Abstract
Mentha haplocalyx Briq. (M. haplocalyx), a notable member of the Lamiaceae family, occupies a significant role in the realm of health foods and botanical medicines. Traditionally, it has been employed to address various diseases, including colds, coughs, fever, indigestion, asthma, and influenza. Recent phytochemical investigations have identified the presence of terpenoids, flavonoids, phenolic acids, anthraquinones, alkanes, and polysaccharides in M. haplocalyx, with terpenoids being the primary bioactive constituents. Notably, both in vitro and in vivo studies have demonstrated its diverse health benefits, such as neuroprotective, anti-asthmatic, anti-inflammatory, gut health improvement, hypoglycemic, anti-aging, anti-bacterial, and antioxidant effects. Additionally, M. haplocalyx is a rich source of carbohydrates, dietary fiber, amino acids, minerals, and vitamins, further underscoring its nutritional value. A thorough literature review was conducted using databases like PubMed, Google Scholar, Web of Science, and China National Knowledge Infrastructure (CNKI) to consolidate existing knowledge on M. haplocalyx. This review synthesizes recent advancements in the botany, traditional uses, nutritional value, phytochemistry, health benefits, and research on the edible uses of M. haplocalyx. Furthermore, the commercial potential and future research opportunities for M. haplocalyx are briefly explored, with the goal of fostering continued interest in this multifunctional plant and inspiring future research and commercial endeavors.
Graphical Abstract
Keywords: Mentha haplocalyx Briq., Traditional uses, Nutritional value, Phytochemistry, Health benefits, Applications
Introduction
Mentha haplocalyx Briq. (M. haplocalyx), a notable aromatic herb within the Lamiaceae family, is highly valued for its edible and medicinal properties and is cultivated extensively across the globe [1, 2]. Predominantly found in subtropical and temperate regions of the Northern Hemisphere, it thrives especially in the humid areas of China, Korea, and Japan [3, 4]. In traditional medicine, the aerial parts of M. haplocalyx, particularly the stems and leaves, have been employed as herbal remedies for wounds, swollen glands, colds, coughs, fevers, indigestion, asthma, and influenza [5, 6]. Moreover, it is recognized in the 2020 edition of the Pharmacopoeia of the People’s Republic of China (Ch.P 2020), underscoring its importance in traditional medicinal practices and its integration into various pharmaceutical products [7]. M. haplocalyx is not only renowned for its therapeutic properties but also as a flavorful ingredient with health-promoting benefits [8]. Its leaves are widely used as flavoring agents in chewing gum, candies, beverages, and tobacco, imparting a refreshing aroma and taste. Additionally, M. haplocalyx is a key ingredient in oral hygiene products, cosmetics, herbal teas, and health beverages, attributed to its recognized health benefits [9–11]. Economically, M. haplocalyx is regarded as a valuable crop with significant potential for commercial exploitation, serving as an essential component in agricultural product development [12]. Cultivation has thus become the primary method for meeting the growing demand for M. haplocalyx. With over 2,000 years of cultivation history in ancient China, it has long been considered a significant spice and medicinal crop [13]. Contemporary pharmaceutical research largely concentrates on M. haplocalyx's applications in neurological and respiratory disorders [14, 15], highlighting its contributions to both traditional medicine and modern drug development [16]. Beyond its medicinal attributes, M. haplocalyx offers considerable nutritional benefits, being a rich source of dietary fiber, vitamins, trace minerals, carbohydrates, and other essential nutrients. These qualities have attracted the attention of both nutrition researchers and health-conscious consumers [17, 18].
Throughout its long history of use, M. haplocalyx has been developed as a crucial source of functional foods and bioactive ingredients in traditional Chinese medicine (TCM) [19]. It remains a popular choice among consumers, both as a vegetable and a functional food, due to its refreshing flavor and nutritional richness [8, 20]. Notably, the essential oil derived from M. haplocalyx is a multifunctional substance with extensive medicinal and health benefits [21–24]. Historically, this essential oil has been esteemed for its efficacy in treating central nervous system disorders, leading to a focus on its volatile components in scientific studies [25]. However, recent findings suggest that non-volatile constituents, such as polyphenols and flavonoids, also play a vital role in clinical treatments, particularly for respiratory, reproductive, and digestive system disorders [26]. In summary, M. haplocalyx offers substantial benefits to human health, owing to its rich nutritional profile and therapeutic properties.
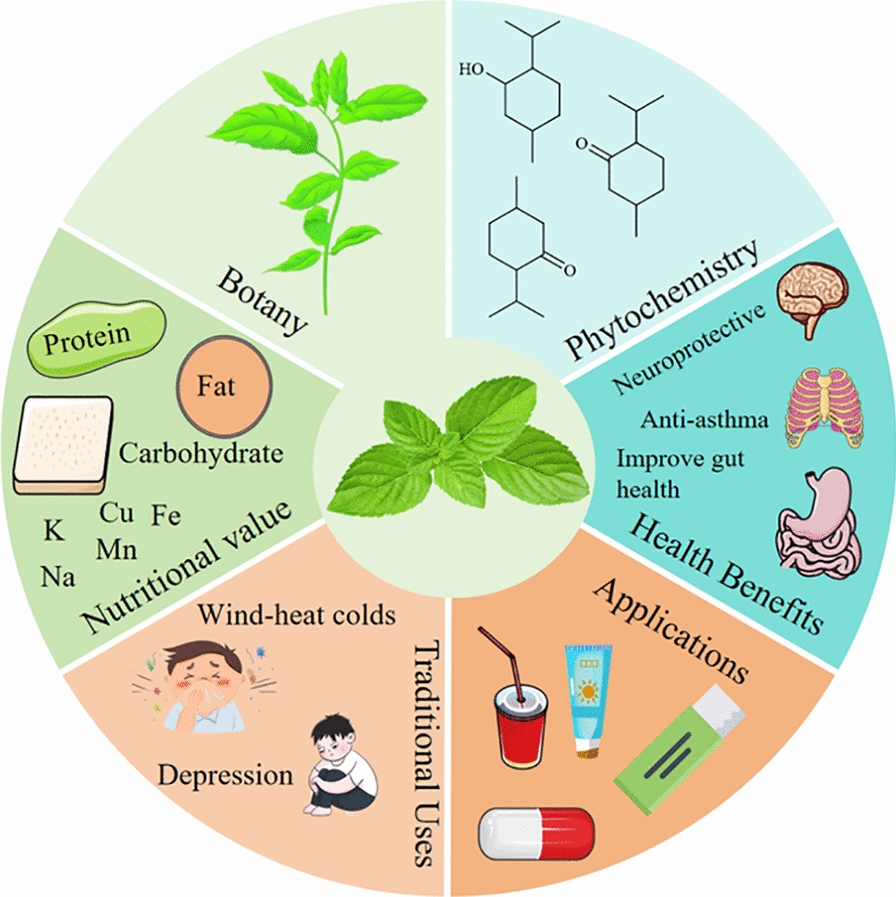
Historically, M. haplocalyx has been associated with numerous health benefits, including neuroprotection, anti-asthmatic effects, gut health improvement, hypoglycemic activity, anti-inflammatory properties, anti-aging effects, anti-bacterial, and antioxidant capabilities [4]. The phytochemical composition of medicinal plants forms the basis for disease prevention and treatment, driving scientists to discover new and more effective therapeutic agents [27–29]. A significant number of phytochemical compounds have been isolated and identified from M. haplocalyx, with terpenoids and other volatile components recognized as their primary bioactive constituents. In addition to terpenoids, M. haplocalyx contains phenols, flavonoids, anthraquinones, polysaccharides, alkanes, and various other phytochemicals [2, 9]. As research advances, there is an increasing focus on the chemical analysis and biological activities of M. haplocalyx. The identification of diverse phytochemicals and the exploration of their pharmacological activities lay a robust foundation for their further exploitation and utilization.
In recent years, research on M. haplocalyx has expanded across multiple disciplines. Despite this, no existing review article comprehensively addresses all facets of this plant. A thorough review of its research progress is essential for optimizing the utilization of this medicinal resource. Thus, this paper aims to present a comprehensive overview of M. haplocalyx research conducted over the past two decades, encompassing aspects such as botany, traditional uses, nutritional value, phytochemistry, health benefits, and potential applications. It is hoped that this paper will provide researchers with a broad understanding of M. haplocalyx's research trajectory and serve as a valuable reference for future studies and applications.
Botany
The Mentha genus, known for its rich species diversity and wide distribution, thrives predominantly in temperate and subtropical regions worldwide. Among its species, several are renowned for their medicinal and culinary value, including M. haplocalyx, M. piperita, M. arvensis, M. longifolia, M. spicata, and M. aquatica. M. piperita, typically growing to a height of 30–100 cm, is characterized by smooth stems and leaves measuring 4–9 cm in length and 1.5–4 cm in width. Its flowers, purple and arranged in whorled clusters, distinguish it from M. haplocalyx, which has rougher stems, smaller leaves, and lighter-colored flowers. M. arvensis, on the other hand, generally reaches a height of 10–60 cm, with paired leaves 2–6 cm long and 1–2 cm wide, and pale purple or pink flowers. M. longifolia can attain heights of 40–120 cm, with long, elliptical leaves measuring 5–10 cm in length and 1.5–3 cm in width, and pale purple or white flowers that grow in dense clusters. M. spicata usually grows to 30–100 cm, with leaves 5–9 cm long and 1.5–3 cm wide, and its flowers, white or pink, are borne on slender spikes. M. aquatica, a perennial herb with rhizomes, can grow up to 90 cm tall, featuring green or purple square stems, a fibrous root system, and small pink or purple flowers [30]. Despite their widespread use across various regions, these Mentha species exhibit significant differences in phytochemical composition and bioactive properties compared to M. haplocalyx. Species of the Mentha genus commonly contain a variety of natural compounds, including terpenoids, flavonoids, and phenolic acids. Specifically, the chemical composition of M. piperita and M. spicata also includes lignans. Additionally, M. longifolia contains cinnamates and ceramides in its chemical profile. These differences in chemical composition contribute to the distinct biological activities of each species. As one of the earliest introduced and cultivated plants in China, M. haplocalyx enjoys broad distribution and a long-standing history of medicinal use, earning its inclusion in the Ch.P 2020. Notably, due to the stringent requirements for clinical safety, efficacy, and quality control, M. haplocalyx remains the only species from the Mentha genus included in Ch.P 2020 to date.
Ch.P 2020 includes M. haplocalyx, specifically its dried aerial parts. M. haplocalyx is a perennial medicinal herb that thrives in humid environments, often found in wetlands near water, and can grow at altitudes up to 3,500 m [31]. According to online records from China’s flora (http://www.cn-flora.ac.cn/index.html, accessed on 25 May 2024), M. haplocalyx features erect stems that reach heights of 30–60 cm, with multiple nodes at the lower part, slender fibrous roots, and horizontally spreading rhizomes. The stems are sharply quadrangular, bearing four grooves, and are covered with inversely pubescent hairs on the upper part, while the lower part is pubescent only along the edges, branching extensively. The leaf blades are oblong-lanceolate, lanceolate, elliptic, or ovate-lanceolate, varying in shape, and measure 3–5 cm in length and 0.8–3 cm in width. They have acute apices, cuneate to subrounded bases, and sparsely coarse dentate margins above the base. The lateral veins number around 5–6 pairs, with a midrib that is slightly concave above and marked below, green on the upper surface. The leaves are sparsely pilose or nearly glabrous except along the veins, which are densely pilose, with petioles 2–10 mm long, ventrally concave, and puberulent. The axillary cymes are globose, approximately 18 mm in diameter, either pedicellate or sessile, with slender pedicels up to 3 mm long, puberulent or nearly glabrous. The calyx is tubular and bell-shaped, approximately 2.5 mm long, with a puberulent and glandular outer surface and a glabrous inner surface. It contains 10 inconspicuous veins and has 5 narrowly triangular subulate calyx teeth with long acute apices, each about 1 mm long. The corolla is lavender, 4 mm long, with a slightly puberulent outer surface and a puberulent inner surface below the throat, featuring a 4-lobed coronal eave. The upper lobe is 2-lobed and larger, while the remaining 3 lobes are subequal, oblong, with obtuse apices. The plant has four stamens, with the anterior pair extending beyond the corolla, approximately 5 mm long, with filamentous and glabrous filaments. The anthers are oval with parallel compartments, and the style slightly exceeds the stamens, with a nearly equal 2-lobed apex and subulate lobes. The flowering period occurs from July to September, with fruiting in October. The plant’s features are illustrated in Fig. 1 (https://ppbc.iplant.cn/). M. haplocalyx is typically harvested during the peak growth of stems and leaves or when flowers are fully bloomed, usually in summer and autumn. Harvesting on sunny days yields a higher volatile oil content, whereas harvesting on rainy days results in minimal content. After harvesting, M. haplocalyx must be promptly washed to remove surface impurities, soil, and pesticide residues, followed by drying in a well-ventilated, cool area until it reaches a semi-dry or dry state, reducing moisture content to prevent mold growth [32]. Post-drying, M. haplocalyx is cut into appropriately sized pieces or slices for further processing or use. It is crucial that harvesting and processing strictly follow operational procedures to ensure the quality and safety of M. haplocalyx products, meeting both culinary and medicinal standards.
Fig. 1.

Plant morphology of M. haplocalyx. A Whole plants, B Dry medicinal parts, C Flowers, D Stems
Traditional uses
M. haplocalyx, a medicinal and edible plant, has been traditionally used in China for over 2,000 years, dating back to the Han Dynasty. It was first documented in Shen Nong's Materia Medica as a treatment for symptoms such as wind-heat colds, headaches, and coughs [22]. Since ancient times, the exploration and development of TCM have been ongoing, strengthening its application in disease treatment and prevention, thereby bolstering confidence in the progress and innovation of TCM [33, 34]. M. haplocalyx was first included in the Ch.P 1963 and has long been recognized in folk culture as a commonly used TCM. According to TCM theory, M. haplocalyx is described as cold in nature, pungent in flavor, and associated with the lung and liver meridians. Its action on these meridians allows it to disperse wind-heat, clear the head, benefit the throat, promote rash eruption, and soothe liver qi. These properties, linked to its flavor and meridian affiliation, are critical in guiding the clinical application of herbal medicine within the TCM framework [35].
In clinical practice, M. haplocalyx is often combined with other herbs to enhance therapeutic effects, with the composition adjusted according to the symptoms being treated. Numerous prescriptions containing M. haplocalyx are currently used in TCM for treating conditions such as depression and atopic dermatitis. For example, Xiaoyaosan (XYS), a renowned classic TCM prescription for depressive disorders, includes M. haplocalyx as a key ingredient. TCM theory interprets depression as "liver qi stagnation," and XYS addresses this by targeting the underlying condition. Studies have demonstrated that M. haplocalyx has a significant effect on liver damage [36], further supporting its role in regulating liver qi and contributing to depression treatment within the XYS formulation [37–39]. Additionally, M. haplocalyx is a key component in a tri-herb formula, used topically in a 1:1:1 ratio with Paeonia suffruticosa Andr. and Calendula officinalis L. for treating atopic dermatitis. Research has confirmed that M. haplocalyx significantly reduces skin irritation in this formulation, making it a promising option for managing specific dermatitis conditions [40]. Beyond China, M. haplocalyx is also a significant element in ethnic medicine in countries such as Japan and Korea, where it is used to treat indigestion and respiratory infections, as noted in the Japanese and Korean Pharmacopoeias [3]. In Europe and America, M. haplocalyx is traditionally employed for treating fever, colds, and digestive issues and is recognized for its antiviral, antifungal, and anti-inflammatory properties, particularly against oral mucosa and throat inflammation [41]. In Africa, the empirical medical system also utilizes M. haplocalyx for various ailments, including influenza, rheumatism, migraines, ulcers, gastrointestinal disorders, diabetes, psychological and cardiac conditions, and constipation [42]. The potential for developing M. haplocalyx in both domestic and international markets is substantial. The diverse therapeutic effects of M. haplocalyx, substantiated by traditional applications and potential future uses, warrant further exploration.
Nutritional value
M. haplocalyx is widely recognized for its nutritional components, essential for a quality diet. Its mature and dried stems and leaves are edible [43], and early studies have highlighted its significant role in dietary intake [44]. Proteins, fats, and carbohydrates—key energy sources in human nutrition—are present in substantial amounts in M. haplocalyx. Specifically, every 100 g of fresh M. haplocalyx contains 6.8 g of protein, 3.9 g of fat, and 67.6 g of carbohydrates (https://www.boohee.com). Additionally, M. haplocalyx is rich in amino acids, fatty acids, organic acids, vitamins (B and C), retinol, carotenoids, various nutrients, and trace elements such as sodium, potassium, and phosphorus [45].
Notably, M. haplocalyx contains 30% dietary fiber, which is known to benefit metabolic health and reduce the risk of cardiovascular events [46, 47]. Both fresh and dried M. haplocalyx also provide relatively high levels of mineral elements, with potassium being the most abundant (135 mg/100 g), followed by iron (4.3 mg/100 g), manganese (5.15 mg/100 g), and zinc (1.64 mg/100 g) (Table 1). These minerals are biologically significant, contributing not only to human metabolic functions and overall health but also supporting the growth, lifecycle, and metabolic functions of plants. Importantly, the minerals in M. haplocalyx also serve as excellent dietary antioxidants.
Table 1.
Nutrition of M. haplocalyx
| Items | Content (/per 100 g) |
|---|---|
| Protein | 6.8 g |
| Crude fat | 3.9 g |
| Carbohydrate | 67.6 g |
| Cellulose | 31.1 g |
| K | 135 mg |
| Fe | 4.3 mg |
| Cu | 2.08 mg |
| Mn | 5.15 mg |
| Zn | 1.64 mg |
| P | 22 mg |
| Na | 17.5 mg |
Proteins, essential for tissue formation and physiological regulation, play a vital role in sustaining human life and health [48]. Amino acids, the building blocks of proteins, form polypeptide chains through peptide bonds [49]. M. haplocalyx contains 15 amino acids, including 7 essential ones (threonine, valine, methionine, isoleucine, leucine, phenylalanine, lysine) and 8 non-essential ones (aspartic acid, serine, glutamic acid, glycine, alanine, tyrosine, histidine, arginine) [50]. As a fresh food source, M. haplocalyx is rich in both essential and non-essential amino acids, which are pivotal in human physiological processes and significantly contribute to maintaining normal bodily functions and development. Therefore, M. haplocalyx can serve as a valuable dietary source of amino acids, supporting the intake of essential amino acids, enhancing bodily functions, and promoting health and quality of life.
M. haplocalyx essential oil contains small amounts of fatty acids, including palmitic acid, linoleic acid, and linolenic acid. Although the fatty acid content is low, it still contributes to the nutritional value of M. haplocalyx. Moreover, M. haplocalyx contains various organic acids, such as caffeic acid, rosmarinic acid, cinnamic acid, citric acid, acetic acid, and malic acid [51]. Rosmarinic acid is the most abundant organic acid, with approximately 156 mg/100 g fresh weight (FW) [52, 53]. These organic acids are important flavor compounds, offering rich taste profiles and being widely used as food flavoring agents in the food industry [54]. The unique taste and aroma of M. haplocalyx, attributed to these organic acids, enhance its appeal for both medicinal and culinary applications.
Phytochemistry
Extensive research has identified that M. haplocalyx primarily contains terpenoids, flavonoids, phenolic acids, anthraquinones, hydrocarbons, polysaccharides, and other phytochemicals. Among these, volatile compounds like terpenoids are recognized as the primary bioactive constituents, with menthol and menthone being particularly notable. Menthol typically constitutes 62.3–87.2% of M. haplocalyx's volatile components, while menthone accounts for approximately 12% [45]. Notably, menthol serves as the key raw material for M. haplocalyx flavoring. These chemical constituents contribute to M. haplocalyx's extensive medicinal properties and nutritional value, making it widely applicable in daily life. Furthermore, the diverse phytochemicals present in M. haplocalyx likely play a significant role in its health benefits, as observed in its effects post-consumption. The identified chemical constituents are summarized in Table 2, with their corresponding structures illustrated in Figs. 2, 3, 4, 5, 6.
Table 2.
Chemical compounds isolated from M. haplocalyx
| No | Chemical component | Molecular formula | Extraction solvent | Plant parts | References |
|---|---|---|---|---|---|
| Terpenoids | |||||
| 1 | Menthol | C10H20O | Water | Leaves | [73] |
| 2 | Isomenthone | C10H8O | Water | Leaves | [73] |
| 3 | Menthone | C10H18O | Water | Leaves | [73] |
| 4 | (4S)-7-hydroxy-carvone 7-O-β-D-glucopyranoside | C16H24O7 | 50% ethanol | Aerial parts | [4] |
| 5 | (4R,6R)-carveol β-D-glucoside | C16H26O6 | 50% ethanol | Aerial parts | [4] |
| 6 | (4R,6S)-carveol β-D-glucoside | C16H26O6 | 50% ethanol | Aerial parts | [4] |
| 7 | Pulegone | C10H16O | Water | Above-ground parts and top leaves | [2] |
| 8 | Carvone | C10H14O | Water | Above-ground parts and top leaves | [2] |
| 9 | Trans-carveol | C10H16O | Water | Above-ground parts and top leaves | [2] |
| 10 | Limonene | C10H16 | Water | Above-ground parts and top leaves | [2] |
| 11 | Trans-isopiperitenol | C10H16O | Water | Above-ground parts and top leaves | [2] |
| 12 | Isopiperitenone | C10H14O | Water | Above-ground parts and top leaves | [2] |
| 13 | Cis-isopulegone | C10H16O | Water | Above-ground parts and top leaves | [2] |
| 14 | Car-3-ene | C10H16 | Water | Aerial parts | [23] |
| 15 | α-Phellandrene | C10H16 | Water | Aerial parts | [23] |
| 16 | Terpinene | C10H16 | Water | Aerial parts | [23] |
| 17 | Isolimonene | C10H16 | Water | Aerial parts | [23] |
| 18 | Camphor | C10H16O | Water | Aerial parts | [23] |
| 19 | Isopulegol | C10H18O | Water | Aerial parts | [23] |
| 20 | α-Terpineol | C10H18O | Water | Aerial parts | [23] |
| 21 | Menthyl acetate | C12H22O2 | Water | Aerial parts | [23] |
| 22 | β-Phellandrene | C10H16 | Water | Aerial parts | [12] |
| 23 | γ-Terpinene | C10H16 | Water | Aerial parts | [12] |
| 24 | Cis-ocimene | C10H16 | Water | Aerial parts | [12] |
| 25 | Piperitone | C10H16O | Water | Aerial parts | [12] |
| 26 | Cinene | C10H16 | Water | Aerial parts | [73] |
| 27 | Linarionoside A | C19H34O7 | 50% ethanol | Aerial parts | [4] |
| 28 | Linarionoside B | C19H34O7 | 50% ethanol | Aerial parts | [4] |
| 29 | Rel-(1R,2S,3R,4R) p-menthane-1,2,3-triol 3-O-β-D-glucopyranoside | C16H30O8 | 70% aqueous acetone | Aerial parts | [5] |
| 30 | Rel-(1S,2R,3S) terpinolene-1,2,3-triol 3-O-β-D-glucopyranoside | C16H28O8 | 70% aqueous acetone | Aerial parts | [5] |
| 31 | Spicatoside A | C16H24O7 | Water | Whole herbs | [109] |
| 32 | Spicatoside B | C16H26O8 | Water | Whole herbs | [109] |
| 33 | Menthofuran | C10H14O | Water | Aerial parts | [12] |
| 34 | Caryophyllene | C15H24 | Water | Aerial parts | [23] |
| 35 | γ-Elemene | C15H24 | Water | Aerial parts | [23] |
| 36 | α-Bourbonene | C15H24 | Water | Aerial parts | [23] |
| 37 | Farnesene | C15H24 | Water | Aerial parts | [23] |
| 38 | Germacrene D | C15H24 | Water | Aerial parts | [23] |
| 39 | β-Bourbonene | C15H24 | Water | Aerial parts | [12] |
| 40 | β-Caryophyllene | C15H24 | Water | Aerial parts | [12] |
| 41 | Germacrene B | C15H24 | Water | Aerial parts | [12] |
| 42 | δ-Cadinene | C15H24 | Water | Aerial parts | [12] |
| 43 | γ-Gurjunene | C15H24 | Water | Aerial parts | [12] |
| 44 | Copaene | C15H24 | Water | Aerial parts | [12] |
| 45 | Aristolon | C15H22O | Water | Aerial parts | [12] |
| 46 | Nerolidol | C15H26O | Water | Aerial parts | [12] |
| 47 | Trans-nerolidol | C15H26O | Water | Aerial parts | [12] |
| 48 | Menthalactone | C10H14O2 | 50% ethanol | Aerial parts | [4] |
| 49 | Isopulegol acetate | C12H22O2 | Water | Aerial parts | [23] |
| 50 | Linaloo | C10H18O | Water | Aerial parts | [12] |
| 51 | Lavandulol | C10H18O | Water | Aerial parts | [12] |
| 52 | Terpineol | C10H18O | Water | Aerial parts | [12] |
| 53 | (1S)-(-)-β-Pinene | C10H16 | Water | Aerial parts | [12] |
| 54 | β-Myrcene | C10H16 | Water | Aerial parts | [12] |
| 55 | β-Thujene | C10H16 | Water | Aerial parts | [12] |
| 56 | 1,5,5-trimethyl-6-methylene-cyclohexene | C10H16 | Water | Aerial parts | [12] |
| 57 | Cinene | C10H16 | Water | Aerial parts | [12] |
| 58 | Lasiodonin | C20H28O6 | Water | Leaves | [9] |
| 59 | (3β,11α)-3-hydroxy-11α-methoxy-olean-12-en-3-yl palmitate | C47H82O3 | 50% ethanol | Aerial parts | [4] |
| 60 | Ursolic acid | C30H48O3 | 50% ethanol | Aerial parts | [4] |
| 61 | Oleanolic acid | C30H48O3 | 80% ethanol | Aerial parts | [110] |
| 62 | Maniladiol | C30H50O2 | 50% ethanol | Aerial parts | [4] |
| 63 | Naphthisoxazol A | C11H9NO2 | 50% ethanol | Aerial parts | [4] |
| Phenolic acids | |||||
| 64 | Protocatechuic acid | C7H6O4 | Water | Leaves | [9] |
| 65 | Ethyl rosmarinate | C20H20O8 | Water | Leaves | [9] |
| 66 | Protocatechuic aldehyde | C7H6O3 | Water | Leaves | [9] |
| 67 | P-coumaric acid | C9H8O3 | Water | Leaves | [9] |
| 68 | Perillic acid | C10H14O2 | Water | Leaves | [9] |
| 69 | Rosmarinic acid | C18H16O8 | Water | Aerial parts | [3] |
| 70 | Eukovoside | C30H38O15 | Water | Leaves | [9] |
| 71 | Chlorogenic acid | C16H18O9 | Water | Leaves | [9] |
| 72 | Caffeic acid | C9H8O4 | Water | Leaves | [9] |
| 73 | Lithospermic acid | C27H22O12 | Water | Leaves | [9] |
| 74 | Cryptochlorogenic acid | C16H18O9 | Water | Leaves | [9] |
| 75 | Cis-salvianolic acid J | C27H22O12 | 70% aqueous acetone | Aerial parts | [8] |
| 76 | Danshensu | C9H10O5 | 70% aqueous acetone | Aerial parts | [8] |
| 77 | Salvianolic acid J | C27H22O12 | 70% aqueous acetone | Aerial parts | [8] |
| 78 | Lithospermic acid B | C36H30O16 | 70% aqueous acetone | Aerial parts | [8] |
| 79 | Eugenol | C10H12O2 | Water | Aerial parts | [12] |
| 80 | Magnesium lithospermate B | C36H28MgO16 | 70% aqueous acetone | Aerial parts | [8] |
| 81 | Sodium lithospermate B | C36H29NaO16 | 70% aqueous acetone | Aerial parts | [8] |
| 82 | Salvianolic acid L | C36H30O16 | 70% aqueous acetone | Aerial parts | [5] |
| 83 | Scopoletin | C10H8O4 | Methanol | Aerial parts | [25] |
| 84 | Vanillylmandelic acid | C9H10O5 | Methanol | Aerial parts | [25] |
| 85 | Salvianolic acid B | C36H30O16 | Methanol | Aerial parts | [25] |
| Flavonoids | |||||
| 86 | Diosmin | C28H32O15 | Water | Leaves | [9] |
| 87 | Hesperidin | C28H34O15 | Water | Leaves | [9] |
| 88 | Linarin | C28H32O14 | Water | Leaves | [9] |
| 89 | Isosakuranetin-7-O-rutinoside | C28H34O14 | Water | Leaves | [9] |
| 90 | Genkwanin | C16H12O5 | Water | Leaves | [9] |
| 91 | Thymusin | C17H14O7 | Water | Leaves | [9] |
| 92 | Thymonin | C18H16O8 | Water | Leaves | [9] |
| 93 | 5,6-dihydroxy-7,3′,4′-methoxyflavone | C18H16O7 | Water | Leaves | [9] |
| 94 | 5,6-dihydroxy-7,8,3′,4′-tetramethoxy flavone | C19H18O8 | Water | Leaves | [9] |
| 95 | 3,4′-dihydroxy-5,6,7-methoxyflavone | C18H16O7 | Water | Leaves | [9] |
| 96 | Syringetin | C17H14O8 | Water | Leaves | [9] |
| 97 | 5-hydroxy-6,7,3′,4′-tetramethoxy flavone | C19H18O7 | Water | Leaves | [9] |
| 98 | 5-hydroxy-6,7,8,3′,4′-pentamethoxy flavone | C20H20O8 | Water | Leaves | [9] |
| 99 | Acacetin | C16H12O5 | Water | Leaves | [9] |
| 100 | Didymin | C28H34O14 | Water | Aerial parts | [3] |
| 101 | Buddleoside | C28H32O14 | Water | Aerial parts | [3] |
| 102 | Luteolin-7-O-rutinoside | C27H30O15 | Methanol | Aerial parts | [25] |
| 103 | Apigenin-7-O-glucoside | C21H20O10 | Methanol | Aerial parts | [25] |
| 104 | Isorhoifolin | C27H30O14 | Methanol | Aerial parts | [25] |
| 105 | Luteolin-7-O-glucoside | C21H20O11 | Methanol | Aerial parts | [25] |
| 106 | Tilianine | C22H22O10 | Methanol | Aerial parts | [25] |
| 107 | 5,4′-dihydroxy-7-methoxyflavone | C16H12O5 | Methanol | Aerial parts | [25] |
| 108 | 5,6,4′-trihydroxy-7-methoxyflavone | C16H12O6 | Methanol | Aerial parts | [25] |
| 109 | 5,6,4′-trihydroxy-7,8,3′-trimethoxyflavone | C18H16O8 | Methanol | Aerial parts | [25] |
| 110 | 5,3′,4′-trihydroxy-6,7,8-trimethoxyflavone | C18H16O8 | Methanol | Aerial parts | [25] |
| 111 | 5,6-dihydroxy-7,8,3′,4′-tetramethoxyflavone | C19H18O8 | Methanol | Aerial parts | [25] |
| 112 | 5-hydroxy-6,7,3′,4′-tetramethoxyflavone | C19H18O7 | Methanol | Aerial parts | [25] |
| 113 | 5-hydroxy-6,7,8,3′,4′-pentamethoxyflavone | C20H20O8 | Methanol | Aerial parts | [25] |
| 114 | Apigenin | C15H10O5 | Methanol | Aerial parts | [25] |
| 115 | Diosmetin | C16H12O6 | Methanol | Aerial parts | [25] |
| 116 | Hesperetin | C16H14O6 | Methanol | Aerial parts | [25] |
| 117 | Xanthomicrol | C18H16O7 | Methanol | Aerial parts | [25] |
| 118 | Gardenin D | C19H18O8 | Methanol | Aerial parts | [25] |
| 119 | Pebrellin | C19H18O8 | Methanol | Aerial parts | [25] |
| 120 | Gardenin B | C19H18O7 | Methanol | Aerial parts | [25] |
| Anthraquinone | |||||
| 121 | Tanshinone I | C18H12O3 | Water | Leaves | [9] |
| 122 | Dihydrotanshinone I | C18H14O3 | Water | Leaves | [9] |
| 123 | Salvianolic acid C | C26H20O10 | Water | Leaves | [9] |
| 124 | Emodin | C15H10O5 | Water | Aerial parts | [111] |
| 125 | Chrysophanic acid | C15H10O4 | Water | Aerial parts | [111] |
| 126 | Physcion | C16H12O5 | Water | Aerial parts | [111] |
| 127 | Aloe-emodin | C15H10O5 | Water | Aerial parts | [111] |
| Alkane | |||||
| 128 | Hexadecane | C16H34 | Water | Leaves | [9] |
| 129 | O-Xylene | C8H10 | Water | Aerial parts | [9] |
| 130 | 2-isopropyltoluene | C10H14 | Water | Aerial parts | [23] |
| 131 | 3,3-dimethylhexane | C8H18 | Water | Aerial parts | [23] |
| Others | |||||
| 132 | Hydroperoxy octadecadienoic acid | C18H32O4 | Water | Leaves | [9] |
| 133 | Diisooctyl phthalate | C24H38O4 | Water | Aerial parts | [23] |
| 134 | Jasmone | C11H16O | Water | Aerial parts | [12] |
| 135 | Cyclohexanol | C6H12O | Water | Aerial parts | [12] |
| 136 | 3-octanol | C8H18O | Water | Aerial parts | [12] |
| 137 | β-Sitosterol | C29H50O | 80% ethanol | Aerial parts | [110] |
| 138 | Cis-3-hexenyl phenyl acetate | C8H14O2 | Water | Aerial parts | [12] |
| 139 | Geranyl acetone | C13H22O | Water | Aerial parts | [12] |
| 140 | Τ-Muurolol | C15H26O | Water | Aerial parts | [12] |
| 141 | α-Cadinol | C15H26O | Water | Aerial parts | [12] |
| 142 | 2-pentadecanone, 6,10,14-trimethyl- | C18H36O | Water | Aerial parts | [12] |
| 143 | Ferulic acid | C10H10O4 | Water | Aerial parts | [112] |
| 144 | Phytol | C20H40O | Water | Aerial parts | [12] |
| 145 | Mono-ethylhexyl phthalate | C16H22O4 | Water | Aerial parts | [12] |
| 146 | Benzoic acid | C7H6O2 | Water | Aerial parts | [111] |
| 147 | Trans-cinnamic acid | C9H8O2 | Water | Aerial parts | [111] |
| 148 | Palmitic acid | C16H32O2 | Water | Aerial parts | [113] |
| 149 | Daucosterol | C35H60O6 | 80% ethanol | Aerial parts | [110] |
| 150 | Tuberonic acid glucoside | C18H28O9 | Methanol | Aerial parts | [25] |
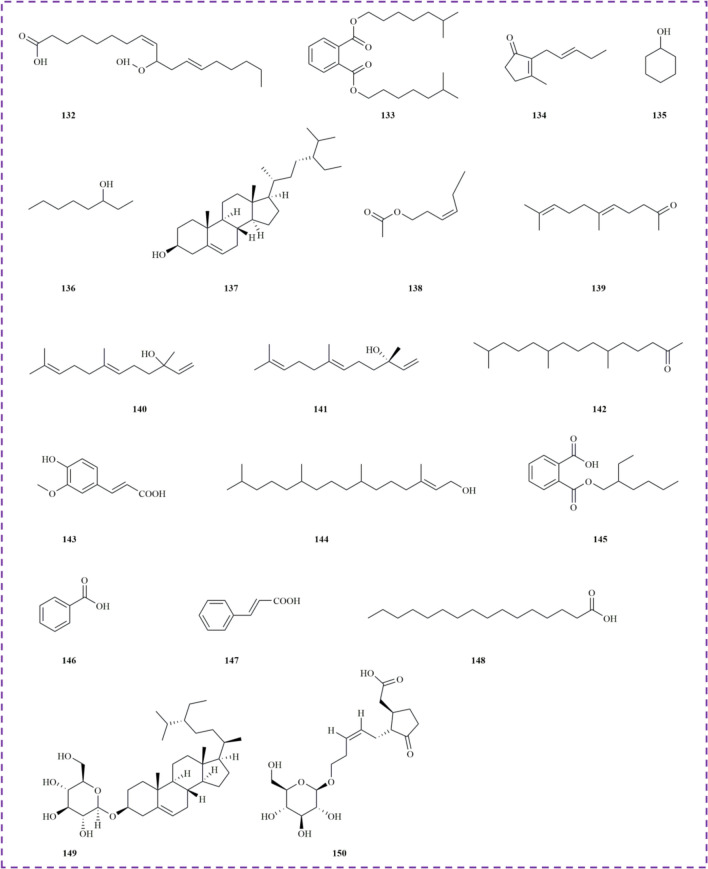
Terpenoids
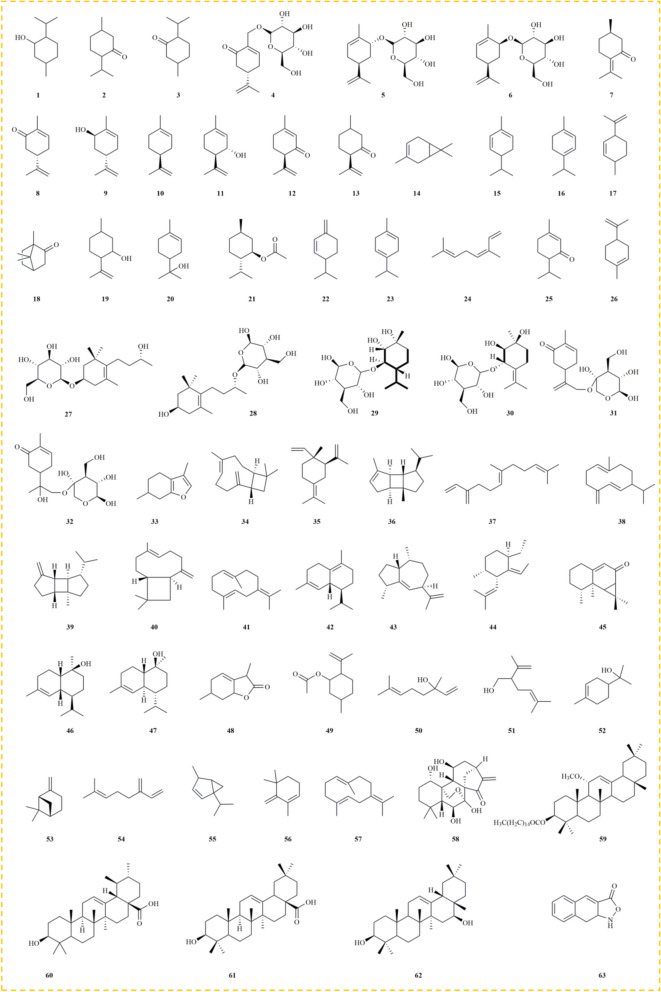
Terpenoids, polymers and derivatives of isoprenes, form the backbone of many essential phytochemical components in M. haplocalyx [55]. These compounds, characterized by their widespread distribution, complex structures, and significant biological activities, are abundant in M. haplocalyx. To date, over 63 terpenoids have been isolated and identified from M. haplocalyx, encompassing a broad spectrum of common terpenoids, including monoterpenes, sesquiterpenes, diterpenes, and triterpenes. Specifically, compounds 1–33 and 49–57 are classified as monoterpenes, 34–47 as sesquiterpenes, 58 as a diterpene, and 59–62 as triterpenes [4, 12, 23]. Notably, terpenoids such as menthol (1) and menthone (3) are the primary bioactive constituents of M. haplocalyx, contributing both to its distinctive aroma and its medicinal properties. For instance, menthol has been shown to significantly reduce neuronal cell death, showcasing neuroprotective effects, while menthone exhibits anti-asthmatic potential. An overview of the terpenoids (1–63) is presented in Fig. 2.
Fig. 2.
The structures of terpenoids in M. haplocalyx
Phenolic acids
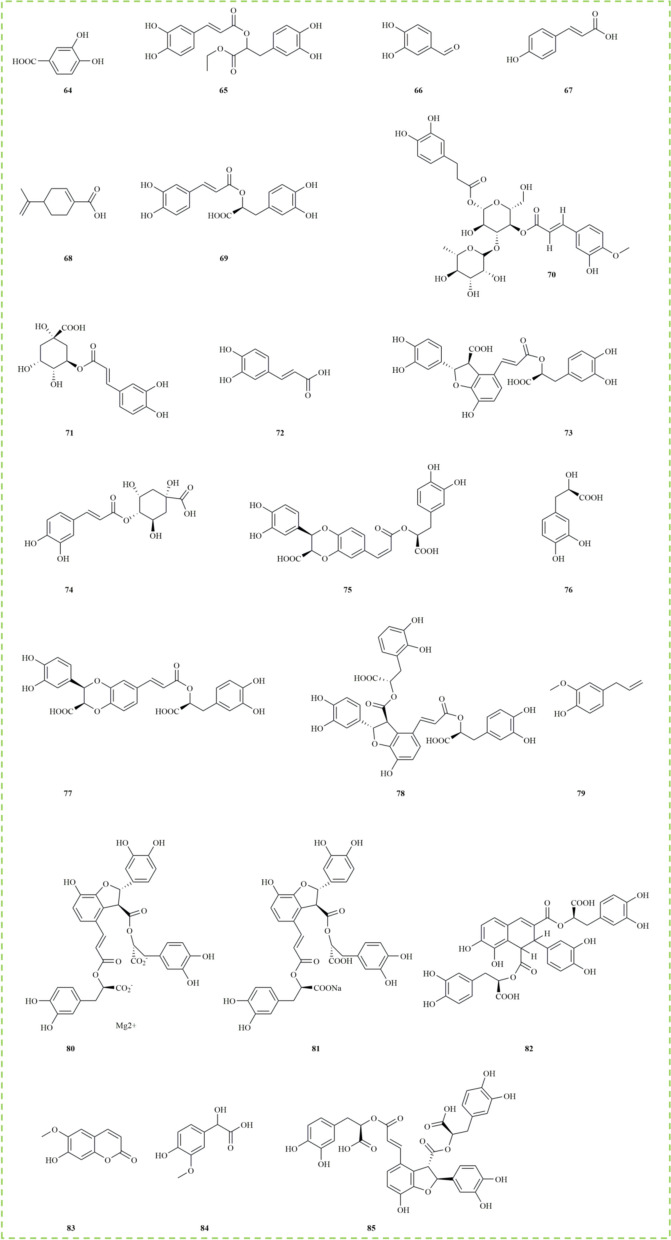
Phenolic acids, a subclass of plant phenols, are important secondary metabolites widely present in fruits, vegetables, and grains, and are prominently found in M. haplocalyx [56]. These compounds are typically conjugated with carbohydrates in the form of glycosides. From a human health perspective, phenolic acids are known to prevent the development of various diseases due to their antioxidant properties [57, 58]. Extensive research indicates that the phenolic acids in M. haplocalyx possess diverse biological activities, making them valuable natural phytochemicals with significant research and application potential. Approximately 22 phenolic acids have been isolated from M. haplocalyx, including common ones such as protocatechuic acid (64), rosmarinic acid (69), chlorogenic acid (71), and caffeic acid (72). Notably, compounds like lithospermic acid B (78), magnesium lithospermate B (80), and sodium lithospermate B (81) were isolated from M. haplocalyx for the first time, demonstrating exceptional antioxidant activity in the DPPH radical scavenging assay, with SC50 values of 15.98 μM, 17.85 μM, and 18.22 μM, respectively [8]. Additionally, rosmarinic acid (69) exhibited not only strong DPPH scavenging activity but also significantly inhibited ovalbumin (OVA)-induced airway inflammation, suggesting its potential in repairing pathological lung damage caused by inflammation [59]. Furthermore, danshensu (76) has been reported to play a critical role in preventing lipid peroxidation and cardiovascular diseases. The structures of these phenolic acids (64–85) are depicted in Fig. 3.
Fig. 3.
The structures of phenolic acids in M. haplocalyx
Flavonoids
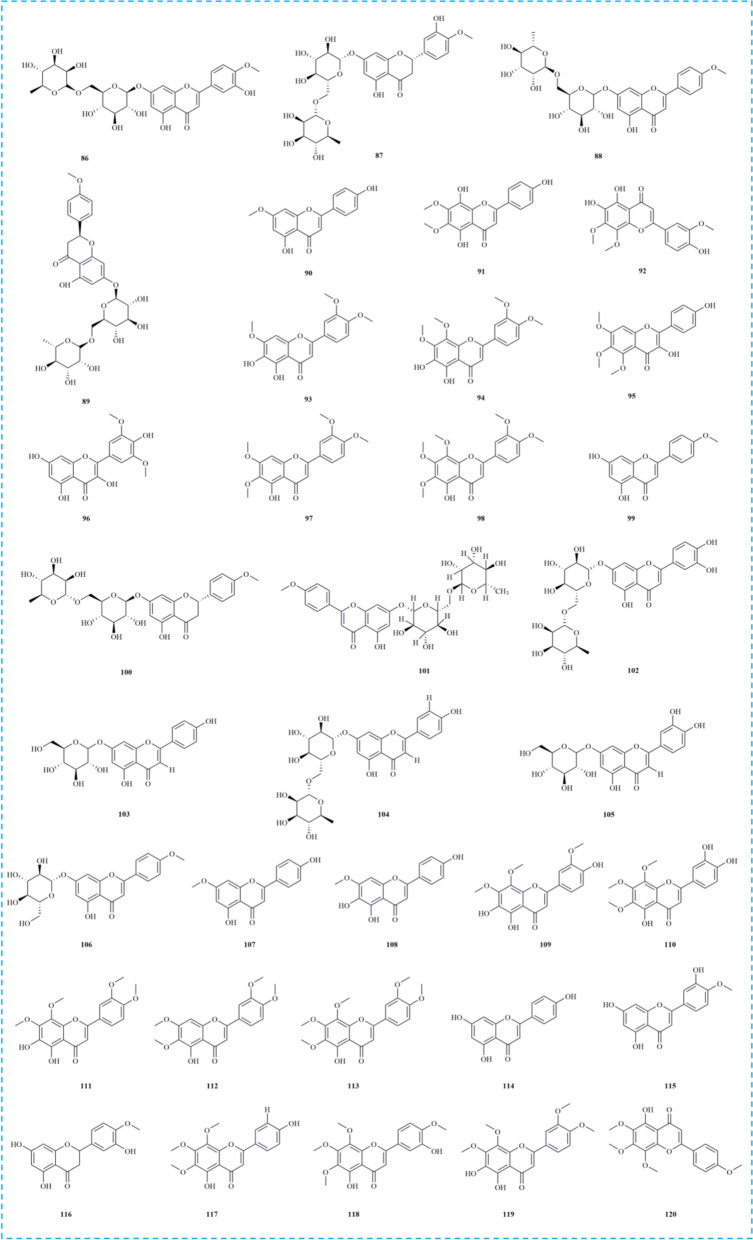
In recent years, flavonoids among the various phytochemical constituents identified in M. haplocalyx have attracted significant attention from researchers due to their unique contributions to the plant's biological properties. Flavonoids are naturally occurring polyphenolic compounds [60], characterized by a 15-carbon skeleton comprising two benzene rings and one heterocyclic ring [61]. Through extensive research, nearly 35 types of flavonoids have been discovered in M. haplocalyx, primarily falling into two major subclasses: flavones and flavonols. The flavonoids present in relatively high concentrations in M. haplocalyx include hesperidin (87), linarin (88), diosmin (86), and luteolin-7-O-glucoside (105). Linarin, in particular, has demonstrated various biological activities in modern pharmacological studies, especially its anti-inflammatory and neuroprotective effects. Additionally, other significant flavonoids in M. haplocalyx include acacetin (99), buddleoside (101), tilianine (106), and diosmetin (115). Flavonoids in M. haplocalyx not only protect the plant from biotic and abiotic stressors but also contribute to the prevention of neurodegenerative diseases in the human diet [9, 25]. The chemical structures of flavonoids 86–120 are depicted in Fig. 4.
Fig. 4.
The structures of flavonoids in M. haplocalyx
Anthraquinone
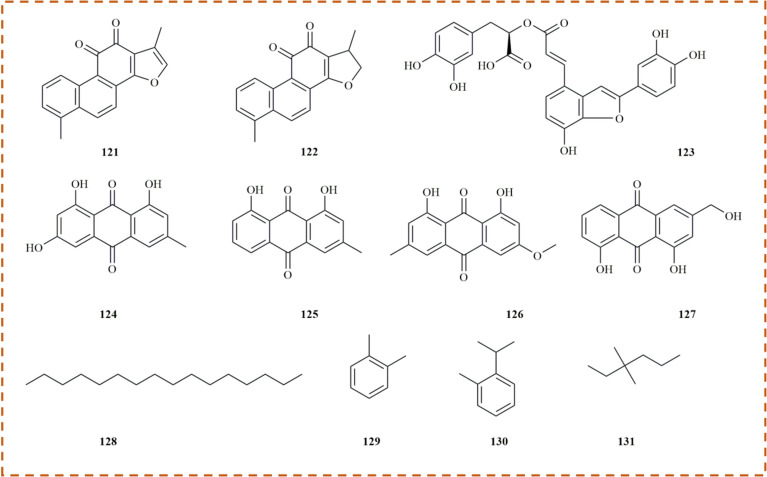
Anthraquinones, another class of natural products with significant biological activities, are commonly found in medicinal herbs and have been extensively studied for their potential applications in various fields [62]. Seven anthraquinone compounds have been isolated and identified from M. haplocalyx, including emodin (124) and aloe-emodin (127), both of which are derivatives of natural anthraquinones. Emodin is recognized as a protein tyrosine kinase inhibitor and an anti-cancer agent, showing efficacy against various tumor cells. Recent studies have highlighted the diverse health benefits of aloe-emodin from M. haplocalyx, garnering global attention (Fig. 5) [63, 64].
Fig. 5.
The structures of anthraquinone and alkane in M. haplocalyx
Alkane
Alkanes, consisting solely of carbon and hydrogen atoms, represent one of the simplest types of organic compounds. In M. haplocalyx, trace amounts of alkane compounds (128–131) have been identified, primarily categorized into cyclic and linear alkanes. These constituents may contribute to the distinctive aromatic characteristics of M. haplocalyx (Fig. 5).
Polysaccharide
Polysaccharides, which are carbohydrate polymers synthesized through dehydration and condensation reactions of multiple monosaccharides, have been increasingly recognized for their significant biological activities and essential roles in various life processes [65, 66]. Extensive research has identified six heteropolysaccharides isolated from M. haplocalyx. Notably, Fang et al. (2020) used solvent extraction techniques to isolate four of these polysaccharides, named MHP-W, MHP-C, MHP-A, and MHP-S [67]. Preliminary analysis, including molecular weight (Mw) determination and monosaccharide composition via high-performance gel permeation chromatography (HPGPC), 1-phenyl-3-methyl-5-pyrazolone (PMP) derivatives, and high-performance liquid chromatography (HPLC), revealed that MHP-A had the highest extraction yield at (9.37 ± 0.24) % but the lowest Mw. Conversely, MHP-W exhibited the highest uronic acid content and the largest Mw. Subsequently, over the next two years, the same group identified an additional polysaccharide, PMHP-3, characterized as an acidic polysaccharide with an Mw of 21.82 kDa. PMP derivative analysis and HPLC determined that PMHP-3 consisted of mannose, rhamnose, glucuronic acid, galacturonic acid, glucose, galactose, and arabinose in molar ratios of 1.01:1.82:4.26:19.29:2.46:55.08:16.08, respectively, and had a high purity with a total sugar content of (90.17 ± 1.41) % [68]. Furthermore, Jiang et al. (2020) isolated an antioxidant polysaccharide (WMP) from M. haplocalyx with an Mw of 26.91 kDa using water extraction, ethanol precipitation, and gel filtration techniques. Detailed structural analysis through HPLC, methylation analysis, gas chromatography-mass spectrometry (GC–MS), and 1D/2D nuclear magnetic resonance spectroscopy revealed that WMP is a heteropolysaccharide primarily composed of galactose (84.2%), glucose (9.8%), mannose (2.8%), and arabinose (3.2%), featuring a main chain of (1 → 6)-α-D-Galp and (1 → 4,6)-α-D-Galp residues, with a side chain comprising (1 → 6)-α-D-Galp and (1 → 6)-α-D-Glcp residues [69]. Collectively, these polysaccharides from M. haplocalyx form the foundation of its notable biological activity, contributing to its extensive health benefits, which continue to attract substantial scientific interest. Table 3 presents a summary of the fundamental characteristics of these polysaccharides.
Table 3.
Polysaccharides of M. haplocalyx plants
| No | Name | Extraction solvent | Composition | Molar ratio | Mw (kDa) | Total yield (%) | Activity | References |
|---|---|---|---|---|---|---|---|---|
| 1 | MHP-W | 95% ethanol | Man, Rib, Rha, GluA, GalA, Glc, Gal, Ara | 3.26:1.06:4.49:1.07:12.34:4.92:43.69:29.18 | 574.76 kDa (23.03%), 22.70 kDa (28.95%), and 12.09 kDa (48.02%) | 6.21 | Antioxidant | [67] |
| 2 | MHP-C | 95% ethanol | Man, Rib, Rha, GluA, GalA, Glc, Gal, Ara | 1.76:N/A:5.51:2.89:7.81:4.6.65:46.60:28.78 | 72.53 kDa (35.06%), 10.88 kDa (13.25%), and 5.84 kDa (51.69%) | 7.28 | Antioxidant | [67] |
| 3 | MHP-A | 95% ethanol | Man, Rib, Rha, GluA, GalA, Glc, Gal, Ara | 2.51:N/A:3.24:1.92:8.54:7.02:44.44:32.34 | 11.73 kDa (15.21%) and 6.21 kDa (84.79%) | 9.37 | Antioxidant | [67] |
| 4 | MHP-S | 95% ethanol | Man, Rib, Rha, GluA, GalA, Glc, Gal, Ara | 1.57:N/A:5.45:2.68:7.80:4.74:44.99:32.76 | 86.75 kDa (26.01%), 18.27 kDa (48.32%), and 6.29 kDa (25.67%) | 7.78 | Antioxidant | [67] |
| 5 | PMHP-3 | Water | Man, Rha, GluA, GalA, Glc, Gal, Ara | 1.01:1.82:4.26:19.29:2.46:55.08:16.08 | 21.82 kDa | 12.73 | Gut health improvement | [68] |
| 6 | WMP | 95% ethanol | Gal, Glc, Man, Ara | 84.2:9.8:2.8:3.2 | 26.91 kDa | 84.33 | Antioxidant and anti-aging | [69] |
mannose, (Man); rhamnose, (Rha); ribose, (Rib); galacturonic acid, (GalA); glucuronic acid, (GluA); glucose, (Glc); galactose, (Gal); arabinose, (Ara)
N/A: information was not available
Others
Beyond these six primary components, an additional 19 compounds (132–150) have also been reported from M. haplocalyx. Among these, cyclohexanol (135), 3-octanol (136), β-sitosterol (137), and phytol (144) are classified as alcohols. Ferulic acid (143) and benzoic acid (146) are aromatic acids, while trans-cinnamic acid (147) and palmitic acid (148) are organic acids. Daucosterol (149), a natural sterol identified in M. haplocalyx, is notable for its neuroprotective and anti-cancer properties (Fig. 6).
Fig. 6.
The structures of other compounds in M. haplocalyx
Biosynthesis
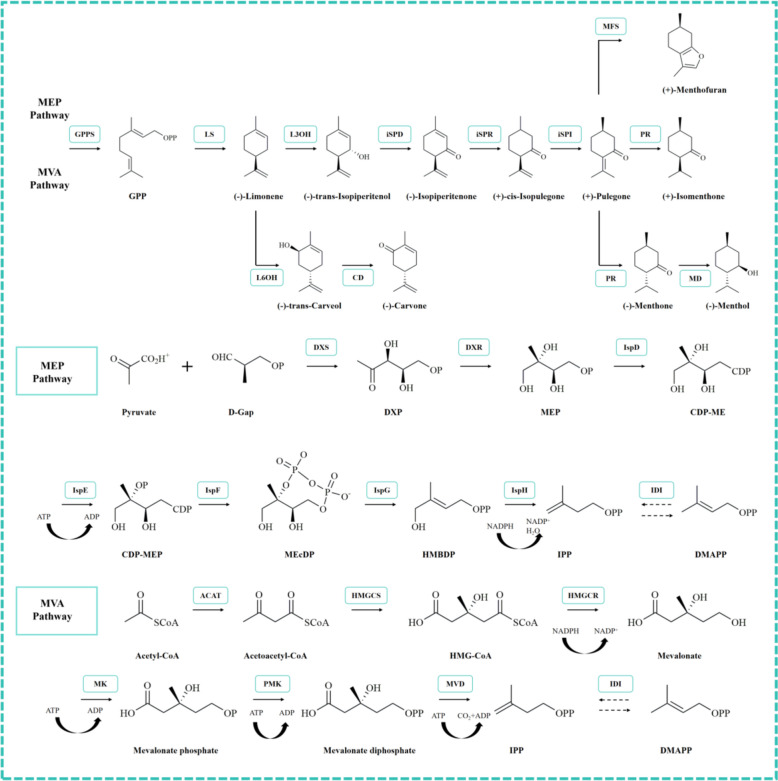
M. haplocalyx is renowned for its abundant bioactive secondary metabolites, with monoterpenes standing out due to their structural diversity and broad pharmacological properties. Over the past few decades, extensive research has documented the wide array of secondary metabolites present in M. haplocalyx, and in recent years, the biosynthesis of these high-value compounds has become a focal point of scientific inquiry. M. piperita and M. haplocalyx, two widely used species of the Mentha genus, play significant roles in the edible and medicinal fields, respectively. Each of these species exhibits distinct characteristics in the biosynthetic pathways of monoterpenes. The biosynthesis of monoterpenes in M. piperita and M. haplocalyx predominantly occurs via the MEP (methylerythritol phosphate) and MVA (mevalonic acid) pathways [21, 70]. As illustrated in Fig. 7, these pathways involve a sequence of enzyme-mediated reactions, starting with the formation of isopentenyl diphosphate (IPP) and dimethylallyl diphosphate (DMAPP) as precursor molecules. These precursors subsequently combine to generate geranyl diphosphate (GPP), a key intermediate leading to the synthesis of various monoterpenes in M. haplocalyx. Notably, the MEP pathway has been identified as the primary route for monoterpene biosynthesis in M. haplocalyx [2]. In M. piperita, the MVA pathway may also play a crucial role, particularly in the synthesis of its characteristic monoterpenes, such as menthol and menthyl acetate. Moreover, variations in enzyme activity and expression levels between the MEP and MVA pathways can influence the yield and composition of specific monoterpenes in the plant. The biosynthesis of monoterpenoids in M. haplocalyx has been extensively studied, and the use of engineering techniques to manipulate their production presents a promising avenue for research [71]. Approaches such as metabolic engineering, genetic engineering, and enzyme engineering hold substantial potential for enhancing monoterpene production in M. haplocalyx, particularly given their extensive applications in the pharmaceutical, food, and fragrance industries. Comprehensive investigation and analysis of the monoterpene biosynthetic pathways in M. haplocalyx not only facilitate the high-purity production of these commercially valuable compounds but also offer critical insights for future optimization of terpenoid production through advanced engineering methods.
Fig. 7.
The biosynthesis pathway of main monoterpenes in M. haplocalyx. (GPPS, Geranylgeranyl pyrophosphate synthase; GPP, Geranyl diphosphate; LS, (−)-limonene synthase; L6OH, (−)-limonene 6-hydroxylase; CD, (−)-trans-carveol-dehydrogenase; L3OH, (−)-limonene 3-hydroxylase; iSPD, isopiperitenol dehydro-genase; iSPR, (−)-isopiperitenone reductase; iSPI, ( +)-cis-isopulegone isomerase; MFS, ( +)-menthofuran synthase; PR, ( +)-pulegone reductase; MD, (−)-menthol d-ehydrogenase; DXS, 1-Deoxy-D-xylulose 5-phosphate synthase; DXP, 1-Deoxy-D-xylulose 5-phosphate; DXR, 1-deoxy-D-xylulose 5-phosphate reductoisomerase; MEP, 2C-Methyl-D-erythritol 4-phosphate; IspD, 2-C-methyl-D-erythritol 4-phos-phate cytidylyltransferase; CDP-ME, 4-Diphosphocytidyl-2-C-methylerythritol; Is-pE, 4-(cytidine 5′-diphospho)-2-C-methyl-D-erythritol kinase; CDP-MEP, 4-Dip-hosphocytidyl-2-C-methyl-D-erythritol 2-phosphate; IspF, 2-C-methyl-D-erythritol-2,4-cyclodiphosphate synthase; MEcDP, 2C-Methyl-D-erythritol-2,4-cyclodiphosp-hate; IspG, 4-hydroxy-3-methylbut-2-en-1-yl diphosphate synthase; HMBDP, 1-H-ydroxy-2-methyl-2-(E)-butenyl-4-diphosphate; IspH, 4-hydroxy-3-methylbut-2-eny-ldiphosphate reductase; IPP, Isopentenyl diphosphate; IDI, Isopentenyl diphosph-ate isomerase; DMAPP, Dimethylallyl diphosphate; ACAT, Acetyl-coenzyme A acetyltransferases (Thiolase); HMGCS, Hydroxymethylglutaryl-CoA synthase; H-MGCR, 3-Hydroxy-3-methylglutaryl-coenzyme A reductase; MK, Mevalonate kin-ase; PMK, Phosphomevalonate kinase; MVD, Mevalonate diphosphate decarbox-ylase)
Health benefits
M. haplocalyx is abundant in nutrients and bioactive components, offering a broad spectrum of health benefits, including neuroprotective, anti-asthma, anti-inflammatory, gut health improvement, hypoglycemic, anti-aging, anti-bacterial, and antioxidant activities. Table 4 and Fig. 8 provide an overview of these health benefits.
Table 4.
Summary of health benefits of M. haplocalyx extracts/compounds
| Health benefits | Study design | Models | Results/mechanisms | Dosages | References |
|---|---|---|---|---|---|
| Neuroprotective | In vitro | H2O2-induced rat hippocampal neuronal cells | Significantly reduced hydrogen H2O2-induced neuronal cell death | 400 μM | [14] |
| In vitro | H2O2-induced rat hippocampal neuronal cells |
The flotation product of menthol could alleviate H2O2-induced oxidative stress |
200 μL | [73] | |
| Anti-asthma | In vivo | Female BALB/c mice (OVA-induced mouse model of allergic asthma) | Significantly inhibited increases in immunoglobulin (Ig) E and T-helper 2 (Th2)-type cytokines such as IL-4 and IL-5 in bronchoalveolar lavage fluid (BALF) and lung tissue | 100 mg/kg | [15] |
| In vivo | OVA-induced mouse models |
Significantly reduced the levels of inflammatory mediators, eosinophil infiltration, and mast cell degranulation in the BALF ↓CC receptor 3 and CXC receptor 1, ↑Th1 cytokine levels |
40 mg/kg | [76] | |
| Anti-inflammatory | In vitro | LPS-induced RAW264.7 cells | ↓NO, ↓TNF-α, ↓IL-1β, ↓IL-6 |
50–200 μg/mL 5–20 μM |
[79] |
| Gut health improvement | In vitro | Fresh saliva of healthy volunteers | Reducing the ratio of Firmicutes/Bacteroidetes, promoting the proliferation of beneficial bacteria such as Bacteroidaceae and Bifidobacteriaceae, and inhibiting harmful bacteria such as Lachnospiraceae and Enterobacteriaceae | Not detected | [68] |
| Hypoglycemic | In vitro | α-Glucosidase and α-Amylase | The inhibition rates for α-glucosidase and α-amylase activities were (65.34 ± 2.48) % and (45.97 ± 1.13) % | 5 mg/mL | [67] |
| In vitro | α-Glucosidase | IC50: 21.0 μg/mL | Not detected | [4] | |
| Anti-aging | In vivo | D-Gal-induced mouse model | ↑SOD, ↑CAT, ↑GSH-Px, ↓MDA | 50 mg/kg | [69] |
| In vivo | Mouse and Caenorhabditis elegans models | ↑TRPM8, ↑Nanog, ↓TRPV1, ↓P53, ↓NF-κB | Not detected | [16] | |
| Anti-bacterial | In vitro | Fusarium oxysporum |
Inhibition of Fusarium oxysporum spore germination and mycelial growth MIC: 0.58 mg/mL |
0.5 mg/mL | [51] |
| Antioxidant | In vivo | D-Gal-induced mouse model | ↑SOD, ↑CAT, ↑GSH-Px, ↓MDA | 10, 50, and 100 mg/kg | [69] |
| In vitro | WMP |
↑DPPH radical scavenging ability ↑Hydroxyl radical scavenging ability ↑Ferrous ion chelating activity |
1–50 μg/mL 0.5–3.5 mg/mL 1 mg/mL |
[69] | |
| In vitro | MHPs |
↑Superoxide radical scavenging activity ↑DPPH radical scavenging activity ↑Hydroxyl radical scavenging activity |
0.125, 0.25, 0.5, 1, 2, 3, and 4 mg/mL | [67] | |
| In vivo | Genetically improved farmed tilapia | ↑SOD, ↑CAT, ↑GPx, ↓GSH | 0.2, 2.0, 20, and 200 µg/L | [91] |
(↑): improve or promote
(↓): inhibit or reduce
Fig. 8.
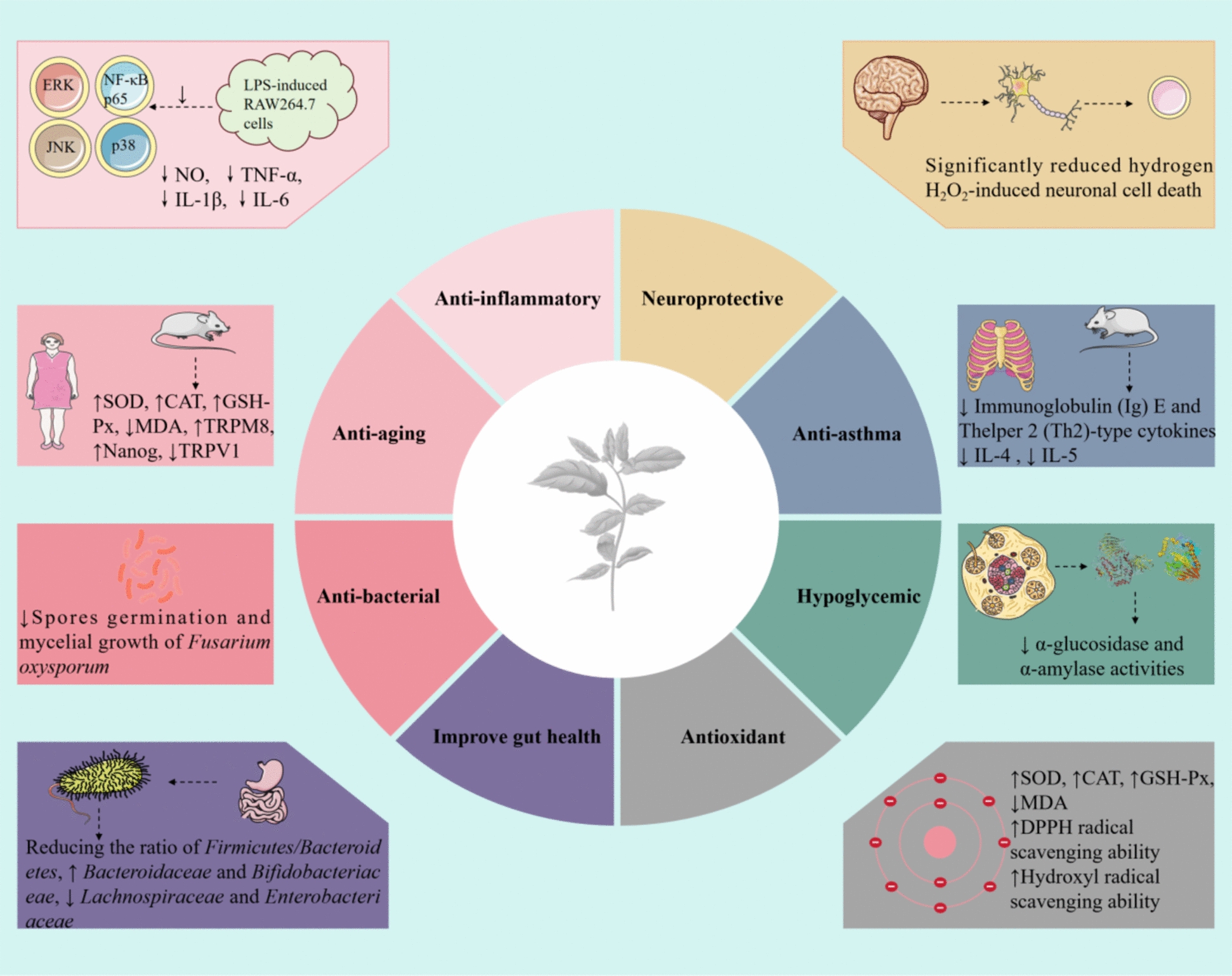
The health benefits of M. haplocalyx. (↑): improve or promote. (↓): inhibit or reduce
Neuroprotective properties
Neuroprotection involves maintaining nerve tissue function and network integrity while preventing damage caused by pathogens and neurodegenerative diseases. Linarin, a flavonoid glycoside naturally found in M. haplocalyx, has garnered attention for its diverse biological effects, particularly in inhibiting the progression of neurodegenerative diseases [72]. Evidence suggests that linarin exerts neuroprotective effects, as demonstrated in a study using H2O2-induced oxidative stress in rat hippocampal neurons. The oxidative stress model was effectively established by treating cells with 400 μM H2O2, leading to a marked decrease in cell viability. Neuronal apoptosis was assessed using the DAPI method, revealing that 400 μM H2O2 significantly increased the number of apoptotic neurons. However, neurons cultured in a medium containing linarin showed a notable reduction in H2O2-induced neuronal death. These results indicate that linarin in M. haplocalyx has a substantial neuroprotective effect, mitigating oxidative stress induced by H2O2 [14]. Thus, linarin emerges as a promising natural neuroprotective agent, offering valuable insights into potential treatments for nervous system diseases.
Furthermore, research has shown that menthol, another component of M. haplocalyx, also exhibits neuroprotective effects against H2O2-induced oxidative stress in rat hippocampal neurons. A similar oxidative stress model was established with 400 μM H2O2, which significantly diminished cell viability. The DAPI method revealed a considerable increase in apoptotic neurons under H2O2 treatment, whereas pre-incubation with menthol significantly reduced neuronal death [73]. These results suggest that menthol in M. haplocalyx may possess neuroprotective properties, potentially paving the way for new therapeutic approaches to related diseases. However, it is important to note that most of the current research on the neuroprotective properties of active compounds in M. haplocalyx is conducted in vitro, which limits the prospects for future clinical applications. Therefore, further in vivo studies are crucial to validate these neuroprotective effects and explore the safety and efficacy of these compounds in humans.
Anti-asthma properties
Asthma, a prevalent respiratory disease affecting both children and adults globally, is associated with substantial morbidity, mortality, and economic burden. As a chronic inflammatory immune disorder, asthma is primarily characterized by excessive mucus production in the lungs and inflammatory responses involving various cell types [74, 75]. Recent studies on M. haplocalyx have increasingly recognized its anti-asthmatic properties. Lee et al. [15] explored the protective effects of M. haplocalyx ethanol extract in an OVA-induced allergic asthma mouse model. Mice were administered M. haplocalyx ethanol extract orally at a dose of 100 mg/kg, with montelukast (30 mg/kg) serving as a positive control. The study revealed that M. haplocalyx ethanol extract significantly reduced the levels of immunoglobulin (Ig) E and IgG2a in bronchoalveolar lavage fluid (BALF) and lung tissue, as well as the expression of T-helper 2 (Th2)-type cytokines, including IL-4 and IL-5. This inhibition of Th2 cytokines consequently suppressed the infiltration of inflammatory cells into the airways. Additionally, the extract demonstrated antioxidant capacity by reducing reactive oxygen species (ROS) levels in BALF. Histological analysis corroborated these findings, showing reduced eosinophil and macrophage infiltration, alongside decreased mucus cell proliferation and secretion. These results suggest that M. haplocalyx ethanol extract may offer therapeutic potential in allergic asthma by modulating immune responses and mitigating oxidative stress [15].
Menthone, a prominent monoterpene in M. haplocalyx, also exhibits promising therapeutic effects against allergic asthma. At a dosage of 40 mg/kg, menthone significantly lowered the levels of inflammatory mediators, eosinophil infiltration, and mast cell degranulation in the BALF of an OVA-induced mouse model. Furthermore, it downregulated the gene expression of CC receptor 3 and CXC receptor 1, both closely linked to allergic inflammation, while promoting the restoration of alveolar macrophage proportions. Menthone's ability to regulate the Th1/Th2 immune balance and reduce the ratio of pro-inflammatory to anti-inflammatory cytokines in BALF underscores its potential as an anti-asthmatic agent [76]. Moreover, other constituents of M. haplocalyx have demonstrated anti-asthmatic activity. Rosmarinic acid, a natural phenolic compound found in M. haplocalyx, has shown significant inhibitory effects on OVA-induced airway inflammation and contributes to the repair of pathological lung damage. Rosmarinic acid exerts its anti-asthmatic effects by significantly reducing mRNA levels of key inflammatory mediators in lung tissue, such as AMCase, CCL11, CCR3, Ym2, and E-selectin. Its therapeutic potential is further enhanced by its regulation of cellular signaling pathways, particularly through the inhibition of extracellular regulated protein kinases (ERK), c-Jun N-terminal kinase (JNK), and p38 mitogen-activated protein kinase (p38 MAPK) phosphorylation, while activating the nuclear factor kappa B (NF-κB) signaling pathway [59]. Taken together, these results highlight the potential of M. haplocalyx as a source of new therapeutic strategies for the clinical treatment of allergic asthma and underscore the significant value of this traditional medicinal plant in modern drug development.
Anti-inflammatory properties
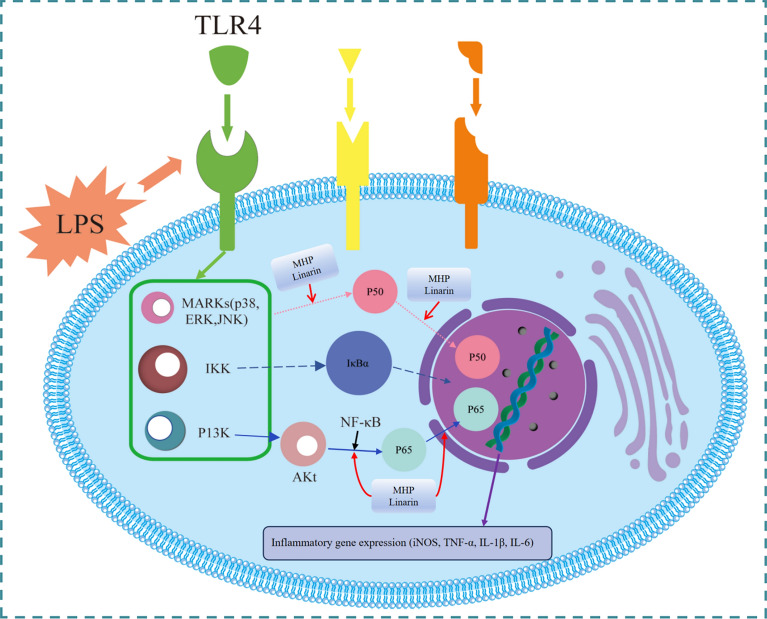
Uncontrolled inflammation, triggered by various factors, is one of the most prevalent health issues and can, in severe cases, lead to fatal outcomes. Consequently, the search for effective anti-inflammatory treatments remains a critical focus of medical research [77, 78]. M. haplocalyx is recognized for its potent anti-inflammatory properties, which align with its traditional use in heat-clearing and detoxification. Chen et al. [79] demonstrated that the phenolic fraction of M. haplocalyx, particularly its active component linarin, exhibits significant inhibitory effects on the production of inflammatory mediators. In a lipopolysaccharide (LPS)-induced RAW264.7 cell model, these compounds, within a dose range of 50–200 µg/mL and 5–20 µM, markedly reduced the levels of nitric oxide (NO), tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and IL-6 in a dose-dependent manner. This reduction was closely associated with the downregulation of inducible nitric oxide synthase (iNOS) mRNA expression. Notably, the anti-inflammatory effects of the phenolic fraction and linarin were comparable to those of dexamethasone, a well-known anti-inflammatory drug. Further mechanistic investigations revealed that the phenolic fraction of M. haplocalyx and linarin significantly inhibited the phosphorylation of critical proteins such as p65 and inhibitor kappa B α (IκBα) in the LPS-induced NF-κB signaling pathway, as well as the activation of ERK, JNK, and p38, which are members of the MAPK family. However, these compounds did not significantly affect the phosphorylation of the Akt signaling pathway. These results suggest that the anti-inflammatory effects of the phenolic fraction and linarin are mediated through the inactivation of the NF-κB and MAPK signaling pathways [79]. Figure 9 illustrates the relationship between these effects and the suppression of LPS-triggered NF-κB and MAPKs signaling. This research not only elucidates the molecular mechanisms underlying the anti-inflammatory actions of M. haplocalyx but also highlights its potential as a therapeutic agent for the prevention and treatment of inflammatory conditions [80].
Fig. 9.
Possible roles of M. haplocalyx and linarin in LPS-induced inflammatory responses in RAW264.7 cells. TLR4: Toll-like receptor 4; IKK: IκB kinase; PI3K: phosphoinositide 3-k
Gut health improvement properties
Disruptions in the balance of the intestinal ecosystem can lead to immune system dysregulation, diminishing the body's disease resistance and potentially triggering various adverse effects, such as metabolic disorders that negatively impact both physical and mental health [81, 82]. Recent research has highlighted the beneficial effects of the polysaccharide PMHP-3, derived from M. haplocalyx, on gut health. In an in vitro simulated digestion experiment, PMHP-3 exhibited remarkable stability, as evidenced by the unchanged Mw, total sugar content, and uronic acid content throughout the digestion process, indicating its resistance to digestion. Moreover, in an in vitro fermentation model, PMHP-3 at a concentration of 12.5 mg/mL significantly lowered the pH of the fermentation broth, a change that correlated with enhanced gut microbiota diversity. Specifically, PMHP-3 from M. haplocalyx stimulated the growth of beneficial bacteria such as Bacteroidaceae and Bifidobacteriaceae while inhibiting the proliferation of potentially harmful bacteria like Lachnospiraceae and Enterobacteriaceae. Additionally, during fermentation, PMHP-3 significantly increased the levels of short-chain fatty acids (SCFAs), including acetic acid, propionic acid, and n-butyric acid, which are essential for maintaining gut health and modulating the host's immune response [68].
In another groundbreaking study, M. haplocalyx extract, utilized as a key component in a feed additive, demonstrated positive regulatory effects on the gut microbiota of fattening sheep. The extract notably increased the relative abundance of beneficial bacteria such as Paraprevotella and Alloprevotella, while reducing the abundance of potentially harmful bacteria like Blautia [83]. These results further corroborate the role of M. haplocalyx extract in regulating gut microbial balance and promoting the growth of beneficial bacteria. Collectively, these studies provide a strong scientific foundation for the potential application of M. haplocalyx in maintaining intestinal function and promoting overall gut health.
Hypoglycemic properties
Hyperglycemia has been reported to accelerate endothelial cell senescence, thereby contributing to the development of diabetic complications. In critically ill patients, hyperglycemia is also associated with increased mortality. Natural plant polysaccharides, which are high molecular weight substances abundantly found in plants, exhibit a wide array of biological activities [84, 85]. Among these, the polysaccharides derived from M. haplocalyx (MHPs) have recently garnered significant attention within the scientific community due to their unique health benefits, particularly their hypoglycemic effects. Further purification of MHPs has yielded four distinct polysaccharides: MHP-W, MHP-C, MHP-S, and MHP-A. In vitro studies assessing the inhibitory activity of these purified polysaccharides on α-glucosidase and α-amylase revealed that different extraction solvents significantly influence their bioactivity. MHP-W, obtained through hot water extraction, demonstrated a (64.42 ± 1.44) % inhibition rate against α-glucosidase and a (44.16 ± 0.96) % inhibition rate against α-amylase at a concentration of 5 mg/mL, underscoring its strong potential as a hypoglycemic agent. Similarly, MHP-A, extracted using 5% NaOH/0.05% NaBH4, showed a (60.64 ± 1.01) % inhibition rate against α-glucosidase and a (42.64 ± 1.19) % inhibition rate against α-amylase at the same concentration, displaying a clear dose-dependent response. Although MHP-S, extracted with 0.9% NaCl, exhibited slightly weaker inhibition against α-glucosidase at (56.11 ± 1.52) %, it still achieved a (42.16 ± 1.44) % inhibition rate against α-amylase. Notably, MHP-C, obtained through citric acid extraction, presented the highest inhibition rates at 5 mg/mL, with (65.34 ± 2.48) % against α-glucosidase and (45.97 ± 1.13) % against α-amylase. Although the IC50 values of MHP-C were 1.96 mg/mL and 10.14 mg/mL—higher than those of the reference drug acarbose—these findings nonetheless highlight the significant hypoglycemic potential of MHP-C [67].
Additionally, other compounds in M. haplocalyx have demonstrated hypoglycemic effects. For instance, M. haplocalyx extract has shown significant inhibitory activity against α-glucosidase in vitro, with an IC50 value of 21.0 μg/mL, exhibiting a stronger dose-dependent effect compared to acarbose. Further bioactivity-guided separation led to the identification of a key active compound, (3R,9S)-megastigman-5-en-3,9-diol 3-O-β-D-glucopyranoside, which displayed an IC50 value of 83.4 μM, indicating its notable efficacy in inhibiting α-glucosidase [4]. Overall, the active components in M. haplocalyx offer valuable insights and serve as key guidance for the development of diabetes treatments.
Anti-aging properties
Organismal aging is characterized by the gradual decline in cellular function and systemic deterioration across multiple tissues, leading to impaired function and an increased susceptibility to mortality. This physiological aging process is complex, often resulting in diminished enzymatic oxidative capacity, heightened production of free radicals, and the accumulation of their by-products [86, 87]. Research has demonstrated that the plant polysaccharide WMP, derived from M. haplocalyx, has the capability to activate the body's antioxidant enzyme systems, thereby delaying the aging process. Jiang et al. [69] highlighted that prolonged administration of D-galactose (D-Gal) induces free radical accumulation and impairs antioxidant enzyme activity, making the D-Gal-induced aging model widely used in aging research and in screening substances with anti-aging properties. In this D-Gal-induced aging model, WMP from M. haplocalyx significantly enhanced the activity of antioxidant enzymes in both the serum and liver of mice at doses of 10 mg/kg, 50 mg/kg, and 100 mg/kg. These enzymes include superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), and catalase (CAT). Notably, the 50 mg/kg dose of WMP exhibited superior effects in restoring the antioxidant enzyme system. Compared to the positive control, vitamin C (VC, 100 mg/kg), WMP significantly increased SOD activity by 59.18%, GSH-Px activity by 13.61%, and CAT activity by 26.52%. Additionally, WMP reduced serum malondialdehyde (MDA) levels by 26.81%, further underscoring its significant potential in anti-aging applications [69]. Moreover, M. haplocalyx and its active component, menthol, may exert anti-aging effects by modulating members of the transient receptor potential (TRP) channel family, particularly TRPV1 and TRPM8. Through the inhibition of the heat-sensitive TRPV1 channel and activation of the cold-sensitive TRPM8 channel, M. haplocalyx and menthol may positively influence healthy longevity. This has been evidenced in models such as mice and Caenorhabditis elegans, where TRPM8 activation is associated with extended lifespan. Additionally, studies on rat mesenchymal stem cells (MSCs) have shown that M. haplocalyx extract can upregulate the expression of the anti-aging gene Nanog while downregulating aging-related genes such as P53 and NF-κB [16]. These effects likely contribute to enhanced cellular regeneration, reduced oxidative stress, and diminished inflammatory responses. In conclusion, this study provides valuable insights into the potential of M. haplocalyx in promoting longevity and extending healthy lifespan.
Anti-bacterial properties
M. haplocalyx is renowned for its potent anti-bacterial properties [88]. Numerous studies have confirmed that extracts and essential oils derived from M. haplocalyx exhibit significant anti-bacterial activity against a wide spectrum of both gram-positive and gram-negative bacteria [16]. Of particular interest is the M. haplocalyx essential oil nanoemulsion (MNEO), which is prepared using ultrasound with Tween 80 and anhydrous ethanol as stabilizers. MNEO, with an average particle size of just 26.07 nm, presents exceptional promise in antimicrobial applications. Compared to traditional M. haplocalyx essential oil solutions, MNEO has shown superior efficacy in inhibiting spore germination and mycelial growth of Fusarium oxysporum. This enhanced effectiveness is evident in the minimum inhibitory concentration (MIC) comparison: MNEO's MIC is 0.58 mg/mL, significantly lower than the 3.51 mg/mL required by the traditional essential oil solution, indicating that MNEO can effectively inhibit Fusarium oxysporum at much lower concentrations. Further molecular studies have elucidated the mechanisms underlying MNEO's anti-bacterial action. MNEO disrupts critical metabolic pathways and biological processes within Fusarium oxysporum, including energy metabolism, meiosis, and ribosome function. Specifically, MNEO markedly reduces the expression of genes involved in glycolysis/gluconeogenesis and starch and sucrose metabolism, leading to a decrease in the accumulation of key metabolites within these pathways, thereby disrupting energy metabolism and arresting fungal growth. Additionally, MNEO impacts genes associated with meiosis and ribosome biogenesis, further inhibiting the reproductive capacity of the fungus [51]. Consequently, M. haplocalyx is increasingly recognized as a potential adjunctive treatment for the prevention and management of various infectious diseases.
Antioxidant properties
Antioxidants play a pivotal role in maintaining human health by reducing the risk of cellular damage caused by free radicals. Free radicals are unstable molecules generated through oxidative reactions that can damage cell membranes, proteins, and DNA, potentially leading to various diseases, including cancer and cardiovascular conditions. Adequate levels of antioxidants are essential for lowering the risk of these illnesses and maintaining normal bodily functions [89, 90]. The antioxidant potential of M. haplocalyx extract has been validated through various testing methods [91].
The polysaccharide WMP derived from M. haplocalyx has demonstrated notable antioxidant properties. In a DPPH radical scavenging assay, WMP achieved a maximum scavenging rate of (71.49 ± 0.84) % at a concentration of 50 μg/mL, with an IC₅₀ value of 6.21 μg/mL, surpassing the standard set by vitamin C (VC) and indicating superior radical scavenging efficiency. Additionally, in a hydroxyl radical scavenging assay, WMP exhibited excellent dose-dependent scavenging activity across a concentration range of 0.5–3.5 mg/mL, with the scavenging rate increasing significantly from 20% to (86.90 ± 2.56) %. The IC₅₀ value was 1.03 mg/mL, slightly higher than VC's 0.98 mg/mL, further confirming WMP's potent antioxidant capabilities. Moreover, WMP also demonstrated impressive results in the Fe2⁺ chelating ability test, achieving 64% chelation efficiency at a concentration of 2 mg/mL, with an IC₅₀ value of 1.58 mg/mL. This suggests WMP's strong potential in preventing iron-induced oxidative stress [69].
Similarly, the four polysaccharides (MHPs) from M. haplocalyx—MHP-A, MHP-C, MHP-S, and MHP-W—not only exhibit hypoglycemic effects but also significant antioxidant properties. Among these, MHP-C stood out in the DPPH radical scavenging assay, achieving the highest scavenging rate of (79.31 ± 0.70) % at a concentration of 4 mg/mL, with an IC₅₀ value of 1.16 mg/mL, indicating its superior efficacy compared to other polysaccharides. MHP-C also demonstrated the strongest performance in the reducing power test, nearly matching the reducing capacity of ascorbic acid. In contrast, although MHP-A had the highest extraction yield and showed strong superoxide anion radical scavenging ability, its performance in the DPPH radical scavenging assay was less prominent, likely due to its lower molecular weight and higher protein and total phenolic content, which may be more effective in scavenging superoxide anion radicals rather than DPPH radicals. Collectively, these results highlight the potent antioxidant properties of M. haplocalyx polysaccharides, establishing them as powerful natural antioxidants [67]. Furthermore, the crude acetone–water extract of M. haplocalyx has been validated for its antioxidant activity in the DPPH radical scavenging assay, with an IC₅₀ value of 45.67 μg/mL. Chemical analysis revealed that the phenolic acid compounds isolated from this extract effectively neutralize DPPH radicals through their abundant phenolic hydroxyl groups, thereby inhibiting radical-mediated oxidative stress [8]. It is recommended to further investigate the safety and reliability of the phenolic acids in M. haplocalyx for their potential development as antioxidant drugs.
Application and commercialization potential of M. haplocalyx products
Application of M. haplocalyx products
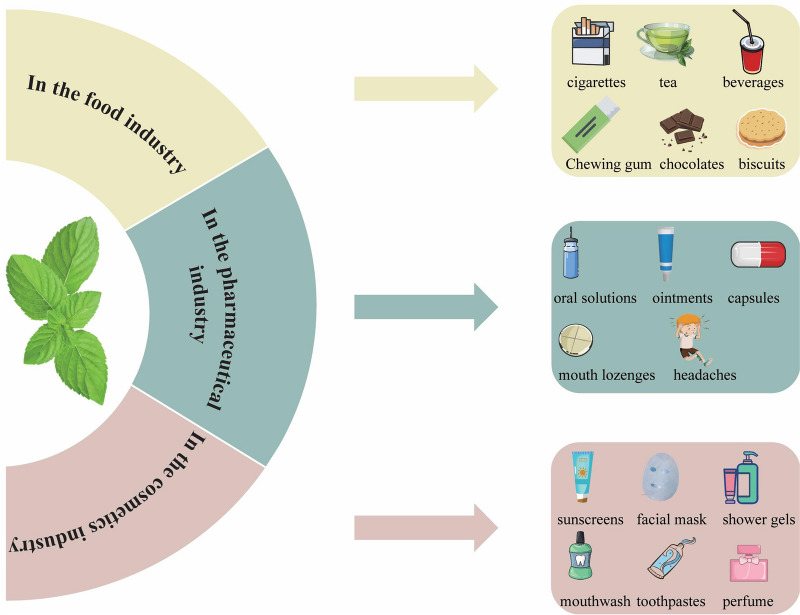
M. haplocalyx holds significant medicinal and nutritional value and exhibits broad potential across various industries. Currently, there are 572 patents related to M. haplocalyx worldwide (https://www.lens.org/), primarily focused on functional foods, medicine, cosmetics, and other applications. In the food industry, the distinctive aroma and health-promoting properties of M. haplocalyx leaves have garnered considerable attention. As a key ingredient in food flavoring, M. haplocalyx leaves are used not only in fresh foods but also as a raw material for producing volatile extracts, which are then incorporated into a wide range of beverages and confectioneries [3, 31, 92]. Additionally, M. haplocalyx is used in desserts, biscuits, chocolates, and ice cream, imparting a refreshing taste and aroma. Moreover, by combining M. haplocalyx with other ingredients such as Fagopyrum tataricum, Poria cocos, Pueraria lobata, and Glycyrrhiza uralensis, and processing them through soaking, extraction, filtration, filling, and sterilization, a health tea can be produced. Guided by TCM principles, this tea is believed to aid digestion and provide soothing effects, making it particularly suitable for individuals with hypertension, hyperlipidemia, and hyperglycemia, thus presenting promising industrial prospects. Furthermore, the terpenes and their oxidized derivatives in M. haplocalyx essential oil exhibit significant inhibitory effects on mold toxin formation [93], suggesting its potential as a flavoring agent to inhibit the growth of Aspergillus flavus and the production of aflatoxin in food.
In the pharmaceutical industry, M. haplocalyx is increasingly valued for its therapeutic potential. A study revealed that an herbal combination of M. haplocalyx, Coptis chinensis, Paeonia lactiflora, and Ligusticum chuanxiong effectively improves or prevents symptoms related to headaches [94]. Notably, extracts and essential oils of M. haplocalyx possess analgesic, sedative, and anti-bacterial properties, making them common ingredients in pharmaceutical formulations such as mouth lozenges, oral solutions, and ointments. For instance, mouth lozenges containing M. haplocalyx are highly effective in treating and preventing oral ulcers [95]. Additionally, M. haplocalyx ointment can be used to some extent in treating mild skin burns; its active ingredients offer anti-itch and analgesic effects, helping to alleviate discomfort in the affected area [96]. The cooling sensation provided by M. haplocalyx also helps reduce inflammation and swelling, offering relief to the skin. Furthermore, M. haplocalyx essential oil has shown remarkable benefits in aromatherapy. When used in methods such as aromatherapy lamps and massage oils, it can invigorate the mind and soothe muscles [97, 98]. As holistic health continues to gain emphasis, the market demand for M. haplocalyx in aromatherapy is likely to grow, further expanding its industrial applications.
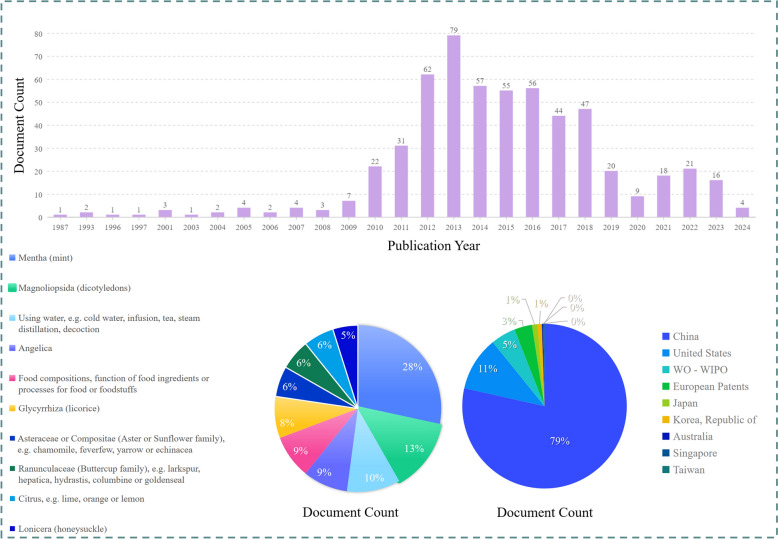
In the cosmetics industry, M. haplocalyx has gained considerable popularity due to its potent antioxidant properties and the tyrosinase inhibition activity of its essential oil. This essential oil effectively enhances the efficacy of sunscreens, largely attributed to its high sun protection factor (SPF) value [99]. Studies suggest that blemish creams formulated with M. haplocalyx extract and Pueraria lobata extract exhibit notable whitening effects. Specifically, M. haplocalyx extract comprises 45–99.9% of the total mass of tyrosinase inhibitors in these formulations, while Pueraria lobata extract makes up 0.1–55%. This formulation, due to its gentle yet effective inhibition of tyrosinase activity, provides a remarkable whitening effect. Additionally, M. haplocalyx extract is a common ingredient in oral care products such as toothpaste and mouthwash, where it contributes to fresh breath and oral hygiene [100]. The cool and refreshing fragrance of M. haplocalyx is also leveraged in perfume blending, enhancing the scent's freshness. Furthermore, M. haplocalyx essential oil is frequently incorporated into skincare products such as facial cleansers, shampoos, and shower gels. In conclusion, with the ongoing research into the unique nutritional and cosmetic properties of M. haplocalyx, its potential for further development in the cosmetics industry is significant. In the field of animal husbandry, the application of M. haplocalyx as an animal feed additive is gaining attention [101, 102]. There is an increasing trend of incorporating M. haplocalyx extracts into animal feed to combat microbial infections and inflammation, particularly in addressing post-weaning diarrhea in livestock and poultry. More importantly, M. haplocalyx extracts have been shown to significantly improve gut health and enhance growth performance in animals by modulating gut microbiota and boosting antioxidant capacity [83]. This positions M. haplocalyx extracts as a promising natural feed additive for future animal husbandry, particularly in promoting sustainable and healthy breeding practices. As illustrated in Fig. 10, the current status of related patented inventions of M. haplocalyx in recent years reveals that China and the United States dominate the patent landscape, accounting for 79% and 11% of the patents, respectively. In contrast, the World Intellectual Property Organization (WO-WIPO) and Europe represent a smaller portion of the patents, with 5% and 3% respectively. Overall, research and development related to M. haplocalyx products are still in their early stages. Nonetheless, M. haplocalyx is already widely used across diverse fields, including functional foods, medicine, cosmetics, and animal husbandry, as depicted in Fig. 11.
Fig. 10.
Current situation of patent inventions related to M. haplocalyx. A Document numbers, B Application of patents, C Patent distribution
Fig. 11.
Practical and potential applications of M. haplocalyx
Commercialization potential of M. haplocalyx products
As previously noted, M. haplocalyx, a multifunctional medicinal plant with a long history of cultivation in China, has now been widely introduced to many countries, including the United Kingdom, the United States, Japan, South Korea, India, and South Africa [103]. Initially cultivated for ornamental purposes, M. haplocalyx was not considered a commercial medicinal plant. However, in recent years, its pleasant aroma, refreshing flavor, and nutritional value have elevated it to the status of a commercial crop [104]. Consequently, the global export value of M. haplocalyx has seen continuous growth. With its widespread application in diverse fields such as food, medicine, cosmetics, and agriculture, global demand for M. haplocalyx products is on the rise. Many countries that produce M. haplocalyx have further stimulated export growth by enhancing cultivation, extraction, and processing technologies while expanding their international markets. These products include M. haplocalyx essential oil, tea, chewing gum, and oral care items. As a versatile and nutritious medicinal plant, M. haplocalyx appeals to health-conscious consumers. Countries like Mexico, Indonesia, Nigeria, and Turkey have substantial import and export scales for M. haplocalyx, reflecting the strong demand in their domestic and foreign markets [30, 105]. M. haplocalyx is a commercially viable crop that is easy to cultivate, maintain, and care for. It is rarely affected by diseases or pests, making it a popular secondary crop often grown in orchards and alongside other local crops [106, 107]. As the demand for fresh M. haplocalyx products and essential oils continues to rise both domestically and internationally, the cultivation area of M. haplocalyx has expanded accordingly. Notably, M. haplocalyx cultivation is cost-effective and can be grown as a secondary crop alongside other plants, making it ideal for small-scale farmers in areas with low land occupancy. Cultivating M. haplocalyx offers significant economic and social benefits, aligning with the principles of the bio-circular-green (BCG) economic model. As a natural, renewable medicinal plant, M. haplocalyx cultivation not only avoids the depletion of natural resources but also promotes sustainable production through proper management and cultivation techniques. The plant's strong photosynthetic capabilities also contribute to mitigating the impacts of climate change. Furthermore, the applications of M. haplocalyx extracts and essential oils in various industries have been proven to meet green product standards, thereby contributing to environmental preservation and supporting local economic development by creating jobs and income for farmers and communities [108]. In summary, the cultivation of M. haplocalyx presents numerous advantages, including low costs, high yields, and strong market demand. Its potential in both domestic and international markets holds significant practical value for increasing farmers' income and promoting regional economic development.
Conclusion and prospects
In recent years, M. haplocalyx has garnered significant attention from nutritionists, food researchers, and natural plant and herbal research institutions, leading to a thorough exploration and application of its potential value. M. haplocalyx is abundant in various nutrients, including essential and non-essential amino acids, organic acids, fatty acids, vitamins, trace elements, high-quality dietary fiber, and structurally diverse phytochemicals. To date, 150 constituents have been successfully isolated from M. haplocalyx, with terpenoids, phenolic acids, and flavonoids being the most prominent. These phytochemical compounds contribute to the wide range of health benefits associated with M. haplocalyx, such as neuroprotective, anti-asthmatic, anti-inflammatory, hypoglycemic, gut health improvement, and anti-bacterial properties. This article provides the first comprehensive review of M. haplocalyx, encompassing its botanical morphology, traditional uses, nutritional value, phytochemistry, health benefits, and practical applications across the food, cosmetics, and pharmaceutical industries.
Despite the significant progress in M. haplocalyx research, several issues remain unresolved. Firstly, the abundance of Mentha species has led to frequent misidentification and mixing of plants within the genus. Accurately identifying the species of M. haplocalyx is a pressing issue that requires resolution. Enhancing the clinical efficacy of M. haplocalyx necessitates distinguishing between different species based on plant morphology, component types and concentrations, pharmacological activities, and genetic characteristics. Furthermore, the current Ch.P 2020 uses menthol content (no less than 0.20%) as the sole indicator for assessing the quality of M. haplocalyx. However, relying on a single component is insufficient to fully reflect the plant's quality and does not align with the holistic principles of TCM. Therefore, establishing new detection methods or bridging the gaps in quality control from a biological activity perspective is essential.
Secondly, the identification of chemical constituents in M. haplocalyx remains incomplete. While recent studies have focused primarily on isolating and identifying terpenoids and volatile essential oils, emerging pharmacological research highlights the potential value of non-volatile components in treating respiratory, reproductive, and digestive system diseases. The development and application of advanced separation techniques are essential for comprehensively identifying and purifying the active compounds in M. haplocalyx. This will not only substantiate its potential in clinical treatments but also provide a robust scientific foundation for future drug development. Additionally, it is important to establish systematic methods to evaluate the synergistic effects of multiple active components in M. haplocalyx to achieve multi-target drug therapy strategies.
Thirdly, while recent studies have highlighted the neuroprotective and anti-inflammatory potential of M. haplocalyx, most of this research has been conducted in vitro or using animal models. The mechanisms of action are not well understood, and no clinical trials of M. haplocalyx extracts have been reported to date. Thus, further investigation into the active components of M. haplocalyx and their mechanisms of action is necessary to advance its therapeutic applications and translate these findings into clinical practice.
Fourthly, given the diversity of high-value compounds in M. haplocalyx and the complexity of its interactions with biological systems, the current research on the plant's potential toxicity is insufficient. Assessing the safety of M. haplocalyx is particularly critical, not only because of its widespread use in clinical treatments but also due to its common application in functional foods, cosmetics, and animal feed additives. Therefore, it is imperative to thoroughly investigate any potential adverse effects, determine the safe dosage range, and elucidate the mechanisms underlying its toxic effects to ensure its safety across various applications.
In conclusion, the reviewed literature provides compelling evidence that M. haplocalyx possesses significant nutritional value and functional characteristics. There is an urgent need for further research on M. haplocalyx in both in vitro and in vivo settings, and even in clinical practice, to develop new therapeutic modalities for various diseases. This paper offers a systematic and comprehensive review of M. haplocalyx, aiming to establish a foundation for further investigation into its mechanisms of action and future applications. Additionally, we hope this review will uncover new and intriguing insights about M. haplocalyx and provide valuable guidance for the continued development of this natural medicinal plant.
Acknowledgements
Not applicable.
Abbreviations
- BALF
Bronchoalveolar lavage fluid
- Ch.P 2020
2020 Edition of the Pharmacopoeia of the People’s Republic of China
- DAPI
4′,6-Diamidino-2-Phenylindole
- D-Gal
D-galactose
- ERK
Extracellular signal-regulated kinase
- FOS
Fructo-oligosaccharides
- GSH-Px
Glutathione peroxidase
- HPLC
High-performance liquid chromatography
- iNOS
Induced nitric oxide synthase
- IPP
Isopentenyl diphosphate
- JNK
C-Jun NH2-terminal kinase
- Mw
Molecular weights
- MEP
Methylerythritol phosphate
- MSCs
Mesenchymal stem cells
- MIC
Minimum inhibitory concentration
- OVA
Ovalbumin
- qRT-PCR
Quantitative real-time polymerase chain reaction
- SOD
Superoxide dismutase
- SCFAs
Short-chain fatty acids
- TNF-α
Tumor necrosis factor-α
- VC
Vitamin C
- BCG
Bio-circular-green
- CAT
Catalase
- DCF
Dichlorofluorescein
- DMAPP
Dimethylallyl diphosphate
- FW
Fresh weight
- GC-MS
Gas chromatography-mass spectrometry
- GPP
Generate geranyl diphosphate
- HPGPC
High-performance gel permeation chromatography
- IL-1β
Interlenkin-1β
- IκBα
Inhibitor kappa B α
- LPS
Lipopolysaccharide
- MDA
Malondialdehyde
- MVA
Mevalonic acid
- MNEO
M. haplocalyx essential oil nanoemulsion
- NF-κB
Nuclear factor kappa B
- PMP
1-phenyl-3-methyl-5-pyrazolone
- ROS
Reactive oxygen species
- SPF
Sun protection factor
- TCM
Traditional Chinese medicine
- TRP
Transient receptor potential
- XYS
Xiaoyaosan
Author contributions
Meng Wang and Hai-Xue Kuang proposed the framework of this paper. Hai-Peng Tang, En-Lin Zhu and Qian-Xiang Bai drafted the manuscript. Hai-Peng Tang, Shuang Wang and Zhi-Bin Wang made the figures and tables. All authors read and approved the final manuscript.
Funding
This work was supported by Heilongjiang Provincial Administration of Traditional Chinese Medicine TCM Scientific Research Project (No. ZHY2024-023), Basic Research Support Program for Outstanding Young Teachers in Heilongjiang Provincial Universities Major Project (No. YQJH2023148), Chief Scientist of Qi-Huang Project of National Traditional Chinese Medicine Inheritance and Innovation "One Hundred Million" Talent Project ([2021] No.7), Heilongjiang Touyan Innovation Team Program ([2019] No.5).
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
The manuscript is approved by all authors for publication.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Meng Wang, Email: wangmeng06tcm@163.com.
Hai-Xue Kuang, Email: hxkuang@hljucm.edu.cn.
References
- 1.He SL, Yang Y, Tian Y. Characteristic and phylogenetic analyses of chloroplast genome for Mentha haplocalyx (Lamiaceae). Mitochondrial DNA B Resour. 2020;5(3):2099–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.An X, Wan J, Jiang H, et al. Transcriptome analysis of transcription factors and enzymes involved in monoterpenoid biosynthesis in different chemotypes of Mentha haplocalyx Briq. PeerJ. 2023;11:e14914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhao BT, Kim TI, Kim YH, et al. A comparative study of Menthaarvensis L. and Menthahaplocalyx Briq. by HPLC. Nat Prod Res. 2018;32(2):239–42. [DOI] [PubMed] [Google Scholar]
- 4.He XF, Geng CA, Huang XY, et al. Chemical constituents from Menthahaplocalyx Briq. (Menthacanadensis L.) and their α-glucosidase inhibitory activities. Nat Prod Bioprospect. 2019;9(3):223–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.She GM, Xu C, Liu B. New monocyclic monoterpenoid glycoside from Mentha haplocalyx Briq. Chem Cent J. 2012;6(1):37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park YJ, Baskar TB, Yeo SK, et al. Composition of volatile compounds and in vitro antimicrobial activity of nine Mentha spp. Springerplus. 2016;5(1):1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dong W, Ni Y, Kokot S. A novel near-infrared spectroscopy and chemometrics method for rapid analysis of several chemical components and antioxidant activity of mint (Menthahaplocalyx Briq.) samples. Appl Spectrosc. 2014;68(2):245–54. [DOI] [PubMed] [Google Scholar]
- 8.She GM, Xu C, Liu B, et al. Polyphenolic acids from mint (the aerial of Menthahaplocalyx Briq.) with DPPH radical scavenging activity. J Food Sci. 2010;75(4):C359–62. [DOI] [PubMed] [Google Scholar]
- 9.Liu R, Wang Y, Liang C, et al. Morphology and mass spectrometry-based chemical profiling of peltate glandular trichomes on Mentha haplocalyx Briq leaves. Food Res Int. 2023;164:112323. [DOI] [PubMed] [Google Scholar]
- 10.Dong W, Ni Y, Kokot S. Differentiation of mint (Menthahaplocalyx Briq.) from different regions in China using gas and liquid chromatography. J Sep Sci. 2015;38(3):402–9. [DOI] [PubMed] [Google Scholar]
- 11.Cao G, Shan Q, Li X, et al. Analysis of fresh Mentha haplocalyx volatile components by comprehensive two-dimensional gas chromatography and high-resolution time-of-flight mass spectrometry. Analyst. 2011;136(22):4653–61. [DOI] [PubMed] [Google Scholar]
- 12.Shu Y, Chen Y, Qin K, et al. Effect of different drying methods on the essential oils of mint (Mentha haplocalyx). Nat Prod Commun. 2013;8(10):1479–80. [PubMed] [Google Scholar]
- 13.Dorman HJ, Koşar M, Kahlos K, et al. Antioxidant properties and composition of aqueous extracts from Mentha species, hybrids, varieties, and cultivars. J Agric Food Chem. 2003;51(16):4563–9. [DOI] [PubMed] [Google Scholar]
- 14.Zeng J, Hu W, Li H, et al. Purification of linarin and hesperidin from Mentha haplocalyx by aqueous two-phase flotation coupled with preparative HPLC and evaluation of the neuroprotective effect of linarin. J Sep Sci. 2021;44(12):2496–503. [DOI] [PubMed] [Google Scholar]
- 15.Lee MY, Lee JA, Seo CS, et al. Protective effects of Mentha haplocalyx ethanol extract (MH) in a mouse model of allergic asthma. Phytother Res. 2011;25(6):863–9. [DOI] [PubMed] [Google Scholar]
- 16.Kazemi A, Iraji A, Esmaealzadeh N, Salehi M, Hashempur MH. Peppermint and menthol: a review on their biochemistry, pharmacological activities, clinical applications, and safety considerations. Crit Rev Food Sci Nutr. 2024. 10.1080/10408398.2023.2296991. [DOI] [PubMed] [Google Scholar]
- 17.Rajkumar V, Gunasekaran C, Paul CA, et al. Development of encapsulated peppermint essential oil in chitosan nanoparticles: characterization and biological efficacy against stored-grain pest control. Pestic Biochem Physiol. 2020;170:104679. [DOI] [PubMed] [Google Scholar]
- 18.Dimidi E, Whelan K. Food supplements and diet as treatment options in irritable bowel syndrome. Neurogastroenterol Motil. 2020;32(8):e13951. [DOI] [PubMed] [Google Scholar]
- 19.Xia X, Lin Z, Shao K, et al. Combination of white tea and peppermint demonstrated synergistic antibacterial and anti-inflammatory activities. J Sci Food Agric. 2021;101(6):2500–10. [DOI] [PubMed] [Google Scholar]
- 20.Kamatou GP, Vermaak I, Viljoen AM, et al. Menthol: a simple monoterpene with remarkable biological properties. Phytochemistry. 2013;96:15–25. [DOI] [PubMed] [Google Scholar]
- 21.Liu C, Gao Q, Shang Z, et al. Functional characterization and structural insights into stereoselectivity of pulegone reductase in menthol biosynthesis. Front Plant Sci. 2021;12:780970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin R, Tian J, Huang G, et al. Analysis of menthol in three traditional Chinese medicinal herbs and their compound formulation by GC-MS. Biomed Chromatogr. 2002;16(3):229–33. [DOI] [PubMed] [Google Scholar]
- 23.Zhang WJ, Yang K, You CX, et al. Contact toxicity and repellency of the essential oil from Menthahaplocalyx Briq. against Lasioderma serricorne. Chem Biodivers. 2015;12(5):832–9. [DOI] [PubMed] [Google Scholar]
- 24.Zhao H, Ren S, Yang H, et al. Peppermint essential oil: its phytochemistry, biological activity, pharmacological effect and application. Biomed Pharmacother. 2022;154:113559. [DOI] [PubMed] [Google Scholar]
- 25.Shan QY, Cao G, Cai H, et al. Novel software-based method to classify structurally similar compounds combined with high performance liquid chromatography-quadrupole time of flight mass spectrometry to identify complex components of herbal medicines. J Chromatogr A. 2012;1264:13–21. [DOI] [PubMed] [Google Scholar]
- 26.Dorman HJ, Koşar M, Başer KH, et al. Phenolic profile and antioxidant evaluation of Mentha x piperita L. (peppermint) extracts. Nat Prod Commun. 2009;4(4):535–42. [PubMed] [Google Scholar]
- 27.Keskin M, Thiruvengadam M. Phytochemicals from natural products for the prevention and treatment of non-communicable diseases. Curr Top Med Chem. 2022;22(23):1907–8. [DOI] [PubMed] [Google Scholar]
- 28.Santa K, Watanabe K, Kumazawa Y, et al. Phytochemicals and vitamin D for a healthy life and prevention of diseases. Int J Mol Sci. 2023;24(15):12167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muscolo A, Mariateresa O, Giulio T, et al. Oxidative stress: the role of antioxidant phytochemicals in the prevention and treatment of diseases. Int J Mol Sci. 2024;25(6):3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anwar F, Abbas A, Mehmood T, et al. Mentha: A genus rich in vital nutra-pharmaceuticals-a review. Phytother Res. 2019;33(10):2548–70. [DOI] [PubMed] [Google Scholar]
- 31.Mimica-Dukic N, Bozin B. Mentha L. species (Lamiaceae) as promising sources of bioactive secondary metabolites. Curr Pharm Des. 2008;14(29):3141–50. [DOI] [PubMed] [Google Scholar]
- 32.Guo HL, Chen Y, Xu W, et al. Assessment of drying kinetics, textural and aroma attributes of Mentha haplocalyx leaves during the hot air thin-layer drying process. Foods. 2022;11(6):784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang Y, Wu C. The linkage of gut microbiota and the property theory of traditional Chinese medicine (TCM): cold-natured and sweet-flavored TCMs as an example. J Ethnopharmacol. 2023;306:116167. [DOI] [PubMed] [Google Scholar]
- 34.Xiang S, Jian Q, Chen W, et al. Pharmacodynamic components and mechanisms of ginger (Zingiber officinale) in the prevention and treatment of colorectal cancer. J Ethnopharmacol. 2024;324:117733. [DOI] [PubMed] [Google Scholar]
- 35.Shen D, Feng Y, Zhang X, et al. Antiosteoporosis studies of 20 medicine food homology plants containing quercetin, rutin, and kaempferol: tcm characteristics, in vivo and in vitro activities, potential mechanisms, and food functions. Evid Based Complement Alternat Med. 2022;2022:5902293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen C, Yin Q, Tian J, et al. Studies on the potential link between antidepressant effect of Xiaoyao San and its pharmacological activity of hepatoprotection based on multi-platform metabolomics. J Ethnopharmacol. 2020;249:112432. [DOI] [PubMed] [Google Scholar]
- 37.Liu X, Liu C, Tian J, et al. Plasma metabolomics of depressed patients and treatment with Xiaoyaosan based on mass spectrometry technique. J Ethnopharmacol. 2020;246:112219. [DOI] [PubMed] [Google Scholar]
- 38.Dai Y, Li Z, Xue L, et al. Metabolomics study on the anti-depression effect of xiaoyaosan on rat model of chronic unpredictable mild stress. J Ethnopharmacol. 2010;128(2):482–9. [DOI] [PubMed] [Google Scholar]
- 39.Jing LL, Zhu XX, Lv ZP, et al. Effect of Xiaoyaosan on major depressive disorder. Chin Med. 2015;10:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu XX, Siu WS, Wat CL, et al. Effects of topical application of a tri-herb formula on inflammatory dry-skin condition in mice with oxazolone-induced atopic dermatitis. Phytomedicine. 2021;91:153691. [DOI] [PubMed] [Google Scholar]
- 41.Mahendran G, Verma SK, Rahman LU. The traditional uses, phytochemistry and pharmacology of spearmint (Menthaspicata L.): a review. J Ethnopharmacol. 2021;278:114266. [DOI] [PubMed] [Google Scholar]
- 42.Amtaghri S, Slaoui M, Eddouks M. Mentha pulegium: a plant with several medicinal properties. Endocr Metab Immune Disord Drug Targets. 2023. 10.2174/1871530323666230914103731. [DOI] [PubMed] [Google Scholar]
- 43.Mikaili P, Mojaverrostami S, Moloudizargari M, et al. Pharmacological and therapeutic effects of MenthaLongifolia L. and its main constituent, menthol. Anc Sci Life. 2013;33(2):131–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Saqib S, Ullah F, Naeem M, et al. Mentha: nutritional and health attributes to treat various ailments including cardiovascular diseases. Molecules. 2022;27(19):6728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang ZF, Ding ZM, He J, et al. Progress in chemical constituents, pharmacological activities and product development of Mentha haplocalyx Briq. Mod Chin Med. 2020;22(06):979–84. [Google Scholar]
- 46.Hever J. Plant-based diets: a physician’s guide. Perm J. 2016;20(3):15–082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barnard ND, Goldman DM, Loomis JF, et al. Plant-based diets for cardiovascular safety and performance in endurance sports. Nutrients. 2019;11(1):130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chauhan P, Ragendu V, Kumar M, et al. Chemical technology principles for selective bioconjugation of proteins and antibodies. Chem Soc Rev. 2024;53(1):380–449. [DOI] [PubMed] [Google Scholar]
- 49.Zhou M, Feng Z, Zhang X. Recent advances in the synthesis of fluorinated amino acids and peptides. Chem Commun. 2023;59(11):1434–48. [DOI] [PubMed] [Google Scholar]
- 50.Velička A, Tarasevičienė Ž, Hallmann E, et al. Impact of foliar application of amino acids on essential oil content, odor profile, and flavonoid content of different mint varieties in field conditions. Plants. 2022;11(21):2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liao H, Wen J, Nie H, et al. Study on the inhibitory activity and mechanism of Mentha haplocalyx essential oil nanoemulsion against Fusarium oxysporum growth. Sci Rep. 2024;14(1):16064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Aldoghachi FEH, Noor Al-Mousawi UM, Shari FH. Antioxidant activity of rosmarinic acid extracted and purified from Mentha piperita. Arch Razi Inst. 2021;76(5):1279–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gholamnia A, Mosleh Arani A, Sodaeizadeh H, et al. Expression profiling of rosmarinic acid biosynthetic genes and some physiological responses from Menthapiperita L. under salinity and heat stress. Physiol Mol Biol Plants. 2022;28(3):545–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shi Y, Pu D, Zhou X, et al. Recent progress in the study of taste characteristics and the nutrition and health properties of organic acids in foods. Foods. 2022;11(21):3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen R, Wang M, Keasling JD, et al. Expanding the structural diversity of terpenes by synthetic biology approaches. Trends Biotechnol. 2024;42(6):699–713. [DOI] [PubMed] [Google Scholar]
- 56.Qin M, Huang Q, Yang X, et al. Taxilluschinensis (DC.) Danser: a comprehensive review on botany, traditional uses, phytochemistry, pharmacology, and toxicology. Chin Med. 2022;17(1):136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.de Araújo FF, de Paulo FD, Neri-Numa IA, et al. Polyphenols and their applications: an approach in food chemistry and innovation potential. Food Chem. 2021;338:127535. [DOI] [PubMed] [Google Scholar]
- 58.Biela M, Kleinová A, Klein E. Phenolic acids and their carboxylate anions: thermodynamics of primary antioxidant action. Phytochemistry. 2022;200:113254. [DOI] [PubMed] [Google Scholar]
- 59.Wang W, Yao Q, Teng F, et al. Active ingredients from Chinese medicine plants as therapeutic strategies for asthma: overview and challenges. Biomed Pharmacother. 2021;137:111383. [DOI] [PubMed] [Google Scholar]
- 60.Chen L, Chen S, Sun P, et al. Psoraleacorylifolia L.: a comprehensive review of its botany, traditional uses, phytochemistry, pharmacology, toxicology, quality control and pharmacokinetics. Chin Med. 2023;18(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Keylani K, Arbab Mojeni F, Khalaji A, et al. Endoplasmic reticulum as a target in cardiovascular diseases: is there a role for flavonoids? Front Pharmacol. 2023;13:1027633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhao L, Zheng L. A Review on bioactive anthraquinone and derivatives as the regulators for ROS. Molecules. 2023;28(24):8139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dong X, Zeng Y, Liu Y, et al. Aloe-emodin: a review of its pharmacology, toxicity, and pharmacokinetics. Phytother Res. 2020;34(2):270–81. [DOI] [PubMed] [Google Scholar]
- 64.Semwal RB, Semwal DK, Combrinck S, et al. Emodin - a natural anthraquinone derivative with diverse pharmacological activities. Phytochemistry. 2021;190:112854. [DOI] [PubMed] [Google Scholar]
- 65.Gaikwad D, Sutar R, Patil D. Polysaccharide mediated nanodrug delivery: a review. Int J Biol Macromol. 2024;261(Pt 1):129547. [DOI] [PubMed] [Google Scholar]
- 66.Fu YL, Shi L. Methods of study on conformation of polysaccharides from natural products: a review. Int J Biol Macromol. 2024;263(Pt 1):130275. [DOI] [PubMed] [Google Scholar]
- 67.Fang C, Chen G, Kan J. Comparison on characterization and biological activities of Mentha haplocalyx polysaccharides at different solvent extractions. Int J Biol Macromol. 2020;154:916–28. [DOI] [PubMed] [Google Scholar]
- 68.Fang C, Chen G, Kan J. Characterization and in vitro simulated gastrointestinal digestion and fermentation of Mentha haplocalyx polysaccharide. Int J Biol Macromol. 2022;222(Pt A):360–72. [DOI] [PubMed] [Google Scholar]
- 69.Jiang P, Meng W, Shi F, et al. Structural characteristics, antioxidant properties and antiaging activities of galactan produced by Mentha haplocalyx Briq. Carbohydr Polym. 2020;234:115936. [DOI] [PubMed] [Google Scholar]
- 70.An X, Liao Y, Yu Y, et al. Effects of MhMYB1 and MhMYB2 transcription factors on the monoterpenoid biosynthesis pathway in l-menthol chemotype of Mentha haplocalyx Briq. Planta. 2024;260(1):3. [DOI] [PubMed] [Google Scholar]
- 71.Fuchs LK, Holland AH, Ludlow RA, et al. Genetic manipulation of biosynthetic pathways in mint. Front Plant Sci. 2022;13:928178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hajialyani M, Hosein Farzaei M, Echeverría J, et al. Hesperidin as a neuroprotective agent: a review of animal and clinical evidence. Molecules. 2019;24(3):648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zeng J, Wang W, Lin J, et al. Purification of menthone and menthol from Mentha haplocalyx by suspension particle assisted solvent sublation, neuroprotective effect in vitro and molecular docking of menthol on amyloid-β. J Chromatogr A. 2022;1674:463125. [DOI] [PubMed] [Google Scholar]
- 74.Wang R, Sui X, Dong X, et al. Integration of metabolomics and transcriptomics reveals the therapeutic mechanism underlying Chelidoniummajus L. in the treatment of allergic asthma. Chin Med. 2024;19(1):65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bruton A, Lee A, Yardley L, et al. Physiotherapy breathing retraining for asthma: a randomised controlled trial. Lancet Respir Med. 2018;6(1):19–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Su YH, Lin JY. Menthone supplementation protects from allergic inflammation in the lungs of asthmatic mice. Eur J Pharmacol. 2022;931:175222. [DOI] [PubMed] [Google Scholar]
- 77.Yu H, Gao R, Liu Y, et al. Stimulus-responsive hydrogels as drug delivery systems for inflammation targeted therapy. Adv Sci. 2024;11(1):e2306152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang Q, Wei W, Jin X, et al. Traditional uses, phytochemistry, pharmacology, quality control and clinical studies of Cimicifugae Rhizoma: a comprehensive review. Chin Med. 2024;19(1):66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chen X, Zhang S, Xuan Z, et al. The phenolic fraction of mentha haplocalyx and its constituent linarin ameliorate inflammatory response through inactivation of NF-κB and MAPKs in lipopolysaccharide-induced RAW264.7 cells. Molecules. 2017;22(5):811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Goudarzi MA, Radfar M, Goudarzi Z. Peppermint as a promising treatment agent in inflammatory conditions: a comprehensive systematic review of literature. Phytother Res. 2024;38(1):187–95. [DOI] [PubMed] [Google Scholar]
- 81.Fassarella M, Blaak EE, Penders J, et al. Gut microbiome stability and resilience: elucidating the response to perturbations in order to modulate gut health. Gut. 2021;70(3):595–605. [DOI] [PubMed] [Google Scholar]
- 82.Gao X, Zhu Z, Bao Y, et al. Chrysanthemum morifolium Ramat extract and probiotics combination ameliorates metabolic disorders through regulating gut microbiota and PPARα subcellular localization. Chin Med. 2024;19(1):76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yi M, Cao Z, Zhou J, et al. Multi-omics analysis of the mechanism of Mentha Haplocalyx Briq on the growth and metabolic regulation of fattening sheep. Animals. 2023;13(22):3461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Meng X, Liu X, Tan J, et al. From Xiaoke to diabetes mellitus: a review of the research progress in traditional Chinese medicine for diabetes mellitus treatment. Chin Med. 2023;18(1):75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pasquel FJ, Lansang MC, Dhatariya K, et al. Management of diabetes and hyperglycaemia in the hospital. Lancet Diabetes Endocrinol. 2021;9(3):174–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Amorim JA, Coppotelli G, Rolo AP, et al. Mitochondrial and metabolic dysfunction in ageing and age-related diseases. Nat Rev Endocrinol. 2022;18(4):243–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nadeeshani H, Li J, Ying T, et al. Nicotinamide mononucleotide (NMN) as an anti-aging health product-promises and safety concerns. J Adv Res. 2021;37:267–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mamadalieva NZ, Hussain H, Xiao J. Recent advances in genus Mentha: phytochemistry, antimicrobial effects, and food applications. Food Front. 2020;1(4):435–58. [Google Scholar]
- 89.Halliwell B. Understanding mechanisms of antioxidant action in health and disease. Nat Rev Mol Cell Biol. 2024;25(1):13–33. [DOI] [PubMed] [Google Scholar]
- 90.Kim YG, Lee Y, Lee N, et al. Ceria-based therapeutic antioxidants for biomedical applications. Adv Mater. 2024;36(10):e2210819. [DOI] [PubMed] [Google Scholar]
- 91.Jing X, Liu T, Fateh B, et al. Effect of methomyl on water quality, growth performance and antioxidant system in liver of GIFT (Oreochromis niloticus) in the presence of peppermint (Menthahaplocalyx Briq.) as a floating bed. Sci Prog. 2022;105(3):368504221124047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.McKay DL, Blumberg JB. A review of the bioactivity and potential health benefits of peppermint tea (Menthapiperita L.). Phytother Res. 2006;20(8):619–33. [DOI] [PubMed] [Google Scholar]
- 93.Dwivedy AK, Prakash B, Chanotiya CS, et al. Chemically characterized Menthacardiaca L. essential oil as plant based preservative in view of efficacy against biodeteriorating fungi of dry fruits, aflatoxin secretion, lipid peroxidation and safety profile assessment. Food Chem Toxicol. 2017;106:175–84. [DOI] [PubMed] [Google Scholar]
- 94.Firdaus A, Yunus MH, Izhar SK, et al. Medicinal plants in the treatment of respiratory diseases and their future aspects. Recent Pat Biotechnol. 2024. 10.2174/0118722083278561231212072408. [DOI] [PubMed] [Google Scholar]
- 95.Morton CA, Garioch J, Todd P, et al. Contact sensitivity to menthol and peppermint in patients with intra-oral symptoms. Contact Dermatitis. 1995;32(5):281–4. [DOI] [PubMed] [Google Scholar]
- 96.Sousa C, Leitão AJ, Neves BM, et al. Standardised comparison of limonene-derived monoterpenes identifies structural determinants of anti-inflammatory activity. Sci Rep. 2020;10(1):7199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Soleimani M, Kashfi LS, Mirmohamadkhani M, et al. The effect of aromatherapy with peppermint essential oil on anxiety of cardiac patients in emergency department: a placebo-controlled study. Complement Ther Clin Pract. 2022;46:101533. [DOI] [PubMed] [Google Scholar]
- 98.Karsten M, Prince D, Robinson R, et al. Effects of peppermint aromatherapy on postoperative nausea and vomiting. J Perianesth Nurs. 2020;35(6):615–8. [DOI] [PubMed] [Google Scholar]
- 99.Ćavar Zeljković S, Šišková J, Komzáková K, et al. Phenolic compounds and biological activity of selected Mentha Species. Plants. 2021;10(3):550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Paiva M, Piedade S, Gaspar A. Toothpaste-induced anaphylaxis caused by mint (Mentha) allergy. Allergy. 2010;65(9):1201–2. [DOI] [PubMed] [Google Scholar]
- 101.Abd El-Hack ME, Kamal M, Altaie HAA, et al. Peppermint essential oil and its nano-emulsion: potential against aflatoxigenic fungus Aspergillus flavus in food and feed. Toxicon. 2023;234:107309. [DOI] [PubMed] [Google Scholar]
- 102.Masouri L, Bagherzadeh-Kasmani F, Mehri M, et al. Mentha piperita as a promising feed additive used to protect liver, bone, and meat of Japanese quail against aflatoxin B1. Trop Anim Health Prod. 2022;54(5):254. [DOI] [PubMed] [Google Scholar]
- 103.Mahendran G, Rahman LU. Ethnomedicinal, phytochemical and pharmacological updates on Peppermint (Mentha × piperita L.)-a review. Phytother Res. 2020;34(9):2088–139. [DOI] [PubMed] [Google Scholar]
- 104.Yu X, Qi X, Li S, et al. Transcriptome analysis of light-regulated monoterpenes biosynthesis in leaves of Mentha canadensis L. Plants. 2021;10(5):930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Negishi H, Ito M. Comparison of Menthae Herba written with the same kanji characters () in Japan and China. J Nat Med. 2024. 10.1007/s11418-024-01822-1. [DOI] [PubMed] [Google Scholar]
- 106.Li J, Tian B. Peppermint essential oil toxicity to the Pear Psylla (Hemiptera: Psyllidae) and potential applications in the field. J Econ Entomol. 2020;113(3):1307–14. [DOI] [PubMed] [Google Scholar]
- 107.Zhao M, Sun Y, Dong M, et al. Hexose/pentose ratio in rhizosphere exudates-mediated soil eutrophic/oligotrophic bacteria regulates the growth pattern of host plant in young apple-aromatic plant intercropping systems. Front Microbiol. 2024;15:1364355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ludwiczuk A, Kieltyka-Dadasiewiczb A, Sawicki R, et al. Essential oils of some Mentha species and cultivars, their chemistry and bacteriostatic activity. Nat Prod Commun. 2016;11(7):1015–8. [PubMed] [Google Scholar]
- 109.Zheng J, Wu LJ, Zheng L, et al. Two new monoterpenoid glycosides from Mentha spicata L. J Asian Nat Prod Res. 2003;5(1):69–73. [DOI] [PubMed] [Google Scholar]
- 110.Zeng JW, Qian SH, Wu JZ, et al. Studies on antiviral constituents in stems and leaves of Pihecellibium clypearia. Zhongguo Zhong Yao Za Zhi. 2006;31(5):400–2. [PubMed] [Google Scholar]
- 111.Liu Y, Zhang YH, Shi RB. Study on the active protein fractions from scorpii tegument. Zhongguo Zhong yao za zhi. 2005;30(14):1086–8. [PubMed] [Google Scholar]
- 112.Zhu SQ, Zhu ZH, Guo S, et al. Effect of drying methods on monoterpenes, phenolie acids and flavonoids in Mentha haplocalyx. Zhongguo Zhong Yao Za Zhi. 2015;40(24):4860–7. [PubMed] [Google Scholar]
- 113.Xu YM, Yue W, Sang MR, et al. Analysis on quality and differences of Mentha haplocalyx from different regions. Zhongguo Zhong Yao Za Zhi. 2017;42(17):3391–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.