Abstract
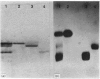
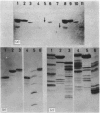
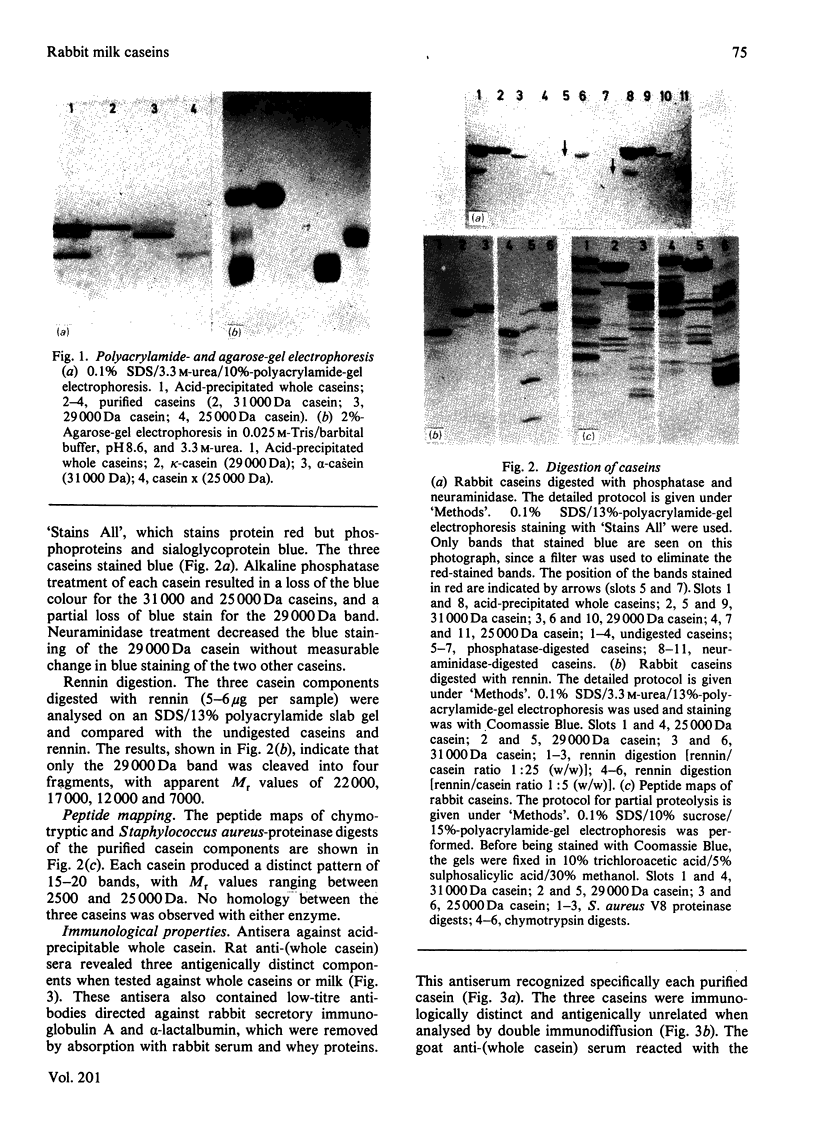
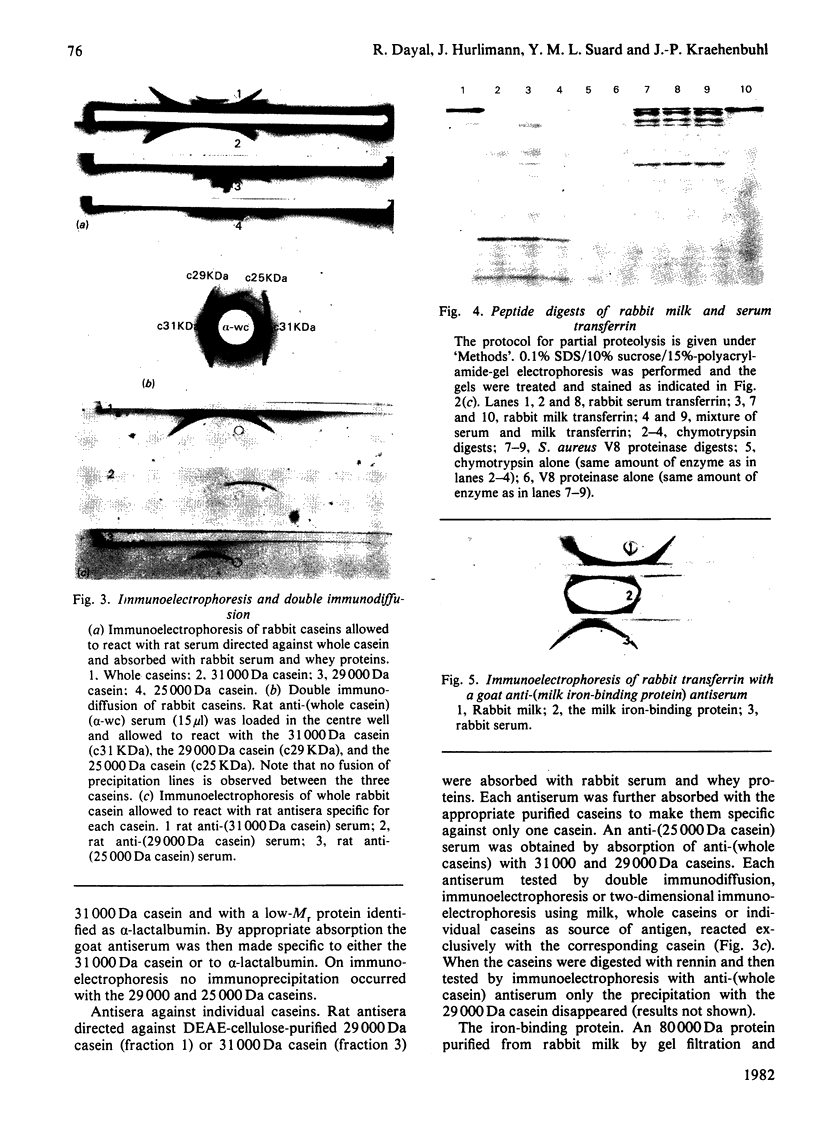
Caseins were separated from whey proteins by acid precipitation of skimmed rabbit milk. Whole casein was resolved by sodium dodecyl sulphate/polyacrylamide-gel electrophoresis into three major bands with apparent relative molecular masses (Mr of 31 000, 29 000 and 25 000. On agarose/urea-gel electrophoresis whole casein gave three bands with electrophoretic mobilities alpha, beta and gamma. The three components were purified by DEAE-cellulose chromatography under denaturing and reducing conditions. Each was shown to have a different amino acid, hexose and phosphorus content, as well as non-identical peptide fragments after proteinase digestion. The 31 000 Da (dalton) protein, of alpha-electrophoretic mobility, had a high phosphorus content (4.38%, w/w); the 29 000 Da peptide, of gamma-mobility, had the highest hexose content (2.2%, w/w), contained 0.8 cysteine residue per 100 amino acid residues and was susceptible to chymosin digestion corresponding thus to kappa-casein; the 25 000 Da protein migrated to the beta-position. The rabbit casein complex is composed of at least three caseins, two of which (alpha- and kappa-caseins) are analogous to the caseins from ruminants. Although caseins are poor immunogens, specific antibodies were raised against total and purified polypeptides. The antiserum directed against whole casein recognized each polypeptide, each casein corresponding to a distinct precipitation line. The antisera directed against each casein polypeptide reacted exclusively with the corresponding casein and no antiserum cross-reaction occurred between the three polypeptides. From whey, several proteins were isolated, characterized and used as antigens to raise specific antibodies. An iron-binding protein with an apparent Mr of 80 000 was shown to be immunologically and structurally identical with serum transferrin.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Al-Sarraj K., White D. A., Mayer R. J. Immunochemical characterization of casein from rabbit mammary gland. Biochem J. 1978 Sep 1;173(3):877–883. doi: 10.1042/bj1730877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Sarraj K., White D. A., Mayer R. J. Purification and properties of casein from mammary gland of lactating rabbits. Int J Biochem. 1978;9(4):269–277. doi: 10.1016/0020-711x(78)90009-5. [DOI] [PubMed] [Google Scholar]
- Alais C., Jollès P. Isolation, purification, and analysis of two k-casein-like fractions from sheep casein. J Dairy Sci. 1967 Oct;50(10):1555–1561. doi: 10.3168/jds.s0022-0302(67)87672-0. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Craig R. K., McIlreavy D., Hall R. L. Separation and partial characterization of guinea-pig caseins. Biochem J. 1978 Aug 1;173(2):633–641. doi: 10.1042/bj1730633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devinoy E., Houdebine L. M., Delouis C. Role of prolactin and glucocorticoids in the expression of casein genes in rabbit mammary gland organ culture. Quantification of casein mRNA. Biochim Biophys Acta. 1978 Feb 16;517(2):360–366. doi: 10.1016/0005-2787(78)90202-2. [DOI] [PubMed] [Google Scholar]
- Devinoy E., Houdebine L. M. Effects of glucocorticoids on casein gene expression in the rabbit. Eur J Biochem. 1977 May 16;75(2):411–416. doi: 10.1111/j.1432-1033.1977.tb11542.x. [DOI] [PubMed] [Google Scholar]
- Enami J., Nandi S. A sensitive radioimmunoassay for a component of mouse casein. J Immunol Methods. 1977;18(3-4):235–244. doi: 10.1016/0022-1759(77)90177-6. [DOI] [PubMed] [Google Scholar]
- Feldman M. K. A direct radioimmunoassay for mouse casein. Comp Biochem Physiol A Comp Physiol. 1974 Sep 1;49(1A):127–135. doi: 10.1016/0300-9629(74)90548-9. [DOI] [PubMed] [Google Scholar]
- Feldman M. K., Ceriani R. L. A comparative immunologic and electrophoretic analysis of rat and mouse caseins. Comp Biochem Physiol. 1970 Dec 1;37(3):421–427. doi: 10.1016/0010-406x(70)90570-0. [DOI] [PubMed] [Google Scholar]
- Green M. R., Pastewka J. V. Identification of sialic acid-rich glycoproteins on polyacrylamide gels. Anal Biochem. 1975 May 12;65(1-2):66–72. doi: 10.1016/0003-2697(75)90491-1. [DOI] [PubMed] [Google Scholar]
- Green M. R., Pastewka J. V. Molecular weights of three mouse milk caseins by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and kappa-like characteristics of a fourth casein. J Dairy Sci. 1976 Oct;59(10):1738–1745. doi: 10.3168/jds.S0022-0302(76)84431-1. [DOI] [PubMed] [Google Scholar]
- Green M. R., Pastewka J. V., Peacock A. C. Differential staining of phosphoproteins on polyacrylamide gels with a cationic carbocyanine dye. Anal Biochem. 1973 Nov;56(1):43–51. doi: 10.1016/0003-2697(73)90167-x. [DOI] [PubMed] [Google Scholar]
- Groves M. L., Gordon W. G. The major component of human casein: a protein phosphorylated at different levels. Arch Biochem Biophys. 1970 Sep;140(1):47–51. doi: 10.1016/0003-9861(70)90008-1. [DOI] [PubMed] [Google Scholar]
- Hopp T. P., Woods K. R. Primary structure of rabbit alpha-lactalbumin. Biochemistry. 1979 Nov 13;18(23):5182–5191. doi: 10.1021/bi00590a024. [DOI] [PubMed] [Google Scholar]
- Houdebine L. M., Djiane J. Effects of lysomotropic agents, and of microfilament- and microtubule-disrupting drugs on the activation of casein-gene expression by prolactin in the mammary gland. Mol Cell Endocrinol. 1980 Jan;17(1):1–15. doi: 10.1016/0303-7207(80)90099-4. [DOI] [PubMed] [Google Scholar]
- Houdebine L. M. Effects of prolactin and progesterone on expression of casein genes. Titration of casein mRNA by hybridization with complementary DNA. Eur J Biochem. 1976 Sep;68(1):219–225. doi: 10.1111/j.1432-1033.1976.tb10781.x. [DOI] [PubMed] [Google Scholar]
- Hurlimann J., Lichaa M. Local immunization in the mammary glands of the rabbit. J Immunol. 1976 May;116(5):1295–1301. [PubMed] [Google Scholar]
- Jollès J., Alais C., Jollès P. Chemical structure of cow kappa-casein: study of the soluble tryptic peptides. Helv Chim Acta. 1970;53(7):1918–1926. doi: 10.1002/hlca.19700530743. [DOI] [PubMed] [Google Scholar]
- Kraehenbuhl J. P. Dispersed mammary gland epithelial cells. I. Isolation and separation procedures. J Cell Biol. 1977 Feb;72(2):390–405. doi: 10.1083/jcb.72.2.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraehenbuhl J. P., Racine L., Galardy R. E. Localization of secretory IgA, secretory component, and alpha chain in the mammary gland of lactating rabbits by immunoelectron microscopy. Ann N Y Acad Sci. 1975 Jun 30;254:190–202. doi: 10.1111/j.1749-6632.1975.tb29169.x. [DOI] [PubMed] [Google Scholar]
- Kühn L. C., Kraehenbuhl J. P. Interaction of rabbit secretory component with rabbit IgA dimer. J Biol Chem. 1979 Nov 10;254(21):11066–11071. [PubMed] [Google Scholar]
- Kühn L. C., Kraehenbuhl J. P. Role of secretory component, a secreted glycoprotein, in the specific uptake of IgA dimer by epithelial cells. J Biol Chem. 1979 Nov 10;254(21):11072–11081. [PubMed] [Google Scholar]
- Masson P. L., Heremans J. F. Lactoferrin in milk from different species. Comp Biochem Physiol B. 1971 May 15;39(1):119–129. doi: 10.1016/0305-0491(71)90258-6. [DOI] [PubMed] [Google Scholar]
- Mayes R. W., Mason R. M., Griffin D. C. The composition of cartilage proteoglycans. An investigation using high- and low-inonic-strength extraction procedures. Biochem J. 1973 Mar;131(3):541–553. doi: 10.1042/bj1310541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKnight G. S., Lee D. C., Hemmaplardh D., Finch C. A., Palmiter R. D. Transferrin gene expression. Effects of nutritional iron deficiency. J Biol Chem. 1980 Jan 10;255(1):144–147. [PubMed] [Google Scholar]
- McMEEKIN T. L., HIPP N. J., GROVES M. L. The separation of the components of alpha-casein. I. The preparation of alpha 1-casein. Arch Biochem Biophys. 1959 Jul;83(1):35–43. doi: 10.1016/0003-9861(59)90007-4. [DOI] [PubMed] [Google Scholar]
- Mercier J. C., Gaye P. Study of secretory lactoproteins: primary structures of the signals and enzymatic processing. Ann N Y Acad Sci. 1980;343:232–251. doi: 10.1111/j.1749-6632.1980.tb47255.x. [DOI] [PubMed] [Google Scholar]
- Morgan E. H. Factors affecting the synthesis of transferrin by rat tissue slices. J Biol Chem. 1969 Aug 10;244(15):4193–4199. [PubMed] [Google Scholar]
- Mostov K. E., Kraehenbuhl J. P., Blobel G. Receptor-mediated transcellular transport of immunoglobulin: synthesis of secretory component as multiple and larger transmembrane forms. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7257–7261. doi: 10.1073/pnas.77.12.7257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagasawa T., Ryoki T., Kiyosawa I., Kuwahara K. Studies on human casein. I. Fractionation of human casein by diethylaminoethyl cellulose column chromatography. Arch Biochem Biophys. 1967 Aug;121(2):502–507. doi: 10.1016/0003-9861(67)90105-1. [DOI] [PubMed] [Google Scholar]
- Nardacci N. J., Lee J. W., McGuire W. L. Differential regulation of alpha-lactalbumin and casein messenger RNA's in mammary tissue. Cancer Res. 1978 Sep;38(9):2694–2699. [PubMed] [Google Scholar]
- Palmiter R. D. Properties of lactose synthetase from mouse mammary gland: role of a proposed third component. Biochim Biophys Acta. 1969 Mar 18;178(1):35–46. doi: 10.1016/0005-2744(69)90129-6. [DOI] [PubMed] [Google Scholar]
- Pich A., Bussolati G., Carbonara A. Immunocytochemical detection of casein and casein-like proteins in human tissues. J Histochem Cytochem. 1976 Aug;24(8):940–947. doi: 10.1177/24.8.822100. [DOI] [PubMed] [Google Scholar]
- Ribadeau Dumas B., Brignon G., Grosclaude F., Mercier J. C. Structure primaire de la caséine beta bovine. Séquence complète. Eur J Biochem. 1972 Feb;25(3):505–514. doi: 10.1111/j.1432-1033.1972.tb01722.x. [DOI] [PubMed] [Google Scholar]
- Rosen J. M., Woo S. L., Comstock J. P. Regulation of casein messenger RNA during the development of the rat mammary gland. Biochemistry. 1975 Jul;14(13):2895–2903. doi: 10.1021/bi00684a016. [DOI] [PubMed] [Google Scholar]
- Shiu R. P., Friesen H. G. Solubilization and purification of a prolactin receptor from the rabbit mammary gland. J Biol Chem. 1974 Dec 25;249(24):7902–7911. [PubMed] [Google Scholar]
- Suard Y. M., Tosi M., Kraehenbuhl J. P. Characterization of the translation products of the major mRNA species from rabbit lactating mammary glands and construction of bacterial recombinants containing casein and alpha-lactalbumin complementary DNA. Biochem J. 1982 Jan 1;201(1):81–90. doi: 10.1042/bj2010081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry P. M., Ganguly R., Ball E. M., Banerjee M. R. Murine mammary gland RNA directed synthesis of casein in a heterologous cell-free protein synthesis system. Cell Differ. 1975 May;4(2):113–122. doi: 10.1016/0045-6039(75)90023-8. [DOI] [PubMed] [Google Scholar]
- Testud M., Ribadeau Dumas B. Etude des caséines du lait de lapine. Biochimie. 1973;55(9):1085–1093. doi: 10.1016/s0300-9084(73)80447-x. [DOI] [PubMed] [Google Scholar]
- Thompson M. P. DEAE-cellulose-urea chromatography of casein in the presence of 2-mercaptoethanol. J Dairy Sci. 1966 Jul;49(7):792–795. doi: 10.3168/jds.S0022-0302(66)87947-X. [DOI] [PubMed] [Google Scholar]