Abstract
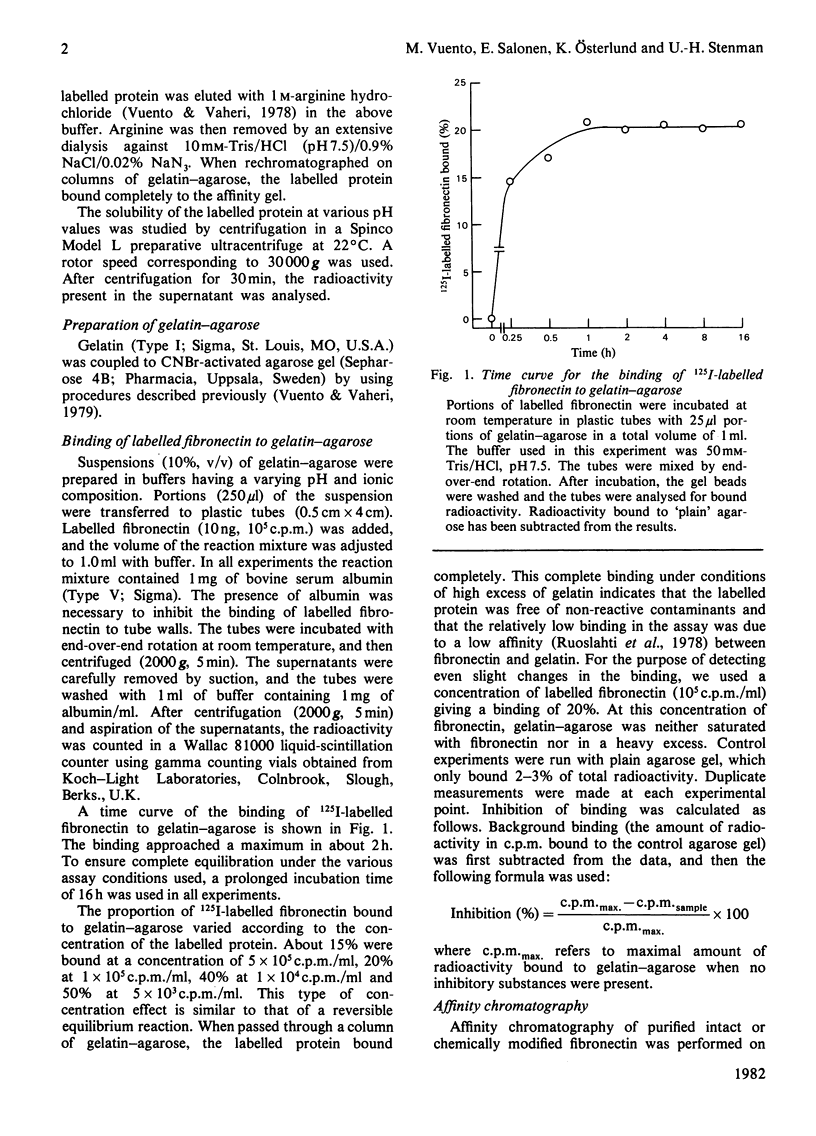
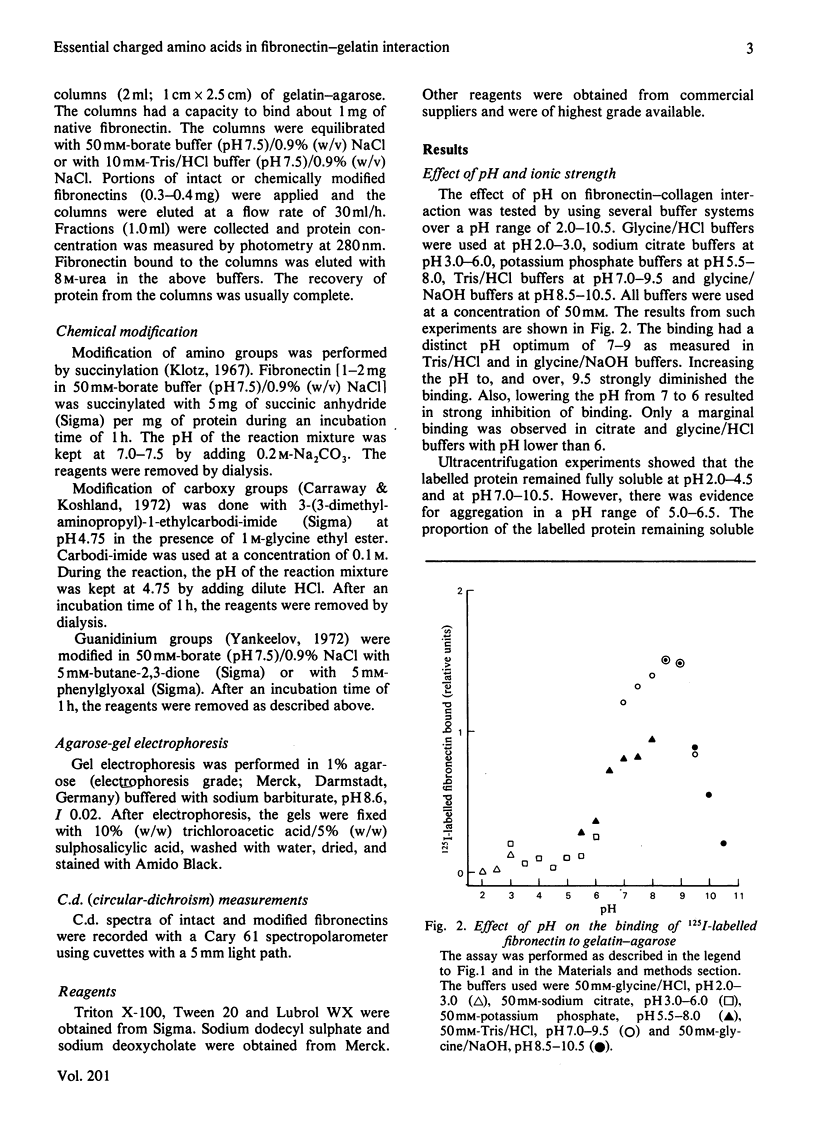
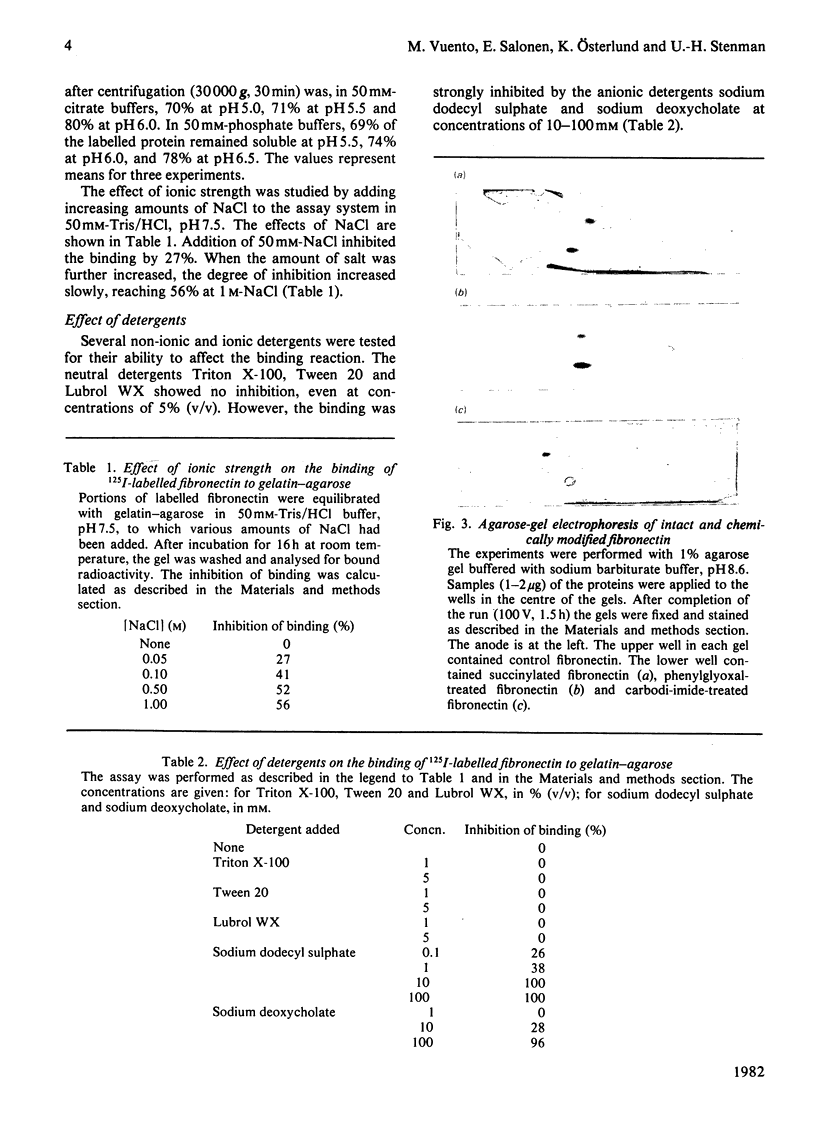
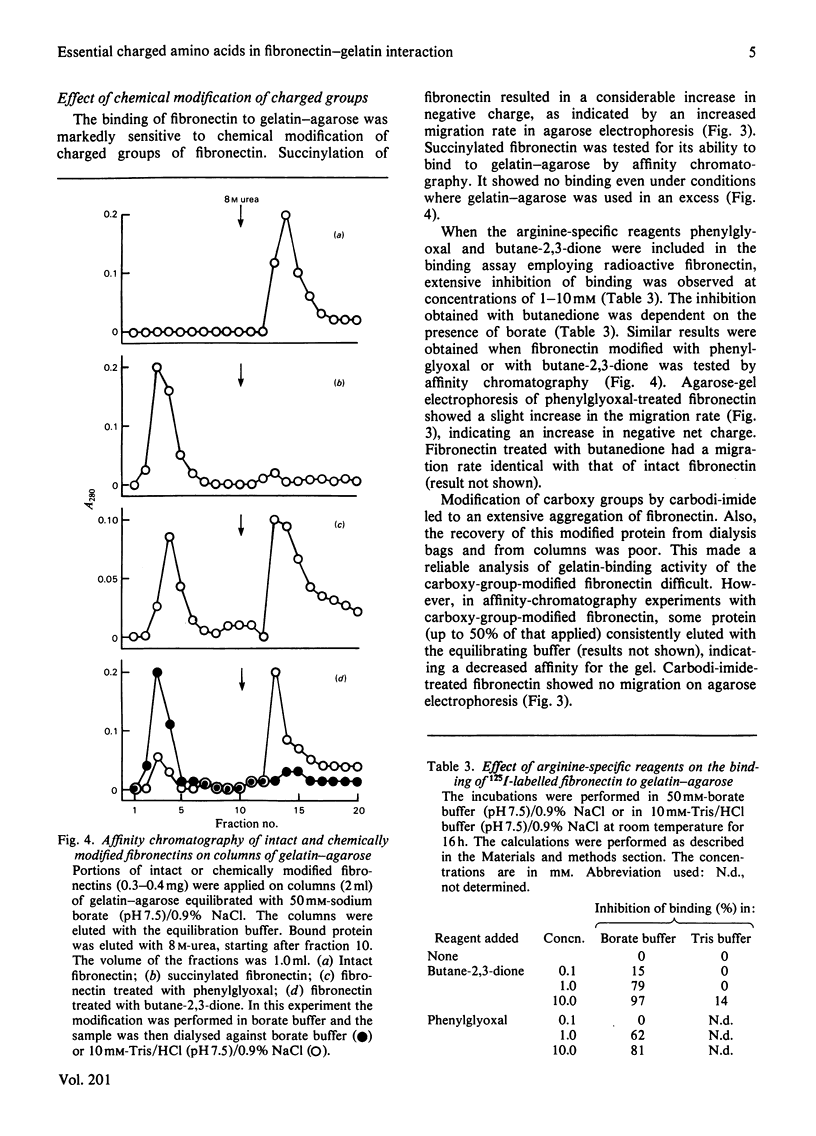
The binding of fibronectin to gelatin-agarose was strictly dependent on pH, having a pH optimum of 7-9. The binding was strongly inhibited by increasing ionic strength. A chemical modification of lysyl and arginyl groups of fibronectin abolished the binding activity. The anionic detergents sodium dodecyl sulphate and sodium deoxycholate in concentrations of 10-100mM had the same effect. The binding was not affected by the non-ionic detergents Triton X-100, Tween 20 or Lubrol WX. The results demonstrate an important role of ionic interactions in the binding of fibronectin to gelatin. Absence of inhibition by non-ionic detergents suggests that hydrophobic interactions contribute relatively little to the binding of fibronectin to gelatin.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balian G., Click E. M., Bornstein P. Location of a collagen-binding domain in fibronectin. J Biol Chem. 1980 Apr 25;255(8):3234–3236. [PubMed] [Google Scholar]
- Colonna G., Alexander S. S., Jr, Yamada K. M., Pastan I., Edelhoch H. The stability of cell surface protein to surfactants and denaturants. J Biol Chem. 1978 Nov 10;253(21):7787–7790. [PubMed] [Google Scholar]
- Engvall E., Ruoslahti E. Binding of soluble form of fibroblast surface protein, fibronectin, to collagen. Int J Cancer. 1977 Jul 15;20(1):1–5. doi: 10.1002/ijc.2910200102. [DOI] [PubMed] [Google Scholar]
- Engvall E., Ruoslahti E., Miller E. J. Affinity of fibronectin to collagens of different genetic types and to fibrinogen. J Exp Med. 1978 Jun 1;147(6):1584–1595. doi: 10.1084/jem.147.6.1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold L. I., Pearlstein E. Fibronectin-collagen binding and requirement during cellular adhesion. Biochem J. 1980 Feb 15;186(2):551–559. doi: 10.1042/bj1860551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold L. I., Pearlstein E. Stability of fibronectin biological activity following chemical modification. Biochim Biophys Acta. 1979 Dec 14;581(2):237–251. doi: 10.1016/0005-2795(79)90243-5. [DOI] [PubMed] [Google Scholar]
- Grinnell F., Minter D. Cell adhesion and spreading factor: chemical modification studies. Biochim Biophys Acta. 1979 Jan 5;550(1):92–99. doi: 10.1016/0005-2736(79)90117-2. [DOI] [PubMed] [Google Scholar]
- Hahn L. H., Yamada K. M. Identification and isolation of a collagen-binding fragment of the adhesive glycoprotein fibronectin. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1160–1163. doi: 10.1073/pnas.76.3.1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jilek F., Hörmann H. Cold-insoluble globulin (fibronectin), IV[1-35 affinity to soluble collagen of various types. Hoppe Seylers Z Physiol Chem. 1978 Feb;359(2):247–250. doi: 10.1515/bchm.1978.359.1.247. [DOI] [PubMed] [Google Scholar]
- Lange L. G., 3rd, Riordan J. F., Vallee B. L. Functional arginyl residues as NADH binding sites of alcohol dehydrogenases. Biochemistry. 1974 Oct 8;13(21):4361–4370. doi: 10.1021/bi00718a019. [DOI] [PubMed] [Google Scholar]
- Mosesson M. W., Amrani D. L. The structure and biologic activities of plasma fibronectin. Blood. 1980 Aug;56(2):145–158. [PubMed] [Google Scholar]
- Mosesson M. W., Chen A. B., Huseby R. M. The cold-insoluble globulin of human plasma: studies of its essential structural features. Biochim Biophys Acta. 1975 Apr 29;386(2):509–524. doi: 10.1016/0005-2795(75)90294-9. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E., Engvall E. Immunochemical and collagen-binding properties of fibronectin. Ann N Y Acad Sci. 1978 Jun 20;312:178–191. doi: 10.1111/j.1749-6632.1978.tb16802.x. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E., Hayman E. G., Kuusela P., Shively J. E., Engvall E. Isolation of a tryptic fragment containing the collagen-binding site of plasma fibronectin. J Biol Chem. 1979 Jul 10;254(13):6054–6059. [PubMed] [Google Scholar]
- Ruoslahti E., Hayman E. G. Two active sites with different characteristics in fibronectin. FEBS Lett. 1979 Jan 15;97(2):221–224. doi: 10.1016/0014-5793(79)80088-5. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E., Vuento M., Engvall E. Interaction of fibronectin with antibodies and collagen in radioimmunoassay. Biochim Biophys Acta. 1978 Jun 21;534(2):210–218. doi: 10.1016/0005-2795(78)90003-x. [DOI] [PubMed] [Google Scholar]
- Saba T. M., Jaffe E. Plasma fibronectin (opsonic glycoprotein): its synthesis by vascular endothelial cells and role in cardiopulmonary integrity after trauma as related to reticuloendothelial function. Am J Med. 1980 Apr;68(4):577–594. doi: 10.1016/0002-9343(80)90310-1. [DOI] [PubMed] [Google Scholar]
- Sekiguchi K., Hakomori S. Functional domain structure of fibronectin. Proc Natl Acad Sci U S A. 1980 May;77(5):2661–2665. doi: 10.1073/pnas.77.5.2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaheri A., Kurkinen M., Lehto V. P., Linder E., Timpl R. Codistribution of pericellular matrix proteins in cultured fibroblasts and loss in transformation: fibronectin and procollagen. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4944–4948. doi: 10.1073/pnas.75.10.4944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaheri A., Mosher D. F. High molecular weight, cell surface-associated glycoprotein (fibronectin) lost in malignant transformation. Biochim Biophys Acta. 1978 Sep 18;516(1):1–25. doi: 10.1016/0304-419x(78)90002-1. [DOI] [PubMed] [Google Scholar]
- Vuento M., Salonen E., Salminen K., Pasanen M., Stenman U. K. Immunochemical characterization of human plasma fibronectin. Biochem J. 1980 Dec 1;191(3):719–727. doi: 10.1042/bj1910719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuento M., Vaheri A. Dissociation of fibronectin from gelatin-agarose by amino compounds. Biochem J. 1978 Oct 1;175(1):333–336. doi: 10.1042/bj1750333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuento M., Vaheri A. Purification of fibronectin from human plasma by affinity chromatography under non-denaturing conditions. Biochem J. 1979 Nov 1;183(2):331–337. doi: 10.1042/bj1830331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuento M., Vartio T., Saraste M., von Bonsdorff C. H., Vaheri A. Spontaneous and polyamine-induced formation of filamentous polymers from soluble fibronectin. Eur J Biochem. 1980 Mar;105(1):33–42. doi: 10.1111/j.1432-1033.1980.tb04471.x. [DOI] [PubMed] [Google Scholar]
- Vuento M., Wrann M., Ruoslahti E. Similarity of fibronectins isolated from human plasma and spent fibroblast culture medium. FEBS Lett. 1977 Oct 15;82(2):227–231. doi: 10.1016/0014-5793(77)80590-5. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]



