Abstract
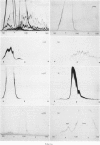
The chromatographic behaviour of 27 different plasma proteins on fractionation of human plasma on immobilized Cibacron Blue F3-GA was studied. The column was eluted by using a three-step procedure. First, a low-molarity buffer (30 mM-H3PO4/Na3PO4, pH 7.0, I0.053) was used, then a linear salt gradient (0-1 M-NaCl in the buffer above) was applied, followed by a wash with two bed volumes of 1.0 M-NaCl. Finally, bound proteins were 'stripped' with 0.5 M-NaSCN. Up to 1 ml of whole plasma could be loaded per 5 ml bed volume. No denaturation of proteinase inhibitors or complement fractions was observed. The recovery of individual proteins ranged between 52 and greater than 95%. Enrichment of four individual plasma components (alpha 1-antitrypsin, caeruloplasmin, antithrombin III and haemopexin) was between 10-fold and 75-fold. These results indicate that chromatography on immobilized Cibacron Blue F3-GA can be a useful initial step in the purification of plasma proteins.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angal S., Dean P. D. The effect of matrix on the binding of albumin to immobilized Cibacron Blue. Biochem J. 1977 Oct 1;167(1):301–303. doi: 10.1042/bj1670301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angal S., Dean P. D. The use of immobilized cibacron blue in plasma fractionation. FEBS Lett. 1978 Dec 15;96(2):346–348. doi: 10.1016/0014-5793(78)80433-5. [DOI] [PubMed] [Google Scholar]
- Bouillon R., Van Baelen H., Rombauts W., De Moor P. The purification and characterisation of the human-serum binding protein for the 25-hydroxycholecalciferol (transcalciferin). Identity with group-specific component. Eur J Biochem. 1976 Jul 1;66(2):285–291. doi: 10.1111/j.1432-1033.1976.tb10518.x. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Crawford I. P. Purification and properties of normal human alpha 1-antitrypsin. Arch Biochem Biophys. 1973 May;156(1):215–222. doi: 10.1016/0003-9861(73)90359-7. [DOI] [PubMed] [Google Scholar]
- GRABAR P., WILLIAMS C. A. Méthode permettant l'étude conjuguée des proprietés électrophorétiques et immunochimiques d'un mélange de protéines; application au sérum sanguin. Biochim Biophys Acta. 1953 Jan;10(1):193–194. doi: 10.1016/0006-3002(53)90233-9. [DOI] [PubMed] [Google Scholar]
- Ganrot P. O. Determination of alpha-2-macroglobulin as trypsin-protein esterase. Clin Chim Acta. 1966 Oct;14(4):493–501. doi: 10.1016/0009-8981(66)90037-4. [DOI] [PubMed] [Google Scholar]
- Görg A., Postel W., Westermeier R., Gianazza E., Righetti P. G. Gel gradient electrophoresis, isoelectric focusing and two-dimensional techniques in horizontal, ultrathin polyacrylamide layers. J Biochem Biophys Methods. 1980 Nov;3(5):273–284. doi: 10.1016/0165-022x(80)90008-1. [DOI] [PubMed] [Google Scholar]
- HJERTEN S. "Molecular sieve" chromatography on polyacrylamide gels, prepared according to a simplified method. Arch Biochem Biophys. 1962 Sep;Suppl 1:147–151. [PubMed] [Google Scholar]
- Harpel P. C. Studies on human plasma alpha 2-macroglobulin-enzyme interactions. Evidence for proteolytic modification of the subunit chain structure. J Exp Med. 1973 Sep 1;138(3):508–521. doi: 10.1084/jem.138.3.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopperschläger G., Freyer R., Diezel W., Hofmann E. Some kinetic and molecular properties of yeast phosphofructokinase. FEBS Lett. 1968 Aug;1(3):137–141. doi: 10.1016/0014-5793(68)80041-9. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laurell C. B. Quantitative estimation of proteins by electrophoresis in agarose gel containing antibodies. Anal Biochem. 1966 Apr;15(1):45–52. doi: 10.1016/0003-2697(66)90246-6. [DOI] [PubMed] [Google Scholar]
- Leatherbarrow R. J., Dean P. D. Studies on the mechanism of binding of serum albumins to immobilized cibacron blue F3G A. Biochem J. 1980 Jul 1;189(1):27–34. doi: 10.1042/bj1890027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe C. R., Small D. A., Atkinson A. Some preparative and analytical applications of triazine dyes. Int J Biochem. 1981;13(1):33–40. doi: 10.1016/0020-711x(81)90133-6. [DOI] [PubMed] [Google Scholar]
- Manolis A., Cox D. W. Purification of rat ceruloplasmin. characterization and comparison with human ceruloplasmin. Prep Biochem. 1980;10(2):121–132. doi: 10.1080/00327488008061728. [DOI] [PubMed] [Google Scholar]
- Measurement of alpha 1-antitrypsin in serum, by immunodiffusion and by enzymatic assay. Clin Chem. 1974 Mar;20(3):396–399. [PubMed] [Google Scholar]
- Morii M., Odani S., Koide T., Ikenaka T. Human alpha1-antitrypsin. Characterization and N- and C-terminal sequences. J Biochem. 1978 Jan;83(1):269–277. doi: 10.1093/oxfordjournals.jbchem.a131900. [DOI] [PubMed] [Google Scholar]
- Noyer M., Dwulet F. E., Hao Y. L., Putnam F. W. Purification and characterization of undegraded human ceruloplasmin. Anal Biochem. 1980 Mar 1;102(2):450–458. doi: 10.1016/0003-2697(80)90181-5. [DOI] [PubMed] [Google Scholar]
- Subramanian S., Kaufman B. T. Dihydrofolate reductases from chicken liver and Lactobacillus casei bind Cibacron blue F3GA in different modes and at different sites. J Biol Chem. 1980 Nov 25;255(22):10587–10590. [PubMed] [Google Scholar]
- Svendsen P. J. Fused rocket immunoelectrophoresis. Scand J Immunol Suppl. 1973;1:69–70. doi: 10.1111/j.1365-3083.1973.tb03781.x. [DOI] [PubMed] [Google Scholar]
- Thompson S. T., Cass K. H., Stellwagen E. Blue dextran-sepharose: an affinity column for the dinucleotide fold in proteins. Proc Natl Acad Sci U S A. 1975 Feb;72(2):669–672. doi: 10.1073/pnas.72.2.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis J., Bowen J., Tewksbury D., Johnson D., Pannell R. Isolation of albumin from whole human plasma and fractionation of albumin-depleted plasma. Biochem J. 1976 Aug 1;157(2):301–306. doi: 10.1042/bj1570301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis J., Garner D., Bowen J. Human alpha-1-antichymotrypsin: purification and properties. Biochemistry. 1978 Dec 26;17(26):5647–5651. doi: 10.1021/bi00619a010. [DOI] [PubMed] [Google Scholar]
- Travis J., Pannell R. Selective removal of albumin from plasma by affinity chromatography. Clin Chim Acta. 1973 Nov 23;49(1):49–52. doi: 10.1016/0009-8981(73)90341-0. [DOI] [PubMed] [Google Scholar]
- Virca G. D., Travis J., Hall P. K., Roberts R. C. Purification of human alpha-2-macroglobulin by chromatography on Cibacron Blue Sepharose. Anal Biochem. 1978 Aug 15;89(1):274–278. doi: 10.1016/0003-2697(78)90750-9. [DOI] [PubMed] [Google Scholar]