ABSTRACT
The rising global demand for sustainable and eco-friendly practices has led to a burgeoning interest in circular bioeconomy, wherein waste materials are repurposed into valuable resources. Lignocellulosic waste, abundant in agricultural residues and forestry by-products, represents a significant untapped resource. This article explores the potential of fungal-mediated processes for the valorisation of lignocellulosic waste, highlighting their role in transforming these recalcitrant materials into bio-based products. The articles delve into the diverse enzymatic and metabolic capabilities of fungi, which enable them to efficiently degrade and metabolise lignocellulosic materials. The paper further highlights key fungal species and their mechanisms involved in the breakdown of complex biomass, emphasising the importance of understanding their intricate biochemical pathways for optimising waste conversion processes. The key insights of the article will significantly contribute to advancing the understanding of fungal biotechnology for circular bioeconomy applications, fostering a paradigm shift towards a more resource-efficient and environmentally friendly approach to waste management and bio-based product manufacturing.
KEYWORDS: Bioconversion, waste management, fungal enzymes, bioproducts, fungal technology
1. Lignocellulosic waste: An untapped resource
The quality of human life is being negatively impacted by the excessive consumption of global resources, which also poses a significant threat to the environment (Mujtaba et al. 2023). To promote sustainable growth and development, the concepts of the bioeconomy and the circular economy have been promoted (Ferreira Gregorio et al. 2018). Worldwide, lignocellulosic waste is acknowledged as an essential part of the planet’s renewable biomass. It is mostly composed of hemicelluloses (25%–30%), cellulose (40%–50%), and lignin (15%–20%) (Yang et al. 2023). Cellulose is a polysaccharide composed of glucose molecules joined by β-(1/4)-glycosidic linkages, creating a robust and inflexible structure. In addition to pentoses like D-xylose and L-arabinose, hexoses like D-glucose, D-mannose, and D-galactose; and sugar acids like uronic acids like D-glucuronic, D-galacturonic, and methyl galacturonic acids are all found in hemicellulose, which is a complex heteropolymer combination. Predominantly, D-xylose (90%) and L-arabinose (10%) make up the hemicellulose backbone chain (Kocher et al. 2018). Next to cellulose, lignin is the most prevalent component found in plant matter. Both aliphatic and aromatic structures are present in this high molecular-weight polymer. Guaiacol (G), syringe (S), and p-hydroxyphenyl (H) units are the phenyl propane units that make up this polymer. The two primary groups of lignin are guaiacyl lignins and guaiacyl-syringyl lignins. Softwoods contain guaiacyl lignin, while hardwoods contain guaiacyl-syringyl lignin (Kumar and Chandra 2020). Lignocellulose biomass (LCB) is a complex bioresource that emerges from a variety of sources, including agricultural and forest residues, organic solid waste collected from recycling stations, and the byproducts of paper, wood, and pulp industries. This diverse range of origins contributes to the complex structure of lignocellulose, which consists of three main components: Cellulose, hemicellulose, and lignin (Haq et al. 2021). Large amounts of lignocellulosic biomass, a sustainable resource material for the development of a broad range of bioproducts, are provided by agricultural waste (Mujtaba et al. 2023). Researchers and businesses are becoming more interested in lignocell biorefineries because they may provide renewable feedstock for a variety of application areas, including energy, food, nutrition, the chemical industry, and so on (Banu et al. 2021a). Recent data indicates that about 181.5 billion tons of lignocellulose biomass are produced annually worldwide (Singh et al. 2022). Only 8.2 billion tons of this biomass made up of lignocellulose are used by various application areas (Ashokkumar et al. 2022). This illustrates the unexplored potential of plant-based biomass to be converted into a diverse range of bioproducts for nearly every application area (Mujtaba et al. 2023). Lignocellulosic waste, primarily comprising agricultural residues, forestry by-products, and various forms of plant biomass, has long been considered a waste problem rather than a resource (Adewuyi 2022). The vast quantities of lignocellulose discarded annually contribute to environmental pollution and resource wastage. Nonetheless, because it is abundant in cellulose, hemicellulose, and lignin – all of which can be transformed into biofuels, biochemicals, and bio-based materials – this garbage offers an underutilised gold mine (Koul et al. 2022). Three primary components make up lignocellulose, a complex organic substance present in plant cell walls: Cellulose, hemicellulose, and lignin (Zoghlami and Paës 2019). This waste stream primarily includes agricultural residues, forestry byproducts, and various types of organic waste. Lignocellulosic waste is a ubiquitous byproduct of numerous industries, including agriculture, forestry, and food processing, and it has traditionally been disposed of in landfills or left to decay, contributing to environmental problems (Chukwuma et al. 2020). However, recent research and advancements in biotechnology and sustainable practices are shedding light on the untapped potential of lignocellulosic waste. One of the primary motivations behind recognising lignocellulosic waste as a valuable resource is its environmental significance. When lignocellulosic waste is left to decompose in landfills or openly burned, it generates greenhouse gases and poses a risk to air and water quality (Bilal et al. 2020). Converting lignocellulosic waste into useful products through sustainable processes can significantly reduce the environmental impact associated with its disposal (Blasi et al. 2023).
2. Fungal biodegradation: A sustainable approach in LCB valorisation
According to Andlar et al. (2018), fungi are a broad class of microorganisms that can break down lignocellulose, a complex polymer that makes up plant cell walls. The synthesis of several enzymes, such as cellulases, hemicellulases, and lignin peroxidases, is what gives this capacity (Sanches 2009; Kracher and Ludwig 2016; Rathner et al. 2017). Fungal-mediated lignocellulosic waste valorisation can be divided into two main categories: Fungi can be employed in two ways: Either directly, producing value goods like ethanol from lignocellulosic waste, or indirectly, producing intermediates or enzymes that can be used to further manufacture valuable products from the waste. Fungi have evolved complex enzymatic machinery to decompose lignocellulosic materials, making them nature’s recyclers (Andlar et al. 2018). Species like Trichoderma, Aspergillus, and Pleurotus are renowned for their lignocellulose-degrading capabilities. The diverse enzymes that these fungi release, including hemicellulases, cellulases, and ligninases, combine to break down the intricate lignocellulosic structure (Andlar et al. 2018). This enzymatic toolbox allows fungi to efficiently break down lignocellulosic waste into its constituent sugars and aromatic compounds. As they have the remarkable ability to convert complicated lignocellulosic materials into useful products, fungi are essential to achieving the goal of a circular bioeconomy (Figure 1) (Meyer et al. 2020). In the realm of increasing environmental concerns and the need for sustainable resource management, the transition towards a circular bioeconomy has garnered substantial attention. Lignocellulosic biomass derived from agricultural waste has been the focal point of several review studies, that have covered its pretreatment, processing, chemical composition, conversion, and other aspects. However, reports providing a comprehensive overview of the fungi’s significant role in lignocellulose biomass from pretreatment to applications, and the impact they have on the circular economy are scanty, however, hard to come by. This review explores the significance of fungal technology in the valorisation of lignocellulosic waste to multiple value products and its impact on the transition towards a circular bioeconomy.
Figure 1.
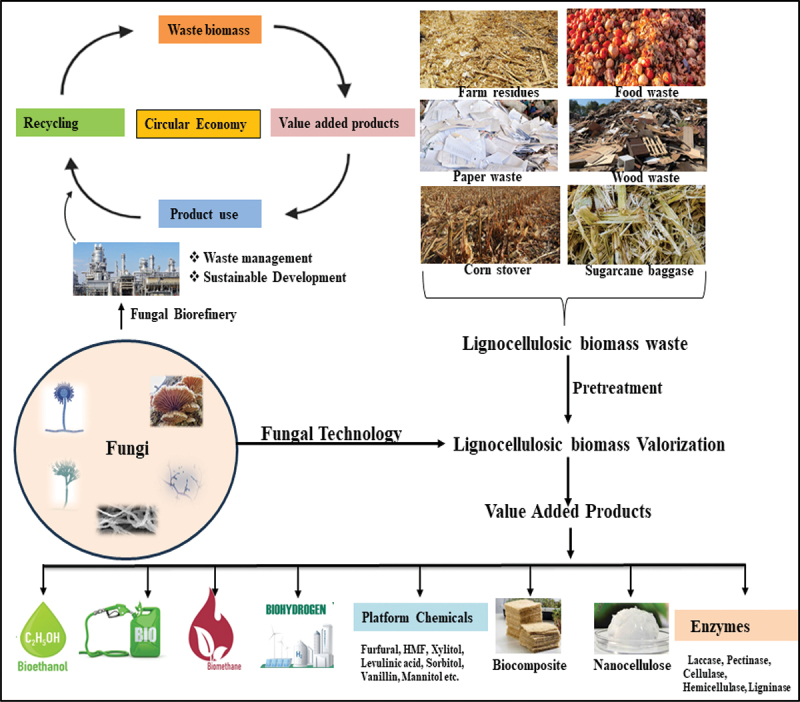
Lignocellulosic biomass valorization to value-added products through fungal technology.
3. Impact of fungi on LCB degradation
Many bacterial and fungal species have been isolated and characterised which can potentially degrade lignocellulosic waste degradation by their enzymatic activities (Kumar and Chandra 2020). Fungi are far more efficient than bacteria at breaking down lignin due to better enzyme secretion capacity and faster lignin breakdown rates (Atiwesh et al. 2022). The fungi offer several other advantages such as (a) growth on different types of lignocellulosic materials, (b) production of a diverse set of lignocellulolytic enzymes, (c) breaking down recalcitrant substances found in lignocellulosic substances, (d) survivability in adverse conditions, (e) amenability to genetic modification (Andlar et al. 2018; Yang et al. 2023). They may be categorised into three main groups: White rot, brown rot, and soft rot fungus, depending on how they look and the enzymes that break down the lignin mechanism (Table 1). Even while lignin can be broken down by all three families, only Basidiomycota – specifically, aerobic white rot fungus – can break down lignin entirely into CO2 and H2O (Civzele et al. 2023).
Table 1.
Diverse types of rot fungi for lignocellulose biomass (LCB) degradation.
| Type | Example | Substrate | Outcome | Reference |
|---|---|---|---|---|
| Soft rot | Parascedosporium putredinis NO1 | Wheat straw | Expansion of CaZymes by monooxygenase, oxidative enzymes, and laccase. | Scott et al. 2023 |
|
Xylaria species [Xylaria 1 (OR052384), Xylaria 2 (OR052385), Xylaria 3 (X. curta) (OR052386)] |
Ochroma pyramidale (Balsa), Gmelina arborea (melina), Samanea saman (saman), and Chlorophora tinctoria (mora) | Significant biomass loss; 60% and 25% of Ochroma and Gmelina respectively. | Rajtar et al. 2023 | |
| Brown rot | Gloeophyllum trabecum, Rhodonia placenta | Dendrocalamus sinicus | Hemicellulose and cellulose degradation by xylanase and mannase. | Qi et al. 2022 |
| Gloeophyllum trabecum | Pinus massoniana (Masson pine) | Depolymerisation of cellulose and hemicellulose by non-enzymatic chelator-mediated Fenton (CMF) system resulting in 15.7% biomass loss. | Zhu et al. 2022 | |
| Coniophora puteana, Rhodonia placenta | Scots pine sapwood | Depolymerisation of crystallisation carbohydrates, as well as a rise in lignin’s functionality and aromatic carbonyl content. | Belt et al. 2022 | |
| Rhodonia placenta | Picea abies (spruce wood) | Accumulation of oxalate within the cell wall, depolymerisation of hemicellulose. | Füchtner et al. 2023 | |
| White rot | Trametes versicolor, Gloeophyllum trabeum, and Rhodonia placenta | Dendrocalamus sinicus | T. versicolor degraded starch and pectin, followed by hemicellulose and lignin, G. trabeum and R. placenta degradation occur in the middle stage, with preferential degradation of hemicellulose and cellulose, respectively. | Qi et al. 2022 |
| Coriolopsis trogii | Industrial lignin (C. trogii TS01), Kraft lignin, EHL (Enzyme hydrolysis lignin) | The activity of C. trogii TS01‘s laccase and manganese-dependent peroxidase activity was raised by 24.8%, 164.1%, and 200%, respectively, by 1% EHL + 1% glucose. | Qiu and Liu 2022 | |
| Lentinula edodes | Grape stalks | 52% delignification in 42 days of incubation. | Costa-Silva et al. 2022 | |
| Ganoderma lucidium | Cocoa pods | Low capacity to digest cellulose and hemicellulose, high lignin degradation. | Mustabi et al. 2023 |
3.1. Soft rot fungi
The laccases and peroxidases produced by soft-rot fungus break down plant polysaccharides, softening and darkening the wood in the process. These enzymes, however, are less adaptable than those found in other species of fungi. Even in the latter phases of soft rot, they mostly demolish cellulose and hemicellulose but not lignin, breaking down carbohydrate polymers but leaving significant amounts of lignin intact. It is still necessary to thoroughly investigate their lignocellulosic degradation process (Andlar et al. 2018). According to Kocher et al. (2018) and Sista Kameshwar and Qin (2018), Aspergillus niger, Penicillium chrysogenum, Trichoderma reesei, Chaetomium cellulolyticum, and Fusarium oxysporum sp. are a few examples of soft rot fungi.
3.2. Brown rot fungi
Fungi that cause brown rot may break down cellulose and hemicellulose quickly, but they can also slightly change lignin. They lack particular enzymes for degrading lignin, and instead depend on tiny reactive chemicals to depolymerise it. As the degradation process advances, oxidised lignin gives the wood residue a cube-like form and a brownish hue (Andlar et al. 2018). During the initial stages of decay, brown-rot fungi have a remarkable propensity to break down hemicellulose, which efficiently destroys the complex structure of cell wall carbohydrates. Many lytic polysaccharide monooxygenase genes found in these fungi are engaged in the hydrolysis of lignocellulosic polysaccharides. These genes are mostly classified as members of the AA9 and AA14 families of auxiliary activity (AAs) under the carbohydrate-active enzymes (CAZy) classification. The breakdown of xylan and cellulose is caused by AA9 and AA14, respectively. Some examples of brown rot fungus include Gloeophyllum trabeum, Rhodonia placenta, Coniophora puteana, Meruliporia incrassate, and Fomitopsis pinicola (Li et al. 2023; Mali et al. 2023; Silva et al. 2023).
The chelator-mediated Fenton system (CMF) effectively illustrates the brown-rot fungus’s degradative process. The CMF method for employing oxygen radicals to break down cellulose non-enzymatically is shown in Figure 2a. As the brown-rot fungal hyphae proliferate inside the plant cell lumen, they emit hydrogen peroxide (H2O2), oxalic acid, and iron-reducing chemicals (RC). The complex that is created when oxalic acid binds to a Fe3+ ion dissolves into the cell wall along with H2O2 and RC. Fe3+ is taken out of the Fe-oxalate complex by RC, which lowers it to Fe2+ when the pH fluctuates. The hydroxyl radicals (−OH) created by this Fe2+ reaction with H2O2 later damage the lignocellulose matrix (Li et al. 2023).
Figure 2.
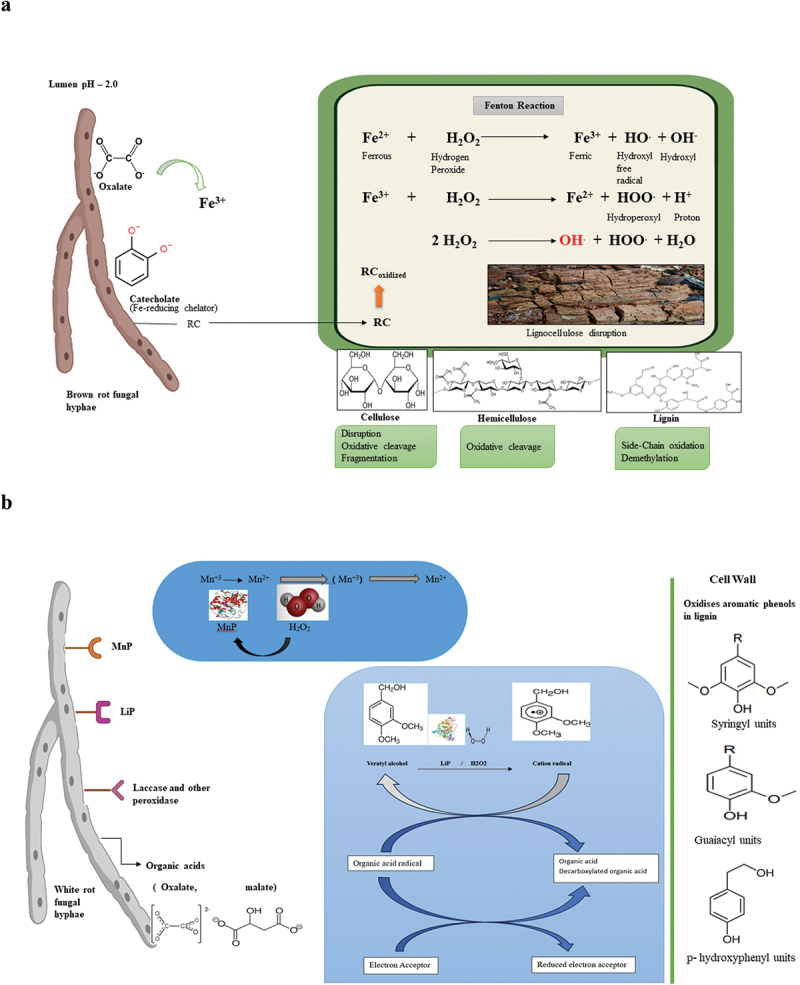
Degradation of lignocellulose biomass (LCB) by brown rot fungi and white rot fungi. (a) Degradation by brown rot fungi. (b) Degradation by white rot fungi.
3.3. White rot fungi
White rot fungi are efficient LCB degraders that specialise in cleaving aromatic compounds, which is why rotten wood has a characteristic white colour (Sista Kameshwar and Qin 2018). The key feature that sets white rot fungi apart is their ability to target and break down lignin, which is resistant to degradation by many other microorganisms. White-rot fungi can break down a variety of cell wall components, including sugars, lignin, and aromatic compounds (Sista Kameshwar and Qin 2018; Hou et al. 2020; Del Cerro et al. 2021; Kijpornyongpan et al. 2022). Notable examples of these fungi are Ceriporiopsis subvermispora, Phlebia radiata, Rigidoporous lignosus, Dichornitus sqiualenis, Lenzites betulina, Phellinus pini, Trametes versicolor, Lentinula edodes, and Pleurotus arthii. Multicomplex enzymes produced by white rot fungi include laccase, lignin peroxidase (LiP), manganese peroxidase (MnP), versatile peroxidase (VP), and dye-decolourising peroxidase (DyP). These enzymes convert lignin into hydrogen peroxide (H2O2) and carbon dioxide (CO2), which are widely used in bioprocessing processes like biopulping and the production of bioethanol, among other industrial uses (Zhang et al. 2023). In the difficult breakdown of lignocellulosic biomass, white rot fungi are essential to the process. They serve a pivotal role in the development of sustainable biotechnological processes due to their distinct enzymatic machinery, which includes laccases, manganese peroxidases, and lignin peroxidases. White rot fungi are positioned to play a major role in realising a more sustainable and environmentally friendly use of lignocellulosic biomass as studies into their intricate properties progress and technology improvements allow for their optimum.
4. LCB degradation - role of fungal lignocellulolytic enzymes
The effective decomposition of lignocellulosic biomass is essential for several industrial processes, such as waste management, the pulp and paper industries, and the production of biofuels. To break down these complex compounds, fungi release ligamyl-CoA-lyase. Enzymes that degrade cellulose, hemicellulose, and lignin are especially significant because they may be used in a wide range of biotechnological procedures. It is the unique and potent enzyme system of fungi that allows them to decompose lignocellulosic material. There are ligninolytic enzymes in their enzymatic system that depolymerise lignin in addition to cellulases and hemicellulases, which break down the components of polysaccharides. The key enzymes involved in the breakdown of LCB elements are discussed in the section that follows, along with their degradative processes.
4.1. Lignin degradation
Plant cell walls are made stiff by the complex and heterogeneous aromatic polymer lignin, which also acts as a key inhibitor of biomass decomposition. To break down lignin, fungi, particularly white-rot fungi like Phanerochaete chrysosporium, have developed an amazing variety of ligninolytic enzymes. The primary enzymes involved in the breakdown of lignin are laccases, manganese peroxidases, and lignin peroxidases. Lignin peroxidases and manganese peroxidases generate highly reactive radicals that can break down lignin into smaller fragments. Laccases, on the other hand, oxidise phenolic compounds in lignin, facilitating its breakdown. In addition to providing access to the embedded cellulose and hemicellulose, the breakdown of lignin is essential for yielding high-value products including aromatic chemicals and biofuels. Peroxidase and laccase are the two sets of enzymes that break down lignin in an oxidative process (Chukwuma et al. 2020; Huang et al. 2024). Peroxidases are important because they help oxidise lignin at the start of the breakdown process. Furthermore, they oxidise additional phenolic compounds using hydrogen peroxide (H2O2) and a mediator. The peroxidases use H2O2 as a co-substratum and are primarily haem proteins with an extraordinary ability to interact with a diverse spectrum of substrates, including organic and inorganic molecules (Lopes et al. 2018). The white rot fungus Phanerochaete chrysosporium is thought to be a model lignin-degrading organism because of its rapid development. In contrast to laccase and volatile peroxidase (VP), the fungus is known to generate manganese peroxidase (MnP) and lignin peroxidase (LiP) (Martinez et al. 2004). Research on P. chrysosporium‘s delignification of lignocellulosic biomass is reported in several publications. Pretreatment of Water Hyacinth with P. chrysosporium led to 42.44% delignification after 16 days of incubation, according to Sari et al. (2015). Pleurotus ostreatus and P. chrysosporium formed a fungal consortium formulated by Kaur et al. (2019) for the rice straw, and under shaking circumstances, they reported 80.9% delignification. According to Wittner et al. (2021), P. chrysosporium MUCL 19343 delignified poplar wood by 24.2%. According to Dao et al. (2023), Trametes versicolor and P. chrysosporium use a solid-state fermentation process to break down the lignin in camelina straw and switchgrass. According to Huang et al. (2024), ligninolytic enzymes may also be produced by marine fungi such Cladosporium cladosporioides CBMAI 857, Aspergillus sclerotiorum CBMAI 849, and Mucor racemosus CBMAI 847. According to Figure 2b, lignin may be broken down by peroxidases such as LiP, MnP, VP, and DyP. Plants and microbes include peroxidases such as LiP, MnP, and VP. During secondary metabolism, the white-rot fungus produces them.
LiP: These were identified in 1983 in the fungus P. chrysosporium and have an active role in the lignin breakdown process (Niladevi 2009). The catalytic activity of LiP is dependent upon H2O2. Lignin depolymerisation is a multistep process that is mediated by enzymes. These include coupling reactions of aromatic compounds, side-chain cleavage, single-bond cleavage, demethylation, and aromatic ring opening. For lignin to depolymerise, a mediator is not necessarily due to its high redox potential. Lignin peroxidases are frequently evaluated by measuring the oxidation of a lignin monomer known as veratryl alcohol to veratraldehyde (Kumar and Chandra 2020; Huang et al. 2024) including Phanerochaete sordida, P. chrysosporium, and Trametes versicolor as examples of fungi that produce LiP.
MnP: They were found by Kuwahara in batch cultures of P. chrysosporium in 1984. These glycoproteins, which include haem, oxidise Mn2+ to Mn3+, which oxidises a lot of other phenolic compounds. MnP’s catalytic cycle is similar to LiP’s, but unlike LiP, it is unable to oxidise non-phenolic molecules that are resistant to oxidation. The creation of an iron-peroxide complex from natural ferric acid and H2O2 starts the catalytic cycle. When Mn3+ is fixed as a chelating agent, its function is to liberate active sites such as oxalate and acetate. Some MnP-producing fungal species are P. chrysosporium, Phlebia radiata, Ceriporiopsis subvermispora, Dichomeris squalens, and P. sordida, etc. (Kocher et al. 2018; Chukwuma et al. 2020; Kumar and Chandra 2020).
VP: They can catalyse MnP and LiP reactions, they are often referred to as hybrid peroxidase or polyvalent peroxidase. They can oxidise nonphenolic substances with a high redox potential, such as LiP, and Mn2+, like MnP. In the absence of manganese, the VP enzyme is capable of oxidising phenolic, nonphenolic, and lignin derivatives without the need for a mediator. Regarding their catalytic mechanism, the VPs are more adaptable to Mn3+-mediated or Mn-independent reactions on aromatic substrates with low or high redox potential (Weng et al. 2021). Examples of VP-producing fungi are Bjerkandera adusta, Pleurotus eryngii, and Pleurotus ostreatus (Ravichandran and Sridhar 2016; Hernández-Bueno et al. 2021).
Laccase: These oxidases, which include copper, use molecular oxygen as an oxidant to convert phenolic rings into phenoxy radicals. They are usually inducible enzymes with a broad range of phenols, heterocyclic compounds, and aromatic amines that they may oxidise. In phenolic β-1 and β-O-4 lignin model dimers, they can catalyse the rupture of the Cα–Cβ bond by oxidising the Cα carbon and splitting the aryl-alkyl link. It cannot directly oxidise nonphenolic lignin units due to their comparatively low redox potential. On the other hand, substantial delignification and oxidation of big molecules are possible with oxidation mediator compositions. Their potential uses include the pulp, paper, and bioremediation industries, as well as the generation of biofuel (Chukwuma et al. 2020). Some of the laccase laccase-producing fungi are Dichomitus squalens, Cerrena maxima, Pleurotus ostreatus, and Irpex lacteus, etc. (Kumar and Chandra 2020; Huang et al. 2024).
DyP: These are haem peroxidases that oxidise different compounds with hydrogen peroxide. The mechanism begins with the ferric enzyme [Fe3+] resting. When it interacts with hydrogen peroxide, it produces the high-valence molecule I [Fe4+ = O]+. Compound II can then be formed by this molecule interacting with an electron from a reducing substrate. Compound II can be reduced with an extra substrate molecule to return to its resting state (Chen et al. 2015; Kocher et al. 2018; Chukwuma et al. 2020). Some examples of DyP fungal producers are Thermomonospora curvata, Bjerkandera adusta, Auricularia auricula-judae, and Pleurotus sapidus, etc. (Chen et al. 2015; Xu et al. 2021).
4.2. Cellulose degradation
Cellulose, a linear polymer of glucose, forms the major component of plant cell walls. The enzymatic degradation of cellulose is a complex process that involves the synergistic action of multiple enzymes. Fungi, particularly filamentous fungi like Aspergillus, Trichoderma, and Phanerochaete, produce a variety of cellulases that act in concert to break down cellulose into soluble sugars. These cellulases include endo-β-1,4-glucanases, exo-β-1,4-glucanases, and β-glucosidases. Exoglucanases break down cellulose strands from the ends, whereas endoglucanases randomly break connections within the chains. Endo-β-1,4-glucanases cleave internal β-1,4-glycosidic bonds in cellulose chains, generating smaller fragments. Exo-β-1,4-glucanases then act on the ends of the cellulose chains, releasing cellobiose or glucose. β-Glucosidases further hydrolyse cellobiose into glucose (Lyagin et al. 2023). The synergy between these enzymes facilitates efficient cellulose degradation. Literature reports various cellulolytic fungi such as Aspergillus sp., Penicillium canescens, P. chrysosporium, Sclerotium rolfsii, Penicillium sp., Schizophyllum sp., Trichoderma reesei, and Trichoderma sp. (Andlar et al. 2018; Ilić et al. 2023).
4.3. Hemicellulose degradation
The cellulose fibres in the plant cell wall are surrounded by hemicellulose, a heterogeneous polymer made of several sugar monomers. Hemicellulases are a broad group of enzymes secreted by fungi that break down hemicellulose into its component sugars. Xylanases, mannanases, and xyloglucanases are examples of hemicellulases produced by fungi. Xylanases, for instance, hydrolyse the β-1,4-glycosidic linkages in xylan, a major component of hemicellulose, releasing xylose. The concerted action of hemicellulases contributes to the complete breakdown of hemicellulose into simpler sugars that can be further utilised by the fungus or other microorganisms in the ecosystem. These enzymes are implicated in the degradation of hemicelluloses such as arabans, galactans, mannans, and xylans. The main catalytic module consists of glycoside hydrolases for the hydrolysis of glycosidic bonds. The other module consists of carbohydrate esterase for the hydrolysis of ester linkages of acetate or ferulic acid side groups. Mannanases break down β-1,4-glycosidic bonds. These enzymes effectively hydrolyse glucomannan and galactomannan chains. Fungal endomannanases are widely used for the enzymatic hydrolysis of steam-pretreated softwood (Malgas and Pletschke 2020; Eugenio et al. 2023). Esterases are accessorial enzymes that support other enzymes to efficiently hydrolyse wood xylans and mannans, e.g. cetylxylan esterase, feruloyl esterase, and p‐coumaroyl esterase.
4.4. Pectin degradation
Pectin is a complex polysaccharide abundantly present in the primary cell walls of both dicotyledonous and monocotyledonous plants. It is primarily composed of four subclasses: Homogalacturonan (HG), rhamnogalacturonan (RG-I), RG-II, and xylogalacturonan (Mohnen 2008). Pectins are mostly soluble in water and break down more readily than other components of walls. Fruit and vegetable waste residues are rich in pectins, which could be utilised as feedstocks to produce ethanol. Pectinases are essential for pectin degradation and are majorly obtained from the fungal species (Schimpf et al. 2013). Pectinases belong to the hydrolase group and are further classified as protopectinase, pectin methylesterase, and polygalacturonase. Some of pectin lyase fungal species are Aspergillus niger, Aspergillus giganteus, Aspergillus sojae, Aspergillus tubingensis, Penicillium griseoroseum, and Botrytis cinerea etc. (Maran and Prakash 2015; Wong et al. 2017; Gunjal et al. 2020; Zeuner et al. 2020).
The breakdown of lignocellulosic biomass is essential for many industrial uses as well as environmental processes, and it is mostly facilitated by fungus-derived lignocellulolytic enzymes. The complex interactions between ligninolytic enzymes, cellulases, and hemicellulases that are generated by fungi demonstrate how adaptable these microbes are to a wide range of biological niches. The secret to creating environmentally sound solutions for waste management, bioenergy generation, and the use of renewable resources is to fully use the potential of these enzymes. Undoubtedly, more investigation into this area will aid in the creation of innovative biotechnological procedures that use the enzymatic capabilities of fungi in the breakdown of lignocellulosic biomass.
5. Valorization of lignocellulosic waste to valuable products through fungal technology
The constraints posed by increased waste output and a finite supply of natural resources have led to a growing understanding in the 21st century of the need for eco-friendly and sustainable solutions. Lignocellulosic waste, comprised of agricultural residues, forestry by-products, and municipal solid waste, has emerged as a rich yet underutilised resource (Adewuyi 2022). Utilising fungi’s special qualities to increase the value of lignocellulosic waste offers a viable way to turn it into useful goods with major positive effects on the environment and the economy (Meyer et al. 2020). At the heart of fungal-mediated lignocellulosic waste valorisation lies the remarkable enzymatic machinery possessed by fungi (Andlar et al. 2018). These microbes, which include cellulolytic species and white-rot fungi, release a series of enzymes called ligninases, hemicellulases, and cellulases that combine to degrade the intricate structure of lignocellulose (Ferdeș et al. 2020; Corbu et al. 2023; Nargotra et al. 2023). This enzymatic breakdown is an essential phase because it liberates the building blocks required to produce platform chemicals, biofuels, and other useful products later on. One of the most prominent applications of fungal-mediated lignocellulosic waste valorisation is the production of biofuels. Fungi, especially yeast and filamentous fungi, possess the ability to ferment the sugars derived from cellulose and hemicellulose into bioethanol and biobutanol (Verardi et al. 2020; Nongthombam et al. 2022). By lowering the greenhouse gas emissions linked to conventional fuel sources, this technique not only offers a sustainable substitute for fossil fuels but also helps to mitigate climate change. Beyond biofuels, fungal-mediated processes generate platform chemicals that serve as crucial building blocks for a diverse range of bioproducts (Meyer et al. 2020). These consist of organic acids, alcohols, and other important substances that are used in the manufacturing of specialised chemicals, medicines, and bioplastics. The adaptability of fungal enzymatic systems allows for tailoring product profiles based on the specific composition of lignocellulosic substrates, opening avenues for a wide array of industrial applications (Gupta et al. 2016). Fungi, particularly white-rot fungi, play a vital role in bioremediation efforts by efficiently degrading lignin – a challenging component of lignocellulosic waste. This unique capability not only facilitates waste valorisation but also enables the fungi to detoxify pollutants, offering a dual benefit for sustainable waste management (Latif et al. 2023). Integrating fungal-mediated processes into environmental clean-up strategies aligns with the principles of a circular economy, where waste is transformed into a valuable resource. The fungal-mediated valorisation of lignocellulosic waste aligns with the core tenets of the circular economy by promoting efficient resource utilisation and reducing dependence on finite raw materials (Wikandari et al. 2022; Blasi et al. 2023). Fungal-mediated lignocellulosic waste valorisation represents a transformative approach to converting abundant waste materials into valuable products, offering solutions to both environmental challenges and the pressing need for sustainable resource utilisation. Fungal-mediated processes have the potential to significantly influence the development of a more resource-efficient and sustainable future as long as technology and research in this area continue to progress. We are getting closer to a circular economy where trash is valued as a resource for making necessities, and this is a big step towards a more resilient and sustainable society, thanks to the discovery of fungi’s potential in waste valorisation. Table 2 illustrates how fungi contribute to the conversion of lignocellulosic waste into a variety of products with added value.
Table 2.
Valorisation of lignocellulosic feedstocks by various fungal species.
| Fungal species | Lignocellulosic feedstock | Value-added product/significance | Reference |
|---|---|---|---|
| Penicillium aurantiogriseum | Corn stover | Biogas | Kovács et al. 2022 |
| Phanerochaete chrysosporium | Apple pomace | Phenolic antioxidants | Ajila et al. 2011 |
| Trametes versicolor | Barley husk | Laccase | Tišma et al. 2020 |
| Microbial consortia [white rot fungi + bacteria (28%)], Phlebia sp. MG-60-P2 + C. saccharoperbutylacetonicum | Hardwood Kraft pulp | Butanol | Tri and Kamei 2020 |
| Actinomucor elegans, Umbelopsis isabellina | Grape pomace | γ-linolenic acid and carotenoids | Dulf et al. 2020 |
| Aspergillus niger ITV02 | Wheat straw | Glucose yield 24.58 ± 0.08 g/L with a conversion rate of 40.2 ± 0.14%; xylose 8.32 ± 0.02 g/L with a conversion rate of 77.54 ± 0.2% | Infanzón-Rodríguez et al. 2022 |
| Pleurotus ostreatus | Apple bagasse, agave mezcalero bagasse | Phenolic compounds, flavonoids, triterpenes | Ibarra-Cantún et al. 2020 |
| Rhizopus oligosporus | Apricot pomace | Neochlorogenic and chlorogenic acids, rutin, quercetin-3 (6“acetyl-glucoside) | Dulf et al. 2017 |
| Trichoderma asperellum BPLMBT1 | Sweet sorghum stover | Lignin removal of 76.93%; biohydrogen production 402.01 mL | Shanmugam et al. 2018 |
| Bjerkandera adusta | Wheat bran | Carboxymethyl cellulase, manganese peroxidase, laccase, xylanase | Elisashvili et al. 2009 |
| Aspergillus niger | Plum fruit by-products | Higher lipid recovery, isoquercitrin | Dulf et al. 2016 |
| T. hirsute, P. ostreatus, P. chrysosporium, T. versicolor, F. fomentarius | Tobacco stalk, organic fraction of municipal solid waste | Compost | Voběrková et al. 2017; Yang et al. 2020 |
| Aspergillus niger, Rhizopus oligosporus | Chokeberry pomace | Cinnamic acid, flavonols | Dulf et al. 2018 |
| Pycnoporus sanguineus MCA 16 | Sugarcane bagasse | Glucose yield, 7.32 g/L; total phenolic reduction, 82.3% | Pereira Scarpa et al. 2019 |
| Marasmiellus palmivorus VE111, Penicillium echinulatum S1M29 | Eucalyptus globulus wood | 31% decrease in the lignin content; 10% increase in the glucose yield; 15% increase in the xylose yield | Schneider et al. 2020 |
| C. cinereus, C. subvermispora, P. ostreatus | Poplar bark sawdust | Phenols and alkylphenols | Xie et al. 2020 |
| Thermomyces lanuginosus | Hull-less pumpkin oil pomace | Lipase | Šibalic et al. 2020 |
| Trametes hirsuta F13 | Beechwood sawdust | 63.58 ± 1.47 mg/mL fermentable sugar from 18 days treated substrate | Jović et al. 2022 |
| Clostridium acetobutylicum ATCC 824 | Corn stover | Biobutanol | Luo et al. 2023 |
| Trametes sp. W-4 | Barley straw | Biogas | Jin and Wei 2023 |
6. Lignocellulosic biomass-based circular bioeconomy concept
Human society has relied significantly on finite fossil fuels, posing economic, societal, and environmental concerns. Renewable energy must replace fossil fuels, and new non-petroleum-based material systems must be established. The linear economic paradigm is unsustainable, contributing to resource depletion, waste generation, and pollution (Pandey et al. 2021).
A shift to a circular economy is recommended as a potential solution to achieve sustainable development that aims to balance resource consumption, economic growth, and social equality. The circular bioeconomy that works on the concept of prevention, reuse, recycling, and recovery, emphasises the effective use of biological resources, exploiting the function of microorganisms in waste valorisation (Branduardi 2021). Within the framework of the integrated biorefinery plan, the waste resources may be valued and converted into a range of goods. Currently, only 9% of the global economy is circular (Yadav et al. 2021).
Due to its widespread availability and numerous uses in a variety of industries, such as the food, chemical, and energy sectors, lignocell biorefinery technology is gaining traction among researchers and industry participants. Fractionating lignin, cellulose, and hemicellulose can yield a variety of compounds with additional value. Life cycle analysis and economic evaluation come next, and integration and optimised technologies are needed for this. Using integrated biorefinery processes, a single source material might create many end products, including biopolymers, acids, and pigments (Lee et al. 2019). The lignocellulosic biomass (LCB) contains a cellulosic component that can yield ethanol, sorbitol, laevulinic acid (LA), succinic acid (SA), levoglucosan, hydroxyacetaldehyde, and other compounds; the hemicelluloses can yield lactic acid, xylitol, xylo-oligosaccharides, furfural, hydroxymethylfurfural (HMF), and polyhydroxyalkanoates; and the lignin component can primarily be used as a fuel for boilers that produce pulp and as a substitute for petroleum-based products in the production of industrial coatings, gels, and emulsifiers (Devi et al. 2022). However, the current processing and product development strategies need good investments from the commercialisation viewpoint. As LCB is complex and recalcitrant an efficient pretreatment approach is essential for maximum recovery of products as well as mass balances of cellulose, hemicellulose, and lignin. Additionally, cost-intensive technology for the valorisation of LCB is integral so that the generated products compete well in the market (Banu et al. 2021b).
The kingdom fungi, which is diverse and rich in valuable enzymes, has traditionally contributed to different industries such as food and beverage. Fungi are essential in ecological systems because they live in soil and contribute to nutrient recycling, which is critical for ecosystems. As seen by the tenfold increase in fungal biotechnology literature since the 1990s, fungi are extremely significant from the perspective of biotechnological applications (Wikandari et al. 2022; Kržišnik and Gonçalves 2023; Roth et al. 2023). A variety of industries, industrial and pharmaceutical in particular benefit from fungi due to their genetic plasticity and ability to survive in various conditions. In especially, mycelial fungi not only yield durable supplies of food, fuel, chemicals, textiles, and materials for the construction, automotive, and transportation sectors, but they also offer ecologically beneficial friendly alternatives to leather, textiles, and plastics. Consequently, the seamless transition from a petroleum-based economy to a bio-based circular economy may be greatly aided by fungal biotechnology (Troiano et al. 2020; Molelekoa et al. 2021; Chai et al. 2022). Traditionally, ethanol was produced from simple sugars found in maize and sugarcane. However, advances in yeast engineering allow for direct starch fermentation. Engineered yeast strains that incorporate genes from both fungi and bacteria can help in sustainable ethanol synthesis (Roth et al. 2023). Trichoderma reesei and Thermus thermophilus are known to produce cellulases and hemicellulases in appreciable quantities which are required for converting agricultural and forestry byproducts such as lignocellulose into biofuel. The thermostable enzymes of T. thermophilus improve lignocellulose breakdown in biorefineries and by genetic reprogramming, it can generate valuable sustainable alternatives to petroleum-based methods. Other uses for this genetic flexibility include the synthesis of synthetic resins, biodegradable polymers, and more. Enzymes such as ligninases, which degrade lignin and other resistant organic compounds, are produced by Pleurotus ostreatus and Phanerodontia chrysosporium and are important in the cleanup of contaminated soil and water (Huang et al. 2006; Purnomo et al. 2010). Oleaginous fungi that produce lipids, such as Aspergillus fumigatus, Umbelopsis isabelline, Thermothelomyces thermophilus, and Thermothielavioides terrestris, have been researched and utilised to make biofuels (Ma et al. 2018; Asci et al. 2020; Satti and Shah 2020). These fungi can grow on a variety of carbon sources, including organic waste or low-grade biomass. A competitive route for the manufacture of bio-oil may be established by combining a vigorous oleaginous yeast, Cryptococcus sp., with an inexpensive feedstock, such as banana peel (Han et al. 2019). A. oryzae, F. venenatum, M. purpureus, and N. intermedia are among the ascomycetes that have demonstrated great potential as biocatalysts for converting a variety of waste materials into useful products. Their capacity to generate lignocellulose-breaking enzymes makes them excellent options for low-cost bioprocesses, especially those that produce ethanol, organic acids, and enzymes. The protein and lipid-rich biomass of fungi hold the potential for expanding biorefineries and improving the existing ones (Ferreira et al. 2016). These industrially relevant fungi demonstrate how genetic innovations can promote a circular economy, highlighting their critical role in sustainable industry operations (Ilica et al. 2019; Meyer et al. 2020). However, a lack of molecular understanding and scaling-up are the bottlenecks that need immediate addressal. The integration of fungal-mediated lignocellulosic biomass valorisation into circular bioeconomy models opens up exciting opportunities. Beyond biofuel production, the generation of bio-based materials, chemicals, and pharmaceuticals presents avenues for diversifying product portfolios. The adaptability of fungi to diverse biomass sources and environmental conditions provides flexibility in application. Moreover, advancements in synthetic biology and genetic engineering offer prospects for tailoring fungi to enhance their enzymatic capabilities and broaden their applicability in circular bioeconomy initiatives. Fungi-mediated lignocellulosic biomass valorisation holds great promise within the context of circular bioeconomy, aligning with the principles of sustainable resource utilisation and waste reduction. As research continues to unravel the complexities of fungal enzymatic systems and technologies evolve, the integration of fungi into circular bioeconomy models is likely to become more efficient and economically viable. Realising the full potential of fungus in converting lignocellulosic biomass into valuable resources and assisting in the creation of a more sustainable and circular bioeconomy will require overcoming obstacles and seizing opportunities.
7. Challenges, limitations, and possible solutions for efficient LCB valorisation
Lignocellulosic waste is a huge and underutilised resource that is made up of plant-derived materials such as forestry byproducts, municipal solid refuse, and agricultural residues. The use of fungal-mediated processes to value lignocellulosic waste has garnered significant interest as a sustainable and eco-friendly method. Thanks to their special enzymatic machinery, fungi can efficiently convert complicated lignocellulosic structures into useful compounds. Nevertheless, there are several difficulties and restrictions related to fungal-mediated lignocellulosic waste valorisation, even with its encouraging promise.
7.1. Substrate diversity and complexity
Lignocellulosic waste is a heterogeneous mixture of cellulose, hemicellulose, and lignin. Each component has a distinct structure and requires specific enzymatic activities for efficient degradation. Different sources of lignocellulosic biomass exhibit considerable variation in their chemical composition and physical properties. Agricultural residues may differ from forestry residues in terms of cellulose content, lignin structure, and the presence of inhibitory compounds. The diversity and complexity of lignocellulosic substrates often require tailored fungal strains and optimisation of culture conditions, leading to increased research and development costs. Further, the process of lignocellulose hydrolysis often releases inhibitory compounds, such as furfural and acetic acid, which can negatively impact fungal growth and enzyme activity. Adapting fungi to tolerate and metabolise these inhibitors is essential for maintaining process efficiency. Overcoming the challenges associated with inhibitory compounds is a crucial aspect of fungal-mediated valorisation. Fungal strains exhibit unique responses to lignocellulosic substrates, further complicating the challenge. While some strains may excel in degrading certain types of biomass, their performance can vary when faced with a different substrate. Tailoring fungal strains for optimal performance across a spectrum of lignocellulosic feedstocks requires a deep understanding of strain-specific responses.
7.2. Enzyme production and regulation
Lignocellulolytic enzymes produced by fungi play a pivotal role in the efficient degradation of lignocellulosic biomass, offering tremendous potential for sustainable bio-based industries. However, despite significant strides in the field, the production and optimisation of these enzymes face challenges that impede their widespread industrial application. One of the primary challenges lies in the diverse and complex nature of lignocellulosic substrates. Fungi encounter various lignin, cellulose, and hemicellulose structures, requiring a sophisticated array of enzymes for effective degradation. The challenge is to identify and tailor fungal strains capable of producing the right combination of enzymes suitable for different types of biomass. Despite the inherent enzymatic prowess of fungi, achieving high enzyme yields at an economically viable scale remains a significant hurdle. Fungal cultures often produce enzymes at levels that fall short of the demands of large-scale industrial applications. Enhancing enzyme productivity and optimising fermentation conditions are essential for overcoming this limitation. Fungi are sensitive to carbon catabolite repression, a regulatory mechanism that prioritises the utilisation of easily metabolisable carbon sources over complex substrates like lignocellulose. Overcoming this repression and ensuring efficient enzyme production on lignocellulosic feedstocks requires a nuanced understanding of the underlying metabolic pathways. The choice of inducers and their concentration significantly influences lignocellulolytic enzyme production. Identifying suitable inducers for specific enzymes and optimising their concentration is a delicate balancing act. Achieving an optimal induction strategy is crucial for maximising enzyme yields while minimising production costs. Enzyme recovery and purification during downstream processing present additional challenges. The heterogeneity of fungal cultures and the presence of other cellular components can complicate purification processes, affecting enzyme purity and overall process efficiency. Developing cost-effective and scalable purification methods is crucial for the commercial viability of lignocellulolytic enzymes. Bridging the gap between laboratory-scale success and large-scale implementation requires continuous innovation and technology transfer. Collaborations between academia, industry, and research institutions play a pivotal role in advancing fungal technology, optimising processes, and overcoming challenges associated with scale-up. Furthermore, the economic viability of fungal-mediated lignocellulosic waste valorisation processes is also linked to regulatory frameworks and market dynamics. Government incentives, subsidies, and policy support for sustainable practices can significantly impact the economic feasibility of such ventures.
7.3. Lignin degradation
Lignin, a complex and irregular polymer, poses a formidable barrier to efficiently valorise lignocellulosic waste. It forms a protective sheath around cellulose and hemicellulose, rendering these polysaccharides less susceptible to enzymatic degradation. This innate defence mechanism evolved by plants to provide structural integrity poses a significant hurdle for fungi trying to break down these biomass components. The challenge further intensifies due to the diversity in lignin structure among different plant species and even within the same plant. The presence of various linkages, branching patterns, and monomeric compositions contribute to the heterogeneity of lignin. Fungi engaged in waste valorisation must adapt to this diversity, necessitating a tailored enzymatic action capable of addressing the unique attributes of different lignin structures. Fungi employ ligninolytic enzymes, such as peroxidases, laccases, and other oxidoreductases, to initiate lignin degradation. However, the intricate nature of lignin necessitates a coordinated action of multiple enzymes. Achieving the right balance and synergy among these ligninolytic enzymes is a challenging task, and developing fungal strains capable of producing an effective lignin-degrading enzyme cocktail is pivotal for successful waste valorisation. The recalcitrance of lignin may have a cascading effect on the overall efficiency of fungal-mediated lignocellulosic waste valorisation processes. Incomplete lignin breakdown can hinder access to cellulose and hemicellulose, reducing the efficiency of subsequent hydrolysis steps. This bottleneck hampers the release of fermentable sugars and limits the production of valuable bio-based products, such as biofuels and biochemicals. Overcoming lignin recalcitrance requires innovative approaches, such as genetic engineering and metabolic pathway optimisation. Tailoring fungal strains to express enhanced ligninolytic enzymes or introducing pathways for more efficient lignin degradation are some of the avenues that are being explored to address the challenge.
7.4. Fungal strain selection and genetic modification
Identifying and selecting suitable fungal strains for specific lignocellulosic waste streams is a critical step in the valorisation process. The fungal kingdom boasts an immense diversity of species, each with its unique characteristics. Selecting the right fungal strain for lignocellulosic waste valorisation requires an understanding of the diverse responses exhibited by different strains. The strain that works effectively for one substrate may not translate to success with another. The challenge lies in navigating this biodiversity to identify strains that exhibit robust lignocellulolytic activities across various waste materials. Fungal strains secrete an array of lignocellulolytic enzymes, each playing a specific role in biomass degradation. Determining the enzymatic requirements for efficient lignocellulosic waste conversion poses a significant challenge. It requires a deep understanding of the specific enzymes needed to break down cellulose, hemicellulose, and lignin in a particular substrate. Strain selection must align with the enzymatic profile necessary for the targeted waste valorisation process. Genetic modification offers a powerful tool for tailoring fungal strains to specific lignocellulosic waste valorisation processes. However, ethical considerations, public perception, and regulatory frameworks surrounding genetically modified organisms pose challenges. Striking a balance between the potential benefits of genetic modification and societal acceptance is vital for the successful deployment of engineered fungal strains. Researchers must consider the potential impacts of their work on the environment, ecosystems, and society, emphasising ethical conduct throughout the entire research process. Open communication and transparency are immensely important in addressing ethical considerations. Researchers engaging in genetic modification should provide clear explanations of their intentions, methodologies, and potential outcomes. Accountability for the ethical implications of their work will foster trust among stakeholders. When genetic modification involves the release of modified strains into the environment, obtaining informed consent from affected communities and engaging the public in decision-making processes become ethical imperatives. Public participation ensures that diverse perspectives are considered, promoting inclusivity and ethical governance. The release of genetically modified fungal strains into the environment raises concerns about unintended ecological consequences. Altered fungal strains may interact with native species, potentially leading to ecological imbalances. Implementing robust containment protocols is essential to prevent the unintended release of modified fungal strains into the environment. This includes controlled laboratory environments, physical barriers, and stringent biosecurity measures to minimise the risk of accidental spread. Modified fungal strains may exhibit unintended effects on non-target organisms. Assessing the potential impact on beneficial microbes, plants, and other organisms in the ecosystem is crucial. Conducting thorough environmental risk assessments is a critical step in understanding and mitigating potential ecological risks. These assessments should evaluate the impact on biodiversity, ecosystem functions, and the persistence of modified traits in the environment. Employing precise gene editing techniques may reduce the likelihood of unintended alterations and minimise the risk of undesirable genetic changes in modified strains. Establishing continuous monitoring and surveillance programmes can allow the researchers to detect any unexpected effects and implement corrective measures promptly. Ethical oversight by institutional review boards and compliance with regulatory frameworks are crucial elements in ensuring responsible research practices. Researchers should adhere to ethical guidelines, obtain necessary approvals, and comply with relevant regulations governing genetic modification. Economic considerations play a pivotal role in the successful implementation of lignocellulosic waste valorisation processes. High-performing strains generated through genetic modification may come with increased production costs. Balancing the enhanced performance with economic viability requires optimising fermentation conditions, substrate utilisation, and downstream processes.
7.5. Scale-up and economic viability
While fungal-mediated lignocellulosic waste valorisation processes have shown promise at the laboratory scale, scaling up these processes for industrial applications presents significant challenges. Scaling up fungal-mediated lignocellulosic waste valorisation requires meticulous optimisation to ensure process efficiency. Factors such as mass transfer limitations, substrate homogeneity, and reactor design become critical considerations. Ensuring a seamless transition from small-scale experiments to industrial processes requires understanding and addressing these efficiency challenges. Identification and adaption of fungal strains for large-scale operations is a key determinant of success. Fungi performing efficiently in laboratory settings may exhibit different behaviours under industrial conditions. Selecting strains with robust lignocellulolytic capabilities, high productivity, and resilience to varying environmental conditions is crucial for achieving consistent results at scale. The economic viability of fungal-mediated processes heavily relies on the availability and cost-effectiveness of lignocellulosic feedstocks. Ensuring a sustainable and reliable supply chain for waste biomass is essential. Moreover, exploring alternative feedstocks and waste streams can contribute to cost reduction and enhance the overall economic feasibility of the valorisation process. The production of lignocellulolytic enzymes, a key component of fungal-mediated valorisation, contributes significantly to overall costs. Optimising fermentation conditions, improving fungal strains for enhanced enzyme productivity, and exploring novel enzyme production techniques are crucial for mitigating these costs and enhancing the economic viability of the process. Efficient downstream processing is immensely important in maximising product yields and minimising costs. Developing scalable and cost-effective methods for separating and purifying the desired bio-based products, such as biofuels or biochemicals, is essential. Streamlining these processes can contribute to the overall economic feasibility of fungal-mediated lignocellulosic waste valorisation. Bridging the gap between laboratory-scale success and large-scale implementation requires continuous innovation and technology transfer. Collaborations between academia, industry, and research institutions play a pivotal role in advancing fungal technology, optimising processes, and overcoming challenges associated with scale-up. The high capital and operational costs associated with large-scale fungal-based processes currently hinder widespread implementation.
7.6. Product inhibition and toxic by-products
During lignocellulosic degradation, fungi produce various by-products, some of which can be inhibitory to their own growth and enzyme activity. Product inhibition occurs when the accumulation of end-products, such as biofuels or biochemicals, hinders the enzymatic activity of fungi. This can reduce the overall efficiency of lignocellulosic waste conversion. One possible solution is implementing continuous fermentation strategies instead of batch processes can help alleviate product inhibition. In continuous systems, product concentrations are kept at lower levels, allowing fungi to maintain higher metabolic rates and sustained enzymatic activity. Employing techniques for in-situ or ex-situ product removal can mitigate inhibition. Techniques like membrane separation or adsorption can selectively remove end-products, preventing their accumulation and maintaining an environment conducive to fungal activity. Genetic modification of fungal strains to enhance their tolerance to specific end-products is a promising avenue. By engineering strains to withstand higher concentrations of inhibitory compounds, product inhibition can be mitigated, leading to more efficient lignocellulosic waste valorisation. Toxic by-products, such as furfural and acetic acid, can accumulate during lignocellulosic waste degradation, impacting fungal growth and enzymatic activity. Optimising pretreatment and hydrolysis conditions can minimise the formation of toxic by-products. Modulating parameters such as temperature and pH during pretreatment can influence the types and concentrations of by-products generated. Implementing in-situ detoxification strategies involves introducing specific microorganisms or enzymes capable of metabolising toxic by-products during the lignocellulosic waste valorisation process. This approach reduces the overall accumulation of harmful compounds. Fine-tuning bioprocess parameters, such as aeration and agitation rates, can enhance fungal resistance to toxic by-products. These adjustments contribute to improved strain performance and ensure a more favourable environment for lignocellulosic waste conversion. Identifying or developing fungal strains with inherent tolerance to toxic by-products is a proactive approach. Screening for strains that exhibit resilience to specific inhibitory compounds ensures a more robust and efficient valorisation process. Leveraging multi-omics technologies, including genomics, transcriptomics, and metabolomics, provides a comprehensive understanding of the molecular mechanisms underlying product inhibition and by-product accumulation. This knowledge can guide targeted strategies for mitigation. Adopting integrated biorefinery concepts can help in diversifying the product portfolio to include multiple value-added outputs. By redirecting metabolic pathways, the impact of product inhibition on specific end-products can be reduced, allowing for a more versatile and sustainable valorisation process.
8. Policy framework
Fungal-mediated lignocellulosic waste valorisation, through processes like enzymatic hydrolysis and fungal fermentation, offers a promising solution to convert this waste into valuable products. However, the successful implementation of fungal-mediated waste valorisation requires a robust policy framework to support research, development, and commercialisation. This section explores the key components of a policy framework for fungal-mediated lignocellulosic waste valorisation.
8.1. Research and development incentives
To promote fungal-mediated waste valorisation, governments, and relevant agencies should provide incentives for research and development. This can include grants, tax incentives, and funding for research projects. Supporting scientific research will help advance fungal biotechnology and improve the efficiency of lignocellulosic waste conversion processes.
8.2. Regulation and standards
Governments should establish clear regulations and standards for fungal-mediated lignocellulosic waste valorisation. These regulations can cover environmental, safety, and quality control aspects. Ensuring the safety of workers and the environmental impact of the processes is crucial. Standards can also help maintain product quality and consistency.
8.3. Financial incentives and subsidies
Financial incentives and subsidies can help drive the adoption of fungal-mediated lignocellulosic waste valorisation technologies by industries. These incentives may include feed-in tariffs for bioenergy production, subsidies for the installation of biorefineries, and preferential pricing for lignocellulosic feedstocks. By reducing the financial barriers to entry, these measures encourage businesses to invest in waste valorisation technologies.
8.4. Education and workforce development
A skilled workforce is essential for the successful implementation of fungal-mediated lignocellulosic waste valorisation. Governments can support education and workforce development programmes that focus on fungal biotechnology, bioprocess engineering, and related fields. This will ensure a steady supply of qualified professionals to drive innovation and growth in the industry.
8.5. Public awareness and outreach
Raising public awareness about the environmental and economic benefits of fungal-mediated waste valorisation is crucial. Governments can support public outreach campaigns to educate citizens and businesses about the potential of these technologies. Encouraging the separation of lignocellulosic waste at the source and promoting the use of fungal-mediated valorisation can contribute to a more sustainable waste management system.
8.6. Intellectual property protection
Intellectual property protection is essential to incentivise innovation and investment in fungal-mediated lignocellulosic waste valorisation. Governments should establish clear guidelines and procedures for patenting and protecting intellectual property related to these technologies. This will encourage private sector participation and investment in research and development.
8.7. International collaboration
Collaboration on fungal-mediated waste valorisation should not be limited to individual countries. Governments should encourage international cooperation, knowledge sharing, and technology transfer. The foundation of international collaboration lies in establishing robust networks of researchers, institutions, and industry stakeholders. Engaging in collaborative projects and initiatives fosters the exchange of ideas, expertise, and resources. Platforms like international conferences, workshops, and collaborative research programmes provide opportunities to build and strengthen these networks. Joint research projects can serve as catalysts for international collaboration. Collaborators can identify specific research themes, allocate responsibilities, and pool resources to address complex challenges in fungal technology. Establishing platforms for sharing research findings, datasets, and methodologies facilitates a dynamic exchange of knowledge. Open-access publications and collaborative databases contribute to a collective knowledge base, accelerating progress in fungal-mediated lignocellulosic waste valorisation. Many governmental and non-governmental agencies offer grants and funding for collaborative research initiatives. Securing financial support enhances the scalability and impact of collaborative projects, enabling the implementation of ambitious research goals. Cross-cultural training and workshops can help collaborators understand and appreciate each other’s perspectives, fostering effective communication and collaboration. Clear legal and ethical frameworks are essential to navigate international collaborations. Collaborators should establish agreements regarding intellectual property rights, data sharing, and publication policies. Clarity on these aspects can help build trust among partners and ensure equitable outcomes from collaborative efforts. Collaborators should focus on building enduring relationships beyond individual projects. Long-term partnerships contribute to the continuous exchange of ideas, resources, and expertise, fostering a culture of innovation and mutual support. Collaboration should extend beyond academia to involve industry stakeholders and policymakers. Engaging these key players ensures that research outcomes align with industry needs and regulatory frameworks. These efforts can help accelerate collaboration on a global scale.
8.8. Incentives for circular economy practices
Incentivising circular economy practices that prioritise waste valorisation can reduce the environmental impact of waste disposal. Policies can promote the use of fungal-mediated technologies to close the loop on materials, transforming waste into resources.
9. Industrial scale-up considerations
Scaling up fungal-mediated lignocellulosic waste valorisation from laboratory-scale processes to industrial-scale production is a complex undertaking that demands meticulous planning, optimisation, and consideration of various factors. Successful scale-up relies on adapting and optimising processes, embracing innovation, and addressing challenges at each stage of the production chain. By carefully navigating these considerations, industrial-scale lignocellulosic waste valorisation can contribute significantly to the development of sustainable bio-based economies. To successfully scale up fungal-mediated lignocellulosic waste valorisation, several key considerations and steps must be addressed:
9.1. Strain selection and optimization
Strain selection and optimisation are pivotal steps in enhancing the efficiency of fungal-mediated lignocellulose valorisation processes at the industrial scale. However, these processes come with inherent risks and challenges that need careful consideration and strategic approaches to ensure successful implementation and scalability. Genetic instability in fungal strains may lead to unpredictable changes in enzymatic and metabolic activities over time. The expression of desired lignocellulolytic enzymes may vary under different growth conditions, affecting the efficiency of lignocellulose degradation. Maintaining genetic stability over prolonged fermentation periods at the industrial scale poses a challenge. Ensuring consistent and high-level expression of lignocellulolytic enzymes across diverse lignocellulosic feedstocks requires meticulous optimisation. Fungal strains optimised for specific lignocellulosic substrates may exhibit limited adaptability to variations in feedstock composition. Substrate-specific strains may face challenges in efficiently degrading heterogeneous lignocellulosic materials encountered at the industrial scale. Developing strains with broad substrate specificity is essential for accommodating diverse feedstocks, but achieving this versatility without compromising efficiency presents a challenge. Balancing adaptability and specificity requires a comprehensive understanding and fine-tuning of the genetic traits governing substrate utilisation. Genetically modified organisms (GMOs) used in industrial-scale processes may face regulatory hurdles and public concerns. Ensuring biosafety and compliance with regulatory frameworks is crucial to avoid legal and societal challenges.
Navigating the regulatory landscape for GMOs in different regions requires extensive documentation and adherence to diverse standards. Public perception and acceptance of genetically modified fungi may pose challenges, emphasising the importance of transparent communication and public engagement. Strains optimised in laboratory-scale experiments may not exhibit the same performance and efficiency when scaled up to industrial fermenters. Challenges in maintaining consistent strain behaviour and performance at larger scales can lead to unpredictable outcomes. Addressing scale-dependent factors, such as mass transfer limitations, nutrient distribution, and heat dissipation, is crucial for successful strain adaptation to industrial-scale bioreactors. Conducting pilot-scale studies and leveraging computational modelling can help predict and address challenges associated with scale-up. Furthermore, optimising the growth conditions and fermentation processes is essential to maximise enzyme production.
9.2. Pretreatment methods
Lignocellulosic materials often require pretreatment to make them more accessible to fungal enzymes. Various physical, chemical, and biological pretreatment methods can be employed to enhance the efficiency of lignocellulose degradation. Mechanical methods such as milling or grinding consume significant energy, contributing to elevated operational costs. The abrasive nature of physical pretreatment can result in the generation of fine particulate matter, leading to potential workplace health and safety concerns. Excessive physical forces during pretreatment can result in the loss of valuable biomass components. Energy-intensive physical pretreatment methods contribute to the carbon footprint of lignocellulosic valorisation processes. The disposal of residual biomass after physical pretreatment may require careful management to avoid environmental harm. Chemical pretreatment methods often involve the use of harsh chemicals such as acids, alkalis, or organic solvents, which can pose safety risks and require careful handling. The release of chemical residues into the wastewater may lead to water pollution and necessitate additional treatment processes. The high operational costs associated with chemical pretreatment can be a limiting factor for large-scale industrial applications. Chemical pretreatment processes may contribute to the generation of hazardous by-products and emissions, contributing to air and water pollution. The potential for soil contamination arises when the waste from chemical pretreatment is not managed properly. Biological pretreatment methods often necessitate longer processing times compared to other pretreatment approaches. The need for specific microbial strains or cultures can lead to challenges in maintaining process consistency. The potential release of fungal spores into the environment may raise concerns related to human health and ecosystem impacts. The introduction of non-native microbial strains for biological pretreatment may lead to unintended ecological consequences, potentially outcompeting native species. The release of volatile organic compounds (VOCs) during biological pretreatment can contribute to air pollution. Continuous research and development efforts should focus on optimising pretreatment methods to minimise drawbacks and improve overall efficiency. Developing more energy-efficient and environmentally friendly pretreatment technologies is essential for sustainable lignocellulosic waste valorisation. Efficient waste management practices, such as the reuse or recycling of pretreatment by-products, can mitigate the environmental impact of these processes. Implementation of closed-loop systems can help minimise the release of pollutants into the environment. Advancements in pretreatment technologies, such as the development of green solvents or more efficient mechanical processes, can contribute to reducing environmental footprints. Exploring innovative methods for recycling or recovering chemicals used in pretreatment can enhance the sustainability of the overall process. Conducting comprehensive life cycle assessments (LCA) can provide a holistic understanding of the environmental impacts associated with pretreatment methods. LCA can guide decision-making processes to optimise and select pretreatment methods that align with environmental sustainability goals.
9.3. Enzyme production
Enzyme production is a critical component of fungal-mediated lignocellulose valorisation processes at the industrial scale, where lignocellulosic biomass is converted into valuable products through the action of enzymes secreted by fungi. While enzymes play a pivotal role in breaking down complex lignocellulosic structures, several challenges are associated with their production at the industrial scale, impacting the overall efficiency and economic viability of lignocellulosic waste valorisation. Selecting and optimising fungal strains for high enzyme production at the industrial scale is a complex task. Balancing the trade-off between lignocellulolytic enzyme activity and fungal growth rates poses a challenge in achieving optimal enzyme yields. Utilising cost-effective lignocellulosic substrates for enzyme production is essential for economic viability. Availability, consistency, and cost of lignocellulosic feedstocks present challenges, especially considering fluctuations in market conditions. Identifying suitable inducers and optimising their concentrations for maximising enzyme production is a challenge. The choice of inducers must consider their impact on fungal metabolism, growth, and overall enzyme yield. Another challenge is achieving and maintaining optimal fermentation conditions, including temperature, pH, and nutrient availability, which is crucial for efficient enzyme production. Large-scale fermentations introduce challenges in ensuring uniform conditions throughout the bioreactor, impacting enzyme production consistency. Fungal growth and enzyme production are oxygen-dependent processes, and efficient oxygen transfer is essential for maintaining high metabolic rates. Scaling up from small-scale laboratory fermenters to large industrial bioreactors introduces challenges in achieving adequate aeration and oxygen distribution. Achieving economic viability in industrial-scale enzyme production involves optimising resource utilisation, minimising operational costs, and maximising enzyme yields. Balancing the costs associated with raw materials, energy, and downstream processing with the value of the enzyme products is a complex challenge. Keeping pace with technological advancements and continuously optimising bioprocesses for enzyme production is essential for staying competitive. Integrating new technologies, such as high-throughput screening and synthetic biology, into industrial-scale enzyme production introduces challenges in terms of scalability and process integration.
9.4. Fermentation and product formation
Fermentation is a key stage in fungal-mediated lignocellulose valorisation processes at the industrial scale, where lignocellulosic biomass is converted into valuable products through the action of fungi. While fungal fermentation offers promising solutions for sustainable bio-based production, several challenges are associated with this crucial stage, encompassing both the process itself and the formation of desired end-products. At the industrial scale, lignocellulosic substrates can exhibit significant heterogeneity due to variations in feedstock composition and quality. Addressing substrate heterogeneity involves developing robust fungal strains capable of adapting to diverse feedstocks and optimising fermentation conditions accordingly. Large-scale fermentation processes are susceptible to microbial contamination from unwanted fungi, bacteria, or other microorganisms. Contamination can lead to decreased product yields, altered fermentation profiles, and compromised product quality. Transitioning from small-scale bioreactors to large industrial fermenters requires careful consideration of mixing, aeration, and heat transfer capabilities. Efficient scaling of bioreactor systems ensures uniform fungal growth, nutrient distribution, and effective lignocellulosic degradation. Achieving uniform fungal growth and nutrient distribution throughout larger bioreactor volumes is a challenge that demands advanced engineering solutions.
9.5. Downstream processing
Downstream processing is a critical phase in fungal-mediated lignocellulose valorisation, aimed at recovering valuable products from the complex mixture generated during enzymatic degradation. While downstream processing is essential for separating and purifying desired components, it introduces challenges and potential inefficiencies that must be addressed to ensure the overall success and sustainability of lignocellulosic waste valorisation. Enzymes utilised in lignocellulose degradation are valuable but can be challenging to recover efficiently from the complex reaction mixtures. Denaturation or degradation of enzymes during the process may limit their reusability, necessitating additional enzyme supplementation. Inefficient enzyme recovery can contribute to increased operational costs and waste generation. The need for frequent enzyme supplementation due to reduced reusability may impact the economic viability of the overall process. Lignocellulosic degradation results in a mixture of products, including sugars, lignin fragments, and fermentation by-products, making their separation and purification challenging. The presence of inhibitory compounds, such as furfurals and organic acids, complicates the separation process. Incomplete separation and purification can lead to impurities in the final product, affecting its quality and market value. The complexity of the mixture may require multiple separation steps, contributing to increased energy consumption and operational costs. Downstream processing generates waste streams containing residual biomass, solvents, and by-products that require proper management. The presence of inhibitory compounds in the waste stream may pose challenges for environmentally friendly disposal or utilisation. Inadequate waste management can lead to environmental pollution and compromise the sustainability of the overall lignocellulose valorisation process. Developing efficient strategies for waste utilisation or treatment is crucial to minimise environmental impact. Transitioning from lab-scale to industrial-scale downstream processing poses challenges in maintaining efficiency and cost-effectiveness. Integrating downstream processing with other stages of lignocellulose valorisation requires careful optimisation to ensure a seamless and economically viable workflow. Inefficient scaling up may lead to increased capital and operational costs, impacting the economic feasibility of large-scale lignocellulosic waste valorisation processes. Poor integration between downstream processing and other stages may result in bottlenecks and reduced overall efficiency. Investing in advanced separation technologies, such as membrane filtration, chromatography, and adsorption, can improve the efficiency of product separation and purification. Exploring environmentally friendly solvents and adopting sustainable process design principles contribute to minimising waste generation and environmental impact. Incorporating principles of green chemistry in downstream processing aligns with the broader goals of sustainable lignocellulosic waste valorisation. Conducting comprehensive life cycle assessments helps identify and mitigate potential inefficiencies and environmental impacts throughout the downstream processing stage. LCA facilitates informed decision-making for optimising processes and selecting more sustainable alternatives.
10. Prospects of industrial scale-up
The prospects of industrial scale-up in fungal-mediated lignocellulose valorisation processes hold significant promise for the development of sustainable and economically viable bio-based industries. As technology advances and the demand for eco-friendly alternatives increases, the transition from laboratory-scale experiments to large-scale industrial operations offers numerous opportunities and potential benefits. Industrial scale-up of fungal-mediated lignocellulosic waste valorisation can significantly contribute to the development of sustainable and eco-friendly bioproducts, including biofuels (such as bioethanol and biodiesel), bioplastics, biochemicals, and biomaterials. Achieving economic growth concerning sustainable bioproducts in fungal-mediated lignocellulose valorisation processes at the industrial scale poses several potential challenges. One significant hurdle lies in the high upfront costs associated with implementing cutting-edge technologies and infrastructure required for large-scale fungal-mediated processes. The complexity of lignocellulosic biomass and the need for specialised equipment add to the initial investment, potentially impacting the overall economic feasibility. Moreover, the fluctuating costs and availability of lignocellulosic feedstocks, regulatory compliance requirements, and market uncertainties can introduce variability in production costs, affecting the profitability of the industrial processes. Balancing the economic viability of sustainable bioproducts with the imperative to meet environmental and social sustainability goals is a delicate challenge. Collaborative efforts between industry, researchers, and policymakers are essential to navigate these challenges, ensuring that fungal-mediated lignocellulose valorisation processes not only contribute to economic growth but also foster long-term sustainability in the bio-based industry. By converting lignocellulosic waste into valuable products, the circular economy approach may aid in closing the loop on waste management, reducing environmental pollution, and conserving natural resources. The conversion of lignocellulosic waste into biofuels reduces reliance on fossil fuels, mitigates greenhouse gas emissions, and contributes to the generation of renewable energy. The industrial scale-up of fungal-mediated lignocellulosic waste valorisation has the potential to create jobs, support rural development, and stimulate local economies, particularly in regions with abundant lignocellulosic resources. The integration of new technologies in fungal-mediated lignocellulose valorisation processes at the industrial scale represents a significant leap towards sustainability and efficiency. However, the adoption of these technologies is accompanied by potential risks and challenges that necessitate careful consideration to ensure successful implementation and mitigate adverse consequences. Continued research and development in this field may open up opportunities for technological advancements, strain improvement, and process optimisation, driving innovation in the biotechnology and bioenergy sectors. The rapid adoption of high-throughput screening techniques may lead to data overload, making it challenging to discern relevant information and optimise processes effectively. The initial cost and complexity of implementing these screening technologies may pose economic challenges. Investing in robust data analysis tools and artificial intelligence algorithms may help in streamlining and interpreting large datasets efficiently. Conducting a cost-benefit analysis may help to ensure the economic feasibility of implementing high-throughput screening technologies. The application of omics technologies introduces challenges related to data standardisation, interpretation, and integration. Privacy concerns may arise when dealing with large-scale genomic data, particularly in the context of personalised strain engineering. The establishment of data governance frameworks and adherence to privacy regulations may ensure the responsible handling of genomic information. The implementation of novel bioreactor designs may encounter technical challenges related to scale-up, mixing, and heat transfer. High capital costs and maintenance expenses associated with advanced bioreactors may impact overall project economics. Conducting thorough pilot-scale studies to validate the scalability and performance of advanced bioreactor designs may help to alleviate the issue. Overreliance on artificial intelligence (AI) and machine learning (ML) models may lead to biased decision-making and misinterpretation of complex biological systems. The lack of interpretability in AI/ML models may pose challenges in understanding and trusting the predictions generated. Implementation of explainable AI/ML models to enhance transparency and interpretability and combining AI/ML predictions with expert knowledge to ensure a more comprehensive understanding of the biological processes may help in encountering the interpretability challenge.
11. Conclusions
Fungal-mediated lignocellulosic waste valorisation holds great promise for converting lignocellulosic waste into valuable products on an industrial scale. While it holds great potential, some several challenges and limitations need to be addressed to realise the full benefits of this technology. One challenge is that fungi can be slow to grow and produce enzymes. Choosing fungal strains with inherently faster growth rates is a fundamental strategy. Certain fungi, such as Trichoderma and Aspergillus species, are known for their rapid growth and robust lignocellulolytic capabilities. Screening and selecting strains based on growth characteristics are crucial for improving overall process efficiency. Further, genetic modification techniques offer a powerful tool for enhancing fungal growth characteristics. Engineered strains with improved tolerance to inhibitory compounds, increased metabolic efficiency, and optimised nutrient utilisation can overcome the challenges of slow growth. Fungal growth is heavily dependent on nutrient availability. Fine-tuning the composition and concentration of nutrient sources, as well as the carbon-to-nitrogen ratio, can significantly impact fungal growth rates. A balanced nutrient environment fosters optimal enzymatic activity and accelerates lignocellulosic waste degradation. Another challenge is that fungi can be sensitive to changes in pH, temperature, and other environmental conditions. The choice of growth media and fermentation conditions plays a crucial role in fungal performance. Adjusting the parameters such as pH, temperature, and aeration levels to create an environment conducive to rapid fungal growth is essential. Continuous monitoring and optimisation of these conditions throughout the fermentation process are key to overcoming these challenges. Implementing real-time monitoring and control systems can enhance the ability to respond to changes in environmental conditions promptly. Automated systems that adjust pH, temperature, and nutrient levels based on real-time data may contribute to maintaining optimal conditions for fungal growth throughout the process. Some lignocellulosic waste valorisation processes involve elevated temperatures due to thermophilic pretreatments. Exploring thermotolerant fungi that can thrive under these conditions offers a potential solution. Thermophilic fungi, adapted to higher temperature ranges, can contribute to faster lignocellulosic waste degradation. Furthermore, researchers are working to address these challenges by developing new strains of fungi that are more efficient and robust. They are also developing new technologies to improve the fermentation process and make it more cost-effective. Despite the challenges, there are several opportunities for the use of fungi in lignocellulosic waste valorisation. The global market for biofuels and bioproducts is expected to grow significantly in the coming years. This growth will create new opportunities for the use of fungi in the production of these products. In addition, the increasing demand for sustainable products and processes is creating opportunities for the use of fungi in lignocellulosic waste valorisation. Fungi can be used to produce biofuels, bioproducts, and other products sustainably. This can help to reduce our reliance on fossil fuels and reduce our environmental impact. Industrial scale-up of fungal-mediated lignocellulosic waste valorisation represents a promising pathway to unlock the untapped potential of lignocellulosic waste as a valuable resource for sustainable, eco-friendly products and bioenergy. As technology advances and research continues, this approach has the potential to play a pivotal role in building a more sustainable and circular economy, while reducing our reliance on fossil fuels and addressing environmental challenges. The industrial scale-up in this field offers exciting opportunities for innovation, economic growth, and environmental sustainability.
Acknowledgments
The authors are grateful to the University Institute of Biotechnology, Chandigarh University, Gharuan for providing a platform for study and valuable guidance, and Dr Manikant Tripathi acknowledges to Biotechnology Program, Dr. Rammanohar Lohia Avadh University, India.
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Adewuyi A. 2022. Underutilized lignocellulosic waste as sources of feedstock for biofuel production in developing countries. Front Energy Res. 10:741570. doi: 10.3389/fenrg.2022.741570. [DOI] [Google Scholar]
- Ajila CM, Brar SK, Verma M, Tyagi RD, Valéro JR. 2011. Solid-state fermentation of apple pomace using Phanerocheate chrysosporium – liberation and extraction of phenolic antioxidants. Food Chemistry. 126(3):1071–1080. doi: 10.1016/j.foodchem.2010.11.129. [DOI] [Google Scholar]
- Andlar M, Rezić T, Marđetko N, Kracher D, Ludwig R, Šantek B. 2018. Lignocellulose degradation: An overview of fungi and fungal enzymes involved in lignocellulose degradation. Eng Life Sci. 18(11):768–778. doi: 10.1002/elsc.201800039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asci F, Aydin B, Akkus GU, Unal A, Erdogmus SF, Korcan SE, Jahan I. 2020. Fatty acid methyl ester analysis of Aspergillus fumigatus isolated from fruit pulps for biodiesel production using GC-MS spectrometry. Bioengineered. 1(1):408–415. doi: 10.1080/21655979.2020.1739379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashokkumar V, Venkatkarthick R, Jayashree S, Chuetor S, Dharmaraj S, Kumar G, Chen WH, Ngamcharussrivichai C. 2022. Recent advances in lignocellulosic biomass for biofuels and value-added bioproducts - A critical review. Bioresour Technol. 344:26195. doi: 10.1016/j.biortech.2021.126195. [DOI] [PubMed] [Google Scholar]
- Atiwesh G, Parrish CC, Banoub J, Le TT. 2022. Lignin degradation by microorganisms: A review. Biotechnol Prog. 38(2):e3226. doi: 10.1002/btpr.3226. [DOI] [PubMed] [Google Scholar]
- Banu JR, Kavitha S, Tyagi VK, Gunasekaran M, Karthikeyan OP, Kumar G. 2021a. Lignocellulosic biomass based biorefinery: A successful platform towards circular bioeconomy. Fuel. 302:121086. doi: 10.1016/j.fuel.2021.121086. [DOI] [Google Scholar]
- Banu JR, Kavitha S, Tyagi VK, Gunasekaran M, Karthikeyan OP, Kumar G. 2021b. Lignocellulosic biomass pretreatment for enhanced bioenergy recovery: Effect of lignocelluloses recalcitrance and enhancement strategies. Front Energy Res. 9:646057. doi: 10.3389/fenrg.2021.646057. [DOI] [Google Scholar]
- Belt T, Awais M, Mäkelä M. 2022. Chemical characterization and visualization of progressive brown rot decay of wood by near infrared imaging and multivariate analysis. Front Plant Sci. 13:13. doi: 10.3389/fpls.2022.940745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilal M, Wang Z, Cui J, Ferreira LFR, Bharagava RN, Iqbal HM. 2020. Environmental impact of lignocellulosic wastes and their effective exploitation as smart carriers–A drive towards greener and eco-friendlier biocatalytic systems. Sci Total Environ. 722. doi: 10.1016/j.scitotenv.2020.137903. [DOI] [PubMed] [Google Scholar]
- Blasi A, Verardi A, Lopresto CG, Siciliano S, Sangiorgio P. 2023. Lignocellulosic agricultural waste valorization to obtain valuable products: An overview. Recycling. 8(4):61. doi: 10.3390/recycling8040061. [DOI] [Google Scholar]
- Branduardi P. 2021. Closing the loop: The power of microbial biotransformations from traditional bioprocesses to biorefineries, and beyond. Micro Biotech. 14(1):68–73. doi: 10.1111/1751-7915.13713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai Y, Bai M, Chen A, Peng L, Shao J, Luo S, Deng Y, Yan B, Peng C. 2022. Valorization of waste biomass through fungal technology: Advances, challenges, and prospects. Ind Crops Prod. 188:115608. doi: 10.1016/j.indcrop.2022.115608. [DOI] [Google Scholar]
- Chen C, Shrestha R, Jia K, Gao PF, Geisbrecht BV, Bossmann SH, Shi J, Li P. 2015. Characterization of dye-decolorizing peroxidase (DyP) from Thermomonospora curvata reveals unique catalytic properties of A-type DyPs. J Biol Chem. 290(38):23447–23463. doi: 10.1074/jbc.M115.658807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chukwuma OB, Rafatullah M, Tajarudin HA, Ismail N. 2020. Lignocellulolytic enzymes in biotechnological and industrial processes: A review. Sustainability. 12(18):7282. doi: 10.3390/su12187282. [DOI] [Google Scholar]
- Civzele A, Stipniece-Jekimova AA, Mezule L. 2023. Fungal ligninolytic enzymes and their application in biomass lignin pretreatment. J Fungi. 9(7):780. doi: 10.3390/jof9070780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbu VM, Gheorghe-Barbu I, AȘ D, Vrâncianu CO, Șesan TE. 2023. Current insights in fungal importance - A comprehensive review. Microorganisms. 11(6):1384. doi: 10.3390/microorganisms11061384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa-Silva V, Anunciação M, Andrade E, Fernandes L, Costa A, Fraga I, Barros A, Marques G, Ferreira L, Rodrigues M. 2022. Biovalorization of grape stalks as animal feed by solid state fermentation using white-rot fungi. App Sci. 12(13):6800. doi: 10.3390/app12136800. [DOI] [Google Scholar]
- Dao CN, Tabil LG, Mupondwa E, Dumonceaux T. 2023. Modeling the microbial pretreatment of camelina straw and switchgrass by Trametes versicolor and Phanerochaete chrysosporium via solid-state fermentation process: A growth kinetic sub-model in the context of biomass-based biorefineries. Front Microbiol. 14:1130196. doi: 10.3389/fmicb.2023.1130196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Cerro C, Erickson E, Dong T, Wong AR, Eder EK, Purvine SO, Mitchell HD, Weitz KK, Markillie LM, Burnet MC, et al. 2021. Intracellular pathways for lignin catabolism in white-rot fungi. Proc Natl Acad Sci USA. 118(9): doi: 10.1073/pnas.2017381118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devi A, Bajar S, Kour H. 2022. Lignocellulosic biomass valorization for bioethanol production: A circular bioeconomy approach. Bioenerg Res. 15(4):1820–1841. doi: 10.1007/s12155-022-10401-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulf FV, Vodnar DC, Dulf EH. 2017. Phenolic compounds, flavonoids, lipids and antioxidant potential of apricot (Prunus armeniaca L.) pomace fermented by two filamentous fungal strains in solid state system. Chem Cent J. 11(1):92. doi: 10.1186/s13065-017-0323-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulf FV, Vodnar DC, Dulf EH, Diaconeasa Z, Socaciu C. 2018. Liberation and recovery of phenolic antioxidants and lipids in chokeberry (Aronia melanocarpa) pomace by solid-state bioprocessing using Aspergillus Niger and Rhizopus oligosporus strains. LWT. 87:241–249. doi: 10.1016/j.lwt.2017.08.084. [DOI] [Google Scholar]
- Dulf FV, Vodnar DC, Socaciu C. 2016. Effects of solid-state fermentation with two filamentous fungi on the total phenolic contents, flavonoids, antioxidant activities and lipid fractions of plum fruit (Prunus domestica L.) by-products. Food Chem. 209:27–36. doi: 10.1016/j.foodchem.2016.04.016. [DOI] [PubMed] [Google Scholar]
- Dulf FV, Vodnar DC, Toşa MI, Dulf EH. 2020. Simultaneous enrichment of grape pomace with γ-linolenic acid and carotenoids by solid-state fermentation with zygomycetes fungi and antioxidant potential of the bioprocessed substrates. Food Chem. 310:125927. doi: 10.1016/j.foodchem.2019.125927. [DOI] [PubMed] [Google Scholar]
- Elisashvili V, Kachlishvili E, Tsiklauri N. 2009. Lignocellulose-degrading enzyme production by white-rot Basidiomycetes isolated from the forests of Georgia. World J Microbiol Biotechnol. 25(2):331–339. doi: 10.1007/s11274-008-9897-x. [DOI] [Google Scholar]
- Eugenio ME, Domínguez G, Molina-Guijarro JM, Hernández M, Arias ME, Ibarra D. 2023. Boosting enzymatic hydrolysis of steam-pretreated softwood by laccase and endo-β-mannanase enzymes from Streptomyces ipomoeae CECT 3341. Wood Sci Technol. 57(4):965–987. doi: 10.1007/s00226-023-01481-7. [DOI] [Google Scholar]
- Ferdeș M, Dincă MN, Moiceanu G, Zăbavă BȘ, Paraschiv G. 2020. Microorganisms and enzymes used in the biological pretreatment of the substrate to enhance biogas production: A review. Sustainability. 12(17):7205. doi: 10.3390/su12177205. [DOI] [Google Scholar]
- Ferreira Gregorio V, Pié L, Terceño A. 2018. A systematic literature review of bio, green and circular economy trends in publications in the field of economics and business management. Sustainability. 10(11):4232. doi: 10.3390/su10114232. [DOI] [Google Scholar]
- Ferreira JA, Mahboubi A, Lennartsson PR, Taherzadeh MJ. 2016. Waste biorefineries using filamentous ascomycetes fungi: Present status and future prospects. Biores Tech. 215:334–345. doi: 10.1016/j.biortech.2016.03.018. [DOI] [PubMed] [Google Scholar]
- Füchtner S, Alfredsen G, Thygesen LG. 2023. Oxalate found in wood cell wall during incipient brown rot degradation. Int Biodeterior Biodegrade. 177:105531. doi: 10.1016/j.ibiod.2022.105531. [DOI] [Google Scholar]
- Gunjal AB, Patil NN, Shinde SS. 2020. Enzymes in degradation of the lignocellulosic wastes. Cham, Switzerland: Springer. [Google Scholar]
- Gupta VK, Kubicek CP, Berrin JG, Wilson DW, Couturier M, Berlin A, Filho EXF, Ezeji T. 2016. Fungal enzymes for bio-products from sustainable and waste biomass. Trends Biochem Sci. 41(7):633–645. doi: 10.1016/j.tibs.2016.04.006. [DOI] [PubMed] [Google Scholar]
- Han S, Kim GY, Han JI. 2019. Biodiesel production from oleaginous yeast, Cryptococcus sp. by using banana peel as carbon source. Ener Rep. 5:1077–1081. doi: 10.1016/j.egyr.2019.07.012. [DOI] [Google Scholar]
- Haq IU, Qaisar K, Nawaz A, Akram F, Mukhtar H, Zohu X, Xu Y, Mumtaz MW, Rashid U, Ghani WA, et al. 2021. Advances in valorization of lignocellulosic biomass towards energy generation. Catalysts. 11(3):309. doi: 10.3390/catal11030309. [DOI] [Google Scholar]
- Hernández-Bueno NS, Suárez-Rodríguez R, Balcázar-López E, Folch-Mallol JL, Ramírez-Trujillo JA, Iturriaga G. 2021. A versatile peroxidase from the fungus Bjerkandera adusta confers abiotic stress tolerance in transgenic tobacco plants. Plants. 10(5):859. doi: 10.3390/plants10050859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou L, Ji D, Dong W, Yuan L, Zhang F, Li Y, Zang L. 2020. The synergistic action of electro-Fenton and white-rot fungi in the degradation of lignin. Front Biotechnol Bioeng. 8:99. doi: 10.3389/fbioe.2020.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Wang J, Liu S. 2024. Advances in the production of fungi-derived lignocellulolytic enzymes using agricultural wastes. Mycology. 15(4):523–537. doi: 10.1080/21501203.2023.2253827. [DOI] [Google Scholar]
- Huang DL, Zeng GM, Jiang XY, Feng CL, Yu HY, Huang GH, Liu HL. 2006. Bioremediation of Pb-contaminated soil by incubating with Phanerochaete chrysosporium and straw. J Hazard Mater. 134(1–3):268–276. doi: 10.1016/j.jhazmat.2005.11.021. [DOI] [PubMed] [Google Scholar]
- Ibarra-Cantún D, Ramos-Cassellis ME, Marín-Castro MA, Castelán-Vega RDC. 2020. Secondary metabolites and antioxidant activity of the solid-state fermentation in apple (Pirus malus L.) and agave mezcalero (Agave angustifolia H.) bagasse. J Fungi. 6(3):137. doi: 10.3390/jof6030137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilica RA, Kloetzer L, Galaction AI, Caşcaval D. 2019. Fumaric acid: Production and separation. Biotechnol Lett. 41(1):47–57. doi: 10.1007/s10529-018-2628-y. [DOI] [PubMed] [Google Scholar]
- Ilić N, Milić M, Beluhan S, Dimitrijević-Branković S. 2023. Cellulases: From lignocellulosic biomass to improved production. Energies. 16(8):3598. doi: 10.3390/en16083598. [DOI] [Google Scholar]
- Infanzón-Rodríguez MI, Ragazzo-Sánchez JA, Del Moral S, Calderón-Santoyo M, Aguilar-Uscanga MG. 2022. Enzymatic hydrolysis of lignocellulosic biomass using native cellulase produced by Aspergillus niger ITV02 under liquid state fermentation. Biotechnol Appl Biochem. 69(1):198–208. doi: 10.1002/bab.2097. [DOI] [PubMed] [Google Scholar]
- Jin X, Wei S. 2023. Efficient short time pretreatment on lignocellulosic waste using an isolated fungus Trametes sp. W-4 for the enhancement of biogas production. Heliyon. 9(3):e14573. doi: 10.1016/j.heliyon.2023.e14573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jović J, Hao J, Kocić-Tanackov S, Mojović L. 2022. Improvement of lignocellulosic biomass conversion by optimization of fungal ligninolytic enzyme activity and molasses stillage supplementation. Biomass Conv Bioref. 12(7):2749–2765. doi: 10.1007/s13399-020-00929-1. [DOI] [Google Scholar]
- Kaur P, Kocher GS, Taggar MS. 2019. Development of fungal consortium for the pretreatment of rice straw under optimized solid state and shake flask conditions. Environ Prog Sustain Energy. 38(2):635–646. doi: 10.1002/ep.12954. [DOI] [Google Scholar]
- Kijpornyongpan T, Schwartz A, Yaguchi A, Salvachúa D. 2022. Systems biology-guided understanding of white-rot fungi for biotechnological applications: a review. IScience. 25(7):104640. doi: 10.1016/j.isci.2022.104640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocher GS, Kaur P, Taggar MS. 2018. An overview of pretreatment processes with special reference to biological pretreatment for rice straw delignification. Curr Biochem Eng. 4(3):151–163. doi: 10.2174/2212711903666161102141859. [DOI] [Google Scholar]
- Koul B, Yakoob M, Shah MP. 2022. Agricultural waste management strategies for environmental sustainability. Environ Res. 206:2285. doi: 10.1016/j.envres.2021.112285. [DOI] [PubMed] [Google Scholar]
- Kovács E, Szűcs C, Farkas A, Szuhaj M, Maróti G, Bagi Z, Rákhely G, Kovács KL. 2022. Pretreatment of lignocellulosic biogas substrates by filamentous fungi. J Biotechnol. 360:160–170. doi: 10.1016/j.jbiotec.2022.10.013. [DOI] [PubMed] [Google Scholar]
- Kracher D, Ludwig R. 2016. Cellobiose dehydrogenase: An essential enzyme for lignocellulose degradation in nature – A review / Cellobiosedehydrogenase: Ein essentielles Enzym für den Lignozelluloseabbau in der Natur – Eine Übersicht. J Land Manag Food Enviro. 67(3):145–163. doi: 10.1515/boku-2016-001. [DOI] [Google Scholar]
- Kržišnik D, Gonçalves J. 2023. Environmentally conscious technologies using fungi in a climate-changing world. Earth. 4(1):69–77. doi: 10.3390/earth4010005. [DOI] [Google Scholar]
- Kumar A, Chandra R. 2020. Ligninolytic enzymes and its mechanisms for degradation of lignocellulosic waste in environment. Heliyon. 6(2):e03170. doi: 10.1016/j.heliyon.2020.e03170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latif W, Ciniglia C, Iovinella M, Shafiq M, Papa S. 2023. Role of white rot fungi in industrial wastewater treatment: A review. Appl Sci. 13(14):8318. doi: 10.3390/app13148318. [DOI] [Google Scholar]
- Lee SY, Kim HU, Chae TU, Cho JS, Kim JW, Shin JH. 2019. A comprehensive metabolic map for production of bio-based chemicals. Nat Catal. 2(1):18–33. doi: 10.1038/s41929-018-0212-4. [DOI] [Google Scholar]
- Li F, Liu Y, Zhao H, Liu X, Yu H. 2023. Lytic polysaccharide monooxygenases from Serpula lacrymans as enzyme cocktail additive for efficient lignocellulose degradation. Fermentation. 9(6):506. doi: 10.3390/fermentation9060506. [DOI] [Google Scholar]
- Lopes AM, Ferreira Filho EX, Moreira LRS. 2018. An update on enzymatic cocktails for lignocellulose breakdown. J Appl Microbiol. 125(3):632–645. doi: 10.1111/jam.13923. [DOI] [PubMed] [Google Scholar]
- Luo H, Zhou T, Cao J, Gao L, Wang S, Gui Z, Shi Y, Xie F, Yang R. 2023. Utilization of lignocellulosic biomass by glycerol organosolv pretreatment for biobutanol production integrated with bioconversion of residual glycerol into value-added products. Bioresour Technol. 387:129661. doi: 10.1016/j.biortech.2023.129661. [DOI] [PubMed] [Google Scholar]
- Lyagin I, Aslanli A, Domnin M, Stepanov N, Senko O, Maslova O, Efremenko E. 2023. Metal nanomaterials and hydrolytic enzyme-based formulations for improved antifungal activity. IJMS. 24(14):11359. doi: 10.3390/ijms241411359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Gao Z, Wang Q, Liu Y. 2018. Biodiesels from microbial oils: Opportunity and challenges. Biores Technol. 263:631–641. doi: 10.1016/j.biortech.2018.05.028. [DOI] [PubMed] [Google Scholar]
- Malgas S, Pletschke BI. 2020. Combination of CTec2 and GH5 or GH26 endo-mannanases for effective lignocellulosic biomass degradation. Catalysts. 10(10):1193. doi: 10.3390/catal10101193. [DOI] [Google Scholar]
- Mali T, Laine K, Hamberg L, Lundell T. 2023. Metabolic activities and ultrastructure imaging at late-stage of wood decomposition in interactive brown rot - white rot fungal combinations. Fungal Ecol. 61:101199. doi: 10.1016/j.funeco.2022.101199. [DOI] [Google Scholar]
- Maran JP, Prakash KA. 2015. Process variables influence on microwave assisted extraction of pectin from waste Carcia papaya L. peel. Int J Biol Macromol. 73:202–206. doi: 10.1016/j.ijbiomac.2014.11.008. [DOI] [PubMed] [Google Scholar]
- Martinez D, Larrondo LF, Putnam N, Sollewijn Gelpke MD, Huang K, Chapman J, Helfenbein KG, Ramaiya P. 2004. Genome sequence of the lignocellulose degrading fungus Phanerochaete chrysosporium strain RP78. Nat Biotechnol. 22(6):695–700. doi: 10.1038/nbt967. [DOI] [PubMed] [Google Scholar]
- Meyer V, Basenko EY, Benz JP, Braus GH, Caddick MX, Csukai M, de Vries RP, Endy D, Frisvad JC, Gunde-Cimerman N, et al. 2020. Growing a circular economy with fungal biotechnology: A white paper. Fungal Biol Biotechnol. 7(1):5. doi: 10.1186/s40694-020-00095-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohnen D. 2008. Pectin structure and biosynthesis. Curr Opin Plant Biol. 11:266–277. doi: 10.1016/j.pbi.2008.03.006. [DOI] [PubMed] [Google Scholar]
- Molelekoa TBJ, Regnier T, da Silva LS, Augustyn W. 2021. Production of pigments by filamentous fungi cultured on agro-industrial by-products using submerged and solid-state fermentation methods. Fermentation. 7(4):295. doi: 10.3390/fermentation7040295. [DOI] [Google Scholar]
- Mujtaba M, Fernandes Fraceto L, Fazeli M, Mukherjee S, Savassa SM, Araujo de Medeiros G, Do Espírito Santo Pereira A, Mancini SD, Lipponen J, Vilaplana F. 2023. Lignocellulosic biomass from agricultural waste to the circular economy: a review with focus on biofuels, biocomposites and bioplastics. J Clean Prod. 402:136815. doi: 10.1016/j.jclepro.2023.136815. [DOI] [Google Scholar]
- Mustabi J, Zhalilal SZ, Natsir A. 2023. The use of rot fungi isolated from wood to degradate fiber cocoa POD. AIP Conf Proc. 2628(1):030017. doi: 10.1063/5.0144098. [DOI] [Google Scholar]
- Nargotra P, Sharma V, Lee YC, Tsai YH, Liu YC, Shieh CJ, Tsai ML, Dong CD, Kuo CH. 2023. Microbial lignocellulolytic enzymes for the effective valorization of lignocellulosic biomass: A review. Catalysts. 13(1):83. doi: 10.3390/catal13010083. [DOI] [Google Scholar]
- Niladevi KN. 2009. Ligninolytic enzymes. In: Nigam P, Pandey A, editors. Biotechnology for agro-industrial residues utilisation. Dordrecht, Netherlands: Springer; p. 397–414. [Google Scholar]
- Nongthombam GD, Sarangi PK, Singh TA, Sharma CK, Talukdar NC. 2022. Bioethanol production from ficus fruits (Ficus cunia) by Fusarium oxysporum through consolidated bioprocessing system. 3 Biotech. 12(9):178. doi: 10.1007/s13205-022-03234-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey A, Tyagi RD, Varjani S. 2021. Circular bioeconomy—Current developments and future outlook. Cambridge, MA, USA: Elsevier. [Google Scholar]
- Pereira Scarpa JDC, Paganini Marques N, Alves Monteiro D, Martins GM, Paula AVD, Boscolo M, da Silva R, Gomes E, Alonso Bocchini D. 2019. Saccharification of pretreated sugarcane bagasse using enzymes solution from Pycnoporus sanguineus MCA 16 and cellulosic ethanol production. Ind Crops Prod. 141:111795. doi: 10.1016/j.indcrop.2019.111795. [DOI] [Google Scholar]
- Purnomo AS, Mori T, Kamei I, Nishii T, Kondo R. 2010. Application of mushroom waste medium from Pleurotus ostreatus for bioremediation of DDT-contaminated soil. Int Biodeterior Biodegradation. 64:397–402. doi: 10.1016/j.ibiod.2010.04.007. [DOI] [Google Scholar]
- Qi J, Jia L, Liang Y, Luo B, Zhao R, Zhang C, Wen J, Zhou Y, Fan M, Xia Y. 2022. Fungi’s selectivity in the biodegradation of Dendrocalamus sinicus decayed by white and brown rot fungi. Ind Crops Prod. 188:115726. doi: 10.1016/j.indcrop.2022.115726. [DOI] [Google Scholar]
- Qiu W, Liu J. 2022. Fermenting and lignin degradability of a white-rot fungus Coriolopsis trogii using industrial lignin as substrate. Appl Biochem Biotech. 194(11):5220–5235. doi: 10.1007/s12010-022-04004-5. [DOI] [PubMed] [Google Scholar]
- Rajtar NN, Kielsmeier-Cook JC, Held BW, Toapanta-Alban CE, Ordonez ME, Barnes CW, Blanchette RA. 2023. Diverse Xylaria in the Ecuadorian Amazon and their mode of wood degradation. Bot Stud. 64(1):30. doi: 10.1186/s40529-023-00403-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathner R, Petz S, Tasnádi G, Koller M. 2017. Monitoring the kinetics of biocatalytic removal of the endocrine disrupting compound 17α-ethinylestradiol from differently polluted wastewater bodies. J Environ Chem Engin. 5(2):1920–1926. doi: 10.1016/j.jece.2017.03.034. [DOI] [Google Scholar]
- Ravichandran A, Sridhar M. 2016. Versatile peroxidases: Super peroxidases with potential biotechnological applications - A mini review. J Dairy Vet Anim Res. 4(2):277‒280. doi: 10.15406/jdvar.2016.04.00116. [DOI] [Google Scholar]
- Roth MG, Westrick NM, Baldwin TT. 2023. Fungal biotechnology: From yesterday to tomorrow. Front Fungal Biol. 4:1135263. doi: 10.3389/ffunb.2023.1135263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanches C. 2009. Lignocellulosic residues: Biodegradation and bioconversion by fungi. Biotechnol Adv. 27(2):185–194. doi: 10.1016/j.biotechadv.2008.11.001. [DOI] [PubMed] [Google Scholar]
- Sari E, Syamsiah S, Sulistyo H, Hidayat M. 2015. Effect of Mn2+ addition on delignification of water hyacinth using Phanerochaete chrysosporium. Mod Appl Sci. 9(2):228–235. doi: 10.5539/mas.v9n2p228. [DOI] [Google Scholar]
- Satti SM, Shah AA. 2020. Polyester‐based biodegradable plastics: An approach towards sustainable development. Lett Appl Microbiol. 70(6):413–430. doi: 10.1111/lam.13287. [DOI] [PubMed] [Google Scholar]
- Schimpf U, Hanreich A, Mähnert P, Unmack T, Junne S, Renpenning J, Lopez-Ulibarri R. 2013. Improving the efficiency of large-scale biogas processes: Pectinolytic enzymes accelerate the lignocellulose degradation. J Sustain Energy Environ. 4(53):53–60. [Google Scholar]
- Schneider WDH, Fontana RC, Baudel HM, de Siqueira FG, Rencoret J, Gutiérrez A, de Eugenio LI, Prieto A, Martínez MJ, Martínez ÁT. 2020. Lignin degradation and detoxification of Eucalyptus wastes by on-site manufacturing fungal enzymes to enhance second-generation ethanol yield. Appl Energy. 262:114493. doi: 10.1016/j.apenergy.2020.114493. [DOI] [Google Scholar]
- Scott CJR, Leadbeater DR, Oates NC, James SR, Newling K, Li Y, McGregor NGS, Bird S, Bruce NC. 2023. Whole genome structural predictions reveal hidden diversity in putative oxidative enzymes of the lignocellulose-degrading ascomycete Parascedosporium putredinis NO1. Microbiol Spectr. 11(6):e0103523. doi: 10.1128/spectrum.01035-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanmugam S, Hari A, Ulaganathan P, Yang F, Krishnaswamy S, Wu YR. 2018. Potential of biohydrogen generation using the delignified lignocellulosic biomass by a newly identified thermostable laccase from Trichoderma asperellum strain BPLMBT1. Int J Hydrog Energy. 43(7):3618–3628. doi: 10.1016/j.ijhydene.2018.01.016. [DOI] [Google Scholar]
- Šibalic D, Šalić A, Tušek AJ, Sokač T, Brekalo K, Zelić B, Tran NN, Hessel V, Tišma M. 2020. Sustainable production of lipase from Thermomyces lanuginosus: Process optimization and enzyme characterization. Ind Eng Chem Res. 59(48):21144–21154. doi: 10.1021/acs.iecr.0c04329. [DOI] [Google Scholar]
- Silva A, Ticona A, Lopes F, Gouveia F, Silveira M, Oliveira J, Garcia L, Noronha E, Vale H. 2023. Characterization of white- and brown-rot fungi applied to the decay of caatinga biome wood (Swartzia psilonema harms) from Brazil. Curr Res Environ Appl Mycol. 13(1):104–122. doi: 10.5943/cream/13/1/8. [DOI] [Google Scholar]
- Singh N, Singhania RR, Nigam PS, Dong CD, Patel AK, Puri M. 2022. Global status of lignocellulosic biorefinery: Challenges and perspectives. Bioresour Technol. 344:126415. doi: 10.1016/j.biortech.2021.126415. [DOI] [PubMed] [Google Scholar]
- Sista Kameshwar AK, Qin W. 2018. Comparative study of genome-wide plant biomass-degrading CAZymes in white rot, brown rot and soft rot fungi. Mycology. 9(2):93–105. doi: 10.1080/21501203.2017.1419296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tišma M, Šalić A, Planinić M, Zelić B, Potočnik M, Šelo G, Bucić-Kojić A. 2020. Production, characterisation and immobilization of laccase for an efficient aniline-based dye decolourization. J Water Process Eng. 36:101327. doi: 10.1016/j.jwpe.2020.101327. [DOI] [Google Scholar]
- Tri CL, Kamei I. 2020. Butanol production from cellulosic material by anaerobic co-culture of white-rot fungus phlebia and bacterium clostridium in consolidated bioprocessing. Bioresour Technol. 305:123065. doi: 10.1016/j.biortech.2020.123065. [DOI] [PubMed] [Google Scholar]
- Troiano D, Orsat V, Dumont MJ. 2020. Status of filamentous fungi in integrated biorefineries. Renew Sust Energ Rev. 117:109472. doi: 10.1016/j.rser.2019.109472. [DOI] [Google Scholar]
- Verardi A, Lopresto CG, Blasi A, Chakraborty A, Calabrò V. 2020. Bioconversion of lignocellulosic biomass to bioethanol and biobutanol. In: Yousuf A, Pirozzi D, and Sannino F, editors. Lignocellulosic biomass to liquid biofuels. Cambridge, MA, USA: Academic Press; p. 67–125. [Google Scholar]
- Voběrková S, Vaverková MD, Burešová A, Adamcová D, Vršanská M, Kynický J, Brtnický M, Adam V. 2017. Effect of inoculation with white-rot fungi and fungal consortium on the composting efficiency of municipal solid waste. Waste Manag. 61:157–164. doi: 10.1016/j.wasman.2016.12.039. [DOI] [PubMed] [Google Scholar]
- Weng C, Peng X, Han Y. 2021. Depolymerization and conversion of lignin to value-added bioproducts by microbial and enzymatic catalysis. Biotechnol Biofuels. 14(1):84. doi: 10.1186/s13068-021-01934-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikandari R, Hasniah N, Taherzadeh MJ. 2022. The role of filamentous fungi in advancing the development of a sustainable circular bioeconomy. Bioresour Technol. 345:126531. doi: 10.1016/j.biortech.2021.126531. [DOI] [PubMed] [Google Scholar]
- Wittner N, Broos W, Bauwelinck J, Slezsák J, Vlaeminck SE, Cornet I. 2021. Enhanced fungal delignification and enzymatic digestibility of poplar wood by combined CuSO4 and MnSO4 supplementation. Process Biochem. 108:129–137. doi: 10.1016/j.procbio.2021.06.002. [DOI] [Google Scholar]
- Wong LY, Saad WZ, Mohamad R, Tahir PM. 2017. Optimization of cultural conditions for polygalacturonase production by a newly isolated Aspergillus fumigatus R6 capable of retting kenaf. Ind Crops Prod. 97:175–183. doi: 10.1016/j.indcrop.2016.12.019. [DOI] [Google Scholar]
- Xie P, Fan L, Huang L, Zhang C. 2020. An innovative co-fungal treatment to poplar bark sawdust for delignification and polyphenol enrichment. Ind Crops Prod. 157:112896. doi: 10.1016/j.indcrop.2020.112896. [DOI] [Google Scholar]
- Xu L, Sun J, Qaria MA, Gao L, Zhu D. 2021. Dye decoloring peroxidase structure, catalytic properties and applications: Current advancement and futurity. Catalysts. 11(8):955. doi: 10.3390/catal11080955. [DOI] [Google Scholar]
- Yadav B, Atmakuri A, Chavan S, Tyagi RD, Drogui P, Pilli S. 2021. Role of bioeconomy in circular economy. In: Pandey A, Tyagi R, and Varjani S, editors Biomass, Biofuels, biochemicals. Cambridge, MA, USA: Elsevier; p. 163–195. [Google Scholar]
- Yang L, Yuan H, Yang Y, Wang R, Wang C, Wei X, Chen S, Yu J, Ma X. 2020. Enhanced lignin degradation in tobacco stalk composting with inoculation of white-rot fungi Trametes hirsuta and Pleurotus ostreatus. Waste Biomass Valorization. 11(7):3525–3535. doi: 10.1007/s12649-019-00692-z. [DOI] [Google Scholar]
- Yang J, Yue HR, Pan LY, Feng JX, Zhao S, Suwannarangsee S, Champreda V, Liu CG, Zhao XQ. 2023. Fungal strain improvement for efficient cellulase production and lignocellulosic biorefinery: Current status and future prospects. Bioresour Technol. 385:129449. doi: 10.1016/j.biortech.2023.129449. [DOI] [PubMed] [Google Scholar]
- Zeuner B, Thomsen TB, Stringer MA, KBRM K, Meyer AS, Holck J. 2020. Comparative characterization of Aspergillus pectin lyases by discriminative substrate degradation profiling. Front Bioeng Biotechnol. 8:873. doi: 10.3389/fbioe.2020.00873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Sun T, Wu T, Li J, Hu D, Liu D, Li J, Tian C. 2023. Consolidated bioprocessing for bioethanol production by metabolically engineered cellulolytic fungus Myceliophthora thermophila. Metabolic Eng. 78:192–199. doi: 10.1016/j.ymben.2023.06.009. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Li W, Meng D, Li X, Goodell B. 2022. Non-enzymatic modification of the crystalline structure and chemistry of Masson pine in brown-rot decay. Carbohydr Polym. 286:119242. doi: 10.1016/j.carbpol.2022.119242. [DOI] [PubMed] [Google Scholar]
- Zoghlami A, Paës G. 2019. Lignocellulosic biomass: Understanding recalcitrance and predicting hydrolysis. Front Chem. 7:874. doi: 10.3389/fchem.2019.00874. [DOI] [PMC free article] [PubMed] [Google Scholar]


