Abstract
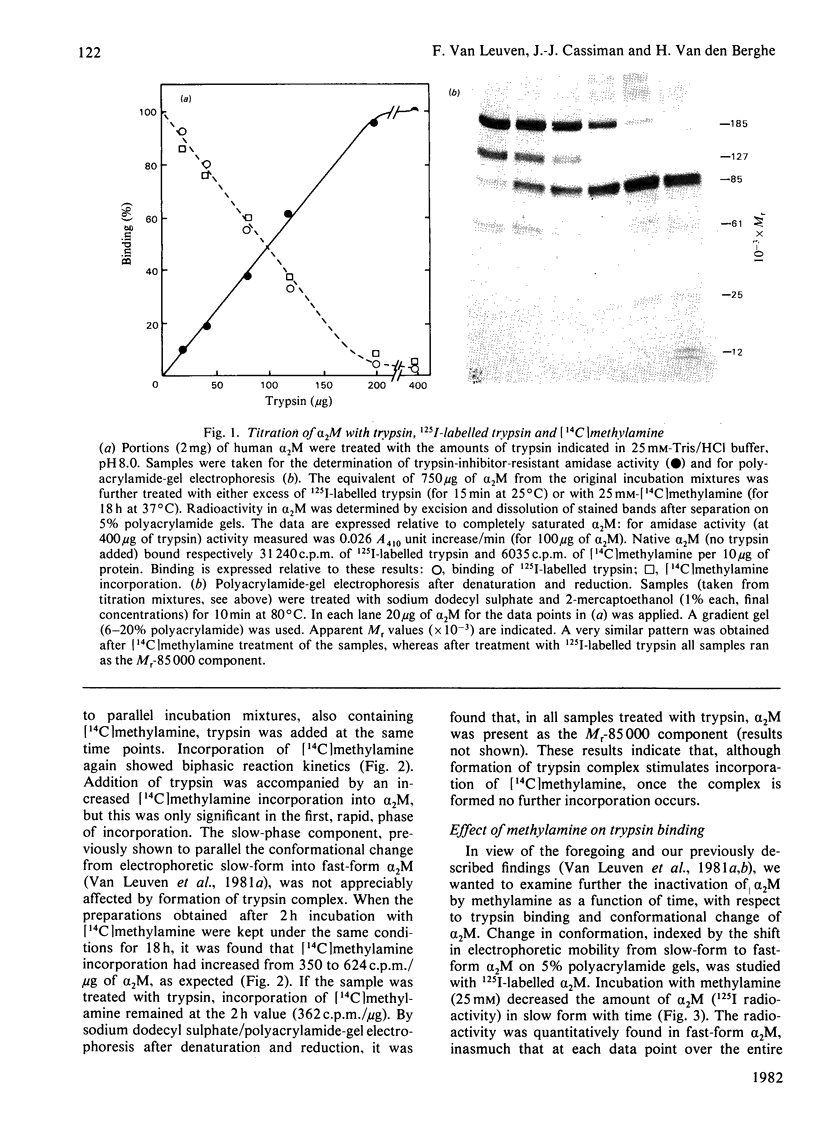
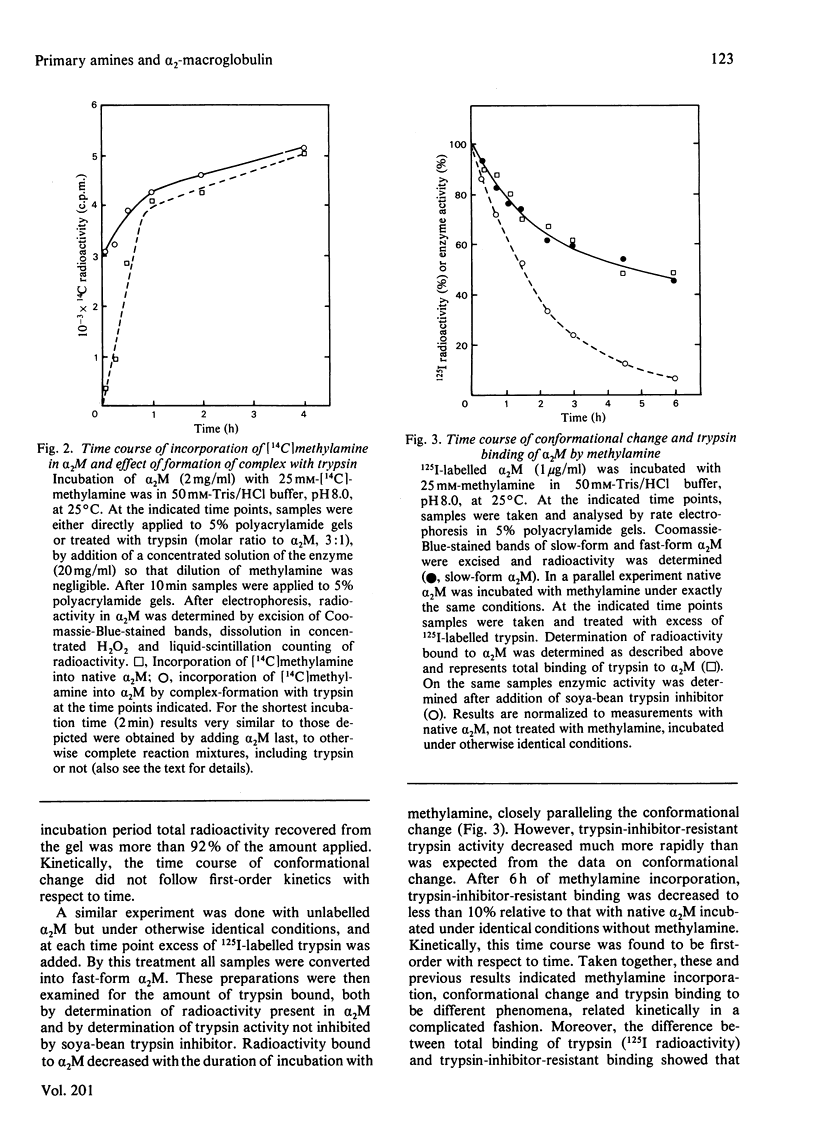
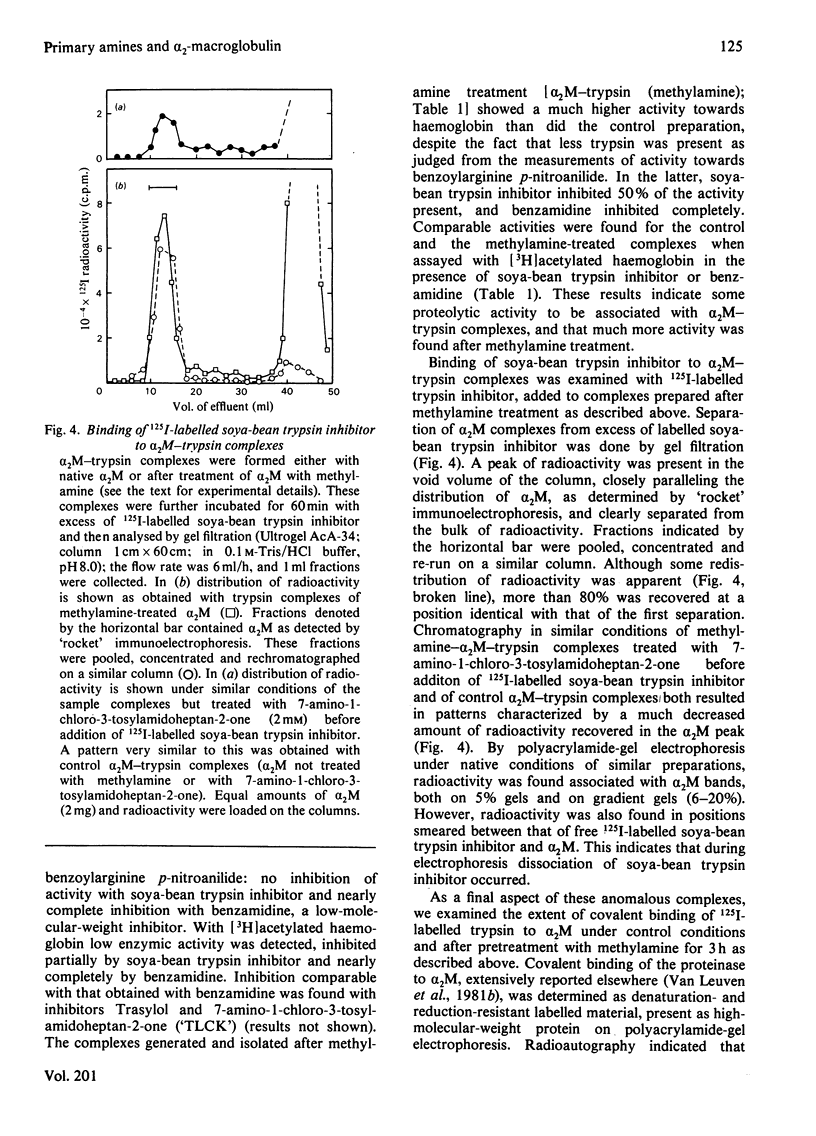
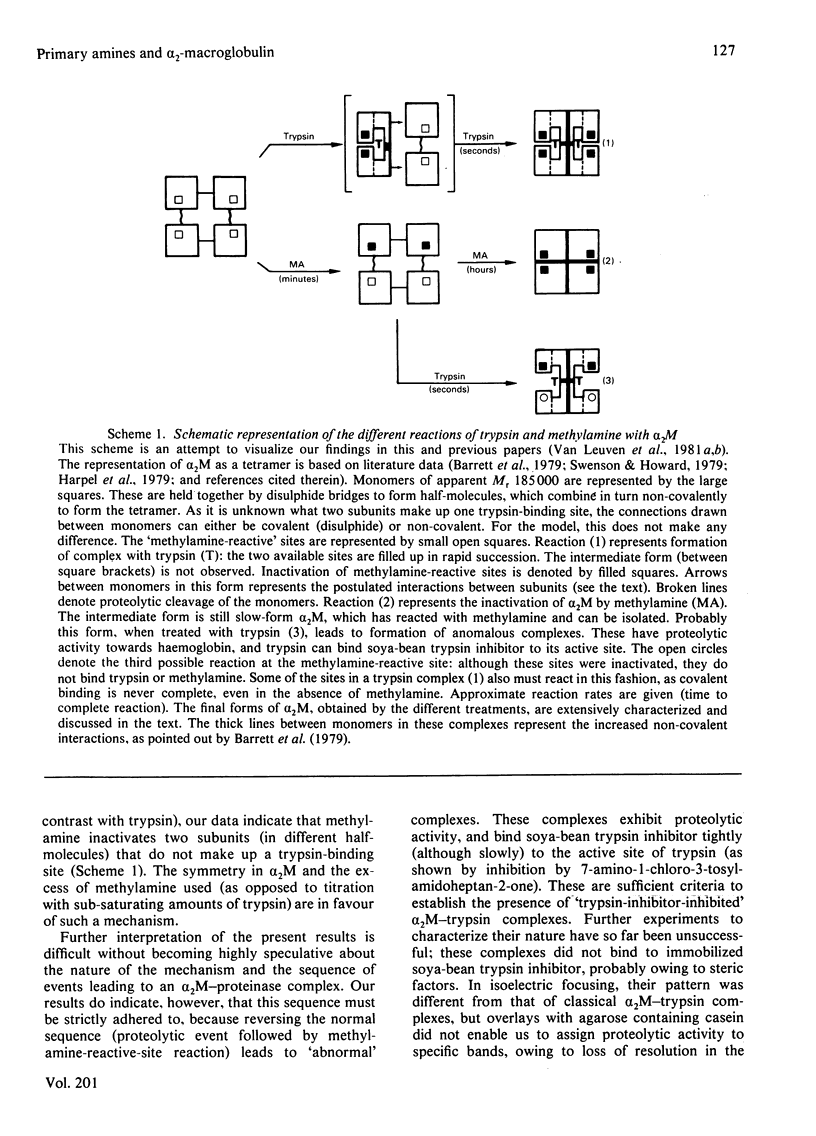
The unique steric inhibition of endopeptidases by human α2M (α2-macroglobulin) and the inactivation of the latter by methylamine were examined in relation to each other. Progressive binding of trypsin by α2M was closely correlated with the loss of the methylamine-reactive sites in α2M: for each trypsin molecule bound, two such sites were inactivated. The results further showed that, even at low proteinase/α2M ratios, no unaccounted loss of trypsin-binding capacity occurred. As α2M is bivalent for trypsin binding and no trypsin bound to electrophoretic slow-form α2M was observed, this indicates that the two sites must react (bind trypsin) in rapid succession. Reaction of [14C]methylamine with α2M was biphasic in time; in the initial rapid phase complex-formation with trypsin caused a largely increased incorporation of methylamine. In the subsequent slow phase trypsin had no such effect. These results prompted further studies on the kinetics of methylamine inactivation of α2M with time of methylamine treatment. It was found that conformational change of α2M and decrease in trypsin binding (activity resistant to soya-bean trypsin inhibitor) showed different kinetics. The latter decreased rapidly, following pseudo-first-order kinetics. Conformational change was much slower and followed complex kinetics. On the other hand, binding of 125I-labelled trypsin to α2M did follow the same kinetics as the conformational change. This discrepancy between total binding (125I radioactivity) and trypsin-inhibitor-resistant binding of trypsin indicated formation of anomalous complexes, in which trypsin could still be inhibited by soya-bean trypsin inhibitor. Further examination confirmed that these complexes were proteolytically active towards haemoglobin and bound 125I-labelled soya-bean trypsin inhibitor to the active site of trypsin. The inhibition by soya-bean trypsin inhibitor was slowed down as compared with reaction with free trypsin. The results are discussed in relation to the subunit structure of α2M and to the mechanism of formation of the complex.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrett A. J., Brown M. A., Sayers C. A. The electrophoretically 'slow' and 'fast' forms of the alpha 2-macroglobulin molecule. Biochem J. 1979 Aug 1;181(2):401–418. doi: 10.1042/bj1810401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debanne M. T., Bell R., Dolovich J. Uptake of proteinase-alpha-macroglobulin complexes by macrophages. Biochim Biophys Acta. 1975 Dec 5;411(2):295–304. doi: 10.1016/0304-4165(75)90309-8. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Anderson R. G., Brown M. S. Coated pits, coated vesicles, and receptor-mediated endocytosis. Nature. 1979 Jun 21;279(5715):679–685. doi: 10.1038/279679a0. [DOI] [PubMed] [Google Scholar]
- Harpel P. C., Hayes M. B., Hugli T. E. Heat-induced fragmentation of human alpha 2-macroglobulin. J Biol Chem. 1979 Sep 10;254(17):8669–8678. [PubMed] [Google Scholar]
- Harpel P. C. Plasmin inhibitor interactions. The effectiveness of alpha2-plasmin inhibitor in the presence of alpha2-macroglobulin. J Exp Med. 1977 Oct 1;146(4):1033–1040. doi: 10.1084/jem.146.4.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard J. B. Reactive site in human alpha 2-macroglobulin: circumstantial evidence for a thiolester. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2235–2239. doi: 10.1073/pnas.78.4.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan J., Nielsen M. L. Analysis of macrophage surface receptors. I. Binding of alpha-macroglobulin . protease complexes to rabbit alveolar macrophages. J Biol Chem. 1979 Aug 10;254(15):7323–7328. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Ohlsson K., Skude G. Demonstration and semiquantitative determination of complexes between various proteases and human alpha2-macroglobulin. Clin Chim Acta. 1976 Jan 2;66(1):1–7. doi: 10.1016/0009-8981(76)90365-x. [DOI] [PubMed] [Google Scholar]
- Parsons M., Romeo G. Cystic fibrosis alpha 2-macroglobulin protease interaction in vitro. Clin Chim Acta. 1980 Jan 31;100(3):215–224. doi: 10.1016/0009-8981(80)90269-7. [DOI] [PubMed] [Google Scholar]
- Sottrup-Jensen L., Petersen T. E., Magnusson S. A thiol-ester in alpha 2-macroglobulin cleaved during proteinase complex formation. FEBS Lett. 1980 Dec 1;121(2):275–279. doi: 10.1016/0014-5793(80)80361-9. [DOI] [PubMed] [Google Scholar]
- Steinbuch M., Pejaudier L., Quentin M., Martin V. Molecular alteration of alpha-2-macroglobulin by aliphatic amines. Biochim Biophys Acta. 1968 Jan 22;154(1):228–231. doi: 10.1016/0005-2795(68)90277-8. [DOI] [PubMed] [Google Scholar]
- Swenson R. P., Howard J. B. Characterization of alkylamine-sensitive site in alpha 2-macroglobulin. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4313–4316. doi: 10.1073/pnas.76.9.4313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tack B. F., Harrison R. A., Janatova J., Thomas M. L., Prahl J. W. Evidence for presence of an internal thiolester bond in third component of human complement. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5764–5768. doi: 10.1073/pnas.77.10.5764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Leuven F., Cassiman J. J., Van Den Berghe H. Demonstration of an alpha2-macroglobulin receptor in human fibroblasts, absent in tumor-derived cell lines. J Biol Chem. 1979 Jun 25;254(12):5155–5160. [PubMed] [Google Scholar]
- Van Leuven F., Cassiman J. J., Van Den Berghe H. Primary amines inhibit recycling of alpha 2M receptors in fibroblasts. Cell. 1980 May;20(1):37–43. doi: 10.1016/0092-8674(80)90232-9. [DOI] [PubMed] [Google Scholar]
- Van Leuven F., Cassiman J. J., Van den Berghe H. Functional modifications of alpha 2-macroglobulin by primary amines. I. Characterization of alpha 2 M after derivatization by methylamine and by factor XIII. J Biol Chem. 1981 Sep 10;256(17):9016–9022. [PubMed] [Google Scholar]
- Van Leuven F., Cassiman J. J., Van den Berghe H. Functional modifications of alpha 2-macroglobulin by primary amines. II. Inhibition of covalent binding of trypsin to alpha 2 M by methylamine and other primary amines. J Biol Chem. 1981 Sep 10;256(17):9023–9027. [PubMed] [Google Scholar]
- Van Leuven F., Cassiman J. J., Van den Berghe H. Uptake and degradation of alpha2-macroglobulin-protease complexes in human cells in culture. Exp Cell Res. 1978 Dec;117(2):273–282. doi: 10.1016/0014-4827(78)90141-6. [DOI] [PubMed] [Google Scholar]



