Abstract
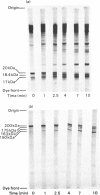
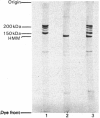
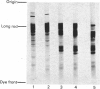
1. Hydrolysis of the myosins from smooth and from skeletal muscle by a rat trypsin-like serine proteinase and by bovine trypsin at pH 7 is compared. 2. Proteolysis of the heavy chains of both myosins by the rat enzyme proceeds at rates approx. 20 times faster than those obtained with bovine trypsin. Whereas cleavage of skeletal-muscle myosin heavy chain by both enzymes results in the generation of conventional products i.e. heavy meromyosin and light meromyosin, the heavy chain of smooth-muscle myosin is degraded into a fragment of mol. wt. 150000. This is dissimilar from heavy meromyosin and cannot be converted into heavy meromyosin. It is shown that proteolysis of the heavy chain takes place in the head region. 3. The 'regulatory' light chain (20kDa) of smooth-muscle myosin is degraded very rapidly by the rat proteinase. 4. The ability of smooth-muscle myosin to have its ATPase activity activated by actin in the presence of a crude tropomyosin fraction on introduction of Ca2+ is diminished progressively during exposure to the rat proteinase. The rate of loss of the Ca2+-activated actomyosin ATPase activity is very similar to the rate observed for proteolysis of the heavy chain and 3-4 times slower than the rate of removal of the so-called 'regulatory' light chain. 5. The significance of these findings in terms of the functional organization of the smooth muscle myosin molecule is discussed. 6. Since the degraded myosin obtained after exposure to very small amounts of the rat proteinase is no longer able to respond to Ca2+, i.e. the functional activity of the molecule has been removed, the implications of a similar type of proteolysis operating in vivo are considered for myofibrillar protein turnover in general, but particularly with regard to the initiation of myosin degradation, which is known to take place outside the lysosome (i.e. at neutral pH).
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aksoy M. O., Williams D., Sharkey E. M., Hartshorne D. J. A relationship between Ca2+ sensitivity and phosphorylation of gizzard actomyosin. Biochem Biophys Res Commun. 1976 Mar 8;69(1):35–41. doi: 10.1016/s0006-291x(76)80268-9. [DOI] [PubMed] [Google Scholar]
- Beynon R. J., Kay J. The inactivation of native enzymes by a neutral proteinase from rat intestinal muscle. Biochem J. 1978 Jul 1;173(1):291–298. doi: 10.1042/bj1730291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney I. T., Beynon R. J., Kay J., Birket N. The susceptibility of muscle phosphorylases a and b to digestion by a neutral proteinase from rat intestinal muscle. Comparison with the effects produced by pancreatic trypsin and chymotrypsin. Biochem J. 1978 Oct 1;175(1):105–113. doi: 10.1042/bj1750105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney I. T., Curtis C. G., Kay J. K., Birket N. A low-molecular-weight inhibitor of the neutral proteinase from rat intestinal smooth muscle. Biochem J. 1980 Feb 1;185(2):423–433. doi: 10.1042/bj1850423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabrowska R., Aromatorio D., Sherry J. M., Hartshorne D. J. Composition of the myosin light chain kinase from chicken gizzard. Biochem Biophys Res Commun. 1977 Oct 24;78(4):1263–1272. doi: 10.1016/0006-291x(77)91429-2. [DOI] [PubMed] [Google Scholar]
- Dayton W. R., Goll D. E., Zeece M. G., Robson R. M., Reville W. J. A Ca2+-activated protease possibly involved in myofibrillar protein turnover. Purification from porcine muscle. Biochemistry. 1976 May 18;15(10):2150–2158. doi: 10.1021/bi00655a019. [DOI] [PubMed] [Google Scholar]
- Dayton W. R., Reville W. J., Goll D. E., Stromer M. H. A Ca2+-activated protease possibly involved in myofibrillar protein turnover. Partial characterization of the purified enzyme. Biochemistry. 1976 May 18;15(10):2159–2167. doi: 10.1021/bi00655a020. [DOI] [PubMed] [Google Scholar]
- Dean R. T. Macrophage protein turnover. Evidence for lysosomal participation in basal proteolysis. Biochem J. 1979 May 15;180(2):339–345. doi: 10.1042/bj1800339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebashi S. A simple method of preparing actin-free myosin from smooth muscle. J Biochem. 1976 Jan;79(1):229–231. doi: 10.1093/oxfordjournals.jbchem.a131052. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Gerard K. W., Schneider D. L. Protein turnover in muscle: inhibitions of the calcium activated proteinase by mersalyl without inhibition of the rate of protein degradation. Biochem Biophys Res Commun. 1980 Jun 30;94(4):1353–1361. doi: 10.1016/0006-291x(80)90568-9. [DOI] [PubMed] [Google Scholar]
- Grinde B., Seglen P. O. Differential effects of proteinase inhibitors and amines on the lysosomal and non-lysosomal pathways of protein degradation in isolated rat hepatocytes. Biochim Biophys Acta. 1980 Sep 17;632(1):73–86. doi: 10.1016/0304-4165(80)90250-0. [DOI] [PubMed] [Google Scholar]
- Gröschel-Stewart U. Comparative studies of human smooth and striated muscle myosins. Biochim Biophys Acta. 1971 Feb 16;229(2):322–334. doi: 10.1016/0005-2795(71)90191-7. [DOI] [PubMed] [Google Scholar]
- Górecka A., Aksoy M. O., Hartshorne D. J. The effect of phosphorylation of gizzard myosin on actin activation. Biochem Biophys Res Commun. 1976 Jul 12;71(1):325–331. doi: 10.1016/0006-291x(76)90286-2. [DOI] [PubMed] [Google Scholar]
- Hegyvary C., Kang K., Bandi Z. Automated assay of phosphohydrolases by measuring the released phosphate without deproteinization. Anal Biochem. 1979 Apr 15;94(2):397–401. doi: 10.1016/0003-2697(79)90380-4. [DOI] [PubMed] [Google Scholar]
- Ikebe M., Aiba T., Onishi H., Watanabe S. Calcium sensitivity of contractile proteins from chicken gizzard muscle. J Biochem. 1978 Jun;83(6):1643–1655. doi: 10.1093/oxfordjournals.jbchem.a132077. [DOI] [PubMed] [Google Scholar]
- Kar N. C., Pearson C. M. Elevated activity of a neutral proteinase in human muscular dystrophy. Biochem Med. 1980 Dec;24(3):238–243. doi: 10.1016/0006-2944(80)90018-6. [DOI] [PubMed] [Google Scholar]
- Kay J. Intracellular protein degradation. Biochem Soc Trans. 1978;6(4):789–797. doi: 10.1042/bst0060789. [DOI] [PubMed] [Google Scholar]
- Kay J. Regulation of proteolysis: implications for the control of protein breakdown within the cell. Biochem Soc Trans. 1980 Aug;8(4):415–417. doi: 10.1042/bst0080415. [DOI] [PubMed] [Google Scholar]
- Kay J., Siemankowski L. M., Siemankowski R. F., Greweling J. A., Goll D. E. Degradation of myofibrillar proteins by trypsin-like serine proteinases. Biochem J. 1982 Feb 1;201(2):279–285. doi: 10.1042/bj2010279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles S. E., Ballard F. J. Selective control of the degradation of normal and aberrant proteins in Reuber H35 hepatoma cells. Biochem J. 1976 Jun 15;156(3):609–617. doi: 10.1042/bj1560609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikawa T., Nonomura Y., Ebashi S. Does phosphorylation of myosin light chain have direct relation to regulation in smooth muscle? J Biochem. 1977 Dec;82(6):1789–1791. doi: 10.1093/oxfordjournals.jbchem.a131878. [DOI] [PubMed] [Google Scholar]
- Mikawa T., Nonomura Y., Hirata M., Ebashi S., Kakiuchi S. Involvement of an acidic protein in regulation of smooth muscle contraction by the tropomyosin-leiotonin system. J Biochem. 1978 Dec;84(6):1633–1636. doi: 10.1093/oxfordjournals.jbchem.a132290. [DOI] [PubMed] [Google Scholar]
- Mrwa U., Hartshorne D. J. Phosphorylation of smooth muscle myosin and myosin light chains. Fed Proc. 1980 Apr;39(5):1564–1568. [PubMed] [Google Scholar]
- Okamoto Y., Sekine T. Effects of tryptic digestion on the enzymatic activites of chicken gizzard myosin. J Biochem. 1978 May;83(5):1375–1379. doi: 10.1093/oxfordjournals.jbchem.a132046. [DOI] [PubMed] [Google Scholar]
- Okitani A., Matsukura U., Kato H., Fujimaki M. Purification and some properties of a myofibrillar protein-degrading protease, cathepsin L, from rabbit skeletal muscle. J Biochem. 1980 Apr;87(4):1133–1143. [PubMed] [Google Scholar]
- Onishi H., Watanabe S. Chicken gizzard heavy meromyosin that retains the two light-chain components, including a phosphorylatable one. J Biochem. 1979 Feb;85(2):457–472. doi: 10.1093/oxfordjournals.jbchem.a132352. [DOI] [PubMed] [Google Scholar]
- Persechini A., Mrwa U., Hartshorne D. J. Effect of phosphorylation on the actin-activated ATPase activity of myosin. Biochem Biophys Res Commun. 1981 Feb 12;98(3):800–805. doi: 10.1016/0006-291x(81)91182-7. [DOI] [PubMed] [Google Scholar]
- Poole B., Ohkuma S., Warburton M. J. The accumulation of weakly basic substances in lysosomes and the inhibition of intracellular protein degradation. Acta Biol Med Ger. 1977;36(11-12):1777–1788. [PubMed] [Google Scholar]
- Riebow J. F., Young R. B. Effect of leupeptin on protein turnover in normal and dystrophic chicken skeletal muscle cells in culture. Biochem Med. 1980 Jun;23(3):316–323. doi: 10.1016/0006-2944(80)90042-3. [DOI] [PubMed] [Google Scholar]
- Seidel J. C. Activation by actin of ATPase activity of chemically modified gizzard myosin without phosphorylation. Biochem Biophys Res Commun. 1979 Aug 13;89(3):958–964. doi: 10.1016/0006-291x(79)91871-0. [DOI] [PubMed] [Google Scholar]
- Seidel J. C. Chymotryptic heavy meromyosin from gizzard myosin: a proteolytic fragment with the regulatory properties of the intact myosin. Biochem Biophys Res Commun. 1978 Nov 14;85(1):107–113. doi: 10.1016/s0006-291x(78)80017-5. [DOI] [PubMed] [Google Scholar]
- Shapiro A. L., Viñuela E., Maizel J. V., Jr Molecular weight estimation of polypeptide chains by electrophoresis in SDS-polyacrylamide gels. Biochem Biophys Res Commun. 1967 Sep 7;28(5):815–820. doi: 10.1016/0006-291x(67)90391-9. [DOI] [PubMed] [Google Scholar]
- Shaw E., Dean R. T. The inhibition of macrophage protein turnover by a selective inhibitor of thiol proteinases. Biochem J. 1980 Feb 15;186(2):385–390. doi: 10.1042/bj1860385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siemankowski R. F., Dreizen P. Canine cardiac myosin with special referrence to pressure overload cardiac hypertrophy. I. Subunit composition. J Biol Chem. 1978 Dec 10;253(23):8648–8658. [PubMed] [Google Scholar]
- Siemankowski R. F., Zobel C. R. Comparative studies on the structure and aggregative properties of the myosin molecule. I. The structure of the lobster myosin molecule. J Mechanochem Cell Motil. 1976 Mar;3(3):171–184. [PubMed] [Google Scholar]
- TAUSSKY H. H., SHORR E. A microcolorimetric method for the determination of inorganic phosphorus. J Biol Chem. 1953 Jun;202(2):675–685. [PubMed] [Google Scholar]
- Vedeckis W. V., Freeman M. R., Schrader W. T., O'Malley B. W. Progesterone-binding components of chick oviduct: partial purification and characterization of a calcium-activated protease which hydrolyzes the progesterone receptor. Biochemistry. 1980 Jan 22;19(2):335–343. doi: 10.1021/bi00543a014. [DOI] [PubMed] [Google Scholar]
- Wildenthal K., Wakeland J. R., Ord J. M., Stull J. T. Interference with lysosomal proteolysis fails to reduce cardiac myosin degradation. Biochem Biophys Res Commun. 1980 Sep 30;96(2):793–798. doi: 10.1016/0006-291x(80)91424-2. [DOI] [PubMed] [Google Scholar]
- Woodbury R. G., Everitt M., Sanada Y., Katunuma N., Lagunoff D., Neurath H. A major serine protease in rat skeletal muscle: evidence for its mast cell origin. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5311–5313. doi: 10.1073/pnas.75.11.5311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodbury R. G., Gruzenski G. M., Lagunoff D. Immunofluorescent localization of a serine protease in rat small intestine. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2785–2789. doi: 10.1073/pnas.75.6.2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K., Sekine T. Interaction of myosin subfragment-1 with actin. III. Effect of cleavage of the subfragment-1 heavy chain on its interaction with actin. J Biochem. 1979 Dec;86(6):1869–1881. doi: 10.1093/oxfordjournals.jbchem.a132710. [DOI] [PubMed] [Google Scholar]
- Yasogawa N., Sanada Y., Katunuma N. Susceptibilities of various myofibrillar proteins to muscle serine protease. J Biochem. 1978 May;83(5):1355–1360. doi: 10.1093/oxfordjournals.jbchem.a132043. [DOI] [PubMed] [Google Scholar]
- Zobel C. R. Proteolytic fragments from the lobster myosin molecule. Biochim Biophys Acta. 1978 Sep 26;536(1):142–155. doi: 10.1016/0005-2795(78)90060-0. [DOI] [PubMed] [Google Scholar]