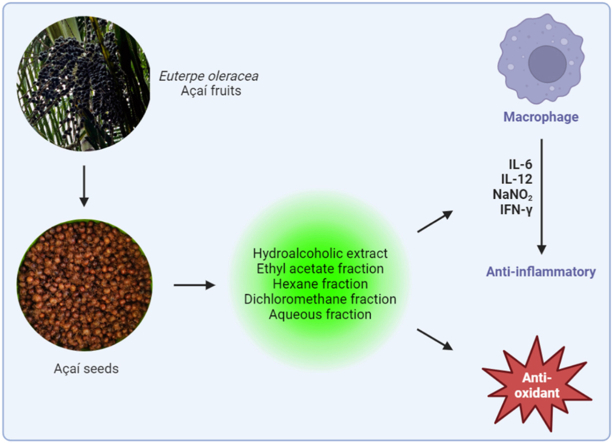
Abstract
Euterpe oleracea Mart. is a tropical palm tree native to the Amazon region. Its fruit, commonly known as açaí, has gained widespread recognition for its therapeutic potential, driving the expansion of pharmacological studies to validate its traditional uses. Leveraging açaí seeds in research not only mitigates environmental impacts but also enables the identification of bioactive compounds with potential pharmacological applications, including drug development. Thus, the present work aims to investigate the antioxidant and anti-inflammatory activities of the hydroalcoholic extract and the ethyl acetate, hexane, dichloromethane and aqueous fractions of açaí seeds in vitro. The extract/fractions from açaí contained a significant amount of flavonoids, such as catechins and procyanidins, according to LC-MS/MS. These bioproducts showed significant antioxidant activity, with emphasis on the ethyl acetate fraction (FRAP: 4516.00 ± 58.07 Eq Trolox/g compound; DPPH: IC50 3.93 ± 0.26 μg/. mL; ABTS•+: IC50 34.65 ± 0.35 μg/mL). In addition, the compounds exhibited an anti-inflammatory action on LPS-stimulated peritoneal macrophages, with the dichloromethane fraction showing the more comprehensive inhibitory effects on NO, IL-12 and IFN-γ production, especially in the concentration of 500 μg/mL. E. oleracea seeds extract/fractions had no cytotoxic effect on peritoneal macrophages (IC50 > 500 μg/mL). These findings suggest that E. oleracea seed-derived bioproducts hold significant promise as safe and effective agents for the development of novel antioxidant and anti-inflammatory therapies targeting a variety of inflammatory pathologies.
Keywords: Antioxidant, Anti-inflammatory, Açaí, Euterpe oleracea
Graphical abstract
1. Introduction
Inflammation is a physiological response to non-self-components, including pathogens, and allergens [1]. Chronic inflammation induces oxidative stress and generates free radicals, which can lead the progression of chronic diseases/disorders such as those caused by parasites (Leishmaniasis, Chagas disease), diabetes, cancer, cardiovascular diseases, arthritis, obesity, autoimmune diseases, among others [2]. In these contexts, the impact of inflammation and its associated factors has become a crucial topic in the study of human illness [3].
Despite the availability of numerous anti-inflammatory and antioxidant drugs, their high costs and significant side effects pose considerable challenges. Incorporating natural antioxidant and anti-inflammatory compounds into the daily diet offers a promising, cost-effective alternative for managing inflammatory diseases. Recent studies have highlighted a strong correlation between the consumption of antioxidant-rich foods and a reduced prevalence of human illnesses [3]. Over the years, humans have used natural products (NPs), particularly plant-derived ones, as a valuable tool for discovering and developing new molecules with therapeutic potential. The importance of NPs in contemporary society is well-known. NPs played a role in the development of approximately 65 % of the drugs introduced in the pharmaceutical market, either directly or indirectly, according to a recent review study by Newman and Cragg [4,5]. Considering the diverse nature of NPs, there are certain classes of substances that are noteworthy in the search for new drug candidates, such as those found in açai seeds. Euterpe oleracea Mart. is a tropical palm tree typically found in the Amazon region and its fruit is popularly known as açaí. This fruit is globose and contains a seed whose diameter varies from 1 to 2 cm and its weight ranges from 0.7 to 1.9 g, representing up to 90 % of its size. According to the Brazilian Institute of Geography and Statistics (IBGE), approximately 1.7 million tons of açaí were produced in Brazil in 2022 [6,7]. The market has explored açaí pulp in various ways, including food products, food supplements, and high-performance product formulations [2], but most of the seeds are transformed into waste causing a negative environmental and social impact. It is estimated that approximately 80 % of the total açaí processed is often discarded irregularly in the environment, without any treatment [8].
The use of açaí seeds in research not only reduces environmental impacts but also opens avenues for discovering biologically active compounds with therapeutic potential. Popular use of açaí for treating various pathologies had led to development and diversity of pharmacological trials aimed at validating its applications. Most studies evaluate the anti-inflammatory and antioxidant activities of compounds present in both the pulp and the seed. The literature describes that the açaí seed has an important pharmacological profile, with antioxidant properties, without side effects, and anti-inflammatory activity [[9], [10], [11], [12]].
Studies have been reported that plant polyphenols act as nuclear factor kB (NF-kB) inhibitors. Dias and collaborators (2014) demonstrated that açaí polyphenolic extract decreases NF-kB mRNA levels, highlighting their potential as therapeutic agents for inflammatory diseases [[13], [14], [15]].
Furthermore, Melo and collaborators (2016) observed that açaí seed extract has high antioxidant capacity in relation to the inhibition of lipid oxidation [16]. Additional studies have shown that hydroalcoholic extract of the açaí seeds has potent endothelium-dependent vasodilatory effects and induces the effect of nitric oxide (NO) from endothelial cells in culture [13]. It was also observed that chronic treatment with açaí seed extract prevents the development of hypertension, endothelial dysfunction, vascular structural changes and oxidative damage in experimental renovascular hypertension [[17], [18], [19]].
Another study demonstrated that treatment with the ethyl acetate fraction of Euterpe oleracea seeds reduced carrageenan-induced paw edema, as well as the production of pro-inflammatory cytokines such as IL-1β, IL-6 and IL-12, by RAW 264.7 macrophages stimulated by LPS, indicating its anti-inflammatory potential, in addition to not exerting cytotoxic action on the host cell [20].
Based on these studies and considering that chronic inflammation and the oxidative stress caused by it are essential factors for the development of many inflammatory diseases, the present work aims to investigate the antioxidant and anti-inflammatory activity of bioproducts from Euterpe oleracea Mart. (Açaí) seeds, in vitro, in order to identify the one with the best activity to develop a new drug with lower cost and without side effects to treat inflammatory diseases.
2. Material and methods
2.1. Chemicals and culture media
Fetal bovine serum (FBS) was purchased from Nova Biotecnologia (São Paulo, Brazil). 3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide) (MTT) (M6494) was purchased from ThermoFisher Scientific (Massachusetts, U.S.A.). Quercetin (Q4951), 2,2-Diphenyl-1-picrylhydrazyl (DPPH) (D9132), (±)-6-Hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid (Trolox) (238813), 2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS) (A1888), 2,4,6-Tris(2-pyridyl)-s-triazine (TPTZ) (93285), Phosphate buffered saline (PBS) (P4417), RPMI 1640 medium (R8755), Brewer thioglycollate medium (B2551), Lipopolysaccharides from Escherichia coli O111:B4 (LPS) (L4391), Dexamethasone (D4902), Dimethylsulfoxide (DMSO) (D5879), Polyoxyethylene Sorbitan Monolaurate (Tween 20) (P1379), Sulphanilamide (C6H8N2O2S) (86060), N-(1-Naphthyl)ethylenediamine dihydrochloride (C12H14N2) (N9125) and sodium nitrite (NaNO2) were purchased from Sigma-Aldrich Chemical Co. (Missouri, U.S.A.). Penicillin–Streptomycin Solution was purchased from LGC Biotecnologia (São Paulo, Brazil). Phosphoric acid PA (H3PO4) (145) was purchased from Vetec (Rio de Janeiro, Brazil). Acetonitrile, methanol, n-hexane, dichloromethane, ethyl acetate (HPLC-grade, Tedia, Rio de Janeiro, Brazil). Iron (III) chloride (FeCl3 -157740), Hydrochloric acid, aluminum chloride 98 %, acetic acid, Methanol ACS, ISO, Reag. Ph Eur were purchased from Merck Millipore (São Paulo, Brazil). BD OptEIA™ Mouse IL-6 ELISA Set (cat. 555240) and BD OptEIA™ Mouse IFN-γ (AN-18) ELISA Set (cat. 551866) were purchased from BD Biosciences Pharmingen (San Diego, CA, USA). Mouse IL-12 p70 DuoSet ELISA (cat: DY419) was purchased from R&D Systems, Inc. (Minneapolis, MN, USA). Absolute ethyl alcohol 99,8 % was purchased from Êxodo Científica (São Paulo, Brazil). All reagents were of analytical grade or superior.
2.2. Plant material
Euterpe oleracea seeds were collected in April 2021, at Sepetiba district, Rio de Janeiro, Brazil (22° 58′ 7″ S, 43° 41′ 22″ W). The plant material, including leaves and flowers, were authenticated by the RBR Herbarium from the Rural Federal University of Rio de Janeiro, where a voucher specimen was registered (RBR 58053). The access for the genetic heritage material was granted by the National System for the Management of Genetic Heritage and Associated Traditional Knowledge (Sistema Nacional de Gestão do Patrimônio Genético e do Conhecimento Tradicional Associado – SisGen), from Ministry of the Environment of Brazil, voucher number AAE018A.
2.3. Obtention of hydroalcoholic extract and their fractions
Euterpe oleracea seeds (1000 g) were washed in running water, distilled water, and boiled in distilled water for 5 min. After, seeds were dried at 47 °C in an air circulation oven (FANEM Orion 520) for 3 or 4 days. Subsequently, after manual removal of the fibers, the seeds were ground in a High-Speed Industrial Blender (KD Eletro). The hydroalcoholic extracts were obtained by maceration in 1000 mL of ethanol:water solution (7:3), for 5 days, at room temperature with occasional stirring and two solvent changes. The liquid extract obtained was concentrated in a rotary evaporator (Buchi, Brazil), bath temperature of 30 °C and dehydrated in a lyophilizer (Christ, Germany) for 72 h at −101 °C, 23 mmHg, and kept at −20 °C, until the day of use. The hydroalcoholic extract (HE; 50g) was resuspended in a methanol:water solution (8:2) fractionated by liquid-liquid partition, using n-hexane (3 × 200 mL), dichloromethane (3 × 200 mL) and ethyl acetate (3 × 200 mL). After fractionation, all obtained fractions [hexane fraction (HF), dichloromethane fraction (DCMF), ethyl acetate fraction (EAF)] were concentrated under reduced pressure, at 30–40 °C, in a rotary evaporator, until complete evaporation of the solvent. The residual fraction, named aqueous fraction (AQF), was lyophilized.
2.4. Chemical characterization of hydroalcoholic extract and fractions ofEuterpe oleracea(seeds) by LC/MS
Hydroalcoholic extract and their fractions samples (10 mg) were dissolved in methanol certified HPLC grade (1 mL) using an ultrasonic bath (Unique, Brazil) for 20 min. LC/MS analyses were performed in a system composed of an LC Shimadzu Nexera UFLC coupled to a Bruker Daltonics Amazon SL ion trap. The analysis conditions were as follows: (a) room temperature; (b) 100 mm × 2.1 mm x 1.8 μm Aquity HSS T3 C18 column; (c) mobile phase composed by (A) 2.5 % acetic acid in water and (B) HPLC-grade acetonitrile. The elution gradient established was 10–85 % B in 24 min using a flow rate of 3.0 mL/min and returning to 10 % B in 10 min. The mass detection was performed in a negative ion mode with a capillary voltage of 2500 V; end plate offset: 2000 V; capillary output 110 V, skimmer 1 (20 V), skimmer 2 (10 V), dry gas (N2) temperature 325 °C and flow 1 mL/min, nebulizer 60 psi, sweep range from 200 to 800 m/z, temperature set at 25 °C.
2.5. Flavonoids quantification
Flavonoids quantification was conducted according to methodology adapted from Acácio et al. (2016) [21]. The hydroalcoholic extract and fractions of Euterpe oleracea seeds were diluted in methanol (500 μg/mL). The flavonoids quantification was assayed in Nunc™ MicroWell™ 96-Well, Nunclon Delta-Treated, Flat-Bottom Microplate (Thermo Fisher Scientific Inc.) where 200 μL of each methanolic solution (hydroalcoholic extract and fractions) and 100 μL of 2 % methanolic aluminum chloride solution were added to each well, in triplicate. The standard curve was performed with the flavonoid quercetin, at concentrations ranging from 30 μg/mL to 0.47 μg/mL. In the negative control wells, 200 μL of methanol and 100 μL of 2 % methanolic aluminum chloride solution were added. The plate was kept in the dark for 30 min and then the absorbance was read on a spectrophotometer (SpectraMax M2, Molecular Devices) at 405 nm.
2.6. Antioxidant activity
2.6.1. DPPH method
The antioxidant activity was determined through the consumption of the radical 2,2-diphenyl-1-picrylhydrazyl (DPPH), in a Nunc™ MicroWell™ 96-Well, Nunclon Delta-Treated, Flat-Bottom Microplate (Thermo Fisher Scientific Inc.), according to methodology adapted Rufino et al. (2010) [22].
Hydroalcoholic extract and fractions, diluted in methanol (500 μg/mL), were prepared for test dilutions, at concentrations ranging from 500 μg/mL to 7.81 μg/mL. Then, 100 μL of each concentration and 40 μL of 0.3 mM DPPH in methanol, were added to each well, in triplicate. For the negative control, 100 μL of methanol and 40 μL of 0.3 mM DPPH in methanol were added. The standard curve was performed with the antioxidant Trolox (±)-6-Hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid, at concentrations ranging from 50 μg/mL to 0.78 μg/mL. The plate was kept in the dark for 30 min, and finally, the absorbance was read at 492 nm. The concentration necessary to reduce the initial concentration of the DPPH radical by 50 % (IC50) was calculated by the linear regression.
2.6.2. ABTS method
The antioxidant activity was achieved through the capture of the radical 2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS), which reduces ABTS•+ to ABTS, with the addition of antioxidant, according to methodology adapted from Rufino et al. (2010) [22].
Hydroalcoholic extract and fractions, diluted in methanol (500 μg/mL), were prepared for test dilutions, in concentrations ranging from 500 μg/mL to 7.81 μg/mL. Then, in each well of a 96-well microplate, 20 μL of each concentration and 280 μL of ABTS•+ radical solution were added, in triplicate. For the negative control, 20 μL of methanol and 280 μL of ABTS•+ radical solution were added. The standard curve was performed with the antioxidant Trolox at concentrations ranging from 500 μM/mL to 50 μM/mL. The plate was kept in the dark for 20 min and then the absorbance was read at 734 nm. The concentration necessary to reduce the initial concentration of the ABTS radical by 50 % (IC50) was calculated by linear regression.
2.6.3. Iron reduction method (FRAP)
The Ferric Reduction Ability Power (FRAP) method determines the reducing power of antioxidants, through the reduction of Fe3+ into Fe2+, according to methodology adapted from Rufino et al. (2010) [22].
FRAP reagent was prepared by mixing 25 mL of a 0.3 M acetate buffer solution (pH 3.6) with 2.5 mL of 10 mM TPTZ solution (2,4,6-Tris (2-pyridyl)-s-triazine in 40 mM HCl) and 2.5 mL of 20 mM FeCl3. In conical tubes, 30 μL (hydroalcoholic extract and fractions, diluted in methanol, 500 μg/mL), 90 μL of distilled water and 900 μL of the FRAP reagent solution were added. The mixture was homogenized by vortexing. Subsequently, 250 μL of each sample mixture was added, in triplicate, into 96-well microplates. Only the FRAP solution was added to the control wells. A Trolox calibration curve was performed at concentrations ranging from 500 μM to 0.48 μM. The plate was incubated at 37 °C, kept away from light, for 30 min. Absorbance was performed in a spectrophotometer at 595 nm.
2.7. Animals
Swiss Webster mice, four-to-six weeks old, female or male, were obtained from the Institute of Science and Technology in Biomodels (ICTB/FIOCRUZ) and maintained in pathogen-free conditions, at a controlled temperature and with water and food ad libitum (CEUA Nº: L-004/2022).
2.8. Collection and culture of peritoneal macrophages
To obtain peritoneal macrophages, 3 mL of a 0.5 % thioglycolate solution in sterile phosphate buffered saline (PBS) was injected into the animals intraperitoneally. After 72h, cells were obtained by washing the peritoneal cavity using 10 mL of ice-cold PBS. Then, the cells were centrifuged (500 g/5 min/4 °C) and resuspended in RPMI 1640 medium without phenol red supplemented with 10 % fetal bovine serum (FBS), penicillin (100U/mL) and streptomycin (100 μg/mL).
2.9. Cytotoxicity assay
For the cytotoxicity assay, peritoneal macrophages (2 × 105 cells/well) were plated in 96-well Flat Bottom TC-treated Microplates (TCP011096) (JetBiofil) and treated with different concentrations of Euterpe oleracea seeds hydroalcoholic extract and fractions (500–31.25 μg/mL), in a final volume of 100 μL of RPMI 1640 medium. Untreated control wells (containing cells, culture medium and 1 % DMSO) and blank (culture medium, without cells) were also used. The plates were incubated at 37 °C with 5 % CO2 in a humidified incubator (Panasonic – MCO-19AICUV-PA) and, after 24h and 48h, cell viability was analyzed using MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide) colorimetric assay [23]. Briefly, 10 μL of MTT solution (5 mg/mL) was added to each well 2 h before of analysis time. At the end of the 2 h incubation, formazan crystals formed by the reduction of the MTT salt were solubilized by adding 50 μL of DMSO to each well. Absorbance was measured in a spectrophotometer using a wavelength of 540 nm. The concentration that inhibits 50 % of cell growth (CC50) was determined by linear regression.
2.10. Anti-inflammatory activity of Euterpe oleracea seed hydroalcoholic extract and fractions on peritoneal macrophages stimulated with LPS
Peritoneal macrophages (2 × 106 cells/well/mL) were seeded in Corning® Falcon® Cell Culture 24-weel Plate (CLS353047) and incubated at 37 °C with 5 % CO2 in a humidified incubator for 1 h. After, cells were treated with different concentrations (0–500 μg/mL) of hydroalcoholic extract and fractions of Euterpe oleracea seeds, or with dexamethasone (100 μM), for 1 h and, then stimulated, or not, with LPS (10 μg/mL) and incubated at 37 °C, with an atmosphere of 5 % CO2. Untreated and unstimulated cells and untreated and stimulated cells were used as controls. All treatment dilutions were carried out in RPMI 1640 medium without phenol red. Supernatants were collected after 48 h of treatment to measure nitrite and cytokines.
2.11. Nitrite and cytokines quantification
Cytokine levels (IFN-γ, IL-6 and IL-12p40 were quantified according to the manufacturer's instructions, using in 96-well microplates, ps, half area, high binding (Greiner Bio-One, São Paulo, Brazil).
Briefly, 50 μL of diluted specific capture antibodies for each cytokine were added to each well. The plates were incubated overnight. After the incubation period, all wells were washed with wash buffer (0.05 % PBS and Tween 20). A blocking solution was added to the wells and incubated for 1 h. Subsequently, the wells were washed again. The supernatant obtained from the macrophage infection assay and standards (50 μL) were added to the respective wells. The plates were maintained at room temperature (RT) for 2 h. Following this incubation, additional washes were performed. Then, 50 μL of detector complex (Detection Ab + SAv-HRP) was added to the wells. The plates were incubated for an additional time at RT. After another round of washing, the substrate solution was added to each well (50 μL) and the plates were incubated at room temperature in dark. To stop the reaction, 50 μL of Stop Solution were added to each well. The absorbance was measured in a plate reader at a wavelength of 450 nm within 30 min with λ correction 570 nm. The concentration of cytokines in the supernatants was determined by interpolation from the standard curve.
NO levels were determined indirectly through nitrite quantification using the Griess reaction [24]. Briefly, 50 μL of the supernatants obtained were placed in each well of Nunc™ MicroWell™ 96-Well, Nunclon Delta-Treated, Flat-Bottom Microplate (Thermo Fisher Scientific Inc.). After, an equal volume of Griess reagent (0,1 % N-(1-Naphthyl)ethylenediamine dihydrochloride and 1 % Sulfanilamide in 2,5 % H3PO4) was added to each well. In parallel, the same procedure was carried out for standard curve, using known concentrations of sodium nitrite (NaNO2) ranging from 200 to 0,1μM, which were prepared in RPMI medium. The plates were then incubated at room temperature for 10 min. Subsequently, the absorbance was measured in a plate reader at a wavelength of 570 nm. The concentration of nitrite in the supernatants was determined by interpolating the absorbance values into the standard curve.
2.12. Statistical analysis
IC50 was calculated by linear regression. The data were expressed by mean ± standard deviation (SD) and analyzed statistically by Brown–Forsythe and Welch ANOVA multiple comparison test. The analyses were performed with the software GraphPad Prism 8.2.1 and differences were considered significant when p < 0.05.
3. Results and discussion
3.1. Chemical characterization of Euterpe oleracea seed hydroalcoholic extract and fractions
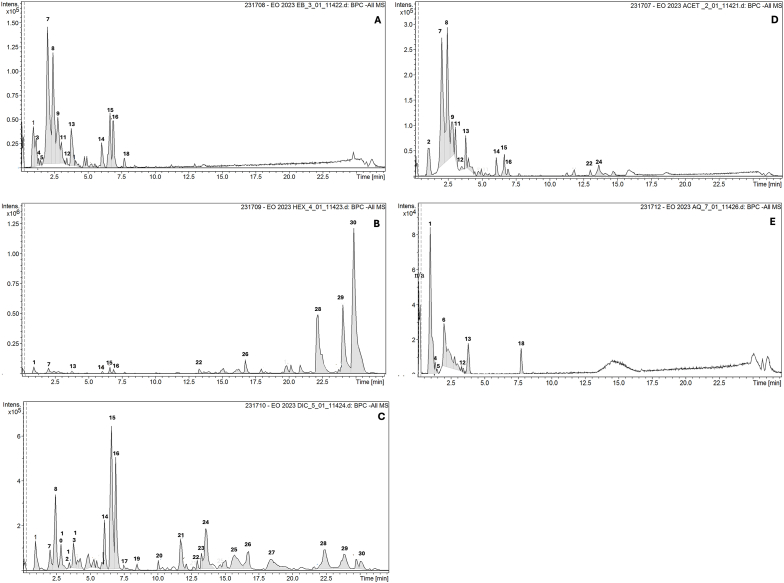
The general characterization of Euterpe oleracea seed hydroalcoholic extract and its fractions was made by LC/MS analyses. Compounds were tentatively identified by similarity of molecular ions with literature data [9,11], and presented in Table 1 and Fig. 1.
Table 1.
Mass spectrometry of Euterpe oleracea seeds hydroalcoholic extract and fractions.
| Rt | Molecular formula | m/z | Tentative identification | HE | HF | DCMF | EAF | AQF | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.9 | –--------- | 377,1140 | n.i. | + | + | + | – | + |
| 2 | 1.0 | C45H38O18 | 865,2579 | Procyanidin trimer C1 | – | – | – | + | – |
| 3 | 1.1 | –--------- | 439,1127 | n.i. | + | – | – | – | – |
| 4 | 1.3 | C11H12O3 | 191,0350 | p-Coumaric acid ethyl ester | + | – | – | – | + |
| 5 | 1.4 | C16H12O6 | 299,0986 | Chrysoeriol | + | – | – | – | + |
| 6 | 1.9 | C45H38O18 | 865,2595 | Procyanidin trimer C1 | – | – | – | – | + |
| 7 | 2.0 | C30H26O12 | 577,1757 | Procyanidin dimer B1 | + | + | + | + | – |
| 8 | 2.4 | C15H14O6 | 289,0919 | (+)-Catechin | + | – | + | + | – |
| 9 | 2.7 | C45H38O18 | 865,2601 | Procyanidin trimer C1 | + | – | – | + | – |
| 10 | 2.8 | C15H14O6 | 289,0927 | (−)-Epicatechin | – | – | + | – | – |
| 11 | 3.0 | C30H26O12 | 577,1748 | Procyanidin dimer B1 | + | – | – | + | – |
| 12 | 3.4 | C28H33O15 | 609,1892 | Peonidin 3-O-rutinoside | + | – | + | + | + |
| 13 | 3.7 | C31H29O14 | 623,2056 | Petunidin 3-O-(6″-p-coumaroyl-glucoside) | + | + | + | + | + |
| 14 | 6.0 | C45H38O19 | 881,3127 | Prodelphinidin trimer | + | + | + | + | – |
| 15 | 6.6 | –--------- | 923,3289 | n.i | + | + | + | + | – |
| 16 | 6.8 | –--------- | 923,3306 | n.i. | + | + | + | + | + |
| 17 | 7.5 | C17H14O7 | 329,2570 | 3,7-Dimethylquercetin | – | – | + | – | – |
| 18 | 7.7 | –--------- | 426,9986 | n.i. | + | – | – | – | + |
| 19 | 8.5 | C18H16O7 | 343,1080 | 5,4′ -Dihydroxy-7,3′,5′ -trimethoxyflavone | – | – | + | – | – |
| 20 | 10.0 | C17H14O6 | 313,2618 | velutin | – | – | + | – | – |
| 21 | 11.7 | –--------- | 737.4494 | n.i. | – | – | + | + | – |
| 22 | 12.9 | C33H40O19 | 739,4654 | Kaempferol 3-O-rhamnosyl-rhamnosyl-glucoside | – | + | + | + | – |
| 23 | 13.2 | C13H12O8 | 295,2494 | p-Coumaroyl tartaric acid | – | – | + | – | – |
| 24 | 13.5 | C33H40O19 | 739,4645 | Kaempferol 3-O-rhamnosyl-rhamnosyl-glucoside | – | – | + | + | – |
| 25 | 15.7 | C15H18O8 | 325,2078 | p-Coumaric acid 4-O-glucoside | – | – | + | – | – |
| 26 | 16.7 | –--------- | 299,2807 | n.i. | – | + | + | – | – |
| 27 | 18.3 | –--------- | 339,2239 | n.i. | – | – | + | – | – |
| 28 | 22.1 | C13H12O7 | 279,2536 | p-Coumaroyl malic acid | – | + | + | – | – |
| 29 | 23.6 | C22H18O11 | 457,3669 | (+)-Gallocatechin 3-O-gallate | – | + | + | – | – |
| 30 | 24.7 | –--------- | 281,2692 | n.i. | – | + | + | – | – |
Note: Rt = retention time, m/z = mass/charge relationship. Signals (+) and (−) indicate the presence or absence of the compound. HE = hydroalcohoolic extract; HF = hexane fraction, DCMF = dichloromethane fraction; EAF = Ethyl acetate fraction, AQF = aqueous fraction.
Fig. 1.
Chromatograms. LC-MS profiles of (A) hydroalcoholic extract (HE), (B) hexane fraction, (C) dichlorometane fraction (DCMF), (D) ethyl acetate fraction (EAF) and (E) aqueous fraction (AQF) of Euterpe oleracea seeds.
The chromatograms of hydroalcoholic extract (Fig. 1A), hexane fraction (Fig. 1B), dichlorometane fraction (Fig. 1C), ethyl acetate fraction (Fig. 1D) and aqueous fraction (Fig. 1E) of Euterpe oleracea seeds showed the predominance of polar compounds in hydroalcoholic extract, ethyl acetate fraction, and aqueous fraction. On the other hand, dichlorometane fraction and hexane fraction chromatograms showed a predominance of medium and low polarity compounds, respectively. This is due to the polarity separation made in the liquid-liquid partition performed. The hydroalcoholic extract and the ethyl acetate fraction showed the same two predominant compounds, procyanidin B1 dimer and catechin. The dichloromethane fraction was the richest fraction and presented several compounds of medium polarity, of which we can highlight the flavonoids velutina, 5,4′-Dihydroxy-7,3′,5′-trimethoxyflavone and 3,7-dimethylquercetin and some coumaric acid derivatives [9]. The hexane fraction showed some derivatives of coumaric acid and gallocatechin as predominant compounds (Table 1).
Chemical profile of the hydroalcoholic extract and seed fractions of Euterpe oleracea, obtained in this work, is similar to those described by other publications in the literature [20,25]. Xavier et al. (2021) showed mainly the presence of catechins and pelargonidin in the ethyl acetate fraction of Euterpe oleracea seeds obtained at São Luis/MA, Brazil. In this same work, the chemical profiles of the hydroalcoholic extract and chloroform and aqueous fractions were also investigated and presented compositions different from those found in our work. These differences are attributed to factors such as geographic location, soil and climate conditions, extracting solvents, extraction methods, etc [26,27].
The presence of catechins, epicatechins and procyanidins (dimers and trimers), in Euterepe oleracea seeds was observed in the present work, as well as, in other studies [9,11]. Filho et al. (2023), studying the chemical composition of the hydroalcoholic extract of Euterpe oleracea seeds, showed that (+)-catechin, caffeic acid-3-glucoside and (−)-epicatechin were the main compounds found. These compounds have been correlated to antioxidant, anti-inflammatory and anticarcinogenic activities [25].
3.2. Euterpe oleracea seeds hydroalcoholic extract and fractions are rich in flavonoids
Flavonoids are plant origin compounds and constitute a large group of polyphenolic compounds. The main classes of flavonoids are: anthocyanidins, isoflavones, chalcones, flavonols, flavones, flavonones, among others [28]. Several studies describe different biological actions of flavonoids, such as antioxidant, anti-inflammatory, anti-tumor activity, in addition to being used in the treatment of neurodegenerative diseases, cardiovascular diseases and diseases caused by protozoa [29,30].
Total flavonoids quantification in hydroalcoholic extract and fractions of Euterpe oleracea seeds revealed that the aqueous fraction (26.60 ± 0.23) presented the highest concentration of flavonoids (Eq Quercetin/g compound) followed by the dichloromethane fractions (16 0.35 ± 0.16), ethyl acetate (12.56 ± 0.07), hexane fraction (7.14 ± 0.10) and hydroalcoholic extract (3.07 ± 0.09), respectively, as seen in Table 2.
Table 2.
Quantification of total flavonoids in hydroalcoholic extract and fractions of Euterpe oleracea seeds
| Euterpe oleracea seeds | Flavonoids (Eq Quercetin/g compound) |
|---|---|
| Hydroalcoholic extract | 3,07 ± 0,09 |
| Hexane fraction | 7,14 ± 0,10 |
| Dichloromethane fraction | 16,35 ± 0,16 |
| Ethyl acetate fraction | 12,56 ± 0,07 |
| Aqueous fraction | 26,60 ± 0,23 |
Note: Flavonoids: μMol of quercetin equivalent per gram sample.
Numerous studies demonstrate the presence of flavonoids in açaí pulp (Earling et al., 2019; Gironés-Vilaplana et al., 2014; Kang et al., 2010; Rodrigues et al., 2006) [11,[31], [32], [33]]. Furthermore, other publications corroborate the present work and indicate that Euterpe oleracea seeds are also rich in flavonoids [11,34].
3.3. Antioxidant power of Euterpe oleracea seed hydroalcoholic extract and fractions
Antioxidants are substances that protect cells and tissues against oxidative stress, preventing or reducing damage caused by free radicals. Normally, they have electron or hydrogen donor substituents in their structure, the ability to complex with metals and the ability to carry out resonance through the radical formed. This class of compounds is often found in many natural products, such as green tea, grapes and açaí. There is a great interest among researchers in the discovery of antioxidant substances mainly for their potential health benefits [35,36].
In this work, three different methods were used to determine the antioxidant capacity, in vitro, of substances present in the hydroalcoholic extract and fractions of Euterpe oleracea seeds: DPPH, ABTS and FRAP. The DPPH method is based on the antioxidant capacity of the hydroalcoholic extract and fractions of Euterpe oleracea seeds to reduce DPPH radicals. In ABTS method, the antioxidant capacity of the hydroalcoholic extract and fractions of Euterpe oleracea seeds was evaluated through the neutralization of ABTS•+ radicals and the formation of ABTS. Finally, in the FRAP method, the antioxidant capacity is determined through the reduction of iron ions Fe3+ to Fe2+. The use of different methods allows us to achieve a more comprehensive and reliable antioxidant assessment because, in each methodology, the assessment of antioxidant capacity occurs through different chemical substances/species [22,37].
In FRAP method, EAF was the compound that showed the highest antioxidant activity (4516.00 ± 58.07 μMol equivalent of trolox/gram of sample), followed by AQF (3542.75 ± 93.16), HE (2553.67 ± 0.01), HF (1865.42 ± 5.90) and DCMF (933.83 ± 0.44). Similarly, this same fraction showed the best antioxidant activity when DPPH and ABTS free radical scavenging assays were employed. The ethyl acetate fraction showed the lowest IC50 (amount of antioxidant required to reduce the initial concentration of DPPH or ABTS radicals by 50 %) 3.93 ± 0.26 μg/mL in the DPPH assay and 34.65 ± 0.35 μg/mL in ABTS, followed by the AQF (IC50 5.0 ± 0.25 μg/mL in the DPPH assay and 61.16 ± 0.31 μg/mL in ABTS) and HE (IC50 of 6.03 ± 0.28 μg/mL in the DPPH assay and 69.84 ± 0.32 μg/mL in the ABTS, compared to the Trolox standard which presented an IC50 of 23.30 ± 0.17 μg/mL in the DPPH assay and 73.53 ± 0.33 μg/mL in the ABTS. All tested samples showed a lower IC50 in the DPPH test than the Trolox standard. In the ABTS test, hexane fractions (83.94 ± 0.31 μg/mL) and dichloromethane fraction (156.53 ± 0.22 μg/mL), presented IC50 values greater than the Trolox standard (73,53 ± 0,33 μg/mL). The results regarding the antioxidant assays can be seen in Table 3.
Table 3.
Antioxidant activity of hydroalcoholic extract and fractions of Euterpe oleracea seeds according to the free radical scavenging assays (ABTS and DPPH) and Fe3+ reducing power (FRAP).
| Euterpe oleracea seeds | FRAP (Eq Trolox/g compound) | DPPH IC50 (μg/mL) |
ABTS IC50 (μg/mL) |
|---|---|---|---|
| Hydroalcoholic extract | 2553,67 ± 0,01 | 6,03 ± 0,28 | 69,84 ± 0,32 |
| Hexane fraction | 1865,42 ± 5,90 | 9,92 ± 0,30 | 83,94 ± 0,31 |
| Dichloromethane fraction | 933,83 ± 0,44 | 17,50 ± 0,30 | 156,53 ± 0,22 |
| Ethyl acetate fraction | 4516,00 ± 58,07 | 3,93 ± 0,26 | 34,65 ± 0,35 |
| Aqueous fraction | 3542,75 ± 93,16 | 5,0 ± 0,25 | 61,16 ± 0,31 |
| Trolox | --------------- | 23,30 ± 0,17 | 73,53 ± 0,33 |
Note: FRAP: μMol of Trolox equivalent per gram sample; DPPH: amount of antioxidant required to reduce the initial concentration of DPPH radical by 50 %; ABTS: amount of antioxidant necessary to reduce the initial concentration of ABTS•+ radical by 50 %.
The results obtained in this work corroborate the results presented by Martinez et al. (2018), in which a high antioxidant activity of the compounds present in the hydroalcoholic extract of Euterpe oleracea seeds was observed, when evaluated by the FRAP, ABTS and DPPH methods [6]. In the same way, Melo et al. (2021) also demonstrated that Euterpe oleracea seeds hydroalcoholic extract have strong antioxidant activity, in vitro, against DPPH and ABTS free radicals [38].
3.4. Treatment with hydroalcoholic extract and fractions of Euterpe oleracea seeds is no toxic for peritoneal macrophages
Conducting cytotoxicity studies allows us to screen compounds, including extracts, fractions and bioactive substances isolated from different natural products, to determine the potential toxicity of these molecules. It is essential that a compound has no or minimal toxicity [39].
In the present study, the treatment with hydroalcoholic extract/fractions of Euterpe oleracea seeds did not show cytotoxicity on peritoneal macrophages of Swiss Webster mice after 24 h and 48 h (CC50 > 500 μg/mL).
A similar result was described by Xavier et al. (2021) when evaluating the cytotoxicity of the ethyl acetate fraction of Euterpe oleracea seeds, against RAW 264.7 macrophages, not observing a significant change in the percentage of viable cells after 48 h of treatment [20]. In another work, however, with cells derived from human fibroblasts (GM cells), Silva et al. (2021) reported that the hydroalcoholic extract of Euterpe oleracea seed, tested at different concentrations (7–1000 μg/mL), demonstrated a reduction cell viability only from 500 μg/mL [40]. These observations indicate the safe use of açaí bioproducts in the treatment of various diseases.
3.5. Anti-inflammatory activity
Recently, numerous studies have been published seeking to investigate the anti-inflammatory activities of compounds present in different natural products [41,42].
The inflammatory process is a host defense response that can be triggered by several factors, including bacterial components such as lipopolysaccharide (LPS), and involves the recruitment of various cells, cytokines, and regulatory mediators, such as nitric oxide (NO). NO production occurs in mammalian cells from L-arginine and oxygen and is associated with a series of enzymes known as NO synthase (NOS), which are highly expressed by macrophages [43].
The beginning of the inflammatory process occurs after the detection of antigens in tissues by macrophages, which, once activated, release TNF-α, attracting neutrophils to the infected area soon after recognition, in addition to the release of IL-12 and IFN-γ. Although neutrophil mobilization is beneficial in terms of protection, their excessive migration is linked to tissue damage in several chronic inflammatory diseases. According to Bueno-Silva et al. (2017), this is due to the release of metalloproteinases, lysosomal proteolytic enzymes and reactive oxygen and nitrogen species. In addition to macrophages, monocytes and dendritic cells are the most important sources of interleukin-12 (IL-12). This cytokine is closely linked to the immune response necessary to resolve infection by bacteria and viruses. It is known that, when stimulated by LPS, the toll-like receptor 4 (TLR4) in monocytes and macrophages induce the synthesis of IL-12 and stimulate macrophages to produce pro-inflammatory cytokines, generating inflammation. IL-12 is composed of the p35 and p40 subunits, which, when combined, form the bioactive IL-12p70 [43,44].
Interleukin-6 (IL-6) is also a pro-inflammatory cytokine that is associated with the recruitment and maturation of neutrophils, the induction of acute inflammatory response, chronic inflammation, and autoimmune diseases [45]. IL-6 is an important modulator for the transition from the acute to the chronic phase of inflammation [46].
Interferon-gamma (IFN-ɣ) can promote inflammation by activating macrophages and inducing the production of other pro-inflammatory cytokines, such as TNF-α, IL-6, as well as reactive oxygen and nitrogen species. IFN-ɣ is one of the main cytokines produced by activated macrophages and exerts several important functions during the inflammatory response [47].
In this sense, macrophages are the main defense cells, essential for the development of the inflammatory response. In vitro experiments using peritoneal macrophages is a widely used tool, since they are collected from the peritoneum of animals, such as mice, and are more like the physiological condition in vivo, being important to simulate specific conditions of the immune response and inflammation [43].
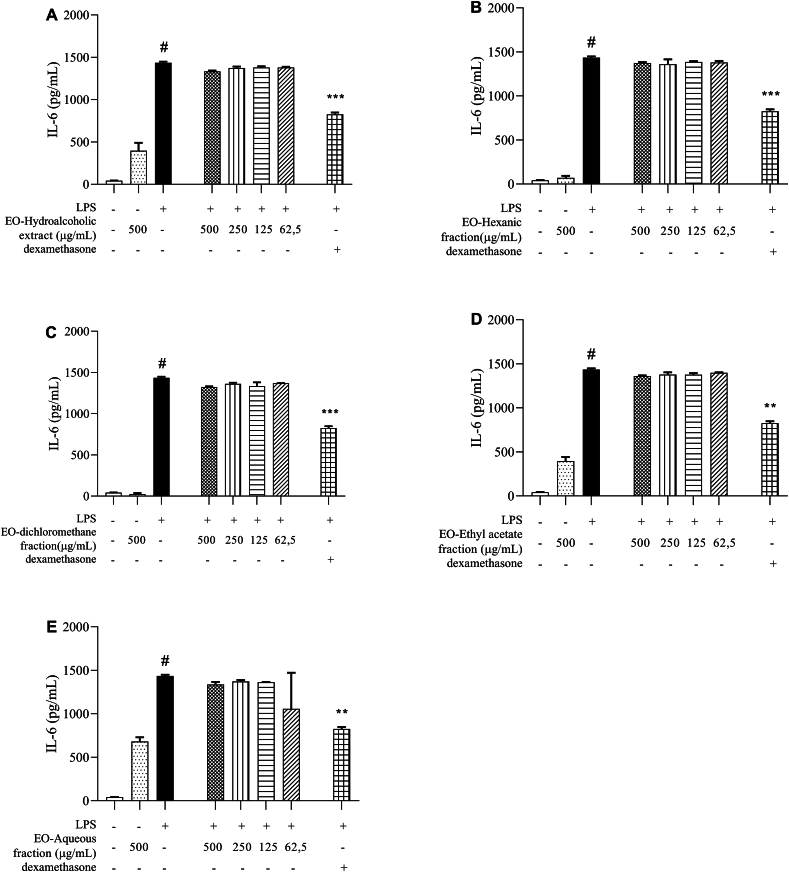
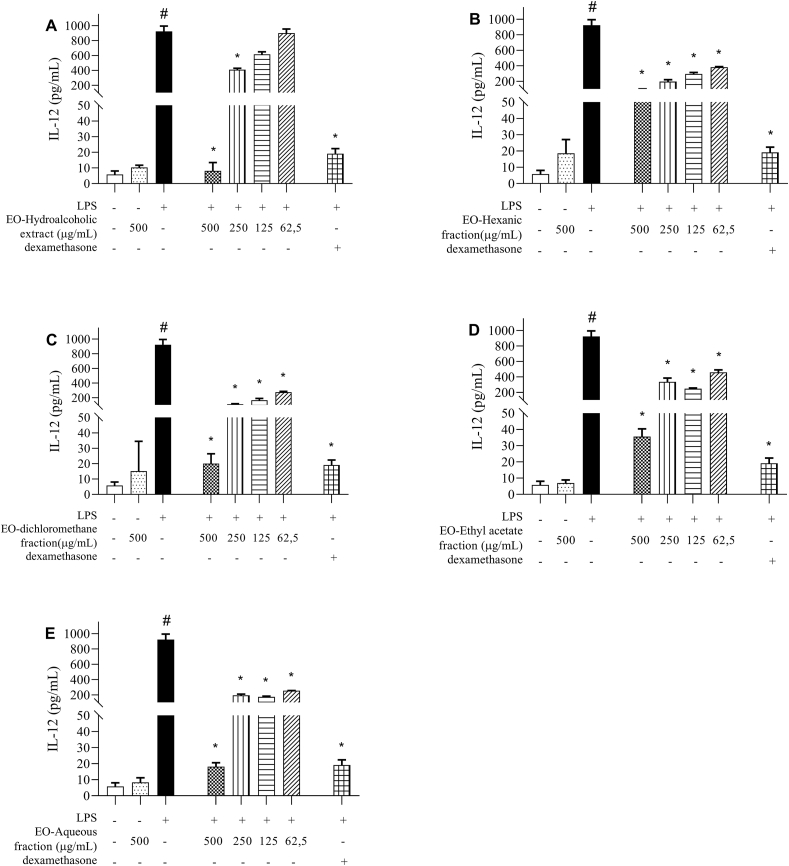
When we analyzed the production of pro-inflammatory cytokines and NO in culture supernatants of peritoneal macrophages stimulated by LPS and treated with different concentrations of E. oleracea extract/fractions, we observed that the treatment was not able to reduce IL-6 levels (Fig. 2A–E), when compared to untreated cells stimulated with LPS. On the other hand, all compounds were efficient in reducing IL-12 production, especially at a concentration of 500 μg/mL (Fig. 3A–E).
Fig. 2.
IL-6 quantification. Measurement of IL-6 in supernatants of peritoneal macrophage cultures stimulated by LPS, treated with 100 μM dexamethasone or (A) hydroalcoholic extract, (B) hexane fraction, (C) dichlorometane fraction, (D) ethyl acetate fraction and (E) aqueous fraction, obtained from Euterpe oleracea (EO) seeds. Data represents the mean ± standard deviation of the experiment performed in triplicate. #p < 0.001 compared to the group without stimulation or treatment (white column); ∗∗p < 0.01, ∗∗∗p < 0.001 when compared with the LPS-stimulated and untreated group, after Brown–Forsythe analysis and Welch, followed by Dunnett's T3 multiple comparison tests.
Fig. 3.
IL-12quantification Measurement of IL-12 in supernatants of peritoneal macrophage cultures stimulated by LPS, treated with 100 μM dexamethasone or (A) hydroalcoholic extract, (B) hexane fraction, (C) dichlorometane fraction, (D) ethyl acetate fraction and (E) aqueous fraction, obtained from Euterpe oleracea (EO) seeds. Data represents the mean ± standard deviation of the experiment performed in triplicate. #p < 0.001 compared to the group without stimulation or treatment (white column); ∗p < 0.05 when compared with the LPS-stimulated and untreated group, after Brown Forsythe analysis and Welch, followed by Dunnett's T3 multiple comparison tests.
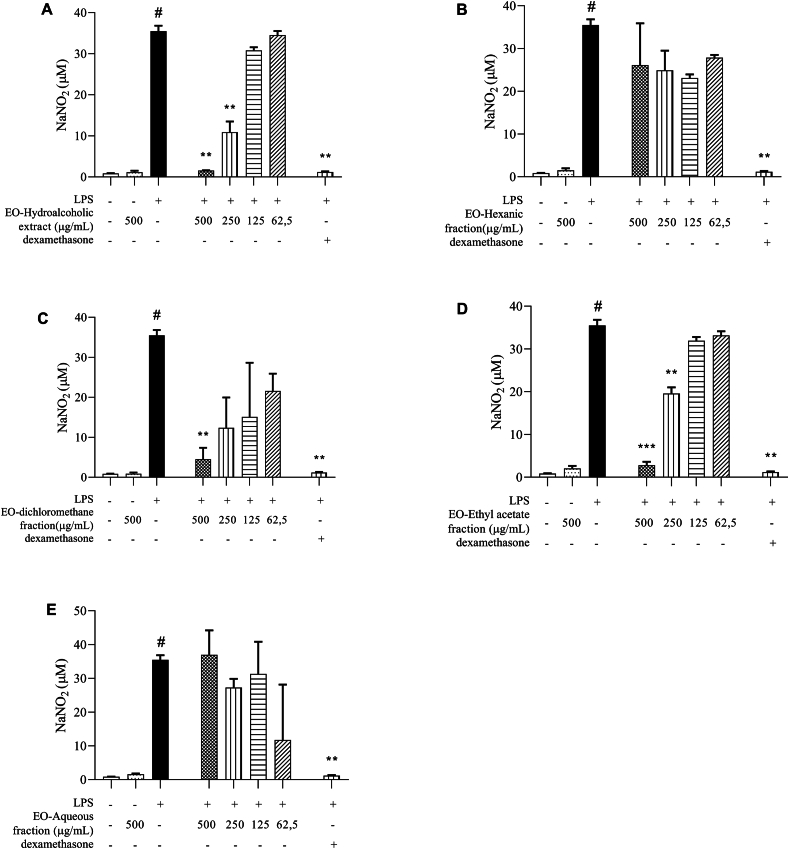
When we analyzed the effect of treatment on decreasing NO production, we observed that cells stimulated with LPS and treated with HE, DCMF and AEF showed low levels of NaNO2 when compared to untreated peritoneal macrophages stimulated with LPS, especially in concentration of 500 μg/mL (Fig. 4A–C and D).
Fig. 4.
NO quantification. Measurement of NaNO2 in supernatants of peritoneal macrophage cultures stimulated by LPS, treated with 100 μM dexamethasone or (A) hydroalcoholic extract, (B) hexane fraction, (C) dichlorometane fraction, (D) ethyl acetate fraction and (E) aqueous fraction, obtained from Euterpe oleracea (EO) seeds. Data represents the mean ± standard deviation of the experiment performed in triplicate. #p < 0.001 compared to the group without stimulation or treatment (white column); ∗∗p < 0.01, ∗∗∗p < 0.001 when compared with the LPS-stimulated and untreated group, after Brown–Forsythe analysis and Welch, followed by Dunnett's T3 multiple comparison tests.
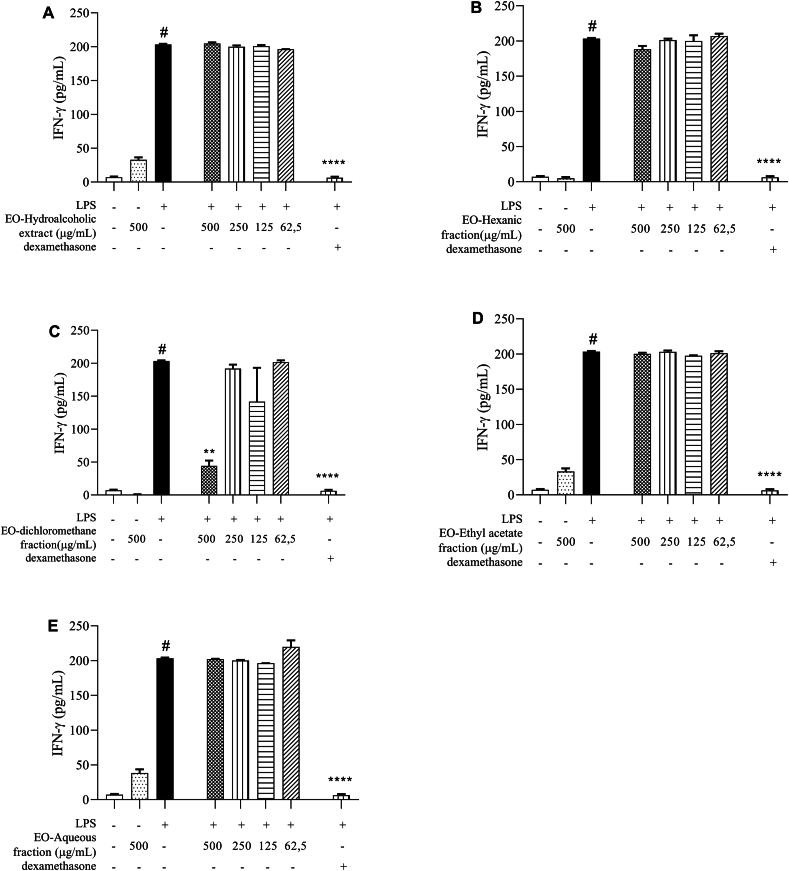
Regarding the quantification of IFN-ɣ, an inhibitory effect was only observed in treatment with DCMF, at a concentration of 500 μg/mL (Fig. 5C). This fraction showed the best anti-inflammatory activity, reducing the NO, IFN-ɣ and IL-12 production.
Fig. 5.
IFN-ɣ quantification. Measurement of IFN-ɣ in supernatants of peritoneal macrophage cultures stimulated by LPS, treated with 100 μM dexamethasone (A) hydroalcoholic extract, (B) hexane fraction, (C) dichlorometane fraction, (D) ethyl acetate fraction and (E) aqueous fraction, obtained from Euterpe oleracea (EO) seeds. Data represents the mean ± standard deviation of the experiment performed in triplicate. #p < 0.001 compared to the group without stimulation or treatment (white column); ∗∗p < 0.01, ∗∗∗∗p < 0.0001 when compared with the LPS-stimulated and untreated group, after Brown–Forsythe analysis and Welch, followed by Dunnett's T3 multiple comparison tests.
Treatment with hydroalcoholic extract/fractions from Euterpe oleracea seeds does not induce changes in the levels of nitrite IL-12 and IFN-ɣ in the supernatants of peritoneal macrophage cultures not stimulated with LPS, as can be seen in Fig. 3, Fig. 4, Fig. 5. Despite not being statistically significant, there is a rise in IL-6 levels in cells treated with HE, EAF, and AF (Fig. 2). The increase in IL-6 induced by treatments with hydroalcoholic extracts can be related to the bioactive properties of the plants that compose these extracts.
As expected, treatment with the reference drug dexamethasone was effective in reducing nitrite levels and cytokines IFN-ɣ, IL-6 and IL-12 (Fig. 2, Fig. 3, Fig. 4, Fig. 5).
Similar results can be found in the literature, although experimental models different from those used in this research were used. Xavier et al. (2021), evaluated the inhibitory effect of treatment with Euterpe oleracea seed extract, rich in catechin, on RAW 264.7 macrophages stimulated by LPS and on the reduction of carrageenan-induced paw edema. In that study, the treatment had an inhibitory effect on the production of the pro-inflammatory marker's nitrite, IL-1β, IL-6 and IL-12 by macrophages, as well as reducing carrageenan-induced paw edema [20].
Filho et al. (2023) demonstrated that treatment with açaí seed extract using an in vivo breast cancer model based on Ehrlich's solid tumor had immunomodulatory effects on the pro-inflammatory cytokines production in mouse serum, supporting the cytotoxic immune response and decreasing the chronic inflammation [25].
The results obtained in this work, involving treatment with hydroalcoholic extract/fractions of Euterpe oleracea seeds, demonstrate an interesting anti-inflammatory activity of these bioproducts.
4. Conclusions
The study successfully characterized the chemical composition and biological activities of the hydroalcoholic extract and fractions of Euterpe oleracea seeds, highlighting their significant rich flavonoid content, and notable antioxidant and anti-inflammatory properties.
In terms of antioxidant capacity, the ethyl acetate fraction demonstrated the highest activity in all assays tested, surpassing the activity of the other fractions and the hydroalcoholic extract. These findings show that the ethyl acetate fraction contains highly potent antioxidant compounds, which are essential for neutralizing free radicals and reducing oxidative stress. Furthermore, the fractions exhibited anti-inflammatory activity, with the dichloromethane fraction showing the most significant inhibitory effects on NO, IL-12 and IFN-γ production, especially at the concentration of 500 μg/mL. The study also demonstrated that treatment with the hydroalcoholic extract and fractions of Euterpe oleracea seeds was not toxic to peritoneal macrophages, which ensures the safety of these bioproducts for potential therapeutic use in traditional medicine.
This study suggest that the therapeutic benefits of Euterpe oleracea seed bioproducts could be attributed due to their antioxidant properties and their ability to down-regulate the immune response, providing data to support further research into using compounds found in Euterpe oleracea seeds in potential treatments for a variety of chronic inflammatory pathologies.
CRediT authorship contribution statement
Henrique Previtalli-Silva: Writing – review & editing, Writing – original draft, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Daiana de Jesus Hardoim: Writing – review & editing, Methodology. Raphael de Lucena Banaggia: Methodology. Carla Junqueira Moragas-Tellis: Writing – review & editing, Writing – original draft, Methodology, Formal analysis, Data curation. Maria do Socorro dos Santos Chagas: Methodology. Maria Dutra Behrens: Writing – review & editing. Thiago de Souza Dias-Silva: Writing – review & editing, Writing – original draft, Methodology, Formal analysis, Data curation. Kátia da Silva Calabrese: Writing – review & editing, Supervision, Resources, Funding acquisition. Flávia de Oliveira Cardoso: Writing – review & editing, Writing – original draft, Validation, Supervision, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization.
Ethics statement
All procedures involving the use of animals were conducted following the guidelines for experimental procedures of the National Council for the Control of Animal Experimentation (CONCEA) and were submitted, evaluated, and approved by the Ethics Committee for the use of Laboratory Animals at FIOCRUZ (CEUA) No.: L-007/2021.
Data availability statement
All data sets obtained in this study are included in the article.
Data availability
Data will be made available on request.
Funding
This work was supported by Oswaldo Cruz Institute and Carlos Chagas Filho Foundation for Research Support of Rio de Janeiro State (FAPERJ) [grant number E−26/210.408/2024]. HSP is doctoral research fellow by Coordination for the Improvement of Higher Education Personnel (CAPES 88887.475529/2020-00), KSC is research productivity fellow by National Scientific and Technical Research Council (CNPq) [grant number 315225/2021-1].
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Cardoso reports equipment, drugs, or supplies was provided by Carlos Chagas Filho Foundation for Research Support of Rio de Janeiro State. Calabrese reports financial support, administrative support, article publishing charges, and equipment, drugs, or supplies were provided by Oswaldo Cruz Institute. Previtalli-Silva reports financial support was provided by Coordination for the Improvement of Higher Education Personnel (CAPES, Brazil). Calabrese reports that is research productivity fellow by National Scientific and Technical Research Council (CNPq). If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We would like to thank the Farmanguinhos Analytical methods platform, Dr. Mirian Claudia de Souza Pereira (Cellular Ultrastructure Laboratory/IOC/FIOCRUZ) for the spectrophotometer use and Dr. Marco Edilson Freire de Lima (Medicinal Chemistry Laboratory/UFRRJ) for preparing and crushing the seeds.
Contributor Information
Henrique Previtalli-Silva, Email: henriqueprevitalli89@gmail.com.
Kátia da Silva Calabrese, Email: calabrese@ioc.fiocruz.br.
Flávia de Oliveira Cardoso, Email: flaviaoc09@gmail.com.
References
- 1.Arulselvan P., et al. Role of antioxidants and natural products in inflammation. Oxid. Med. Cell. Longev. Jan. 2016;2016(1) doi: 10.1155/2016/5276130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ravipati A.S., et al. Antioxidant and anti-inflammatory activities of selected Chinese medicinal plants and their relation with antioxidant content. BMC Compl. Alternative Med. 2012;12(1):173. doi: 10.1186/1472-6882-12-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krishnaiah D., Sarbatly R., Nithyanandam R. A review of the antioxidant potential of medicinal plant species. Food Bioprod. Process. 2011;89(3):217–233. doi: 10.1016/j.fbp.2010.04.008. [DOI] [Google Scholar]
- 4.Atanasov A.G., et al. Discovery and resupply of pharmacologically active plant-derived natural products: a review. Biotechnol. Adv. Dec. 2015;33(8):1582–1614. doi: 10.1016/J.BIOTECHADV.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Newman D.J., Cragg G.M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. Mar. 2020;83(3):770–803. doi: 10.1021/acs.jnatprod.9b01285. [DOI] [PubMed] [Google Scholar]
- 6.Martinez R.M., et al. Açai (Euterpe oleracea Mart.) seed extract induces cell cycle arrest and apoptosis in human lung carcinoma cells. Foods. 2018;7(11) doi: 10.3390/foods7110178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.IBGE, “Produção Agrícola Municipal,” IBGE. Accessed: August. 01, 2024. [Online]. Available: https://sidra.ibge.gov.br/tabela/1613#resultado.
- 8.Farinas C.S., dos Santos R.R.M., Bertucci Neto V., Pessoa J.D.C., Aproveitamento do Caroço do Açaí como Substrato para a Produção de Enzimas por Fermentação em Estado Sólido 30 (2009) 1-15p. [Online]. Available: https://www.infoteca.cnptia.embrapa.br/bitstream/doc/658280/1/BPD302009.pdf. (Accessed 31 July 2024).
- 9.Barros L., et al. The powerful in vitro bioactivity of Euterpe oleracea Mart. seeds and related phenolic compounds. Ind. Crops Prod. 2015;76:318–322. doi: 10.1016/j.indcrop.2015.05.086. [DOI] [Google Scholar]
- 10.Miranda L. de V.A., Mochiutti S., da Cunha A.C., Cunha H.F.A. Discarding and final destination of açaí in the oriental Amazon - Brazil. Ambiente Sociedade. 2022;25 [Google Scholar]
- 11.Rodrigues R.B., et al. Total oxidant scavenging capacity of Euterpe oleracea Mart. (Açaí) seeds and identification of their polyphenolic compounds. J. Agric. Food Chem. Jun. 2006;54(12):4162–4167. doi: 10.1021/jf058169p. [DOI] [PubMed] [Google Scholar]
- 12.Silva D.F., et al. Cytotoxic effects of Euterpe oleracea Mart. in malignant cell lines. BMC Compl. Alternative Med. 2014;14(1):175. doi: 10.1186/1472-6882-14-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dias M.M., Noratto G., Martino H.S., Arbizu S., Peluzio M.doC., Talcott S., Ramos A.M., Mertens-Talcott S.U. Pro-apoptotic activities of polyphenolics from açai (Euterpe oleracea martius) in human SW-480 colon cancer cells. Nutr. Cancer. 2014;66(8):1394–1405. doi: 10.1080/01635581.2014.956252. [DOI] [PubMed] [Google Scholar]
- 14.Noratto G.D., Angel-Morales G., Talcott S.T., Mertens-Talcott S.U. Polyphenolics from açaí (Euterpe oleracea Mart.) and red muscadine grape (vitis rotundifolia) protect human umbilical vascular endothelial cells (HUVEC) from glucose- and lipopolysaccharide (LPS)-Induced inflammation and target MicroRNA-126. J. Agric. Food Chem. Jul. 2011;59(14):7999–8012. doi: 10.1021/jf201056x. [DOI] [PubMed] [Google Scholar]
- 15.Bera A., Roy B. In: Polyphenols in Health and Diseases. Yadav L., Singh S., Maurya N.K., editors. 2022. Chapter 16: polyphenols in medicinal plants: an overview; pp. 253–266. (Rasipuram, India: Darshan Publishers). ch. Chapter 16. [Google Scholar]
- 16.Melo P.S., Arrivetti L. de O.R., de Alencar S.M., Skibsted L.H. Antioxidative and prooxidative effects in food lipids and synergism with α-tocopherol of açaí seed extracts and grape rachis extracts. Food Chem. 2016;213:440–449. doi: 10.1016/j.foodchem.2016.06.101. [DOI] [PubMed] [Google Scholar]
- 17.da Costa C.A., et al. Euterpe oleracea Mart.-derived polyphenols prevent endothelial dysfunction and vascular structural changes in renovascular hypertensive rats: role of oxidative stress. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2012;385(12):1199–1209. doi: 10.1007/s00210-012-0798-z. [DOI] [PubMed] [Google Scholar]
- 18.de Moraes Arnoso B.J., et al. Açaí seed extract (ASE) rich in proanthocyanidins improves cardiovascular remodeling by increasing antioxidant response in obese high-fat diet-fed mice. Chem. Biol. Interact. 2022;351 doi: 10.1016/j.cbi.2021.109721. [DOI] [PubMed] [Google Scholar]
- 19.Machado da Rocha A., et al. Antihypertensive effects and antioxidant action of a hydro-alcoholic extract obtained from fruits of Euterpe oleracea Mart. (Acai) J. Pharmacol. Toxicol. Jun. 2008;3:435–448. doi: 10.3923/jpt.2008.435.448. [DOI] [Google Scholar]
- 20.Xavier G.S., et al. Inhibitory effect of catechin-rich açaí seed extract on LPS-stimulated RAW 264.7 cells and carrageenan-induced paw edema. Foods. 2021;10(5) doi: 10.3390/foods10051014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Acácio R.D.S., Franco S.P.B., Costa Filho W.S., da Costa J.G., dos Santos A.F., Goulart H.F. Avaliação da atividade antioxidante do extrato etanólico de Melochia tomentosa Linaeus (1735) Diversitas Journal. Sep. 2018;3(2):412. doi: 10.17648/diversitas-journal-v3i2.643. [DOI] [Google Scholar]
- 22.Rufino M. do S.M., Alves R.E., de Brito E.S., Pérez-Jiménez J., Saura-Calixto F., Mancini-Filho J. Bioactive compounds and antioxidant capacities of 18 non-traditional tropical fruits from Brazil. Food Chem. 2010;121(4):996–1002. doi: 10.1016/j.foodchem.2010.01.037. [DOI] [Google Scholar]
- 23.Almeida-Souza F., et al. Nitric oxide induction in peritoneal macrophages by a 1,2,3-triazole derivative improves its efficacy upon leishmania amazonensis in vitro infection. J. Med. Chem. Sep. 2021;64(17):12691–12704. doi: 10.1021/acs.jmedchem.1c00725. [DOI] [PubMed] [Google Scholar]
- 24.Green L.C., Wagner D.A., Glowski J., Skipper P.L., Wishnnok J.S., Tannenbaum S.R. Analysis of nitrate, nitrite, and nitrate in biological fluids. Anal. Biochem. 1982;26(February 2017):131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- 25.Filho W.E.M., et al. Antitumor effect of açaí (Euterpe oleracea Mart.) seed extract in LNCaP cells and in the solid Ehrlich carcinoma model. Cancers. May 2023;15(9) doi: 10.3390/cancers15092544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.da Costa N.B., Teles A.M., Oliveira M.V. da S., Oliveira É.S., Mouchrek A.N. Obtenção do perfil químico de extratos das folhas do cajueiro (Anacardium occidentale) a partir de diferentes solventes. Research, Society and Development. Jul. 2021;10(8) doi: 10.33448/rsd-v10i8.17473. [DOI] [Google Scholar]
- 27.Caldeira Morzelle M., Peters L.P., Angelini B.G., Roberto De Camargo E Castro P., Cabrera A.C., Mendes M. 2017. Agroquímicos estimulantes, extratos vegetais e metabólitos microbianos na agricultura. Série no 63., vol. ISSN 1414-4530, no. 1414–4530. Piracicaba. [Google Scholar]
- 28.Beecher G.R. Overview of dietary flavonoids: nomenclature, occurrence and intake. J. Nutr. 2003;133(10):3248S–3254S. doi: 10.1093/jn/133.10.3248S. [DOI] [PubMed] [Google Scholar]
- 29.Saúde-Guimarães D.A., Faria A.R. Substâncias da natureza com atividade anti-Trypanosoma cruzi. Revista Brasileira de Farmacognosia. 2007;17 [Google Scholar]
- 30.Adegbola P., Aderibigbe I., Hammed W., Omotayo T. Antioxidant and anti-inflammatory medicinal plants have potential role in the treatment of cardiovascular disease: a review. Am J Cardiovasc Dis. 2017;7(2):19. [PMC free article] [PubMed] [Google Scholar]
- 31.Earling M., Beadle T., Niemeyer E.D. Açai berry (Euterpe oleracea) dietary supplements: variations in anthocyanin and flavonoid concentrations, phenolic contents, and antioxidant properties. Plant Foods Hum. Nutr. Sep. 2019;74(3):421–429. doi: 10.1007/s11130-019-00755-5. [DOI] [PubMed] [Google Scholar]
- 32.Gironés-Vilaplana A., Baenas N., Villaño D., Speisky H., García-Viguera C., Moreno D.A. Evaluation of Latin-American fruits rich in phytochemicals with biological effects. J. Funct.Foods. 2014;7:599–608. doi: 10.1016/j.jff.2013.12.025. [DOI] [Google Scholar]
- 33.Kang J., Li Z., Wu T., Jensen G.S., Schauss A.G., Wu X. Anti-oxidant capacities of flavonoid compounds isolated from acai pulp (Euterpe oleracea Mart.) Food Chem. 2010;122(3):610–617. doi: 10.1016/j.foodchem.2010.03.020. [DOI] [Google Scholar]
- 34.Martins G.R., et al. Chemical characterization, antioxidant and antimicrobial activities of açaí seed (Euterpe oleracea Mart.) extracts containing A- and B-type procyanidins. LWT. 2020;132 doi: 10.1016/j.lwt.2020.109830. [DOI] [Google Scholar]
- 35.Kim M.R. MDPI; Apr. 01, 2021. Antioxidants of Natural Products. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sánchez-Villamil J.P., Bautista-Ninõ P.K., Serrano N.C., Rincon M.Y., Garg N.J. Hindawi Limited; 2020. Potential Role of Antioxidants as Adjunctive Therapy in Chagas Disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oliveira G.L.S. Determinação da capacidade antioxidante de produtos naturais in vitro pelo método do DPPH•: estudo de revisão. Rev. Bras. Plantas Med. 2015;17 [Google Scholar]
- 38.Melo P.S., Selani M.M., Gonçalves R.H., Paulino J. de O., Massarioli A.P., de Alencar S.M. Açaí seeds: an unexplored agro-industrial residue as a potential source of lipids, fibers, and antioxidant phenolic compounds. Ind. Crops Prod. 2021;161 doi: 10.1016/j.indcrop.2020.113204. [DOI] [Google Scholar]
- 39.McGaw L.J., Elgorashi E.E., Eloff J.N. In: Toxicological Survey of African Medicinal Plants. Kuete V., editor. Elsevier; 2014. 8 - cytotoxicity of african medicinal plants against normal animal and human cells; pp. 181–233. [DOI] [Google Scholar]
- 40.da Silva M.A.C.N., et al. Açai (Euterpe oleracea Mart.) seed extract induces ROS production and cell death in MCF-7 breast cancer cell line. Molecules. 2021;26(12) doi: 10.3390/molecules26123546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Batista J.E.C., et al. Plant lectins ConBr and CFL modulate expression toll-like receptors, pro-inflammatory cytokines and reduce the bacterial burden in macrophages infected with Salmonella enterica serovar Typhimurium. Phytomedicine. 2017;25:52–60. doi: 10.1016/j.phymed.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 42.de Alcântara Almeida I., Mancebo Dorvigny B., Souza Tavares L., Nunes Santana L., Vitor Lima-Filho J. Anti-inflammatory activity of caffeine (1,3,7-trimethylxanthine) after experimental challenge with virulent Listeria monocytogenes in Swiss mice. Int. Immunopharm. 2021;100 doi: 10.1016/j.intimp.2021.108090. [DOI] [PubMed] [Google Scholar]
- 43.Bueno-Silva B., et al. Brazilian red propolis effects on peritoneal macrophage activity: nitric oxide, cell viability, pro-inflammatory cytokines and gene expression. J. Ethnopharmacol. 2017;207:100–107. doi: 10.1016/j.jep.2017.06.015. [DOI] [PubMed] [Google Scholar]
- 44.Gee K., Guzzo C., Che Mat N.F., Ma W., Kumar A. The IL-12 family of cytokines in infection, inflammation and autoimmune disorders. Inflamm. Allergy - Drug Targets. 2009;8(1):40–52. doi: 10.2174/187152809787582507. [DOI] [PubMed] [Google Scholar]
- 45.De Filippo K., et al. Mast cell and macrophage chemokines CXCL1/CXCL2 control the early stage of neutrophil recruitment during tissue inflammation. Blood. Jun. 2013;121(24):4930–4937. doi: 10.1182/blood-2013-02-486217. [DOI] [PubMed] [Google Scholar]
- 46.Kaur S., Bansal Y., Kumar R., Bansal G. A panoramic review of IL-6: structure, pathophysiological roles and inhibitors. Bioorg. Med. Chem. 2020;28(5) doi: 10.1016/j.bmc.2020.115327. [DOI] [PubMed] [Google Scholar]
- 47.Mühl H., Pfeilschifter J. Anti-inflammatory properties of pro-inflammatory interferon-γ. Int. Immunopharm. 2003;3(9):1247–1255. doi: 10.1016/S1567-5769(03)00131-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data sets obtained in this study are included in the article.
Data will be made available on request.