Abstract
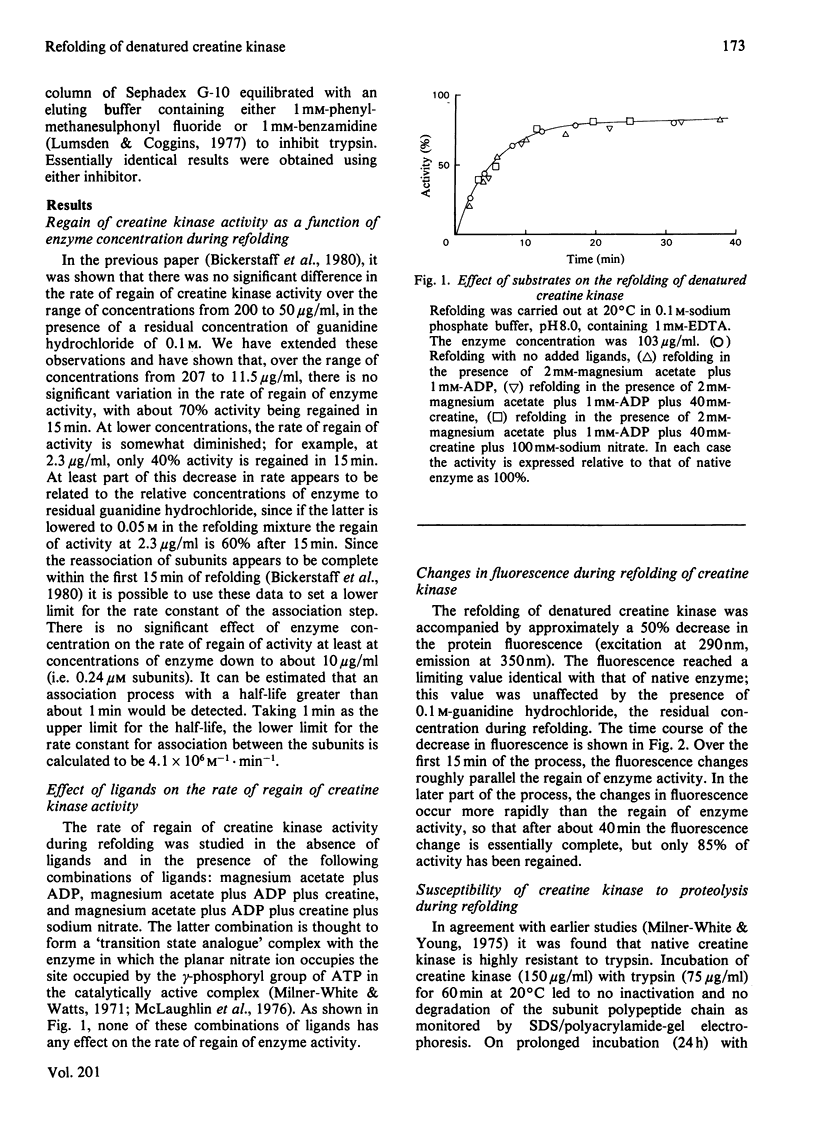
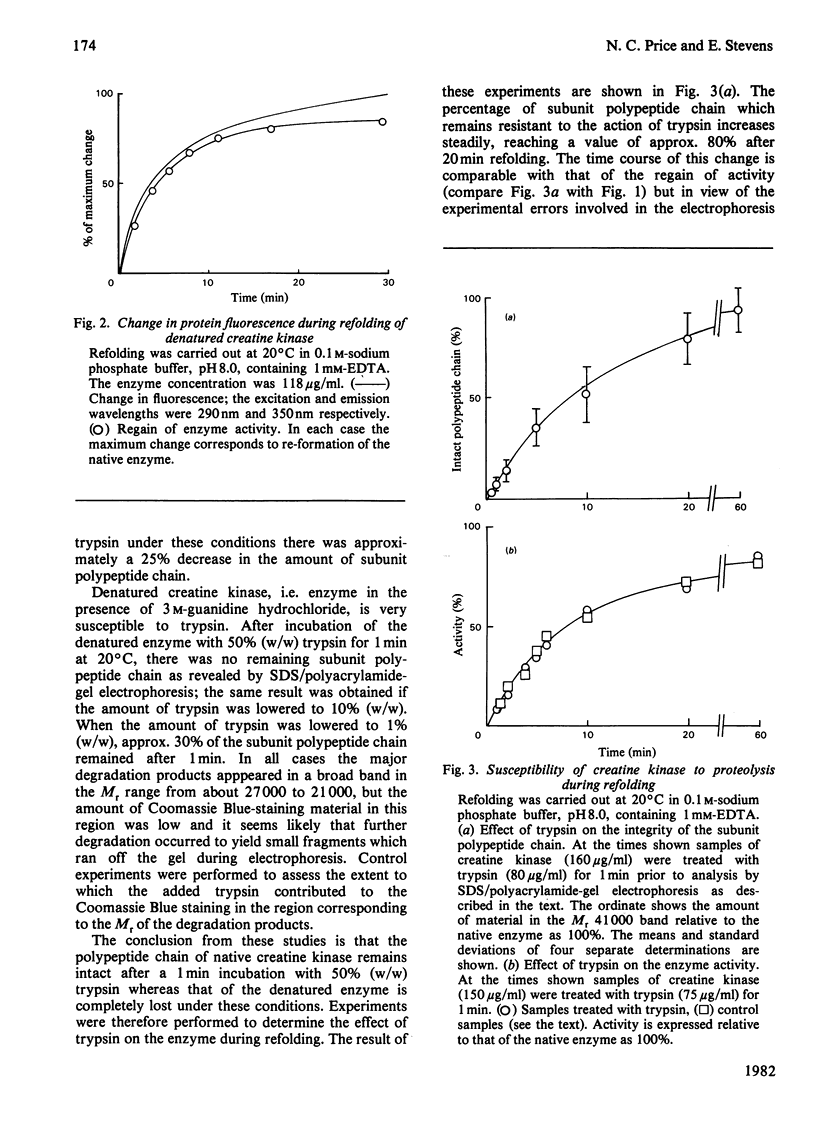
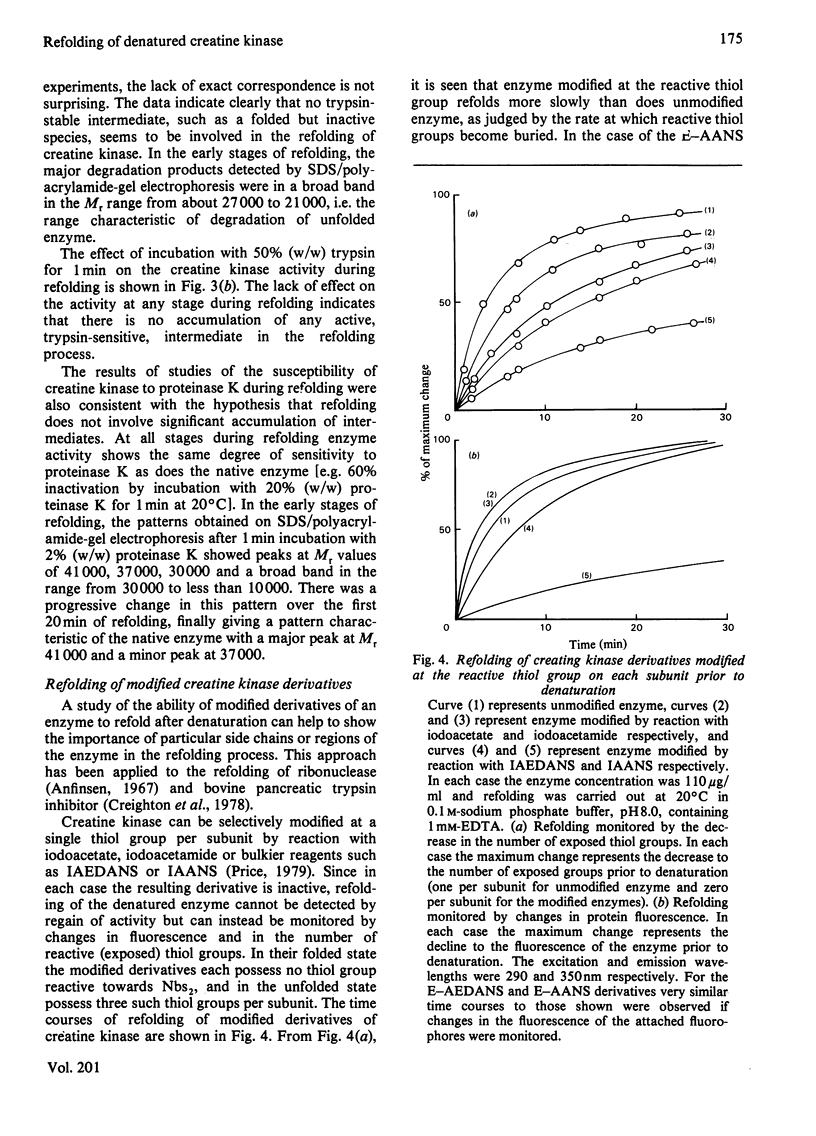
A number of aspects of the refolding of denatured rabbit muscle creatine kinase have been studied. Addition of substrates has no effect on the rate or extent of regain of activity. The changes in protein fluorescence during refolding broadly parallel the regain of activity. A study of the susceptibility of the enzyme to proteolysis during refolding indicates that there is no significant accumulation of folded, but inactive, intermediates in the folding process. Modification of the reactive thiol group on each subunit of the enzyme by small reagents such as iodoacetate or iodoacetamide prior to denaturation has only a small effect on the rate of subsequent refolding. However, modification by the bulky reagent 6-(4-iodoacetamidophenyl)aminonaphthalene-2-sulphonate has a very large effect on the ability of the enzyme to refold after denaturation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anfinsen C. B. The formation of the tertiary structure of proteins. Harvey Lect. 1967;61:95–116. [PubMed] [Google Scholar]
- Bickerstaff G. F., Paterson C., Price N. C. The refolding of denatured rabbit muscle creatine kinase. Biochim Biophys Acta. 1980 Feb 27;621(2):305–314. doi: 10.1016/0005-2795(80)90182-8. [DOI] [PubMed] [Google Scholar]
- Chan W. W., Mort J. S., Chong D. K., Macdonald P. D. Studies on protein subunits. 3. Kinetic evidence for the presence of active subunits during the renaturation of muscle aldolase. J Biol Chem. 1973 Apr 25;248(8):2778–2784. [PubMed] [Google Scholar]
- Creighton T. E., Dyckes D. F., Sheppard R. C. Refolding of bovine pancreatic trypsin inhibitor modified at methionine-52. J Mol Biol. 1978 Mar 15;119(4):507–518. doi: 10.1016/0022-2836(78)90199-7. [DOI] [PubMed] [Google Scholar]
- Kato I., Anfinsen C. B. On the stabilization of ribonuclease S-protein by ribonuclease S-peptide. J Biol Chem. 1969 Feb 10;244(3):1004–1007. [PubMed] [Google Scholar]
- Lumsden J., Coggins J. R. The subunit structure of the arom multienzyme complex of Neurospora crassa. A possible pentafunctional polypeptide chain. Biochem J. 1977 Mar 1;161(3):599–607. doi: 10.1042/bj1610599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggio E. T., Kenyon G. L. Properties of a CH3-blocked creatine kinase with altered catalytic activity. Kinetic consequences of the presence of the blocking group. J Biol Chem. 1977 Feb 25;252(4):1202–1207. [PubMed] [Google Scholar]
- McLaughlin A. C., Leigh J. S., Jr, Cohn M. Magnetic resonance study of the three-dimensional structure of creatine kinase-substrate complexes. Implications for substrate specificity and catalytic mechanism. J Biol Chem. 1976 May 10;251(9):2777–2787. [PubMed] [Google Scholar]
- Milner-White E. J., Watts D. C. Inhibition of adenosine 5'-triphosphate-creatine phosphotransferase by substrate-anion complexes. Evidence for the transition-state organization of the catalytic site. Biochem J. 1971 May;122(5):727–740. doi: 10.1042/bj1220727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner-White E. J., Young D. Effect of transition-state analogue complexes on trypsin susceptibility of creatine kinase. Biochem Soc Trans. 1975;3(4):554–556. doi: 10.1042/bst0030554. [DOI] [PubMed] [Google Scholar]
- Pohl F. M. Kinetics of reversible denaturation of trypsin in water and water--ethanol mixtures. Eur J Biochem. 1968 Dec;7(1):146–152. doi: 10.1111/j.1432-1033.1968.tb19585.x. [DOI] [PubMed] [Google Scholar]
- Price N. C., Hunter M. G. Non-identical behaviour of the subunits of rabbit muscule creatine kinase. Biochim Biophys Acta. 1976 Sep 14;445(2):364–376. doi: 10.1016/0005-2744(76)90090-5. [DOI] [PubMed] [Google Scholar]
- Price N. C., Murray S., Milner-White E. J. The effect of limited proteolysis on rabbit muscle creatine kinase. Biochem J. 1981 Oct 1;199(1):239–244. doi: 10.1042/bj1990239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price N. C. The reaction of rabbit muscle creatine kinase with some derivatives of iodoacetamide. Biochem J. 1979 Feb 1;177(2):603–612. doi: 10.1042/bj1770603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson B., Pain R. H. The mechanism of folding of globular proteins. Equilibria and kinetics of conformational transitions of penicillinase from Staphylococcus aureus involving a state of intermediate conformation. Biochem J. 1976 May 1;155(2):331–344. doi: 10.1042/bj1550331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniuchi H. Formation of randomly paired disulfide bonds in des-(121-124)-ribonuclease after reduction and reoxidation. J Biol Chem. 1970 Oct 25;245(20):5459–5468. [PubMed] [Google Scholar]
- Weber K., Pringle J. R., Osborn M. Measurement of molecular weights by electrophoresis on SDS-acrylamide gel. Methods Enzymol. 1972;26:3–27. doi: 10.1016/s0076-6879(72)26003-7. [DOI] [PubMed] [Google Scholar]
- Williamson J., Greene J., Chérif S., Milner-White E. J. Heterogeneity of rabbit muscle creatine kinase and limited proteolysis by proteinase K. Biochem J. 1977 Dec 1;167(3):731–737. doi: 10.1042/bj1670731. [DOI] [PMC free article] [PubMed] [Google Scholar]


