Abstract
Herrin, three Gemini cationic surfactants related to benzo[d]thiazol-3-ium bromide with variable hydrocarbon chain lengths (TBC n = 6, 12, and 18) were synthesized successfully and confirmed by using IR and 1HNMR spectroscopies. Critical micelle concentration and different thermodynamic properties of all surfactants under study were measured using conductivity, density, molal volume, and refractive index techniques. The Critical micelle concentration of TBC 6, TBC 12, and TBC 18 surfactants measured from the different techniques shows an acceptable agreement. The molecular weight of the investigated surfactants was decreased with the order: TBC 18 > TBC 12 > TBC 6. An increase in the magnitudes of the association constant, Gibbs free energy of micellization, molar refraction, polarizability, and binding constant proved the effect of hydrocarbon chain length on increasing surfactant’s micellization as follows: TBC 18 < TBC 12 < TBC 6. The enhancement in surfactant properties was also indicated under the effect of different concentrations of inorganic salts (NaI, NaBr, NaCl, MnCl2, CuCl2, and CoCl2). This effect was measured using conductivity and refractive index measurements. Different salts were indicated to adsorb on head groups of micelles, leading to an increase in the degree of ionization of the surfactant solution and improved aggregation of the surfactant at lower concentrations. The increase in the negative value of Gibbs free energy of association in the presence of salts proved an increase in the stability of micelles formed in a 15% DMSO-water solvent at 298.15 K.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13065-024-01334-9.
Keywords: Gemini cationic surfactants, Micellization, Conductivity, Polarizability
Introduction
Gemini cationic surfactants have gained much attention in several fields, including detergents, coatings, biocides, pharmacy, food processing, enhanced oil recovery, and environmental protection [1–4]. By doubling the hydrophilic head and hydrophobic tail, Gemini cationic surfactants have higher surface activity and a lower critical micelle concentration [5]. The hydrophilic heads were separated with spacers with different compositions [6]. Aggregation is induced by attraction between the hydrophobic tails, while an interfacial area is ensured by repulsion between the hydrophilic head groups [7]. By altering the hydrophobic tail's length and the head groups and adjusting this force balance between opposing forces, the micellization of surfactant can be tuned [8, 9].
The critical micelle concentration (CMC) is what determines how effective surfactants are. When any of the physicochemical properties are plotted versus surfactant concentration, an inflection point can be found that represents the minimal range of concentration at which the surfactant monomer begins to self-associate [10–12]. Several direct techniques used to measure critical micelle concentrations of surfactants include surface tension, conductivity, osmotic pressure, refractive index, and viscosity [13–18]. Also, CMC may be measured indirectly by volumetric and spectrometric methods [16, 19].
Adding inorganic salts to surfactant solutions increases their importance in several emulsifications in the industry. Salting-out frequently occurs when surfactant and salt are combined in a solution. The preferential migration of water molecules from the coordination shells of surfactant molecules to those of salts, which immobilizes and quenches their activity as solvents, is the cause of salting-out, according to hydration theory [20–24]. The inorganic salts affect the morphology of surfactants leading to increased hydrophobicity [9].
Thermodynamic parameters were indicated, such as the degree of ionization, binding constant, Gibbs free energy of micellization and association, molar refraction, atomic polarizability, Van der Waals volume, and electrostriction volume [25–27]. Properties of all surfactants were indicated to change under the effect of different inorganic salts at different concentrations [20, 23, 28]. Salts are indicated to improve micellization of surfactants in solution at a lower concentration [28–31]. Improvements in the properties of surfactants were computed related to solvation parameters, including the binding constant of counter ions and Gibbs free energy of micellization and association from conductivity technique [32, 33]. Changes in refractive indices proved the enhancement in the micellization process in the presence of salts.
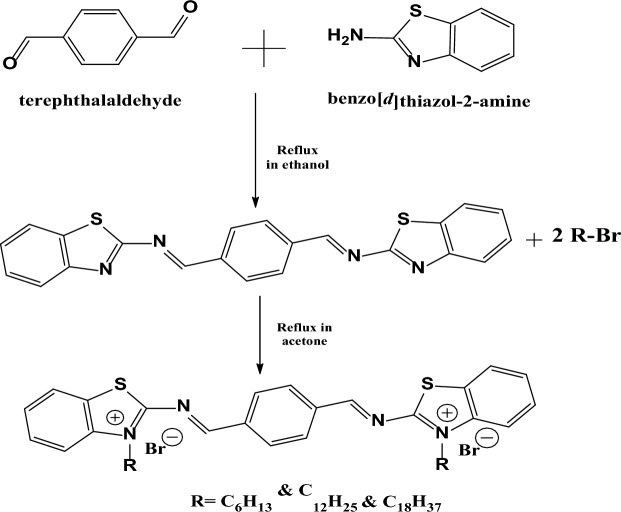
In this study, the synthesis of three new cationic Gemini surfactants from the reaction between terephthalaldehyde and benzo[d]thiazole-2amine, which followed a reaction with various alkyl halides, was discussed. The structure of all surfactants was confirmed using 1H-NMR and IR spectroscopy. The critical micelle concentration of all surfactants was measured using conductivity, refractive index, density, and molar volume techniques. Various thermodynamic parameters related to the mentioned techniques were discussed. For all surfactants for which parameter changes were explained, the effect of two different concentrations of six inorganic salts was reported. All measurements were reported in 15% dimethyl sulfoxide (DMSO)-water solvent.
Experimental
Materials
All materials used in this study were selected with a high purity value indicated in a mass percent as follows: terephthaladehyde (99%), benzo[d]thiazol-2-amine (99%), bromohexane (98%), bromododecane (97%), bromooctadecane (97%), acetone (99.9%), ethanol (99.9%), sodium chloride (98%), sodium bromide (98%), sodium iodide (98%), cobalt chloride (98%), manganese chloride (98%) and copper chloride (98%). All chemicals were purchased from the international Sigma Aldrich company, United States without needing any purification. Distilled water used in this study had a conductivity of less than 2 µS cm−1.
Synthesis of TBC Gemini cationic surfactant
Terphtalaldehye (0.1 mol) was refluxed with 0.1 mol of benzo[d]thiazol-2amine for 12 h in the presence of ethanol (100 mL) as a solvent and 0.01% (by weight) of p-toluene sulphonic acid as a dehydrating agent. The reaction mixture was allowed to cool overnight and then filtered. The products were recrystallized twice from ethanol, washed with water, and dried under vacuum at 60 °C to afford crystals of (1E,1′E)-1,1′-(1,4-phenylene)bis(N-(benzo[d]thiazol-2-yl)methanimine). Which was then refluxed with (0.2 mol) of different alkyl halides (R = C6H13 & C12H25 & C18H37) in acetone for 24 h. The reaction mixture was allowed to cool overnight and then filtered. The products were recrystallized twice from acetone, washed with water, and dried under vacuum at 60 °C to afford crystals of 2,2′-(1E,1′E)-1,4-phenylenebis(methaneylylidene) bis(azaneylylidene))bis(3-hexylbenzo[d]thiazol-3-ium)bromide (TBC6); 2,2′-(((1E,1′E)-1,4 phenylenebis(methaneylylidene))bis(azaneylylidene))bis(3-dodecylbenzo[d]thiazol-3-ium) bromide (TBC12) and 2,2′-(((1E,1′E)-1,4-phenylenebis(methaneylylidene))bis(azaneylylidene)) bis(3-octa decylbenzo[d]thiazol-3-ium) bromide (TBC18) as shown in Scheme 1.
Scheme 1.
Synthetic Route of TBC Gemini Cationic Surfactants
Characterization of TCB Gemini cationic surfactants
The structure of all synthesized surfactants (TBC6, TBC12 and TBC 18) was confirmed using a Thermo-Fisher Scientific Nicolet iS10 FT-IR spectrometer, United States with a range of 400–4000 cm−1. All solid surfactants were combined with potassium bromide pellets. Additionally, 1HNMR spectroscopy was used to support the conformation of all surfactant structures, where the solid surfactants are dissolved in DMSO solvent as part of sample preparation. Chemical shifts in ppm of nuclear spin of functional groups were then calculated using a 500 MHz JNM-ECA series FT-NMR, United States.
Solvation measurements
Stock solutions of all studied surfactants (TBC6, TBC12, and TBC18) were prepared at a concentration of 0.001 M in 15% DMSO-water solvent. Salt solutions including NaCl, NaBr, NaI, CoCl2, MnCl2, and CuCl2 were prepared at two concentrations (0.01 M and 0.001 M) in 15% DMSO-water solvent.
Conductivity measurement
4510 JENWAY CONDUCTIVITY METER 0–1.999S, United Kingdom (± 0.050 µS cm−1) and temperature accuracy ± 0.1 °C was calibrated using standard KCl solution and measured cell constant equal to 1 cm−1 [34]. The temperature was maintained and remained constant with the aid of the recirculation thermostat “ultraterm 200” adjustable temperatures from ambient + 5 °C to 200 °C (± 0.2 °C) (JP Selecta, Spain). A specific volume of each surfactant (0.5 mL or 1 mL) was added to 15% DMSO-water solvent in the absence and presence of different concentrations of inorganic salts with homogeneous mixing. Conductivity measurement was performed using an epoxy-bodied Conductivity Electrode at 298.15 K.
Refractive index
After the addition of a specific volume of all studied surfactants (0.5 mL or 1 mL) to 15% DMSO-water solvent in the absence and presence of different concentrations (0.001 M and 0.01 M) of inorganic salts at 298.15 K. The Mettler Toledo refractometer (± 0.0001), United States was used to determine the refractive index of the solutions by putting one drop of the solution on the center of the prism and then determining the displayed results [35].
Density
Density measurements were performed using one milliliter from the pure 15% DMSO-water solvent and the 15% DMSO-water solvent after adding a specific volume of each surfactant (1 mL). A Balance Digital Electronic (± 0.1 mg), United States was used to measure the weight of all previously mentioned solutions.
Molal volume
Molal volume measurement is a theoretical measurement of CMC of all surfactants from the data observed from density for pure solvent 15% DMSO-water solvent in the absence and the presence of different surfactant volumes [36].
Surface tension
Attention optical tensiometer theta lite, Biolin scientific, China (Measuring range 0.01–2000) (Accuracy ± 0.01 mN/m) with one attention software was used to measure surface tension (γ) through the contact angle of different concentrations of all examined surfactants. By detecting the diameter of the pendant drop of each surfactant solution for 30 s and then automatically measuring its surface tension [37].
Results and discussion
Structure confirmation of synthesized Gemini cationic surfactants
The chemical structure of the synthesized TBC Gemini cationic surfactants was confirmed using 1HNMR spectroscopy and IR spectroscopies.
1HNMR chemical shift
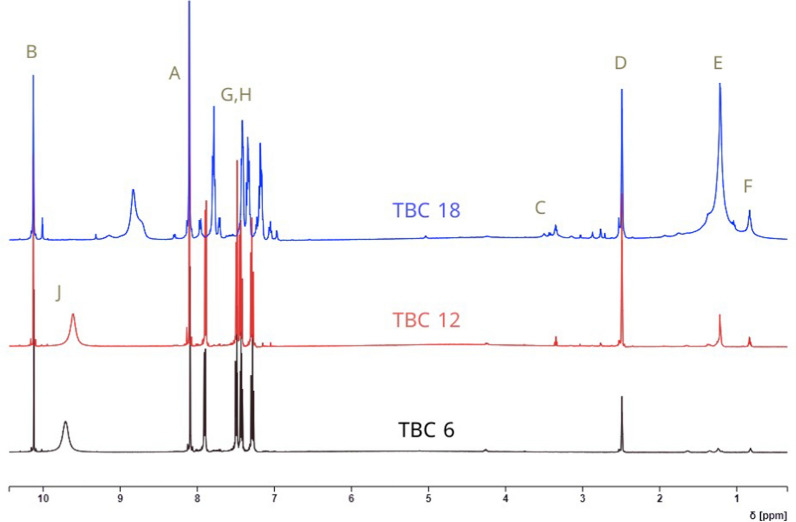
The chemical shifts in ppm for all surfactants were shown in Fig. 1 while the functional groups appeared in Structure 1 with symbol (a–j). The peaks at 0.84–0.85 (s, 1H, (f) –CH3); 1.02–1.36 (t, 6H, (e) –CH2); 2.49 (d, 4H, (d) –CH2); 3.49–3.52 (s, 2H, (c) –CH2); 10.12–10.15 (s, 1H, (b) –N = CH-R); 8.02–8.1 (s, 2H, (a) –CH); 7.48–7.88 (t, 3H, (g, h) –CH); 9.61–9.71 (s, H, (j) –CH).
Fig. 1.
1HNMR spectrum of all surfactants in DMSO solvent
Structure 1.
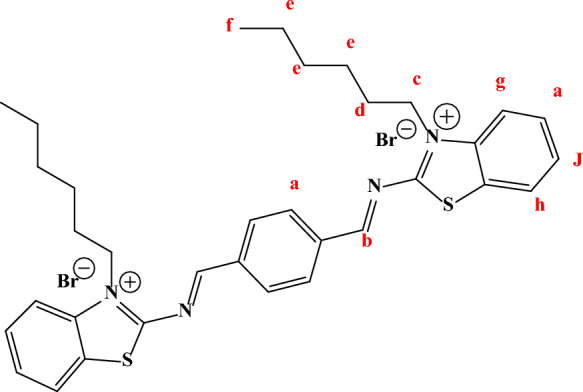
Chemical structure of TBC 6 with numbered at different positions (a–h)
IR analysis
The IR spectrum for (TBC6, TBC12, and TBC18) Gemini cationic surfactants is represented in Fig. 2. The absorption band of C–Br at 620–623 cm−1, aliphatic symmetric CH at 2850 cm−1, symmetric bending CH2 at 1464 cm−1, symmetric bending CH3 at 1375 cm−1, rock –(CH2)n– at 752 cm−1, aromatic amine C–N+ at 1202 cm−1, aromatic imine C=N 1691 cm−1, aromatic bending CH at 3042 cm−1, aromatic C=C at 1775 cm−1. While DMSO used as a solvent in sample preparation showed an absorption band at 1010 cm−1.
Fig. 2.
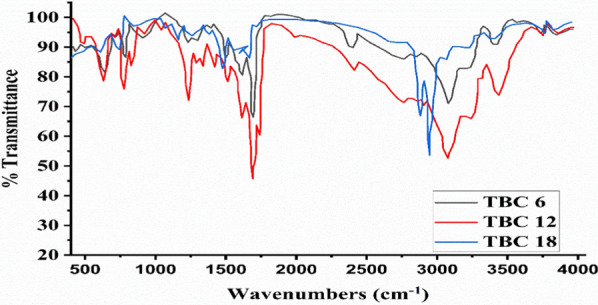
IR spectra for All Gemini cationic surfactants TBC 6, TBC 12, and TBC 18
Solvation indication
Critical micelle concentration measurement
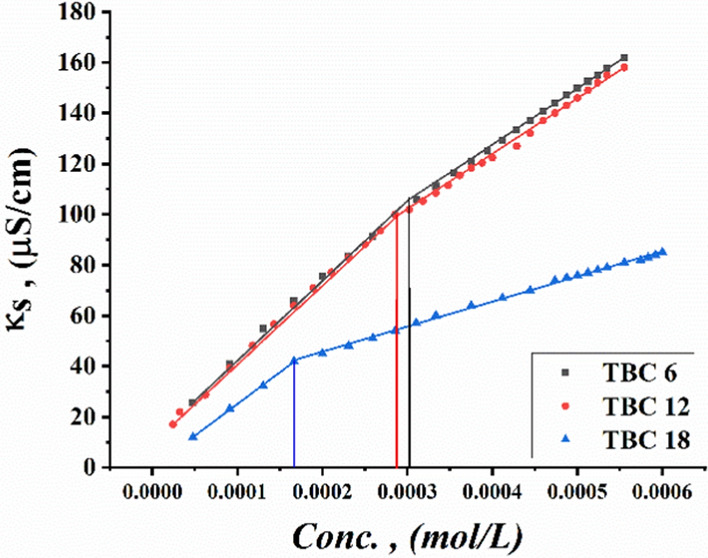
Critical micelle concentrations of all synthesized surfactants TBC 6, TBC 12, and TBC 18 were measured by plotting various parameters, including specific conductivity, refractive index, density, molal volume, and surface tension against the concentration of the solutions after each addition of 0.001 M of each surfactant to 15% DMSO-water solvent at 298.15 K as shown in Figs. 3, 4, 5, 6, 7. The CMC values reported by each technique are summarized in Table 1.
Fig. 3.
Conductivity vs. Molar Concentration for all surfactants TBC 6, TBC 12, and TBC 18 in 15% DMSO-water at 298.15K
Fig. 4.
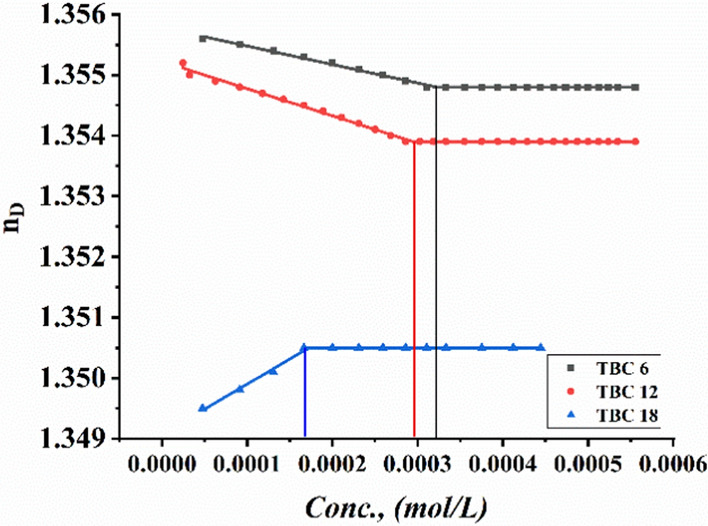
Refractive index vs. Molar Concentration for all surfactants TBC 6, TBC 12, and TBC 18 in 15% DMSO-water at 298.15K
Fig. 5.
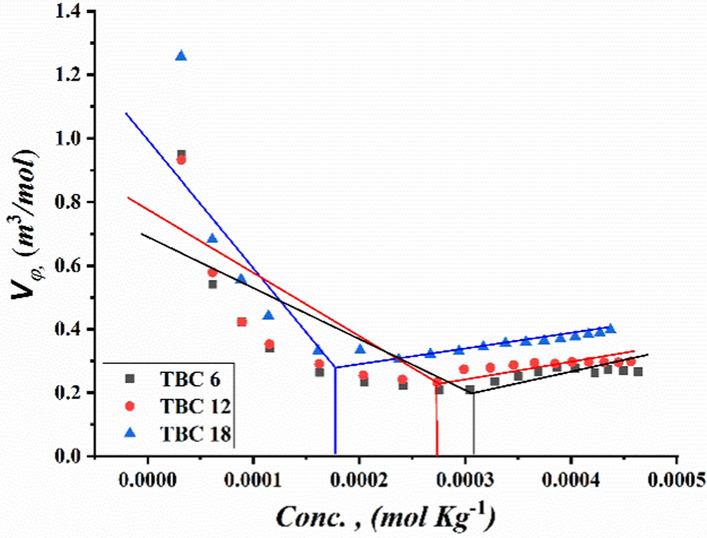
Molal Volume vs. Molal Concentration for all surfactants TBC 6, TBC 12, and TBC 18 in 15% DMSO-water at 298.15K
Fig. 6.
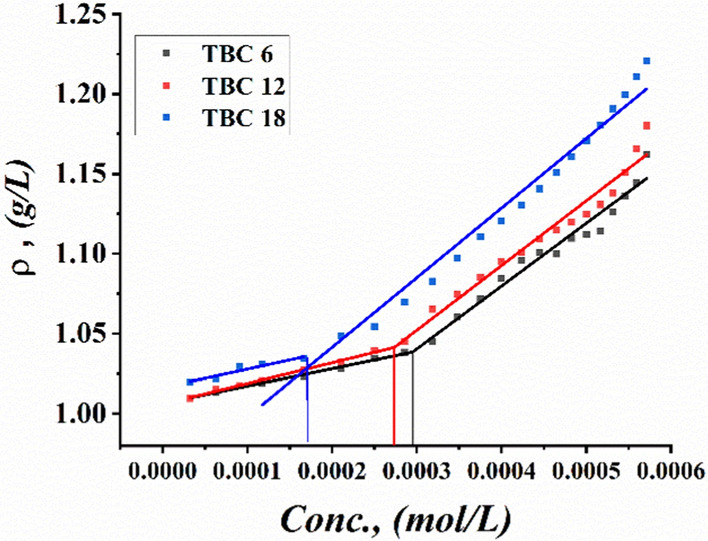
Densities vs. Molar Concentration for all surfactants TBC 6, TBC 12, and TBC 18 in 15% DMSO-water at 298.15K
Fig. 7.
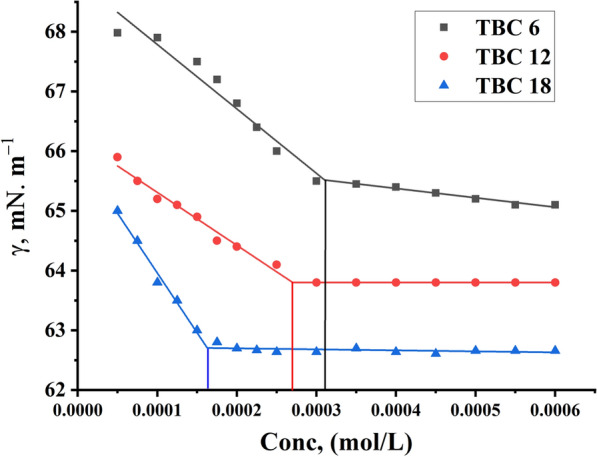
Surface tension vs. Molar Concentration for all surfactants TBC 6, TBC 12, and TBC 18 in 15% DMSO-water at 298.15K
Table 1.
CMC values of all surfactants in 15% DMSO-water solvent
| Surfactant abbreviation | CMC | ||||
|---|---|---|---|---|---|
| Conductivity (mol L−1) | Refractive index (mol L−1) | Density (mol L−1) | Molal volume (mol kg−1) | Surface tension (mol L−1) | |
| TBC 6 | 0.000302 | 0.000309 | 0.000294 | 0.000300 | 0.000312 |
| TBC 12 | 0.000282 | 0.000286 | 0.000270 | 0.000265 | 0.000270 |
| TBC 18 | 0.000166 | 0.000167 | 0.000171 | 0.000177 | 0.000164 |
Standard uncertainties (u) of CMC is = 0.00002 mol L−1
The Specific conductivity tends to increase with increasing mobility of the free monomers and dimers of each surfactant solvated in a 15% DMSO-water solvent [38]. There is a sharp decrease in the conductivities of all studied surfactant solutions which may be related to a decrease in the diffusion rate of each surfactant under study solvated in the same solvent. This is due to the onset of micelle formation for all surfactants TBC 6, TBC 12, and TBC 18 as shown in Fig. 3. The decrease in refractive index of TBC 6 and TBC 12 in 15% DMSO-water solvent shown in Fig. 4 is related to an increase in hydrophobic solvation between hydrocarbon chains bound to surfactant and 15% DMSO-water [39].
The molal volume of all surfactant solutions was indicated to decrease with the addition of each surfactant to 15% DMSO-water solvent before CMC. This is related to a decrease in solvent molecules bound to the hydrocarbon chains of each surfactant. After reaching CMC, it was observed that the molal volume of all studied surfactants was observed to be constant as shown in Fig. 5. This is related to the formation of micelles between the individual surfactant molecules. In contrast, the solvent molecules surrounding each surfactant are removed [40].
The densities of all studied surfactants tend to increase with each addition of all surfactants under study to 15% DMSO-water solvent as shown in Fig. 6. This is related to an increase in the molecular weight of each surfactant added to 15% DMSO-water solvent. The molecular weights of all surfactants examined were arranged in the following order: TBC 18 < TBC 12 < TBC 6 [41].
Surface tension measurement serves as a common method for determining the CMC where Gemini cationic surfactants adhere to the solution-air interface forming a monolayer. This layer is used to reduce the cohesive forces among solution molecules [42]. As illustrated in Fig. 7, the surface tension coefficient of the surfactant solutions decreases with increasing surfactant concentration till reaching their CMC [43]. However, this technique was revealed ineffectively to accurately measuring the aggregation properties of all studied Gemini cationic surfactants. This restriction might result from the solvent composition, a 15% DMSO-water mixture, that is utilized to dissolve the surfactants TBC 6, TBC 12, and TBC 18 under investigation. Polar aprotic solvent dimethyl sulfoxide (DMSO) with a high Dielectric constant may reduce the surface activity of surfactants [44]. Furthermore, disables the surface activity of surfactants by damaging their micellar structure [45].
Comparison between the critical micelle concentration of all investigated surfactants TBC 6, TBC 12, and TBC 18 in 15% DMSO-water solvent at 298.15 K was indicated as shown in Fig. 8. There was good agreement between the measuring of CMC all surfactants in the study indicated by all techniques. It was stated that the critical micelle concentration of all techniques is arranged in order TBC18 < TBC12 < TBC6.
Fig. 8.
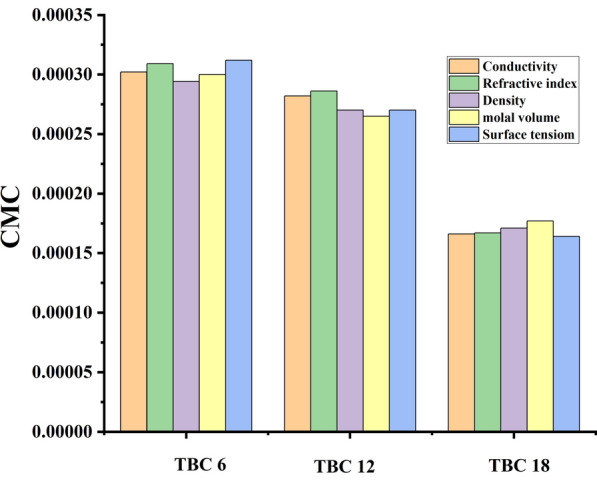
Comparison between CMC measuring from different techniques
Thermodynamics parameters from conductivity technique
Molar conductivity (ʌ) for all examined surfactants in 15% DMSO-water solvent at 298.15 K was calculated from Eq. (1)
| 1 |
While limiting molar conductivity ( was estimated from Eq. (2)
| 2 |
binding constant for Counterions (β) was calculated by measuring the degree of ionization (α) of all surfactants in pure 15% DMSO-water solvent according to Eq. (3).
| 3 |
where S2/S1 represents the ratio between the slope of post-micelles to pre-micelles.
Calculation of Gibbs free energies of micellization () for monomer and dimer in 15% DMSO-water solvent were indicated with Eqs. (4, 5).
| 4 |
| 5 |
where; R is gas constant and T is absolute temperature under study = 298.15K.
Gibbs free energies for an association of all surfactants under study () in 15% DMSO-water solvent were indicated using Eq. (6).
| 6 |
where; the Ka association constant for all surfactants under different conditions was calculated with helping of Shedlovsky Eq. (7) [46].
| 7 |
where; 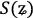 is a Shedlovsky function that can be calculated from Eq. 8 and Eq. 9, is the activity coefficient, (Λ) is molar conductivity, (C) is a CMC concentration of all studied surfactants and (Λ0) is limiting molar conductivity.
is a Shedlovsky function that can be calculated from Eq. 8 and Eq. 9, is the activity coefficient, (Λ) is molar conductivity, (C) is a CMC concentration of all studied surfactants and (Λ0) is limiting molar conductivity.
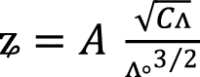 |
8 |
| 9 |
where; (A) is the Onsager coefficient [47] equal , (ε) is the dielectric constant, (T) is the absolute temperature, and is the viscosity of the surfactant solution.
The counterion binding constant and association constant of surfactants in 15% DMSO-water solvent tended to increase in the order TBC 6 > TBC 12 > TBC 18 as shown in Table 2. This increase follows the increase in the hydrocarbon chain length of the surfactants [48]. Gemini cationic surfactant TBC 18 was indicated to have the highest values of association and binding constant and so the largest micelle in 15% DMSO-water solvent [49, 50].
Table 2.
Thermodynamics parameters from conductivity measurement
| Sur. name | (kJ mol−1) | (kJ mol−1) | (kJ mol−1) | |||
|---|---|---|---|---|---|---|
| TBC 6 | 0.7135 | 0.2865 | − 25.826 | − 31.60 | 5653.880 | − 22.50 |
| TBC 12 | 0.5620 | 0.4380 | − 29.073 | − 38.01 | 12,586.78 | − 24.58 |
| TBC 18 | 0.3989 | 0.6011 | − 34.519 | − 47.51 | 307,900.6 | − 32.91 |
Standard uncertainties (u) of α and β are = 0.002
Gibbs free energies of association and micellization of all studied surfactants in 15% DMSO-water solvent showed an increase in the following arrangement: TBC 18 > TBC 12 > TBC 6. This may be related to an increase in micelle formation with an increase in the hydrocarbon chain length of surfactants [51]. The negative value of the Gibbs free energies for micellization and association was found to be spontaneous for these processes [52]. Different models were used to measure Gibbs free energies for studied Gemini cationic surfactants including the phase separation model [53] and pseudo-phase separation model [54]. Both models proved that the increase in hydrocarbon chain length led to an increase in micellization as a following arrangement TBC 18 < TBC 12 < TBC 6.
The decrease in the degree of ionization (α) has been attributed to a shift in charge density at the micelle surface as the chain length increases. A longer chain length results in a higher degree of aggregate compactness, and the “head groups” tend to approach more, indicating that more counter ions are drawn to the Stern layer surrounding the “heads” and lowering the micelle ionization degree [55].
Molal volume
From the measurement of the density of the pure solvent () and the density of the solution after each addition of all surfactants to 15% DMSO-water solvent (), the molal volume of all investigated surfactants was calculated as in Eq. (10).
| 10 |
where; M is the molecular weight of each surfactant and m is the molal concentration of each surfactant in the solvent.
The packing density () had a constant value of 0.661 for the large molecules. The van der Waals volume () and electrostriction volume () were calculated from Eq. (11).
| 11 |
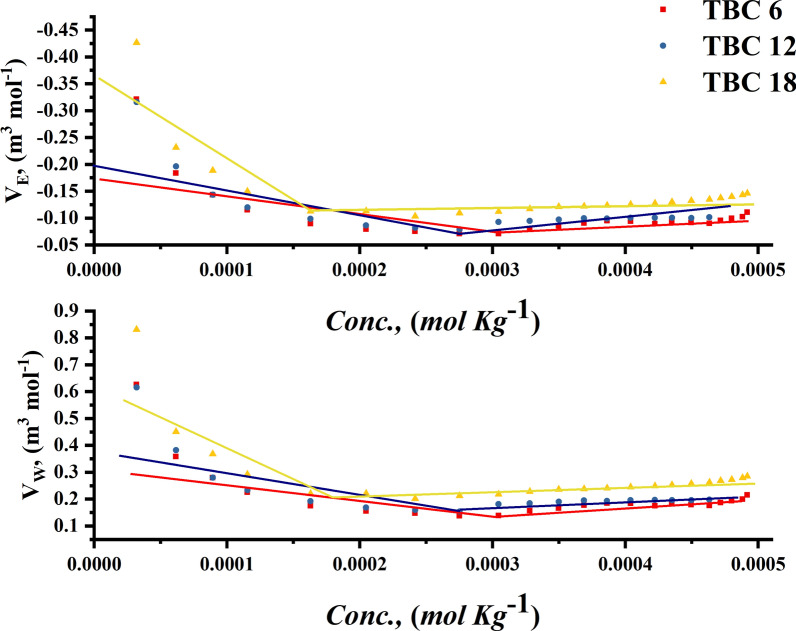
The changes in electrostriction volume and van der Waals volume for all investigated surfactants are shown in Fig. 9.
Fig. 9.
The relationship between molal concentration against Van der Waals Volume (Vw) and electrostriction Volume (VE) for all surfactants
The interaction between all studied surfactants (TBC6, TBC12, and TBC18) and 15% DMSO-water solvent was shown to decrease with increasing surfactant concentrations [56]. This may be related to a decrease in solvation between the hydrophobic hydrocarbon chains of surfactants and the solvent molecules surrounding these chains [57, 58]. The decrease in solvation leads to a reduce the surface area of interaction and enhanced steric hindrance between surfactant molecules leading to decreasing in the Van der Waals volume [59, 60]. The chemical structure of surfactants, including the nature of their hydrophilic and hydrophobic groups and the high polarity of 15% DMSO-water solvent influences how Gemini cationic surfactants respond to electric fields [61]. The presence of an electric field can alter the size, shape, and stability of these aggregates. As shown in Fig. 9, the electrostriction volume of all studied surfactant solutions decreases which may be related to that the micelles become more stable and reduce their ability to undergo significant volume changes in response to electric fields [62].
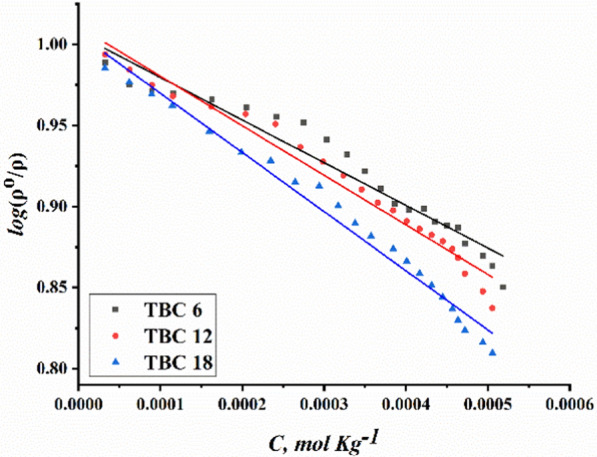
Modeling of densities
From the calculation, the density and molal volumes of all surfactants in 15% DMSO-water at different concentrations at 298.15K. This modeling is indicated according to the Setschenow relationship shown in Eq. (12).
| 12 |
where; is the ratio between the density of 15% DMSO-water solvent and each addition of all surfactants (TBC6, TBC12, and TBC18) into the solvent.
The densities of the surfactant solutions (TBC6, TBC12, and TBC18) were reported to increase with the addition of each surfactant in 15% DMSO-water solvent at 298.15 K as shown in Fig. 10. This could be related to the increase in molecular weight of surfactant in the following arrangement: TBC18 < TBC12 < TBC6. It is stated that the Setschenow constant increases with increasing hydrocarbon chain length as summarized in Table 3.
Fig. 10.
Modeling changes in densities of all surfactants TBC 6, TBC 12, and TBC 18 in 15% DMSO-water solvent
Table 3.
The Setschenow parameter
| Surfactant abbreviation | K | R2 |
|---|---|---|
| TBC 6 | − 263.09 | 0.96 |
| TBC 12 | − 305.64 | 0.98 |
| TBC 18 | − 364.84 | 0.96 |
The negative values of the Setschenow constant indicate an increase in the density of the solutions after adding the investigated surfactants TBC 6, TBC 12, and TBC 18 to 15% DMSO-water solvent. An increase in the values of the setschenow constant indicates the direction of density arrangement in the following TBC18 < TBC12 < TBC6. While the Gemini cationic surfactant TBC18 has the greatest value as it has the largest molecular weight [63].
Refractive index
Molar refraction () and atomic polarization () for all newly Gemini cationic surfactants TBC 6, TBC 12, and TBC 18 were calculated from data observed from refractive indices measurement at a fixed concentration in 15% DMSO-water solvent at 298.15K by using Eq. (13).
| 13 |
The polarizability () of all surfactants TBC 6, TBC 12, and TBC 18 in 15% DMSO-water solvent at 298.15K was calculated from Eq. (14) by using the Avogadro number (N).
| 14 |
when comparing the molar refraction and polarizability values of all surfactants (TBC6, TBC12, and TBC18) as shown in Table 4, it was observed that the data increased as the hydrocarbon chain of the surfactants increased. This is related to the increasing strength of the hydrophobic interaction between the hydrocarbon chain of surfactants and the surrounding 15% DMSO-water solvent molecules. The surfactant TBC 18 was reported to have the highest values in molar refraction and polarizability, thus ensuring the strongest hydrophobic solvation [64].
Table 4.
refractive index (nD), molar refraction (), atomic polarizability (PA), and the polarizability () of TBC 6, TBC 12, and TBC 18 at the same concentration in 15% DMSO-water at 298.15K
| Molal Conc. | Surfactants | nD | (m3 mol−1) | (m3 mol−1) | (m3) E-26 |
|---|---|---|---|---|---|
| 0.00013 | TBC 6 | 1.3553 | 0.0742 | 1.9287 | 2.94 |
| TBC 12 | 1.3548 | 0.0769 | 1.9273 | 3.05 | |
| TBC 18 | 1.3505 | 0.0951 | 1.9150 | 3.77 |
Standard uncertainties (u) of nD = 0.02, Rm=0.001, PA = 0.03, αD = 0.04
Enhancing aggregation properties under salts effect
Detection of CMC
Conductometric techniques
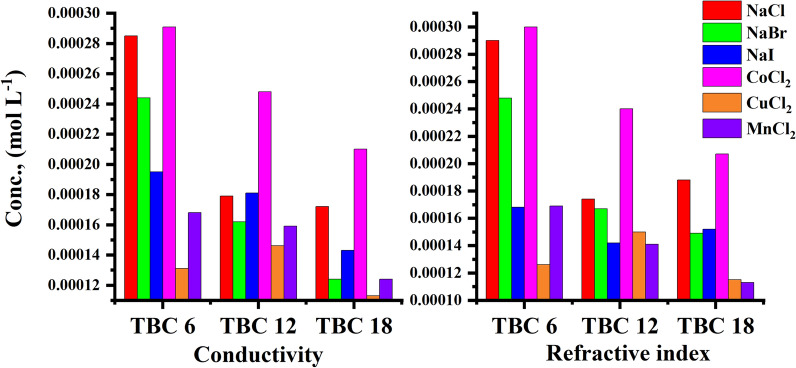
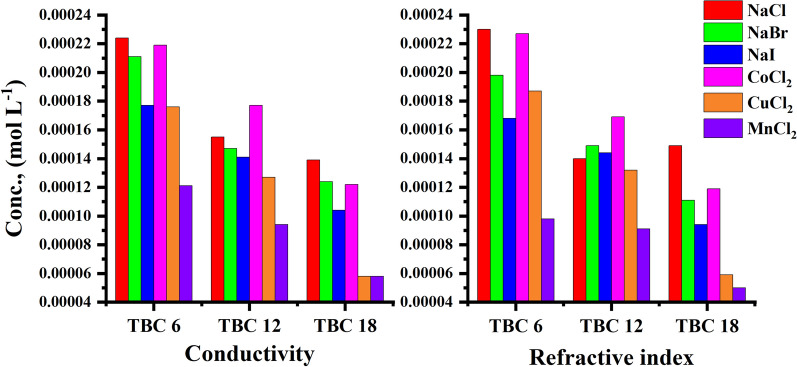
The effect of the addition of various inorganic salts on the CMC of all studied surfactants was indicated by conductometric measurement as shown in Figs. 11, 12 for TBC 6 surfactant and Figs. S1-S4 for both TBC 12 and TBC 18 respectively. The relationship between solution conductivity and concentration of all studied surfactants in 15% DMSO-water solvent in the presence of two different concentrations (0.001 and 0.01 M) of six different inorganic salts, including NaCl, NaBr, NaI, CoCl2, CuCl2, and MnCl2. The effect of different salts on the critical micelle concentration of all examined surfactants is summarized in Table 5.
Fig. 11.
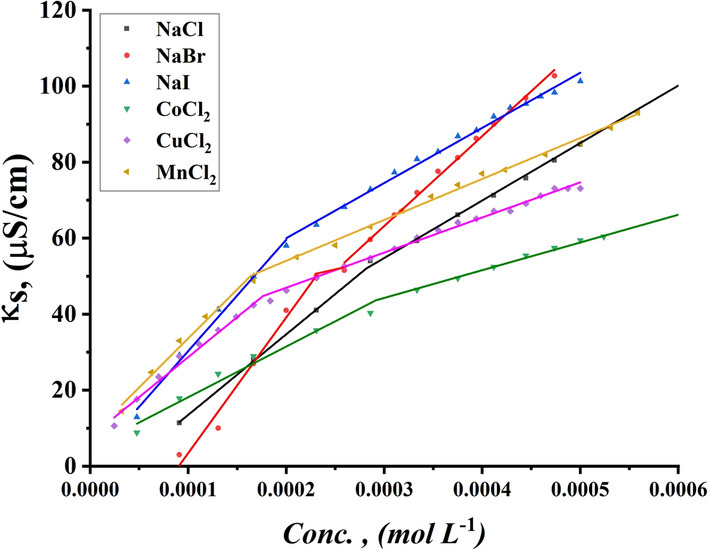
Conductivity vs. concentration of 0.001 M solution of different salts solution after addition of TBC 6 surfactant at 298.15 K
Fig. 12.
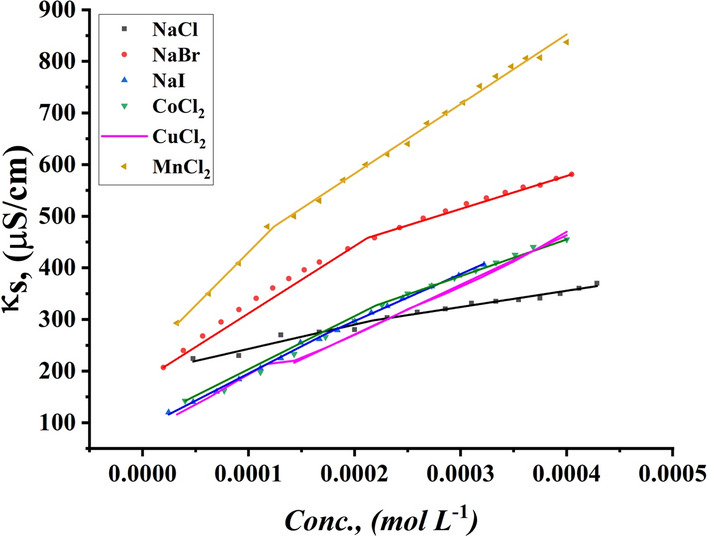
Conductivity vs. concentration of 0.01 M solution of different salts solution after addition of TBC 6 surfactant at 298.15 K
Table 5.
CMC of all surfactants TBC 6, TBC 12, and TBC 18 with different salt concentrations (0.001M and 0.01M) in 15% DMSO-water solvent at 298.15K with conductometric technique
| Salt Conc. mol L−1 | CMC (mol L−1) | |||||||
|---|---|---|---|---|---|---|---|---|
| Solvent | 15% DMSO-water without the addition of salts | 15% DMSO-water with the addition of different salts | ||||||
| Additives | NaCl | NaBr | NaI | CoCl2 | CuCl2 | MnCl2 | ||
| 0.001 | TBC 6 | 0.000302 | 0.000285 | 0.000244 | 0.000195 | 0.000291 | 0.000131 | 0.000168 |
| TBC 12 | 0.000282 | 0.000179 | 0.000162 | 0.000181 | 0.000248 | 0.000146 | 0.000159 | |
| TBC 18 | 0.000166 | 0.000172 | 0.000124 | 0.000143 | 0.000210 | 0.000113 | 0.000124 | |
| 0.01 | TBC 6 | 0.000302 | 0.000224 | 0.000211 | 0.000177 | 0.000219 | 0.000176 | 0.000121 |
| TBC 12 | 0.000282 | 0.000155 | 0.000147 | 0.000141 | 0.000177 | 0.000127 | 0.000094 | |
| TBC 18 | 0.000166 | 0.000139 | 0.000124 | 0.000104 | 0.000122 | 0.000058 | 0.000058 | |
Standard uncertainties (u) of CMC = 0.00002 mol l−1
The CMC values of all surfactants tended to decrease with the addition of salts compared to their values in 15% DMSO-water solvent as shown in Table 5. The addition of salts increased the micelle formation rate for all surfactants studied due to the salting out effect, where added salts interacted with 15% DMSO-water solvent leaving surfactants with lesser solvent molecules around them [65]. Due to the salting-out effect, the hydrocarbon chains of all investigated surfactants TBC 6, TBC 12, and TBC 18 can interact more easily to form micelles at lower concentrations [66]. According to this concept, a larger cation size has a larger solvated shell which reduces the effective size and charge density of the counter ions available for interaction with the surfactant head groups [67]. The radius of cations in (A°) Mn2+ = 0.83, Co2+ = 0.75, and Cu2+ = 0.73. It is stated that the effect of reducing CMC follows the following trend CuCl2 > MnCl2 > CoCl2.
The radius of salts influences the internuclear separation, ionic strength, ionization potential, lattice energy, and solubility of salts in various media [68]. Salts with higher lattice energy have more ability to attract solvent molecules and so increase micellization. The lattice energy for cations was indicated to be 2532, 2688, and 2804 kJ/mol for MnCl2, CoCl2, and CuCl2. It is stated that copper chloride has the greatest ability to reduce the CMC of all studied surfactants.
Furthermore, the presence of salts in the solvent increases the ionic strength of surfactant solutions [69]. This increase led to a reduction in the repulsion forces in surfactant head groups [70]. By comparing the aggregation behavior of all surfactants under the influence of all salts used, the decrease in the CMC of all surfactants is associated with an increase in the radius of the counter ions. The radii of the anions used in this study had values of Clˉ = 1.81 A°, Brˉ = 1.96 A°, and Iˉ = 2.2 A° while for [71–73]. As the size of the anions or cations increased, the ionic strength of the solution increased. This can lead to a reduction in the repulsion force between the head groups of all surfactants. This effect in turn causes the micellization of all surfactants to increase at lower concentrations. CMC for all investigated surfactants is given in ascending order as follows NaI > NaBr > NaCl.
Refractive index measurement
The CMC of all studied surfactants was given by adding a specific volume of all surfactants to a specific volume of two different concentrations of six inorganic salts. The CMC of all indications was performed using a refractometer to determine the refractive index for each addition of surfactant to different media, including all six inorganic salts, with the refractive index of all surfactants being 0.001 and 0.01 M NaI, NaBr, NaCl, MnCl2, CuCl2, and CoCl2 plotted against the concentration of the surfactant solution, as shown in Figs. 13, 14 for TBC 6 surfactant and Figs. S5-S8 for TBC 12 and TBC 18 respectively.
Fig. 13.
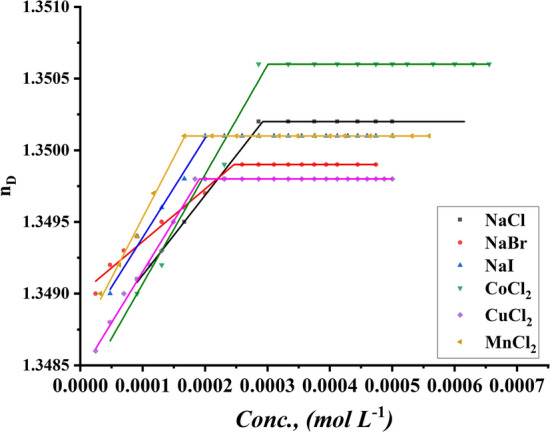
Refractive index vs. concentration of 0.001 M solution of different salts solution after addition of TBC 6 surfactant at 298.15 K
Fig. 14.
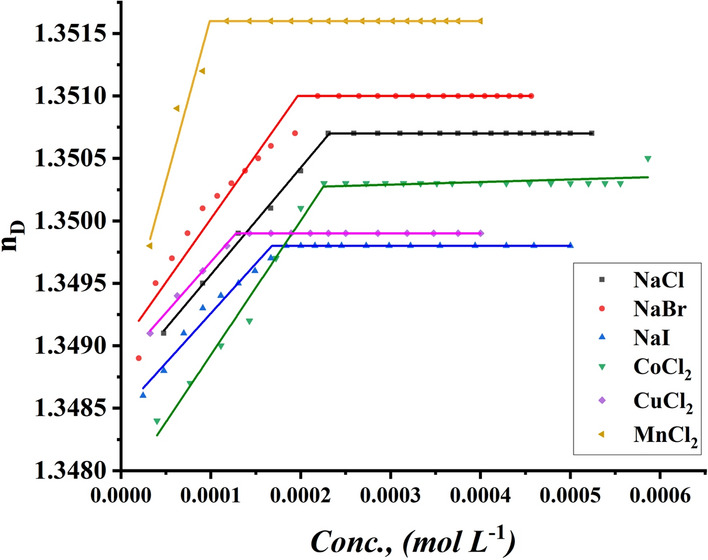
Refractive index vs. concentration of 0.01 M solution of different salts solution after addition of TBC 6 surfactant at 298.15 K
Various inorganic salts were shown to have the same effect on lowering the CMC of all surfactants studied as previously demonstrated in conductometric measurements, proving the salting out of the studied surfactant from 15% DMSO-water solvent at 298.15 K as shown in Table 6. It is stated that various inorganic salts condense in the stern layer around the head groups of each surfactant [74].
Table 6.
CMC of all surfactants in different salt solutions with 0.001M and 0.01M solution with 15% DMSO-water solvent at 298.15K with refractive index techniques
| Salt Conc. mol L−1 | CMC (mol L−1) | |||||||
|---|---|---|---|---|---|---|---|---|
| Solvent | 15% DMSO-water without the addition of salts | 15% DMSO-water with the addition of different salts | ||||||
| Additives | NaCl | NaBr | NaI | CoCl2 | CuCl2 | MnCl2 | ||
| 0.001 | TBC 6 | 0.000309 | 0.000290 | 0.000248 | 0.000168 | 0.000300 | 0.000126 | 0.000169 |
| TBC 12 | 0.000286 | 0.000174 | 0.000167 | 0.000142 | 0.000240 | 0.000150 | 0.000141 | |
| TBC 18 | 0.000167 | 0.000188 | 0.000149 | 0.000152 | 0.000207 | 0.000115 | 0.000113 | |
| 0.01 | TBC 6 | 0.000309 | 0.000230 | 0.000198 | 0.000168 | 0.000227 | 0.000187 | 0.000098 |
| TBC 12 | 0.000286 | 0.000140 | 0.000149 | 0.000144 | 0.000169 | 0.000132 | 0.000091 | |
| TBC 18 | 0.000167 | 0.000149 | 0.000111 | 0.000094 | 0.000119 | 0.000059 | 0.000050 | |
Standard uncertainties (u) of CMC = 0.00002
The size of the micelle formed depends on the size of the counter ions adsorbed on the surface of the head groups of each surfactant. Iodide counter anions were shown to have the greatest effect when each surfactant was salted out in 15% DMSO-water solvent at 298.15 K. The iodide anion was reported to have the largest radius with the largest CMC reduction [28].
The CMC of all surfactants decreased with increasing salt concentration used in this study. It was indicated that the surfactants in 15% DMSO-water solvent with a salt concentration of 0.01M were indicated to have lower CMC than the same surfactants in 15% DMSO-water solvent with a salt concentration of 0.001M using techniques that were measured. This effect proved the relationship between the increase of multiple counter ions condensing around the head groups of all surfactants and the size of the formed micelle [23].
Thermodynamic parameters from conductivity measurements under the effect of salts.
Several thermodynamic parameters, including the degree of ionization, the counter ion binding constant, the association constant, and the Gibbs free energy of micellization and association were calculated as previously mentioned in Eqs. (1–9).
All degrees of ionization of each surfactant (TBC 6, TBC 12, and TBC 18) were reported to increase with the addition of all studied salts at different concentrations (0.01 M and 0.001 M) as listed in Table 7. The increase in the degree of ionization of the surfactant solutions is related to the increase in the ionic strength of the surfactant solution in the presence of all six inorganic salts. It was found that the degree of ionization of each surfactant in the presence of 0.01 M of all salts was higher than that of the same surfactant in the presence of 0.001 M of all salts [75].
Table 7.
The degree of ionization, (α) the counter ion binding, (β) and the standard free energy of micellization, the limiting molar conductivity (Λ0), association constant (Ka), and the standard free energy change of association (ΔGa) and micellization (ΔGmic.) for the surfactants understudy in 0.001M and 0.01M solutions of different inorganic salts 15% DMSO –water solvent at 298.15K
| Sur. name | Salt Conc. mol L−1 | Salt name | (kJ/mol) | S.m2.mol−1 | (kJ/mol) | |||
|---|---|---|---|---|---|---|---|---|
| TBC 6 | 0.001 | NaCl | 0.7136 | 0.2864 | − 26.030 | 2326 | 1,093,447 | − 34.47 |
| NaBr | 0.7467 | 0.2533 | − 25.843 | 2133 | 1,498,273 | − 35.25 | ||
| NaI | 0.7934 | 0.2066 | − 25.549 | 2791 | 1,653,112 | − 35.50 | ||
| CoCl2 | 0.7829 | 0.2171 | − 24.565 | 5150 | 2,553,115 | − 36.58 | ||
| CuCl2 | 0.7666 | 0.2334 | − 26.431 | 3524 | 1,012,979 | − 34.28 | ||
| MnCl2 | 0.7807 | 0.2193 | − 26.269 | 3619 | 1,982,866 | − 35.95 | ||
| 0.01 | NaCl | 0.9556 | 0.0444 | − 27.756 | 21,579 | 2,518,357 | − 36.54 | |
| NaBr | 0.7950 | 0.2050 | − 26.280 | 23,756 | 1,846,096 | − 35.77 | ||
| NaI | 0.9548 | 0.0452 | − 26.384 | 23,378 | 4,386,258 | − 37.91 | ||
| CoCl2 | 0.8591 | 0.1409 | − 26.832 | 32,209 | 5,341,669 | − 38.40 | ||
| CuCl2 | 0.8258 | 0.1742 | − 26.021 | 39,994 | 6,861,714 | − 39.02 | ||
| MnCl2 | 0.9197 | 0.0803 | − 25.154 | 41,314 | 2,600,565 | − 36.62 | ||
| TBC 12 | 0.001 | NaCl | 0.6702 | 0.3298 | − 28.441 | 2326 | 1,266,818 | − 34.84 |
| NaBr | 0.6674 | 0.3326 | − 28.830 | 2433 | 1,666,505 | − 35.52 | ||
| NaI | 0.7642 | 0.2358 | − 26.398 | 2791 | 1,599,675 | − 35.42 | ||
| CoCl2 | 0.6662 | 0.3338 | − 27.449 | 4950 | 2,958,284 | − 36.94 | ||
| CuCl2 | 0.7118 | 0.2882 | − 28.201 | 3724 | 1,072,670 | − 34.43 | ||
| MnCl2 | 0.8348 | 0.1652 | − 25.264 | 2919 | 2,573,418 | − 36.6 | ||
| 0.01 | NaCl | 0.7396 | 0.2604 | − 29.406 | 16,000 | 4,832,914 | − 38.15 | |
| NaBr | 0.7384 | 0.2616 | − 29.598 | 19,000 | 2,556,325 | − 36.55 | ||
| NaI | 0.9111 | 0.0889 | − 29.933 | 14,000 | 5,799,868 | − 38.61 | ||
| CoCl2 | 0.8077 | 0.1923 | − 29.534 | 32,000 | 9,683,245 | − 39.88 | ||
| CuCl2 | 0.5618 | 0.4382 | − 31.984 | 31,000 | 4,700,581 | − 38.08 | ||
| MnCl2 | 0.8884 | 0.1116 | − 29.550 | 39,000 | 3,767,181 | − 37.54 | ||
| TBC 18 | 0.001 | NaCl | 0.5694 | 0.4306 | − 34.737 | 1326 | 1,854,616 | − 35.78 |
| NaBr | 0.6055 | 0.3945 | − 34.094 | 1433 | 1,954,509 | − 35.91 | ||
| NaI | 0.7421 | 0.2579 | − 33.604 | 1791 | 2,474,822 | − 36.50 | ||
| CoCl2 | 0.7336 | 0.2664 | − 33.585 | 3050 | 3,010,138 | − 36.98 | ||
| CuCl2 | 0.7580 | 0.2420 | − 33.979 | 3524 | 1,673,414 | − 35.53 | ||
| MnCl2 | 0.8995 | 0.1005 | − 33.538 | 2919 | 2,895,114 | − 36.89 | ||
| 0.01 | NaCl | 0.7593 | 0.2407 | − 34.314 | 12,000 | 5,628,836 | − 38.53 | |
| NaBr | 0.6344 | 0.3656 | − 34.449 | 12,000 | 4,114,195 | − 37.75 | ||
| NaI | 0.8050 | 0.1950 | − 35.166 | 18,000 | 9,697,917 | − 39.80 | ||
| CoCl2 | 0.7995 | 0.2005 | − 36.817 | 34,000 | 14,099,602 | − 40.81 | ||
| CuCl2 | 0.8108 | 0.1892 | − 38.756 | 22,000 | 22,244,399 | − 41.94 | ||
| MnCl2 | 0.9026 | 0.0974 | − 36.536 | 25,000 | 9,185,123 | − 39.75 |
Standard uncertainties (u) of α and β are = 0.002
It was shown that the Gibbs free energy of association and micellization increase with increasing the addition of all examined salts at the same concentration. This indicated that the formation of the micelle generated much more energy indicating the stability of the formed micelle [29]. The negative magnitude of the Gibbs free energy of both micellization and association indicates spontaneous processes [76]. The increase in the negativity of Gibbs free energy proved the increase in the formation of more stable micelles [77].
The effect of different inorganic salts on all surfactants under study TBC 6, TBC 12, and TBC 18 from different techniques including conductivity and refractive index measurement were compared to indicate the acceptability agreement in proving results as shown in Fig. 15 at salts concentration equal 0.001M and Fig. 16 at salts concentration equal 0.01M.
Fig. 15.
Comparison between CMC of different surfactants TBC 6, TBC 12, and TBC 18 under the effect of different salts at 0.001 M using conductivity and refractive index measurement
Fig. 16.
Comparison between CMC of different surfactants TBC 6, TBC 12, and TBC 18 under the effect of different salts at 0.01 M using conductivity and refractive index measurement
Conclusion
The CMC values of a new series of Gemini cationic surfactants with different hydrocarbon chain lengths were detected with different techniques including conductivity, refractive index, surface tension, density, and molal volume in 15% DMSO-water solvent at 298.15 K. Raw data from different techniques indicated to have good compatibility to explain the micellization process. Counter ions binding constant (β) and association constant (Ka) demonstrated the formation of a more stable micelle with increased surfactant concentration and hydrocarbon chain length. An increase in the negativity of micellization (ΔGmic) and association (ΔGass) Gibbs free energies verified the increasing spontaneity of surfactant aggregation TBC 18 > TBC 12 < TBC 6. Micellization of Gemini cationic surfactants was verified by a decrease in the molal volume (), van der Waals volume (), and electrostriction volume (), indicating a decrease in the interaction between surfactants and surrounding solvent till reaching CMC and formation of more stable micelle. The CMC of all studied surfactants in the presence of different concentrations of salts was determined by conductivity and refractive index measurement. A decrease in CMC was indicated due to adsorption on the stern layer and a decrease in repulsion force between head groups of surfactants which indicated the following CuCl2 > MnCl2 > CoCl2 > NaI > NaBr > NaCl. An increase in the concentration of salts verified increasing in micellization of each surfactant as the following 0.01mol L−1 < 0.001 mol L−1. Degrees of ionization (α) for all studied surfactants indicated an increase in the presence of different inorganic salts, indicating the formation of micelle at lower concentrations. An increase in the negativity of Gibbs free energies of micellization (ΔGmic) and association (ΔGass) in the presence of salts compared to the absence of salts indicated an increase in the stability of the micelle formed. This study contributes to understanding the design of surfactants by correlating hydrocarbon chain length, concentration, and addition of salts with surfactant properties and tailoring it to specific industrial applications such as detergency, emulsification, and corrosion inhibitor applications.
Supplementary Information
Acknowledgements
“Not applicable” in this section.
Author contributions
Farid I. El-Dossoki: contributed to writing and reviewed the manuscript Samir A. Abd El-Maksoud: contributed to writing and reviewed the manuscript Mohamed A. Migahed: preparation and characterized of the studied compounds Mahmoud M. Gouda: make the other practical measurements in the manuscript.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). This study was carried out in a research project number 46361/2021 funded by STDF.
Data availability
Raw data were generated at Faculty of Science, Port-Said University, Egypt. Derived data supporting the findings of this study are available from the corresponding author, Prof. Dr. Farid I. El-Dossoki, on request.
Declarations
Ethics approval and consent to participate
Not applicable in this section.
Consent for publication
Not applicable in this section.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Brycki BE, Kowalczyk IH, Szulc A, OlgaKaczerewska, Pakiet M, Brycki BE, et al. Multifunctional Gemini surfactants: structure, synthesis, properties and applications. Appl Charact Surfactants. 2017. 10.5772/INTECHOPEN.68755. [Google Scholar]
- 2.Raffa P, Broekhuis AA, Picchioni F. Polymeric surfactants for enhanced oil recovery: a review. J Pet Sci Eng. 2016;145:723–33. [Google Scholar]
- 3.Tornero V, Hanke G. Chemical contaminants entering the marine environment from sea-based sources: a review with a focus on European seas. Mar Pollut Bull. 2016;112:17–38. [DOI] [PubMed] [Google Scholar]
- 4.Abd El-Maksoud SA, El-Dossoki FI, Migahed MA, Gouda MM, El-Gharkawy ES. New imidazol-1-ium bromide derivative surfactants as corrosion inhibitors for carbon steel in 1 M HCl solutions: experimental and theoretical studies. J Bio- Tribo-Corrosion. 2021;7:1–15. [Google Scholar]
- 5.Shabestary N, Rensing DT, Reed DN, Austiff AF, Cox MD, Shabestary N, et al. Triphase catalysis based on Gemini surfactant-clay intercalates. Mod Res Catal. 2014;3:26–34. [Google Scholar]
- 6.Labena A, Hegazy MA, Sami RM, Hozzein WN. Multiple applications of a novel cationic Gemini surfactant: anti-microbial, anti-biofilm, biocide, salinity corrosion inhibitor, and biofilm dispersion (Part II). Molecules. 2020;25:1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.In M. Gemini surfactants and surfactant oligomers. 2001. 10.1201/9780203908662-8.
- 8.Krimm S. The hydrophobic effect: formation of micelles and biological membranes, Charles Tanford, Wiley‐Interscience, New York, 1980, 233 pp. price: $18.50. J Polym Sci Polym Lett Ed. 1980;18:687–687. [Google Scholar]
- 9.Israelachvili JN, Mitchell DJ, Ninham BW. Theory of self-assembly of hydrocarbon amphiphiles into micelles and bilayers. 1976;72:1525–68.
- 10.Rai SR, Sarkar A, Pandey A, Dasgupta S, Majumder I, Das D, et al. Photometric study of the interaction of zinc (II) complexes of schiff bases with cetyltrimethyl ammonium bromide. Macromol Symp. 2019;388.
- 11.Sachin KM, Karpe S, Singh M, Bhattarai A. Physicochemical properties of dodecyltrimethylammonium bromide (DTAB) and sodiumdodecyl sulphate (SDS) rich surfactants in aqueous medium, at T = 293.15, 298.15, and 303.15 K. Macromol Symp. 2018;379.
- 12.Azum N, Kumar D. Kinetic study of the metal-dipeptide complex with ninhydrin facilitated by gemini (m-s-m) surfactant micelles. Sci Rep. 2020;10:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.El-Dossoki FI, Abd El-Maksoud SA, Migahed MA, Gouda MM. Micellization and solvation properties of newly synthesized imidazolium- and aminium-based surfactants. ACS Omega. 2020;5:9429–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perinelli DR, Cespi M, Lorusso N, Palmieri GF, Bonacucina G, Blasi P. Surfactant self-assembling and critical micelle concentration: one approach fits all? Langmuir. 2020;36:5745–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tan CH, Huang ZJ, Huang XG. Rapid determination of surfactant critical micelle concentration in aqueous solutions using fiber-optic refractive index sensing. 2010;401:144–7. [DOI] [PubMed]
- 16.Nesměrák K, Němcová I. Determination of critical micelle concentration by electrochemical means. Anal Lett. 2006;39:1023–40. [Google Scholar]
- 17.Becherová L, Prokopec V, Čejková J. Vibrational spectroscopic analysis of critical micelle concentration in sodium decanoate solutions. Spectrochim Acta Part A Mol Biomol Spectrosc. 2021;250: 119387. [DOI] [PubMed] [Google Scholar]
- 18.Sah MK, Edbey K, Ettarhouni ZO, Bhattarai A, Kumar D. Conductometric and spectral analyses of dye-surfactant interactions between indigo carmine and N-alkyltrimethylammonium chloride. J Mol Liq. 2024;399: 124413. [Google Scholar]
- 19.Mabrouk MM, Hamed NA, Mansour FR. Spectroscopic methods for determination of critical micelle concentrations of surfactants; a comprehensive review. Appl Spectrosc Rev. 2023;58:206–34. [Google Scholar]
- 20.Akhlaghi N, Riahi S. Salinity effect on the surfactant critical micelle concentration through surface tension measurement. 2019;8:50–63.
- 21.Zhu Y, Free ML, Yi G. The effects of surfactant concentration, adsorption, aggregation, and solution conditions on steel corrosion inhibition and associated modeling in aqueous media. Corros Sci. 2016;102:233–50. [Google Scholar]
- 22.Rafique AS, Khodaparast S, Poulos AS, Sharratt WN, Robles ESJ, Cabral JT. Micellar structure and transformations in sodium alkylbenzenesulfonate (NaLAS) aqueous solutions: effects of concentration, temperature, and salt. Soft Matter. 2020;16:7835–44. [DOI] [PubMed] [Google Scholar]
- 23.Ren ZH. Mechanism of the salt effect on micellization of an aminosulfonate amphoteric surfactant. Ind Eng Chem Res. 2015;54:9683–8. [Google Scholar]
- 24.Walker JRD, Keppel RA, Cosper MB, Funk JJ, Meister MJ. Salting-out and multivalent cation precipitation of anionic surfactants. Am Chem Soc, Div Pet Chem, Prepr; (United States). 26:1.
- 25.Pokhrel DR, Sah MK, Gautam B, Basak HK, Bhattarai A, Chatterjee A. A recent overview of surfactant–drug interactions and their importance. RSC Adv. 2023;13:17685–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zakharova LY, Vasilieva EA, Mirgorodskaya AB, Zakharov SV, Pavlov RV, Kashapova NE, et al. Hydrotropes: solubilization of nonpolar compounds and modification of surfactant solutions. J Mol Liq. 2023;370:120923. [Google Scholar]
- 27.Li J, Du B, Wang F, Yao W, Yao S. The effect of nanoparticle surfactant polarization on trapping depth of vegetable insulating oil-based nanofluids. Phys Lett A. 2016;380:604–8. [Google Scholar]
- 28.Miyagishi S, Okada K, Asakawa T. Salt effect on critical micelle concentrations of nonionic surfactants, N-Acyl-N-methylglucamides (MEGA-n). J Colloid Interface Sci. 2001;238:91–5. [DOI] [PubMed] [Google Scholar]
- 29.Akram M, Bhat IA, Kabir-ud-Din. Effect of salt counterions on the physicochemical characteristics of novel green surfactant, ethane-1,2-diyl bis(N, N-dimethyl-N-tetradecylammoniumacetoxy) dichloride. Colloids Surfaces A Physicochem Eng Asp. 2016;493:32–40. [Google Scholar]
- 30.Bhuiyan HA, Anis-Ul-Haque KM, Joy MTR, Rana S, Khan JM, Kumar D, et al. Aggregation phenomena and physico-chemical properties of tetradecyltrimethylammonium bromide and protein (bovine serum albumin) mixture: influence of electrolytes and temperature. Int J Biol Macromol. 2023;253: 127101. [DOI] [PubMed] [Google Scholar]
- 31.Sultana AA, Rahman MH, Joy MTR, Rana S, Khan JM, Kumar D, et al. Interaction of sodium alginate biopolymer with sodium dodecyl sulfate in aqueous medium and different additive solutions at several temperatures. Chem Eng Commun. 2024;211:510–25. [Google Scholar]
- 32.Alghamdi YG, Rub MA, Kumar D, Asiri AM. Effects of various media on micellization, adsorption and thermodynamic behaviour of imipramine hydrochloride and antimicrobial surfactant mixtures. R Soc Open Sci. 2021;8. [DOI] [PMC free article] [PubMed]
- 33.Šarac B, Bešter-Rogač M. Temperature and salt-induced micellization of dodecyltrimethylammonium chloride in aqueous solution: a thermodynamic study. J Colloid Interface Sci. 2009;338:216–21. [DOI] [PubMed] [Google Scholar]
- 34.Wu YC, Koch WF, Feng D, Holland LA, Juhasz E, Arvay E, et al. A dc method for the absolute determination of conductivities of the primary standard KCl solutions from 0 °C to 50 °C. J Res Natl Inst Stand Technol. 1994;99:241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dodd LE. Calibration of abbe refractometer with compensating prisms, to measure refractive index for any wave length. Rev Sci Instrum. 1931;2:466–501. [Google Scholar]
- 36.Yalkowsky SH, Zografi G. Calculation of partial molal volume in micellar systems. J Pharm Sci. 1972;61:793–5. [DOI] [PubMed] [Google Scholar]
- 37.Law K-Y, Zhao H. Determination of solid surface tension by contact angle. Surf Wetting. 2016;:135–48.
- 38.Cui X, Mao S, Liu M, Yuan H, Du Y. Mechanism of surfactant micelle formation. Langmuir. 2008;24:10771–5. [DOI] [PubMed] [Google Scholar]
- 39.Tao C, Yan H, Yuan X, Yin Q, Zhu J, Ni W, et al. Hydrophobic antireflective coatings with ultralow refractive index synthesized by deposition of methylated hollow silica nanoparticles. Mater Lett. 2016;183:374–7. [Google Scholar]
- 40.Kale KM, Zana R. Effect of the nature of the counterion on the volume change upon micellization of ionic detergents in aqueous solutions. J Colloid Interface Sci. 1977;61:312–22. [Google Scholar]
- 41.Firetto V, Floriane MA, Panagiotopoulos AZ. Effect of stiffness on the micellization behavior of model H4T4 surfactant chains. Langmuir. 2006;22:6514–22. [DOI] [PubMed] [Google Scholar]
- 42.Song Y, Sun R, Zhao K, Pan X, Zhou H, Li D. An induction current method for determining the critical micelle concentration and the polarity of surfactants. Colloid Polym Sci. 2015;293:1525–34. [Google Scholar]
- 43.Liu K, Yang L, Peng X, Gong H, Wang J, Lu JR, et al. Effects of conventional surfactants on the activity of designed antimicrobial peptide. Langmuir. 2020;36:3531–9. [DOI] [PubMed] [Google Scholar]
- 44.Golmaghani-Ebrahimi E, Bagheri A, Fazli M. The influence of temperature on surface concentration and interaction energy between components in binary liquid systems. J Chem Thermodyn. 2020;146: 106105. [Google Scholar]
- 45.Das S, Mondal S, Ghosh S. Physicochemical studies on the micellization of cationic, anionic, and nonionic surfactants in water-polar organic solvent mixtures. J Chem Eng Data. 2013;58:2586–95. [Google Scholar]
- 46.Daggett HM. The Shedlovsky extrapolation function. J Am Chem Soc. 1951;73:4977. [Google Scholar]
- 47.Onsager L. Theories of concentrated electrolytes. Chem Rev. 1933;13:73–89. [Google Scholar]
- 48.Mahbub S, Rub MA, Hoque MA, Khan MA, Kumar D. Micellization behavior of cationic and anionic surfactant mixtures at different temperatures: effect of sodium carbonate and sodium phosphate salts. J Phys Org Chem. 2019;32: e3967. [Google Scholar]
- 49.Sharker KK, Yusa S, Phan CM. Micellar formation of cationic surfactants. Heliyon. 2019;5:e02425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Anis-Ul-Haque KM, Hossain MA, Uddin N, Jonayed M, Gatasheh MK, Kumar D, et al. Effects of short-chain alcohols and urea on the association of tetradecyltrimethylammonium bromide surfactant and cefixime trihydrate antibiotic: conductometric and physicochemical analysis. Colloids Surfaces A Physicochem Eng Asp. 2024;692: 133972. [Google Scholar]
- 51.Bhardwaj V, Sharma P, Chauhan MS, Chauhan S. Micellization, interaction and thermodynamic study of butylated hydroxyanisole (synthetic antioxidant) and sodium dodecyl sulfate in aqueous-ethanol solution at 25, 30 and 35 °C. J Saudi Chem Soc. 2016;20:S109–14. [Google Scholar]
- 52.Pires JM, De Moura AF, Freitas LCG. Investigating the spontaneous formation of SDS micelle in aqueous solution using a coarse-grained force field. Quim Nova. 2012;35:978–81. [Google Scholar]
- 53.Esan OS, Bankole OM, Olanrewaju O, Moses AA. Conductometric approach to the thermodynamic of micellization of anionic surfactants in the presence of procaine hydrochloride. Adv J Chem A. 2021;4:258–69. [Google Scholar]
- 54.Shinoda K, Hutchinson E. Pseudo-phase separation model for thermodynamic calculations on micellar solutions. J Phys Chem. 1962;66:577–82. [Google Scholar]
- 55.Bai G, Lopes A, Bastos M. Thermodynamics of micellization of alkylimidazolium surfactants in aqueous solution. J Chem Thermodyn. 2008;40:1509–16. [Google Scholar]
- 56.Calderón SM, Prisle NL. Composition dependent density of ternary aqueous solutions of ionic surfactants and salts: capturing the effect of surfactant micellization in atmospheric droplet model solutions. J Atmos Chem. 2021;78:99–123. [Google Scholar]
- 57.Bhattarai A, Chatterjee SK, Jha K. Density and partial molar volume of cetyltrimethylammonium bromide in the presence and absence of KCl and NaCl in aqueous media at room temperature. Phys Chem. 2015;5:1–5. [Google Scholar]
- 58.Chauhan S, Chauhan MS, Chauhan GS, Sonika, Jyoti J. Sound speed and density studies of interactions between cationic surfactants and aqueous gelatin solution. Int J Thermophys. 2012;33:279–88. [Google Scholar]
- 59.Ali A, Tariq M, Patel R, Ittoo FA. Interaction of glycine with cationic, anionic, and nonionic surfactants at different temperatures: a volumetric, viscometric, refractive index, conductometric, and fluorescence probe study. Colloid Polym Sci. 2008;286:183–90. [Google Scholar]
- 60.Domínguez-Arca V, Sabín J, Taboada P, García-Río L, Prieto G. Micellization thermodynamic behavior of gemini cationic surfactants. Modeling its adsorption at air/water interface. J Mol Liq. 2020;308:113100. [Google Scholar]
- 61.Sharma VD, Ilies MA. Heterocyclic cationic Gemini surfactants: a comparative overview of their synthesis, self-assembling, physicochemical, and biological properties. Med Res Rev. 2014;34:1–44. [DOI] [PubMed] [Google Scholar]
- 62.Chauhan S, Sharma K, Rana DS, Kumar G, Umar A. Conductance, apparent molar volume and compressibility studies of cetyltrimethylammonium bromide in aqueous solution of leucine. J Mol Liq. 2012;175:103–10. [Google Scholar]
- 63.Ni N, Yalkowsky SH. Prediction of Setschenow constants. Int J Pharm. 2003;254:167–72. [DOI] [PubMed] [Google Scholar]
- 64.Urbina-Villalba G, Reif I, Márquez ML, Rogel E. Theoretical study on the structure and interfacial areas of nonyl phenol ethoxylated surfactants. Colloids Surfaces A Physicochem Eng Asp. 1995;99:207–20. [Google Scholar]
- 65.Aslam J. Cationic gemini surfactant as corrosion inhibitor for mild steel in 1 M HCl and synergistic effect of organic salt (sodium tosylate). J Adhes Sci Technol. 2019;33:1989–2009. [Google Scholar]
- 66.Poša M, Pilipović A, Lalić M. The influence of NaCl on hydrophobicity of selected, pharmacologically active bile acids expressed with chromatographic retention index and critical micellar concentration. Colloids Surfaces B Biointerfaces. 2010;81:336–43. [DOI] [PubMed] [Google Scholar]
- 67.Bhattarai A, Shah SK, Limbu K. Conductance of sodium dodecylsulfate in presence and absence of Na2SO4 AND ZnSO4 in aqueous media at room temperature. Sci World. 2015;12:41–3. [Google Scholar]
- 68.Park BJ, Pantina JP, Furst EM, Oettel M, Reynaert S, Vermant J. Direct measurements of the effects of salt and surfactant on interaction forces between colloidal particles at water-oil interfaces. Langmuir. 2008;24:1686–94. [DOI] [PubMed] [Google Scholar]
- 69.Rezaie A, Ghasemi H, Eslami F. An in-depth investigation of the impact of salt nature on the formulation of microemulsion systems. Sci Rep. 2023;13:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vatanparast H, Shahabi F, Bahramian A, Javadi A, Miller R. The role of electrostatic repulsion on increasing surface activity of anionic surfactants in the presence of hydrophilic silica nanoparticles. Sci Rep. 2018;8:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mu L, Feng C, He H. Topological research on lattice energies for inorganic compounds. MATCH Commun Math Comput Chem MATCH Commun Math Comput Chem. 2006;56:97–111. [Google Scholar]
- 72.Ouyang R. Exploiting ionic radii for rational design of halide perovskites. Chem Mater. 2020;32:595–604. [Google Scholar]
- 73.Lundberg D. The coordination chemistry of solvated metal ions in DMPU: a study of a space-demanding solvent. Acta Univ Agric Sueciae. 2006;2006:23. [Google Scholar]
- 74.Aswal VK, Goyal PS. Role of different counterions and size of micelle in concentration dependence micellar structure of ionic surfactants. Chem Phys Lett. 2003;368:59–65. [Google Scholar]
- 75.Haq ZU, Rehman N, Ali F, Ullah H. Effect of electrolyte (NaCl) and temperature on the mechanism of cetyl trimethylammonium bromide micelles (Kesan Elektrolit (NaCl) dan Suhu terhadap Mekanisme Setil Trimetilammonium Bromida Misel). Sains Malaysiana. 2017;46:733–41. [Google Scholar]
- 76.Fu D, Gao X, Huang B, Wang J, Sun Y, Zhang W, et al. Micellization, surface activities and thermodynamics study of pyridinium-based ionic liquid surfactants in aqueous solution. RSC Adv. 2019;9:28799–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cookey GA, Obunwo CC. Effects of sodium bromide salt and temperature on the behaviour of aqueous solution of cetyltrimethylammonium bromide. IOSR J Appl Chem IOSR-JAC. 2014;7:34–8. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw data were generated at Faculty of Science, Port-Said University, Egypt. Derived data supporting the findings of this study are available from the corresponding author, Prof. Dr. Farid I. El-Dossoki, on request.