Abstract
There is increasing interest in identifying how posttraumatic growth (PTG) impacts emotional processing following traumatic events (e.g., the COVID‐19 pandemic). Previous research suggests that high PTG levels may lead to enduring changes in positive emotional processing. Despite this fact, little is known regarding brain activation and responses to stressful emotional stimuli. The present study utilised event‐related potentials (ERPs) to investigate whether individual differences in emotional responses toward neutral and negative emotional stimuli related to COVID‐19 are related to self‐reported PTG levels. A total of 77 participants were analysed: 21 in the high PTG group and 56 in the control group. The amplitude of the N2 was smaller in the high PTG group compared to the control group under both negative and neutral conditions. When viewing the negative emotion pictures (vs. neutral pictures), the N2 amplitude significantly decreased for the high PTG group in the right occipital and frontal‐parietal areas, whereas no significant change was observed among the control group. In the time window Late Positive Potential (LPP) 600–1000 ms, emotional stimuli and the group interaction were significant. Viewing negative pictures (vs. neutral pictures) decreased the LPP 600–1000 ms amplitudes for the control group, mainly originating from the brain's frontal regions. However, there were no such significant differences for the PTG group. Due to the limited sample size and cultural differences, the applicability of these results to other regions or countries needs to be verified. The presented findings suggest that the impact of PTG during emotional response is reflected in both bottom‐up (evidenced by the early ERP components) and top‐down (evidenced by the later ERP components) processes. Individuals with high PTG may use a meditation‐related emotional regulation strategy of acceptance at the basic stage and non‐judgement at a later stage.
Keywords: COVID‐19, emotional response, event‐related potentials, post‐traumatic growth
1. INTRODUCTION
The experience of life crises such as pandemics or natural disasters (e.g., the coronavirus disease 2019, short for the COVID‐19 pandemic) may result in posttraumatic stress disorder or other mental health problems. Nevertheless, traumatic events such as the COVID‐19 pandemic have also led to positive psychological reactions labelled as posttraumatic growth (PTG; Lau et al., 2021). Given the role of PTG in psychological functioning and well‐being, abundant studies have focused on the impact and outcomes of PTG, indicating that it affects a series of psychosocial factors, including its enduring and quantitative change of emotional regulation, interpersonal relationships, and social functioning (Gil‐González et al., 2022; Vloet et al., 2017; Zhou et al., 2016). This study centred on the impact of high PTG levels on individuals' emotional responses during the COVID‐19 pandemic by utilising event‐related potentials (ERPs) as indexed by N200 (N2) and Late Positive Potential (LPP) amplitude.
1.1. The COVID‐19 pandemic and posttraumatic growth (PTG)
The COVID‐19 pandemic was an unprecedented global public health crisis that can be deemed to be a compounding traumatic event (Kamranvand et al., 2022; Lau et al., 2021; Liu, Ng, et al., 2021). Research on the adverse sequelae of COVID‐19 pandemic trauma has emerged rapidly during the past years (Lau et al., 2021; Miragall et al., 2021), and rapid development in our understanding of adverse outcomes of the COVID‐19 pandemic has come from the study of neural and other biological correlates of these phenomena (Dell’Acqua et al., 2022; Kamranvand et al., 2022; Povero et al., 2022).
Although it is essential to investigate the negative impact of COVID‐19, it is also necessary to examine the protective factors and positive changes that explain how people coped with the pandemic and confinement. Moreover, it is useful to analyse whether positive changes could prevent emotional and mental health problems and promote growth and resilience in such circumstances (Miragall et al., 2021). However, only in recent years have positive changes following trauma been studied systematically, as well as positive outcomes after the COVID‐19 pandemic (Povero et al., 2022). These positive changes have been labelled posttraumatic growth (PTG), referring to positive psychological changes from significant traumatic events (e.g., the COVID‐19 pandemic, earthquakes, and car accidents). Personal positive coping strategies, meaningful intimate relationships, an increase in appreciation of life, changes in priorities, and openness to new possibilities are typical PTG changes (Tedeschi et al., 1998). When an individual's perceptions of themselves, others, and the meaning of the crisis are positively reconstructed in the aftermath of a crisis, successful PTG occurs. This positive growth is associated with various positive outcomes, such as increased life satisfaction, decreased distress over time, and better emotional responses evoked by traumatic events (Gil‐González et al., 2022; Vloet et al., 2017; Zhou et al., 2016).
A traumatic event is an event that causes actual or threatened harm to oneself or others, challenging an individual's core belief about themselves, others, and the world (Tedeschi & Calhoun, 2004). Like a psychological earthquake, the impact of the COVID‐19 pandemic could shake one's core beliefs and allow individuals to rebuild their belief system and make sense of the traumatic event in a way that fosters posttraumatic growth (PTG) and development. Prior research has shown that individuals experiencing post‐traumatic growth (PTG) during the COVID‐19 pandemic tend to place greater emphasis on the well‐being of significant others and their own emotions, as well as to adopt healthier coping strategies for dealing with negative emotions (Petrocchi et al., 2023). Furthermore, they may better appreciate their daily lives and maintain a positive belief system (Xie & Kim, 2022). The bulk of research on the COVID‐19 pandemic experience and PTG has emphasised the importance of understanding the mechanisms and antecedents of PTG, including the association between resources, personality traits, coping strategies, and the barriers to PTG (e.g., Cao et al., 2022; Dell’Acqua et al., 2022; Kalaitzaki, 2021; Petrocchi et al., 2023; Povero et al., 2022; Xie & Kim, 2022). For example, a study conducted among 181 adult participants (Mean age = 24 years, mainly Chinese and Swedish) found that 50% of the participants reported PTG experiences during the COVID‐19 pandemic, and their coping strategies (e.g., emotion‐focused coping and social support coping) were positively correlated with PTG (Xie & Kim, 2022). In addition, a longitudinal study conducted in Switzerland with 4934 adult participants (Mean age = 57.81 years) found that a sense of control and self‐mastery may account for antecedents of PTG, and the balanced emotions mediate the relationships between personality traits and PTG (Petrocchi et al., 2023). However, there has been limited research on the positive effects of PTG, particularly how it modulates negative emotions, with a scarcity of studies employing cognitive neuroscience methodologies, such as ERPs (ERP) and functional magnetic resonance imaging (Miragall et al., 2021). More evidence is needed to further clarify the possibilities of positive emotional regulation and recovery brought by PTG during/after the traumatic experience of the COVID‐19 pandemic.
1.2. PTG and emotional response
Hence, in order to fill the gap, this study placed a particular focus on the relationship between COVID‐19‐ related PTG and negative emotional processing. Specifically, negative emotional processing is defined as either increasing or decreasing responses that result from either or both the internal emotional state and the external stimuli (Rachman, 1980). It is a process by which individuals modify their negative emotional experiences, expressions, and subsequent physiological responses. Three commonly used emotional strategies are reappraisal, distraction, and suppression (Gross, 2014; Kira et al., 2019). Reappraisal is one of the positive strategies of negative emotion processing that changes the interpretation of an emotionally evocative stimulus (Gross, 2014). Reappraisal is recognized as a facet of coping that is central to trauma recovery and represents a significant ability to manage, experience, and express intense negative feelings associated with traumatic events and thereby cope with trauma (Orejuela‐Dávila et al., 2019).
Several empirical studies have found that PTG can benefit negative emotional processing (Larsen & Berenbaum, 2015; Liu, Ng, et al., 2021; Orejuela‐Dávila et al., 2019). Researchers argue that PTG can help traumatised individuals increase positive emotional regulation, such as reappraisal, and create positive attention bias. For instance, an experimental study conducted among 109 undergraduate students in the United States who had experienced traumatic events reported that when recording their emotional response to negative pictures of varying intensity, an increase in reappraisal strategies was associated with higher PTG (Orejuela‐Dávila et al., 2019). In addition, a study conducted among 107 adult women who had experienced traumatic events within the past three years found that PTG was positively associated with meaning‐creating but not expressive suppression during emotional processing (Larsen & Berenbaum, 2015). Moreover, an experimental study among 202 injured patients in China indicated that higher PTG levels positively correlated with attention bias toward positive stimuli but were not significantly correlated with attention bias toward negative stimuli (Liu, Ng, et al., 2021).
It is worth noting that several theoretical research proposed that individuals with higher PTG levels may utilise a particular meditation‐oriented reappraisal strategy of emotional regulation (Haspolat & Çırakoğlu, 2021; Tedeschi & Blevins, 2015), which could even lead to enduring and quantitative change (Hanley et al., 2017; Jayawickreme et al., 2021). In the immediate aftermath of a traumatic event, intrusive ruminations enter unwittingly into an individual's mind, causing possible experiences of stress, depression and anxiety. The set‐shifting feature of PTG mind‐set may allow individuals to practice direct engagement with their thoughts and feelings while observing the higher order functions of awareness and consciousness from a nonjudgmental perspective. Through positive mediation‐oriented regulation, individuals with high PTG levels learn to modulate reactivity but control their involvement in self‐referential processing. Individuals with high levels of PTG could be more efficient in implementing attentional resources and modifying automatic activity in the presence of negative stimulation than distraction or suppression. In other words, people with high PTG levels seem to foster non‐avoidance of stimuli processing and acceptance without over‐engagement in its regulation. However, empirical evidence regarding the relationship between high PTG level and emotional response is scarce.
1.3. Emotional processing: ERP evidence
Negative emotion processing occurs in different brain processing stages. ERP has high time resolution and can record the underlying neural mechanisms of emotional response (Langeslag & Van Strien, 2010; Liu et al., 2012). The N2 and LPP are the typical ERP indices of emotional processing that are related to modulations in the early attentional process (e.g., the relationship between the N2 components with selective attention) and the later regulatory processes (e.g., the relationship between LPP to continuous attention and emotional regulation; Deng et al., 2019; Liu et al., 2012).
N2 is an essential early component observed at the frontal‐parietal electrodes peaking between 200 and 300 ms after a stimulus presentation, reflecting increased bottom‐up selective attention to emotional stimulus (Kaunhoven & Dorjee, 2020). It is suggested that N2 indexes an early automatic component related to attention to emotions and is associated with inhibitory control when perceiving emotions (Huster et al., 2013). An individual's emotional regulation level also influences N2 components, but previous empirical evidence regarding the direction of these results is inconsistent. For example, some ERP studies found that N2 was decreased in participants after practicing 10 min of body‐mind meditation compared with participants in control groups (Deng et al., 2019; Fan et al., 2015). However, some studies reported increased amplitudes of N2 components among participants with better emotional regulation strategies (e.g., meditation, chanters; Gao et al., 2017). In general, ERP N2 components can be modulated by personal emotional status.
Late Positive Potential is a broad superior posterior positive signal that reflects increased and continuous attention to emotional stimuli. Researchers found that the late LPP time window (e.g., 600–1000 ms) reflected the deeper processing related to the evaluation of emotional meaning (Yuan et al., 2015). Therefore, during the late time window of LPP, the change in potential amplitude changed from the increase in intentional attention caused by emotion perception to the intentional redistribution of attention caused by using emotional regulation strategies (Yang et al., 2021). Regarding the relationship between LPP and negative emotion regulation, previous studies indicated increased LPP amplitudes under negative emotional response conditions compared to neutral stimuli (Yang et al., 2021). However, some researchers reported significantly decreased electrophysiological activity of LPP when using suppression strategies regarding negative emotional stimuli (Moser et al., 2006).
Furthermore, evidence from neuropsychological studies suggests that several regions may be crucial for emotional processing. Specifically, previous studies on source localization reported that emotional processing was linked to changes in N2 and LPP at frontal, posterior‐parietal, and occipital regions (Raz et al., 2014; Sobolewski et al., 2011).
1.4. The present study
During the past decades, research on PTG has mainly focused on the correlation with self‐report constructs, such as emotion regulation, personality traits, and social support (Orejuela‐Dávila et al., 2019; Vloet et al., 2017; Zhou et al., 2016). As a result, some researchers argue that some individuals may not develop positive coping strategies and growth after trauma, and consider PTG to be an illusory concept (Börner, 2016). Hence, evidence from cognitive neuroscience‐related studies can further alleviate such concerns about PTG and its impact (Dell’Acqua et al., 2022; Kamranvand et al., 2022). Despite theoretical argument on high PTG and quantitative change of emotional processing being of great interest, this topic has received far less attention and associated research is scarce.
Therefore, the ERP method was used in this study to explore the relationship between COVID‐19 pandemic‐related PTG and emotional response toward negative pictures. Based on previous research and related theory, we compared early (N2) and late (LPP) ERP components in adult participants who reported a high level of PTG with a control group of their counterparts. We hypothesised that individuals with high PTG would have fewer suppressed responses and more neutralised or positive attitudes toward negative pictures than the participants in the control group. Due to the scarcity of previous evidence, we did not have a clear hypothesis regarding the N2 and LPP amplitude changes and differences among high PTG and control groups.
2. MATERIALS AND METHODS
2.1. Participants and measures
Participants were recruited through snowballing via social media (e.g., Facebook, Telegram, WhatsApp) and email lists. Eligible participants were Hong Kong Chinese adult residents aged 18 or above. Participants unable to read traditional Chinese were excluded. People with neurological, psychiatric, or other mental disorders were also excluded from the research. The consent form approved by the Human Research Ethics Committee at the University of Hong Kong was signed by each participant prior to the experiment, and they were rewarded with $200 HKD (The exchange rate is approximately 1 USD = 7.8 HKD) for taking part in the study. Participants were asked to complete questionnaires before the experiment to assess their PTG during the COVID‐19 pandemic and were instructed to answer based on their experience during the pandemic. Specifically, they were asked to complete the 21‐item posttraumatic growth inventory (Ho et al., 2004; Tedeschi & Calhoun, 1996). The response format used in the scale was a 6‐point Likert scale running from 0 (none) to 5 (very much). Responses to the 21 items were averaged to form the total scores of PTG. The Cronbach alpha was 0.95, indicating good reliability.
In total, 82 participants were recruited. Data from five participants were excluded due to either missing questionnaire data or poor‐quality EEG data. The mean PTG score was 53.34, with SD 20.45. We then grouped participants by their PTG scores. Those with a PTG level above the 3rd quartile (PTG score ≥67) were assigned to the PTG group, with the remainder assigned to the control group. As a result, 21 participants were assigned to the high PTG group, and 56 participants were assigned to the control group.
2.2. Experimental design
The study was conducted in a quiet room at an EEG laboratory. Participants were seated in a comfortable chair and were instructed to relax while the EEG cap was placed on their heads. They were then presented with a series of visual stimuli related to COVID‐19, and their neural responses were recorded.
E‐prime (Psychology Software Tools, Inc., USA) was utilised to display images in the affective task. The COVID‐related emotional pictures, sourced from the Internet using Google search with the terms “covid,” covid daily life,” ‘covid news,’ “Covid death,” and “Covid hospital,” fell into two categories: negative and neutral emotional stimulus. These images were obtained and classified by three senior researchers through several times' discussions and then reviewed by two other senior researchers to further validate their classification. Negative emotional images depicted sad and miserable scenes, inducing COVID‐19 pandemic‐related negative emotions, while neutral emotional images displayed scenes during the COVID‐19 pandemic, such as people walking on the street wearing masks, not eliciting any specific emotion. Participants first viewed a fixation (+) that randomly lasted between 1000 and 2000 ms in steps of 200 ms, followed by a COVID‐related emotional picture displayed for 1500 ms. We randomly presented 120 trials of the same type of image in each condition. The type of images was also chosen randomly for presentation. The continuous EEG was recorded throughout the whole experiment.
Next, we then asked participants to evaluate the valence or arousal of each picture using a Likert scale from one to nine and an arousal level from 1 to 9. Participants had 20 s to respond (the average response time was about 7 seconds). They rated the valence of negative pictures with an average score of 2.62 (SD = 1.43), while neutral pictures received an average score of 4.87 (SD = 1.09). Similarly, negative pictures had an average arousal score of 5.70 (SD = 2.32), compared to 2.63 (SD = 1.77) for neutral pictures. Each participant experienced the evaluation conditions in a random order (either arousal or valence evaluation), and the entire ERP experiment lasted approximately 35 min. The overall duration of the experiment, including the setup of the EEG cap and the administration of questionnaires, was approximately an hour and a half.
The procedure of each trial is shown in Figure 1, and the examples of emotional stimulus are shown in Figure 2.
FIGURE 1.
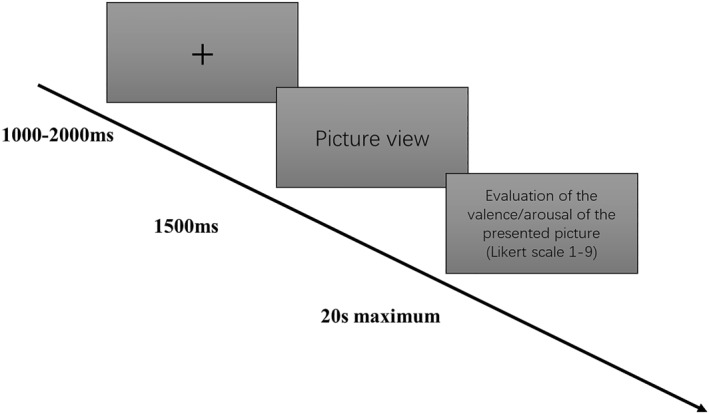
The procedure of each trial.
FIGURE 2.

Examples of neutral/negative emotional stimulus.
2.3. EEG data collection and pre‐processing
EEG data were recorded using a 128‐channel EEG cap and a Net‐station machine with a sampling rate of 1000 Hz. The default reference was set at the Cz electrode. The recorded data were then converted from Net‐station raw format to EEGLAB readable data for further processing.
EEG data preprocessing involved the following steps: (1) resampling the data from 1000 to 250 Hz, (2) filtering the data using a 1–30 Hz band‐pass filter, (3) manually cleaning noise by scrolling through each dataset, (4) interpolating bad channels identified visually with neighbour channels, and (5) applying independent component analysis to remove channel noise, muscle, and eye movement components.
2.4. ERP data analysis
Event‐related potential (ERP) data were obtained based on the stimulus onset time using a time window of −500 to 1000 ms. The ERP data were then re‐referenced based on the average of all channels. Statistical analyses were performed using t‐tests and ANOVA to compare the ERP data between the high PTG and control groups.
The main goal of the ERP analysis was to examine the neural correlates of psychological responses to the COVID‐19 pandemic in the two groups, the high PTG group and the control group.
The specific ERP components of interest and their corresponding time windows were determined based on prior literature on emotion regulation and cognitive processing. These components may include early components such as N2, reflecting attentional processes, as well as later components such as LPP600‐1000 ms, reflecting cognitive processing during emotion regulation.
3. RESULTS
The EEG data revealed significant findings in specific channels and time windows. At the frontal channel E16 (near the channel Fz of the standard 10–20 system), within the ERP time window of 600–1000 ms, a 2 x 2 ANOVA showed a significant group and emotional stimuli interaction (p = 0.019) (as shown in Figure 3). For the control group, the negative condition had a significantly lower amplitude than the neutral condition, t (55) = −2.10, p = 0.040, with mean amplitudes of 0.85 (0.80) uV for the negative condition and 1.12 (0.10) uV for the neutral condition (as shown in Figure 4). However, no significant difference was found for the high PTG group (as shown in Figure 5).
FIGURE 3.
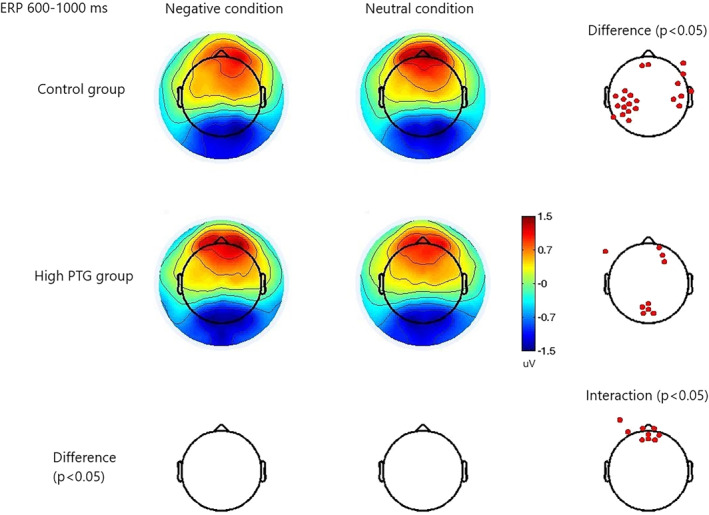
Significant interaction of group and emotional stimuli (negative and neutral). Red dots: channels with significant difference or interaction (p < 0.05).
FIGURE 4.
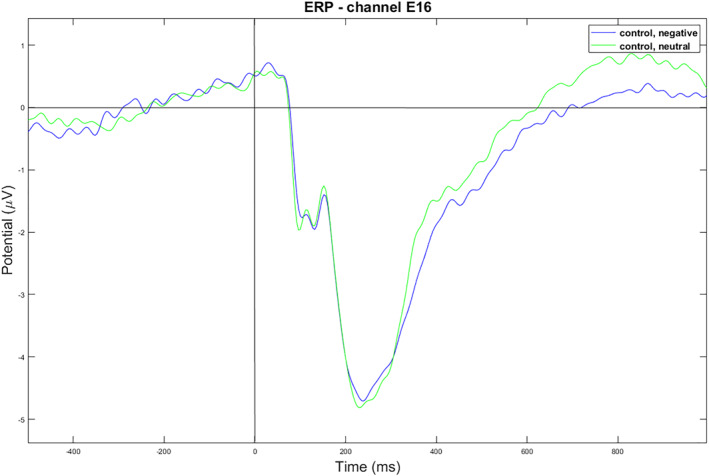
Negative versus neutral condition for the control group. E16 is a channel located near Fz of the standard 10–20 system.
FIGURE 5.
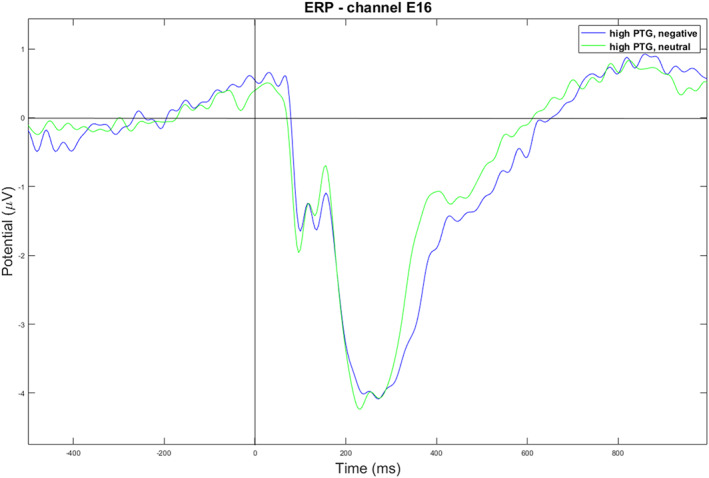
Negative versus neutral condition for the high PTG group. E16 is a channel located near Fz of the standard 10–20 system.
At the right occipital channel E84, within the time window of 200–300 ms, no significant interaction of group and stimuli was found. Between‐group comparisons revealed the control group (4.84 (2.79) uV) had higher amplitude than the high PTG group (3.52 (1.65) uV) under the negative condition, t (60.66) = −2.54, p = 0.014. The control group also had higher amplitude (4.97 (2.92) uV) than the high PTG group (3.76 (1.71) uV) under the neutral condition, t (61.21) = −2.25, p = 0.028. For the high PTG group, the negative condition had significantly lower amplitude than the neutral condition, t (20) = −2.28, p = 0.034, with mean amplitudes of 3.52 (1.65) uV for the negative condition and 3.76 (1.71) uV for the neutral condition. No significant difference was found when comparing the negative condition with the neutral condition in the control group.
At channel E106 located by the frontal‐parietal, within the time window of 200–300 ms, no significant interaction of group and stimuli was found. For the high PTG group, the negative condition has significantly lower amplitude than the neutral condition, t (20) = 4.29, p = 0.00036, with mean amplitudes of −1.62 (1.52) uV for the negative condition and −1.95 (1.53) uV for the neutral condition. No significant difference was found for the control group.
4. DISCUSSION
As indicated earlier, N2 may be related to emotional attention. The amplitude of N2 was smaller in the PTG group compared to the control group under both negative and neutral conditions. Our results indicated that PTG might help participants reduce their primary intentional attention when facing emotional stimuli, in comparison with their counterparts in the control group, thereby reducing the cognitive resources that need to be recruited and reducing cognitive load at the basic perceptual phase. These results were in line with our expectations and showed that PTG could effectively help individuals control the perceptual load required when they face negative emotions, thereby reducing the impact of negative emotions at the early perceptual phase.
In addition, our ERP study found that relative to neutral emotional stimuli, N2 amplitudes for negative emotional stimuli were smaller among the high PTG group, whereas no such significant difference was found in the control group. It is possible that PTG hampered the processing of negative stimuli. Most of the negative stimuli concerned fear and painful scenarios related to COVID‐19, and it seemed that the participants in the high PTG group appeared less reactive when witnessing other people in stressful situations. Humans tend to use the self as a reference point to perceive the world and gain information about other people's emotions. This self‐referential projection mechanism can sometimes result in egocentrically biased judgements. When a person has a positive meaning‐seeking tendency, such as people with high PTG, their reactions to negative emotions would be moderated. Accordingly, their less reactive response to negative emotions might bias their primary perception of these emotions. Hence, people with high levels of PTG tend to temper or downregulate their perceptions of negative emotions in the early phase of emotional processing.
In addition, the present study demonstrated no significant change of electro‐cortical activation (as indicated by the LPP) in response to negative emotional stimuli (compared with neutral stimuli) among the high PTG group. These results suggest no extra recruitment of executive processing and cortical resources during the later stage of emotional processing among individuals with high PTG levels. As stated in previous literature on LPP and emotional response (Yang et al., 2021; Yuan et al., 2015), high PTG may facilitate emotional regulation by decreasing initial reactivity to emotionally complicated stimuli. Therefore, the insignificant change in response to negative versus neutral stimuli in the high PTG group indicates their neutralised attitude and peaceful status. This counterbalancing effect on the response to negative stimuli among the high PTG group was quite prominent. Our findings suggest that the neutralised attitude towards negative stimuli following a traumatic event may be a key sign of developing PTG. People with PTG attempt to process their emotions in a neutral and nonjudgmental way, allowing them to engage with trauma‐related emotions with less activation (Haspolat & Çırakoğlu, 2021; Tedeschi & Blevins, 2015). In addition, since the main sources seem to involve frontal cortical regions, it is reasonable to assume that individuals with high PTG can hamper afferent emotional arousal triggered by negative picture stimuli.
The present findings indicate that the participants' PTG levels were crucial in processing negative emotions and acted in different roles in the early bottom‐up and later top‐down phases. Combining the findings of N2 and LPP, we found that PTG can control early affective arousal to negative emotions (N2 200–300 ms time windows) and maintain a non‐judgemental and less‐active attitude during the latter cognitive evaluation process (LPP 600–1000 ms time window). This is similar to meditation—acceptance but nonjudgement––a particular strategy of emotional processing (Gao et al., 2017; Lin et al., 2022; Taylor et al., 2011). Those who have practiced meditation for a while have learnt to develop the capability to accept and experience aroused emotion at the fundamental stage without being overly engaged with it, such as by non‐association or no self‐referential thought interference (Lin et al., 2022). Based on our findings, it is possible that individuals with high PTG use the meditation‐related strategy to regulate their response when viewing negative stimuli. These interpretations are particularly interesting because they show some similarities between meditators and individuals with high levels of PTG. Further studies evaluating the relationship between meditation, PTG, and emotion processing are clearly necessary.
Interestingly, in the LPP early time window, the amplitude of negative emotions among the control group was smaller than that under the condition of neutral stimuli, indicating that people might use an effective cognitive regulation strategy in that time window to save neurological and cognitive resources when facing negative emotional challenges. The lack of increment of LPP amplitude may be a function of emotional strategies, such as distraction, suppression, or avoidance (Kelley et al., 2019). As an emotional processing strategy, although distraction can decrease people's responses toward emotional stimuli in the short term, it can be costly in the long term as it may promote belief disruption and habitual avoidance (Orejuela‐Dávila et al., 2019). Our findings align with previous research in that the amplitudes of LPP were significantly lower under suppression regulation strategies than those in no‐regulation conditions (Moser et al., 2006; Yuan et al., 2015). Thus, together with the previous findings, our results indicate that decreasing emotional response to negative stimuli in the LPP time window decreases an individual's cognitive activity of peripheral and central systems.
5. LIMITATIONS AND FUTURE DIRECTION
Although the current findings add to an understanding of PTG's impact on emotional response, there are several limitations. First, East Asian cultures stress social harmony and group connection, whereas Western cultures emphasise self‐fulfilment, personal rights, and liberty (Markus & Kitayama, 2010). There could be cross‐cultural differences regarding their neural response towards emotional stimulus within social contexts. For example, some researchers argue that Chinese participants showed higher neural activity when viewing social‐related emotions than Caucasians (Deng et al., 2022). Also, in the current study, the high PTG group only consists of 21 participants, which precludes us from drawing robust conclusions due to the relatively small sample size. Hence, due to the limited sample size and cultural differences argument, the applicability of current findings to other regions or countries needs to be further verified. Second, as noted above, although current research provided several interpretations of regulation strategies during emotional processing, more detailed neural evidence of the relationship between PTG and emotional regulation strategies remains an avenue for future research and an important topic to explore. Third, the findings were based on a basic picture‐viewing experiment design. Such a short induction may lead to only temporary state changes. Since PTG represents a progressive change that involves changes in perceptions, feelings, thoughts, and even decision‐making, future research should explore the impact of PTG with a longer duration and experiment design with a more profound cognitive process (e.g., decision‐making). Fourth, since the LPP is affected by other factors, such as positive priming or personal characteristics, a better‐designed experiment is needed to delineate neural evidence‐based pictures of PTG. Fifth, since our findings are based on correlational data analysis, which fails to draw a causal relation between PTG and neural emotional response, and thus limits the strength of the rigorous conclusion. We recommend that future studies utilise advanced analytic methods to reach causal results (e.g., time‐locked analysis, spatial distribution analysis, and condition comparison).
6. CONCLUSIONS
The current study illustrates the effects of PTG on emotional response when viewing different types of emotional picture stimuli. The results showed that participants with high levels of PTG were less active during the basic perceptual procedure and showed more neutral attitudes toward negative emotional stimuli during later emotional processing. Specifically, in comparison with participants in the control group, viewing negative stimuli (vs. neutral emotional stimulus) induced smaller N2 amplitudes. Additionally, when viewing negative stimuli (vs. neutral emotional stimulus), LPP amplitudes induced non‐significant change among high PTG groups, whereas LPP amplitudes were significantly smaller among the control group. Our study enriches our understanding of PTG and its impact on emotional response and provides support to the notion that PTG might impact emotional processing by using a meditation‐related strategy. When confronting negative emotions, PTG benefits an individual's emotional processing by controlling perceptions in the early phase and being less active and more neutralised in the later cognitive regulation phase. A possible alternative explanation is that individuals with high PTG may use a meditation‐related emotional regulation strategy of acceptance at the basic stage and non‐judgement at a later stage. We call for further research to validate our findings with a larger sample size from multiple cultural backgrounds by using more rigorous analytic methods and longitudinal datasets.
CONFLICT OF INTEREST STATEMENT
The authors report there are no competing interests to declare.
ACKNOWLEDGEMENT
This research received no specific grant from funding agencies in the public, commercial, or not‐for‐profit sectors.
Ng, S. , Xie, W. , Gao, J. , Wang, M. , Leung, H. , Li, H. , Sik, H. H. , Lau, B. H. P. , & Chan, C. L. W. (2024). Posttraumatic growth modulates the response to negative emotions related to COVID‐19: An event‐related potentials study. Stress and Health, 40(6), e3488. 10.1002/smi.3488
DATA AVAILABILITY STATEMENT
The participants of this study did not give written consent for their data to be shared publicly, so due to the sensitive nature of the research supporting data is not available.
REFERENCES
- Börner, M. (2016). Real and illusory reports of posttraumatic growth and their correlation with well‐being: An empirical examination with special focus on defence mechanisms. [PhD Thesis]. University of Nottingham. [Google Scholar]
- Cao, C. , Wang, L. , Fang, R. , Liu, P. , Bi, Y. , Luo, S. , Grace, E. , & Olff, M. (2022). Anxiety, depression, and PTSD symptoms among high school students in China in response to the COVID‐19 pandemic and lockdown. Journal of Affective Disorders, 296, 126–129. 10.1016/j.jad.2021.09.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell’Acqua, C. , Moretta, T. , Dal Bò, E. , Messerotti Benvenuti, S. , & Palomba, D. (2022). Emotional processing prospectively modulates the impact of anxiety on COVID‐19 pandemic‐related post‐traumatic stress symptoms: An ERP study. Journal of Affective Disorders, 303, 245–254. 10.1016/j.jad.2022.02.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng, X. , An, S. , & You, Y. (2022). Cross‐cultural differences in the processing of social and non‐social positive emotions: An ERP study. Current Psychology, 42(16), 1–12. 10.1007/s12144-021-02604-8 36468159 [DOI] [Google Scholar]
- Deng, X. , Zhang, J. , Hu, L. , & Zeng, H. (2019). Neurophysiological evidences of the transient effects of mindfulness induction on emotional processing in children: An ERP study. International Journal of Psychophysiology, 143, 36–43. 10.1016/j.ijpsycho.2019.06.014 [DOI] [PubMed] [Google Scholar]
- Fan, Y. , Tang, Y.‐Y. , Tang, R. , & Posner, M. I. (2015). Time course of conflict processing modulated by brief meditation training. Frontiers in Psychology, 6. 10.3389/fpsyg.2015.00911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao, J. , Fan, J. , Wu, B. W. , Halkias, G. T. , Chau, M. , Fung, P. C. , Chang, C. , Zhang, Z. , Hung, Y.‐S. , & Sik, H. (2017). Repetitive religious chanting modulates the late‐stage brain response to fear‐ and stress‐provoking pictures. Frontiers in Psychology, 7. 10.3389/fpsyg.2016.02055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil‐González, I. , Martín‐Rodríguez, A. , Conrad, R. , & Pérez‐San‐Gregorio, M. Á. (2022). Coping strategies furthering post‐traumatic growth in multiple sclerosis: A longitudinal study. International Journal of Environmental Research and Public Health, 19(19), 12679. 10.3390/ijerph191912679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross, J. J. (2014). Emotion regulation: Conceptual and empirical foundations. Handbook of Emotion Regulation, 2, 3–20. [Google Scholar]
- Hanley, A. W. , Garland, E. L. , & Tedeschi, R. G. (2017). Relating dispositional mindfulness, contemplative practice, and positive reappraisal with posttraumatic cognitive coping, stress, and growth. Psychological Trauma: Theory, Research, Practice, and Policy, 9(5), 526–536. 10.1037/tra0000208 [DOI] [PubMed] [Google Scholar]
- Haspolat, A. , & Çırakoğlu, O. C. (2021). Mindfulness as a moderator in the relation among core belief disruption, rumination, posttraumatic symptoms, and growth. Mindfulness, 12(1), 186–197. 10.1007/s12671-020-01511-6 [DOI] [Google Scholar]
- Ho, S. M. , Chan, C. L. , & Ho, R. T. (2004). Posttraumatic growth in Chinese cancer survivors. Psycho‐Oncology. Journal of the Psychological, Social and Behavioral Dimensions of Cancer, 13(6), 377–389. 10.1002/pon.758 [DOI] [PubMed] [Google Scholar]
- Huster, R. J. , Enriquez‐Geppert, S. , Lavallee, C. F. , Falkenstein, M. , & Herrmann, C. S. (2013). Electroencephalography of response inhibition tasks: Functional networks and cognitive contributions. International Journal of Psychophysiology, 87(3), 217–233. 10.1016/j.ijpsycho.2012.08.001 [DOI] [PubMed] [Google Scholar]
- Jayawickreme, E. , Infurna, F. J. , Alajak, K. , Blackie, L. E. , Chopik, W. J. , Chung, J. M. , Dorfman, A. , Fleeson, W. , Forgeard, M. J. C. , Frazier, P. , Furr, R. M. , Grossmann, I. , Heller, A. S. , Laceulle, O. M. , Lucas, R. E. , Luhmann, M. , Luong, G. , Meijer, L. , McLean, K. C. , & Zonneveld, R. (2021). Post‐traumatic growth as positive personality change: Challenges, opportunities, and recommendations. Journal of Personality, 89(1), 145–165. 10.1111/jopy.12591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalaitzaki, A. (2021). Posttraumatic symptoms, posttraumatic growth, and internal resources among the general population in Greece: A nation‐wide survey amid the first COVID‐19 lockdown. International Journal of Psychology, 56(5), 766–771. 10.1002/IJOP.12750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamranvand, M. , Dehghani‐Arani, F. , Rostami, R. , Sadeghniat, K. , & Farahani, H. (2022). Predicting posttraumatic growth in COVID‐19 patients using electroencephalogram signals. Frontiers in Biomedical Technologies. 10.18502/fbt.v9i3.9643 [DOI] [Google Scholar]
- Kaunhoven, R. J. , & Dorjee, D. (2020). How does mindfulness modulate self‐regulation in preadolescent children. An Integrative Neuro Cognitive Review. Neuroscience & Biobehavioral Reviews, Р, 163–184. 10.1016/j.neubiorev.2017.01.007 [DOI] [PubMed] [Google Scholar]
- Kelley, N. J. , Glazer, J. E. , Pornpattananangkul, N. , & Nusslock, R. (2019). Reappraisal and suppression emotion‐regulation tendencies differentially predict reward‐responsivity and psychological well‐being. Biological Psychology, 140, 35–47. 10.1016/j.biopsycho.2018.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kira, I. A. , Shuwiekh, H. , Al Ibraheem, B. , & Aljakoub, J. (2019). Appraisals and emotion regulation mediate the effects of identity salience and cumulative stressors and traumas, on PTG and mental health: The case of Syrian’s IDPs and refugees. Self and Identity, 18(3), 284–305. 10.1080/15298868.2018.1451361 [DOI] [Google Scholar]
- Langeslag, S. J. , & Van Strien, J. W. (2010). Comparable modulation of the late positive potential by emotion regulation in younger and older adults. Journal of Psychophysiology, 24(3), 186–197. 10.1027/0269-8803/a000009 [DOI] [Google Scholar]
- Larsen, S. E. , & Berenbaum, H. (2015). Are specific emotion regulation strategies differentially associated with posttraumatic growth versus stress? Journal of Aggression, Maltreatment & Trauma, 24(7), 794–808. 10.1080/10926771.2015.1062451 [DOI] [Google Scholar]
- Lau, B. H. P. , Chan, C. L. W. , & Ng, S. M. (2021). Post‐traumatic growth in the first COVID outbreak in Hong Kong. Frontiers in Psychology, 12, 675132. 10.3389/fpsyg.2021.675132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, Y. , Tang, R. , & Braver, T. S. (2022). Investigating mindfulness influences on cognitive function: On the promise and potential of converging research strategies. Psychonomic Bulletin & Review, 29(4), 1–25. 10.3758/s13423-021-02008-6 [DOI] [PubMed] [Google Scholar]
- Liu, X. , Liu, Y. , Shi, H. , Li, L. , & Zheng, M. (2021). Regulation of mindfulness‐based music listening on negative emotions related to COVID‐19: An ERP study. International Journal of Environmental Research and Public Health, 18(13), 7063. 10.3390/ijerph18137063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, X. , Ng, S.‐M. , Xing, Y. Y. , Liu, X. , Li, H. Y. , Fung, M. H. Y. , & Lai Wan Chan, C. (2021). A Strength‐Based Online Community Intervention (SOCI) for promoting resilience among adults in Hubei province, China, during COVID‐19 lockdown. Asia Pacific Journal of Social Work and Development, 31(4), 253–270. 10.1080/02185385.2021.1923560 [DOI] [Google Scholar]
- Liu, Y. , Huang, H. , McGinnis‐Deweese, M. , Keil, A. , & Ding, M. (2012). Neural substrate of the late positive potential in emotional processing. Journal of Neuroscience, 32(42), 14563–14572. 10.1523/JNEUROSCI.3109-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markus, H. R. , & Kitayama, S. (2010). Cultures and selves: A cycle of mutual constitution. Perspectives on Psychological Science, 5(4), 420–430. 10.1177/1745691610375557Menezes [DOI] [PubMed] [Google Scholar]
- Miragall, M. , Herrero, R. , Vara, M. D. , Galiana, L. , & Baños, R. M. (2021). The impact of strict and forced confinement due to the COVID‐19 pandemic on positive functioning variables, emotional distress, and posttraumatic growth in a Spanish sample. European Journal of Psychotraumatology, 12(1), 1918900. 10.1080/20008198.2021.1918900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser, J. S. , Hajcak, G. , Bukay, E. , & Simons, R. F. (2006). Intentional modulation of emotional responding to unpleasant pictures: An ERP study. Psychophysiology, 43(3), 292–296. 10.1111/j.1469-8986.2006.00402.x [DOI] [PubMed] [Google Scholar]
- Orejuela‐Dávila, A. I. , Levens, S. M. , Sagui‐Henson, S. J. , Tedeschi, R. G. , & Sheppes, G. (2019). The relation between emotion regulation choice and posttraumatic growth. Cognition & Emotion, 33(8), 1709–1717. 10.1080/02699931.2019.1592117 [DOI] [PubMed] [Google Scholar]
- Pereira, M. G. , & Bizarro, L. (2012). Sitting and silent meditation as a strategy to study emotion regulation. Psychology & Neuroscience, 5, 027–036. 10.3922/j.psns.2012.1.05 [DOI] [Google Scholar]
- Petrocchi, S. , Pellegrino, S. A. , Manoni, G. , Petrovic, G. , & Schulz, P. J. (2023). What does not kill you… mutates and tries again.” A study on personality determinants of post‐traumatic growth during the COVID‐19 pandemic. Current Psychology, 42(23), 20134–20148. 10.1007/s12144-023-04415-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Povero, M. , Turco, P. , & Dal Negro, R. W. (2022). The emotional response to pandemic of middle‐ and high‐school students of an Italian northern province: The ERP study. Children, 9(1), 59. Article 1. 10.3390/children9010059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachman, S. (1980). Emotional processing. Behaviour Research and Therapy, 18(1), 51–60. 10.1016/0005-7967(80)90069-8 [DOI] [PubMed] [Google Scholar]
- Raz, S. , Dan, O. , & Zysberg, L. (2014). Neural correlates of emotional intelligence in a visual emotional oddball task: An ERP study. Brain and Cognition, 91, 79–86. 10.1016/j.bandc.2014.09.003 [DOI] [PubMed] [Google Scholar]
- Sobolewski, A. , Holt, E. , Kublik, E. , & Wróbel, A. (2011). Impact of meditation on emotional processing—A visual ERP study. Neuroscience Research, 71(1), 44–48. 10.1016/j.neures.2011.06.002 [DOI] [PubMed] [Google Scholar]
- Taylor, V. A. , Grant, J. , Daneault, V. , Scavone, G. , Breton, E. , Roffe‐Vidal, S. , Courtemanche, J. , Lavarenne, A. S. , & Beauregard, M. (2011). Impact of mindfulness on the neural responses to emotional pictures in experienced and beginner meditators. NeuroImage, 57(4), 1524–1533. 10.1016/j.neuroimage.2011.06.001 [DOI] [PubMed] [Google Scholar]
- Tedeschi, R. G. , & Blevins, C. L. (2015). From mindfulness to meaning: Implications for the theory of posttraumatic growth. Psychological Inquiry, 26(4), 373–376. 10.1080/1047840x.2015.1075354 [DOI] [Google Scholar]
- Tedeschi, R. G. , & Calhoun, L. G. (1996). The posttraumatic growth inventory: Measuring the positive legacy of trauma. Journal of Traumatic Stress, 9(3), 455–471. 10.1007/bf02103658 [DOI] [PubMed] [Google Scholar]
- Tedeschi, R. G. , & Calhoun, L. G. (2004). Posttraumatic growth: Conceptual foundations and empirical evidence. Psychological Inquiry, 15(1), 1–18. 10.1207/s15327965pli1501_01 [DOI] [Google Scholar]
- Tedeschi, R. G. , Park, C. L. , & Calhoun, L. G. (1998). Posttraumatic growth: Positive changes in the aftermath of crisis. Routledge. [Google Scholar]
- Vloet, T. D. , Vloet, A. , Burger, A. , & Romanos, M. (2017). Post‐traumatic growth in children and adolescents. Journal of Traumatic Stress Disorders & Treatment, 06(04). 10.4172/2324-8947.1000178 [DOI] [Google Scholar]
- Xie, C.‐S. , & Kim, Y. (2022). Post‐traumatic growth during COVID‐19: The role of perceived social support, personality, and coping strategies. Healthcare, 10(2), 224. 10.3390/healthcare10020224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, M. , Deng, X. , & An, S. (2021). The immediate and lasting effect of emotion regulation in adolescents: An ERP study. International Journal of Environmental Research and Public Health, 18(1), 1–2. 10.3390/ijerph2004010001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan, J. , Long, Q. , Ding, N. , Lou, Y. , Liu, Y. , & Yang, J. (2015). Suppression dampens unpleasant emotion faster than reappraisal: Neural dynamics in a Chinese sample. Science China Life Sciences, 58(5), 480–491. 10.1007/s11427-014-4739-6 [DOI] [PubMed] [Google Scholar]
- Zhou, X. , Wu, X. , Zeng, M. , & Tian, Y. (2016). The relationship between emotion regulation and PTSD/PTG among adolescents after the Ya’an earthquake: The moderating role of social support. Acta Psychology Sinica, 48(8), 969. 10.3724/SP.J.1041.2016.00969 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The participants of this study did not give written consent for their data to be shared publicly, so due to the sensitive nature of the research supporting data is not available.


