Abstract
Patchy global data on belowground litter decomposition dynamics limit our capacity to discern the drivers of carbon preservation and storage across inland and coastal wetlands. We performed a global, multiyear study in over 180 wetlands across 28 countries and 8 macroclimates using standardized litter as measures of “recalcitrant” (rooibos tea) and “labile” (green tea) organic matter (OM) decomposition. Freshwater wetlands and tidal marshes had the highest tea mass remaining, indicating a greater potential for carbon preservation in these ecosystems. Recalcitrant OM decomposition increased with elevated temperatures throughout the decay period, e.g., increase from 10 to 20 °C corresponded to a 1.46-fold increase in the recalcitrant OM decay rate constant. The effect of elevated temperature on labile OM breakdown was ecosystem-dependent, with tidally influenced wetlands showing limited effects of temperature compared with freshwater wetlands. Based on climatic projections, by 2050 wetland decay constants will increase by 1.8% for labile and 3.1% for recalcitrant OM. Our study highlights the potential for reduction in belowground OM in coastal and inland wetlands under increased warming, but the extent and direction of this effect at a large scale is dependent on ecosystem and OM characteristics. Understanding local versus global drivers is necessary to resolve ecosystem influences on carbon preservation in wetlands.
Keywords: blue carbon, macroclimate, TeaComposition H2O, tea bags, teal carbon
Short abstract
A multiyear decomposition incubation of standardized litter in wetlands reveals global impacts of ecosystem and climate on soil carbon preservation.
1. Introduction
Inland and coastal wetland ecosystems, including a range of freshwater and saline ecosystems with different inundation regimes,1 have a high capacity for soil carbon storage due to anaerobic waterlogged soils and the accumulation of internal and external carbon sources.2,3 Within these ecosystems, the vast majority of carbon is stored belowground (e.g., 62–99% in coastal wetlands and 50–93% in freshwater wetlands).4−6 The development of belowground carbon pools is largely dependent on inputs of carbon into the system and the microbial processes that moderate the decomposition of carbon-rich organic matter (OM). In addition to being fundamentally important for soil fertility and supporting biodiversity,7 the decomposition process in wetland soils is vital for soil carbon (trans)formation and sequestration in wetlands,8 and thus the global carbon cycle itself.9,10
Identifying the drivers of wetland litter decomposition processes is essential for predicting feedbacks to environmental and climate change. Elevated temperature can enhance OM breakdown through increased microbial metabolism, which in turn might increase the decay rate for a range of wetland types.10−12 Changes in soil water content (e.g., through inundation patterns, wetland drainage, tidal cycles, and interannual variability in precipitation) can control much of the decay process in wetland soils, influencing initial mass loss through leaching and providing access to nutrients that support microbial metabolism, while also influencing oxygen availability, potentially limiting microbial mineralization under anaerobic conditions.2,9,13−15 Anaerobic conditions caused by waterlogging can dampen the enhanced decomposition caused by elevated temperature during the later stages of decay when decay processes are dominated by enzymatic breakdown of plant structural compounds,2,16 thereby complicating the impacts of warming temperatures in the long term. Further, variability in litter chemical composition (e.g., nitrogen concentration, tissue type, phylogenetic history) can be a major influence on the decomposition dynamics in wetlands and can result in litter-specific responses to external factors like salinity, inundation, and warming.10,17−22
Although there is greater potential for belowground OM deposits (e.g., roots, rhizomes) to contribute to wetland soil carbon, decomposition studies in wetlands often focus on aboveground litter decomposition,10,23,24 despite high litter export rates and potential for herbivory.25−27 Additionally, existing global models of long-term decomposition are primarily based on terrestrial ecosystems and do not represent belowground wetland decay well due to different decomposition dynamics (e.g., wet conditions).28 Long-term field datasets on belowground OM decomposition may help develop paradigms on the controls of wetland decay, including sea-level rise,29,30 soil water content, climate, and decomposers.31,32 Global wetland decay datasets may also help parametrize global carbon cycling through earth system models33,34 and by means of satellite-based models.35
Since litter chemical composition strongly affects the decomposition process, it is difficult to draw broader conclusions when a range of litter types are used over regional and global scales.36,37 A promising approach to advance our knowledge of belowground decomposition across ecosystems and climates is to use standardized substrates in lieu of local plant litter.38,39 Standardized green tea and rooibos tea “litters” have water-soluble-dominant (labile, rapid leaching) and lignin-dominant (recalcitrant, stable) compositions, respectively.39,40 Tea litter has been valuable at revealing short-term, 3 month drivers of belowground litter decay at regional and global scales, although a limited collection of studies for wetland and aquatic ecosystems exists.41,42 There are limitations and challenges in extrapolating the drivers of short-term incubations to inform longer-term predictions and processes of decomposition, i.e., linked to carbon storage and sequestration. Longer standardized tea litter incubations (e.g., ∼1 year) show how differences in inundation and oxygen conditions and temperature may be driving wetland OM decay, particularly for the more recalcitrant rooibos tea litter,43,44 suggesting different ecosystem responses to anthropogenic changes in the future.
To improve our understanding of belowground litter decomposition dynamics in wetlands, we performed a global, up to four-year decomposition experiment using standardized tea litter. By burying the litter bags, we are subjecting the proxy substrates to the conditions and processes that influence belowground OM turnover processes. Labile and recalcitrant OM sources, represented by green and rooibos tea litters, respectively, were incubated in the soils of inland and coastal wetlands, as well as lotic (e.g., stream) and lentic (e.g., pond/lake) ecosystems for approximately three years across eight macroclimates. First, we compared short-term (3 months) and long-term (≥24 months) decomposition across ecosystem types. Next, we applied asymptotic and single exponential decay rate models45 to the entire time series at each site to produce site-level decay parameters that describe an early leaching-influenced decay rate constant, the proportion of stable mass remaining at the end of the incubation, and an overall decay rate constant. We used these parameters to explore the impacts of climatic and ecosystem properties on different stages of standardized litter decomposition. We hypothesize that warmer and higher precipitation climates will enhance decomposition but have litter-specific impacts.46 We also expect that ecosystem type, comprised of a range of varying factors including inundation, would significantly influence longer-term decay dynamics for both litter types.43,44 Lastly, we used the decay parameters to project decay responses under future climatic conditions, expecting temperature to be a key driver of decomposition.10 Together, this study aims to expand our knowledge of wetland OM decay and soil carbon preservation in global wetlands to improve global climate and carbon cycle modeling, as well as to provide a catalyst for future long-term wetland decomposition studies using both natural and standardized litter.
2. Materials and Methods
2.1. TeaComposition H2O Initiative and Decomposition Experiment
The standardized litters are Lipton© (Unilever) green and rooibos teas, packaged in the original nylon mesh. Green tea represents labile forms of OM (high water-soluble compound content) and the rooibos tea represents recalcitrant/stable (high fiber/lignin content) forms of OM (Camellia sinensis, EAN no.: 8 722700 055525; Aspalathus linearis, EAN no.: 8 722700 188438).39 Generally, the definitions of labile and recalcitrant are context dependent and sit along a spectrum depending on substrate type and chemical characteristics, spatiotemporal frame of observation, and microbial characteristics.47 For the purpose of this study in which initial chemical characteristics are well-known,39,40 we will refer to the tea litter OM in terms of its inherent chemical characteristics,48 that is, labile and recalcitrant OM for green and rooibos teas, respectively.
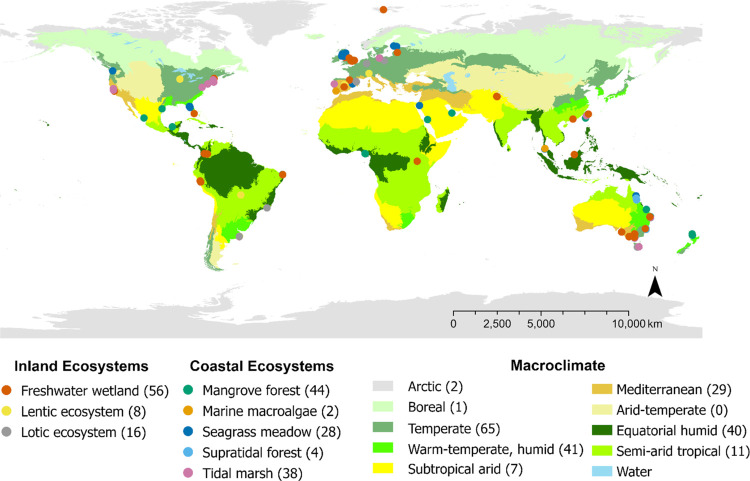
This work was performed within TeaComposition H2O, a global initiative to collect long-term decomposition data from wetlands and aquatic ecosystems using standardized litter methods. We defined wetlands as inclusive of freshwater/aquatic and coastal/marine marshes, peatlands, and waters that do not exceed 6 m depth at low tide.1 We focused on vegetated sites that did not receive experimental manipulations, resulting in data from 196 sites (Figure 1). See Supporting Methods for details on information collected for each site (Figure 1 and Table S1). The “freshwater wetland” category comprised a diversity of wetland types, so we further categorized using the IUCN Ecosystem Typology 2.0 for additional statistical analysis (Table S1, Supporting Methods).49 Monthly mean temperature and total precipitation from local weather stations were calculated for each month from deployment to the final sampling. Monthly mean temperature variation was calculated as the standard deviations of the monthly temperatures during the incubation period. Subtidal sites used in the final analyses represented <10% of the sites, most of which were lagoonal/estuarine (e.g., near intertidal). Therefore, local air temperatures were used for all sites.50
Figure 1.
Map of TeaComposition H2O sites across eight macroclimatic zones. Numbers next to ecosystem and macroclimate types indicate the site number. See Table S1 for more details on the sites. Climatic zones are from Walter and Breckle.55
At each site, green and rooibos tea litter bags were buried 10–15 cm deep into the soil by hand in two plots at least 1 m apart. This depth generally captures the rhizosphere or surficial OM layers of our inland and coastal wetlands while at a depth that is easily retrievable. Within each plot, there were two replicates for each tea type (i.e., n = 4 for each tea type at each sampling time and site).43 Deployment occurred in the summer of 2017 for the northern hemisphere (e.g., ∼June to August) and summer of 2017/2018 for the southern hemisphere (e.g., ∼December to February). Tea bags were collected 3, 6, 12, 24, and 36 months after deployment. As sites varied in accessibility and time constraints, some sites ended the experiment earlier at 12 or 24 months. Further, the 3-year sampling in 2020 occurred during the global coronavirus pandemic, resulting in 19 sites with delayed final samplings between 36 and 48 months. The initial mass was calculated by weighing the tea in the bag and then subtracting the mean bag mass of 0.20 g (±0.002 g S.E.M, averaged over 40 empty bags). Post-incubation samples were cleaned of soil and dried at 60–70 °C until a constant weight. Contaminating root biomass (i.e., root in-growth) was removed before weighing the final dry tea mass without the bag.
2.2. Decay Modeling
As the study focused on quantifying the long-term tea litter decay, we calculated decay parameters for sites that had incubations for 1 year or longer. Only sites with at least two replicates across three sampling times (i.e., six data points over the first year) were included. After filtering, 181 sites remained for labile green tea, and 184 sites remained for recalcitrant rooibos tea. See Supporting Methods for details on data cleaning.
For our decay modeling approach, we fit the site-level data with single exponential and asymptotic decay functions following Gill et al.45
Asymptotic exponential decay
| 1 |
where A is the asymptote (A), t is the time (days), and ka is the early decay rate constant (day−1). The asymptotic decay function uses a negative exponential function approaching a nonzero horizontal asymptote. This formulation partitions the tea litter between early- and late-stage decay. The early stage is characterized by the initial rapid decay (1 – A, as a proportion) at rate ka (d–1). The later stage is characterized by very slow or negligible decay after reaching the asymptote, i.e., the proportion of stable OM (A).
Single exponential decay
| 2 |
The single exponential decay function describes the tea litter as a single pool that decomposes at a constant rate (ks, d–1) over time (t, days).
By using the parameters from both the asymptotic and the single exponential decay models, we were able to describe tea litter decay in the following ways: (1) the negative exponential rate before reaching the asymptote quantifies the early decay rate constant (ka) and is linked to abiotic leaching of water-soluble compounds (eq 1), (2) the asymptote (A) is the proportion of stable mass remaining under a long-term decay constant and has the potential to contribute to soil carbon stocks (eq 1), and (3) the overall negative exponential decay rate constant (ks) quantifies the overall decay rate in each time series (eq 2).45 See Supporting Methods for details on model fits.
2.3. Statistical Analyses and Prediction Modeling
Using linear models, we tested the effects of ecosystem type and climate on litter decay parameters (i.e., A, ka, and ks) for each OM type (Table S2, Supporting Methods). Ecosystem type and climate were important factors in previous shorter-term tea litter decay studies (3–12 months).43,51 Therefore, the first model included the following terms: precipitation, temperature, temperature variability (as standard deviation), ecosystem type, and two-way interactions between ecosystem type and each of the three local climate terms to compare the sensitivity of the ecosystem types to the climatic factors. The macroalgal ecosystems were represented by only two sites each, so they were removed for this analysis. Model selection using the Bayesian information criterion (BIC) was performed for each OM type and decay parameter combination.52 The second and third models for macroclimate and freshwater wetland IUCN typologies were analyzed in single-factor models separately. For all models, significant interactions between factors were explored with Tukey posthoc pairwise comparisons using the emmeans package.53 All analyses were performed using the lm() function in R version 4.1.3.54
We generated worldwide spatial predictions of decay parameters (i.e., A, ka, and ks) based on linear models using only local climate without accounting for ecosystem type due to incomplete geospatial coverage of each ecosystem type in this study. We sourced from Copernicus Climate Data Store spatially explicit climate factors for temperature, precipitation, and temperature variability using eight IPCC global climate models from the IPCC’s Fifth Assessment Report (i.e., Coupled Model Intercomparison Project Phase 5; CMIP5), assuming a Representative Concentration Pathway of 4.5 (RCP 4.5). See Supporting Methods and Table S3 for the model details. While we are mostly interested in wetland projections for inland and coastal regions of the globe, we kept the open ocean in the scope of the projections to capture changes in decomposition parameters for wetlands of small islands. Each climate model was used to estimate yearly averages for present (January 2018 to December 2021) and future (January 2048 to December 2051) conditions, allowing us to take into consideration seasonal and interannual climate cycles. The best-fitting model was used to generate spatial predictions on decay parameters for 2020 and 2050 based on simulated conditions of rainfall and temperature extracted from each CMIP5.
3. Results
3.1. Total Proportion Mass Remaining Across Ecosystem and OM Types
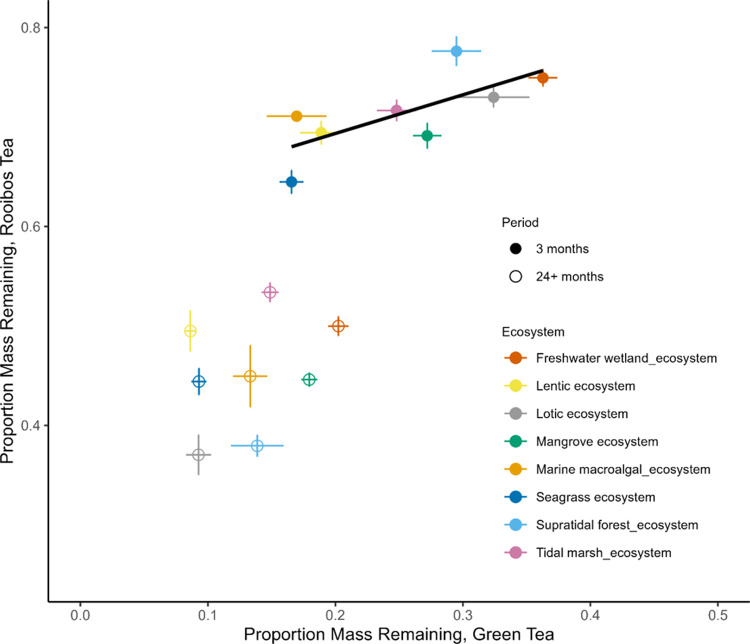
As expected, mass loss differed for labile and recalcitrant OM. The final remaining mass was on average 15 ± 0.6% for labile OM and 47 ± 1% for recalcitrant OM across the sites. The patterns of mass loss among ecosystem types differed between the early (3 months) and late (≥24 months) stages of decay (Figure 2). Lotic and supratidal forest ecosystems had the lowest mass loss, and seagrass ecosystems had the highest mass loss for both OM types after three months of decay (Figure 2 and Table S4). However, as decay progressed, lotic and supratidal forest ecosystems experienced high mass loss for both OM types, resulting in the least mass remaining across the ecosystems (e.g., ∼35 to 40% remaining for recalcitrant OM; Figure 2 and Table S4). By comparison, freshwater wetlands had the highest proportion of mass remaining for labile OM (19–21%), while tidal marsh ecosystems had the highest proportion of mass remaining for recalcitrant OM (∼50 to 55%, empty circles; Figure 2 and Table S4). After 24 months, decomposition dynamics differed between OM types and between early (3 month) and late (≥24 months) stages of decay (Figure 2). Decomposition rates of labile and recalcitrant OM were positively correlated with each other after three months (Pearson’s, p-value = 0.04, R2 = 0.72). This relationship was not significant after 24+ months of decomposition (p-value = 0.4, R2 = 0.32).
Figure 2.
Total proportion of mass remaining of labile (green tea) and recalcitrant (rooibos tea) organic matter (OM) in each ecosystem type at early (3 months) and later (≥24 months) stages of decay. Values are means ± standard errors. Ecosystem-level values for each sampling period can be found in Table S4.
3.2. Effect of Ecosystem Type and Local Climate on Decomposition
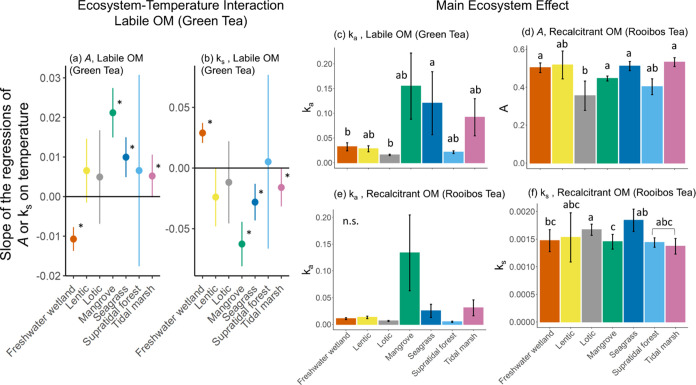
Six independent models for each OM type and decay parameter combination were run for the full-factorial ecosystem-climate models, and after BIC selection, we found that ecosystem and temperature best explained OM decomposition (Table 1 and Figure 3). A statistically significant ecosystem and temperature interaction was found for stable mass remaining (asymptotic A) and overall decay rate constant (ks) for labile OM, but not for the early decay rate constant for labile OM (ka; Table 1). A significant temperature effect was detected for freshwater wetlands, mangroves, seagrass, and tidal marsh ecosystems (see confidence intervals (CIs) not crossing zero in Figure 3a,b). In freshwater wetlands, increasing temperatures caused lower stable mass remaining and higher overall decay constant, while temperature had the opposite effect on these two parameters within mangrove, tidal marsh, and seagrass ecosystems (Figure 3a,b). Here, mangrove ecosystems had 2-fold and 4-fold greater effects of temperature on decomposition relative to seagrass and tidal marsh ecosystems, respectively (Figure 3a,b).
Table 1. Main and Interaction Effects from Linear Models of Decay Parameters and Environmental Variablesa,b.
| labile
OM (green tea) |
recalcitrant
OM (rooibos tea) |
||||||
|---|---|---|---|---|---|---|---|
| model | explanatory variables | A | ka | ks | A | ka | ks |
| ecosystem & climate | ecosystem:precipitation | ||||||
| ecosystem:temperature | 8.49*** | 8.57*** | |||||
| ecosystem:temperature variation | |||||||
| ecosystem | 170.99*** | 319.6*** | 2404.6*** | 273.15*** | 449.65*** | 5444.0*** | |
| precipitation | |||||||
| temperature mean | 18.45*** | 18.34*** | 15.84*** | 37.34*** | |||
| temperature variation | 0.47 | 0.908 | |||||
| days of incubation | 2.64 | 7.21** | 7.10** | 12.48*** | |||
| macroclimate | macroclimate | 6.51*** | 3.21** | 7.30*** | 4.02*** | 1.46 | 13.03*** |
| days of incubation | 3.30* | 2.51 | 3.05* | 5.25** | 8.32*** | 18.0*** | |
| freshwater wetland | IUCN Typologies 2.0 | 4.91*** | 0.48 | 5.06*** | 2.45* | 1.02 | 8.16*** |
| days of incubation | 0.291 | 2.26 | 1.31 | 3.01 | 2.42 | 0.77 | |
Each model is run independently for the six OM type and decay parameter combinations. For the ecosystem and climate model, the starting model included all eight variables. We used the Bayesian information criterion (BIC) to identify the best-fitting model, for which we report F-values and significance for each variable. Missing F-values in the table indicate variables selected against by BIC model selection. The macroclimate categories are presented in Figure 1. The freshwater wetland model uses the IUCN Typologies 2.0 that incorporate climate, inundation, and vegetation characteristics. Temperature variation represents the mean monthly variation during the timeframe of incubation. Two decay equations were applied to site-level time series to provide information on different stages of decay. An asymptotic exponential model (eq 1) provides A (proportion of stable mass remaining) and ka (rate constant of early decay, i.e., leaching). A single exponential decay model (eq 2) provides ks values that represent the overall decay rate constant. Values represent F-values, and significant p-values are in bold. Precipitation is in mm units.
p-values: *** < 0.001, ** 0.001 ≤ to ≤ 0.01, * 0.01 < to ≤ 0.05.
Figure 3.
Ecosystem effects on the decay parameters. Each panel is the result of an independent modelrun on each OM type and decay parameter combination (Table 1). (a, b) Strength of interactions between ecosystem type and temperature for the labile organic matter (OM) type. Values represent the slope of the relationship between decomposition parameter and temperature ± the 95% confidence intervals (CIs). Asterisks indicate significant temperature effect on ecosystems (p < 0.05, CIs do not cross zero). (c–f) Significant main effects of the ecosystem type. Different letters indicate significant differences among the means according to Tukey’s posthoc comparisons (n.s. = not significant, p > 0.05). Decay parameter definitions: asymptotic A represents the proportion of stable mass remaining (eq 1), ka represents the rate constant of early decay, i.e., leaching (eq 1), and single exponential ks represents the overall decay rate constant (eq 2). Data represent means ± standard errors.
The main effects of the ecosystem type were found for early decay rate constants (ka) of the labile OM, as well as all decay parameters for the recalcitrant OM (Figure 3c–f). For labile OM, early decay rates were significantly higher in seagrass ecosystems than in lotic and freshwater wetland ecosystems (Figure 3c). For recalcitrant OM, the remaining stable mass (A) was significantly lower in lotic ecosystems (Figure 3d). The overall decay rate of recalcitrant OM was lower in freshwater wetlands and mangroves than in seagrass and lotic ecosystems (Figure 3f). Tukey’s posthoc analyses for early decay rate constants of recalcitrant OM showed only a nonsignificant effect between lotic and mangrove ecosystems (p = 0.09), likely due to the conservative nature of the posthoc test and the high variation in mangrove ecosystems (Figure 3e).
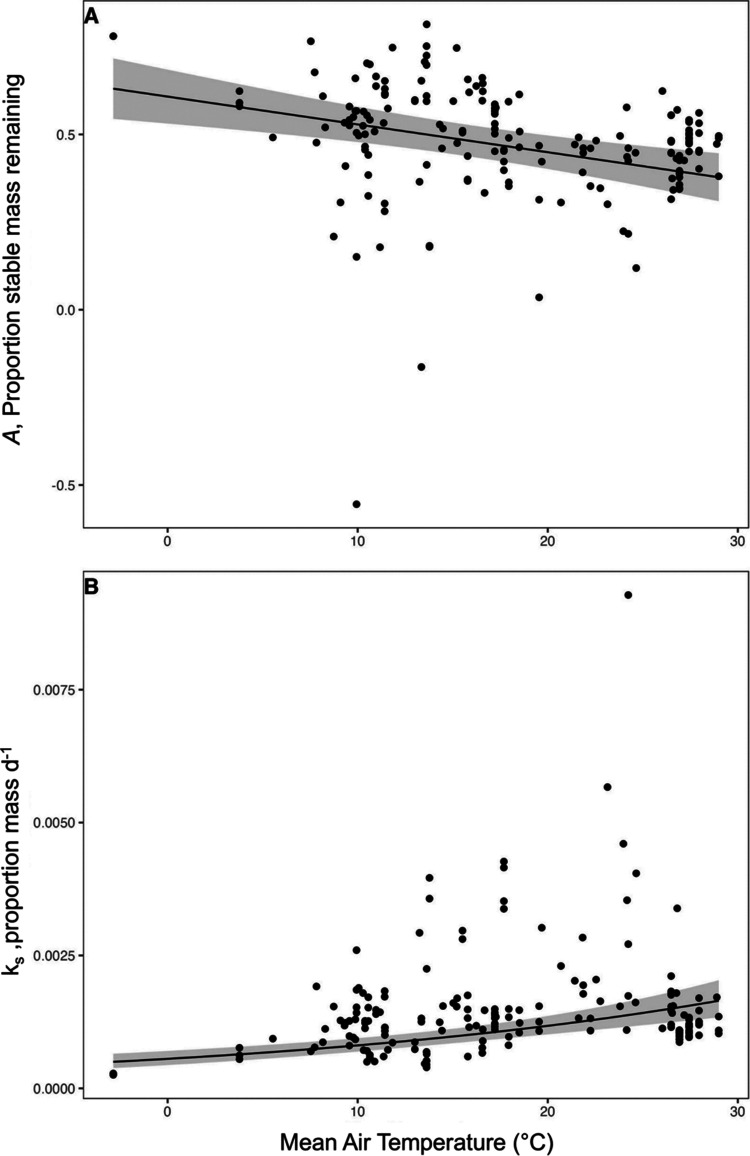
Increasing temperatures were found to enhance decomposition for recalcitrant OM (main temperature effect; Table 1). Higher temperatures reduced the stable mass remaining (A) and increased the overall decay constant (ks) for recalcitrant OM (Figure 4) but did not significantly affect the early decay constant (ka). The temperature effect was linear for A and indicated a 4% decrease in stable mass remaining for every 5 °C increase (e.g., from 52.8% at 10 °C to 44.9% at 20 °C; Figure 4A). The temperature effect on the overall decay constant (ks) of recalcitrant OM was nonlinear. An increase from 10 to 20 °C corresponded to a 1.46-fold increase in ks (from 0.00081 d–1 at 10 °C to 0.00118 d–1 at 20 °C; Figure 4B).
Figure 4.
Temperature main effects on recalcitrant OM (rooibos tea) decay parameters. Decay parameter asymptotic A represents the proportion of stable mass remaining (eq 1) (A) and single exponential ks represents the overall decay rate constant (eq 2) (B; d–1). Raw data are plotted with a regression line with 95% confidence intervals.
3.3. Effect of Macroclimate on Decomposition
Macroclimate significantly influenced decay parameters of both OM (tea) types, except for the early decay rate constant (ka) for recalcitrant OM (Table 1). Arctic and boreal macroclimates generally had a higher proportion of stable mass remaining (A) and slower overall decay rate constant (ks) than the other macroclimates (Figure S1a). However, arctic and boreal macroclimates were represented by only three sites, limiting our ability to make robust interpretations of the impacts of these cold macroclimates on wetland OM decay. Therefore, we focus on posthoc comparisons across the remaining six macroclimates.
The decay parameters of the two OM types generally showed that the temperate climate experienced a higher stable mass remaining (A) and slower overall decay rate constant (ks) compared to warmer macroclimates. For labile OM, the lowest stable mass remaining values were from warm temperate and semiarid tropical climates (means 15 and 9%, respectively; Figure S1a), while the higher stable mass values (mean 21%) and slower overall decay constant (mean 0.0088 d–1) were from equatorial humid climates (Figure S1a,c). For recalcitrant OM, the lowest stable mass remaining values were from warm temperate, subtropical arid, equatorial humid, and semiarid tropical climates (means 36–45%; Figure S1d). Overall decay constants of the recalcitrant OM were 2 to 3.5-fold higher (p > 0.05) in the semiarid tropical climate (mean 0.0038 d–1) compared to temperate, warm-temperate, Mediterranean, and equatorial humid climates (means 0.0011–0.0019 d–1; Figure S1e).
3.4. Effects of Freshwater Wetland IUCN Typologies on Decomposition
We used the palustrine wetland IUCN typology groupings to identify any effect of the ecosystem type on tea litter decay within the broad freshwater wetland category. The stable mass remaining of labile OM in tundras was more than 2-fold higher than that of most typologies, with the exception of boreal and temperate fens (Figure S2a). This highly stable mass remaining for the tundra coincided with a significantly slower overall decay rate constant (Figure S2e), but there was no significant difference in early decay rate constant (ka) for the labile OM across typologies (Figure S2c). For the recalcitrant OM, permanent marshes had 4- to 24-fold and significantly higher overall decay rate constants than those of the other ecosystem types (Figure S2f). For recalcitrant OM, no significant differences across freshwater wetland typologies were found for early decay constants or the stable mass remaining (Figure S2b,d).
3.5. Projections of OM Decay Parameters
In our ecosystem-independent climate predictions, the stable mass remaining of labile OM decreased with increasing precipitation and temperature variation, while early and overall decay constants increased with temperature variation (Table S5). In contrast for recalcitrant OM, stable mass remaining decreased with increasing mean temperature and precipitation. Early decay rate constants for recalcitrant OM increased with increasing temperature variability, and overall decay rate constants were found to increase with increases in all three climatic variables (Table S5). These climatic variables were used to predict changes in each decay parameter between 2020 and 2050 by using eight CMIP5 climate models (Figures 5 and S4). The uncertainties of the predicted percent change were relatively low throughout for both OM (tea) types (Figure S4).
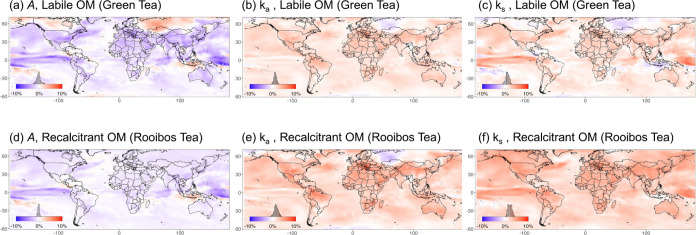
Figure 5.
Percentage change of decay parameters across the globe from 2020 to 2050. Colors in the maps indicate the expected relative change in values from 2020 to 2050, with red indicating decreases, blue increases, and white no change. Model projections include open oceans to capture inland and coastal wetland habitats on the islands. The histograms show the distributions of the values in the map. The models were based on IPCC climate models (Coupled Model Intercomparison Project Phase 5, CMIP5) with an assumed representative concentration pathway (RCP) of 4.5. Decay parameter asymptotic A (a, d) represents the proportion of stable mass remaining (eq 1), ka (b, e) represents the early decay rate constants (d–1; eq 1), and single exponential ks (c, f) represents the overall decay rate constants (d–1; eq 2).
Overall for labile OM, the stable mass remaining (A) was predicted to decrease by 1.7% from 2020 to 2050, while early (ka) and overall (ks) decay rate constants were predicted to increase by 1.7 and 1.8%, respectively (Figure S4). Across the globe, the range of change was highest for labile OM stable mass remaining A, varying from approximately 4% decreases to 3% increases with extremes to ±10% (Figure 5a). Similarly, the overall decay constant, ks, for labile OM was also predicted to vary, with overall changes ranging from 2% decrease to 4% increase (Figure 5c). In contrast, early decay for labile OM had generally predicted increases of <5% globally (Figure 5b). Specifically, for labile OM, hotspots for enhanced decomposition (i.e., decreased A and increased ka and ks) included parts of North America (particularly south/southeast), eastern Europe, and northern South America (Figure 5a–c). In contrast, parts of SE Asia and arctic regions were predicted to have an increased stable mass remaining and a decreased overall decay rate constant.
For recalcitrant OM, future climatic conditions were predicted to generally increase decomposition globally. The proportion of stable mass remaining (A) was predicted to decrease by 1%, with 2.5 and 3.1% increases in early (ka) and overall (ks) decay rate constants, respectively (Figure S4). For A, most of the change was predicted to occur in the range of ∼0 to −2% (Figure 5d), while early and overall decay rate constants were predicted to have greater relative increases than labile OM, in some cases >4% (Figure 5e,f). Recalcitrant OM had hotspots of enhanced decomposition similar to those of labile OM, although with greater predicted increases in both decay constant parameters (Figure 5d–f). Small pockets of reduced decomposition were predicted for SE Asia and Central America (Figure 5d–f).
4. Discussion
4.1. Impacts of Climate and Ecosystem on Long-Term OM Decomposition
Our global long-term study comprising over 180 wetland sites showed that temperature was a key factor in belowground litter decomposition in wetlands. The long-term decomposition of recalcitrant OM (rooibos tea) was enhanced by higher mean annual temperatures and within warmer macroclimates independent of ecosystem type, similar to previous tea litter studies (∼12-month incubation).43,46 This temperature effect was found for late-stage stable mass remaining and overall decay parameters (A, ks), but not for early decay rate constant ka that are often associated with the passive leaching of OM (Table 1 and Figure 4). A similar persistent temperature effect has been shown for aboveground litter and belowground rhizome/root decay in coastal wetlands across similar temperature ranges as in this study.10,25 Increased aboveground decay rates in coastal wetlands were found in warmer climates (over ∼25 °C),10 although the positive effect of warmer temperature on recalcitrant OM decay found here (Figure 4) was not as pronounced in comparison. A possible explanation is that the belowground environment considered in this study comprises a different set of external factors that influence decomposition compared to above-soil conditions (e.g., slower oxygen diffusion, presence of microbial activity and root exudates, porewater salinity, porewater pumping),10,13,25 thereby causing a slightly different realized temperature response.
Ecosystem-dependent effects of temperature were found for labile OM; here, decay was enhanced with increasing temperatures in freshwater wetlands, while tidal wetlands showed the opposite trend. Some ecosystems (e.g., seagrass meadows) had as low as 5% mass remaining (Table S4), losing much of its nonhydrolyzable fraction (fresh green tea litter has ∼16% lignin and ash).39 Perhaps, these labile OM residues are less susceptible to temperature effects on further decomposition compared to ecosystems that have relatively more OM remaining (e.g., freshwater wetlands), due to lack of accessible OM, insensitivity of microbes to temperature differences, or physicochemical conditions limiting microbial attack within tidal wetland soils.3,16,56,57 As such, the remaining highly decomposed OM from green tea litter in this study may be less impacted by parameters that typically enhance microbial metabolism (e.g., temperature, moisture, solubility of nutrients). Labile OM, such as that represented by green tea, may not make substantial contributions to long-term carbon stocks in wetland soils, but certain hydrologic conditions may promote the preservation or stabilization of the remaining OM. We could not resolve if longer incubations beyond 3 years of the recalcitrant rooibos tea litter would also show an inundation-inhibited temperature effect observed for labile OM.
Ecosystem-type differences across decomposition parameters indicate that the driving forces of ecosystem characteristics on decay, such as moisture and inundation, change throughout the decomposition process. For both OM types, early decay rate constant (ka) and mass loss at three months were generally higher for tidally influenced ecosystem types (Figure 3). This is consistent with soil moisture and inundation enhancing abiotic, leaching-driven mass loss of tea litter in short-term cross- and within-ecosystem studies for aquatic and wetland ecosystems.42,43,58 In contrast, the ecosystem differences were variable across the parameters that represented longer-term decay (asymptotic A, overall decay constant ks, and mass loss at 24+ months; Figures 2 and 3). Freshwater wetlands had double the proportion of mass remaining for labile OM compared to lentic, lotic, and seagrass ecosystems. Such ecosystem differences were less pronounced for recalcitrant OM, but tidal marshes and freshwater wetlands had 3–10% more mass remaining compared to the other ecosystem types at the end of the incubation (Figure 3 and Table S4). Additionally, within the freshwater wetland typologies, permanently inundated marshes had enhanced decomposition compared to that in other freshwater wetland typologies (Figure S2). We suspect that inundation or flow rates are influencing a range of conditions that moderate biotic microbial processes at multiple scales that can increase or decrease recalcitrant OM breakdown, including soil temperature, nutrient availability, salinity, and oxygen.59−63
4.2. OM Decomposition under Future Climate Scenarios
Our model based on RCP 4.5 predicts that decomposition will increase under future climate scenarios for both labile and recalcitrant OM. Labile OM (e.g., green tea litter) will have a higher percent change for loss of stable OM (parameter A) under future conditions compared to recalcitrant OM (e.g., rooibos tea litter). However, since the labile OM type is relatively low in mass and largely represented by OM residues at this stage of decay, the net change in labile OM will likely be small. By comparison, decomposition of recalcitrant OM occurs over a longer period and will likely be more susceptible to increasing temperatures in global wetlands, which is important for future predictions of soil carbon stocks and flux (i.e., blue and teal carbon). Overall, our results for recalcitrant OM are consistent with the trends of climate change-induced warming increasing plant decomposition in terrestrial (14–27% by 2070s)64 and coastal ecosystems (2–3-fold increase in carbon loss between 2050 and 2100).10 Ouyang and colleagues10 also found that the potential for plant biomass production of carbon in the future to out-pace carbon remineralization (i.e., net carbon gains), in addition to ecosystem-specific differences for predicted enhancement of decomposition (mangrove > tidal marsh > seagrass). Warming can also impact wetland plant diversity and composition,65 which could in turn influence the production of recalcitrant OM, e.g., lignocellulose, in a wetland.66 While ecosystem type was not included in the model in our study, studies on natural litter and the ecosystem-climate model responses of the different OM types from this study using standardized litter highlight the importance of resolving ecosystem-specific climate responses of standardized OM, to better predict the effects of climate change on wetlands at regional or global scales.
There are uncertainties and gaps in our approach and data set that can be addressed in future studies to increase the breadth and scope of long-term tea decay datasets for improved prediction and interpretation, including a greater sampling of poorly studied ecosystems (macroalgal, brackish ecosystem) and macroclimate types (Arctic, boreal, and arid temperate). There are uncertainties associated with modeling based on future air temperatures instead of future soil temperatures, which may impact the variability of temperature change the litter would experience.67 Additionally, our study was designed to explore the global-scale drivers of decay. Measuring additional environmental metrics at the site level, such as salinity, inundation/flooding period and groundwater metrics, vegetation, nutrient concentrations, and soil mineral composition, will provide further insight into local-scale drivers of belowground litter decay in wetlands in the context of regional- and global-scale drivers (e.g., micro- vs macroclimate controls),25,68,69 as well as vulnerability to droughts and sea-level rise.70−72
4.3. Environmental Implications
This study provides the first empirical comparison of multiyear wetland decomposition using standardized OM, providing new insight on the benefits and limitations of using tea litter to resolve longer-term controls on belowground litter turnover. By deriving multiple decay parameters that describe different stages of decomposition, we show that few climatic- or ecosystem-type variables influenced early leaching-dominated decay (ka) for either OM (tea) type. We also observed relative changes in mass loss among ecosystem types between 3 and ≥24 months time points (Figure 2). These results highlight that short-term decomposition incubations capture less of the processes occurring during OM turnover and are likely not suitable for making inferences about soil carbon preservation and long-term storage (blue carbon and teal carbon). Rather, the stable portion of mass (A) and overall decay rate constant (ks) derived from multiyear time series datasets reveal that increased temperatures and ecosystem traits, aggregated at a global scale, can significantly increase belowground litter remineralization.
Additionally, our study reveals a significant positive relationship between modeled stable mass remaining (A, eq 1) and raw final proportion mass remaining values at the end of the incubation for both OM types, as well as a consistent inverse relationship between A and ks across all our statistical models (Figure S5). Only in a few sites was the asymptote not reached, resulting in negative A values (Figure S5). While model-fitting of time-series datasets is recommended for short-term 3 months incubations,73 these correlative relationships in this study suggest that the final mass remaining data from longer-term incubations (e.g., >1 year) are representative of wetland long-term decomposition processes. As longer-term tea litter studies become more common,44,74 these findings highlight how the final mass remaining data of standardized OM may be useful as a simple metric for long-term ecosystem decomposition modeling without the need for gathering time- and cost-intensive time-series datasets.
We propose that standardized tea litter decay data could contribute to addressing the limited long-term decay datasets for wetlands, which represents a current barrier in predicting wetland carbon-cycling at the scale and biogeographic levels available for terrestrial ecosystems. Multiyear and cross-ecosystem decay time series data (e.g., LIDET) and modeling frameworks, such as WARMER-2, have an immense value for evaluating the drivers of decomposition from local and regional to global scales now and in the future.28,29,37,75 Since we have shown that the final mass remaining can be used as a predictor of longer-term decay rates, standardized litter datasets can contribute to existing efforts to fill this gap in wetland ecosystems, including their value as translatable datasets across existing networks and databases.76 A key limitation of tea litter studies is their limited chemical diversity compared to natural litter, and this may limit our interpretation of OM turnover.77 Transplant and long-term studies that directly compare local wetland OM and tea litters78 would help reveal if any and what uncertainties exist and the ecosystems where tea litters are less representative in characterizing natural OM turnover (e.g., homefield advantage),77,79 as well as allowing for the quantification of such uncertainties in predictive analyses. Future studies measuring carbon fluxes (e.g., respiration, loss of carbon with decay) to estimate potential changes in greenhouse case emissions, as well as microbial community and function, can complement standardized decay metrics to predict net change in carbon and parametrize system models under future climate change.10,32,34,80 Together, addressing these unknowns concerning OM preservation in wetlands, using multiple approaches including both standardized and natural litters, will advance our knowledge on potential threats to soil carbon sequestration capacity of wetlands now and in the future.
Acknowledgments
Thank you Nidhi Yogesh for your assistance with creating the graphical abstract. We also would like to acknowledge collaborators, volunteers, and assistants who have contributed to the field and lab work, including R. Gregg, B. Fuentealba, J. Petch, S.K. Thamilselva, P. Gondola, C. George, S. Naham, A. Driskill, J. M. Booth, R. Marasco, L. Yang, M. Reiss, B. Proemse, R. Hughes, A. Murphy, M. Farina, L. Bohlman, L.J. Simmons, A. Bruder, M. Alsafran, C. Freitas, A. Silva, E.S. Dias, C.B. Iwuji, B. Greenfield, J. Bailey, V. Bennion, N. Sanmartí Boixeda, Y. Ontoria, L.H. PerezBernal, M.J. Simon, and T. Hernandez.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.est.4c02116.
Detailed methodology; descriptions of sites and models used in the study; decay parameters from different incubation periods, local climate conditions, macroclimates, and freshwater wetland IUCN typologies; predicted change in litter decay and coefficient of variables for predicted change between 2020 and 2025; and regressions between decay parameters (PDF)
Author Contributions
S.M.T.-T.: Conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, project administration, visualization, and writing—original draft preparation; S.K.-R.: Conceptualization, formal analysis, methodology, visualization, and writing—review and editing; M.M.: Formal analysis, methodology, visualization, and writing—review and editing; P.I.M.: Conceptualization, funding acquisition, and writing—review and editing; I.D.: Conceptualization, methodology, resources, and writing—review and editing; and co-authors of TeaComposition H2O (J.Z. to F.A.): Investigation and writing—review and editing.
This study and S.M.T-T. were supported by an ARC DECRA (DE210101029) and an ADPRF Fellowship. Tea litter was provided in-kind by Unilever via the TeaComposition initiative. P.I.M. was supported by an ARC Discovery Project (DP200100575). Foundation for Science and Technology (Portugal) supported projects for C.B.d.l.S. and R.S. (UIDB/04326/2020 (DOI:10.54499/UIDB/04326/2020), UIDP/04326/2020 (DOI:10.54499/UIDP/04326/2020), and LA/P/0101/2020 (DOI:10.54499/LA/P/0101/2020)), A.I.S., A.I.L., and D.J. (UIDB/50017/2020 + UIDP/50017/2020 + LA/P/0094/2020 to CESAM) and A.I.S. (DOI: 10.54499/CEECIND/00962/2017/CP1459/CT0008). E.G. (Spain) was supported by eLTSER Research Infrastructure H2020, ref 999452014-EAA, CEE 2015–2019. T.J.M. was supported by NSF-DEB-2051602. D.D. (Kingdom of Saudi Arabia) was supported by KAUST baseline funding.
The authors declare no competing financial interest.
Notes
† R. Payne co-authorship is posthumous (June 2019).
Supplementary Material
References
- Matthews G. V. T.(Ramsar Convention Bureau Gland).
- Brinson M. M.; Lugo A. E.; Brown S. Primary productivity, decomposition and consumer activity in freshwater wetlands. Annu. Rev. Ecol. Syst. 1981, 12, 123–161. 10.1146/annurev.es.12.110181.001011. [DOI] [Google Scholar]
- Spivak A. C.; Sanderman J.; Bowen J. L.; Canuel E. A.; Hopkinson C. S. Global-change controls on soil-carbon accumulation and loss in coastal vegetated ecosystems. Nat. Geosci. 2019, 12, 685–692. 10.1038/s41561-019-0435-2. [DOI] [Google Scholar]
- Serrano O.; Lovelock C. E.; Atwood T. B.; Macreadie P. I.; Canto R.; Phinn S.; Arias-Ortiz A.; Bai L.; Baldock J.; Bedulli C.; Carnell P.; Connolly R. M.; Donaldson P.; Esteban A.; Ewers Lewis C. J.; Eyre B. D.; Hayes M. A.; Horwitz P.; Hutley L. B.; Kavazos C. R. J.; Kelleway J. J.; Kendrick G. A.; Kilminster K.; Lafratta A.; Lee S.; Lavery P. S.; Maher D. T.; Marbà N.; Masque P.; Mateo M. A.; Mount R.; Ralph P. J.; Roelfsema C.; Rozaimi M.; Ruhon R.; Salinas C.; Samper-Villarreal J.; Sanderman J.; Sanders C. J.; Santos I.; Sharples C.; Steven A. D. L.; Cannard T.; Trevathan-Tackett S. M.; Duarte C. M. Australian vegetated coastal ecosystems as global hotspots for climate change mitigation. Nat. Commun. 2019, 10, 4313 10.1038/s41467-019-12176-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulter B.; Fluet-Chouinard E.; Hugelius G.; Koven C.; Fatoyinbo L.; Page S. E.; Rosentreter J. A.; Smart L. S.; Taillie P. J.; Thomas N.. et al. A Review of Global Wetland Carbon Stocks and Management Challenges. In Wetland Carbon and Environmental Management; Krauss K. W.; Zhu Z.; Stagg C. L., Eds.; American Geophysical Union (AGU), 2021; pp 1–20. [Google Scholar]
- Sorensen K. W. Indonesian peat swamp forests and their role as a carbon sink. Chemosphere 1993, 27, 1065–1082. 10.1016/0045-6535(93)90068-G. [DOI] [Google Scholar]
- Laegdsgaard P. Ecology, disturbance and restoration of coastal saltmarsh in Australia: a review. Wetlands Ecol. Manage. 2006, 14, 379–399. 10.1007/s11273-005-8827-z. [DOI] [Google Scholar]
- Prescott C. E.; Vesterdal L. Decomposition and transformations along the continuum from litter to soil organic matter in forest soils. For. Ecol. Manage. 2021, 498, 119522 10.1016/j.foreco.2021.119522. [DOI] [Google Scholar]
- Page S. E.; Baird A. Peatlands and global change: response and resilience. Annu. Rev. Environ. Resour. 2016, 41, 35–57. 10.1146/annurev-environ-110615-085520. [DOI] [Google Scholar]
- Ouyang X.; Kristensen E.; Zimmer M.; Thornber C.; Yang Z.; Lee S. Y. Response of macrophyte litter decomposition in global blue carbon ecosystems to climate change. Global Change Biol. 2023, 29, 3806–3820. 10.1111/gcb.16693. [DOI] [PubMed] [Google Scholar]
- Leng L. Y.; Ahmed O. H.; Jalloh M. B. Brief review on climate change and tropical peatlands. Geosci. Front. 2019, 10, 373–380. 10.1016/j.gsf.2017.12.018. [DOI] [Google Scholar]
- Kayranli B.; Scholz M.; Mustafa A.; Hedmark Å. Carbon storage and fluxes within freshwater wetlands: a critical review. Wetlands 2010, 30, 111–124. 10.1007/s13157-009-0003-4. [DOI] [Google Scholar]
- Yarwood S. A. The role of wetland microorganisms in plant-litter decomposition and soil organic matter formation: a critical review. FEMS Microbiol. Ecol. 2018, 94, fiy175 10.1093/femsec/fiy175. [DOI] [PubMed] [Google Scholar]
- Kirwan M. L.; Langley J.; Guntenspergen G. R.; Megonigal J. The impact of sea-level rise on organic matter decay rates in Chesapeake Bay brackish tidal marshes. Biogeosciences 2013, 10, 1869–1876. 10.5194/bg-10-1869-2013. [DOI] [Google Scholar]
- Holgerson M. A.; Raymond P. A. Large contribution to inland water CO2 and CH4 emissions from very small ponds. Nat. Geosci. 2016, 9, 222–226. 10.1038/ngeo2654. [DOI] [Google Scholar]
- Trevathan-Tackett S. M.; Seymour J.; Nielsen D.; Macreadie P. I.; Jeffries T. C.; Sanderman J.; Baldock J.; Howes J.; Steven A. D. L.; Ralph P. Sediment anoxia limits microbial-driven seagrass carbon remineralization under warming conditions. FEMS Microbiol. Ecol. 2017, 93, fix033 10.1093/femsec/fix033. [DOI] [PubMed] [Google Scholar]
- Trevathan-Tackett S. M.; Kelleway J. J.; Macreadie P. I.; Beardall J.; Ralph P.; Bellgrove A. Comparison of marine macrophytes for their contributions to blue carbon sequestration. Ecology 2015, 96, 3043–3057. 10.1890/15-0149.1. [DOI] [PubMed] [Google Scholar]
- Enríquez S.; Duarte C. M.; Sand-Jensen K. Patterns in decomposition rates among photosynthetic organisms: the importance of detritus C:N:P content. Oecologia 1993, 94, 457–471. 10.1007/BF00566960. [DOI] [PubMed] [Google Scholar]
- Rejmánková E.; Houdková K. Wetland plant decomposition under different nutrient conditions: what is more important, litter quality or site quality?. Biogeochemistry 2006, 80, 245–262. 10.1007/s10533-006-9021-y. [DOI] [Google Scholar]
- Stagg C. L.; Schoolmaster D. R.; Krauss K. W.; Cormier N.; Conner W. H. Causal mechanisms of soil organic matter decomposition: deconstructing salinity and flooding impacts in coastal wetlands. Ecology 2017, 98, 2003–2018. 10.1002/ecy.1890. [DOI] [PubMed] [Google Scholar]
- LeRoy C. J.; Hipp A. L.; Lueders K.; Follstad Shah J. J.; Kominoski J. S.; Ardón M.; Dodds W. K.; Gessner M. O.; Griffiths N. A.; Lecerf A.; et al. Plant phylogenetic history explains in-stream decomposition at a global scale. J. Ecol. 2020, 108, 17–35. 10.1111/1365-2745.13262. [DOI] [Google Scholar]
- Yin M.; Liu L.; Wu Y.; Sheng W.; Ma X.; Du N.; Zhu P.; Wang C.; Cui Z.; Brix H.; et al. Effects of litter species and genetic diversity on plant litter decomposition in coastal wetland. Ecol. Indic. 2022, 144, 109439 10.1016/j.ecolind.2022.109439. [DOI] [Google Scholar]
- Trevathan-Tackett S. M.; Jeffries T. C.; Macreadie P. I.; Manojlovic B.; Ralph P. Long-term decomposition captures key steps in microbial breakdown of seagrass litter. Sci. Total Environ. 2020, 705, 135806 10.1016/j.scitotenv.2019.135806. [DOI] [PubMed] [Google Scholar]
- Simpson L. T.; Chapman S. K.; Simpson L. M.; Cherry J. A. Do global change variables alter mangrove decomposition? A systematic review. Glob. Ecol. Biogeogr. 2023, 32, 1874–1892. 10.1111/geb.13743. [DOI] [Google Scholar]
- Ouyang X.; Lee S. Y.; Connolly R. M. The role of root decomposition in global mangrove and saltmarsh carbon budgets. Earth-Sci. Rev. 2017, 166, 53–63. 10.1016/j.earscirev.2017.01.004. [DOI] [Google Scholar]
- Cebrián J. Patterns in the fate of production in plant communities. Am. Nat. 1999, 154, 449–468. 10.1086/303244. [DOI] [PubMed] [Google Scholar]
- Stoler A. B.; Relyea R. A. Reviewing the role of plant litter inputs to forested wetland ecosystems: leafing through the literature. Ecol. Monogr. 2020, 90, e01400 10.1002/ecm.1400. [DOI] [Google Scholar]
- Adair E. C.; Parton W. J.; Del Grosso S. J.; Silver W. L.; Harmon M. E.; Hall S. A.; Burke I. C.; Hart S. C. Simple three-pool model accurately describes patterns of long-term litter decomposition in diverse climates. Global Change Biol. 2008, 14, 2636–2660. 10.1111/j.1365-2486.2008.01674.x. [DOI] [Google Scholar]
- Buffington K. J.; Janousek C. N.; Dugger B. D.; Callaway J. C.; Schile-Beers L. M.; Borgnis Sloane E.; Thorne K. M. Incorporation of uncertainty to improve projections of tidal wetland elevation and carbon accumulation with sea-level rise. PLoS One 2021, 16, e0256707 10.1371/journal.pone.0256707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne K. M.; Buffington K. J.; Jones S. F.; Largier J. L. Wetlands in intermittently closed estuaries can build elevations to keep pace with sea-level rise. Estuarine, Coastal Shelf Sci. 2021, 257, 107386 10.1016/j.ecss.2021.107386. [DOI] [Google Scholar]
- Bradford M. A.; Veen G. C.; Bonis A.; Bradford E. M.; Classen A. T.; Cornelissen J. H. C.; Crowther T. W.; Jonathan R.; Freschet G. T.; Kardol P.; et al. A test of the hierarchical model of litter decomposition. Nat. Ecol. Evol. 2017, 1, 1836–1845. 10.1038/s41559-017-0367-4. [DOI] [PubMed] [Google Scholar]
- Wieder W. R.; Bonan G. B.; Allison S. D. Global soil carbon projections are improved by modelling microbial processes. Nat. Clim. Change 2013, 3, 909–912. 10.1038/nclimate1951. [DOI] [Google Scholar]
- Bonan G. B.; Hartman M. D.; Parton W. J.; Wieder W. R. Evaluating litter decomposition in earth system models with long-term litterbag experiments: an example using the Community Land Model version 4 (CLM 4). Global Change Biol. 2013, 19, 957–974. 10.1111/gcb.12031. [DOI] [PubMed] [Google Scholar]
- Luo Y.; Ahlström A.; Allison S. D.; Batjes N. H.; Brovkin V.; Carvalhais N.; Chappell A.; Ciais P.; Davidson E. A.; Finzi A.; et al. Toward more realistic projections of soil carbon dynamics by Earth system models. Global Biogeochem. Cycles 2016, 30, 40–56. 10.1002/2015GB005239. [DOI] [Google Scholar]
- Malerba M. E.; de Paula Costa M. D.; Friess D. A.; Schuster L.; Young M. A.; Lagomasino D.; Serrano O.; Hickey S. M.; York P. H.; Rasheed M.; et al. Remote sensing for cost-effective blue carbon accounting. Earth-Sci. Rev. 2023, 238, 104337 10.1016/j.earscirev.2023.104337. [DOI] [Google Scholar]
- Freschet G. T.; Aerts R.; Cornelissen J. H. Multiple mechanisms for trait effects on litter decomposition: moving beyond home-field advantage with a new hypothesis. J. Ecol. 2012, 100, 619–630. 10.1111/j.1365-2745.2011.01943.x. [DOI] [Google Scholar]
- Currie W. S.; Harmon M. E.; Burke I. C.; Hart S. C.; Parton W. J.; Silver W. Cross-biome transplants of plant litter show decomposition models extend to a broader climatic range but lose predictability at the decadal time scale. Global Change Biol. 2010, 16, 1744–1761. 10.1111/j.1365-2486.2009.02086.x. [DOI] [Google Scholar]
- Latter P.; Walton D.. The Cotton Strip Assay for Cellulose Decomposition Studies in Soil: History of the Assay and Development. In Cotton Strip Assay: An Index of Decomposition in Soils; NERC/ITE Symposium No. 24; Harrison A. F.; Latter P. M.; Walton D. W. H., Eds.; NERC/ITE: Grange-over-Sands, 1988; pp 7–10. [Google Scholar]
- Keuskamp J. A.; Dingemans B. J.; Lehtinen T.; Sarneel J. M.; Hefting M. M. Tea Bag Index: a novel approach to collect uniform decomposition data across ecosystems. Methods Ecol. Evol. 2013, 4, 1070–1075. 10.1111/2041-210X.12097. [DOI] [Google Scholar]
- Duddigan S.; Shaw L. J.; Alexander P. D.; Collins C. D. Chemical Underpinning of the Tea Bag Index: An Examination of the Decomposition of Tea Leaves. Appl. Environ. Soil Sci. 2020, 2020 (1), 6085180 10.1155/2020/6085180. [DOI] [Google Scholar]
- Mueller P.; Schile-Beers L. M.; Mozdzer T. J.; Chmura G. L.; Dinter T.; Kuzyakov Y.; Groot A. V. d.; Esselink P.; Smit C.; D’Alpaos A.; Ibanez C.; Lazarus M.; Neumeier U.; Johnson B. J.; Baldwin A. H.; Yarwood S. A.; Montemayor D. I.; Yang Z.; Wu J.; Jensen K.; Nolte S. Global-change effects on early-stage decomposition processes in tidal wetlands–implications from a global survey using standardized litter. Biogeosciences 2018, 15, 3189–3202. 10.5194/bg-15-3189-2018. [DOI] [Google Scholar]
- Petraglia A.; Cacciatori C.; Chelli S.; Fenu G.; Calderisi G.; Gargano D.; Abeli T.; Orsenigo S.; Carbognani M. Litter decomposition: effects of temperature driven by soil moisture and vegetation type. Plant Soil 2019, 435, 187–200. 10.1007/s11104-018-3889-x. [DOI] [Google Scholar]
- Trevathan-Tackett S. M.; Kepfer-Rojas S.; Engelen A. H.; York P. H.; Ola A.; Li J.; Kelleway J. J.; Jinks K. I.; Jackson E. L.; Adame M. F.; Pendall E.; Lovelock C. E.; Connolly R. M.; Watson A.; Visby I.; Trethowan A.; Taylor B.; Roberts T. N. B.; Petch J.; Farrington L.; Djukic I.; Macreadie P. I. Ecosystem type drives tea litter decomposition and associated prokaryotic microbiome communities in freshwater and coastal wetlands at a continental scale. Sci. Total Environ. 2021, 782, 146819 10.1016/j.scitotenv.2021.146819. [DOI] [PubMed] [Google Scholar]
- Marley A. R.; Smeaton C.; Austin W. E. An assessment of the tea bag index method as a proxy for organic matter decomposition in intertidal environments. J. Geophys. Res.: Biogeosci. 2019, 124, 2991–3004. 10.1029/2018JG004957. [DOI] [Google Scholar]
- Gill A. L.; Adler P. B.; Borer E. T.; Buyarski C. R.; Cleland E. E.; D’Antonio C. M.; Davies K. F.; Gruner D. S.; Harpole W. S.; Hofmockel K. S.; et al. Nitrogen increases early-stage and slows late-stage decomposition across diverse grasslands. J. Ecol. 2022, 110, 1376–1389. 10.1111/1365-2745.13878. [DOI] [Google Scholar]
- Kwon T.; Shibata H.; Kepfer-Rojas S.; Schmidt I. K.; Larsen K. S.; Beier C.; Berg B.; Verheyen K.; Lamarque J.-F.; Hagedorn F.; Nico E.; Ika D.; Network T. Effects of climate and atmospheric nitrogen deposition on early to mid-term stage litter decomposition across biomes. Front. For. Glob. Change 2021, 4, 678480 10.3389/ffgc.2021.678480. [DOI] [Google Scholar]
- Lehmann J.; Hansel C.; Kaiser C.; Kleber M.; Maher K.; Manzoni S.; Nunan N.; Reichstein M.; Schimel J.; Torn M.; et al. Persistence of soil organic carbon caused by functional complexity. Nat. Geosci. 2020, 13, 529–534. 10.1038/s41561-020-0612-3. [DOI] [Google Scholar]
- Kleber M. What is recalcitrant soil organic matter?. Environ. Chem. 2010, 7, 320–332. 10.1071/EN10006. [DOI] [Google Scholar]
- Keith D. A.; Ferrer-Paris J. R.; Nicholson E.; Kingsford R. T.. IUCN Global Ecosystem Typology 2.0. In Descriptive Profiles for Biomes and Ecosystem Functional Groups; International Union for Conservation of Nature: Gland, 2020. [Google Scholar]
- Young M. A.; Serrano O.; Macreadie P. I.; Lovelock C. E.; Carnell P.; Ierodiaconou D. National scale predictions of contemporary and future blue carbon storage. Sci. Total Environ. 2021, 800, 149573 10.1016/j.scitotenv.2021.149573. [DOI] [PubMed] [Google Scholar]
- Djukic I.; Kepfer-Rojas S.; Schmidt I. K.; Larsen K. S.; Beier C.; Berg B.; Verheyen K.; Caliman A.; Paquette A.; Gutiérrez-Girón A.; et al. Early stage litter decomposition across biomes. Sci. Total Environ. 2018, 628–629, 1369–1394. 10.1016/j.scitotenv.2018.01.012. [DOI] [PubMed] [Google Scholar]
- Burnham K.; Anderson D.. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach, 2nd ed.; Springer-Verlag: New York, NY, 2002. [Google Scholar]
- Lenth R.; Singmann H.; Love J.; Buerkner P.; Herve M.. Emmeans: Estimated Marginal Means, Aka LEAST-SQUARES Means. R Package Version 1:3, 2018.
- R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022.
- Walter H.; Breckle S.-W.. Vegetation und Klimazonen; Ulmer: Stuttgart, 1999; Vol. 544. [Google Scholar]
- Prescott C. E. Litter decomposition: what controls it and how can we alter it to sequester more carbon in forest soils?. Biogeochemistry 2010, 101, 133–149. 10.1007/s10533-010-9439-0. [DOI] [Google Scholar]
- Canfield D. E. Factors influencing organic carbon preservation in marine sediments. Chem. Geol. 1994, 114, 315–329. 10.1016/0009-2541(94)90061-2. [DOI] [PubMed] [Google Scholar]
- Sarneel J. M. J.; Veen G. C. Legacy effects of altered flooding regimes on decomposition in a boreal floodplain. Plant Soil 2017, 421, 57–66. 10.1007/s11104-017-3382-y. [DOI] [Google Scholar]
- Röthig T.; Trevathan-Tackett S. M.; Voolstra C. R.; Ross C.; Chaffron S.; Durack P. J.; Warmuth L. M.; Sweet M. Human-induced salinity changes impact marine organisms and ecosystems. Global Change Biol. 2023, 29, 4731–4749. 10.1111/gcb.16859. [DOI] [PubMed] [Google Scholar]
- Janousek C. N.; Buffington K. J.; Guntenspergen G. R.; Thorne K. M.; Dugger B. D.; Takekawa J. Y. Inundation, vegetation, and sediment effects on litter decomposition in Pacific Coast tidal marshes. Ecosystems 2017, 20, 1296–1310. 10.1007/s10021-017-0111-6. [DOI] [Google Scholar]
- Gao G.-F.; Peng D.; Tripathi B. M.; Zhang Y.; Chu H. Distinct community assembly processes of abundant and rare soil bacteria in coastal wetlands along an inundation gradient. Msystems 2020, 5, e01150–e01120. 10.1128/mSystems.01150-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalán N.; Marcé R.; Kothawala D. N.; Tranvik L. J. Organic carbon decomposition rates controlled by water retention time across inland waters. Nat. Geosci. 2016, 9, 501–504. 10.1038/ngeo2720. [DOI] [Google Scholar]
- Wik M.; Varner R. K.; Anthony K. W.; MacIntyre S.; Bastviken D. Climate-sensitive northern lakes and ponds are critical components of methane release. Nat. Geosci. 2016, 9, 99–105. 10.1038/ngeo2578. [DOI] [Google Scholar]
- Mori A. S.; Cornelissen J. H. C.; Fujii S.; Okada K.-i.; Isbell F. A meta-analysis on decomposition quantifies afterlife effects of plant diversity as a global change driver. Nat. Commun. 2020, 11, 4547 10.1038/s41467-020-18296-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin A. H.; Jensen K.; Schönfeldt M. Warming increases plant biomass and reduces diversity across continents, latitudes, and species migration scenarios in experimental wetland communities. Global Change Biol. 2014, 20, 835–850. 10.1111/gcb.12378. [DOI] [PubMed] [Google Scholar]
- Bao T.; Jia G.; Xu X. Warming enhances dominance of vascular plants over cryptogams across northern wetlands. Global Change Biol. 2022, 28, 4097–4109. 10.1111/gcb.16182. [DOI] [PubMed] [Google Scholar]
- Follstad Shah J. J.; Kominoski J. S.; Ardón M.; Dodds W. K.; Gessner M. O.; Griffiths N. A.; Hawkins C. P.; Johnson S. L.; Lecerf A.; LeRoy C. J.; et al. Global synthesis of the temperature sensitivity of leaf litter breakdown in streams and rivers. Global Change Biol. 2017, 23, 3064–3075. 10.1111/gcb.13609. [DOI] [PubMed] [Google Scholar]
- Bradford M. A.; Berg B.; Maynard D. S.; Wieder W. R.; Wood S. A. Understanding the dominant controls on litter decomposition. J. Ecol. 2016, 104, 229–238. 10.1111/1365-2745.12507. [DOI] [Google Scholar]
- Ricciuto D. M.; Yang X.; Wang D.; Thornton P. E. The impacts of model structure, parameter uncertainty and experimental design on Earth system model simulations of litter bag decomposition experiments. Biogeosci. Discuss. 2021, (2021), 1–36. 10.5194/bg-2021-163. [DOI] [Google Scholar]
- Lovelock C. E.; Feller I. C.; Reef R.; Hickey S.; Ball M. C. Mangrove dieback during fluctuating sea levels. Sci. Rep. 2017, 7 (1), 1680 10.1038/s41598-017-01927-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond N. R.; Lake P. S.; Arthington A. H. The impacts of drought on freshwater ecosystems: an Australian perspective. Hydrobiologia 2008, 600, 3–16. 10.1007/s10750-008-9326-z. [DOI] [Google Scholar]
- Rodríguez J. F.; Saco P. M.; Sandi S.; Saintilan N.; Riccardi G. Potential increase in coastal wetland vulnerability to sea-level rise suggested by considering hydrodynamic attenuation effects. Nat. Commun. 2017, 8, 16094 10.1038/ncomms16094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori T. Is the Tea Bag Index (TBI) Useful for Comparing Decomposition Rates among Soils?. Ecologies 2022, 3, 521–529. 10.3390/ecologies3040038. [DOI] [Google Scholar]
- Kominoski J. S.; Weaver C. A.; Armitage A. R.; Pennings S. C. Coastal carbon processing rates increase with mangrove cover following a hurricane in Texas, USA. Ecosphere 2022, 13, e4007 10.1002/ecs2.4007. [DOI] [Google Scholar]
- Harmon M. E.; Silver W. L.; Fasth B.; Chen H.; Burke I. C.; Parton W. J.; Hart S. C.; Currie W. S.; Lidet Long-term patterns of mass loss during the decomposition of leaf and fine root litter: an intersite comparison. Global Change Biol. 2009, 15, 1320–1338. 10.1111/j.1365-2486.2008.01837.x. [DOI] [Google Scholar]
- Djukic I.; Guerra C. A.; Maestre F. T.; Hagedorn F.; Oggioni A.; Bergami C.; Magagna B.; Kwon T.; Shibata H.; Eisenhauer N.; Patoine G.; Bierbaumer M.; Kepfer-Rojas S.; Kappel Schmidt I.; Steenberg Larsen K.; Beier C.; Berg B.; Verheyen K.; Trevathan-Tackett S. M.; Macreadie P. I.; The TeaComposition Initiative: unleashing the power of international collaboration to understand litter decomposition. Soil Org. 2021, 93, 73–78. 10.25674/so93iss1pp73. [DOI] [Google Scholar]
- Joly F.-X.; Scherer-Lorenzen M.; Hättenschwiler S. Resolving the intricate role of climate in litter decomposition. Nat. Ecol. Evol. 2023, 7 (2), 214–223. 10.1038/s41559-022-01948-z. [DOI] [PubMed] [Google Scholar]
- Ferreira V.; Silva J.; Cornut J.; Graça M. A. Microbial colonization and decomposition of commercial tea and native alder leaf litter in temperate streams. Aquat. Sci. 2022, 84, 4. 10.1007/s00027-021-00834-3. [DOI] [Google Scholar]
- Meng Y.; Hui D.; Huangfu C. Site conditions interact with litter quality to affect home-field advantage and rhizosphere effect of litter decomposition in a subtropical wetland ecosystem. Sci. Total Environ. 2020, 749, 141442 10.1016/j.scitotenv.2020.141442. [DOI] [PubMed] [Google Scholar]
- Frolking S.; Roulet N. T.; Moore T. R.; Richard P. J.; Lavoie M.; Muller S. D. Modeling northern peatland decomposition and peat accumulation. Ecosystems 2001, 4, 479–498. 10.1007/s10021-001-0105-1. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.