Highlights
-
•
Humans with complex regional pain syndrome (CRPS) exhibited altered microbiota.
-
•
A diagnosis of CRPS is associated with functional differences compared to controls.
-
•
Longitudinal microbiota changes were seen in a mouse model of CRPS.
-
•
Microbiota changes may reflect pain chronicity.
Keywords: Complex regional pain syndrome, Gut microbiota, Microbiome, Chronic pain, Injury
Abstract
Objective
This study aimed to evaluate pain metrics and gut microbiota differences from human subjects with complex regional pain syndrome (CRPS) compared to cohabitants (HHC) and non-cohabitating (biobank) controls. In addition, we aimed evaluate longitudinal changes of gut microbiota using a mouse model of acute and chronic CRPS.
Methods
In an observational, cross-sectional study, 25 patients with CRPS and 24 household controls (HHC) were recruited, completed pain questionnaires, and submitted stool samples. 23 biobank stool samples were matched to the CRPS group. Additionally, longitudinal stool samples were collected from a mouse model of acute and chronic CRPS. 16S rRNA gene sequencing analysis was performed on all samples.
Results
A diagnosis of CRPS is associated with higher pain, increased pain interference, and decreased physical and social function when compared to HHC. Interestingly, 46% of HHC reported significant daily pain. In the households where HHC were also suffering from pain, there was decreased bacterial richness and diversity when compared to households wherein only the participant with CRPS suffered from pain. Furthermore, when comparing households where the HHC had significant pain, CRPS was clinically more severe. In the mouse model of CRPS, we observed decreased bacterial richness and diversity when compared to non-cohabitating littermate controls.
Conclusions
Both humans living in chronic pain households and mice shared distinct taxa over the time course of disease and pain chronicity. These findings suggest that microbiota changes seen in CRPS as well as in a mouse model of CRPS may reflect pain chronicity and may indicate that pain alone can contribute to microbiota dysbiosis. The trial was registered at ClinicalTrials.gov (NCT03612193).
Introduction
Complex regional pain syndrome (CRPS) is a rare and devastating condition of unknown etiology estimated to affect 5.4–26.2 per 100,000 person-years (Petersen et al., 2018). CRPS usually occurs following trauma or surgery and is characterized by severe pain out of proportion to an initial injury (Iolascon et al., 2015). Per the ‘Budapest Criteria’, CRPS is a diagnosis of exclusion with signs in at least two and symptoms in at least three categories: sensory, vasomotor, sudomotor and motor/trophic (Harden et al., 2010). Most patients will have significant symptomatic recovery when CRPS is identified early (<12 months, acute); however, when CRPS is chronic (> 12 months), recovery and response to treatment is less likely, resulting in great personal and societal costs (van Velzen et al., 2014). The factors contributing to the transition from acute to chronic CRPS remain unclear.
The cause(s) of CRPS remain uncertain, and thus identification of biomarkers for CRPS recovery or chronicity could lead to improvements in clinical care. Previous work has demonstrated that women with chronic CRPS (average duration 5 years) exhibit changes to their intestinal microbiota community structure when compared to non-cohabitating healthy controls (Reichenberger et al., 2013). However, cohabitation alone is known to have a significant effect on gut microbiota taxa (Gacesa et al., 2022). Previous studies have not compared gut microbiota in those with CRPS to cohabitating household members (Crock and Baldridge, 2020).
Numerous areas remain to be explored in the field of CRPS. Why do some patients develop CRPS? Once CRPS has developed, what factors drive acute pain, disability and other associated symptoms of CRPS to become chronic (Crock and Baldridge, 2020)? Does gut microbiota composition influence, or reflect, the transition from acute CRPS to chronic CRPS? Preclinical models of pain have demonstrated that an intact gut microbiota (one prior to high dose antibiotic treatment) is necessary for the development of chemotherapy-induced neuropathic (Shen et al., 2017) and inflammatory pain (Amaral et al., 2008). Recent clinical studies have demonstrated a clinical correlation between gut microbiota and osteoarthritis knee pain (Boer et al., 2019), widespread musculoskeletal pain (Minerbi et al., 2019, Freidin et al., 2021), neuropathic pain (Ellis et al., 2022) and postoperative pain (Brenner et al., 2021, Yao et al., 2021). We hypothesized that differences in gut microbiota composition may contribute to or be reflected in CRPS in both humans and mice (Crock and Baldridge, 2020).
Here, we describe both pain metrics and the gut microbiota composition in an observational cross-sectional study of human participants suffering from acute and chronic CRPS compared to both cohabitants (HHC) and non-cohabitating controls. We observed unusually high rates of chronic pain in the household members of participants with CRPS. No differences were observed when comparing gut microbiota diversity in the full cohort of participants with CRPS to their cohabitating household members (HHC). However, given the high rate of chronic pain in household members, we evaluated the effect of household pain status on gut microbiota metrics. Interestingly, we found a significant decrease in bacterial richness and Shannon diversity when we compared participants with CRPS who live with someone with chronic pain (HHC-pain) versus those with CRPS who live with someone without pain (HHC-NO pain). This study is the first to suggest that cohabitating with individuals with chronic pain is associated with reduced gut microbial diversity. In addition, we observed changes in the composition of the intestinal microbiota in a longitudinal mouse model of acute and chronic CRPS. In mice, chronic injury alone produces gut microbiota changes similar to those observed in humans with chronic pain.
Methods
Human study design and oversight
The study was conducted with informed consent under the Washington University Institutional Review Board (IRB, 201806182). The trial was registered at ClinicalTrials.gov (NCT03612193).
Patient recruitment and clinical evaluation
This was a single-center cross-sectional observational case-controlled study. Adults (18 years or older) with a confirmed diagnosis of complex regional pain syndrome (CRPS) were recruited through the Pain Management Center at Washington University/Barnes Jewish Hospital medical campus in St. Louis, Missouri, United States from 12/19/18–10/4/19. Study size was determined by available patient recruitment prior to the COVID-19 shutdown. Inclusion criteria for patients: confirmed current or previous diagnosis of CRPS by Budapest criteria. Inclusion criteria for household controls: cohabitation with participant in study with a diagnosis of CRPS. Exclusion criteria for both were: current use of probiotics, pregnant or lactating, or extreme (paleo, ketogenic, or vegan) diet. One household control was enrolled per subject with CRPS with the exception of one participant with CRPS who lived alone. One-to-one unrelated age-, sex-, smoking status-, race-, ethnicity- and BMI- matched control Biobank samples were obtained from the Washington University Digestive Disease Research Core Center Biobank Core (Biobank samples, see Table 1).
Table 1.
Characteristics of all participants. Patients mean score on short Brief Pain Inventory ± SD and the percentage of respondents with “substantial interference” as defined by a score of >/= 5/10. Pain catastrophizing was determined by the Pain Catastrophizing Scale (PCS), general health metrics calculated by the RAND-36 short form (SF-36), and cognitive flexibility calculated by Stroop Color and Word T-Score. Mean ± SD, percentage (%) or number (n). CRPS age range 20–74 years, HHC age range 35–75 years, Biobank age range 26–75. Included one participant in CRPS group and HHC group who did not submit a stool sample.
| CRPS | Biobank Controls | HHC | |||
|---|---|---|---|---|---|
| n | 25 | 23 | 24 | ||
| Characteristics | |||||
| Age (years) | 51.7 ± 14.2 | 51.8 ± 12.4 | 0.884 | 57.7 ± 12.1 | 0.13 |
| % Female | 80 | 78 | 1 | 25 | <0.001 |
| BMI (kg/m2) | 29.8 ± 6.6 | 28.9 ± 6.2 | 0.646 | 30.1 ± 5.9 | 0.88 |
| % Caucasian | 100 | 100 | 1 | 100 | 1 |
| % non-Hispanic | 100 | 100 | 1 | 100 | 1 |
| Smokers (n) | 3 | 2 | 0.964 | 2 | 0.68 |
| CRPS Severity Score (0–16) | 9.9 ± 3.2 | n/a | |||
| Location of CRPS | |||||
| Lower extremity CRPS (n) | 15 | n/a | |||
| Upper Extremity CRPS | 8 | n/a | |||
| Both Upper and Lower Extremity CRPS (n) | 2 | n/a | |||
| Current medications (n) | |||||
| Opioid use | 6 | 3 | 0.31 | ||
| NSAID or Acetaminophen use | 7 | 7 | 0.94 | ||
| Anticonvulsant use | 11 | 0 | <0.001 | ||
| Muscle relaxant use | 4 | 2 | 0.42 | ||
| Low-dose naltrexone | 9 | 0 | 0.001 | ||
| Tricyclic antidepressant use | 6 | 0 | 0.01 | ||
| Antidepressant use | 4 | 0 | 0.04 | ||
| No treatments | 1 | 9 | 0.003 | ||
| Bowel habits | |||||
| Cleveland Constipation Score | 5.2 ± 4.7 | 4.5 ± 3.4 | 0.56 | ||
| Bristol stool scale | 3.7 ± 1.3 | 3.6 ± 1.5 | 0.73 | ||
| Pain Metrics | |||||
| Brief Pain Inventory (BPI) | |||||
| Pain more than normal everyday pain (n = yes) | 21, 84 % | 11, 46 % | 0.003 | ||
| Worst Pain 24hr (0–10) | 6.6 ± 2.8 | 2.8 ± 2.9 | <0.001 | ||
| Least Pain 24hr (0–10) | 3.2 ± 2.2 | 1 ± 1.4 | <0.001 | ||
| Average Pain (0–10) | 4.8 ± 2.1 | 1.7 ± 1.9 | <0.001 | ||
| Current pain | 4.4 ± 2.6 | 1.2 ± 1.7 | <0.001 | ||
| Pain Interference | |||||
| Activity (0–10), % substantial interference | 5.8 ± 3.3, 72 | 1.8 ± 3.0, 17 | <0.001 | ||
| Mood (0–10), % substantial interference | 4.6 ± 3.5, 44 | 1.4 ± 2.6, 21 | <0.001 | ||
| Mobility (0–10), % substantial interference | 5.1 ± 3.7, 60 | 0.9 ± 1.6, 4 | <0.001 | ||
| Work (0–10), % substantial interference | 5.8 ± 3.4, 76 | 2.2 ± 3.2, 17 | <0.001 | ||
| Relations (0–10), % substantial interference | 3.2 ± 3.1, 28 | 0.8 ± 2.2, 8 | 0.003 | ||
| Sleep (0–10), % substantial interference | 5.6 ± 3.4, 64 | 1.2 ± 2.3, 8 | <0.001 | ||
| Enjoyment (0–10), % substantial interference | 5.2 ± 3.3, 60 | 1.1 ± 2.1, 13 | <0.001 | ||
| Pain Catastrophizing Scale (PCS) | |||||
| Mean PCS (0–52) | 23.4 ± 15.5 | 13.3 ± 12.1 | 0.02 | ||
| PCS Rumination | 8.1 ± 5.2 | 5.4 ± 4.8 | 0.07 | ||
| PCS Magnification | 4.4 ± 3.8 | 2.1 ± 2.4 | 0.02 | ||
| PCS Helpless | 10.6 ± 7.3 | 5.3 ± 5.8 | 0.01 | ||
| General Health Metrics (SF-36) | |||||
| Physical Functioning | 45.2 ± 27.0 | 88.1 ± 15.0 | <0.001 | ||
| Role limitations due to physical health | 25.0 ± 35.4 | 65.6 ± 45.0 | 0.001 | ||
| Role limitations due to emotional problems | 74.7 ± 34.4 | 84.7 ± 33.3 | 0.31 | ||
| Energy/fatigue | 40.0 ± 24.7 | 59.6 ± 19.0 | 0.004 | ||
| Emotional well-being | 65.6 ± 24.0 | 79.0 ± 14.2 | 0.03 | ||
| Social Functioning | 62.0 ± 27.5 | 84.9 ± 18.0 | 0.002 | ||
| Pain | 33.7 ± 28.5 | 71.8 ± 24.0 | <0.001 | ||
| General Health | 61.0 ± 28.7 | 71.9 ± 20.0 | 0.14 | ||
| Stroop Color and Word T-Score | 51.1 ± 6.9 | 50.7 ± 10.8 | 0.85 | ||
Clinical measurements
Participants underwent a detailed medical history and physical exam. All participants were asked about recent (last 6 months) antibiotic use and current medications. Participants filled out standardized brief pain inventory short form (BPI) (Keller et al., 2004), pain catastrophizing scale (PCS) (Sullivan and Pivik, 1995), 36-item short form survey (RAND SF-36 1.0), and CRPS severity score (CSS, 0–16 scale) (Harden et al., 2017). Bowel habits were evaluated using the Cleveland constipation score (CCS) (Agachan et al., 1996) and the Bristol stool chart (Lewis and Heaton, 1997). Cognitive flexibility was determined by the color-word matching Stroop test (CWMST) (Stoelting Co., Wood Dale, IL, USA). Data managed using REDCap electronic data capture tools hosted at Washington University.
Human sample collection and processing
Stool samples from all participants were collected by the participants at their home and delivered to the lab fresh but cold for aliquoting and storage at −80 degrees Celsius.
Animal ethics statement
All mice were used under regulations stipulated by Stanford University Institutional Animal Care and Use Committee and on protocols approved by the Stanford University Animal Studies Committee which is accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care (AAALAC International) and by the Veterans Affairs Palo Alto Health Care System Institutional Animal Care and Use Committee in accordance with American Veterinary Medical Association guidelines and the International Association for the Study of Pain. The procedures uphold all federal and state regulations governing the humane care and use of laboratory animals. To comply with the Stanford Administrative Panel on Laboratory Animal Care guidelines on minimizing animal numbers, for each experimental paradigm we used the minimum number of mice required to obtain significance after conducting a power analysis based on the expected mean and SD from existing data for a given assay.
Mouse acute and chronic pain model
C57BL/6J male mice (10–11 weeks) were obtained from Jackson Laboratories, Bar Harbor, ME, USA. All animals were maintained under a 12:12 h light/dark cycle in a temperature-controlled environment with ad libitum access to food and water. Littermates were housed 2–5 per cage before surgery and following the tibial fracture CRPS model. Uninjured controls were similarly housed but did not undergo surgery or anesthesia. As previously described (Tajerian et al., 2015), mice were anesthetized with inhalational isoflurane 2–4 % for induction and 1.5–2.5 % for maintenance. While anesthetized with isoflurane, the right hind limb was wrapped in gauze and a hemostat was used to make a closed fracture of the distal tibia. The hind limb was then wrapped in casting tape (ScotchCast™ Plus) from the metatarsals of the hind paw up to a spica formed around the abdomen to ensure that the cast did not slip off. The cast over the paw was applied only to the plantar surface with a window left open over the dorsum of the paw and ankle to prevent constriction when post-fracture edema developed. Mice were inspected throughout the post-operative period of cast immobilization to ensure that the cast was properly positioned. 3 weeks post-fracture, mice were briefly anesthetized, and casts were removed with cast shears. Pain behavior was considered acute at cast removal, chronic after 5 weeks and nearly resolved at 20 weeks following fracture. Mechanical allodynia as determined by Von Frey testing and percentage weight bearing of the injured and uninjured paw was performed as described previously (n = 10) (Huck et al., 2021). Briefly, a female researcher (G.B.P.M) performed all behavioral testing in a blinded fashion. All testing was conducted between 7:00 am and 1:00 pm in an isolated, temperature- and light-controlled room. Mice were acclimated for 30 to 60 min in the testing environment within custom clear plastic cylinders (4″ D) on a raised metal mesh platform (24″ H). Mice were randomized by simple selection from their home cage (2–5 mice per cage) before testing and placed in a cylinder. Stool samples were collected fresh, and frozen and stored at −80 degrees at the following timepoints: baseline (prior to fracture), 3 weeks, 5 weeks, 9 weeks and 16 weeks post-fracture. Two independent sets of experiments were performed.
Processing and taxonomic analysis of human and mouse stool samples Phenol:chloroform-extracted DNA from fecal samples was used for 16S rRNA gene analysis as previously described (Caporaso et al., 2011).
2,556 to 372,376 reads were obtained for each sample and excluded if less than 1000 reads. Samples were rarefied to 2,556 reads for subsequent analyses. Read quality control and the resolution of amplicon sequence variants (ASVs) were performed with the dada2 R package (Callahan et al., 2016). ASVs not assigned to the kingdom Bacteria were filtered out; no additional abundance-based filtering of ASVs was performed nor was any abundance transformation performed prior to analyses. Remaining reads were assigned taxonomy using the Ribosomal Database Project (RDP trainset 16/release 11.5) 16S rRNA gene sequence database (Cole JR, Wang Q, Fish JA, Chai B, McGarrell DM, Sun Y, et al. Ribosomal Database Project: data and tools for high throughput rRNA analysis. Nucleic Acids Res, 2014) or SILVA v138.1. Ecological analyses, such as alpha-diversity (richness, Shannon diversity) and beta-diversity analyses (weighted and unweighted UniFrac distances), were performed using PhyloSeq and additional R packages (McMurdie and Holmes, 2013), and differentially abundant taxa at all phylogenetic levels between sample groups were identified by performing pairwise comparisons using Linear discriminant analysis Effect Size (LEfSe) (Segata et al., 2011). LEfSe has been reported to have a high false discovery rate (Nearing et al., 2022), so discriminant taxa analysis was also performed using DESeq2 to identify differential individual ASVs (Love et al., 2014).
Statistical analysis
Data were analyzed with Prism 9 software (GraphPad Software, San Diego, CA). Group characteristics was assessed using two-sample t-test or Chi square test, or their non-parametric equivalent. Biobank samples were matched with all available data including sex, BMI, and smoking status. All statistical tests are two-sided with a significance level of 0.05. In all graphs, p value was determined by Mann-Whitney test, Wilcoxon, one-way analysis of variance (ANOVA) or Kruskal-Wallis test, or two-way ANOVA with Tukey's multiple-comparison test, as specified in the figure legends. Differences in beta-diversity were determined using Permutational Multivariate Analysis of Variance (ADONIS). R package stats (version 4.1.2) was used for linear regression modeling, and R package lme4 (version 1.1.28) was used for linear fixed-effects modeling.
All data generated as part of this study are included in this published article and its supplementary files. 16S rRNA gene sequencing data have been uploaded to the European Nucleotide Archive (accession no. PRJEB44143). Phyloseq data is available at the following websites: Human: https://github.com/RachelRodgers/201129-Human-CRPS-Combined Mouse: https://github.com/RachelRodgers/201113-Mouse-CRPS-Combined.
Results
Characteristics of study participants
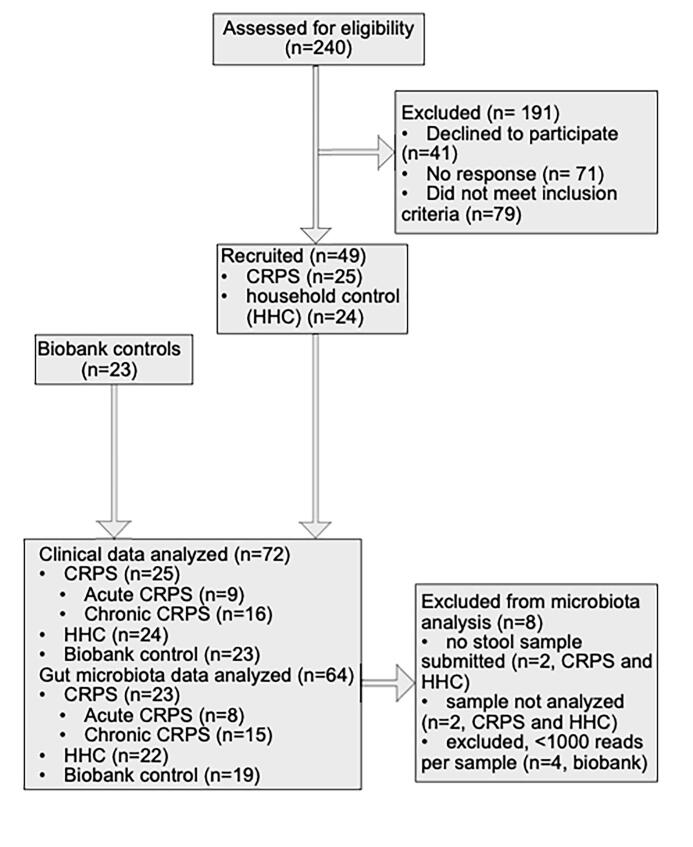
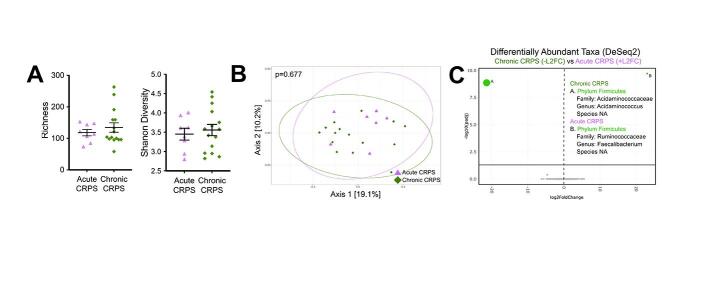
240 participants were screened, and 49 who met eligibility criteria participated. Two participants (a CRPS patient and their cohabitant) decided not to submit stool samples after answering all questionnaires. Their responses were included in Table 1 and Supplementary Table 1A. 23 control Biobank samples were matched to enrolled CRPS participants with stool samples based on available host-intrinsic and extrinsic microbiota factors (Schmidt et al., 2018) (Supplementary Figure 1). Of the 72 participants (Table 1), 25 had CRPS, 24 lived with participants with CRPS (household controls, HHC) and 23 were matched controls (Biobank). Age, BMI and smoking status were similar between participants with CRPS compared to HHC (Table 1). CRPS were more likely to be female (80 %) and take medications for neuropathic pain (anticonvulsants, antidepressants and low-dose naltrexone). HHC had a similar rate of medication use as their afflicted counterparts. Acute versus chronic CRPS were similar (Supplementary Table 1A).
Stool consistency and rate of constipation is similar between participants with CRPS and household members
Rates of constipation and stool consistency were similar between CRPS and HHC (Table 1) but not available for biobank samples. No significant differences in stool consistency or constipation were found between participants with CRPS based on household pain status (HHC-pain vs HHC-NO pain Table 2) or acute and chronic CRPS (Supplementary Table 1A).
Table 2.
Characteristics of participants with CRPS based on household pain status. Patients mean score on short Brief Pain Inventory ± SD and the percentage of respondents with “substantial interference” as defined by a score of >/= 5/10. Pain catastrophizing was determined by the Pain Catastrophizing Scale (PCS), general health metrics calculated by the RAND-36 short form (SF-36), and cognitive flexibility calculated by Stroop Color and Word T-Score. Mean ± SD, percentage (%) or number (n). CRPS age range 20–74 years. Excluded one participant who did not submit a stool sample and one participant who did not have a corresponding household member.
| CRPS w/chronic pain HHC | CRPS w/HHC without chronic pain | ||
|---|---|---|---|
| n | 11 | 12 | |
| Demographics | |||
| Age (years) | 54.9 ± 10.2 | 48.2 ± 17.4 | 0.28 |
| % Female | 82 | 83 | 0.93 |
| BMI (kg/m2) | 30.1 ± 7.8 | 29.7 ± 6.3 | 0.90 |
| Duration of symptoms (m) | 57.2 ± 66.7 | 39.3 ± 41.9 | 0.45 |
| % Acute CRPS | 27.3 | 33.3 | |
| % Caucasian | 100 | 100 | 1 |
| % non-Hispanic | 100 | 100 | 1 |
| Smokers (n) | 0 | 2 | 0.17 |
| CRPS Severity Score (0–16) | 11.5 ± 1.8 | 7.9 ± 3.5 | 0.01 |
| Current Medications (n) | |||
| Opioid use | 5 | 1 | 0.04 |
| NSAID or Acetaminophen use | 2 | 5 | 0.24 |
| Anticonvulsant use | 3 | 7 | 0.15 |
| Muscle relaxant use | 2 | 2 | 0.93 |
| Low-dose naltrexone | 3 | 6 | 0.29 |
| Tricyclic antidepressant use | 4 | 2 | 0.30 |
| Antidepressant use | 2 | 2 | 0.93 |
| No treatments | 0 | 1 | 0.35 |
| Bowel habits | |||
| Cleveland Constipation Score | 6.2 ± 5.4 | 3.3 ± 3.3 | 0.14 |
| Bristol stool scale | 4.3 ± 1.4 | 3.0 ± 1.1 | 0.09 |
| Pain Metrics | |||
| Brief Pain Inventory (BPI) | |||
| Pain more than normal everyday pain (n, %) | 100 | 75 | 0.08 |
| Worst Pain 24hr (0–10) | 7.6 ± 1.5 | 5.5 ± 3.1 | 0.05 |
| Least Pain 24hr (0–10) | 3.9 ± 2.0 | 2.3 ± 2.1 | 0.07 |
| Average Pain (0–10) | 5.6 ± 1.6 | 3.9 ± 2.3 | 0.06 |
| Current pain | 5.0 ± 2.0 | 3.8 ± 2.8 | 0.28 |
| Percent Relief with Treatment | 44.6 ± 18.1 | 51.8 ± 29.3 | 0.49 |
| Pain Interference | |||
| Activity (0–10), % substantial interference | 7.1 ± 2.7 | 4.7 ± 3.8 | 0.09 |
| Mood (0–10), % substantial interference | 5.5 ± 3.4 | 3.4 ± 3.2 | 0.17 |
| Mobility (0–10), % substantial interference | 6.7/- 3.5 | 4.4 ± 3.4 | 0.13 |
| Work (0–10), % substantial interference | 6.7 ± 2.9 | 4.8 ± 4.0 | 0.20 |
| Relations (0–10), % substantial interference | 2.5 ± 3.1 | 3.2 ± 3.1 | 0.64 |
| Sleep (0–10), % substantial interference | 6.0 ± 3.1 | 4.8 ± 4.0 | 0.44 |
| Enjoyment (0–10), % substantial interference | 5.8 ± 3.4 | 4.4 ± 3.7 | 0.35 |
| Pain Catastrophizing Score (PCS) | |||
| Mean PCS (0–52) | 25.6 ± 17.5 | 19.5 ± 14.4 | 0.38 |
| PCS Rumination | 9.0 ± 5.8 | 6.8 ± 5.1 | 0.36 |
| PCS Magnification | 4.1 ± 3.8 | 3.8 ± 4.0 | 0.87 |
| PCS Helpless | 11.8 ± 8.6 | 8.6 ± 6.5 | 0.33 |
| General Health Metrics (SF-36) | |||
| Physical Functioning | 36.4 ± 22.9 | 53.8 ± 30.4 | 0.14 |
| Role limitations due to physical health | 15.9 ± 25.7 | 37.5 ± 43.3 | 0.17 |
| Role limitations due to emotional problems | 63.6 ± 37.9 | 86.1 ± 30.0 | 0.13 |
| Energy/fatigue | 41.5 ± 21.6 | 38.3 ± 27.5 | 0.77 |
| Emotional well-being | 67.6 ± 26.6 | 66.7 ± 21.5 | 0.92 |
| Social Functioning | 60.0 ± 21.1 | 66.7 ± 35.9 | 0.61 |
| Pain | 22.0 ± 22.1 | 44.2 ± 34.3 | 0.09 |
| General Health | 60.5 ± 29.2 | 63.8 ± 32.3 | 0.81 |
| Stroop Color and Word T-Score | 50.5 ± 6.3 | 50.8 ± 7.5 | 0.93 |
CRPS is associated with higher pain, increased pain interference, and decreased physical and social function when compared to household members
CRPS was associated with significantly more pain across all categories (Table 1). A substantial proportion (11/24, 46 %) of HHC reported significant daily pain described as more than normal everyday pain. Of the controls with daily pain, most (7/11) had a diagnosis of a painful medical condition such as low back pain (n = 1), fibromyalgia (n = 1), arthritis (n = 2), rheumatoid arthritis (n = 1), and daily use of naproxen for unspecified pain (n = 1). One HHC had reported depression/anxiety. 4 of 11 HHC-pain had no diagnoses and reported taking no medications for pain relief. Pain interference was significantly higher in those with CRPS vs HHC.
CRPS patients had decreased physical function, higher pain and limitations in their daily life due to physical health, decreased energy, and decreased social functioning. No significant differences in their reported ability to function because of emotional problems or overall assessment of their general health were found when compared to their HHC. CRPS has been associated with neurocognitive dysfunction (Kolb et al., 2012). In our cohort, no differences were found when cognitive flexibility was assessed using the Stroop Color-word Test between participants with CRPS and HHC (Table 1).
Household pain status associated with more severe CRPS, higher opioid use and higher worst pain
Those with CRPS who cohabitate with household members with daily pain (HHC-pain) were not significantly different in age, gender, BMI, bowel habits, duration of CRPS, pain interference, pain catastrophizing, or general health metrics when compared to participants with CRPS who live with a household member without chronic pain (HHC-NO pain). Conversely, participants with CRPS who live with HHC-pain were more likely to use opioids, have more severe CRPS (CSS 11.5 vs 7.9) and have more severe worst pain as assessed by the brief pain inventory (7.6) when compared to participants with CRPS who lived with a HHC-NO pain (5.5) (Table 2).
Chronic CRPS is associated with chronic opioid use, decreased general health compared to participants with acute CRPS
No difference in BPI metrics was observed comparing those with acute versus chronic CRPS. Chronic CRPS was associated with increased opioid use and lower general health compared to acute CRPS (Supplementary Table 1A).
Household controls with chronic pain were more likely to use opioids, suffer from constipation, and to have higher pain scores, higher pain interference, helplessness due to pain and decreased physical functioning.
HHC-pain were not significantly different in age, gender, and BMI when compared to HHC-NO pain. HHC-pain compared to HHC-NO pain were more likely to use daily opioids (n = 3 of 11, 27 % versus n = 0, 0 %) and suffer from constipation (Supplementary Table 1B). Pain interference was higher in HHC-pain (except for interference on relationships) when compared to HHC-NO pain (Supplementary Table 1B). HHC-pain were more likely to have lower physical functioning and limitations due to health and pain (Supplementary Table 1B).
Decreased gut microbiota richness and diversity in chronic pain households
To evaluate gut microbiota of participants with CRPS compared to non-cohabitating matched controls, we analyzed samples from 72 participants (23 CRPS, 22 HHC, 23 Biobank controls) using Illumina-based sequencing of the V4 region of the 16S rRNA gene. 4 samples were excluded due to inadequate read depth, with an average of 60,415 reads per sample for the 68 remaining samples.
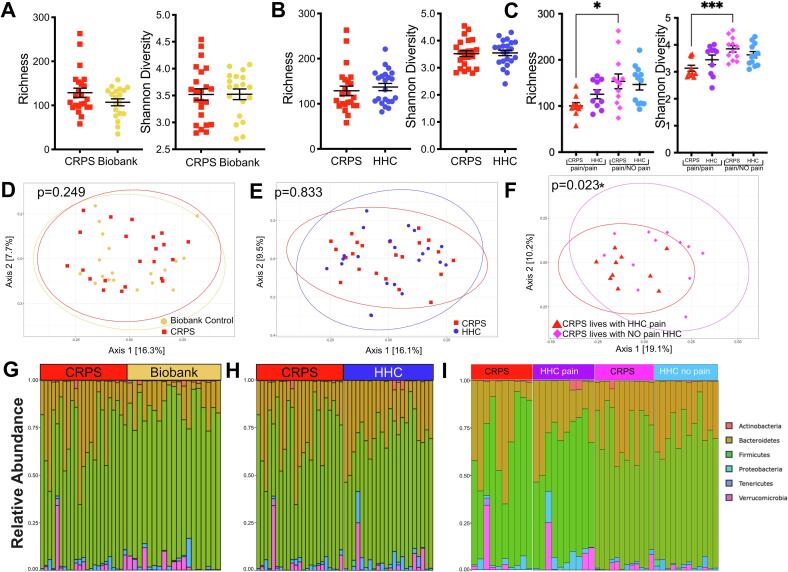
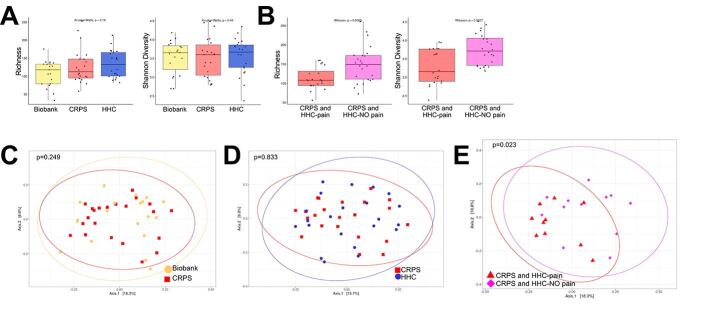
Comparing both the number of distinct taxa (bacterial richness) and the distribution of the abundance of these taxa (Shannon diversity) between participants with CRPS and either Biobank controls (Fig. 1A) or HHC (Fig. 1B), no significant differences were observed. Similarly, no differences were observed when comparing participants with acute versus chronic CRPS (Supplementary Figure 2A,B). Household pain status was associated with decreased bacterial richness and decreased Shannon diversity in those with CRPS (Fig. 1C) as well as pain (HHC-pain and CRPS) households (Fig. 3A, B). Chronic pain in a parent can increase the risk of developing chronic post-surgical pain in children (Rabbitts et al., 2017). These preliminary findings suggest that factors such as the presence of household members with chronic pain and CRPS may synergistically decrease gut microbiota diversity in addition to increasing pain severity.
Fig. 1.
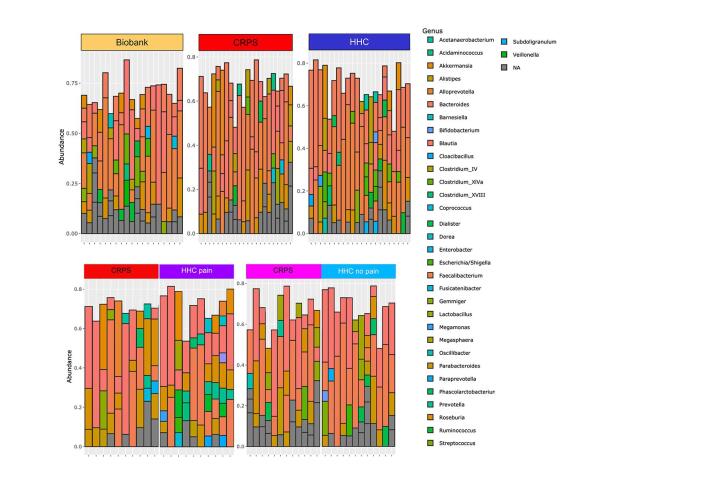
Intestinal microbiota diversity is decreased in participants with CRPS who live in chronic pain households. Sequencing analysis of the V4 region of the 16S rRNA gene was performed, and richness and Shannon diversity analysis were performed for (A) participants with CRPS (n = 23) versus Biobank controls (n = 19), (B) participants with CRPS (n = 23) compared to household controls (HHC, n = 22), and (C) CRPS versus HHC by household pain status. Analyses by Mann-Whitney test with mean +/- SEM shown. Principal components analysis was also performed using unweighted UniFrac distances comparing (D) CRPS (n = 23) versus Biobank controls (n = 19), (E) CRPS (n = 23) compared to HHC (n = 22), and (F) CRPS (n = 10, 12) separated by household control pain status. Analyzed using permutational MANOVA (ADONIS). Phylum-level relative abundance of bacterial taxa shown for (G) CRPS (n = 23) versus Biobank controls (n = 19), (H) CRPS (n = 23) compared to HHC (n = 22), and (I) by household pain status CRPS (CRPS cohabitating with HHC with pain n = 10, CRPS cohabitating with HHC without pain n = 12) vs HHC (with pain n = 10, without pain n = 12).
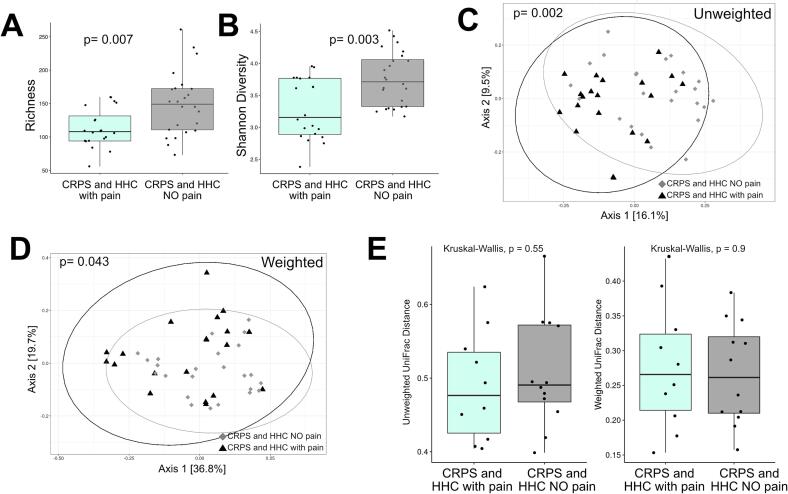
Fig. 3.
Comparison of alpha, beta diversity and phylogenetic distances of households based on HHC pain status. Sequencing analysis of the V4 region of the 16S rRNA gene was performed for households with CRPS based on HHC pain status. (A) Richness alpha diversity metric comparing household of CRPS with HHC pain (n = 20) versus CRPS with HHC NO pain (n = 24), (B) Shannon Diversity comparing household of CRPS with HHC pain (n = 20) versus CRPS with HHC NO pain (n = 24). Analyses by Wilcoxon test with mean ± SEM shown. Principal components analysis was also performed using unweighted UniFrac distances comparing (C) household with CRPS and HHC with pain (n = 20) households with CRPS and HHC with NO pain (n = 24). Principal components analysis was performed using weighted UniFraq distances comparing (D) household with CRPS and HHC with pain (n = 20) households with CRPS and HHC with NO pain (n = 24). Analyzed using permutational MANOVA (ADONIS). To compare overall microbiota structure, we compared phylogenetic distances of CRPS versus their matched HHC using unweighted (E) and weighted (F) pairwise Unifrac distances between samples from households with pain (CRPS and HHC-pain compared to households without pain (CRPS and HHC-NO pain). Analyzed using Kruskal-Wallis test.
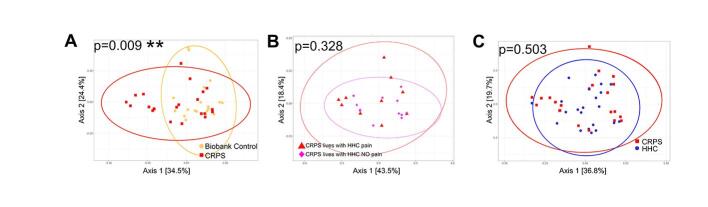
We compared overall bacterial community structures using unweighted and weighted UniFrac distances (Fig. 1D-E, Fig. 3C,D, Supplementary Figure 3). Bacterial community composition was not significantly different between participants with CRPS and Biobank or CRPS and household controls when analyzed by unweighted UniFrac (Fig. 1D, 1E), which considers the presence and absence of taxa in addition to phylogenetic distance. When HHC pain status was considered, a significant difference in beta diversity between patients with CRPS from either chronic or nonchronic pain households (Fig. 1F). These data suggest differences in the presence of taxa based on household pain status. When also taking relative abundance of taxa into account, using weighted UniFrac, overall bacterial community composition was different when comparing CRPS and Biobank controls (Supplementary Figure 3A), comparing CRPS based on household pain status (Supplementary Figure 3B), and CRPS households (CRPS and HHC) based on household pain status (Fig. 3C, D). However weighted UniFrac comparison of CRPS to HHC (Supplementary Figure 3C) demonstrated no difference.
To evaluate if the differences observed based on household pain status were a reflection of changes in the shared microbiota (i.e. do participants with CRPS who live with participants in pain share more microbiota compared to those with CRPS who live with HHC without pain), we performed a paired-sample analysis using unweighted and weighted UniFrac beta diversity based on household pain status. Pairwise weighted and unweighted, UniFrac distances between samples from households with pain (CRPS and HHC-pain) were not significantly different compared to households without pain (CRPS and HHC-NO pain) (Fig. 3E) suggesting that the differences observed based on HHC pain status were unrelated to differences in shared microbiota due to cohabitation.
In sum, these data suggest that households with CRPS who cohabitate with HHC-pain harbor microbiota dysbiosis as reflected by decreased bacterial richness, Shannon diversity and beta diversity driven by altered relative abundance of taxa when compared to participants with CRPS who cohabitate with HHC-NO pain. We confirmed that our results were not dependent on the taxonomic database used by evaluating annotation of our 16S rRNA gene reads using SILVA v138 and we utilized RDP for all remaining analyses (Supplementary Figure 4). Participants with CRPS may harbor microbiota differences, driven by differences in relative abundance but not presence/absence of taxa compared to metadata-matched Biobank controls. In contrast, overall HHC are broadly similar to matched participants with CRPS.
Specific taxonomic differences are associated with CRPS
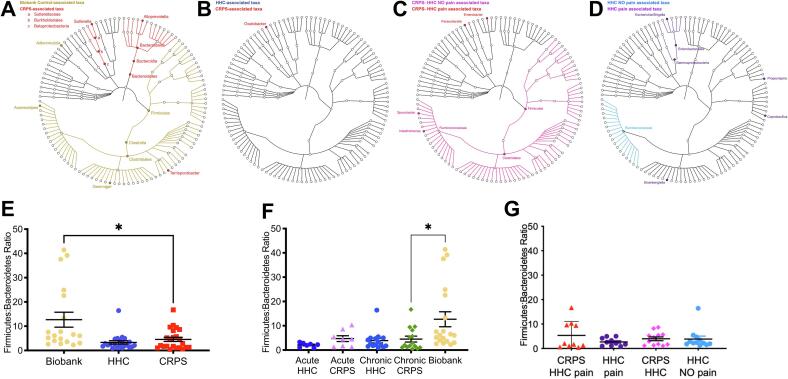
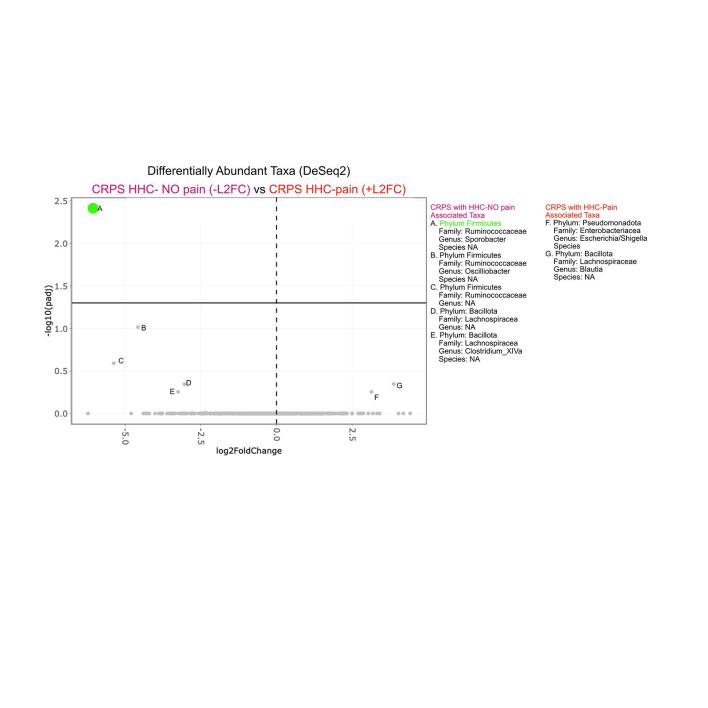
Visualization of phylum- and genus-level composition suggested potential differences in relative proportions of taxa, particularly between patients with CRPS and Biobank controls (Fig. 1G-I, Supplementary Figure 5). To identify taxa with statistically significant differences in relative abundance between groups, we used LEfSe (Segata et al., 2011) (Fig. 2A-D). Participants with CRPS exhibited significantly higher levels of Bacteroidetes, with a corresponding loss of Firmicutes including various taxa in the Clostridiales order when compared to biobank controls (Fig. 2A, 2E). CRPS was associated with higher levels of Sutterella, a Proteobacteria. Comparison of participants with CRPS to HHC revealed substantially fewer differences, with only Oxalobacter, a Proteobacteria, identified as CRPS-associated (Fig. 2B). However, comparison of participants with CRPS based on cohabitation with an individual with chronic pain (HHC-pain) or cohabitation with an individual without chronic pain (HHC-NO pain) demonstrated overall Firmicutes and specifically Clostridiales including Sporobacter and Intestinimonas associated with CRPS/HHC-NO pain households (Fig. 2C). Proteobacteria taxa Parasutterella and Enterobacter were enriched in CRPS/HHC-pain households (Fig. 2C). Clostridiales family Ruminococcaceae was associated with HHC-NO pain, while Gammaproteobacteria, Enterobacteriales and specifically Escherichia/Shigella were enriched in HHC-pain, in addition to several Firmicutes taxa including Coprobacillus and Propionispira (Fig. 2D). CRPS Severity Score did not correlate with microbiota richness or Shannon diversity (data not shown). Thus, a general pattern in which decreased overall Firmicutes and Clostridiales were associated either with all patients with CRPS compared to unrelated controls or with CRPS patients with HHC-pain compared to those with HHC-NO pain emerged. Using DEseq2 to specifically identify differentially abundant amplicon sequence variants (ASVs) (Supplementary Figure 6, 2C), we made the same comparisons (CRPS versus Biobank controls, CRPS versus HHC, acute vs chronic CRPS and comparison based on household pain status). While individual ASVs did not emerge as significantly different in comparisons of CRPS versus Biobank or HHC, in the household pain status comparison, Sporobacter again emerged as associated with household of CRPS/HHC-NO pain (Supplementary Figure 6).
Fig. 2.
Specific microbiota taxa and dysbiosis are associated with CRPS. LEfSe analysis results plotted in cladograms made using GraPhlAn, providing a graphical representation of discovered taxa (all annotated taxa are significantly different) in a taxonomic tree identified comparing (A) CRPS (acute and chronic) to Biobank controls, (B) CRPS (acute and chronic) to household controls (HHC), (C) CRPS living with HHC with pain to CRPS living with HHC with NO pain, and (D) HHC with pain cohabitant with participant with CRPS to HHC with NO pain cohabitating with participant with CRPS. Ratio of relative abundances of Firmicutes to Bacteroidetes phyla in: (E) Biobank controls, HHC and all CRPS (acute and chronic), and (F) Biobank controls; acute versus chronic CRPS and their respective HHC: acute HHC and chronic HHC. (G) separated by household pain status: HHC no pain (HHC, n = 12), HHC pain (n = 10), CRPS living with HHC no pain (CRPS HHC, n = 12), CRPS living with HHC with pain (CRPS HHC pain, n = 10). Analyzed by Mann-Whitney test. *, P < 0.05, mean ± SEM.
Altered Firmicutes:Bacteroidetes (F:B) ratios have been associated with numerous disease states, with increased F:B ratios found in individuals with obesity or metabolic syndrome (Ley et al., 2006), and decreased F:B ratios found in patients with inflammatory bowel diseases (IBD) (Lloyd-Price et al., 2019). Based upon altered relative abundance of Bacteroidetes and Firmicutes between CRPS patients and Biobank controls, we assessed this ratio in our patient groups. We found CRPS was associated with a significantly lower F:B ratios compared to Biobank controls (Fig. 2E), consistent with the broad taxonomic changes observed (Fig. 1G, Fig. 2A). No difference was observed in F:B ratios for participants with CRPS compared to HHC (Fig. 2E) or when comparing CRPS to HHC based on household pain status (Fig. 2G). Thus, CRPS is associated with significant modifications to the taxonomic composition of the intestinal microbiota compared to metadata-matched controls, while overall, cohabitating HHC exhibit similar intestinal microbiota compositions as individuals with CRPS. However, when assessing CRPS patients based on the pain status of their HHC, cohabitation with HHC-pain is associated with specific taxa that track with those generally lost in the microbiota of CRPS patients (Supplementary Figure 6). Overall, these data may suggest that cohabitating with HHC-pain may enhance those microbiota alterations inherent to CRPS.
Overall intestinal microbiota composition is altered in a mouse model of CRPS
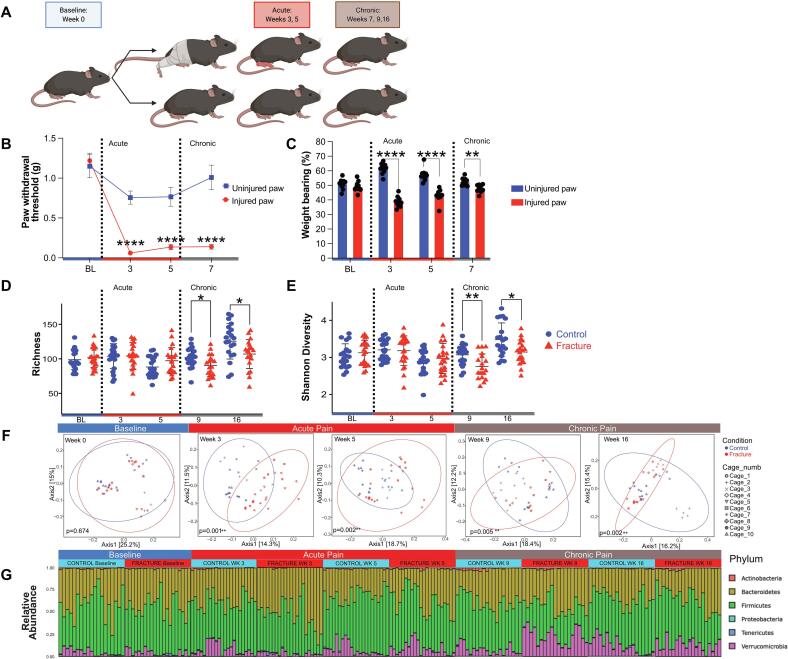
While our data from human participants support an association between CRPS and alterations to the microbiota, we sought to assess whether we could observe longitudinal changes in the gut microbiota associated with injury and associated acute or chronic pain in a mouse model. Thus, we leveraged a well-characterized fracture/casting model of CRPS in mice (Haight et al., 2020, Cropper et al., 2019, Shen et al., 2020) that closely mimics the clinical presentation of CRPS in humans to longitudinally characterize the composition of the intestinal microbiota throughout the acute and chronic phases of the condition. We compared mice that underwent a closed tibial fracture followed by three weeks of casting (Fracture) to non-cohoused littermate controls (Control) (Fig. 4A). Mice are coprophagic and cohousing injured and uninjured mice causes behavioral changes in both (Mogil, 2015); thus uninjured and injured mice were not cohoused. We have previously characterized the time course of behavioral sensitization in the tibial fracture/casting model of CRPS (Huck et al., 2021). We demonstrate again here that profound mechanical allodynia develops in the injured hindlimb at the time of cast removal at 3 weeks and lasts through 20 weeks post-injury (Fig. 4B), well into the chronic phase (Huck et al., 2021). The functional effects of this injury including decreased weight bearing on the injured limb, both during the acute (week 3 and 5) and chronic (week 7) phases of the model (Fig. 4C). Signs of peripheral inflammation, characteristic of the acute phase, including edema and increased temperature of the injured paw, are present at 3 weeks post-fracture and dissipate around 5 weeks (previously published, data not shown) (Huck et al., 2021). To capture potential microbiota changes throughout the acute, transition and chronic phases of CRPS, stool samples were collected at baseline and at weeks 3, 5, 9 and 16, for 16S rRNA gene sequencing.
Fig. 4.
Overall intestinal microbiota composition is altered in a mouse model of CRPS. (A) Schematic depicting mouse model of CRPS, made using BioRender. (B,C) Behavioral testing indicates acute (weeks 3–5) and chronic (week 7) mechanical allodynia (B) and decreased weight bearing (C) on the injured paw when compared to the uninjured paw. Blue = uninjured paw, red = injured paw. Analyzed by 2-way ANOVA as a function of time (P < 0.0001) (C) or Bonferroni’s multiple comparisons test (B). (n = 10) ****, P < 0.0001, **, P < 0.01. Sequencing analysis of the V4 region of the 16S rRNA gene was performed on longitudinally-collected fecal samples. Richness (D) and Shannon diversity analyses (E) were performed. Blue circle = control (n = 20–22), red triangle = fracture. (n = 20–22). Analyzed by Mann-Whitney test. *, P < 0.05. (F) Principal components analysis was performed using unweighted UniFrac distances at the indicated timepoints with cage number indicated in the legend. Analyzed by permutational MANOVA (ADONIS). *, P < 0.05. **, P < 0.01. (G) Relative abundances of bacterial taxa at the phylum level (E) with the acute (weeks 3, 5) to chronic (weeks 9–16) pain transition.
While bacterial richness and Shannon diversity were similar between fracture and non-cohoused littermate control mice at baseline and during the acute phase (weeks 3 and 5 post-fracture), mice in the chronic phase of CRPS following tibial fracture/casting (weeks 9 and 16) exhibited significantly decreased richness and Shannon diversity compared to controls (Fig. 4D, E). Fracture and control samples were highly similar at baseline but exhibited significant disparity in both the acute and chronic phases post-fracture (Fig. 4F) by unweighted UniFrac distance analysis.
“Cage effects” can account for substantial variation in the microbiota, and we observed clustering of samples based on cage of origin (Fig. 4F) (Singh et al., 2021, Moon et al., 2015). To confirm that the microbiota differences observed between fracture and control samples were robust to cage as a potential confounder, we fit a linear regression model including both condition and cage as independent variables for our alpha diversity metrics. When cage was not taken into account, week 9 and 16 were significantly different between fracture and control (P = 0.0202 and P = 0.017, respectively), but when cage was considered, these differences were no longer present (P = 0.317 and P = 0.334, respectively). For Shannon diversity, the week 9 difference (P = 0.003) was robust to cage effect (P = 0.003), but week 16 (P = 0.010) was not (P = 0.432). Interestingly, consideration of cage effects revealed a significant difference in Shannon diversity between fracture and control samples at week 3 (P = 0.045) that was not observed when cage effects were ignored (P = 0.823).
We similarly assessed whether cage of origin influenced the beta-diversity differences we had observed, using a mixed linear model wherein the fixed factor/dependent variable was condition (fracture versus control) and the random factor was cage to assess the association between condition and PC1. After adjusting for cage, fracture and control samples were significantly different at weeks 3 and 9 (P = 0.035697 and P = 0.0002, respectively), but non-significant for weeks 0, 5, and 16 (P = 0.53163, P = 0.0776, and P = 0.0585). Overall, these data support that both acute (week 3) and chronic (week 9) phases of CRPS are associated with substantial modulation to the intestinal microbiota in this model, but also emphasize that cage effects are an important consideration for mouse microbiota analyses.
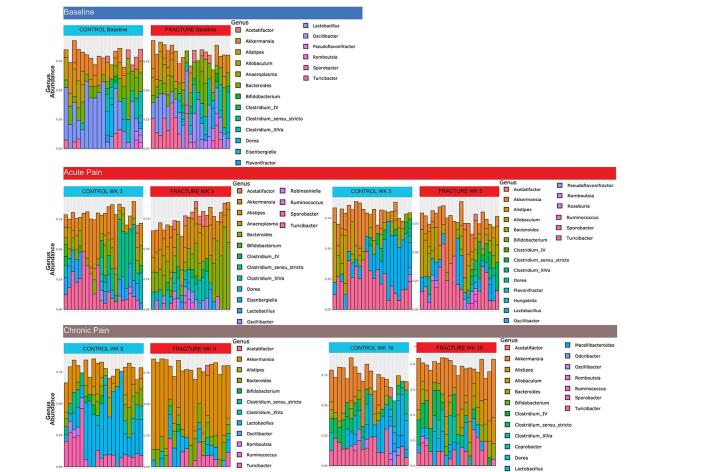
Specific microbiota taxa are observed in a mouse model of CRPS
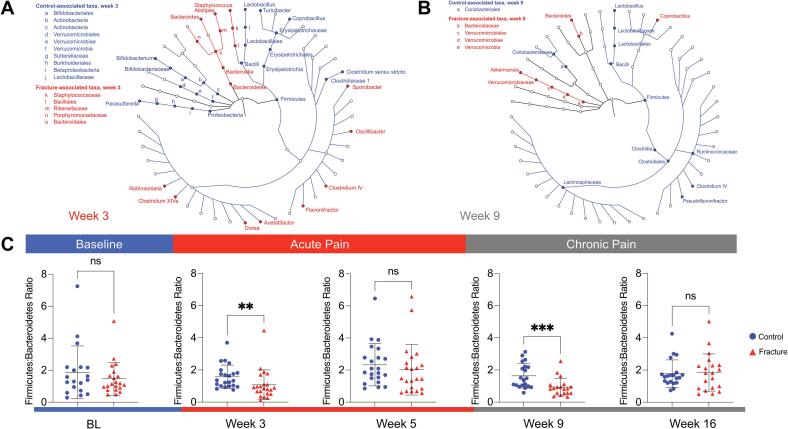
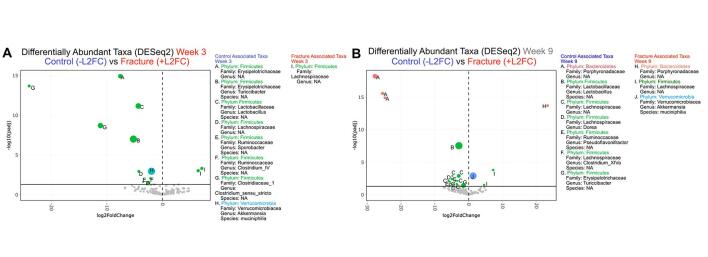
We plotted longitudinal phylum and genus level taxonomic composition of the intestinal microbiota in the fracture and control mice (Fig. 4G, Supplementary Figure 7). At weeks 3 and 9 post-fracture, the most dramatic phylum-level differences were observed between these groups (Fig. 4G), and we thus used LEfSe analysis to identify taxa at these timepoints (Fig. 5A, B). At both timepoints, we observed a loss of Firmicutes including Bacilli in the fracture group, with an increase in taxa in the Bacteroidetes phylum (Fig. 5A, B). However, at week 3, samples from fractured mice exhibited a loss of Parasutterella, Bifidobacterium and Verrucomicrobiales as well as increased Staphylococcus and various Firmicutes genera (Fig. 5A), while at week 9, Coprobacillus, Bacteroides and Akkermansia were increased in fractured mice (Fig. 5B). We also performed an analysis comparing fracture and control groups using DESeq2 at weeks 3 and 9 (Supplementary Figure 8). Numerous ASVs emerged in line with our LEfSe analysis, including loss of numerous Firmicutes taxa including Bacilli at both timepoints, as well as decreased Akkermansia at week 3 and increased Akkermansia at week 9 in the fracture group. Week 9 taxa identified in mice recapitulate aspects of the comparison between pain- and non-pain associated households. Specifically, Firmicutes and Clostridiales, associated with CRPS in HHC-NO pain households, were associated with the taxa in the control mice. Similarly, Ruminococcaceae were associated with HHC-NO pain as well as control mice at week 9, while Coprobacillus was associated with fracture in the mice as well as HHC-pain.
Fig. 5.
Specific microbiota taxa and dysbiosis are associated with the acute and chronic phases of CRPS in mice. LEfSe analysis identified statistically significant bacterial taxa comparing mice after fracture/casting to controls. Taxa in the acute phase following fracture/casting at 3 weeks (A) and at 9 weeks (B). (C) Ratio of relative abundances of Firmicutes to Bacteroidetes phyla in control versus mice that underwent fracture/casting at: baseline (BL), weeks 3 and 5 (acute phase), weeks 9 and 16 (chronic phase). Analyzed by Mann-Whitney test. ***, P < 0.001, **, P < 0.01; *, P < 0.05; ns, not significant.
These mouse data support a consistent loss of Firmicutes associated with acute or chronic CRPS (Fig. 2). Importantly, however, they also reveal key differences dependent upon the chronicity of injury/pain in the specific taxonomic alterations that occur. We analyzed the F:B ratios along the time course, and as suggested by the phylum-level composition (Fig. 4G), observed a significant decrease in the F:B ratio in fractured mice at weeks 3 and 9 (Fig. 5C). In sum, consistent with our observations in samples from a human cohort of participants with CRPS, we observed a loss of Firmicutes and increased Bacteroidetes associated with the acute and chronic phases post-fracture in a mouse model of CRPS.
Discussion
Our study illustrates that a diagnosis of CRPS affects every aspect of a patient’s life and may influence the gut microbiota composition in both humans and mice. Significant differences in the composition of gut microbiota reflect the chronicity of pain or nociceptive behaviors in both humans with CRPS and mice subjected to a model of CRPS. In addition, household member pain status may significantly influence pain from CRPS. Cohabitation with a household member suffering from chronic pain was associated with more severe CRPS and altered alpha and beta diversity when compared to participants with CRPS who live with participants without chronic pain. These households on average consumed more pain medications, such as opioids, as well. Since these and other medications are known to affect gut microbiota composition (Vich Vila et al., 2020), they may have contributed to the differences seen and larger future studies are needed to evaluate these effects. In mice, injury alone resulted in similar gut microbiota dysbiosis compared to non-cohabitating littermate controls.
A diagnosis of CRPS is associated with significantly higher levels of daily pain and neuropathic pain medication use when compared to their cohabitants. A substantial number (46 %) of their cohabitants responded “yes” to having current pain other than normal everyday pain (excluding minor headaches, sprains, and toothaches) on the brief pain inventory (BPI). Originally designed as a screening question for the BPI, the national average of a positive response is currently unclear. However, this is larger than the CDC-reported 20.4 % incidence of US adults with chronic pain, as defined by pain on most days or every day in the past 6 months (Dahlhamer et al., 2018). It is unknown if people with chronic pain tend to cohabitate more frequently or if the presence of chronic pain in a household influences the development or severity of CRPS.
As seen in our study and many others, individuals share a large proportion of their gut microbiota with their cohabitants (regardless of relationship) (Gacesa et al., 2022, Minerbi et al., 2019, Rothschild et al., 2018). Most household controls were not related by blood (91.6 %) and share a large proportion of gut microbiota with their cohabitants. In concordance with a prior study, the overall gut microbiota community structure is distinct in human participants with CRPS compared to nonrelated (Biobank) controls (Reichenberger et al., 2013). Our study differs from the prior clinical study with a larger sample size, participants with both acute and chronic CRPS, male and female participants, and the availability of household controls. Unlike the prior study of chronic CRPS in women (average duration 5 years), we did not observe significant differences in the richness or Shannon diversity of the samples between participants with CRPS (acute and chronic) and Biobank controls. No differences were observed in participants with CRPS and their cohabitants.
The acute to chronic pain transition is difficult to study in humans. A robust mouse model of both the acute to chronic pain transition as well as a phenotype mimicking the condition of CRPS has been well established (Haight et al., 2020). Our work supports the hypothesis that injury and chronic pain mediate a dramatic modulatory effect on the gut microbiota of mice. Significant community structure differences, as reflected by changes in beta diversity metrics are observed during early acute CRPS (3 weeks) and persist into the chronic phase of the model. As pain becomes chronic in these mice, decreases in both richness and Shannon diversity also develop, phenotypes that mimic what has previously been reported during chronic CRPS in human patients (Reichenberger et al., 2013). At week 16, as pain behavior begins to normalize, the overall beta diversity changes persist. Dysbiosis in humans and mice may reflect or influence the chronicity of CRPS. Major limitations of this work are the use of male mice and lack of sham surgery or anesthesia in the controls.
Alterations to the intestinal microbiota in CRPS were reflected in the altered relative abundance of specific taxa. Sutterella, a Proteobacteria in the family Sutterellaceae, is more prevalent in participants with CRPS compared to non-cohabitating matched controls. Interestingly, higher levels of Sutterellaceae are associated with decreased levels of extraintestinal pain in women with irritable bowel syndrome (Hollister et al., 2020). Alloprevotella, from the phylum Bacteroidetes, was also more prevalent in participants with CRPS; the abundance of this taxon has been associated with responsiveness to treatment of rheumatoid arthritis (Hammad et al., 2019) and visceral hypersensitivity in rats (Enqi et al., 2020). Similar to the prior study in humans, we found increased relative abundance of Verrucomicrobia (specifically Akkermansia muciniphila) in mice with chronic CRPS (Fig. 5B) (Reichenberger et al., 2013). Future, larger studies may examine the clinical characteristics and outcomes of these participants with a differential abundance of Akkermansia in greater detail.
Intriguingly, the week 9 taxa identified in mice recapitulate aspects of the comparison between pain- and non-pain associated households. Specifically, Firmicutes and Clostridiales, associated with CRPS households in which HHC had no pain, were also associated with control mice. Similarly, Ruminococcaceae was associated with households with HHC-no pain compared to household where the HHC has pain (Supplementary Fig 6), and also with control mice at week 9, while Coprobacillus was associated with fracture in mice as well as HHC-pain.
Chronic CRPS impacts pain, function, and possibly gut microbiota composition. These data provide initial evidence in both humans and mice that gut microbiota composition reflects pain chronification and specific taxa including Akkermansia as deserving of further focus. Future studies using this mouse CRPS model to elucidate potential gut microbiota mechanisms is an important next step. These studies may evaluate if the gut microbiota primes microglia or other immune system components to facilitate the development of chronic pain from CRPS (Haight et al., 2020, David Clark et al., 2018). Additionally, it will be of substantial interest to examine the pain trajectories in acute CRPS paired with gut microbiota composition and metabolomics to identify biomarkers of pain chronification in participants with acute CRPS. Our current study has some important limitations. Our small, observational study cannot determine causality of microbiota dysbiosis and CRPS. We do not know if an interaction between pain and gut microbiota leads to CRPS or if chronic pain alone influences microbiota composition. Medications such as opioids may affect microbiota composition and we were unable to control for this in this small study (Vich Vila et al., 2020). Although the gut microbiota composition is resilient in the face of dietary changes and weight loss (Fragiadakis et al., 2020), diet was not assessed in this study.
Future work will continue to provide broader insights into the effects of pain chronification on the microbiota and if a CRPS-specific pain microbiota signature exists. Further work on the interaction between household member pain status and the severity of pain from CRPS is needed. As recovery from acute CRPS is relatively common, longitudinal studies of patients with early acute CRPS will be critical to determine if gut microbiota changes predict or reflect recovery.
Author contributions
L.W.C., L.S., D.L., G.M. and N.H. performed the experiments. L.W.C., R.R., L.W. G.M. and M.T.B. analyzed the results. L.W.C, V.L.T. and M.T.B. designed the project. L.W.C. wrote the manuscript. All authors read and edited the manuscript.
CRediT authorship contribution statement
Lara W. Crock: . Rachel Rodgers: Writing – review & editing, Visualization, Investigation, Formal analysis. Nolan A. Huck: . Lawrence A. Schriefer: Writing – review & editing, Validation, Investigation. Dylan Lawrence: Writing – review & editing, Methodology, Investigation. Leran Wang: Writing – review & editing, Methodology, Investigation, Formal analysis. Gabriella P.B. Muwanga: . Vivianne L. Tawfik: Writing – review & editing, Methodology, Investigation, Funding acquisition, Conceptualization. Megan T. Baldridge: Writing – review & editing, Writing – original draft, Visualization, Supervision, Resources, Funding acquisition.
Funding
The authors thank the Departments of Anesthesiology at Washington University and Stanford University for their financial and departmental support. The authors acknowledge the generous financial support from the IARS Mentored Research Award (L.C.W.) and the Goldberg Research Fund (L.W.C.) as well as NIH grant R35 GM137906 (V.L.T).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We would like to thank the participants in the study as well as our research coordinators Kristen Roles, Carrie Burk, Karen Frey and Dannie Tallchief for assistance in patient enrollment. We would like to thank Ellen Fischbach for assistance with IRB compliance. We acknowledge all members of the Baldridge and Tawfik laboratories for helpful discussions. We would like to thank the Washington University Digestive Disease Research Core Center Biobank Core (NIDDK P30 DK052574) for access to biobank samples.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ynpai.2024.100173.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Supplementary Fig. 1.
Supplementary Fig. 2.
Supplementary Fig. 3.
Supplementary Fig. 4.
Supplementary Fig. 5.
Supplementary Fig. 6.
Supplementary Fig. 7.
Supplementary Fig. 8.
Data availability
We have clearly specified where our data is deposited in our manuscript.
References
- Agachan F., Chen T., Pfeifer J., Reissman P., Wexner S.D. A constipation scoring system to simplify evaluation and management of constipated patients. Dis. Colon Rectum. 1996;39(6):681–685. doi: 10.1007/BF02056950. [DOI] [PubMed] [Google Scholar]
- Amaral F.A., Sachs D., Costa V.V., Fagundes C.T., Cisalpino D., Cunha T.M., et al. Commensal microbiota is fundamental for the development of inflammatory pain. PNAS. 2008;105(6):2193–2197. doi: 10.1073/pnas.0711891105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boer C.G., Radjabzadeh D., Medina-Gomez C., Garmaeva S., Schiphof D., Arp P., et al. Intestinal microbiome composition and its relation to joint pain and inflammation. Nat. Commun. 2019;10(1):4881. doi: 10.1038/s41467-019-12873-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner D., Cherry P., Switzer T., Butt I., Stanton C., Murphy K., et al. Pain after upper limb surgery under peripheral nerve block is associated with gut microbiome composition and diversity. Neurobiol. Pain. 2021;10 doi: 10.1016/j.ynpai.2021.100072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan B.J., McMurdie P.J., Rosen M.J., Han A.W., Johnson A.J., Holmes S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods. 2016;13(7):581–583. doi: 10.1038/nmeth.3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporaso J.G., Lauber C.L., Walters W.A., Berg-Lyons D., Lozupone C.A., Turnbaugh P.J., et al. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. PNAS. 2011;108(Suppl 1):4516–4522. doi: 10.1073/pnas.1000080107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole JR, Wang Q, Fish JA, Chai B, McGarrell DM, Sun Y, et al. Ribosomal Database Project: data and tools for high throughput rRNA analysis. Nucleic Acids Res 2014;42(Database issue):D633-42. [DOI] [PMC free article] [PubMed]
- Crock L.W., Baldridge M.T. A role for the microbiota in complex regional pain syndrome? Neurobiol. Pain. 2020;8 doi: 10.1016/j.ynpai.2020.100054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cropper H.C., Johnson E.M., Haight E.S., Cordonnier S.A., Chaney A.M., Forman T.E., et al. Longitudinal translocator protein-18 kDa-positron emission tomography imaging of peripheral and central myeloid cells in a mouse model of complex regional pain syndrome. Pain. 2019;160(9):2136–2148. doi: 10.1097/j.pain.0000000000001607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlhamer J., Lucas J., Zelaya C., Nahin R., Mackey S., DeBar L., et al. Prevalence of Chronic Pain and High-Impact Chronic Pain Among Adults - United States, 2016. MMWR Morb. Mortal. Wkly Rep. 2018;67(36):1001–1006. doi: 10.15585/mmwr.mm6736a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David Clark J., Tawfik V.L., Tajerian M., Kingery W.S. Autoinflammatory and autoimmune contributions to complex regional pain syndrome. Mol. Pain. 2018;14 doi: 10.1177/1744806918799127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis R.J., Heaton R.K., Gianella S., Rahman G., Knight R. Reduced Gut Microbiome Diversity in People With HIV Who Have Distal Neuropathic Pain. J. Pain. 2022;23(2):318–325. doi: 10.1016/j.jpain.2021.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enqi W., Jingzhu S., Lingpeng P., Yaqin L. Comparison of the Gut Microbiota Disturbance in Rat Models of Irritable Bowel Syndrome Induced by Maternal Separation and Multiple Early-Life Adversity. Front. Cell. Infect. Microbiol. 2020;10 doi: 10.3389/fcimb.2020.581974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fragiadakis G.K., Wastyk H.C., Robinson J.L., Sonnenburg E.D., Sonnenburg J.L., Gardner C.D. Long-term dietary intervention reveals resilience of the gut microbiota despite changes in diet and weight. Am. J. Clin. Nutr. 2020;111(6):1127–1136. doi: 10.1093/ajcn/nqaa046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freidin M.B., Stalteri M.A., Wells P.M., Lachance G., Baleanu A.F., Bowyer R.C.E., et al. An association between chronic widespread pain and the gut microbiome. Rheumatology (Oxford) 2021;60(8):3727–3737. doi: 10.1093/rheumatology/keaa847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gacesa R., Kurilshikov A., Vich Vila A., Sinha T., Klaassen M.A.Y., Bolte L.A., et al. Environmental factors shaping the gut microbiome in a Dutch population. Nature. 2022;604(7907):732–739. doi: 10.1038/s41586-022-04567-7. [DOI] [PubMed] [Google Scholar]
- Haight E.S., Johnson E.M., Carroll I.R., Tawfik V.L. Of mice, microglia, and (wo)men: a case series and mechanistic investigation of hydroxychloroquine for complex regional pain syndrome. Pain Rep. 2020;5(5):e841. doi: 10.1097/PR9.0000000000000841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammad D.B.M., Hider S.L., Liyanapathirana V.C., Tonge D.P. Molecular Characterization of Circulating Microbiome Signatures in Rheumatoid Arthritis. Front. Cell. Infect. Microbiol. 2019;9:440. doi: 10.3389/fcimb.2019.00440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harden R.N., Bruehl S., Perez R.S., Birklein F., Marinus J., Maihofner C., et al. Validation of proposed diagnostic criteria (the “Budapest Criteria”) for Complex Regional Pain Syndrome. Pain. 2010;150(2):268–274. doi: 10.1016/j.pain.2010.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harden R.N., Maihofner C., Abousaad E., Vatine J.J., Kirsling A., Perez R., et al. A prospective, multisite, international validation of the Complex Regional Pain Syndrome Severity Score. Pain. 2017;158(8):1430–1436. doi: 10.1097/j.pain.0000000000000927. [DOI] [PubMed] [Google Scholar]
- Hollister E.B., Cain K.C., Shulman R.J., Jarrett M.E., Burr R.L., Ko C., et al. Relationships of Microbiome Markers With Extraintestinal, Psychological Distress and Gastrointestinal Symptoms, and Quality of Life in Women With Irritable Bowel Syndrome. J. Clin. Gastroenterol. 2020;54(2):175–183. doi: 10.1097/MCG.0000000000001107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huck N.A., Siliezar-Doyle J., Haight E.S., Ishida R., Forman T.E., Wu S., et al. Temporal contribution of myeloid-lineage TLR4 to the transition to chronic pain: A focus on sex differences. J. Neurosci. 2021 doi: 10.1523/JNEUROSCI.1940-20.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iolascon G., de Sire A., Moretti A., Gimigliano F. Complex regional pain syndrome (CRPS) type I: historical perspective and critical issues. Clin. Cases Miner. Bone Metab. 2015;12(Suppl 1):4–10. doi: 10.11138/ccmbm/2015.12.3s.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller S., Bann C.M., Dodd S.L., Schein J., Mendoza T.R., Cleeland C.S. Validity of the brief pain inventory for use in documenting the outcomes of patients with noncancer pain. Clin. J. Pain. 2004;20(5):309–318. doi: 10.1097/00002508-200409000-00005. [DOI] [PubMed] [Google Scholar]
- Kolb L., Lang C., Seifert F., Maihofner C. Cognitive correlates of “neglect-like syndrome” in patients with complex regional pain syndrome. Pain. 2012;153(5):1063–1073. doi: 10.1016/j.pain.2012.02.014. [DOI] [PubMed] [Google Scholar]
- Lewis S.J., Heaton K.W. Stool form scale as a useful guide to intestinal transit time. Scand. J. Gastroenterol. 1997;32(9):920–924. doi: 10.3109/00365529709011203. [DOI] [PubMed] [Google Scholar]
- Ley R.E., Turnbaugh P.J., Klein S., Gordon J.I. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444(7122):1022–1023. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- Lloyd-Price J., Arze C., Ananthakrishnan A.N., Schirmer M., Avila-Pacheco J., Poon T.W., et al. Multi-omics of the gut microbial ecosystem in inflammatory bowel diseases. Nature. 2019;569(7758):655–662. doi: 10.1038/s41586-019-1237-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love M.I., Huber W., Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15(12):550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMurdie P.J., Holmes S. phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. PLoS One. 2013;8(4) doi: 10.1371/journal.pone.0061217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minerbi A., Gonzalez E., Brereton N.J.B., Anjarkouchian A., Dewar K., Fitzcharles M.A., et al. Altered microbiome composition in individuals with fibromyalgia. Pain. 2019;160(11):2589–2602. doi: 10.1097/j.pain.0000000000001640. [DOI] [PubMed] [Google Scholar]
- Mogil J.S. Social modulation of and by pain in humans and rodents. Pain. 2015;156(Suppl 1):S35–S41. doi: 10.1097/01.j.pain.0000460341.62094.77. [DOI] [PubMed] [Google Scholar]
- Moon C., Baldridge M.T., Wallace M.A., D CA, Burnham, Virgin HW, Stappenbeck TS. Vertically transmitted faecal IgA levels determine extra-chromosomal phenotypic variation. Nature. 2015;521(7550):90–93. doi: 10.1038/nature14139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nearing J.T., Douglas G.M., Hayes M.G., MacDonald J., Desai D.K., Allward N., et al. Microbiome differential abundance methods produce different results across 38 datasets. Nat. Commun. 2022;13(1):342. doi: 10.1038/s41467-022-28034-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen P.B., Mikkelsen K.L., Lauritzen J.B., Krogsgaard M.R. Risk Factors for Post-treatment Complex Regional Pain Syndrome (CRPS): An Analysis of 647 Cases of CRPS from the Danish Patient Compensation Association. Pain Pract. 2018;18(3):341–349. doi: 10.1111/papr.12610. [DOI] [PubMed] [Google Scholar]
- Rabbitts J.A., Fisher E., Rosenbloom B.N., Palermo T.M. Prevalence and Predictors of Chronic Postsurgical Pain in Children: A Systematic Review and Meta-Analysis. J. Pain. 2017;18(6):605–614. doi: 10.1016/j.jpain.2017.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichenberger E.R., Alexander G.M., Perreault M.J., Russell J.A., Schwartzman R.J., Hershberg U., Rosen G. Establishing a relationship between bacteria in the human gut and complex regional pain syndrome. Brain Behav. Immun. 2013;29:62–69. doi: 10.1016/j.bbi.2012.12.005. [DOI] [PubMed] [Google Scholar]
- Rothschild D., Weissbrod O., Barkan E., Kurilshikov A., Korem T., Zeevi D., et al. Environment dominates over host genetics in shaping human gut microbiota. Nature. 2018;555(7695):210–215. doi: 10.1038/nature25973. [DOI] [PubMed] [Google Scholar]
- Schmidt T.S.B., Raes J., Bork P. The Human Gut Microbiome: From Association to Modulation. Cell. 2018;172(6):1198–1215. doi: 10.1016/j.cell.2018.02.044. [DOI] [PubMed] [Google Scholar]
- Segata N., Izard J., Waldron L., Gevers D., Miropolsky L., Garrett W.S., Huttenhower C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011;12(6):R60. doi: 10.1186/gb-2011-12-6-r60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H., Gardner A.M., Vyas J., Ishida R., Tawfik V.L. Modeling Complex Orthopedic Trauma in Rodents: Bone, Muscle and Nerve Injury and Healing. Front. Pharmacol. 2020;11 doi: 10.3389/fphar.2020.620485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen S., Lim G., You Z., Ding W., Huang P., Ran C., et al. Gut microbiota is critical for the induction of chemotherapy-induced pain. Nat. Neurosci. 2017;20(9):1213–1216. doi: 10.1038/nn.4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh G., Brass A., Cruickshank S.M., Knight C.G. Cage and maternal effects on the bacterial communities of the murine gut. Sci. Rep. 2021;11(1):9841. doi: 10.1038/s41598-021-89185-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan M.J.L. BSR, Pivik J. The Pain Catastrophizing Scale: development and validation. Psychol Assess 1995;7:524-32.
- Tajerian M., Sahbaie P., Sun Y., Leu D., Yang H.Y., Li W., et al. Sex differences in a Murine Model of Complex Regional Pain Syndrome. Neurobiol. Learn. Mem. 2015;123:100–109. doi: 10.1016/j.nlm.2015.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Velzen G.A., Perez R.S., van Gestel M.A., Huygen F.J., van Kleef M., van Eijs F., et al. Health-related quality of life in 975 patients with complex regional pain syndrome type 1. Pain. 2014;155(3):629–634. doi: 10.1016/j.pain.2013.12.017. [DOI] [PubMed] [Google Scholar]
- Vich Vila A., Collij V., Sanna S., Sinha T., Imhann F., Bourgonje A.R., et al. Impact of commonly used drugs on the composition and metabolic function of the gut microbiota. Nat. Commun. 2020;11(1):362. doi: 10.1038/s41467-019-14177-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Z.W., Yang X., Zhao B.C., Deng F., Jiang Y.M., Pan W.Y., et al. Predictive and Preventive Potential of Preoperative Gut Microbiota in Chronic Postoperative Pain in Breast Cancer Survivors. Anesth. Analg. 2021 doi: 10.1213/ANE.0000000000005713. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
We have clearly specified where our data is deposited in our manuscript.