Assays of viruses were performed using cell culture supernatants from virus-producing cells cultured at confluence for 24 h. For assay of viruses on NIH 3T3, A431, and C2C12 cells, 2 × 104 target cells were plated in 12-well culture plates (Nunc). Twenty-four hours later, the culture medium was removed and replaced by 1 ml of fresh culture medium containing serially diluted virus-containing culture supernatants and 8 μg of Polybrene (Sigma)/ml. Infection was allowed to proceed overnight, the culture medium was changed, and an X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) (Eurobio) assay of retrovirally infected cells was performed 48 h later (36). For nonadherent H9 T cells virus assays were performed using the fibronectin method as described in reference 12. For kinetic infection experiments, cells were incubated in the presence of viruses for various periods of time and rapidly washed three times with phosphate-buffered saline (PBS; 0.15 M NaCl, 0.01 M NaPO4, pH 7) before the addition of fresh culture medium. For the assay of viruses produced by cells transiently transfected in the presence of various amounts of DOX, the culture medium was replaced by fresh medium containing the same concentration of DOX 48 h posttransfection and viruses released in culture supernatants were assayed and processed 16 h later. For the assay of viruses produced by stable cell lines, the latter were grown to confluence in the presence of various concentrations of DOX for at least 24 h before replacement of the culture medium by fresh medium containing the same concentration of DOX. Viruses were also assayed and processed 18 h later.
FACS and immunoblotting assays of Env.
Env expression at the cell surface was quantified by fluorescence-activated cell sorter (FACS) analysis as described by Lavilette et al. (29) using the 83A25 anti-Env monoclonal antibody (15). For quantification of virion-associated Env by immunoblotting, 1.2 ml of virus-containing culture supernatants was adjusted to 10 mM CaCl2 and incubated at room temperature for 30 min. Precipitated virions were spun down at 17,600 × g at 4°C for 1 min and resuspended directly in 10 μl of electrophoresis loading buffer. For analysis of cell-associated Env, cells were washed with PBS, scraped off culture dishes, centrifuged at 270 × g at 4°C for 5 min, resuspended in triplex lysis buffer (50 mM Tris-HCl [pH 8], 150 mM NaCl, 0.2% NaN3, 0.1% sodium dodecyl sulfate (SDS), 1% NP-40, 0.5% sodium deoxycholate, 2 mg of leupeptin/ml, 1 mM phenylmethylsulfonyl fluoride), and incubated on ice for 30 min. Cell debris and nuclei were removed by centrifugation of samples at 17,600 × g at 4°C for 10 min. Protein concentrations in supernatants were determined, and 75-μg samples were processed for electrophoresis. Virus and cell protein samples were fractionated through SDS-containing 10% polyacrylamide gels and transferred on Protran nitrocellulose membranes (Schleicher and Schuell), and immunodetections were carried out as described in reference 36 using the anti-gp70 goat antibody from Quality Biotech Inc. For viral particles, normalization of experiments was obtained by reprobing membranes with the R187b monoclonal antibody (8), which recognizes the MuLV p30Gag protein under the conditions described in reference 37. Densitometer analysis of luminograms exposed for appropriate periods of time was performed using the National Institutes of Health IMAGE software.
RESULTS
Infection efficiency of virions expressing various amounts of amphotropic Env in transient-transfection assays.
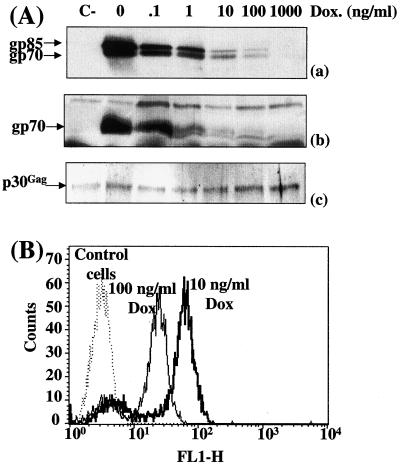
To address whether the amount of Env influences the infection efficiency of MuLVs, we first used a transient-transfection assay for production of homogeneous populations of virions expressing different amounts of Env. TelCeb6 cells produce Env-less noninfectious Moloney MuLV derived retroviral particles expressing the bacterial β-galactosidase reporter gene (11). They were engineered (clone Tel179.9) to constitutively express the tTA transcriptional transactivator, the activity of which is negatively regulated by DOX (19), and then were transiently transfected in the presence of various concentrations of the drug with a plasmid (PM377) carrying a tTA-responsive amphotropic Env gene. This approach was preferred over transfection of various amounts of a constitutive expression plasmid because it permits control of the ectopic gene in each transfected cell whereas the second method has more effect on the number of transfected cells than on the level of expression in each transfected cell. Immunoblotting experiments using a specific anti-MuLV Env antiserum were conducted in duplicate to assay for Env abundance in transfected cells and in viral particles released in culture supernatants which varied in both situations as a (nonlinear) function of DOX (Fig. 1Aa and 1Ab). FACS analysis of transfected cells was also conducted to assay for cell surface-associated Env at the individual-cell level (Fig. 1B). Under the conditions tested (10 and 100 ng of DOX/ml), the vast majority of cells (more than 80%) were found in sharp peaks of fluorescence whereas the remaining cells, probably nontransfected or poorly transfected cells, showed lower or no fluorescence. Overall, these data point to a homogeneous expression of Env at the individual-cell level in the vast majority of transfected cells under the experimental conditions used. The mean fluorescence ratio of these peaks (2.3) was comparable to the ratio of Env abundances (2.5) assayed by immunoblotting, confirming the DOX dose-dependent accumulation of Env.
FIG. 1.
Infection of NIH 3T3 cells by virions expressing various amounts of amphotropic Env in transient-transfection assays. Tel179.9 cells were transiently transfected with the PM377 amphotropic Env-expressing vector in the presence of various concentrations of DOX. (A) Env abundance assayed by immunoblotting. Equal amounts of cell extracts (a) and viral particles (b) were processed for immunoblotting analysis. Chemiluminescence detection was performed using a goat anti-MuLV Env antiserum. Only luminograms corresponding to short exposure times are presented in panels a and b. Longer exposures allowed visualization of Env expression at the highest DOX doses and thus quantification of signals (not shown). Both gp70 and its noncleaved precursor form, gp85, were detected in cellular extracts, and only gp70 was detected in experiments with viral particles. The blot presented in panel b was stripped and reprobed with anti-p30Gag capsid monoclonal antibodies to verify that comparable amounts of viral proteins were analyzed in all tracks. The slight variations in p30Gag protein abundance were taken into account for normalization of gp70 abundance. C-, nontransfected control cells. (B) FACS analysis of Env-expressing cells. Experiments were conducted using the 83A25 anti-Env rat monoclonal antibody.
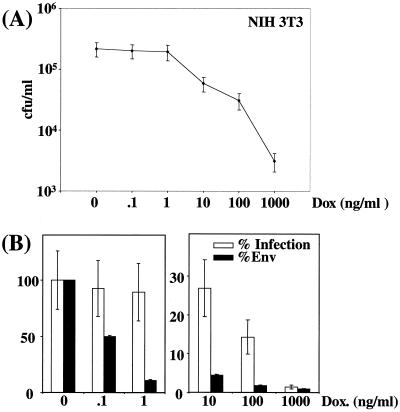
Infectious viruses released in culture supernatants were then assayed in quadruplicate on NIH 3T3 murine embryo fibroblasts (Fig. 2A), and titers were plotted against relative Env abundances deduced from densitometer scanning of immunoblots made with viral particles (Fig. 2B). The most striking observation was the absence of a linear relationship between Env abundance and infection efficiency. At the lowest DOX concentration (0 to 1 ng/ml), a 10-fold variation in Env was not associated with any significant variation in infectious titers, indicating the existence of a threshold above which better incorporation of Env does not lead to any improvement in infection efficiency. At the highest doses of DOX (1,000 and 100 ng/ml) a 2-fold increase in Env abundance resulted in a 10-fold-higher titer, raising the possibility of cooperation between Env molecules occurring at low density. At intermediate DOX concentrations (1 to 100 ng/ml), variations in Env abundance were associated with variations in viral titers, supporting the idea that multiple Env-receptor interactions favor infection.
FIG. 2.
Infection of NIH 3T3 cells by virions expressing various amounts of amphotropic Env in transient-transfection assays. (A) Cell infection assays. Infectious viruses contained in culture supernatants were assayed on NIH 3T3 cells in quadruplicate. (B) Relative Env abundance versus relative infectious titers. Env abundance and infectious titers assayed in the absence of DOX were taken as 100% references. Relative Env abundances were deduced from densitometer scanning analysis of luminograms exposed for various periods of time for accurate comparison of the relative intensities of the various signals. Error bars, standard deviations (A and B).
Infection efficiency of virions expressing various amounts of ecotropic and amphotropic Env in stable-transfection assays.
We then addressed whether the observed effect could be seen with another MuLV Env. Stable Tel179.9 cell-derived clones expressing tTA-responsive ecotropic (clone CPM64) and amphotropic (clone CPM79) Env genes were derived for direct comparison of ecotropic and amphotropic Envs. Virion-associated Envs and infectious particles contained in culture supernatants of CPM64 and CPM79 cells cultured in the presence of various concentrations of DOX were assayed in triplicate under conditions similar to those used in transient-transfection experiments (Fig. 3A and B and 4A and B, respectively). FACS analysis also showed homogenous expression of ecotropic and amphotropic Env at the surfaces of virus-producing cells in the various conditions used (data not shown).
FIG. 3.
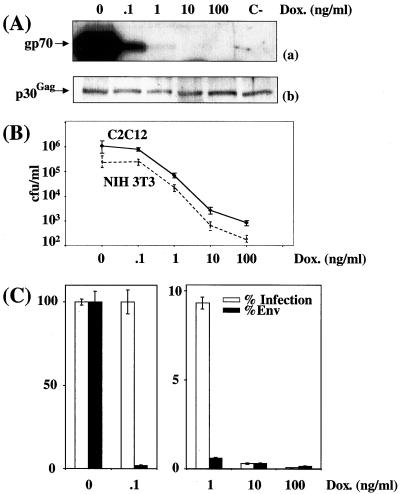
Cell infection by virions expressing various amounts of ecotropic Env in stable-transfection assays. CPM64 cells were cultured in the presence of various concentrations of DOX. (A) Immunoblotting assay of Env (a) and p30Gag (b) associated with viral particles contained in culture supernatant. A luminogram corresponding to a short exposure time is presented in panel a. p30Gag abundance was used to normalize Env abundance. C-, Tel179.9 control cells. (B) Assay of infectious particles on NIH 3T3 cells and on C2C12. (C) Relative Env abundance versus relative infectious titers assayed on NIH 3T3 cells. An immunoblotting assay of Env was performed in duplicate, and relative abundances were deduced from densitometer scanning analysis of luminograms exposed for various periods of time for accurate comparison of signals. Infectious titers were determined in triplicate, and error bars indicate standard deviations (B and C).
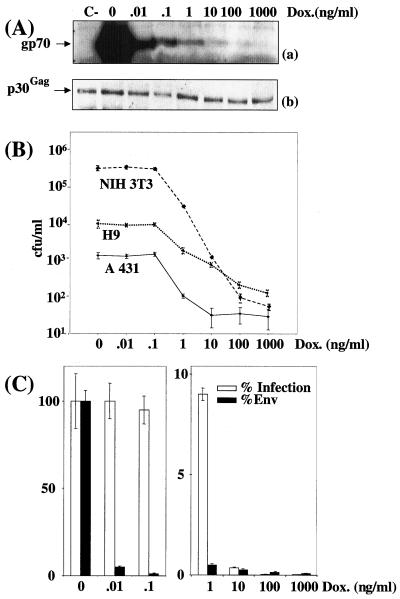
It is worthy of note that the drop in Env abundance observed between 0 and 0.1 ng of DOX/ml was more dramatic than that seen in the transient-transfection experiments. The reason for this effect was not investigated but might simply reflect the differential responsiveness to tTa of chromatin-associated and non-chromatin-associated Tet-responsive genes. This, however, did not change, in their essence, the essential outcomes of the experiments since (i) at the lowest DOX concentrations (0 and 0.1 ng/ml), 50- and 80-fold variations in ecotropic and amphotropic Env, respectively, did not result in any change in infectious virus titers, (ii) at intermediate concentrations, a reduction in Env abundance resulted in less-efficient infection, and (iii) at the highest concentrations used, a synergistic effect between Env molecules was also observed. Thus, for ecotropic Env, the 2- and 3-fold increases in abundances between 10 and 1 ng/ml and between 1 and 0.1 ng/ml resulted in 30- and 10-fold-higher titers, respectively (Fig. 3C). Similarly, for amphotropic Env, the 2-fold increments in amphotropic Env abundance between 100 and 10 ng/ml and between 10 and 1 ng/ml resulted in 10- and 40-fold elevations in infectious titers, respectively (Fig. 4C). Unfortunately, raising the DOX concentration above 100 and 1,000 ng/ml when using CPM64 and CPM79 cells (not shown), respectively, did not allow a further diminishing of Env expression because of the leakiness of the tetracycline expression system, and total infection inhibition could thus not be achieved using these cell lines. Low levels of infection at high DOX concentrations were, however, clearly Env dependent because no infectious viruses were detected in parallel experiments using Tel179.9 cells as a source of viral particles.
FIG. 4.
Cell infection by virions expressing various amounts of amphotropic Env in stable-transfection assays. CPM79 cells were cultured in the presence of various concentrations of DOX. (A) Immunoblotting assay of Env (a) and p30Gag (b) associated with viral particles contained in culture supernatant. A luminogram corresponding to a short exposure time is presented in panel a. p30Gag abundance was used to normalize Env abundance. C-, Tel179.9 control cells. (B) Assay of infectious particles on NIH 3T3, A431, and H9 cells. (C) Relative Env abundance versus relative infectious titers assayed on NIH 3T3 cells. An immunoblotting assay of Env was performed in duplicate, and relative abundances were deduced from densitometer scanning analysis of luminograms exposed for various periods of time. Infectious titers were determined in triplicate, and error bars indicate standard deviations (B and C).
Infection efficiency of virions expressing various amounts of ecotropic and amphotropic Env for various cell types.
Next, we determined whether the observed effect was cell type independent. In one series of experiments, culture supernatant from CPM64 cells grown in the presence of various concentrations of DOX were used to infect murine myogenic C2C12 cells (Fig. 3B), and, in a second series, culture supernatants from CPM79 cells were used to infect human keratinocytic A431 cells and H9 T lymphocytes (Fig. 4B). The final outcomes of these experiments were very similar to those of experiments involving NIH 3T3 cells as target cells. The only noticeable difference was the lower sensitivity of A431 and H9 cells to infection.
Infection kinetics using retroviruses expressing different amounts of ecotropic and amphotropic Env.
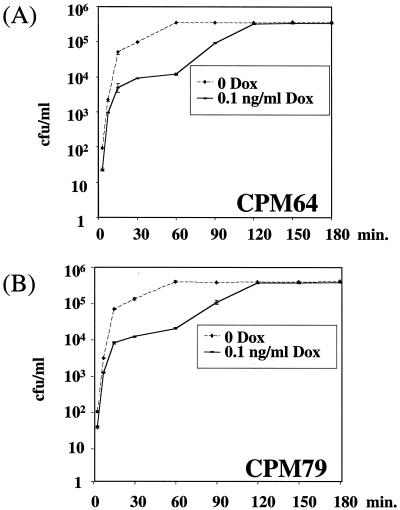
We next addressed whether the amount of Env could influence the kinetics of cell infection by MuLVs by conducting duplicate infection experiments in which NIH 3T3 cells were exposed for various periods of time to culture supernatants of CPM64 and CPM79 cells cultured in the absence or the presence of 0.1 ng of DOX/ml. In parallel, variations in virion-associated Env abundance were controlled by immunoblotting. In an initial phase (0 to 20 min), a rapid increase in the number of infected cells was observed under both conditions, indicating a rapid attachment of virions to cells (also see references 43 and 51) (Fig. 5). In the absence of DOX, a plateau was then rapidly reached (between 30 and 60 min), whereas in the presence of 0.1 ng of DOX/ml, where 50- and 80-fold less ecotropic and amphotropic Env was associated with viruses, respectively, the same plateau as that described above was eventually reached (which is consistent with the experiments presented in Fig. 1 to 3) but it was reached much more slowly (by 120 min). Since adsorption of Env-bearing MuLVs and that of Env-lacking MuLVs to target cells occur equally efficiently and equally rapidly (43), these data suggest that the reduction in the amount of virion-associated Env primarily affects the rate of postadsorption steps of the infection process and permits elimination of adsorbed virions from the cell surface by washing in experiments for infection kinetics (see Discussion). The initial rapid increase in infected cells in the experiments carried out in the presence of 0.1 ng of DOX/ml deserved a comment since it raises the possibility of the presence of a very minor fraction of viruses that incorporated larger amounts of Env and that could bind irreversibly to cells at a high rate. Although this would influence the shape of the curve, it does not change our overall conclusion that diminishing Env density entails a slowing down of infectious attachment to cells.
FIG. 5.
Infection kinetics of NIH 3T3 cells by virions expressing different amounts of Env. CPM64 and CPM79 cells were cultured in the presence of various concentrations of DOX. For infection experiments, which were carried out in triplicate, culture supernatants were added to NIH 3T3 cells for various periods of time and then washed out using PBS. Infected cells were scored 3 days later.
DISCUSSION
It is commonly assumed that many noncooperative Env-receptor interactions are necessary for efficient retrovirus entry into cells. Unexpectedly, for MuLVs a very small amount of Env per viral particle was shown here to be sufficient to permit cell infection since a 100- to 200-fold reduction in Env level dramatically reduces but does not totally abolish infection by MuLV-derived vectors. In line with this conclusion, Zavorotinskaya and Albritton (52) have recently reported that virions incorporating unprocessed precursor molecules as a predominant species because of a deficiency in SU-TM proteolytic processing and processed Env as a very minor species retain part of their infectious potential, indicating that the presence of only a few mature TM proteins (i.e., molecules capable of exposing a free N-terminal fusion peptide) is sufficient to permit fusion of the viral envelope and cell membrane. Because functional interactions between SU-TM heterodimers can occur within Env trimers (46, 53), these authors, however, could not exclude possible cooperation between processed and unprocessed Envs. Our data now support the notion that both the recognition of the receptor and the formation of the fusion pore can be achieved by a limited number of mature Env molecules. Unfortunately, this number could not be quantified exactly in our experiments because the leakiness of the expression system used did not allow the identification of conditions of total cell infection inhibition (not shown). It can, however, be reasonably estimated not to exceed 10 molecules or a few more if it is taken into consideration that natural MuLVs express several hundred (200 to 400) Env molecules (51) by analogy with human immunodeficiency virus type 1 (HIV-1) and -2 retroviruses (18) and that MuLV-based vectors incorporate only slightly larger Env amounts under optimized env gene expression in packaging cell lines. Also supporting the idea that a limited amount of envelope glycoprotein is sufficient for virus entry into cells is the recent observation that as few as one hemagglutinin trimer may trigger fusion of influenza virus with infected cells (20).
Our data show that infection efficiency is dependent on Env density up to a threshold amount above which an apparently large excess of Env can be incorporated without improving infection titers whatever the target cell used. Moreover, they also raise the possibility of cooperation between Envs when small amounts of Env are incorporated in viral particles. Definite proof of cooperativity will, however, have to await techniques comparable to FACS analysis for cells, permitting the assay of small amounts of incorporated Env at the level of individual viral particles to exclude formally the possibility that a fraction of viral particles expressing significantly larger amounts of Env could bias infection assays carried out at the highest concentration of DOX. The experiments for infection kinetics conducted in the presence of 0.1 ng of DOX/ml (Fig. 5), however, suggest that the fraction of viral particles expressing significantly higher amounts of Env, if it exists, is most likely very minor. The mechanisms underlying cooperativity are still elusive and are possibly severalfold. A first possibility could be the existence of functional interactions between Env trimers for either recruiting several receptors or for forming more-efficient fusion pores after the binding of viruses to one receptor or to a very limited number of receptors. Another possible mechanism would rely on the possibility for Env monomers (each monomer comprising SU and TM) to dissociate and reassociate at the surfaces of viral particles; increasing Env density would thus favor reassociation of Env monomers and, consequently, functional cooperation within trimers. Studying the multimerization state of Env molecules incorporated at low density in virions should help to address these two non-mutually exclusive possibilities. One could, for example, envisage that, at low density, increasing the amount of Env initially favors functional interactions within Env trimers and then functional interactions between trimers. A possible mechanism takes into account the recent finding that SU not only permits virion attachment to the viral receptor but also sensitizes cells for infection (30); increasing the amount of Env would thus result not only in a binding to more receptors but also in a better sensitization of target cells to infection.
The observation that virions can incorporate an apparently vast excess of Env is intriguing. Whether incorporation of large amounts of Env is necessary to compensate for the propensity of Env to shed (1) deserves further investigation. Without excluding this possibility, our experiments are consistent with the idea that augmenting Env density accelerates the infection process. Since retroviruses rapidly adsorb onto cells in an Env-independent manner before, most probably, browsing the cell surface until they encounter the viral receptors (43), it is possible that delayed infectious attachment of virions at reduced Env density simply reflects a reduction in the frequency of virus binding to the cognate receptor during the cell surface scanning phase of infection. Alternatively, high Env density might result in a faster sensitization of cells to infection and, as a consequence, in a faster entry of virions into cells. This observation also raises two interesting biological issues. First, our experiments have been conducted exclusively in vitro. The possibility that large amounts of Env are required for efficient viral replication in vivo cannot yet be excluded. Second, we have used eco- and amphotropic MuLVs, which, interestingly, use different receptors for entry into cells although they most probably use a common entry pathway activated by conserved features of their envelope glycoprotein (30). It would now be interesting to establish whether our observations also apply to other MuLV subtypes as well as to other retroviruses or, more generally, to enveloped viruses. From experiments based on the blocking of HIV infection by soluble CD4 molecules, Layne et al. (31) have already concluded that the HIV envelope is covered by a redundant number of gp120 molecules and that efficient CD4-mediated infection requires multiple gp120 molecules. Experiments are under way to address this point on a novel quantitative basis.
Recombinant MuLVs are widely used as gene transfer vectors both in the laboratory and in clinical protocols although this technology still suffers from a number of limitations. More specifically, the development of efficient cell-targeting techniques based on the genetic engineering of Env has met with difficulties since, when it is successful, redefined infection usually occurs at low yields (28, 35, 47). Poor targeting efficiency is attributable, at least in part, to poor fusion activity of reshaped Env (7, 55), but it is commonly thought that reduced incorporation of modified Env into virions is also detrimental to infection. Our work suggests that this might not necessarily be the case, especially when long incubations (hours) with target cells are possible because Env density, at least above a certain threshold, primarily acts on the kinetics of infection and has little, if any, effect on the final infection yield. However, when infection cannot be carried out for long periods of time (1 h or less), our results also suggest that ensuring high incorporation of engineered Env into viral Env is necessary for achieving efficient targeted infection.
ACKNOWLEDGMENTS
This work was supported by grants from the Centre National de la Recherche Scientifique, the Agence Nationale contre le SIDA, the Association de Recherche contre le Cancer, the Ligue contre le Cancer, and the Association Française contre les Myopathies.
We are grateful to J.-L. Battini, F.-L. Cosset, D. Kabat, A. Oates, and M. Sitbon for fruitful discussions and critical reading of the manuscript.
REFERENCES
- 1.Andersen K B. A domain of murine retrovirus surface protein gp70 mediates cell fusion, as shown in a novel SC-1 cell fusion system. J Virol. 1994;68:3175–3182. doi: 10.1128/jvi.68.5.3175-3182.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bae Y, Kingsman S M, Kingsman A J. Functional dissection of the Moloney murine leukemia virus envelope protein gp70. J Virol. 1997;71:2092–2099. doi: 10.1128/jvi.71.3.2092-2099.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Battini J L, Danos O, Heard J M. Definition of a 14-amino-acid peptide essential for the interaction between the murine leukemia virus amphotropic envelope glycoprotein and its receptor. J Virol. 1998;72:428–435. doi: 10.1128/jvi.72.1.428-435.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Battini J L, Danos O, Heard J M. Receptor-binding domain of murine leukemia virus envelope glycoproteins. J Virol. 1995;69:713–719. doi: 10.1128/jvi.69.2.713-719.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Battini J L, Heard J M, Danos O. Receptor choice determinants in the envelope glycoproteins of amphotropic, xenotropic, and polytropic murine leukemia viruses. J Virol. 1992;66:1468–1475. doi: 10.1128/jvi.66.3.1468-1475.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Battini J L, Rodrigues P, Muller R, Danos O, Heard J M. Receptor-binding properties of a purified fragment of the 4070A amphotropic murine leukemia virus envelope glycoprotein. J Virol. 1996;70:4387–4393. doi: 10.1128/jvi.70.7.4387-4393.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benedict C A, Tun R Y, Rubinstein D B, Guillaume T, Cannon P M, Anderson W F. Targeting retroviral vectors to CD34-expressing cells: binding to CD34 does not catalyze virus-cell fusion. Hum Gene Ther. 1999;10:545–557. doi: 10.1089/10430349950018625. [DOI] [PubMed] [Google Scholar]
- 8.Chesebro B, Britt W, Evans L, Wehrly K, Nishio J, Cloyd M. Characterization of monoclonal antibodies reactive with murine leukemia viruses: use in analysis of strains of Friend MCF and Friend ecotropic murine leukemia virus. Virology. 1983;127:134–148. doi: 10.1016/0042-6822(83)90378-1. [DOI] [PubMed] [Google Scholar]
- 9.Choppin J, Schaffar-Deshayes L, Debre P, Levy J P. Lymphoid cell surface receptor for Moloney leukemia virus envelope glycoprotein gp71. I. Binding characteristics. J Immunol. 1981;126:2347–2351. [PubMed] [Google Scholar]
- 10.Coffin J M, Hughes S H, Varmus H E, editors. Retroviruses. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1997. [PubMed] [Google Scholar]
- 11.Cosset F L, Takeuchi Y, Battini J L, Weiss R A, Collins M K. High-titer packaging cells producing recombinant retroviruses resistant to human serum. J Virol. 1995;69:7430–7436. doi: 10.1128/jvi.69.12.7430-7436.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dardalhon V, Noraz N, Pollok K, Rebouissou C, Boyer M, Bakker A Q, Spits H, Taylor N. Green fluorescent protein as a selectable marker of fibronectin-facilitated retroviral gene transfer in primary human T lymphocytes. Hum Gene Ther. 1999;10:5–14. doi: 10.1089/10430349950019147. [DOI] [PubMed] [Google Scholar]
- 13.Davey R A, Zuo Y, Cunningham J M. Identification of a receptor-binding pocket on the envelope protein of Friend murine leukemia virus. J Virol. 1999;73:3758–3763. doi: 10.1128/jvi.73.5.3758-3763.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eiden M V, Farrell K, Warsowe J, Mahan L C, Wilson C A. Characterization of a naturally occurring ecotropic receptor that does not facilitate entry of all ecotropic murine retroviruses. J Virol. 1993;67:4056–4061. doi: 10.1128/jvi.67.7.4056-4061.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Evans L H, Morrison R P, Malik F G, Portis J, Britt W J. A neutralizable epitope common to the envelope glycoproteins of ecotropic, polytropic, xenotropic, and amphotropic murine leukemia viruses. J Virol. 1990;64:6176–6183. doi: 10.1128/jvi.64.12.6176-6183.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fass D, Harrison S C, Kim P S. Retrovirus envelope domain at 1.7 angstrom resolution. Nat Struct Biol. 1996;3:465–469. doi: 10.1038/nsb0596-465. [DOI] [PubMed] [Google Scholar]
- 17.Ganguly K, Kalyanaraman V S, Sarngadharan M G. Analysis of the interaction between Rauscher murine leukemia virus and murine cell membrane receptor by in vitro binding assay. Cancer Lett. 1983;18:79–86. doi: 10.1016/0304-3835(83)90120-9. [DOI] [PubMed] [Google Scholar]
- 18.Gelderblom H R. Assembly and morphology of HIV: potential effect of structure on viral function. AIDS. 1991;5:617–638. [PubMed] [Google Scholar]
- 19.Gossen M, Freundlieb S, Bender G, Muller G, Hillen W, Bujard H. Transcriptional activation by tetracyclines in mammalian cells. Science. 1995;268:1766–1769. doi: 10.1126/science.7792603. [DOI] [PubMed] [Google Scholar]
- 20.Gunther-Ausborn S, Schoen P, Bartoldus I, Wilschut J, Stegmann T. Role of hemagglutinin surface density in the initial stages of influenza virus fusion: lack of evidence for cooperativity. J Virol. 2000;74:2714–2720. doi: 10.1128/jvi.74.6.2714-2720.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Han J Y, Cannon P M, Lai K M, Zhao Y, Eiden M V, Anderson W F. Identification of envelope protein residues required for the expanded host range of 10A1 murine leukemia virus. J Virol. 1997;71:8103–8108. doi: 10.1128/jvi.71.11.8103-8108.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Han J Y, Zhao Y, Anderson W F, Cannon P M. Role of variable regions A and B in receptor binding domain of amphotropic murine leukemia virus envelope protein. J Virol. 1998;72:9101–9108. doi: 10.1128/jvi.72.11.9101-9108.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hunter E. Viral entry and receptors. In: Coffin J M, Hughes S H, Varmus H E, editors. Retroviruses. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1997. pp. 71–120. [PubMed] [Google Scholar]
- 24.Januszeski M M, Cannon P M, Chen D, Rozenberg Y, Anderson W F. Functional analysis of the cytoplasmic tail of Moloney murine leukemia virus envelope protein. J Virol. 1997;71:3613–3619. doi: 10.1128/jvi.71.5.3613-3619.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jones J S, Risser R. Cell fusion induced by the murine leukemia virus envelope glycoprotein. J Virol. 1993;67:67–74. doi: 10.1128/jvi.67.1.67-74.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kadan M J, Sturm S, Anderson W F, Eglitis M A. Detection of receptor-specific murine leukemia virus binding to cells by immunofluorescence analysis. J Virol. 1992;66:2281–2287. doi: 10.1128/jvi.66.4.2281-2287.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kalyanaraman V S, Sarngadharan M G, Gallo R C. Characterization of Rauscher murine leukemia virus envelope glycoprotein receptor in membranes from murine fibroblasts. J Virol. 1978;28:686–696. doi: 10.1128/jvi.28.3.686-696.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karavanas G, Marin M, Salmons B, Gunzburg W H, Piechaczyk M. Cell targeting by murine retroviral vectors. Crit Rev Oncol Hematol. 1998;28:7–30. doi: 10.1016/s1040-8428(98)00007-9. [DOI] [PubMed] [Google Scholar]
- 29.Lavillette D, Maurice M, Roche C, Russell S J, Sitbon M, Cosset F L. A proline-rich motif downstream of the receptor binding domain modulates conformation and fusogenicity of murine retroviral envelopes. J Virol. 1998;72:9955–9965. doi: 10.1128/jvi.72.12.9955-9965.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lavillette D, Ruggieri A, Russell S J, Cosset F L. Activation of a cell entry pathway common to type C mammalian retroviruses by soluble envelope fragments. J Virol. 2000;74:295–304. doi: 10.1128/jvi.74.1.295-304.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Layne S P, Merges M J, Dembo M, Spouge J L, Nara P L. HIV requires multiple gp120 molecules for CD4-mediated infection. Nature. 1990;346:277–279. doi: 10.1038/346277a0. [DOI] [PubMed] [Google Scholar]
- 32.Li X, McDermott B, Yuan B, Goff S P. Homomeric interactions between transmembrane proteins of Moloney murine leukemia virus. J Virol. 1996;70:1266–1270. doi: 10.1128/jvi.70.2.1266-1270.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li Z, Pinter A, Kayman S C. The critical N-linked glycan of murine leukemia virus envelope protein promotes both folding of the C-terminal domains of the precursor polyprotein and stability of the postcleavage envelope complex. J Virol. 1997;71:7012–7019. doi: 10.1128/jvi.71.9.7012-7019.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.MacKrell A J, Soong N W, Curtis C M, Anderson W F. Identification of a subdomain in the Moloney murine leukemia virus envelope protein involved in receptor binding. J Virol. 1996;70:1768–1774. doi: 10.1128/jvi.70.3.1768-1774.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marin M, Noël D, Piechaczyk M. Towards efficient cell targeting by recombinant retroviruses. Mol Med Today. 1997;3:396–403. doi: 10.1016/S1357-4310(97)01095-2. [DOI] [PubMed] [Google Scholar]
- 36.Marin M, Noel D, Valsesia-Wittman S, Brockly F, Etienne-Julan M, Russell S, Cosset F L, Piechaczyk M. Targeted infection of human cells via major histocompatibility complex class I molecules by Moloney murine leukemia virus-derived viruses displaying single-chain antibody fragment-envelope fusion proteins. J Virol. 1996;70:2957–2962. doi: 10.1128/jvi.70.5.2957-2962.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marin M, Pelegrin M, Bachrach E, Noël D, Brockly F, Piechaczyk M. Antiviral activity of an intracellularly expressed single-chain antibody fragment directed against the murine leukemia virus capsid protein. Hum Gene Ther. 2000;11:389–401. doi: 10.1089/10430340050015860. [DOI] [PubMed] [Google Scholar]
- 38.Morgan R A, Nussbaum O, Muenchau D D, Shu L, Couture L, Anderson W F. Analysis of the functional and host range-determining regions of the murine ectropic and amphotropic retrovirus envelope proteins. J Virol. 1993;67:4712–4721. doi: 10.1128/jvi.67.8.4712-4721.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Notter M F, Leary J F, Balduzzi P C. Adsorption of Rous sarcoma virus to genetically susceptible and resistant chicken cells studied by laser flow cytometry. J Virol. 1982;41:958–964. doi: 10.1128/jvi.41.3.958-964.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Opstelten D J, Wallin M, Garoff H. Moloney murine leukemia virus envelope protein subunits, gp70 and Pr15E, form a stable disulfide-linked complex. J Virol. 1998;72:6537–6545. doi: 10.1128/jvi.72.8.6537-6545.1998. . (Erratum, 72:8460.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ott D, Rein A. Basis for receptor specificity of nonecotropic murine leukemia virus surface glycoprotein gp70SU. J Virol. 1992;66:4632–4638. doi: 10.1128/jvi.66.8.4632-4638.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pinter A, Kopelman R, Li Z, Kayman S C, Sanders D A. Localization of the labile disulfide bond between SU and TM of the murine leukemia virus envelope protein complex to a highly conserved CWLC motif in SU that resembles the active-site sequence of thiol-disulfide exchange enzymes. J Virol. 1997;71:8073–8077. doi: 10.1128/jvi.71.10.8073-8077.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pizzato M, Marlow S A, Blair E D, Takeuchi Y. Initial binding of murine leukemia virus particles to cells does not require specific Env-receptor interaction. J Virol. 1999;73:8599–8611. doi: 10.1128/jvi.73.10.8599-8611.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ragheb J A, Anderson W F. pH-independent murine leukemia virus ecotropic envelope-mediated cell fusion: implications for the role of the R peptide and p12E TM in viral entry. J Virol. 1994;68:3220–3231. doi: 10.1128/jvi.68.5.3220-3231.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rein A, Mirro J, Haynes J G, Ernst S M, Nagashima K. Function of the cytoplasmic domain of a retroviral transmembrane protein: p15E-p2E cleavage activates the membrane fusion capability of the murine leukemia virus Env protein. J Virol. 1994;68:1773–1781. doi: 10.1128/jvi.68.3.1773-1781.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rein A, Yang C, Haynes J A, Mirro J, Compans R W. Evidence for cooperation between murine leukemia virus Env molecules in mixed oligomers. J Virol. 1998;72:3432–3435. doi: 10.1128/jvi.72.4.3432-3435.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Russell S J, Cosset F-L. Modifying the host-range properties of retroviral vectors. J Gene Med. 1999;1:300–311. doi: 10.1002/(SICI)1521-2254(199909/10)1:5<300::AID-JGM59>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 48.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 49.Tucker S P, Srinivas R V, Compans R W. Molecular domains involved in oligomerization of the Friend murine leukemia virus envelope glycoprotein. Virology. 1991;185:710–720. doi: 10.1016/0042-6822(91)90542-j. [DOI] [PubMed] [Google Scholar]
- 50.Wu B W, Cannon P M, Gordon E M, Hall F L, Anderson W F. Characterization of the proline-rich region of murine leukemia virus envelope protein. J Virol. 1998;72:5383–5391. doi: 10.1128/jvi.72.7.5383-5391.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yu H, Soong N, Anderson W F. Binding kinetics of ecotropic (Moloney) murine leukemia retrovirus with NIH 3T3 cells. J Virol. 1995;69:6557–6562. doi: 10.1128/jvi.69.10.6557-6562.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zavorotinskaya T, Albritton L M. Failure to cleave murine leukemia virus envelope protein does not preclude its incorporation in virions and productive virus-receptor interaction. J Virol. 1999;73:5621–5629. doi: 10.1128/jvi.73.7.5621-5629.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhao Y, Lee S, Anderson W F. Functional interactions between monomers of the retroviral envelope protein complex. J Virol. 1997;71:6967–6972. doi: 10.1128/jvi.71.9.6967-6972.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhao Y, Zhu L, Benedict C A, Chen D, Anderson W F, Cannon P M. Functional domains in the retroviral transmembrane protein. J Virol. 1998;72:5392–5398. doi: 10.1128/jvi.72.7.5392-5398.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhao Y, Zhu L, Lee S, Li L, Chang E, Soong N W, Douer D, Anderson W F. Identification of the block in targeted retroviral-mediated gene transfer. Proc Natl Acad Sci USA. 1999;96:4005–4010. doi: 10.1073/pnas.96.7.4005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhu N L, Cannon P M, Chen D, Anderson W F. Mutational analysis of the fusion peptide of Moloney murine leukemia virus transmembrane protein p15E. J Virol. 1998;72:1632–1639. doi: 10.1128/jvi.72.2.1632-1639.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]