ABSTRACT
Salmonella enterica is a diverse species that infects both humans and animals. S. enterica subspecies enterica consists of more than 1,500 serovars. Unlike typhoidal Salmonella serovars which are human host-restricted, non-typhoidal Salmonella (NTS) serovars are associated with foodborne illnesses worldwide and are transmitted via the food chain. Additionally, NTS serovars can cause disease in livestock animals causing significant economic losses. Salmonella is a well-studied model organism that is easy to manipulate and evaluate in animal models of infection. Advances in genetic engineering approaches in recent years have led to the development of Salmonella vaccines for both humans and animals. In this review, we focus on current progress of recombinant live-attenuated Salmonella vaccines, their use as a source of antigens for parenteral vaccines, their use as live-vector vaccines to deliver foreign antigens, and their use as therapeutic cancer vaccines in humans. We also describe development of live-attenuated Salmonella vaccines and live-vector vaccines for use in animals.
KEYWORDS: Salmonella, vaccines, anticancer therapy, Salmonella-delivered vaccines, recombinant-DNA technology
INTRODUCTION
Salmonella spp. are Gram-negative, flagellated, intracellular, facultatively anaerobic bacilli classified as members of the family Enterobacteriaceae (1). The genus Salmonella is comprised of two species, Salmonella enterica and Salmonella bongori, of which species S. enterica is associated with global morbidity and mortality in humans (2). The species S. enterica is further classified into six subspecies including S. enterica subspecies enterica which includes more than 1,500 serovars based on typing of somatic (O) and flagellar (H) antigens (3). S. enterica serovars Typhi, Paratyphi A, and Paratyphi B are highly adapted to human hosts and cause typhoid and paratyphoid enteric fevers (4). The Global Burden of Disease Study estimated that there were 14.3 million cases and 135,900 deaths due to typhoid and paratyphoid fevers in 2017 (5). In contrast, non-typhoidal Salmonella (NTS) have broader host range and cause gastroenteritis in both animals and humans (4, 6). NTS serovars are mainly transmitted by animal-based foods such as contaminated raw eggs, beef, pork, and poultry (7–9). While NTS normally causes self-limiting gastroenteritis, certain serovars such as Salmonella Typhimurium and Salmonella Enteritidis are associated with invasive NTS (iNTS) disease in infants in sub-Saharan Africa (10). The global burden of iNTS disease is estimated to be 535,000 cases leading to 77,500 deaths in 2017 with most infections affecting infants and immunocompromised adults (5). Despite the significant burden of disease, there are no licensed vaccines against any NTS serovars for humans. The only vaccines licensed in the United States to protect against salmonellosis are Ty21a (Vivotif), a live oral vaccine, and Vi polysaccharide, which both prevent typhoid fever caused by Salmonella Typhi (11). Elsewhere, Vi conjugate vaccines are also available to prevent typhoid fever (11).
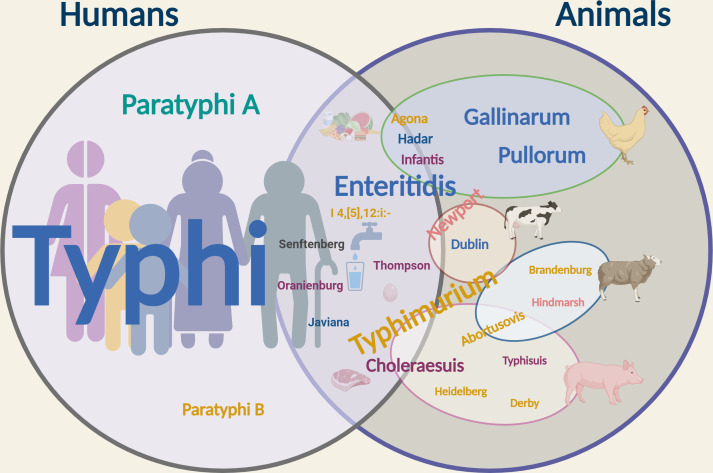
Salmonella can cause a variety of diseases in animals, including diarrhea, enteritis, septicemia, and even death. Among the numerous pathogenic Salmonella serovars, some are host-restricted, affecting a specific animal type, while others, known as generalist serovars, have a broad host range, and others are host-adapted and tend to be isolated only from certain hosts. For example, Salmonella Pullorum and Salmonella Gallinarum are host-restricted and specifically cause systemic infections in chickens; Salmonella Typhimurium is a generalist serovar and infects a variety of animals; Salmonella Choleraesuis and Salmonella Dublin commonly affect pigs and cattle, respectively, but can infect other animals (12, 13). Figure 1 shows the most common human-restricted and animal host-restricted serovars, generalist serovars, and serovars with a broad host range.
Fig 1.
Human- and animal-associated Salmonella serovars. Salmonella Typhi, Salmonella Paratyphi A, and Salmonella Paratyphi B are human host-restricted (indicated in the left circle). NTS infect a wide variety of hosts (indicated in the right circle) including humans (middle). The text color indicates the serogroup: cyan, serogroup O:2; yellow, serogroup O:4; purple, serogroup O:7; light pink, serogroup O:8; blue, serogroup O:9; and dark grey, serogroup O:1,3,19. The font size of the serovar indicates its prevalence relative to other serovars. Common NTS serovars are depicted with the agriculturally important animal they are most closely associated with respect to disease burden. Serovars that cause infection in more than one animal span multiple spheres. Created by Biorender.com.
For host-restricted serovars that cause systemic infections in food animals, vaccines are used to prevent invasive disease, especially when these infections lead to significant production losses and death. Vaccines are particularly effective in mitigating Salmonella colonization and shedding in food animals, helping to limit the spread of NTS on farms and reducing the bacterial load from the animal products coming out of them. This reduction is vital since even stringent measures at slaughterhouses might not wholly prevent contaminated food from reaching the consumer. By reducing Salmonella levels in animals, vaccines can decrease the risk to public health, such as reducing contamination of poultry products and environmental sources (14). In addition, they may indirectly reduce the risk of antimicrobial resistance (AMR) development and spread by diminishing the need for antibiotics to treat salmonellosis. From a “One Health” perspective, vaccines against multiple serotypes of NTS with protective efficacy in multiple susceptible host species are desirable. However, the vaccines currently licensed for use in food animals generally only protect against a single serovar which limits their efficacy in environments where many serovars are circulating.
Salmonella is a well-studied model organism, which is easy to manipulate, and its pathogenic mechanisms are well understood. This has enabled the rational design of live-attenuated Salmonella vaccines (15, 16). Additionally, Salmonella can be genetically engineered to express single or multiple heterologous antigens from other bacteria, viruses, parasites, or eukaryotes, or they can be manipulated to serve as a source of antigen for Salmonella vaccines (17–19). In this review, we will describe genetically engineered Salmonella for use as live vaccines, live-vector vaccines, or reagent strains for purification of components used in parenteral vaccines for use in humans, their use in vaccination against cancer in humans, and their use as vaccines and live-vector vaccines in animals.
LIVE-ATTENUATED SALMONELLA VACCINES FOR HUMAN USE
Live-attenuated Salmonella vaccines are strains which are weakened by stable mutations, allowing them to colonize the target host transiently while eliciting a protective immune response. Live-attenuated vaccines have several advantages over other vaccine formats: (i) they mimic natural infection and therefore induce cell-mediated immunity in addition to antibody responses, (ii) they have the potential to induce mucosal immunity, (iii) they can be orally administered to large communities with no risk of hazardous waste, and (iv) they have relatively lower cost and easy storage (20, 21). Attenuated Salmonella strains can be constructed by well-defined deletions, insertions, or disruptions in virulence- and metabolism-related genes of pathogenic strains using site-directed mutagenesis. However, finding the perfect balance between vaccine immunogenicity and reactogenicity is a challenge (22). Below, we discuss the most widely and extensively characterized attenuation strategies. Candidate Salmonella vaccines related to each strategy are summarized in Table 1.
TABLE 1.
Recombinant Salmonella strains for human vaccines
| Vaccine strategy | Target serovar(s) | Vaccine or strain name | Genetic modification | Phase | Reference |
|---|---|---|---|---|---|
| Live-attenuated Salmonella vaccines | Salmonella Typhi | CVD 908 | Salmonella Typhi Ty2; ΔaroC, ΔaroD | Phase 1 | (23) |
| CVD 908-htrA | Salmonella Typhi Ty2; ΔaroC, ΔaroD ΔhtrA | Phase 2 | (24–26) | ||
| CVD 909 | Salmonella Typhi Ty2; ΔaroC, ΔaroD, ΔrpoS Ptac-tviA, ΔhtrA | Phase 2 | (27) | ||
| χ4073 | Salmonella Typhi Ty2; Δcya Δcrp Δcdt | Phase 1 | (28) | ||
| Ty800 | Salmonella Typhi Ty2; ΔphoP ΔphoQ | Phase 1 | (29) | ||
| M01ZH09 | Salmonella Typhi Ty2; ΔaroC ΔssaV | Phase1/2 | (30, 31) | ||
| Salmonella Paratyphi A | CVD 1902 | ATCC 9150; ΔguaBA ΔclpX | Phase 1 | (32, 33) | |
| Salmonella Paratyphi B | CVD 2005 | CMF 6999; ΔguaBA ΔclpX | Pre-clinical | (34) | |
| Salmonella Typhimurium | WT05 | TML; ΔaroC ΔssaV | Phase 1 | (35) | |
| LH1160 | ATCC 14028; ∆purB ∆phoP/Q | Phase 1 | (36) | ||
| CVD 1931 | D65; ΔguaBA ΔclpX | Pre-clinical | (37) | ||
| Salmonella Enteritidis | CVD 1944 | Salmonella Enteritidis R11; ΔguaBA ΔclpX | Pre-clinical | (37) | |
| Salmonella Newport | CVD 1966 | Salmonella Newport; ΔguaBA ΔhtrA | Pre-clinical | (38) | |
| Live-vector vaccines—expression from plasmids | Helicobacter pylori | Ty21a (pDB1) | Salmonella Typhi Ty21a; ΔthyA; expresses H. pylori Urease A and B antigens from thyA-stabilized expression plasmids | Phase 1 | (39, 40) |
| Streptococcus pneumoniae | χ9640 (pYA4088) | Salmonella Typhi Ty2 RpoS+; ΔPcrp527::TT araC PBAD crp, ΔPfur81::TT araC PBAD fur, Δpmi-2426, Δ(gmd-fcl)−26, ΔrelA198::araC PBAD lacI TT, ΔsopB1925, ΔagfBAC811, ΔtviABCDE10, ΔaraE25, and ΔasdA33; expresses S. pneumoniae surface protein antigen PspA from Asd+ expression plasmid | Phase 1 | (41) | |
| Live-vector vaccines—expression from the chromosome |
Salmonella Typhi and S. sonnei |
Ty21a-Ss (MD77) | Salmonella Typhi Ty21a expressing S. sonnei O-antigen genes integrated into tviD-vexA locus on the chromosome | Pre-clinical | (42) |
|
Salmonella Typhi and S. dysenteriae |
Ty21a-Sd (MD149) | Salmonella Typhi Ty21a expressing S. dysenteriae serotype 1 O-antigen genes integrated into tviD-vexA locus on the chromosome | Pre-clinical | (43) | |
| Salmonella Typhi and S. flexneri | Ty21a-2a (MD194) | Salmonella Typhi Ty21a expressing S. flexneri 2a O-antigen genes integrated into tviD-vexA locus on the chromosome | Pre-clinical | (44) | |
| Ty21a-3a (MD196)D | Salmonella Typhi Ty21a expressing S. flexneri 3a O-antigen genes integrated into tviD-vexA locus on the chromosome | Pre-clinical | (44) | ||
| Salmonella Typhimurium and Salmonella Enteritidis | S1075 | Salmonella Typhimurium S100; (Δabe:prt-tyvD1 Δcrp Δcya) (O9); chromosomal deletion–insertion mutations resulting in O-serotype conversion | Pre-clinical | (45) | |
| Salmonella Typhimurium and Salmonella Choleraesuis | S1157 | Salmonella Typhimurium S100; [Δ(rmlB-wbaP):(wzyC1-wzxC1) Δcrp Δcya] (O7); chromosomal deletion–insertion mutations resulting in O-serotype conversion | Pre-clinical | (45) | |
| Salmonella Typhi, Salmonella Typhimurium, and Salmonella Enteritidis | S1163 | Salmonella Typhimurium S100; (O9, Vi+ , ΔPtviA::PssaG Δcya Δcrp); chromosomal deletion–insertion mutations resulting in O-serotype conversion | Pre-clinical | (46) | |
| Live-vector vaccines mediating directed antigen delivery | S. aureus | MvP728 (p3635) |
Salmonella Typhimurium NCTC 12023 ΔhtrA ΔpurD (carrier strain MvP728); pWSK29 PsseA sseJ::lisA51-363::HA (p3635); T3SS effector protein SseJ fused with Listeria monocytogenes LisA |
Pre-clinical | (47) |
| N19 | Salmonella Typhimurium (ΔaroA ΔpryF) carrying PagC-invB-sipA-SaEsxA (ColE1 ori) | Pre-clinical | (48) | ||
| N20 | Salmonella Typhimurium (ΔaroA ΔpryF) carrying PagC-invB-sipA-SaEsxB (ColE1 ori) | Pre-clinical | (48) | ||
| P. aeruginosa | SV9699 | Salmonella Typhimurium 14028 aroA551::Tn10 ΔaroB::KmR; pWSK29-PsseA-SseJ-PcrV-FLAG (pIZ2267); T3SS effector protein SseJ fused with P. aeruginosa antigen PcrV | Pre-clinical | (49) | |
| Designed to cause regulated delayed lysis | Streptococcus pneumoniae | χ8937 (pYA3685) | Salmonella Typhimurium UK‐1 ΔasdA19::araC PBAD c2 ΔP murA7 ::araC PBAD murA Δ(gmd‐fcl)‐26; Expression plasmid pYA3685 releases pneumococcal surface protein A (PspA) within the lymphoid tissues. | Pre-clinical | (50) |
| M. tuberculosis | RASV χ11021 | Salmonella Typhimurium ΔPmurA25::TT araC PBADmurA ΔasdA27::TT araC PBAD c2 ΔaraBAD23 Δ(gmd-fcl)−26 Δpmi-2426 Δ relA198::TT araC PBAD lacI; Asd+/MurA+ lysis vector expresses M. tuberculosis proteins Ag85A294. | Pre-clinical | (51) | |
| Extraintestinal pathogenic E. coli | χ9558 (pYA4428) |
Salmonella Typhimurium strain UK-1 (χ3761);Δpmi-2426 Δ(gmd-fcl)−26 ΔPfur81:TT araC PBAD
furΔPcrp527:TT araC PBAD crp ΔasdA27:TT araC PBAD c2 ΔaraE25 ΔaraBAD23 ΔrelA198:araC PBAD lacI TT ΔsopB1925 ΔagfBAC811; expresses E. coli EcpA and EcpD recombinant antigens |
Pre-clinical | (52) | |
| Reagent strains for parenteral vaccines |
Salmonella Typhimurium and Salmonella Enteritidis |
CVD 1925 (pSEC10‐wzzB) and CVD 1943 | Salmonella Typhimurium I77 ΔguaBA ΔclpP ΔfliD ΔfljB (pSEC10-wzzB); Salmonella Enteritidis R11 ΔguaBA ΔclpP ΔfliD; OPS and flagellin for conjugate vaccines | Phase 2 | (53, 54) |
| STmG and SEnG | Salmonella Typhimurium 1418 tolR::aph; Salmonella Enteritidis 618 tolR::aph for production of GMMA | Phase1/2 | (55) |
Auxotrophic mutants
Live-attenuated Salmonella vaccine strains with deletions in genes involved in biosynthesis of aromatic amino acids (aroA, aroC, and aroD), purines (pur), or guanine (guaBA) metabolic pathways are auxotrophic as they are unable to synthesize metabolites required for growth and are thus avirulent (56). Several pre-clinical studies have shown that Salmonella vaccine strains with mutations in aro genes have good potential as live-attenuated vaccines (56–58). For example, CVD 908 (Salmonella Typhi ΔaroC and ΔaroD) was well tolerated and highly immunogenic in clinical trials although subjects developed silent vaccinemia after vaccination (23, 24, 59).
Deletion of guaBA, which results in the inability of the bacterium to synthesize the nucleotide guanine, has been used as an attenuating mutation in multiple serovars, including Typhimurium, Enteritidis, Paratyphi A, Paratyphi B, and Newport (32, 34, 37, 38, 53). CVD 1931 (Salmonella Typhimurium D65 ΔguaBA ΔclpX) and CVD 1944 (Salmonella Enteritidis R11 ΔguaBA ΔclpX) are live-attenuated iNTS vaccines with deletion mutations in the guaBA and clpX genes. Both vaccines were proven to be immunogenic and protective against lethal challenge with the homologous serovar in mice (37). The live-attenuated Salmonella Paratyphi A vaccine (CVD 1902), (ΔguaBA ΔclpX), was evaluated in a Phase 1 clinical trial (NCT01129453; ClinicalTrials.gov), and results suggested that CVD 1902 was well tolerated and elicited CD8+ and CD4+ T-cell responses following a single dose [109 or 1010 colony-forming units (CFUs)] in human volunteers (32, 33). The Salmonella Paratyphi B vaccine, CVD 2005, was shown to induce antibody responses (serum IgG) against Salmonella Paratyphi B lipopolysaccharide (LPS) and conferred significant protection from both homologous challenge with Salmonella Paratyphi B sensu stricto (90%) and heterologous challenge (42%) with Salmonella Paratyphi B Java (34). Fuche et al. (38) constructed the Salmonella Newport (O:8) live-attenuated CVD 1966 vaccine (ΔguaBA ΔhtrA) which showed protection against Salmonella Newport infection in a mouse model.
Similarly, deletion of purB (required for de novo synthesis of purine nucleotides) has been used as an attenuating mutation in Salmonella Typhimurium. The Salmonella Typhimurium vaccine LH1160 (∆purB ∆phoPQ) elicited robust vaccine-specific humoral and mucosal immune responses against Salmonella Typhimurium LPS and flagellin in adult volunteers after a single oral dose of 5–8 × 107 CFU (36).
Deletion and/or upregulation of virulence genes
The pathogenic mechanisms of Salmonella have been extensively studied, and multiple virulence genes have been targeted in live-attenuated vaccines. As described above, the Salmonella Typhi vaccine, CVD 908, elicited vaccinemia in volunteers and was attenuated further by deleting the htrA gene, which is a part of the stress response system and encodes a periplasmic serine protease required for survival in macrophages (24, 60–63). The resulting vaccine strain CVD 908-htrA (Ty2 ΔaroC ΔaroD ΔhtrA) was well tolerated and showed robust immunogenicity in human volunteers in a Phase 2 study (25).
Another virulence gene which has been targeted is ssaV, which encodes a structural protein that is part of the Salmonella pathogenicity island 2 (SPI2) type III secretion system (T3SS), which is associated with virulence mechanisms of Salmonella infection (64). SsaV is an essential component of the T3SS machinery required for secretion of SPI2 effector proteins that mediate adhesion, invasion, and intracellular replication in host cells (65–67). The ssaV gene was deleted along with aroC in Salmonella Typhi and Salmonella Typhimurium to create vaccine strains, M01ZH09 and WT05, respectively. Both strains were well tolerated and highly immunogenic in a Phase 1 clinical study (30, 31, 35, 68). However, prolonged excretion of WT05 (up to 23 days) was observed (35).
Live oral Salmonella Typhimurium vaccines that are shed in stool for extended periods pose a significant safety concern as the live vaccine could be transmitted to household contacts. Live Salmonella Typhimurium vaccines with mutations in shdA and misL, which contribute to colonization of the intestine, have reduced shedding in mice but are still immunogenic and effective (69).
Virulence genes have also been upregulated in live-attenuated vaccines to enhance immune responses. For example, the Vi capsular polysaccharide is a key virulence determinant of Salmonella Typhi (70). To improve efficacy of the live Salmonella Typhi vaccine CVD 908-htrA, the strain was genetically engineered by replacement of the native PtviA promoter with the strong constitutive promoter Ptac to constitutively express Vi (71). The resulting vaccine strain, designated as CVD 909, was found to be safe and immunogenic in a Phase 1 clinical trial and elicited serum IgG Vi antibody in addition to anti‐LPS and antiflagellin responses (27).
Deletion of regulatory genes
The adenylate cyclase (cya) and the cyclic AMP receptor protein (crp) genes regulate the expression of many virulence genes in Salmonella. Salmonella Typhi vaccine strains carrying mutations in cya and crp have been shown to be safe and immunogenic in Phase 1 clinical trials (28). The phoP–phoQ two-component regulatory system has also been targeted in a live oral Salmonella Typhi vaccine (Ty800) which was shown to be well tolerated and highly immunogenic in a Phase 1 clinical trial (29). Similarly, Roland et al. (72) constructed the Salmonella Paratyphi A MGN10028 vaccine candidate carrying a deletion mutation in phoPQ and showed that this vaccine was attenuated and immunogenic in an oral rabbit model (72).
RECOMBINANT SALMONELLA AS LIVE-VECTOR VACCINES FOR HUMAN USE
Attenuated Salmonella strains have received attention over the last three decades as prospective carriers to deliver recombinant antigens to the immune system (22, 73). Salmonella can express heterologous antigens from other pathogens, stimulating innate immunity and activating the adaptive immune system (74, 75). Attenuated Salmonella Typhi and Salmonella Typhimurium vaccines have been engineered to express and deliver heterologous antigens from several pathogens including enteric bacterial pathogens, viral pathogens, and pathogenic protozoan parasites (76, 77). However, success with such hybrid vaccines depends on many factors including carrier strain genetics, antigen expression strategies, immunization protocols, and human host factors (22, 75, 78, 79). Therefore, various strategies have been developed for the rational design of Salmonella live-vector candidates expressing heterologous antigens at sufficient levels to elicit an immune response (19, 22); candidate Salmonella vaccines related to these strategies are again summarized in Table 1.
Expression of foreign antigens from plasmids
The use of high-copy number plasmids for antigen expression by a carrier vaccine has been reported to improve foreign antigen-specific immunogenicity of the vaccine, but such plasmid-based expression is often associated with metabolic burden in the carrier vaccine, leading to over-attenuation and plasmid instability (80, 81). To overcome plasmid loss and reduced immunogenicity of the carrier vaccine, various “balanced lethal” plasmid stabilization systems based on thyA (39, 40), asd (41, 82), and purB (83) were developed and evaluated in clinical trials. Here, genes encoding essential factors required for growth are deleted from the bacterial chromosome and complemented with a wild-type copy of the corresponding gene on a plasmid, thereby constituting a balanced lethal combination in all surviving cells (84–86). For example, the enzyme aspartate β-semialdehyde dehydrogenase (Asd) is essential for the synthesis of several amino acids as well as the diaminopimelic acid (DAP) constituent of peptidoglycan in the cell wall of Gram-negative bacteria (85). Consequently, Salmonella asd mutant strains harboring multicopy asd-encoding plasmids constitute a balanced lethal combination in that all surviving cells would have to possess the recombinant asd+ plasmid (77, 87). Using an asd balanced lethal system, highly immunogenic live-attenuated Salmonella Typhimurium strains were constructed for the heterologous expression of a viral peptide from hepatitis B virus (88) and F1-Ag and V-Ag antigens derived from Yersinia pestis (89).
Expression of foreign antigens from the chromosome
Chromosomal integration of expression cassettes has been successfully used to obtain stable expression of the foreign antigen in the absence of selective pressure (90–92). For example, Dharmasena et al. (42) developed a bivalent candidate vaccine against typhoid and shigellosis. Chromosomal integration of the Shigella sonnei O-antigen biosynthetic gene cluster (12 kb) into the genome of Salmonella Typhi Ty21a led to a recombinant Ty21a-Ss (Ty21a expressing S. sonnei O-antigen) strain, which was observed to be 100% genetically stable and elicited robust serum anti-S. sonnei LPS and anti-Salmonella Typhi LPS IgG antibody responses. Mice immunized with the Ty21a-Ss construct showed significant protection against a virulent S. sonnei challenge (42). This group further developed immunogenic candidate Ty21a vaccine vector strains expressing heterologous Shigella dysenteriae 1 O-antigens (Ty21a-Sd) or Shigella flexneri 2a (Ty21a-2a) or 3a O-antigens (Ty21a-3a) (43, 44).
In a study by Li et al. (45), the immunodominant O4 serotype of Salmonella Typhimurium was converted into O9, O7, and O8 serotypes by introducing chromosomal deletion–insertion mutations with an overall goal to develop live-attenuated Salmonella Typhimurium vaccines providing broad-spectrum protection against the major serovars responsible for NTS infections. Live-attenuated Salmonella Typhimurium vaccine strains S1075 (Δabe:prt-tyvD1 Δcrp Δcya) (encoding serotype O9), S1157 [Δ(rmlB-wbaP):(wzyC1-wzxC1) Δcrp Δcya] (encoding serotype O7), and S1116 [Δ(wzxB1-wabN):(wzxC2-wbaZ) Δcrp Δcya] (encoding serotype O8) were constructed using this novel O-serotype conversion strategy. All vaccinated mice survived challenge with 100 times the LD50 dose of the homologous wild-type Salmonella Typhimurium. Mice vaccinated with Salmonella Typhimurium S1075 (O9) also showed 100% protection against a heterologous lethal challenge with Salmonella Enteritidis. Similarly, complete protection was observed against Salmonella Choleraesuis in mice vaccinated with Salmonella Typhimurium S1157 (O7). Co-vaccination with Salmonella Typhimurium S1075 (O9) and Salmonella Typhimurium S1157 (O7) vaccine strains provided broad coverage and protective efficacy against Salmonella Typhimurium, Salmonella Enteritidis, and Salmonella Choleraesuis (45). In a related study, live-attenuated Salmonella Typhimurium vaccine S1163 (O9, Vi+, ΔPtviA::PssaG Δcya Δcrp) that is capable of producing both Vi capsular and O9 O-antigen polysaccharide antigens was shown to be immunogenic and conferred a high level of protection in mice against challenge with wild-type strains of Salmonella Typhimurium and Salmonella Enteritidis (46).
Directed presentation of antigens
Another important aspect for the successful delivery of heterologous antigen from a live carrier vaccine is the presentation of antigen to the appropriate immune inductive site(s), which will influence the type and strength of the immune response induced in the vaccinated host (93). Toward this end, Salmonella T3SS can be used to deliver foreign antigens (94). S. enterica encodes several virulence factors through SPI genes and, in particular, SPI1 and SPI2 that play important roles in different phases of pathogenesis. The SPI1-encoded T3SS delivers at least 13 effector proteins across the host cell membrane and plays an important role in contact and invasion of host intestinal epithelial cells (95). The SPI2 T3SS on the other hand delivers about 30 effector proteins into the host cytoplasm and causes systemic infections and proliferation within host cells (96). Therefore, SPI1 and SPI2 genes encode distinct T3SS that can be used for the efficient delivery of heterologous antigen to the cytosol of antigen-presenting cells leading to desired immune responses (97). T3SS-mediated heterologous antigen delivery by recombinant Salmonella has been shown to produce protective immunity against other bacterial and viral pathogens. However, selection of the appropriate SPI2 T3SS effector protein is a critical parameter for heterologous antigen fusions and construction of efficient recombinant vaccines (97). For example, Hegazy et al. (47) demonstrated that out of five SPI2 T3SS effector proteins tested for their efficiency of translocation of the model antigens ovalbumin and listeriolysin O (Llo) and elicitation of immune responses, including SifA, SteC, SseL, SseJ, and SseF, only an attenuated Salmonella Typhimurium strain construct MvP728 (p3635) in which SseJ was fused to listeriolysin (Llo51–363) elicited potent T-cell responses in vitro as well as in vivo when tested in mice (47). In another study, an Salmonella Typhimurium-attenuated strain SV9699, expressing the SPI2 T3SS effector protein SseJ fused to the Pseudomonas aeruginosa antigen PcrV and placed under the transcriptional control of an in vivo inducible promoter PsseA, produced elevated levels of specific anti-PcrV IgG and protected mice against a lethal challenge with fully virulent P. aeruginosa (49). Finally, mice orally vaccinated with attenuated Salmonella Typhimurium vaccine strains N19 and N20, delivering Staphylococcal antigens SaEsxA and SaEsxB via the SPI-1 T3SS effector protein SipA, elicited both humoral and cell-mediated immune responses protecting mice against challenge with Staphylococcus aureus strains (48).
Programmed delayed cell lysis approach
For the rational design of recombinant attenuated Salmonella vaccine (RASV) strains with improved safety, Curtiss III et al. (50) developed a system of “programmed delayed cell lysis.” Under this system, the Salmonella Typhimurium host vector strain χ8937(pYA3685) was constructed by introducing arabinose-regulated expression of the asdA and murA genes. The Asd enzyme is required for the synthesis of DAP, and the MurA enzyme controls the expression of muramic acid. Both DAP and muramic acid are components of the peptidoglycan layer of the bacterial cell wall. In addition, another deletion mutation Δ(gmd-fel) was introduced to repress the synthesis of colonic acid which is a polysaccharide made in response to stress associated with cell wall damage. Mice orally inoculated with RASV Salmonella strains did not show any lethality even when delivered at high doses; once in the host tissue, these strains undergo several rounds of replication, then programmed lysis occurs due to the absence of arabinose, and the strains are subsequently cleared from the lymphoid tissue (50).
RASV strains have also been used to deliver recombinant antigens to the immune system. Juárez-Rodríguez et al. (51) constructed the RASV strain χ11021 expressing Mycobacterium tuberculosis antigens ESAT-6 and culture filtrate protein 10 (CFP-10) which induced both humoral and cellular immune responses in mice. The RASV χ11021 strain showed regulated delayed lysis and regulated delayed antigen synthesis in vivo and conferred significant protection against mycobacterial infection (51). In another study, a RASV strain was designed to provide protection against extraintestinal pathogenic Escherichia coli infections. Mice orally immunized with RASV χ9558 vaccine strain expressing E. coli major pilin (EcpA) and tip pilus adhesin (EcpD) antigens produced both anti-E. coli EcpA and EcpD IgG and IgA antibodies as well as anti-Salmonella LPS antibodies (52).
Genetically controlled induction of regulated attenuation, delayed antigen expression, and delayed lysis in these RASV strains is regulated at the level of transcription by sugar-inducible promoters that can easily be activated by the addition of one or more sugars to the growth medium during preparation of the live-vector vaccine. Following administration of the vaccine, the availability of these supplemented sugars vanishes in vivo. The vaccine strain gradually becomes attenuated as the strain replicates and intracellular levels of the inducing sugars drop to non-inducing levels; as intracellular sugar levels decrease, foreign antigen expression is derepressed, and high levels of antigen are efficiently released to immune inductive sites through delayed lysis of the vaccine strain.
A particularly remarkable example of this approach is found in a recent report by Wang et al. (98) in which attenuation, antigen synthesis, and delayed lysis of an Salmonella Typhimurium live-vector vaccine strain are simultaneously regulated by supplementation with three exogenous sugars. Delayed attenuation is accomplished at two levels. Chromosomal deletion of the pmi gene encoding phosphomannose isomerase (required for proper synthesis of LPS O-antigen side chains) attenuates the vaccine strain; propagation of the vaccine strain therefore requires supplementation with mannose to properly synthesize full-length LPS. A second level of attenuation is achieved by integrating a rhamnose-regulated promoter into the chromosome of the Salmonella Typhimurium live-vector strain to control expression of WaaL. Since Waal is an O-antigen ligase that is necessary to ligate polysaccharide to the lipid A-LPS core moiety, removal of rhamnose results in attenuation of the strain due to the complete loss of O-antigen. Coordinated regulation of delayed lysis and foreign antigen synthesis is accomplished by arabinose-regulated promoters controlling synthesis of Asd as well as MurA, both involved in biosynthesis of the bacterial cell wall, as well as arabinose-controlled expression of foreign antigen(s) from multicopy plasmids. Therefore, growth of the vaccine strain in medium supplemented with mannose, rhamnose, and arabinose results in metabolically fit live-vector strains displaying full-length LPS and no foreign antigen production in vitro; after immunization, vaccine strains become attenuated upon replication in vivo, with a rise in foreign antigen synthesis and efficient delivery of immunogens to immune inductive sites upon subsequent lysis of the vaccine. This novel vaccine design was applied to the development of a Salmonella Typhimurium-based poultry live-vector vaccine against necrotic enteritis caused by the Gram-positive pathogen Clostridium perfringens Type G (98). This approach could similarly be applied to Salmonella live-vector vaccines for use in humans.
RECOMBINANT SALMONELLA AS REAGENT STRAINS FOR HUMAN VACCINES
The development of various parenteral vaccines may be facilitated using attenuated Salmonella vaccines as a source of purified antigens. These vaccines include conjugate as well as outer membrane vesicle (OMV)-based vaccines. We have previously engineered Salmonella Typhimurium CVD 1925 (pSEC10‐wzzB) and Salmonella Enteritidis CVD 1943 reagent strains which could efficiently produce high concentrations of flagellin monomers and core and O-polysaccharide (COPS) for use in conjugate vaccines (53, 54, 99). Salmonella Typhimurium and Salmonella Enteritidis COPS:FliC glycoconjugate vaccines were constructed using components purified from these reagent strains and were shown to be immunogenic and protective against lethal challenge (99–103). These vaccines have been combined with Bharat Biotech’s Typbar TCV as a trivalent Salmonella conjugate vaccine which is currently being evaluated in a Phase 2 clinical trial (ClinicalTrials.gov ID NCT05784701).
OMVs are spherical exosomes of about ~25–250 nm in size, which are naturally released by Gram-negative bacteria (104). Their composition includes antigens found in the bacterial outer membrane, such as LPS, endogenous proteins, lipoproteins, and peptidoglycan; these vesicles are being investigated as vaccines (105). GSK Vaccines Institute for Global Health has developed OMV-based Salmonella vaccines which they call Generalized Modules for Membrane Antigens (GMMA) (106, 107). They have genetically engineered Salmonella Typhimurium and Salmonella Enteritidis to produce large amounts of GMMA by deletion of the tolR gene. In an elegant study reported by Micoli et al. (55), GMMAs from Salmonella Typhimurium (STmG) or Salmonella Enteritidis (SEnG) were constructed and directly compared to the homologous conjugate vaccine comprised of purified O-antigen conjugated to CRM197. Immunization with purified GMMAs induced higher anti-O-antigen IgG than glycoconjugate administered without Alhydrogel adjuvant; when the glycoconjugate vaccines were administered with Alhydrogel, antibody levels were similar. O-antigen-specific antibody responses were also accompanied by a reduction in bacterial colonization (55). As a result, such OMV-based vaccines against iNTS have now progressed to clinical studies (108).
RECOMBINANT SALMONELLA AS A THERAPEUTIC CANCER VACCINE IN HUMANS
The remarkable versatility of Salmonella used as a prophylactic vaccine against infectious diseases has also been investigated in attempts to develop therapeutic interventions against non-infectious diseases such as cancer (109–113). As opposed to prophylactic vaccines designed to prevent disease, immunotherapeutic Salmonella vaccines are intended to disrupt established disease caused by tumors and to block metastasis of cancer cells to new anatomical sites. Despite recent success with immune checkpoint inhibitors that break tolerance against tumors and reactivate the immune system to target non-solid tumors, attempts to apply this approach to solid tumor tissue have been disappointing in multiple clinical trials. Solid tumors present a daunting challenge to immunotherapeutic approaches due to the robust immunosuppressive microenvironment surrounding established tumors, which blocks productive innate and adaptive tumor-specific immunity. However, it has been shown in experimental murine models that systemic infection with Salmonella can result in specific colonization of tumor tissue and subsequent reactivation of innate immunity, leading to a robust cytotoxic T-cell response targeting the clearance of growing tumors.
Salmonella Typhimurium
Most pre-clinical studies conducted thus far have focused on intravenous (IV) administration of attenuated Salmonella Typhimurium to mice subcutaneously implanted with various tumor cell lines. The IV route has been intensively investigated based on work repeatedly demonstrating that systemic administration of attenuated strains of Salmonella Typhimurium can preferentially colonize tumor tissue at ratios of 1,000:1 versus normal tissues (114, 115), resulting in robust anti-tumor immunity with minimal off-target side effects. Although the mechanisms behind this observation have not been conclusively defined, several factors have been implicated including (i) the hypoxic necrotic environment of tumors preferentially supporting the growth of facultative anaerobic Salmonella Typhimurium strains, (ii) chemotaxis toward necrotic tissues releasing nutrients, and (iii) active invasion of tumor cells to escape immune surveillance (109, 112, 116).
To date, the most extensively studied attenuated strain of Salmonella Typhimurium examined in both pre-clinical mouse studies and subsequent clinical trials is VNP20009, carrying attenuating mutations in purM (conferring a purine auxotrophy) (117, 118), msbB (reducing the acylation of lipid A) (114, 118), and a cryptic 128-kb chromosomal deletion that phenotypically suppresses the salt sensitivity associated with deletion of msbB (119). The msbB deletion is intended to reduce potential endotoxic inflammatory responses associated with IV delivery of therapeutic bacteria. This strategy of using attenuated strains of Salmonella Typhimurium to reduce potentially toxic systemic inflammatory responses associated with IV delivery, while maintaining tumor-specific inflammatory responses triggered by specific colonization of tumor tissues, is central to all systemic Salmonella-based immunotherapeutic approaches. For example, several excellent studies by Kong et al. (120, 121) have demonstrated that detoxification of lipid A through both dephosphorylation and reduced acylation strategies can be achieved while still maintaining the immunogenicity and efficacy of the modified vaccine strain. The most reactogenic form of lipid A is a hexa-acylated species that triggers TLR4-mediated proinflammatory responses through the TLR4-MD2-CD14 pathway (122). Significant reductions in proinflammatory cytokines can be achieved through genetic manipulation of the vaccine strain to synthesize only penta-acylated lipid A. Kong et al. (121) demonstrated that deletion of both msbB (addition of a C14 myristate acyl chain) and pagP (addition of a C16 palmitate acyl chain) reduced acylation of the wild-type Salmonella Typhimurium hepta-acylated lipid A, resulting in synthesis of only a penta-acylated but fully phosphorylated species of lipid A. Significant reduction in proinflammatory responses was demonstrated in vitro using cultured human Mono Mac 6 cells, while impressive antigen-specific immunity and efficacy against challenge were still observed in a mouse model.
Pre-clinical therapeutic efficacy studies in mice experimentally challenged with various tumor cell lines (e.g., B16F10 melanoma cells) and subsequently treated with VNP20009 strains strongly suggested that therapeutic success in clinical trials might be realized (114, 123). Despite such promising results in mice, when VNP20009 was tested intravenously in Phase 1 clinical trials, similar levels of efficacy were not observed. Toso et al. (124) reported that IV infusion of 3 × 108 CFU/m2 of VNP20009 as a single dose into six patients with metastatic cancer during a 30-minute period proved to be remarkably safe, with no serious side effects. However, no clinical benefit from the treatment was reported; modest tumor accumulation was inconsistently observed, and no patient experienced objective tumor regression. It was also noted in this study that VNP20009 was cleared from the blood within 60 minutes of infusion. A second study was therefore initiated in which four additional metastatic cancer patients received infusions of 3 × 108 CFU/m2 of VNP20009, this time administered IV over an extended 4-hour period in an attempt to maintain a high circulating level of bacteria intended to potentially improve tumor-specific accumulation. Again, the treatment was well tolerated, but bacteria were cleared from the bloodstream within 2 hours post-infusion, again with negligible tumor colonization and no objective clinical responses to treatment (125). Subsequent attempts to improve the therapeutic efficacy of engineered VNP20009 involving co-administration with chemotherapeutic agents again proved very promising in mouse models but failed to elicit objective tumor responses in patients (126, 127).
To improve both tumor targeting of therapeutic strains, intratumoral replication, and increased oncolysis, new auxotrophic strains of VNP20009 have been engineered for methionine dependency (128), taking advantage of the methionine metabolic dependence (Hoffman effect) common to cancer cells (129). In mouse models using both syngeneic and xenograft tumor cells, colonizing auxotroph effectively outcompeted tumor cells for methionine, resulting in tumor regression and suppression of metastasis (128). Still other strategies have explored co-administration of therapeutic Salmonella strains along with immune checkpoint PD-L1 inhibitors (130) or using bacteria delivering oncolytic prokaryotic (131) or eukaryotic payloads (132) to targeted tumor tissues. Excellent reviews discussing in greater detail these and other novel engineering strategies have been previously published (111–113, 127).
Salmonella Typhi
One parameter often overlooked in pre-clinical studies is the host specificity of the Salmonella serovar being investigated. Although wild-type Salmonella Typhimurium is pathogenic for humans, it is not human host-adapted and therefore cannot mount a sustained systemic infection in human hosts as it can when infecting mice and other warm-blooded animals. Failure in clinical trials of non-invasive attenuated strains of Salmonella Typhimurium administered intravenously may therefore be partially explained by an inherent inability of even the wild-type strain to persist in deep human tissues. However, the wild-type serovar Salmonella Typhi is exquisitely human host-restricted and can efficiently invade into deep tissues, leading to systemic typhoid disease and a potentially protracted carrier state in which pathogenic organisms are continually shed. This insight has spawned new efforts to explore attenuated strains of Salmonella Typhi, including the licensed typhoid vaccine Ty21a (Vivotif), as therapeutic agents against cancer.
Unmodified Ty21a has been recently tested against non-muscle-invasive bladder cancer (NMIBC) using intravesical introduction of the vaccine strain directly into the bladder. This approach is based on standard-of-care treatment of these cancers based on intravesical administration of the Bacillus–Calmette–Guerin (BCG) tuberculosis vaccine to prevent recurrence and/or progression (133, 134). Pre-clinical testing was conducted in an orthotopic MB49-bladder cancer model that closely reproduces NMIBC in mice (135, 136). Results indicated that Ty21a was more effective than BCG at inducing regression of established bladder tumors after intravesical administration. When tested in Phase 1 clinical trials by intravesical administration of Ty21a into patients with NMBIC, the treatment was found to be safe, with no serious side effects and a lower risk of undesirable bacterial persistence than in patients treated with BCG (137).
Phase 1 clinical trials have also been reported in which Ty21a was used as a DNA vaccine delivery platform (138–140). The therapeutic vaccine strain VXM01 was derived from Ty21a into which a DNA vaccine encoding human vascular endothelial growth factor receptor 2 (VEGFR2) was introduced. VXM01 was designed to compete with rapidly proliferating tumor tissues as an antiangiogenic therapy leading to the breakdown of nascent vasculature and tumor necrosis (141). Of note, no pre-clinical efficacy studies in mice were reported for this candidate therapeutic vaccine. In Phase 1 clinical trials, patients with advanced pancreatic cancer received four oral priming vaccinations of VXM01 at doses up to 1010 CFUs per dose in addition to gemcitabine as standard of care. No dose-limiting toxicities were reported, and modest reductions of tumor perfusion were observed in some patients, potentially linked to an observed reactivation of pre-existing antiangiogenic memory T cells triggered by the vaccine (139). A follow-up study was conducted to potentially improve T-cell responses by again orally priming patients with four doses of vaccine between 106 and 107 CFUs spaced every 2 days, followed by monthly booster doses for a further 6 months; patients also received gemcitabine across the duration of the study. Although some improvement in VEGFR2-specific T cells was again observed, no objective clinical improvements were noted (140).
LIVE-ATTENUATED SALMONELLA VACCINES FOR USE IN ANIMALS
As noted above, NTS cause a wide range of diseases in animals, particularly those of agricultural and economic importance. The range of host adaptation within NTS also makes agriculturally important animals a reservoir for human NTS infections. Vaccine development strategies, similar to those developed for Salmonella vaccines intended for human use, have been employed to limit the burden of NTS disease particularly in chickens, pigs, and cattle. Reduction in the colonization of Salmonella in various organs like the cecum, liver, and spleen and reduced shedding in feces are among the more critical readouts in assessing vaccine efficacy. Live-attenuated Salmonella animal vaccines, developed using various strategies, aim to provoke adaptive immunity while ensuring they cannot revert to a pathogenic form or pose environmental risks (142). While many live Salmonella vaccines with different mutations have undergone efficacy testing in different animals as discussed here and elsewhere (143, 144), only a handful are currently available commercially. Table 2 lists by host species some of the more promising vaccine candidates either commercially available or in the research pipeline. As with the human vaccines, these live-attenuated vaccines can also serve as carriers to boost immunity against various pathogens (142), and future multitarget vaccines could offer wider protection with a single dose (142). Below, we discuss development of NTS vaccines for the most agriculturally important animal species.
TABLE 2.
Candidate and currently available Salmonella vaccines in agriculturally important animals
| Target species | Vaccine serovar(s) | Vaccine name/strain | Target serovar(s) | Parental strains and genetic modification | Target gene class | Effect | Reference |
|---|---|---|---|---|---|---|---|
| Chickens | Enteritidis | Gallivac SE/Salmovac SE | Enteritidis and Typhimurium | Salmonella Enteritidis strain 441/014 adenine-histidine auxotroph | Metabolic pathways | Reduced colonization of liver and cecum following challenge with wild-type nalidixic acid resistant strains of Salmonella Enteritidis and Salmonella Typhimurium | (145) |
| Z11 ΔrfbG | Enteritidis | Salmonella Enteritidis Z11; ΔrfbG | Virulence factor | Reduced colonization in liver, spleen, and cecum following challenge with wild-type Salmonella Enteritidis | (146) | ||
| SE 147N ΔphoP ΔfliC | Enteritidis | Salmonella Enteritidis 147N; ΔphoP ΔfliC | Global regulators | Reduced cecal colonization and dissemination of Salmonella Enteritidis following challenge | (147) | ||
| JOL919 | Enteritidis | Salmonella Enteritidis JOL860; Δlon ΔcpxR | Global regulators | Reduced colonization and protected from lethal challenge with Salmonella Enteritidis | (148) | ||
| Typhimurium | Poulvac ST | Typhimurium | Lyophilized form of Salmonella Typhimurium strain AWC 591; ΔaroA ΔserC | Metabolic pathways | Protected from lethal challenge with Salmonella Heidelberg after multiple doses and partial protection after one dose. It also reduced shedding of wild-type Salmonella Typhimurium | (149, 150) | |
| MT2313 | Typhimurium, Enteritidis, O:6,14,24:e,h:monophasic | Salmonella Typhimurium UK-1; dam102::Mud-Cm | Global regulators | Protected against colonization of homologous and heterologous challenge serovars | (151) | ||
| χ3985 | Typhimurium, Heidelberg, Agona, Bredeney | Salmonella Typhimurium strain X3761; Δcya Δcrp | Metabolic pathways | Protected against colonization of homologous and heterologous challenge serovars | (152) | ||
| Gallinarum | SG-VAC | Gallinarum | Salmonella enterica, subsp. enterica, serovar Gallinarum, Live strain SGP695AV | Information not publicly available | Information not publicly available | (153) | |
| Nobilis SG 9R | Gallinarum | Salmonella Gallinarum rfaJ point mutation | Virulence factor | Protected against fowl typhoid (Salmonella Gallinarum infection) | (154) | ||
| χ11387 | Gallinarum and Enteritidis |
Salmonella Gallinarum 287/91 ΔPcrp527::TT araC PBAD crp |
Metabolic pathways (amino acid synthesis, transporters, and metal uptake) | Reduced colonization of challenge strain in liver and spleen | (155) | ||
| JOL2841 | Gallinarum |
Salmonella Gallinarum JOL 2624 (Δlon ΔcpxR ΔrfaL); ΔarnT |
Global regulators | Reduced colonization in liver and spleen following challenge with Salmonella Gallinarum; reduced liver pathology compared to SG 9R vaccine and protected from lethal challenge | (156) | ||
| χ11797 | Gallinarum |
Salmonella Gallinarum 287/91; Δfur-712 |
Metabolic pathways | Protected from lethal challenge against Salmonella Gallinarum | (157) | ||
| 1009 ΔspiC Δcrp | Gallinarum and Pullorum |
Salmonella Gallinarum 1009; ΔspiC Δcrp |
Virulence factor | Vaccine strain persisted in liver and spleen; reduced clinical symptoms and mortality after lethal challenge with wild-type Salmonella Gallinarum | (158) | ||
| HG212 | Gallinarum | Salmonella Gallinarum strain 9; ΔaroA | Metabolic pathways | Protected from lethal challenge after multiple doses, partially after one dose. Protective by intramuscular delivery but not oral delivery | (159) | ||
| Pullorum | S06004ΔSPI2 | Gallinarum and Pullorum | Salmonella Pullorum S06004; ΔSPI2 | Virulence factor | Vaccine strain persisted in liver and spleen; reduced clinical symptoms and mortality after lethal challenge with the parental Salmonella Pullorum strain and wild-type Salmonella Gallinarum | (160) | |
| S06004 ΔspiC ΔrfaH (DIVA strain) | Pullorum | Salmonella Pullorum S06004; ΔspiC ΔrfaH | Virulence factor | Reduced colonization and protected from lethal challenge against wild-type parental Salmonella Pullorum | (161) | ||
| Enteritidis, Infantis, Typhimurium |
Salmonella Enteritidis 147 SPI1-lon-fliC, Salmonella Typhimurium 16E5 SPI1-lon-fliC-fljB, and Salmonella Infantis 18G6 SPI1-lon-fliC-fljB |
Enteritidis, Infantis, Typhimurium, Agona, Dublin, and Hadar |
Salmonella Enteritidis 147; ΔSPI1 Δlon ΔfliC Salmonella Typhimurium 16E5 and Salmonella Infantis 18G6; ΔSPI1 Δlon ΔfliC ΔfljB |
Virulence factor | Reduced cecal colonization following homologous challenge; some protection observed against heterologous strains (Salmonella Hadar and Salmonella Dublin) | (162) | |
| Turkey | Typhimurium | Elanco AviPro Megan Egg | Hadar and Infantis | Salmonella Typhimurium; live culture | Information not publicly available | Reduced intestinal colonization (cecum, cecal tonsils, and cloaca) and systemic dissemination to spleen following challenge with wild-type Salmonella Infantis and Salmonella Hadar | (163) |
| Swine | Cholerasuis | Enterisol Salmonella T/C | Choleraesuis and Typhimurium | Salmonella Choleraesuis variety Kurzendorf SC-54 strain and Salmonella Typhimurium strain 421/125 | Loss of spv on virulence plasmid by serial passage in neutrophils | Significantly reduced the seroprevalence and Salmonella isolation in pigs at slaughter | (164) |
| NOBL SC-54 | Choleraesuis | Salmonella Choleraesuis variety Kurzendorf SC-54 strain | Loss of spv on virulence plasmid by serial passage in neutrophils | Significantly reduced clinical symptoms and bacterial burden in organs post-challenge with Salmonella Choleraesuis | (165) | ||
| Argus SC/ST | Cholerasuis and Typhimurium | Δcya, Δcrp | Metabolic pathways | Significantly reduced Salmonella infections in swine farm | (166) | ||
| C500 | Choleraesuis | Salmonella Choleraesuis strain C78-1; attenuated by chemical methods | Unknown | Significantly reduced Salmonella infections in swine farm | (167) | ||
| Typhimurium | ΔznuABC | Typhimurium | Salmonella Typhimurium ATCC14028; ΔznuABC | Metabolic pathways | Induced humoral and cellular immune responses; reduced viability of vaccine strain in feces and the environment | (168) | |
| BBS 866 | Typhimurium and Choleraesuis | Salmonella Typhimurium strain BSX 8 (χ4232); ∆rfaH; deletion mutation in multiple sRNA genes (∆omrA, ∆omrB, ∆rybB, ∆micA, and ∆invR) | Virulence factor | Reduced colonization of wild-type strain after challenge, fecal shedding, and reduced clinical symptoms of infection post-challenge | (169–171) | ||
| JOL911 | Typhimurium | Salmonella Typhimurium strain JOL401;ΔcpxR Δlon | Global regulators | Immunogenic in pregnant sows; protected piglets from clinical symptoms of wild-type Salmonella Typhimurium infection | (172) | ||
| Salmoporc | Typhimurium | Salmonella Typhimurium strain 421/125; Δhis Δade | Metabolic pathways | Reduced tissue colonization of Salmonella Typhimurium challenge strain | (173) | ||
| Cattle | Dublin | EnterVene-d | Dublin | Salmonella Dublin aro mutant | Metabolic pathways | Reduced mortality risk in immunized calves | (174) |
| Dublin | Sdu189 ΔspiC ΔaroA | Dublin |
Salmonella Dublin Sdu189; ΔspiC ΔaroA |
Virulence factor, global regulators |
Protected mice from lethal challenge with wild-type parental strain | (175) | |
| Typhimurium | MT2313 | Typhimurium and Newport | Salmonella Typhimurium UK-1; dam102::Mud-Cm | Global regulators | Reduced tissue colonization of Salmonella Newport challenge strain in calf organs; protected calves from clinical symptoms of Salmonella Newport infection | (176) | |
| Sheep | Abortusovis | SSM189 | Abortusovis | Salmonella Abortusovis strain SS44; Δcya Δcrp Δcdt | Metabolic pathways | Improved lambing performance in immunized sheep after challenge with Salmonella Abortusovis | (177) |
| SU304 | Abortusovis | Salmonella Abortusovis strain SS44; ΔaroA | Global regulators | Improved lambing performance in immunized sheep after challenge with Salmonella Abortusovis | (177) | ||
| Typhimurium | MT2313 | Typhimurium | Salmonella Typhimurium UK-1; dam102::Mud-Cm | Global regulators | Reduced tissue colonization of wild-type Salmonella Typhimurium and protected sheep from lethal challenge | (178) |
Poultry
In response to rising demand for poultry products and stricter consumer and regulatory demands, vaccination has become a cornerstone in integrated control programs for poultry including chickens, turkeys, especially for breeders, and layers (179). Vaccination reduces the risk of human foodborne infections by decreasing Salmonella colonization, organ invasion, and environmental contamination (180–182). Additionally, vaccination against host-restricted Salmonella serovars protects the health of chickens and prevents economic losses due to disease or mortality. Diseases caused by Salmonella Pullorum and Salmonella Gallinarum in chickens are effectively managed in high-income countries like the United States through robust eradication and surveillance initiatives implemented within commercial chicken production systems. Consequently, countries adopting this approach do not employ vaccines targeting these host-restricted strains. The poultry industry in resource-poor nations across Africa and Asia encounters persistent challenges posed by these strains. The primary focus of vaccine development efforts against Salmonella Pullorum and Salmonella Gallinarum is therefore directed toward these developing regions, where the impact of these bacterial strains remains a significant concern. Generalist serovars have also been targeted in efforts to reduce disease burden in animals but also limit transmission of strains to humans.
There are several commercially available live-attenuated vaccines for use in poultry (Table 2). Nobilis SG 9R has a long history of use and is a rough mutant of a Salmonella Gallinarum strain (154). However, there have been reports showing high similarity between field strains isolated during outbreaks and the vaccine strain indicating possible reversion to the virulent wild-type (183, 184). Another Salmonella Gallinarum vaccine (SG-VAC) is only available in Poland (153). Salmonella Typhimurium vaccines Poulvac ST (150) and AviPro Megan Egg (163) and the Salmonella Enteritidis vaccine Gallivac SE/Salmovac SE are more widely available commercially for use in poultry (145). Their availability varies in different countries based on the epidemiological context and local regulations.
A variety of vaccines have been engineered by targeting genes linked to bacterial antigens, synthesis pathways, regulatory functions, and virulence factors (Table 2). Generalist serovars like Salmonella Enteritidis are a major concern in poultry flocks not only because of the morbidity and mortality of flocks but also due to the associated food safety risks and the potential for transmission to human hosts. Vaccine candidates targeting this serotype have been engineered to be deficient in global regulators of protein expression and/or deficient in virulence factors. For example, Salmonella Enteritidis strain Z11 ΔrfbG does not make LPS, a key virulence factor for invasion, and was developed as a Differentiating Infected from Vaccinated Animals (DIVA) vaccine strain (146). The deletion of the rfbG gene produces a truncated rough LPS that does not react with O antibodies and can be distinguished from wild-type strains during routine sampling and surveillance; it can also be distinguished from wild-type strains based on morphology. Chickens immunized intramuscularly with Z11 ΔrfbG produced Salmonella Enteritidis-specific IgG, with reduced bacterial colonization of the liver, spleen, and cecum after challenge with wild-type Salmonella Enteritidis Z11. Salmonella Enteritidis strain JOL919 is deficient in both Lon protease and CpxR, a global regulator and response regulator in a two-component signal transduction pathway, respectively (148). Chickens immunized with JOL919 and challenged with wild-type Salmonella Enteritidis had reduced gross lesion scores on the liver and reduced colonization of the wild-type strain in the liver, spleen, and cecum. Live vaccines deficient in genes involved in global gene expression have also been paired with competitive exclusion (CE) cultures which limit the capacity of wild-type infectious strains to colonize the intestinal tract. Oral administration of candidate vaccine SE 147N ΔphoP ΔfliC alone or with subsequent administration of a CE culture induced cytokine responses. After challenge with wild-type Salmonella Enteritidis, chicks receiving SE 147N ΔphoP ΔfliC alone or with CE had reduced cecal colonization of the wild-type strain, and the effect was additive when chicks received both the vaccine and CE (147). Another example of generalist serovars being engineered as vaccine strains for use in animals is the Salmonella Typhimurium strain MT2313. Chicks immunized at 10 hours post-hatching and subsequently challenged with wild-type Salmonella Typhimurium had significantly reduced colonization; moderate protection was also observed following challenge with heterologous Salmonella Enteritidis and Salmonella O:6,14, 24:e,h-monophasic strains (151).
Live-attenuated vaccines targeting host-restricted serovars in chickens like Salmonella Gallinarum and Salmonella Pullorum encompass a range of genetic alterations across multiple strains. For instance, the Salmonella Gallinarum JOL2841 vaccine strain harbors deletions in the cpxR and arnT genes, which has multiple impacts on strain viability and pathogenicity; the absence of cpxR affects stress, intracellular survival, and biofilm formation, while deletion of arnT alters the charge and stoichiometry of lipid A, decreasing toxicity and virulence (156). Chickens immunized with JOL2841 had significant protection from death upon challenge (156).
The T3SS has also been a target for vaccines administered to animals. Vaccine strains targeting poultry-adapted serovars have been engineered to be deficient in T3SS effector proteins. Salmonella Gallinarum 1009 ΔspiC Δcrp, Salmonella Pullorum S06004 ΔSPI2, and Salmonella Pullorum S06004 ΔspiC ΔrfaH (Table 2) lack the SpiC effector protein; intracellular trafficking of host proteins is disrupted and prevents the fusion of Salmonella-containing vacuoles with lysosomes, consequently leading to Salmonella with reduced virulence (160, 161). Chickens immunized with Salmonella Gallinarum 1009 ΔspiC Δcrp had increased antibody and cellular responses to whole Salmonella Gallinarum as an antigen. This vaccine also demonstrated 100% efficacy against lethal challenge with the wild-type strain Salmonella Gallinarum SG9 (158). Salmonella Pullorum S06004 ΔSPI2 also elicited protective cellular and humoral responses and protected chickens from challenge with wild-type Salmonella Pullorum and Salmonella Gallinarum strains (160). The protective efficacy of Salmonella Pullorum S06004 ΔSPI2 was found to be 90% when the birds were challenged with wild-type Salmonella Pullorum. In comparison, vaccine efficacy was 70% in birds challenged with strain Salmonella Gallinarum SG9 (160). Salmonella Pullorum S06004 ΔspiC ΔrfaH refines this strategy by deleting a single gene in the SPI2 region (spiC) and deleting a gene involved in LPS synthesis to generate a DIVA vaccine strain making it distinguishable from wild-type strains during routine sampling and surveillance. Chickens immunized with the Salmonella Pullorum strain S06004 ΔspiC ΔrfaH produced Salmonella Pullorum-specific IgG (assessed against whole bacteria as antigen) and showed increased proliferation of peripheral blood mononuclear cells (PBMCs) and increased cytokine expression compared to non-immunized controls. Upon challenge with wild-type Salmonella Pullorum strain S06004, chickens immunized with Salmonella Pullorum S06004 ΔspiC ΔrfaH had an 86% (13/15) survival rate compared to only 20% survival in non-immunized birds (161).
Auxotrophic and global regulator mutants appear to be highly immunogenic live vaccines against multiple Salmonella serovars including Salmonella Gallinarum (185), Salmonella Abortusequi (186), Salmonella Dublin (187), Salmonella Typhimurium (188), Salmonella Enteritidis (189), and Salmonella Choleraesuis (190) and in different animal species. Early research and development of aroA mutants in host-restricted strains led to promising candidates like HG212, a mutant of Salmonella Gallinarum strain 9; however, this vaccine strain only showed protection from lethal challenge with wild-type Salmonella Gallinarum strain 9 when the vaccine was delivered intramuscularly and not orally (159). Salmonella Typhimurium-based vaccine candidates like χ3985, a Δcya Δcrp mutant, have been able to reduce tissue colonization of challenge strains from homologous and heterologous serovars (152). However, the challenge strains assessed were not chicken-adapted. Candidate vaccine χ11387, a modified Salmonella Gallinarum strain, required arabinose for expression of the crp gene which encodes CRP, a global regulator of virulence genes. This vaccine conferred homologous (vs wild-type Salmonella Gallinarum) and heterologous (vs wild-type Salmonella Enteritidis) protection with reduced bacterial load of the liver and spleen as a readout (155). Another Salmonella Gallinarum candidate vaccine strain developed by this group, χ11797, had the ferric uptake regulator (fur) gene deleted. After challenge, it reduced the enlargement of livers and spleens in vaccinated chickens compared to non-vaccinated controls, but this effect was only observed when the vaccine was delivered intramuscularly and not orally (157).
Notably, genetically modified live-attenuated Salmonella vaccines can offer a degree of cross-protection against multiple serovars due to shared somatic and flagellar antigens (152, 191–193). The commercially available Poulvac ST vaccine is comprised of the Salmonella Typhimurium strain AWC 591 with deletions of aroA and serC; it is marketed as a vaccine that can induce homologous protection against Salmonella Typhimurium and heterologous protection against serovars Enteritidis and Heidelberg (145, 149, 150). Cross-protective efficacy of Poulvac ST has been assessed and verified in studies using locally circulating field strains. In one example, chickens immunized with Poulvac ST and subsequently challenged with a Brazilian field strain of Salmonella Heidelberg had reduced colonization of the liver and spleen 3 days after challenge and reduced cecum colonization 21 days post-challenge (150). However, making a broadly protective vaccine is a challenge. A trivalent vaccine comprised of lon and fliC/fljB mutants of Salmonella Enteritidis 147, Salmonella Typhimurium 16E5, and Salmonella Infantis 18G6 conferred protection in chickens challenged with wild-type versions of the vaccine strains. However, heterologous challenges with serovars Agona, Hadar, and Dublin showed mixed results (162). While the number of animals assessed in this study was small, it indicates that more broadly protective vaccines that target host-restricted and generalist strains could be better developed.
The potential for shedding of live vaccines into the environment remains a concern (194). Live oral Salmonella vaccines activate immunity and can defend poultry against Salmonella colonization, but they need to be cleared or reduced to undetectable levels in poultry before they reach processing for safety and regulatory purposes (142, 182). Oral vaccination post-hatch can offer swift protection via a colonization–inhibition mechanism (195, 196). Methner et al. (196) demonstrated protection against superinfection by Salmonella strains within hours following the administration of live-attenuated vaccines to day-old chicks, showing a colonization inhibition mechanism that provides immediate partial protection before developing an adaptive immune response. The effectiveness varies across vaccine strains, underscoring the importance of carefully choosing strains that hinder colonization while avoiding over-attenuating modifications (142). For instance, pre-treatment of chicks with mutants of Salmonella Typhimurium lacking ompC decreased the ability of the challenge strain to colonize the liver and ceca compared to control strains. While rpoS and phoP deletions (either alone or in combination) in Salmonella Typhimurium and Salmonella Enteritidis also reduced the challenge strain’s ability to colonize these tissues, the resulting vaccine strains were significantly attenuated, limiting the capacity of the vaccine strain to colonize the cecum and more fully inhibit colonization and growth of the challenge strain (196). Some studies suggest the potential benefits of combining live vaccines with probiotics for more comprehensive protection with CE (196, 197).
Swine
The urgency to find effective interventions against Salmonella in swine has also become paramount with the global expansion of food animal production systems and growing restrictions on antimicrobial usage. Among the intervention strategies, vaccination stands out due to its potential to mitigate the spread of Salmonella. This not only reduces dissemination within herds but also diminishes the need for antibiotics against salmonellosis, further reducing the risk of AMR spread (198). In swine, live vaccines can be administered to the sows to enhance passive immunity for piglets or may be administered to the piglets directly (198).
In China, Salmonella Choleraesuis strain C500 has been developed by chemical mutagenesis as a live oral vaccine for swine. This strain has demonstrated both efficacy and safety, improving the prevention and control of piglet paratyphoid in China for over four decades (199). Despite the complete genome characterization of C500 (200), the precise molecular mechanism responsible for its virulence attenuation remains unclear (167). Furthermore, C500 exhibits residual toxicity, which limits its practical utility as a live oral vaccine vector. Therefore, many researchers have explored modifying it to increase its potential as a live oral vaccine vector for delivering heterologous antigen (167, 201–203).
In the European Union, the Salmoporc vaccine was the first licensed live-attenuated vaccine against Salmonella Typhimurium for administration to sows and piglets. It consists of the live Salmonella Typhimurium strain 421/125 with histidine–adenine auxotrophic mutations for attenuation. Several studies have demonstrated its effectiveness in reducing Salmonella concentrations in pigs (204–206). In the United States, the Center for Veterinary Biologics at the United States Department of Agriculture-Animal and Plant Health Inspections Service (USDA-APHIS) has licensed several live Salmonella vaccines for use in swine, though availability varies for administration (198, 207). Some of the prominent vaccines, like Enterisol Salmonella T/C and Argus SC/ST, which consist of attenuated strains of Salmonella Choleraesuis, have yielded encouraging results in reducing the prevalence of Salmonella Choleraesuis and Salmonella Typhimurium in vaccinated pigs (164). Enterisol Salmonella T/C and NOBL SC-54 employ live strains of Salmonella Choleraesuis that lack the spv genes found on the virulence plasmid (164, 165, 208). The spv genes encode proteins that play crucial roles in various intracellular processes and contribute to increased virulence of Salmonella within the host (209). Argus SC/ST utilizes a live-attenuated strain of Salmonella Choleraesuis with deletion mutations in the cya and crp genes, impacting its virulence and growth (210). This vaccine is indicated for preventing pneumonia, diarrhea, septicemia, and mortality caused by Salmonella Choleraesuis and helps in controlling disease and shedding caused by Salmonella Typhimurium.
Similarly, vaccine candidates against the generalist serovar Salmonella Typhimurium have also been developed for use in pigs but are not available commercially. Examples of these are Salmonella Typhimurium JOL911 (172), Salmonella Typhimurium ΔznuABC (168), and the DIVA strains BBS 202 (169) and BBS 866 (170). Strain JOL911 is a cpxR and lon deletion mutant; cpxR is required for invasion of host cells and lon regulates SPI-1 invasion genes. Two orally administered doses of Salmonella Typhimurium JOL911 induced significant LPS-specific IgG and IgA responses in serum and colostrum. Piglets born to sows primed and boosted with Salmonella Typhimurium JOL911 also had increased LPS-specific antibody titers compared to control piglets and more importantly exhibited no clinical signs of infection until 21 days after challenge with a wild-type Salmonella Typhimurium strain (172). Salmonella Typhimurium ΔznuABC lacks the entire znuABC operon, leading to an inability to retrieve metals from the host, which causes a loss of virulence. It generated vaccine-specific antibodies and protected immunized pigs from clinical symptoms of infection (168). Additionally, the environmental presence of this strain remained below detectable levels by day 14 post-inoculation. Salmonella Typhimurium BBS 202 has a mutation in the ΔrfaH gene, and its derivative, BBS 866, has additional mutations in five small regulatory RNA (sRNA) genes—omrA, omrB, rybB, micA, and invR—that negatively regulate conserved outer membrane proteins (169, 170). Vaccination with Salmonella Typhimurium BBS 202 reduced clinical symptoms such as fever and diarrhea, decreased Salmonella fecal shedding, and minimized intestinal colonization upon exposure to virulent Salmonella Typhimurium (169). Moreover, immunization with Salmonella Typhimurium BBS 866 provided broader protection, showing efficacy against challenge with wild-type Salmonella Typhimurium and Salmonella Choleraesuis (170).
Cattle
Like other animals, cattle can be infected by both host-restricted and generalist Salmonella serovars. The host-restricted Salmonella Dublin strain causes various clinical indications in cattle. Calves commonly experience respiratory symptoms, whereas older cattle experience fever; depression; foul-smelling diarrhea containing mucus, blood, and fragments of intestinal lining; and even abortions. In the United States, EnterVene-d, an auxotroph for aromatic amino acids, is a commercially available live-attenuated vaccine specifically targeting Salmonella Dublin and is administered to cattle 2 weeks and older (174). A randomized field study with calves receiving two doses of EnterVene-d orally, intranasally, or no vaccine (control group) determined that while there was no significant difference in the incidence of pneumonia in the calves, vaccinated calves had a significantly reduced risk of mortality (174). A promising live-attenuated vaccine candidate in development is Salmonella Dublin Sdu189 ΔspiC ΔaroA. This strain induced serum IgG antibodies against whole Salmonella Dublin and also stimulated increased cytokine expression in BALB/c mice intramuscularly immunized with the vaccine (175). Mice were also completely protected upon lethal challenge with wild-type Salmonella Dublin Sdu189 (175). Salmonella Dublin is also associated with invasive disease in humans making control of this serovar in cattle important for human health.
Among the generalist Salmonella serovars, Salmonella Typhimurium and Salmonella Newport are frequently associated with cattle and their products. Currently, there are no live-attenuated vaccines available on the market for these strains for use in cattle. However, strain MT2313, which is a Salmonella Typhimurium vaccine deficient in DNA methylation, has been tested against serovars Typhimurium, Dublin, and Newport in a challenge study conducted in Australia. Salmonella Typhimurium MT2313 conferred considerable reduction in clinical symptoms, fecal shedding of the challenge strain, and tissue colonization in calves (151, 176, 211).
Sheep
In sheep flocks, Salmonella outbreaks are commonly linked to serovars Abortusovis, Dublin, and Typhimurium. Salmonella Abortusovis stands as the predominant cause of ovine salmonellosis in southern Europe, often resulting in abortions, as its name implies (212). Currently, there are no commercially available live-attenuated vaccines against salmonellosis for use in sheep in the United States. However, strains such as SSM189 (Δcrp Δcdt Δcya) and SU304 (ΔaroA), both derivatives of wild-type Salmonella Abortusovis strain SS44, have been evaluated in sheep for homologous protection from disease (177). Mutations in aroA alone or crp, cdt, and cya result in avirulence of Salmonella Abortusovis and complete elimination of its ability to induce abortions. Immunization with either strain resulted in reduced abortions and fewer infected ewes at parturition. However, the reduction in pregnancy failures, while promising, was not statistically significant.
Salmonella Typhimurium vaccine strain MT2313 was also evaluated in sheep (178). Animals were fasted prior to being offered water containing the vaccine strain for 24 hours. Sheep were challenged 24 hours, 7 days, or 28 days after in-water immunization. Colonization of the mesenteric lymph nodes, ileum, liver, spleen, and lung was significantly reduced in sheep immunized 28 days before challenge; fewer bacteria were also recovered from the organs of sheep immunized 7 days before challenge. Salmonella Typhimurium MT2313 completely protected sheep immunized 7 and 28 days prior to lethal challenge with a wild-type Salmonella Typhimurium strain (178).
LIVE-VECTOR SALMONELLA VACCINES FOR ANIMAL USE
Many live-attenuated Salmonella vaccines, previously developed and tested against homologous challenge in experimental animal models, are now being further engineered as live vectors to vaccinate against multiple animal pathogens including other bacteria, parasites, and viruses. For example, chickens vaccinated orally with Salmonella Typhimurium live-vector RASV strains c4550 and c3987, expressing the Campylobacter jejuni cjaA gene, encoding an N-glycosylated lipoprotein of the inner membrane, elicited serum IgG and mucosal IgA antibody responses against C. jejuni membrane proteins; this immune response led to reduced colonization of heterologous wild-type C. jejuni in the cecum of immunized chickens (213).
The RASV system has also been used to immunize against Gram-positive infections. An attenuated Salmonella Typhimurium vaccine strain has been employed as an oral live-vector vaccine to protect chickens from necrotic enteritis by expressing the antigens fructose-1,6-bisphosphate aldolase, α-toxoid, and NetB toxoid from C. perfringens (214). More recently, AVERT NE became the only mass-administered commercial vaccine based on RASV technology, licensed for use in chickens in the United States, for controlling necrotic enteritis caused by C. perfringens Type A. It expresses C. perfringens genes coding for a nontoxic C-terminal adhesive part of α-toxin and a fusion of glutathione S-transferase (GST) with NetB toxin (GST-NetB) (98).
Salmonella Choleraesuis has been investigated as a potential live-vector vaccine for delivery of heterologous antigens in swine because of its host restriction and pathogenic mechanisms. Two promising vaccine candidates (SCL 00031 and SCL 00032) with mutations in ΔrpoS and ΔphoP (215) were developed for use in pigs against Salmonella Choleraesuis. However, they were highly attenuated and were unable to colonize deep tissue organs when competing against the wild-type parent strain; these candidates have not advanced in development.
Similar strategies have been applied to combat infections by parasitic and viral pathogens. Another RASV Salmonella Typhimurium vaccine strain was engineered to deliver the Eimeria acervulina merozoite antigen EAMZ250 expressed from plasmid pYA3658 as a peptide fused to the translocation domain of the T3SS effector protein SptP (216). Chickens immunized with the vaccine strain expressing EAMZ250 and challenged with E. acervulina oocysts showed significant weight gain compared to vector control groups. The strain was also able to colonize the bursa, intestines, and spleens of the chickens indicating that long-lasting immunity from Eimeria infection may be possible with an easily administered oral vaccine (216). This strategy has also been used to target Eimeria tenella by expressing its SO7 antigen from an RASV strain. Chickens immunized with this SO7-expressing strain showed a reduction in oocyst shedding and both humoral and cellular immune responses to immunization (217).
New technologies have also broadened the development landscape. A Salmonella Typhimurium strain, modified with deletions in the lon and sifA genes, was employed to deliver a CRISPR/Cas9 plasmid encoding the virulence gene pp38 from Marek’s disease virus (MDV), a herpes virus that causes tumors and paralysis in chickens; pp38 is crucial for the infection and maintenance of B cells by MDV (218). Salmonella-mediated delivery of the CRISPR::pp38 plasmid was confirmed by isolation of PBMCs from vaccinated chickens for analysis by PCR. In vivo plasmid delivery was also confirmed by fluorescence-activated cell sorting analysis of spleen and liver cells from chickens that received a green fluorescent protein-expressing version of the plasmid. This genetic interference strategy was used against highly pathogenic MDV, resulting in significant resistance to MDV infection in Salmonella-vaccinated chickens (219).
CONCLUSION
Comprehensive knowledge of Salmonella pathogenesis, gene regulation, and virulence along with the advent of modern recombinant DNA technology permits the possibility of genetic engineering of Salmonella with great efficiency and efficacy. Many new strategies to develop live-attenuated vaccine strains of Salmonella spp. can be harnessed to reduce the burden of Salmonella infections in both humans and animals. The development of recombinant Salmonella strains as vaccine vectors against heterologous pathogens constitutes a promising approach toward the development of safe, effective, and affordable vaccines against a number of infectious diseases. Recently, genetically engineered Salmonella have also been used to generate components for other vaccine modalities such as conjugate vaccines and OMV-based vaccines. In addition, Salmonella-mediated antitumor therapy is an attractive strategy for the control of cancer. As research progresses, modern technologies that offer safer, effective, and more broadly protective vaccines have the potential to decrease morbidity and mortality due to Salmonella and other pathogens in humans as well as animals.
ACKNOWLEDGMENTS
This work was funded by National Institutes of Health (NIH)/National Institute of Allergy and Infectious Diseases (NIAID) under Project 2: Multivalent vaccines effective against MDR Salmonella (project leader: S.M.T.) of U19 AI142725.
Contributor Information
Sharon M. Tennant, Email: stennant@som.umaryland.edu.
Gregory J. Phillips, University of Georgia, Athens, Georgia, USA
REFERENCES
- 1. Popoff MY, Bockemühl J, Gheesling LL. 2004. Supplement 2002 (no. 46) to the Kauffmann-White scheme. Res Microbiol 155:568–570. doi: 10.1016/j.resmic.2004.04.005 [DOI] [PubMed] [Google Scholar]
- 2. GBDDD . 2018. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of diarrhoea in 195 countries: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect Dis 18:1211–1228. doi: 10.1016/S1473-3099(18)30362-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Grimont PAD, François-Xavier W. 2007. Antigenic formulae of the Salmonella serovars 9th edition, on WHO collaborating centre for reference and research on Salmonella. https://www.pasteur.fr/sites/default/files/veng_0.pdf.
- 4. Gal-Mor O, Boyle EC, Grassl GA. 2014. Same species, different diseases: how and why typhoidal and non-typhoidal Salmonella enterica serovars differ. Front Microbiol 5:391. doi: 10.3389/fmicb.2014.00391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. GBDN-TSID . 2019. The global burden of non-typhoidal salmonella invasive disease: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Infect Dis 19:1312–1324. doi: 10.1016/S1473-3099(19)30418-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Smith SI, Seriki A, Ajayi A. 2016. Typhoidal and non-typhoidal Salmonella infections in Africa. Eur J Clin Microbiol Infect Dis 35:1913–1922. doi: 10.1007/s10096-016-2760-3 [DOI] [PubMed] [Google Scholar]
- 7. Scallan E, Hoekstra RM, Angulo FJ, Tauxe RV, Widdowson MA, Roy SL, Jones JL, Griffin PM. 2011. Foodborne illness acquired in the United States--major pathogens. Emerg Infect Dis 17:7–15. doi: 10.3201/eid1701.p11101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Berger CN, Sodha SV, Shaw RK, Griffin PM, Pink D, Hand P, Frankel G. 2010. Fresh fruit and vegetables as vehicles for the transmission of human pathogens. Environ Microbiol 12:2385–2397. doi: 10.1111/j.1462-2920.2010.02297.x [DOI] [PubMed] [Google Scholar]
- 9. WHO . 2015. WHO estimates of the global burden of foodborne diseases: foodborne disease burden epidemiology reference group 2007-2015. World Health Organization. https://iris.who.int/bitstream/handle/10665/199350/9789241565165_eng.pdf?sequence=1. [Google Scholar]
- 10. Feasey NA, Dougan G, Kingsley RA, Heyderman RS, Gordon MA. 2012. Invasive non-typhoidal Salmonella disease: an emerging and neglected tropical disease in Africa. Lancet 379:2489–2499. doi: 10.1016/S0140-6736(11)61752-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Syed KA, Saluja T, Cho H, Hsiao A, Shaikh H, Wartel TA, Mogasale V, Lynch J, Kim JH, Excler J-L, Sahastrabuddhe S. 2020. Review on the recent advances on typhoid vaccine development and challenges ahead. Clin Infect Dis 71:S141–S150. doi: 10.1093/cid/ciaa504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Huang K, Herrero-Fresno A, Thøfner I, Skov S, Olsen JE. 2019. Interaction differences of the avian host-specific Salmonella enterica serovar Gallinarum, the host-generalist S. Typhimurium, and the cattle host-adapted S. Dublin with chicken primary macrophage. Infect Immun 87:e00552-19. doi: 10.1128/IAI.00552-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jajere SM. 2019. A review of Salmonella enterica with particular focus on the pathogenicity and virulence factors, host specificity and antimicrobial resistance including multidrug resistance. Vet World 12:504–521. doi: 10.14202/vetworld.2019.504-521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hoelzer K, Bielke L, Blake DP, Cox E, Cutting SM, Devriendt B, Erlacher-Vindel E, Goossens E, Karaca K, Lemiere S, Metzner M, Raicek M, Collell Suriñach M, Wong NM, Gay C, Van Immerseel F. 2018. Vaccines as alternatives to antibiotics for food producing animals. Part 1: challenges and needs. Vet Res 49:64. doi: 10.1186/s13567-018-0560-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Abrahams GL, Hensel M. 2006. Manipulating cellular transport and immune responses: dynamic interactions between intracellular Salmonella enterica and its host cells. Cell Microbiol 8:728–737. doi: 10.1111/j.1462-5822.2006.00706.x [DOI] [PubMed] [Google Scholar]
- 16. Hansen-Wester I, Hensel M. 2001. Salmonella pathogenicity islands encoding type III secretion systems. Microbes Infect 3:549–559. doi: 10.1016/s1286-4579(01)01411-3 [DOI] [PubMed] [Google Scholar]
- 17. Datsenko KA, Wanner BL. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A 97:6640–6645. doi: 10.1073/pnas.120163297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Herring CD, Glasner JD, Blattner FR. 2003. Gene replacement without selection: regulated suppression of amber mutations in Escherichia coli. Gene 311:153–163. doi: 10.1016/S0378-1119(03)00585-7 [DOI] [PubMed] [Google Scholar]
- 19. Roland KL, Brenneman KE. 2013. Salmonella as a vaccine delivery vehicle. Expert Rev Vaccines 12:1033–1045. doi: 10.1586/14760584.2013.825454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mastroeni P, Chabalgoity JA, Dunstan SJ, Maskell DJ, Dougan G. 2001. Salmonella: immune responses and vaccines. Vet J 161:132–164. doi: 10.1053/tvjl.2000.0502 [DOI] [PubMed] [Google Scholar]
- 21. Spreng S, Dietrich G, Weidinger G. 2006. Rational design of Salmonella-based vaccination strategies. Methods 38:133–143. doi: 10.1016/j.ymeth.2005.09.012 [DOI] [PubMed] [Google Scholar]
- 22. Galen JE, Curtiss III R. 2014. The delicate balance in genetically engineering live vaccines. Vaccine 32:4376–4385. doi: 10.1016/j.vaccine.2013.12.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tacket CO, Hone DM, Losonsky GA, Guers L, Edelman R, Levine MM. 1992. Clinical acceptability and immunogenicity of CVD 908 Salmonella typhi vaccine strain. Vaccine 10:443–446. doi: 10.1016/0264-410x(92)90392-w [DOI] [PubMed] [Google Scholar]
- 24. Tacket CO, Sztein MB, Losonsky GA, Wasserman SS, Nataro JP, Edelman R, Pickard D, Dougan G, Chatfield SN, Levine MM. 1997. Safety of live oral Salmonella typhi vaccine strains with deletions in htrA and aroC aroD and immune response in humans. Infect Immun 65:452–456. doi: 10.1128/iai.65.2.452-456.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tacket CO, Sztein MB, Wasserman SS, Losonsky G, Kotloff KL, Wyant TL, Nataro JP, Edelman R, Perry J, Bedford P, Brown D, Chatfield S, Dougan G, Levine MM. 2000. Phase 2 clinical trial of attenuated Salmonella enterica serovar Typhi oral live vector vaccine CVD 908-htrA in U.S. volunteers. Infect Immun 68:1196–1201. doi: 10.1128/IAI.68.3.1196-1201.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Levine MM, Galen J, Barry E, Noriega F, Chatfield S, Sztein M, Dougan G, Tacket C. 1996. Attenuated Salmonella as live oral vaccines against typhoid fever and as live vectors. J Biotechnol 44:193–196. doi: 10.1016/0168-1656(95)00094-1 [DOI] [PubMed] [Google Scholar]
- 27. Tacket CO, Pasetti MF, Sztein MB, Livio S, Levine MM. 2004. Immune responses to an oral typhoid vaccine strain that is modified to constitutively express Vi capsular polysaccharide. J Infect Dis 190:565–570. doi: 10.1086/421469 [DOI] [PubMed] [Google Scholar]
- 28. Tacket CO, Kelly SM, Schödel F, Losonsky G, Nataro JP, Edelman R, Levine MM, Curtiss III R. 1997. Safety and immunogenicity in humans of an attenuated Salmonella typhi vaccine vector strain expressing plasmid-encoded hepatitis B antigens stabilized by the Asd-balanced lethal vector system. Infect Immun 65:3381–3385. doi: 10.1128/iai.65.8.3381-3385.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hohmann EL, Oletta CA, Killeen KP, Miller SI. 1996. phoP/phoQ-deleted Salmonella typhi (Ty800) is a safe and immunogenic single-dose typhoid fever vaccine in volunteers. J Infect Dis 173:1408–1414. doi: 10.1093/infdis/173.6.1408 [DOI] [PubMed] [Google Scholar]
- 30. Kirkpatrick BD, Tenney KM, Larsson CJ, O’Neill JP, Ventrone C, Bentley M, Upton A, Hindle Z, Fidler C, Kutzko D, Holdridge R, Lapointe C, Hamlet S, Chatfield SN. 2005. The novel oral typhoid vaccine M01ZH09 is well tolerated and highly immunogenic in 2 vaccine presentations. J Infect Dis 192:360–366. doi: 10.1086/431605 [DOI] [PubMed] [Google Scholar]
- 31. Kirkpatrick BD, McKenzie R, O’Neill JP, Larsson CJ, Bourgeois AL, Shimko J, Bentley M, Makin J, Chatfield S, Hindle Z, Fidler C, Robinson BE, Ventrone CH, Bansal N, Carpenter CM, Kutzko D, Hamlet S, LaPointe C, Taylor DN. 2006. Evaluation of Salmonella enterica serovar Typhi (Ty2 aroC-ssaV-) M01ZH09, with a defined mutation in the Salmonella pathogenicity island 2, as a live, oral typhoid vaccine in human volunteers. Vaccine 24:116–123. doi: 10.1016/j.vaccine.2005.08.008 [DOI] [PubMed] [Google Scholar]
- 32. Gat O, Galen JE, Tennant S, Simon R, Blackwelder WC, Silverman DJ, Pasetti MF, Levine MM. 2011. Cell-associated flagella enhance the protection conferred by mucosally-administered attenuated Salmonella Paratyphi A vaccines. PLoS Negl Trop Dis 5:e1373. doi: 10.1371/journal.pntd.0001373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wahid R, Kotloff KL, Levine MM, Sztein MB. 2019. Cell mediated immune responses elicited in volunteers following immunization with candidate live oral Salmonella enterica serovar Paratyphi A attenuated vaccine strain CVD 1902. Clin Immunol 201:61–69. doi: 10.1016/j.clim.2019.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Higginson EE, Ramachandran G, Hazen TH, Kania DA, Rasko DA, Pasetti MF, Levine MM, Tennant SM. 2018. Improving our understanding of Salmonella enterica serovar paratyphi B through the engineering and testing of a live attenuated vaccine strain. mSphere 3:e00474-18. doi: 10.1128/mSphere.00474-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hindle Z, Chatfield SN, Phillimore J, Bentley M, Johnson J, Cosgrove CA, Ghaem-Maghami M, Sexton A, Khan M, Brennan FR, Everest P, Wu T, Pickard D, Holden DW, Dougan G, Griffin GE, House D, Santangelo JD, Khan SA, Shea JE, Feldman RG, Lewis DJM. 2002. Characterization of Salmonella enterica derivatives harboring defined aroC and Salmonella pathogenicity island 2 type III secretion system (ssaV) mutations by immunization of healthy volunteers. Infect Immun 70:3457–3467. doi: 10.1128/IAI.70.7.3457-3467.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Angelakopoulos H, Hohmann EL. 2000. Pilot study of phoP/phoQ-deleted Salmonella enterica serovar Typhimurium expressing Helicobacter pylori urease in adult volunteers. Infect Immun 68:2135–2141. doi: 10.1128/IAI.68.4.2135-2141.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tennant SM, Schmidlein P, Simon R, Pasetti MF, Galen JE, Levine MM. 2015. Refined live attenuated Salmonella enterica serovar Typhimurium and Enteritidis vaccines mediate homologous and heterologous serogroup protection in mice. Infect Immun 83:4504–4512. doi: 10.1128/IAI.00924-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fuche FJ, Jones JA, Ramachandran G, Higginson EE, Simon R, Tennant SM. 2019. Deletions in guaBA and htrA but not clpX or rfaL constitute a live-attenuated vaccine strain of Salmonella Newport to protect against serogroup C2-C3 Salmonella in mice. Hum Vaccin Immunother 15:1427–1435. doi: 10.1080/21645515.2018.1491499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bumann D, Metzger WG, Mansouri E, Palme O, Wendland M, Hurwitz R, Haas G, Aebischer T, von Specht BU, Meyer TF. 2001. Safety and immunogenicity of live recombinant Salmonella enterica serovar Typhi Ty21a expressing urease A and B from Helicobacter pylori in human volunteers. Vaccine 20:845–852. doi: 10.1016/s0264-410x(01)00391-7 [DOI] [PubMed] [Google Scholar]
- 40. Metzger WG, Mansouri E, Kronawitter M, Diescher S, Soerensen M, Hurwitz R, Bumann D, Aebischer T, Von Specht B-U, Meyer TF. 2004. Impact of vector-priming on the immunogenicity of a live recombinant Salmonella enterica serovar typhi Ty21a vaccine expressing urease A and B from Helicobacter pylori in human volunteers. Vaccine 22:2273–2277. doi: 10.1016/j.vaccine.2003.11.020 [DOI] [PubMed] [Google Scholar]
- 41. Frey SE, Lottenbach KR, Hill H, Blevins TP, Yu Y, Zhang Y, Brenneman KE, Kelly-Aehle SM, McDonald C, Jansen A, Curtiss III R. 2013. A Phase I, dose-escalation trial in adults of three recombinant attenuated Salmonella Typhi vaccine vectors producing Streptococcus pneumoniae surface protein antigen PspA. Vaccine 31:4874–4880. doi: 10.1016/j.vaccine.2013.07.049 [DOI] [PubMed] [Google Scholar]
- 42. Dharmasena MN, Hanisch BW, Wai TT, Kopecko DJ. 2013. Stable expression of Shigella sonnei form I O-polysaccharide genes recombineered into the chromosome of live Salmonella oral vaccine vector Ty21a. Int J Med Microbiol 303:105–113. doi: 10.1016/j.ijmm.2013.01.001 [DOI] [PubMed] [Google Scholar]
- 43. Dharmasena MN, Osorio M, Filipova S, Marsh C, Stibitz S, Kopecko DJ. 2016. Stable expression of Shigella dysenteriae serotype 1 O-antigen genes integrated into the chromosome of live Salmonella oral vaccine vector Ty21a. Pathog Dis 74:ftw098. doi: 10.1093/femspd/ftw098 [DOI] [PubMed] [Google Scholar]
- 44. Dharmasena MN, Osorio M, Takeda K, Stibitz S, Kopecko DJ. 2017. Stable chromosomal expression of Shigella flexneri 2a and 3a O-antigens in the live Salmonella oral vaccine vector Ty21a. Clin Vaccine Immunol 24:e00181-17. doi: 10.1128/CVI.00181-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Li P, Liu Q, Luo H, Liang K, Yi J, Luo Y, Hu Y, Han Y, Kong Q. 2017. O-serotype conversion in Salmonella Typhimurium induces protective immune responses against invasive non-typhoidal Salmonella infections. Front Immunol 8:1647. doi: 10.3389/fimmu.2017.01647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Li P, Liu Q, Luo H, Liang K, Han Y, Roland KL, Curtiss R, Kong Q. 2018. Bi-valent polysaccharides of Vi capsular and O9 O-antigen in attenuated Salmonella Typhimurium induce strong immune responses against these two antigens. NPJ Vaccines 3:1. doi: 10.1038/s41541-017-0041-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hegazy WAH, Xu X, Metelitsa L, Hensel M. 2012. Evaluation of Salmonella enterica type III secretion system effector proteins as carriers for heterologous vaccine antigens. Infect Immun 80:1193–1202. doi: 10.1128/IAI.06056-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Xu C, Zhang BZ, Lin Q, Deng J, Yu B, Arya S, Yuen KY, Huang JD. 2018. Live attenuated Salmonella Typhimurium vaccines delivering SaEsxA and SaEsxB via type III secretion system confer protection against Staphylococcus aureus infection. BMC Infect Dis 18:195. doi: 10.1186/s12879-018-3104-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Aguilera-Herce J, García-Quintanilla M, Romero-Flores R, McConnell MJ, Ramos-Morales F. 2019. A live Salmonella vaccine delivering PcrV through the type III secretion system protects against Pseudomonas aeruginosa. mSphere 4:e00116-19. doi: 10.1128/mSphere.00116-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kong W, Wanda SY, Zhang X, Bollen W, Tinge SA, Roland KL, Curtiss III R. 2008. Regulated programmed lysis of recombinant Salmonella in host tissues to release protective antigens and confer biological containment. Proc Natl Acad Sci U S A 105:9361–9366. doi: 10.1073/pnas.0803801105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Juárez-Rodríguez MD, Yang J, Kader R, Alamuri P, Curtiss III R, Clark-Curtiss JE. 2012. Live attenuated Salmonella vaccines displaying regulated delayed lysis and delayed antigen synthesis to confer protection against Mycobacterium tuberculosis. Infect Immun 80:815–831. doi: 10.1128/IAI.05526-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Maddux JT, Stromberg ZR, Curtiss III R, Mellata M. 2017. Evaluation of recombinant attenuated Salmonella vaccine strains for broad protection against extraintestinal pathogenic Escherichia coli. Front Immunol 8:1280. doi: 10.3389/fimmu.2017.01280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Tennant SM, Wang JY, Galen JE, Simon R, Pasetti MF, Gat O, Levine MM. 2011. Engineering and preclinical evaluation of attenuated nontyphoidal Salmonella strains serving as live oral vaccines and as reagent strains. Infect Immun 79:4175–4185. doi: 10.1128/IAI.05278-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hegerle N, Bose J, Ramachandran G, Galen JE, Levine MM, Simon R, Tennant SM. 2018. Overexpression of O-polysaccharide chain length regulators in Gram-negative bacteria using the Wzx-/Wzy-dependent pathway enhances production of defined modal length O-polysaccharide polymers for use as haptens in glycoconjugate vaccines. J Appl Microbiol 125:575–585. doi: 10.1111/jam.13772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Micoli F, Rondini S, Alfini R, Lanzilao L, Necchi F, Negrea A, Rossi O, Brandt C, Clare S, Mastroeni P, Rappuoli R, Saul A, MacLennan CA. 2018. Comparative immunogenicity and efficacy of equivalent outer membrane vesicle and glycoconjugate vaccines against nontyphoidal Salmonella. Proc Natl Acad Sci U S A 115:10428–10433. doi: 10.1073/pnas.1807655115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hoiseth SK, Stocker BA. 1981. Aromatic-dependent Salmonella typhimurium are non-virulent and effective as live vaccines. Nature 291:238–239. doi: 10.1038/291238a0 [DOI] [PubMed] [Google Scholar]
- 57. Harrison JA, Villarreal-Ramos B, Mastroeni P, Demarco de Hormaeche R, Hormaeche CE. 1997. Correlates of protection induced by live Aro- Salmonella typhimurium vaccines in the murine typhoid model. Immunology 90:618–625. doi: 10.1046/j.1365-2567.1997.00158.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Killar LM, Eisenstein TK. 1985. Immunity to Salmonella typhimurium infection in C3H/HeJ and C3H/HeNCrlBR mice: studies with an aromatic-dependent live S. typhimurium strain as a vaccine. Infect Immun 47:605–612. doi: 10.1128/iai.47.3.605-612.1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hone DM, Harris AM, Chatfield S, Dougan G, Levine MM. 1991. Construction of genetically defined double aro mutants of Salmonella typhi. Vaccine 9:810–816. doi: 10.1016/0264-410x(91)90218-u [DOI] [PubMed] [Google Scholar]
- 60. Bäumler AJ, Kusters JG, Stojiljkovic I, Heffron F. 1994. Salmonella typhimurium loci involved in survival within macrophages. Infect Immun 62:1623–1630. doi: 10.1128/iai.62.5.1623-1630.1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Dilts DA, Riesenfeld-Orn I, Fulginiti JP, Ekwall E, Granert C, Nonenmacher J, Brey RN, Cryz SJ, Karlsson K, Bergman K, Thompson T, Hu B, Brückner AH, Lindberg AA. 2000. Phase I clinical trials of aroA aroD and aroA aroD htrA attenuated S. typhi vaccines; effect of formulation on safety and immunogenicity. Vaccine 18:1473–1484. doi: 10.1016/s0264-410x(99)00424-7 [DOI] [PubMed] [Google Scholar]
- 62. Johnson K, Charles I, Dougan G, Pickard D, O’Gaora P, Costa G, Ali T, Miller I, Hormaeche C. 1991. The role of a stress-response protein in Salmonella typhimurium virulence. Mol Microbiol 5:401–407. doi: 10.1111/j.1365-2958.1991.tb02122.x [DOI] [PubMed] [Google Scholar]
- 63. Pickett TE, Pasetti MF, Galen JE, Sztein MB, Levine MM. 2000. In vivo characterization of the murine intranasal model for assessing the immunogenicity of attenuated Salmonella enterica serovar Typhi strains as live mucosal vaccines and as live vectors. Infect Immun 68:205–213. doi: 10.1128/IAI.68.1.205-213.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Nikolaus T, Deiwick J, Rappl C, Freeman JA, Schröder W, Miller SI, Hensel M. 2001. SseBCD proteins are secreted by the type III secretion system of Salmonella pathogenicity island 2 and function as a translocon. J Bacteriol 183:6036–6045. doi: 10.1128/JB.183.20.6036-6045.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Wael AHH, Hisham AA. 2017. Evaluation of the role of SsaV Salmonella pathogenicity island-2 dependent type III secretion system components on the virulence behavior of Salmonella enterica serovar Typhimurium. Afr J Biotechnol 16:718–726. doi: 10.5897/AJB2016.15852 [DOI] [Google Scholar]
- 66. Browne SH, Hasegawa P, Okamoto S, Fierer J, Guiney DG. 2008. Identification of Salmonella SPI-2 secretion system components required for SpvB-mediated cytotoxicity in macrophages and virulence in mice. FEMS Immunol Med Microbiol 52:194–201. doi: 10.1111/j.1574-695X.2007.00364.x [DOI] [PubMed] [Google Scholar]
- 67. Deiwick J, Salcedo SP, Boucrot E, Gilliland SM, Henry T, Petermann N, Waterman SR, Gorvel J-P, Holden DW, Méresse S. 2006. The translocated Salmonella effector proteins SseF and SseG interact and are required to establish an intracellular replication niche. Infect Immun 74:6965–6972. doi: 10.1128/IAI.00648-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Khan SA, Stratford R, Wu T, Mckelvie N, Bellaby T, Hindle Z, Sinha KA, Eltze S, Mastroeni P, Pickard D, Dougan G, Chatfield SN, Brennan FR. 2003. Salmonella typhi and S. typhimurium derivatives harbouring deletions in aromatic biosynthesis and Salmonella Pathogenicity Island-2 (SPI-2) genes as vaccines and vectors. Vaccine 21:538–548. doi: 10.1016/s0264-410x(02)00410-3 [DOI] [PubMed] [Google Scholar]
- 69. Abd El Ghany M, Jansen A, Clare S, Hall L, Pickard D, Kingsley RA, Dougan G. 2007. Candidate live, attenuated Salmonella enterica serotype Typhimurium vaccines with reduced fecal shedding are immunogenic and effective oral vaccines. Infect Immun 75:1835–1842. doi: 10.1128/IAI.01655-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Hu X, Chen Z, Xiong K, Wang J, Rao X, Cong Y. 2017. Vi capsular polysaccharide: synthesis, virulence, and application. Crit Rev Microbiol 43:440–452. doi: 10.1080/1040841X.2016.1249335 [DOI] [PubMed] [Google Scholar]
- 71. Wang JY, Noriega FR, Galen JE, Barry E, Levine MM. 2000. Constitutive expression of the Vi polysaccharide capsular antigen in attenuated Salmonella enterica serovar Typhi oral vaccine strain CVD 909. Infect Immun 68:4647–4652. doi: 10.1128/IAI.68.8.4647-4652.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Roland KL, Tinge SA, Kochi SK, Thomas LJ, Killeen KP. 2010. Reactogenicity and immunogenicity of live attenuated Salmonella enterica serovar Paratyphi A enteric fever vaccine candidates. Vaccine 28:3679–3687. doi: 10.1016/j.vaccine.2010.03.019 [DOI] [PubMed] [Google Scholar]
- 73. Shata MT, Stevceva L, Agwale S, Lewis GK, Hone DM. 2000. Recent advances with recombinant bacterial vaccine vectors. Mol Med Today 6:66–71. doi: 10.1016/s1357-4310(99)01633-0 [DOI] [PubMed] [Google Scholar]
- 74. Levine MM, Tacket CO, Sztein MB. 2001. Host-Salmonella interaction: human trials. Microbes Infect 3:1271–1279. doi: 10.1016/s1286-4579(01)01487-3 [DOI] [PubMed] [Google Scholar]
- 75. Sztein MB. 2007. Cell-mediated immunity and antibody responses elicited by attenuated Salmonella enterica serovar Typhi strains used as live oral vaccines in humans. Clin Infect Dis 45:S15–S19. doi: 10.1086/518140 [DOI] [PubMed] [Google Scholar]
- 76. da Silva AJ, Zangirolami TC, Novo-Mansur MTM, Giordano R de C, Martins EAL. 2014. Live bacterial vaccine vectors: an overview. Braz J Microbiol 45:1117–1129. doi: 10.1590/s1517-83822014000400001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Curtiss III R, Xin W, Li Y, Kong W, Wanda S-Y, Gunn B, Wang S. 2010. New technologies in using recombinant attenuated Salmonella vaccine vectors. Crit Rev Immunol 30:255–270. doi: 10.1615/CritRevImmunol.v30.i3.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Garmory HS, Brown KA, Titball RW. 2002. Salmonella vaccines for use in humans: present and future perspectives. FEMS Microbiol Rev 26:339–353. doi: 10.1111/j.1574-6976.2002.tb00619.x [DOI] [PubMed] [Google Scholar]
- 79. Galen JE, Levine MM. 2001. Can a 'flawless' live vector vaccine strain be engineered? Trends Microbiol 9:372–376. doi: 10.1016/s0966-842x(01)02096-0 [DOI] [PubMed] [Google Scholar]
- 80. Dunstan SJ, Simmons CP, Strugnell RA. 2003. In vitro and in vivo stability of recombinant plasmids in a vaccine strain of Salmonella enterica var. Typhimurium. FEMS Immunol Med Microbiol 37:111–119. doi: 10.1016/S0928-8244(03)00065-8 [DOI] [PubMed] [Google Scholar]
- 81. Galen JE, Nair J, Wang JY, Wasserman SS, Tanner MK, Sztein MB, Levine MM. 1999. Optimization of plasmid maintenance in the attenuated live vector vaccine strain Salmonella typhi CVD 908-htrA. Infect Immun 67:6424–6433. doi: 10.1128/IAI.67.12.6424-6433.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Nardelli-Haefliger D, Kraehenbuhl JP, Curtiss III R, Schodel F, Potts A, Kelly S, De Grandi P. 1996. Oral and rectal immunization of adult female volunteers with a recombinant attenuated Salmonella typhi vaccine strain. Infect Immun 64:5219–5224. doi: 10.1128/iai.64.12.5219-5224.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. DiPetrillo MD, Tibbetts T, Kleanthous H, Killeen KP, Hohmann EL. 1999. Safety and immunogenicity of phoP/phoQ-deleted Salmonella typhi expressing Helicobacter pylori urease in adult volunteers. Vaccine 18:449–459. doi: 10.1016/s0264-410x(99)00246-7 [DOI] [PubMed] [Google Scholar]
- 84. Curtiss R, Galan JE, Nakayama K, Kelly SM. 1990. Stabilization of recombinant avirulent vaccine strains in vivo. Res Microbiol 141:797–805. doi: 10.1016/0923-2508(90)90113-5 [DOI] [PubMed] [Google Scholar]
- 85. Galán JE, Nakayama K, Curtiss III R. 1990. Cloning and characterization of the asd gene of Salmonella typhimurium: use in stable maintenance of recombinant plasmids in Salmonella vaccine strains. Gene 94:29–35. doi: 10.1016/0378-1119(90)90464-3 [DOI] [PubMed] [Google Scholar]
- 86. Morona R, Yeadon J, Considine A, Morona JK, Manning PA. 1991. Construction of plasmid vectors with a non-antibiotic selection system based on the Escherichia coli thyA+ gene: application to cholera vaccine development. Gene 107:139–144. doi: 10.1016/0378-1119(91)90307-w [DOI] [PubMed] [Google Scholar]
- 87. Zhang T, Stanley Jr SL. 1997. Expression of the serine rich Entamoeba histolytica protein (SREHP) in the avirulent vaccine strain Salmonella typhi TY2 χ4297 (Δcya Δcrp Δasd): safety and immunogenicity in mice. Vaccine 15:1319–1322. doi: 10.1016/S0264-410X(97)00042-X [DOI] [PubMed] [Google Scholar]
- 88. Schödel F, Peterson D, Hughes J, Wirtz R, Milich D. 1996. Hybrid hepatitis B virus core antigen as a vaccine carrier moiety: I. presentation of foreign epitopes. J Biotechnol 44:91–96. doi: 10.1016/0168-1656(95)00118-2 [DOI] [PubMed] [Google Scholar]
- 89. Yang X, Hinnebusch BJ, Trunkle T, Bosio CM, Suo Z, Tighe M, Harmsen A, Becker T, Crist K, Walters N, Avci R, Pascual DW. 2007. Oral vaccination with Salmonella simultaneously expressing Yersinia pestis F1 and V antigens protects against bubonic and pneumonic plague. J Immunol 178:1059–1067. doi: 10.4049/jimmunol.178.2.1059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Husseiny MI, Hensel M. 2008. Construction of highly attenuated Salmonella enterica serovar Typhimurium live vectors for delivering heterologous antigens by chromosomal integration. Microbiol Res 163:605–615. doi: 10.1016/j.micres.2006.10.003 [DOI] [PubMed] [Google Scholar]
- 91. Husseiny MI, Hensel M. 2005. Rapid method for the construction of Salmonella enterica serovar Typhimurium vaccine carrier strains. Infect Immun 73:1598–1605. doi: 10.1128/IAI.73.3.1598-1605.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Husseiny MI, Hensel M. 2009. Evaluation of Salmonella live vaccines with chromosomal expression cassettes for translocated fusion proteins. Vaccine 27:3780–3787. doi: 10.1016/j.vaccine.2009.03.053 [DOI] [PubMed] [Google Scholar]
- 93. Kaufmann SH, Hess J. 1999. Impact of intracellular location of and antigen display by intracellular bacteria: implications for vaccine development. Immunol Lett 65:81–84. doi: 10.1016/s0165-2478(98)00128-x [DOI] [PubMed] [Google Scholar]
- 94. Rüssmann H, Shams H, Poblete F, Fu Y, Galán JE, Donis RO. 1998. Delivery of epitopes by the Salmonella type III secretion system for vaccine development. Science 281:565–568. doi: 10.1126/science.281.5376.565 [DOI] [PubMed] [Google Scholar]
- 95. Lou L, Zhang P, Piao R, Wang Y. 2019. Salmonella pathogenicity island 1 (SPI-1) and its complex regulatory network. Front Cell Infect Microbiol 9:270. doi: 10.3389/fcimb.2019.00270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Figueira R, Holden DW. 2012. Functions of the Salmonella pathogenicity island 2 (SPI-2) type III secretion system effectors. Microbiology (Reading) 158:1147–1161. doi: 10.1099/mic.0.058115-0 [DOI] [PubMed] [Google Scholar]
- 97. Panthel K, Meinel KM, Sevil Domènech VE, Trülzsch K, Rüssmann H. 2008. Salmonella type III-mediated heterologous antigen delivery: a versatile oral vaccination strategy to induce cellular immunity against infectious agents and tumors. Int J Med Microbiol 298:99–103. doi: 10.1016/j.ijmm.2007.07.002 [DOI] [PubMed] [Google Scholar]
- 98. Wang S, Hofacre CL, Wanda S-Y, Zhou J, Callum RA, Nordgren B, Curtiss III R. 2022. A triple-sugar regulated Salmonella vaccine protects against Clostridium perfringens-induced necrotic enteritis in broiler chickens. Poult Sci 101:101592. doi: 10.1016/j.psj.2021.101592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Simon R, Curtis B, Deumic V, Nicki J, Tennant SM, Pasetti MF, Lees A, Wills PW, Chacon M, Levine MM. 2014. A scalable method for biochemical purification of Salmonella flagellin. Protein Expr Purif 102:1–7. doi: 10.1016/j.pep.2014.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Baliban SM, Yang M, Ramachandran G, Curtis B, Shridhar S, Laufer RS, Wang JY, Van Druff J, Higginson EE, Hegerle N, Varney KM, Galen JE, Tennant SM, Lees A, MacKerell Jr AD, Levine MM, Simon R. 2017. Development of a glycoconjugate vaccine to prevent invasive Salmonella Typhimurium infections in sub-Saharan Africa. PLoS Negl Trop Dis 11:e0005493. doi: 10.1371/journal.pntd.0005493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Baliban SM, Curtis B, Toema D, Tennant SM, Levine MM, Pasetti MF, Simon R. 2018. Immunogenicity and efficacy following sequential parenterally-administered doses of Salmonella Enteritidis COPS:FliC glycoconjugates in infant and adult mice. PLoS Negl Trop Dis 12:e0006522. doi: 10.1371/journal.pntd.0006522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Simon R, Tennant SM, Wang JY, Schmidlein PJ, Lees A, Ernst RK, Pasetti MF, Galen JE, Levine MM. 2011. Salmonella enterica serovar Enteritidis core O polysaccharide conjugated to H:g,m flagellin as a candidate vaccine for protection against invasive infection with S. Enteritidis. Infect Immun 79:4240–4249. doi: 10.1128/IAI.05484-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Baliban SM, Lu YJ, Malley R. 2020. Overview of the nontyphoidal and paratyphoidal Salmonella vaccine pipeline: current status and future prospects. Clin Infect Dis 71:S151–S154. doi: 10.1093/cid/ciaa514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Schwechheimer C, Kuehn MJ. 2015. Outer-membrane vesicles from Gram-negative bacteria: biogenesis and functions. Nat Rev Microbiol 13:605–619. doi: 10.1038/nrmicro3525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Mancini F, Micoli F, Necchi F, Pizza M, Berlanda Scorza F, Rossi O. 2021. GMMA-based vaccines: the known and the unknown. Front Immunol 12:715393. doi: 10.3389/fimmu.2021.715393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Meloni E, Colucci AM, Micoli F, Sollai L, Gavini M, Saul A, Di Cioccio V, MacLennan CA. 2015. Simplified low-cost production of O-antigen from Salmonella Typhimurium Generalized Modules for Membrane Antigens (GMMA). J Biotechnol 198:46–52. doi: 10.1016/j.jbiotec.2015.01.020 [DOI] [PubMed] [Google Scholar]
- 107. De Benedetto G, Alfini R, Cescutti P, Caboni M, Lanzilao L, Necchi F, Saul A, MacLennan CA, Rondini S, Micoli F. 2017. Characterization of O-antigen delivered by Generalized Modules for Membrane Antigens (GMMA) vaccine candidates against nontyphoidal Salmonella. Vaccine 35:419–426. doi: 10.1016/j.vaccine.2016.11.089 [DOI] [PubMed] [Google Scholar]
- 108. Hanumunthadu B, Kanji N, Owino N, Ferreira Da Silva C, Robinson H, White R, Ferruzzi P, Nakakana U, Canals R, Pollard AJ, Ramasamy M, Vacc-iNTS Consortium . 2023. Salmonella Vaccine Study in Oxford (SALVO) trial: protocol for an observer-participant blind randomised placebo-controlled trial of the iNTS-GMMA vaccine within a European cohort. BMJ Open 13:e072938. doi: 10.1136/bmjopen-2023-072938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Forbes NS. 2010. Engineering the perfect (bacterial) cancer therapy. Nat Rev Cancer 10:785–794. doi: 10.1038/nrc2934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Gurbatri CR, Arpaia N, Danino T. 2022. Engineering bacteria as interactive cancer therapies. Science 378:858–864. doi: 10.1126/science.add9667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Becerra-Báez EI, Meza-Toledo SE, Muñoz-López P, Flores-Martínez LF, Fraga-Pérez K, Magaño-Bocanegra KJ, Juárez-Hernández U, Mateos-Chávez AA, Luria-Pérez R. 2022. Recombinant attenuated Salmonella enterica as a delivery system of heterologous molecules in cancer therapy. Cancers 14:4224. doi: 10.3390/cancers14174224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Hernández-Luna MA, Luria-Pérez R. 2018. Cancer immunotherapy: priming the host immune response with live attenuated Salmonella enterica. J Immunol Res 2018:2984247. doi: 10.1155/2018/2984247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Howell LM, Forbes NS. 2022. Bacteria-based immune therapies for cancer treatment. Semin Cancer Biol 86:1163–1178. doi: 10.1016/j.semcancer.2021.09.006 [DOI] [PubMed] [Google Scholar]
- 114. Low KB, Ittensohn M, Le T, Platt J, Sodi S, Amoss M, Ash O, Carmichael E, Chakraborty A, Fischer J, Lin SL, Luo X, Miller SI, Zheng L, King I, Pawelek JM, Bermudes D. 1999. Lipid A mutant Salmonella with suppressed virulence and TNFalpha induction retain tumor-targeting in vivo. Nat Biotechnol 17:37–41. doi: 10.1038/5205 [DOI] [PubMed] [Google Scholar]
- 115. Clairmont C, Lee KC, Pike J, Ittensohn M, Low KB, Pawelek J, Bermudes D, Brecher SM, Margitich D, Turnier J, Li Z, Luo X, King I, Zheng LM. 2000. Biodistribution and genetic stability of the novel antitumor agent VNP20009, a genetically modified strain of Salmonella typhimurium. J Infect Dis 181:1996–2002. doi: 10.1086/315497 [DOI] [PubMed] [Google Scholar]
- 116. Chen J, Wei D, Zhuang H, Qiao Y, Tang B, Zhang X, Wei J, Fang S, Chen G, Du P, Huang X, Jiang W, Hu Q, Hua ZC. 2011. Proteomic screening of anaerobically regulated promoters from Salmonella and its antitumor applications. Mol Cell Proteomics 10:M111.009399. doi: 10.1074/mcp.M111.009399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Low KB, Ittensohn M, Luo X, Zheng LM, King I, Pawelek JM, Bermudes D. 2004. Construction of VNP20009: a novel, genetically stable antibiotic-sensitive strain of tumor-targeting Salmonella for parenteral administration in humans. Methods Mol Med 90:47–60. [PubMed] [Google Scholar]
- 118. Broadway KM, Modise T, Jensen RV, Scharf BE. 2014. Complete genome sequence of Salmonella enterica serovar Typhimurium VNP20009, a strain engineered for tumor targeting. J Biotechnol 192:177–178. doi: 10.1016/j.jbiotec.2014.07.006 [DOI] [PubMed] [Google Scholar]
- 119. Murray SR, de Felipe KS, Obuchowski PL, Pike J, Bermudes D, Low KB. 2004. Hot spot for a large deletion in the 18- to 19-centisome region confers a multiple phenotype in Salmonella enterica serovar Typhimurium strain ATCC 14028. J Bacteriol 186:8516–8523. doi: 10.1128/JB.186.24.8516-8523.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Kong Q, Six DA, Roland KL, Liu Q, Gu L, Reynolds CM, Wang X, Raetz CRH, Curtiss III R. 2011. Salmonella synthesizing 1-dephosphorylated [corrected] lipopolysaccharide exhibits low endotoxic activity while retaining its immunogenicity. J Immunol 187:412–423. doi: 10.4049/jimmunol.1100339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Kong Q, Six DA, Liu Q, Gu L, Roland KL, Raetz CRH, Curtiss III R. 2011. Palmitoylation state impacts induction of innate and acquired immunity by the Salmonella enterica serovar Typhimurium msbB mutant. Infect Immun 79:5027–5038. doi: 10.1128/IAI.05524-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Brandenburg K, Wiese A. 2004. Endotoxins: relationships between structure, function, and activity. Curr Top Med Chem 4:1127–1146. doi: 10.2174/1568026043388213 [DOI] [PubMed] [Google Scholar]
- 123. Luo X, Li Z, Lin S, Le T, Ittensohn M, Bermudes D, Runyab JD, Shen SY, Chen J, King IC, Zheng LM. 2001. Antitumor effect of VNP20009, an attenuated Salmonella, in murine tumor models. Oncol Res 12:501–508. doi: 10.3727/096504001108747512 [DOI] [PubMed] [Google Scholar]
- 124. Toso JF, Gill VJ, Hwu P, Marincola FM, Restifo NP, Schwartzentruber DJ, Sherry RM, Topalian SL, Yang JC, Stock F, Freezer LJ, Morton KE, Seipp C, Haworth L, Mavroukakis S, White D, MacDonald S, Mao J, Sznol M, Rosenberg SA. 2002. Phase I study of the intravenous administration of attenuated Salmonella typhimurium to patients with metastatic melanoma. J Clin Oncol 20:142–152. doi: 10.1200/JCO.2002.20.1.142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Heimann DM, Rosenberg SA. 2003. Continuous intravenous administration of live genetically modified Salmonella Typhimurium in patients with metastatic melanoma. J Immunother 26:179–180. doi: 10.1097/00002371-200303000-00011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. King I, Bermudes D, Lin S, Belcourt M, Pike J, Troy K, Le T, Ittensohn M, Mao J, Lang W, Runyan JD, Luo X, Li Z, Zheng LM. 2002. Tumor-targeted Salmonella expressing cytosine deaminase as an anticancer agent. Hum Gene Ther 13:1225–1233. doi: 10.1089/104303402320139005 [DOI] [PubMed] [Google Scholar]
- 127. Nemunaitis J, Cunningham C, Senzer N, Kuhn J, Cramm J, Litz C, Cavagnolo R, Cahill A, Clairmont C, Sznol M. 2003. Pilot trial of genetically modified, attenuated Salmonella expressing the E. coli cytosine deaminase gene in refractory cancer patients. Cancer Gene Ther 10:737–744. doi: 10.1038/sj.cgt.7700634 [DOI] [PubMed] [Google Scholar]
- 128. Zhou S, Lin Y, Zhao Z, Lai Y, Lu M, Shao Z, Mo X, Mu Y, Liang Z, Wang X, Qu J, Shen H, Li F, Zhao AZ. 2023. Targeted deprivation of methionine with engineered Salmonella leads to oncolysis and suppression of metastasis in broad types of animal tumor models. Cell Rep Med 4:101070. doi: 10.1016/j.xcrm.2023.101070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Kaiser P. 2020. Methionine dependence of cancer. Biomolecules 10:568. doi: 10.3390/biom10040568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Al-Saafeen BH, Al-Sbiei A, Bashir G, Mohamed YA, Masad RJ, Fernandez-Cabezudo MJ, Al-Ramadi BK. 2022. Attenuated Salmonella potentiate PD-L1 blockade immunotherapy in a preclinical model of colorectal cancer. Front Immunol 13:1017780. doi: 10.3389/fimmu.2022.1017780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Mai PT, Lim D, So E, Kim HY, Duysak T, Tran TQ, Song M, Jeong JH, Choy HE. 2023. Constitutive expression of a cytotoxic anticancer protein in tumor-colonizing bacteria. Cancers (Basel) 15:1486. doi: 10.3390/cancers15051486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Wen LJ, Gao LF, Jin CS, Zhang HJ, Ji K, Yang JP, Zhao XJ, Wen MJ, Guan GF. 2013. Small interfering RNA survivin and GRIM-19 co-expression salmonella plasmid inhibited the growth of laryngeal cancer cells in vitro and in vivo. Int J Clin Exp Pathol 6:2071–2081. [PMC free article] [PubMed] [Google Scholar]
- 133. Gontero P, Bohle A, Malmstrom P-U, O’Donnell MA, Oderda M, Sylvester R, Witjes F. 2010. The role of bacillus Calmette-Guerin in the treatment of non-muscle-invasive bladder cancer. Eur Urol 57:410–429. doi: 10.1016/j.eururo.2009.11.023 [DOI] [PubMed] [Google Scholar]
- 134. Babjuk M, Burger M, Capoun O, Cohen D, Compérat EM, Dominguez Escrig JL, Gontero P, Liedberg F, Masson-Lecomte A, Mostafid AH, Palou J, van Rhijn BWG, Rouprêt M, Shariat SF, Seisen T, Soukup V, Sylvester RJ. 2022. European association of urology guidelines on non-muscle-invasive bladder cancer (Ta, T1, and carcinoma in situ). Eur Urol 81:75–94. doi: 10.1016/j.eururo.2021.08.010 [DOI] [PubMed] [Google Scholar]
- 135. Domingos-Pereira S, Cesson V, Chevalier MF, Derré L, Jichlinski P, Nardelli-Haefliger D. 2017. Preclinical efficacy and safety of the Ty21a vaccine strain for intravesical immunotherapy of non-muscle-invasive bladder cancer. Oncoimmunology 6:e1265720. doi: 10.1080/2162402X.2016.1265720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Domingos-Pereira S, Sathiyanadan K, La Rosa S, Polák L, Chevalier MF, Martel P, Hojeij R, Derré L, Haefliger J-A, Jichlinski P, Nardelli-Haefliger D. 2019. Intravesical Ty21a vaccine promotes dendritic cells and T cell-mediated tumor regression in the MB49 bladder cancer model. Cancer Immunol Res 7:621–629. doi: 10.1158/2326-6066.CIR-18-0671 [DOI] [PubMed] [Google Scholar]
- 137. Lucca I, Derré L, Cesson V, Bohner P, Crettenand F, Rodrigues-Dias S, Dartiguenave F, Masnada A, Texeira-Pereira C, Benmerzoug S, Chevalier M, Domingos-Pereira S, Nguyen S, Polak L, Schneider A, Roth B, Jichlinski P, Nardelli-Haefliger D. 2022. Intravesical Ty21a treatment of non-muscle-invasive bladder cancer shows a good safety profile. Eur Urol Open Sci 45:55–58. doi: 10.1016/j.euros.2022.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Niethammer AG, Lubenau H, Mikus G, Knebel P, Hohmann N, Leowardi C, Beckhove P, Akhisaroglu M, Ge Y, Springer M, Grenacher L, Buchler MW, Koch M, Weitz J, Haefeli WE, Schmitz-Winnenthal FH. 2012. Double-blind, placebo-controlled first in human study to investigate an oral vaccine aimed to elicit an immune reaction against the VEGF-Receptor 2 in patients with stage IV and locally advanced pancreatic cancer. BMC Cancer 12:361. doi: 10.1186/1471-2407-12-361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Schmitz-Winnenthal FH, Hohmann N, Niethammer AG, Friedrich T, Lubenau H, Springer M, Breiner KM, Mikus G, Weitz J, Ulrich A, et al. 2015. Anti-angiogenic activity of VXM01, an oral T-cell vaccine against VEGF receptor 2, in patients with advanced pancreatic cancer: a randomized, placebo-controlled, phase 1 trial. Oncoimmunology 4:e1001217. doi: 10.1080/2162402X.2014.1001217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Schmitz-Winnenthal FH, Hohmann N, Schmidt T, Podola L, Friedrich T, Lubenau H, Springer M, Wieckowski S, Breiner KM, Mikus G, Büchler MW, Keller A-V, Koc R, Springfeld C, Knebel P, Bucur M, Grenacher L, Haefeli WE, Beckhove P. 2018. A phase 1 trial extension to assess immunologic efficacy and safety of prime-boost vaccination with VXM01, an oral T cell vaccine against VEGFR2, in patients with advanced pancreatic cancer. Oncoimmunology 7:e1303584. doi: 10.1080/2162402X.2017.1303584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Wang X, Bove AM, Simone G, Ma B. 2020. Molecular bases of VEGFR-2-mediated physiological function and pathological role. Front Cell Dev Biol 8:599281. doi: 10.3389/fcell.2020.599281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Barrow PA. 2007. Salmonella infections: immune and non-immune protection with vaccines. Avian Pathol 36:1–13. doi: 10.1080/03079450601113167 [DOI] [PubMed] [Google Scholar]
- 143. Fuche FJ, Sow O, Simon R, Tennant SM. 2016. Salmonella Serogroup C: current status of vaccines and why they are needed. Clin Vaccine Immunol 23:737–745. doi: 10.1128/CVI.00243-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Singh BR. 2009. Salmonella vaccines for animals and birds and their future perspective. Open Vaccine J 2:100–112. doi: 10.2174/1875035400902010100 [DOI] [Google Scholar]
- 145. Springer S, Lindner T, Ahrens M, Woitow G, Prandini F, Selbitz HJ. 2011. Duration of immunity induced in chickens by an attenuated live Salmonella enteritidis vaccine and an inactivated Salmonella enteritidis/typhimurium vaccine. Berl Munch Tierarztl Wochenschr 124:89–93. doi: 10.2376/0005-9366-124-89 [DOI] [PubMed] [Google Scholar]
- 146. Wang X, Kang X, Pan M, Wang M, Zhang J, Song H. 2022. Evaluation of the protective immune response induced by an rfbG-deficient Salmonella enterica serovar Enteritidis strain as a live attenuated DIVA (differentiation of infected and vaccinated animals) vaccine in chickens. Microbiol Spectr 10:e0157422. doi: 10.1128/spectrum.01574-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Methner U, Berndt A, Locke M. 2017. Salmonella Enteritidis with double deletion in phoP fliC and a competitive exclusion culture elicit substantial additive protective effects against Salmonella exposure in newly hatched chicks. Vaccine 35:6076–6082. doi: 10.1016/j.vaccine.2017.09.071 [DOI] [PubMed] [Google Scholar]
- 148. Nandre RM, Matsuda K, Chaudhari AA, Kim B, Lee JH. 2012. A genetically engineered derivative of Salmonella Enteritidis as a novel live vaccine candidate for salmonellosis in chickens. Res Vet Sci 93:596–603. doi: 10.1016/j.rvsc.2011.11.005 [DOI] [PubMed] [Google Scholar]
- 149. Alderton MR, Fahey KJ, Coloe PJ. 1991. Humoral responses and salmonellosis protection in chickens given a vitamin-dependent Salmonella typhimurium mutant. Avian Dis 35:435–442. doi: 10.2307/1591205 [DOI] [PubMed] [Google Scholar]
- 150. Muniz EC, Verdi R, Leão JA, Back A, Nascimento VPD. 2017. Evaluation of the effectiveness and safety of a genetically modified live vaccine in broilers challenged with Salmonella Heidelberg. Avian Pathol 46:676–682. doi: 10.1080/03079457.2017.1348598 [DOI] [PubMed] [Google Scholar]
- 151. Dueger EL, House JK, Heithoff DM, Mahan MJ. 2003. Salmonella DNA adenine methylase mutants prevent colonization of newly hatched chickens by homologous and heterologous serovars. Int J Food Microbiol 80:153–159. doi: 10.1016/s0168-1605(02)00152-6 [DOI] [PubMed] [Google Scholar]
- 152. Hassan JO, Curtiss III R. 1994. Development and evaluation of an experimental vaccination program using a live avirulent Salmonella typhimurium strain to protect immunized chickens against challenge with homologous and heterologous Salmonella serotypes. Infect Immun 62:5519–5527. doi: 10.1128/iai.62.12.5519-5527.1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Agency EM . 2016. SG-VAC Salmonella enterica, subsp. enterica, serovar Gallinarum, strain SGP695AV, Live. Available from: https://medicines.health.europa.eu/veterinary/sl/600000083944. Retrieved 04 Nov 2016.
- 154. Feberwee A, de Vries TS, Hartman EG, de Wit JJ, Elbers ARW, de Jong WA. 2001. Vaccination against Salmonella enteritidis in dutch commercial layer flocks with a vaccine based on a live Salmonella gallinarum 9R strain: evaluation of efficacy, safety, and performance of serologic Salmonella tests. Avian Dis 45:83–91. doi: 10.2307/1593015 [DOI] [PubMed] [Google Scholar]
- 155. Mitra A, Loh A, Gonzales A, Łaniewski P, Willingham C, Curtiss III R, Roland KL. 2013. Safety and protective efficacy of live attenuated Salmonella Gallinarum mutants in Rhode Island Red chickens. Vaccine 31:1094–1099. doi: 10.1016/j.vaccine.2012.12.021 [DOI] [PubMed] [Google Scholar]
- 156. Sivasankar C, Hewawaduge C, Lee JH. 2023. Screening of lipid-A related genes and development of low-endotoxicity live-attenuated Salmonella gallinarum by arnT deletion that elicits immune responses and protection against fowl typhoid in chickens. Dev Comp Immunol 145:104707. doi: 10.1016/j.dci.2023.104707 [DOI] [PubMed] [Google Scholar]
- 157. Laniewski P, Mitra A, Karaca K, Khan A, Prasad R, Curtiss III R, Roland KL. 2014. Evaluation of protective efficacy of live attenuated Salmonella enterica serovar Gallinarum vaccine strains against fowl typhoid in chickens. Clin Vaccine Immunol 21:1267–1276. doi: 10.1128/CVI.00310-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Cheng Z, Yin J, Kang X, Geng S, Hu M, Pan Z, Jiao X. 2016. Safety and protective efficacy of a spiC and crp deletion mutant of Salmonella gallinarum as a live attenuated vaccine for fowl typhoid. Res Vet Sci 107:50–54. doi: 10.1016/j.rvsc.2016.05.007 [DOI] [PubMed] [Google Scholar]
- 159. Griffin HG, Barrow PA. 1993. Construction of an aroA mutant of Salmonella serotype Gallinarum: its effectiveness in immunization against experimental fowl typhoid. Vaccine 11:457–462. doi: 10.1016/0264-410X(93)90288-9 [DOI] [PubMed] [Google Scholar]
- 160. Yin J, Cheng Z, Xu L, Li Q, Geng S, Pan Z, Jiao X. 2015. Immunogenicity and protective efficacy of Salmonella enterica serovar Pullorum pathogenicity island 2 mutant as a live attenuated vaccine candidate. BMC Vet Res 11:162. doi: 10.1186/s12917-015-0497-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Kang X, Yang Y, Meng C, Wang X, Liu B, Geng S, Jiao X, Pan Z. 2022. Safety and protective efficacy of Salmonella Pullorum spiC and rfaH deletion rough mutant as a live attenuated DIVA vaccine candidate. Poult Sci 101:101655. doi: 10.1016/j.psj.2021.101655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Varmuzova K, Faldynova M, Elsheimer-Matulova M, Sebkova A, Polansky O, Havlickova H, Sisak F, Rychlik I. 2016. Immune protection of chickens conferred by a vaccine consisting of attenuated strains of Salmonella Enteritidis, Typhimurium and infantis. Vet Res 47:94. doi: 10.1186/s13567-016-0371-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Bearson SMD, Monson MS, Bearson BL, Whelan SJ, Byrd II JA, Burciaga S. 2024. Commercial vaccine provides cross-protection by reducing colonization of Salmonella enterica serovars infantis and Hadar in turkeys. Vaccine 42:727–731. doi: 10.1016/j.vaccine.2023.12.054 [DOI] [PubMed] [Google Scholar]
- 164. Schwarz P, Kich JD, Kolb J, Cardoso M. 2011. Use of an avirulent live Salmonella Choleraesuis vaccine to reduce the prevalence of Salmonella carrier pigs at slaughter. Vet Rec 169:553. doi: 10.1136/vr.d5510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Roof MB, Doitchinoff DD. 1995. Safety, efficacy, and duration of immunity induced in swine by use of an avirulent live Salmonella choleraesuis-containing vaccine. Am J Vet Res 56:39–44. [PubMed] [Google Scholar]
- 166. Maes D, Gibson K, Trigo E, Saszak A, Grass J, Carlson A, Blaha T. 2001. Evaluation of cross-protection afforded by a Salmonella Choleraesuis vaccine against Salmonella infections in pigs under field conditions. Berl Munch Tierarztl Wochenschr 114:339–341. doi: 10.31274/safepork-180809-1071 [DOI] [PubMed] [Google Scholar]
- 167. Liu G, Li C, Liao S, Guo A, Wu B, Chen H. 2023. C500 variants conveying complete mucosal immunity against fatal infections of pigs with Salmonella enterica serovar Choleraesuis C78-1 or F18+ Shiga toxin-producing Escherichia coli. Front Microbiol 14:1210358. doi: 10.3389/fmicb.2023.1210358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Pesciaroli M, Gradassi M, Martinelli N, Ruggeri J, Pistoia C, Raffatellu M, Magistrali CF, Battistoni A, Pasquali P, Alborali GL. 2013. Salmonella Typhimurium lacking the Znuabc transporter is attenuated and immunogenic in pigs. Vaccine 31:2868–2873. doi: 10.1016/j.vaccine.2013.04.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Bearson BL, Bearson SMD, Kich JD, Lee IS. 2014. An rfaH mutant of Salmonella enterica serovar Typhimurium is attenuated in swine and reduces intestinal colonization, fecal shedding, and disease severity due to virulent Salmonella Typhimurium. Front Vet Sci 1:9. doi: 10.3389/fvets.2014.00009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Bearson BL, Bearson SMD, Kich JD. 2016. A DIVA vaccine for cross-protection against Salmonella. Vaccine 34:1241–1246. doi: 10.1016/j.vaccine.2016.01.036 [DOI] [PubMed] [Google Scholar]
- 171. Papenfort K, Pfeiffer V, Lucchini S, Sonawane A, Hinton JCD, Vogel J. 2008. Systematic deletion of Salmonella small RNA genes identifies CyaR, a conserved CRP-dependent riboregulator of OmpX synthesis. Mol Microbiol 68:890–906. doi: 10.1111/j.1365-2958.2008.06189.x [DOI] [PubMed] [Google Scholar]
- 172. Hur J, Lee JH. 2010. Immunization of pregnant sows with a novel virulence gene deleted live Salmonella vaccine and protection of their suckling piglets against salmonellosis. Vet Microbiol 143:270–276. doi: 10.1016/j.vetmic.2009.11.034 [DOI] [PubMed] [Google Scholar]
- 173. Selke M, Meens J, Springer S, Frank R, Gerlach GF. 2007. Immunization of pigs to prevent disease in humans: construction and protective efficacy of a Salmonella enterica serovar Typhimurium live negative-marker vaccine. Infect Immun 75:2476–2483. doi: 10.1128/IAI.01908-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Cummings KJ, Rodriguez-Rivera LD, Capel MB, Rankin SC, Nydam DV. 2019. Short communication: oral and intranasal administration of a modified-live Salmonella Dublin vaccine in dairy calves: clinical efficacy and serologic response. J Dairy Sci 102:3474–3479. doi: 10.3168/jds.2018-14892 [DOI] [PubMed] [Google Scholar]
- 175. Wang F, Wang L, Ge H, Wang X, Guo Y, Xu Z, Geng S, Jiao X, Chen X. 2022. Safety of the Salmonella enterica serotype Dublin strain Sdu189-derived live attenuated vaccine-A pilot study. Front Vet Sci 9:986332. doi: 10.3389/fvets.2022.986332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Mohler VL, Heithoff DM, Mahan MJ, Walker KH, Hornitzky MA, Shum LWC, Makin KJ, House JK. 2008. Cross-protective immunity conferred by a DNA adenine methylase deficient Salmonella enterica serovar Typhimurium vaccine in calves challenged with Salmonella serovar Newport. Vaccine 26:1751–1758. doi: 10.1016/j.vaccine.2008.01.018 [DOI] [PubMed] [Google Scholar]
- 177. Uzzau S, Marogna G, Leori GS, Curtiss III R, Schianchi G, Stocker BAD, Rubino S. 2005. Virulence attenuation and live vaccine potential of aroA, crp cdt cya, and plasmid-cured mutants of Salmonella enterica serovar Abortusovis in mice and sheep. Infect Immun 73:4302–4308. doi: 10.1128/IAI.73.7.4302-4308.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Mohler VL, Heithoff DM, Mahan MJ, Walker KH, Hornitzky MA, Gabor L, Thomson PC, Thompson A, House JK. 2011. Protective immunity conferred by a DNA adenine methylase deficient Salmonella enterica serovar Typhimurium vaccine when delivered in-water to sheep challenged with Salmonella enterica serovar Typhimurium. Vaccine 29:3571–3582. doi: 10.1016/j.vaccine.2011.03.075 [DOI] [PubMed] [Google Scholar]
- 179. Hofacre CL, Rosales AG, Costa MD, Cookson K, Schaeffer J, Jones MK. 2021. Immunity and protection provided by live modified vaccines against paratyphoid Salmonella in poultry-an applied perspective. Avian Dis 65:295–302. doi: 10.1637/aviandiseases-D-20-00126 [DOI] [PubMed] [Google Scholar]
- 180. Gast RK. 2007. Serotype-specific and serotype-independent strategies for preharvest control of food-borne Salmonella in poultry. Avian Dis 51:817–828. doi: 10.1637/8090-081807.1 [DOI] [PubMed] [Google Scholar]
- 181. Revolledo L, Ferreira AJP. 2012. Current perspectives in avian salmonellosis: vaccines and immune mechanisms of protection. J Appl Poult Res 21:418–431. doi: 10.3382/japr.2011-00409 [DOI] [Google Scholar]
- 182. Gast RK, Porter Jr RE. 2020. Salmonella infections, p 717–753. In Diseases of poultry [Google Scholar]
- 183. Van Immerseel F, Studholme DJ, Eeckhaut V, Heyndrickx M, Dewulf J, Dewaele I, Van Hoorebeke S, Haesebrouck F, Van Meirhaeghe H, Ducatelle R, Paszkiewicz K, Titball RW. 2013. Salmonella Gallinarum field isolates from laying hens are related to the vaccine strain SG9R. Vaccine 31:4940–4945. doi: 10.1016/j.vaccine.2013.08.033 [DOI] [PubMed] [Google Scholar]
- 184. De Carli S, Gräf T, Kipper D, Lehmann FKM, Zanetti N, Siqueira FM, Cibulski S, Fonseca ASK, Ikuta N, Lunge VR. 2017. Molecular and phylogenetic analyses of Salmonella Gallinarum trace the origin and diversification of recent outbreaks of fowl typhoid in poultry farms. Vet Microbiol 212:80–86. doi: 10.1016/j.vetmic.2017.11.001 [DOI] [PubMed] [Google Scholar]
- 185. Barrow PA, Lovell MA, Stocker BAD. 2000. Protection against experimental fowl typhoid by parenteral administration of live SL5828, an aroA-serC (aromatic dependent) mutant of a wild-type Salmonella Gallinarum strain made lysogenic for P22 sie. Avian Pathol 29:423–431. doi: 10.1080/030794500750047171 [DOI] [PubMed] [Google Scholar]
- 186. Alam J, Singh BR, Hansda D, Singh VP, Verma JC. 2009. Evaluation of aroA deletion mutant of Salmonella enterica subspecies enterica serovar Abortusequi for its vaccine candidate potential. Indian J Exp Biol 47:871–879. [PubMed] [Google Scholar]
- 187. Stocker BA, Hoiseth SK, Smith BP. 1983. Aromatic-dependent "Salmonella sp." as live vaccine in mice and calves. Dev Biol Stand 53:47–54. [PubMed] [Google Scholar]
- 188. Smith BP, Reina-Guerra M, Hoiseth SK, Stocker BA, Habasha F, Johnson E, Merritt F. 1984. Aromatic-dependent Salmonella Typhimurium as modified live vaccines for calves. Am J Vet Res 45:59–66. [PubMed] [Google Scholar]
- 189. Copper GL, Venables LM, Nicholas RAJ, Cullen GA, Hormaeche CE. 1992. Vaccination of chickens with chicken-derived Salmonella enteritidis phage type 4 aroA live oral salmonella vaccines. Vaccine 10:247–254. doi: 10.1016/0264-410X(92)90160-L [DOI] [PubMed] [Google Scholar]
- 190. Paulin SM, Jagannathan A, Campbell J, Wallis TS, Stevens MP. 2007. Net replication of Salmonella enterica serovars Typhimurium and Choleraesuis in porcine intestinal mucosa and nodes is associated with their differential virulence. Infect Immun 75:3950–3960. doi: 10.1128/IAI.00366-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Yang Y, Wolfenden A, Mandal RK, Faulkner O, Hargis B, Kwon YM, Bielke L. 2017. Evaluation of recombinant Salmonella vaccines to provide cross-serovar and cross-serogroup protection. Poult Sci 96:4352–4360. doi: 10.3382/ps/pex144 [DOI] [PubMed] [Google Scholar]
- 192. Holt PS, Gast RK. 2004. Effects of prior coinfection with different Salmonella serovars on the progression of a Salmonella enterica serovar enteritidis infection in hens undergoing induced molt. Avian Dis 48:160–166. doi: 10.1637/7101 [DOI] [PubMed] [Google Scholar]
- 193. Heithoff DM, Enioutina EY, Daynes RA, Sinsheimer RL, Low DA, Mahan MJ. 2001. Salmonella DNA adenine methylase mutants confer cross-protective immunity. Infect Immun 69:6725–6730. doi: 10.1128/IAI.69.11.6725-6730.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Johnson TJ, Flores-Figueroa C, Munoz-Aguayo J, Pinho G, Miller E. 2024. Persistence of vaccine origin Salmonella Typhimurium through the poultry production continuum, and development of a rapid typing scheme for their differentiation from wild type field isolates. Poult Sci 103:103707. doi: 10.1016/j.psj.2024.103707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Van Immerseel F, De Zutter L, Houf K, Pasmans F, Haesebrouck F, Ducatelle R. 2009. Strategies to control Salmonella in the broiler production chain. World’s Poult Sci J 65:367–392. doi: 10.1017/S0043933909000270 [DOI] [Google Scholar]
- 196. Methner U, Barrow PA, Gregorova D, Rychlik I. 2004. Intestinal colonisation-inhibition and virulence of Salmonella phoP, rpoS and ompC deletion mutants in chickens. Vet Microbiol 98:37–43. doi: 10.1016/j.vetmic.2003.10.019 [DOI] [PubMed] [Google Scholar]
- 197. Methner U, Berndt A, Steinbach G. 2001. Combination of competitive exclusion and immunization with an attenuated live Salmonella vaccine strain in chickens. Avian Dis 45:631. doi: 10.2307/1592904 [DOI] [PubMed] [Google Scholar]
- 198. Bearson SMD. 2022. Salmonella in swine: prevalence, multidrug resistance, and vaccination strategies. Annu Rev Anim Biosci 10:373–393. doi: 10.1146/annurev-animal-013120-043304 [DOI] [PubMed] [Google Scholar]
- 199. Fang H-W, Li Y-L, Huang C-B, Zheng M, Feng W-T, Sun W. 1981. Live vaccine against swine paratyphoid from the attenuated smooth strain C500 of Salmonella choleraesuis. Chin J Anim Vet Sci 2:99–106. [Google Scholar]
- 200. Li Q, Hu Y, Xu L, Xie X, Tao M, Jiao X. 2014. Complete genome sequence of Salmonella enterica serovar Choleraesuis vaccine strain C500 attenuated by chemical mutation. Genome Announc 2:e01022-14. doi: 10.1128/genomeA.01022-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Zhao Z, Xue Y, Tang X, Wu B, Cheng X, He Q, Zhang C, Guo A, Jin M, Chen H. 2009. Immunogenicity of recombinant protective antigen and efficacy against intranasal challenge with Bordetella bronchiseptica. Vaccine 27:2523–2528. doi: 10.1016/j.vaccine.2008.09.091 [DOI] [PubMed] [Google Scholar]
- 202. Han L, Zhen YH, Liang AX, Zhang J, Riaz H, Xiong JJ, Guo AZ, Yang LG. 2014. Oral vaccination with inhibin DNA delivered using attenuated Salmonella choleraesuis for improving reproductive traits in mice. J Basic Microbiol 54:962–968. doi: 10.1002/jobm.201300052 [DOI] [PubMed] [Google Scholar]
- 203. Xu YD, Guo AZ, Liu WH, Jia AQ, Chen HC. 2006. Construction and characterization of delta crp delta asd mutant host-vector balanced lethal system of Salmonella choleraesuis C500 strain. Sheng Wu Gong Cheng Xue Bao 22:366–372. [PubMed] [Google Scholar]
- 204. Peeters L, Dewulf J, Boyen F, Brossé C, Vandersmissen T, Rasschaert G, Heyndrickx M, Cargnel M, Mattheus W, Pasmans F, Haesebrouck F, Maes D. 2020. Evaluation of group vaccination of sows and gilts against Salmonella Typhimurium with an attenuated vaccine in subclinically infected pig herds. Prev Vet Med 182:104884. doi: 10.1016/j.prevetmed.2020.104884 [DOI] [PubMed] [Google Scholar]
- 205. Peeters L, Dewulf J, Boyen F, Brossé C, Vandersmissen T, Rasschaert G, Heyndrickx M, Cargnel M, Mattheus W, Pasmans F, Haesebrouck F, Maes D. 2020. Bacteriological evaluation of vaccination against Salmonella Typhimurium with an attenuated vaccine in subclinically infected pig herds. Prev Vet Med 182:104687. doi: 10.1016/j.prevetmed.2019.04.016 [DOI] [PubMed] [Google Scholar]
- 206. Theuß T, Ueberham E, Lehmann J, Lindner T, Springer S. 2017. Immunogenic potential of a Salmonella Typhimurium live vaccine for pigs against monophasic Salmonella Typhimurium DT 193. BMC Vet Res 13:343. doi: 10.1186/s12917-017-1271-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. USDA-APHIS . 2013. Veterinary biological products-licensees and permitees. Available from: https://www.aphis.usda.gov/aphis/ourfocus/animalhealth/veterinary-biologics/ct_vb_licensed_products. Retrieved 12 Oct 2013.
- 208. Kramer TT, Roof MB, Matheson RR. 1992. Safety and efficacy of an attenuated strain of Salmonella choleraesuis for vaccination of swine. Am J Vet Res 53:444–448. doi: 10.2460/ajvr.1991.53.04.444 [DOI] [PubMed] [Google Scholar]
- 209. Guiney DG, Fierer J. 2011. The role of the spv genes in Salmonella pathogenesis. Front Microbiol 2:129. doi: 10.3389/fmicb.2011.00129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Kennedy MJ, Yancey Jr RJ, Sanchez MS, Rzepkowski RA, Kelly SM, Curtiss R. 1999. Attenuation and immunogenicity of Δcya Δcrp derivatives of Salmonella choleraesuis in pigs. Infect Immun 67:4628–4636. doi: 10.1128/IAI.67.9.4628-4636.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Mohler V, Heithoff D, Mahan M, Walker K, Hornitzky M, Mcconnell C, Shum L, House J. 2006. Cross-protective immunity in calves conferred by a DNA adenine methylase deficient Salmonella enterica serovar Typhimurium vaccine. Vaccine 24:1339–1345. doi: 10.1016/j.vaccine.2005.09.022 [DOI] [PubMed] [Google Scholar]
- 212. Spickler AR. 2017. Salmonella abortusovis. Available from: https://www.cfsph.iastate.edu/diseaseinfo/factsheets
- 213. Wyszyńska A, Raczko A, Lis M, Jagusztyn-Krynicka EK. 2004. Oral immunization of chickens with avirulent Salmonella vaccine strain carrying C. jejuni 72Dz/92 cjaA gene elicits specific humoral immune response associated with protection against challenge with wild-type Campylobacter. Vaccine 22:1379–1389. doi: 10.1016/j.vaccine.2003.11.001 [DOI] [PubMed] [Google Scholar]
- 214. Wilde S, Jiang Y, Tafoya AM, Horsman J, Yousif M, Vazquez LA, Roland KL. 2019. Salmonella-vectored vaccine delivering three Clostridium perfringens antigens protects poultry against necrotic enteritis. PLoS One 14:e0197721. doi: 10.1371/journal.pone.0197721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Domínguez-Bernal G, Tierrez A, Bartolomé A, Martínez-Pulgarín S, Salguero FJ, Orden JA, de la Fuente R. 2008. Salmonella enterica serovar Choleraesuis derivatives harbouring deletions in rpoS and phoP regulatory genes are attenuated in pigs, and survive and multiply in porcine intestinal macrophages and fibroblasts, respectively. Vet Microbiol 130:298–311. doi: 10.1016/j.vetmic.2008.01.008 [DOI] [PubMed] [Google Scholar]
- 216. Konjufca V, Wanda S-Y, Jenkins MC, Curtiss III R. 2006. A recombinant attenuated Salmonella enterica serovar Typhimurium vaccine encoding Eimeria acervulina antigen offers protection against E. acervulina challenge. Infect Immun 74:6785–6796. doi: 10.1128/IAI.00851-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Kong W, Wang X, Fields E, Okon B, Jenkins MC, Wilkins G, Brovold M, Golding T, Gonzales A, Golden G, Clark-Curtiss J, Curtiss R. 2020. Mucosal delivery of a self-destructing Salmonella-based vaccine inducing immunity against Eimeria. Avian Dis 64:254–268. doi: 10.1637/aviandiseases-D-19-00159 [DOI] [PubMed] [Google Scholar]
- 218. Gimeno IM, Witter RL, Hunt HD, Reddy SM, Lee LF, Silva RF. 2005. The pp38 gene of Marek's Disease Virus (MDV) is necessary for cytolytic infection of B cells and maintenance of the transformed state but not for cytolytic infection of the feather follicle epithelium and horizontal spread of MDV. J Virol 79:4545–4549. doi: 10.1128/JVI.79.7.4545-4549.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Senevirathne A, Hewawaduge C, Lee JH. 2021. Genetic interference exerted by Salmonella-delivered CRISPR/Cas9 significantly reduces the pathological burden caused by Marek's disease virus in chickens. Vet Res 52:125. doi: 10.1186/s13567-021-00995-x [DOI] [PMC free article] [PubMed] [Google Scholar]