Abstract
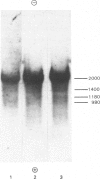
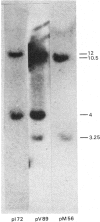
Double-stranded complementary DNA (cDNA) sequences were prepared from day-old chick lens total polysomal RNA and inserted into the unique PstI restriction site of the plasmid pBR322. Colonies containing sequences complementary to abundant lens poly(A)-containing RNA sequences were identified by using lens 32P-labelled cDNA. Some of these clones have been characterized as containing delta-crystallin mRNA coding sequences by genomic DNA blot hybridization and RNA blot hybridizations. Hybridization of labelled DNA from such clones to RNA blots detected four size classes of delta-crystallin RNA sequences, although Southern blots indicated that there are probably only two delta-crystallin genes.
Full text
PDF

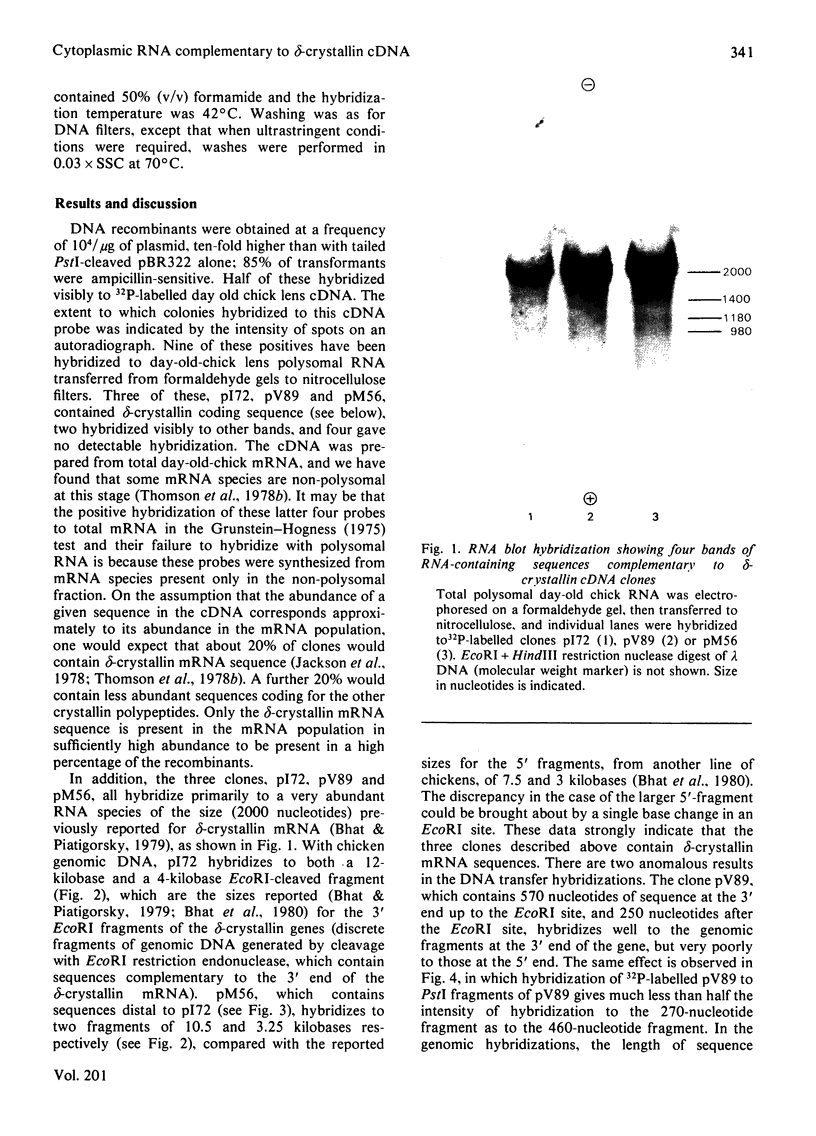
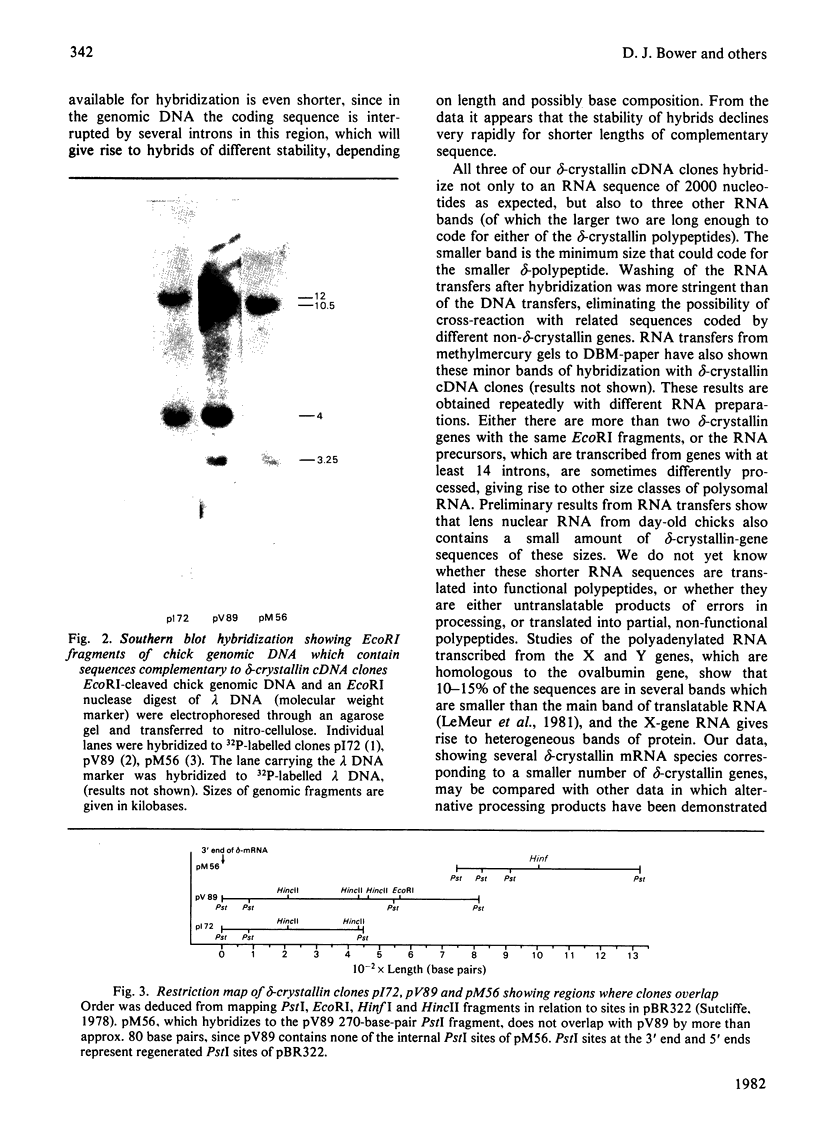

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alwine J. C., Kemp D. J., Stark G. R. Method for detection of specific RNAs in agarose gels by transfer to diazobenzyloxymethyl-paper and hybridization with DNA probes. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5350–5354. doi: 10.1073/pnas.74.12.5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat S. P., Jones R. E., Sullivan M. A., Piatigorsky J. Chicken lens crystallin DNA sequences show at least two delta-crystallin genes. Nature. 1980 Mar 20;284(5753):234–238. doi: 10.1038/284234a0. [DOI] [PubMed] [Google Scholar]
- Bhat S. P., Piatigorsky J. Molecular cloning and partial characterization of delta-crystallin cDNA sequences in a bacterial plasmid. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3299–3303. doi: 10.1073/pnas.76.7.3299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton R. M. Failure of growth regulation of the lens epithelium in strains of fast-growing chicks. Genet Res. 1975 Feb;25(1):79–82. doi: 10.1017/s0016672300015457. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Properties of a supercoiled deoxyribonucleic acid-protein relaxation complex and strand specificity of the relaxation event. Biochemistry. 1970 Oct 27;9(22):4428–4440. doi: 10.1021/bi00824a026. [DOI] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Enea V., Vovis G. F., Zinder N. D. Genetic studies with heteroduplex DNA of bacteriophage fl. Asymmetric segregation, base correction and implications for the mechanism of genetic recombination. J Mol Biol. 1975 Aug 15;96(3):495–509. doi: 10.1016/0022-2836(75)90175-8. [DOI] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries P., Cochet M., Krust A., Gerlinger P., Kourilsky P., Chambon P. Molecular cloning of extensive sequences of the in vitro synthesized chicken ovalbumin structural gene. Nucleic Acids Res. 1977 Jul;4(7):2389–2406. doi: 10.1093/nar/4.7.2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson J. F., Clayton R. M., Williamson R., Thomson I., Truman D. E., de Pomerai D. I. Sequence complexity and tissue distribution of chick lens crystallin mRNAs. Dev Biol. 1978 Aug;65(2):383–395. doi: 10.1016/0012-1606(78)90034-9. [DOI] [PubMed] [Google Scholar]
- LeMeur M., Glanville N., Mandel J. L., Gerlinger P., Palmiter R., Chambon P. The ovalbumin gene family: hormonal control of X and Y gene transcription and mRNA accumulation. Cell. 1981 Feb;23(2):561–571. doi: 10.1016/0092-8674(81)90152-5. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RABAEY M. Electrophoretic and immunoelectrophoretic studies on the soluble proteins in the developing lens of birds. Exp Eye Res. 1962 Jun;1:310–316. doi: 10.1016/s0014-4835(62)80017-7. [DOI] [PubMed] [Google Scholar]
- Roychoudhury R., Jay E., Wu R. Terminal labeling and addition of homopolymer tracts to duplex DNA fragments by terminal deoxynucleotidyl transferase. Nucleic Acids Res. 1976 Apr;3(4):863–877. doi: 10.1093/nar/3.4.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow P., Brutlag D., Kornberg A. Deoxyribonucleic acid polymerase: two distinct enzymes in one polypeptide. I. A proteolytic fragment containing the polymerase and 3' leads to 5' exonuclease functions. J Biol Chem. 1972 Jan 10;247(1):224–231. [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stein J. P., Catterall J. F., Kristo P., Means A. R., O'Malley B. W. Ovomucoid intervening sequences specify functional domains and generate protein polymorphism. Cell. 1980 Oct;21(3):681–687. doi: 10.1016/0092-8674(80)90431-6. [DOI] [PubMed] [Google Scholar]
- Sutcliffe J. G. pBR322 restriction map derived from the DNA sequence: accurate DNA size markers up to 4361 nucleotide pairs long. Nucleic Acids Res. 1978 Aug;5(8):2721–2728. doi: 10.1093/nar/5.8.2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson I., Wilkinson C. E., Burns A. T., Truman D. E., Clayton R. M. Characterization of chick lens soluble proteins and the control of their synthesis. Exp Eye Res. 1978 Mar;26(3):351–362. doi: 10.1016/0014-4835(78)90081-7. [DOI] [PubMed] [Google Scholar]
- Thomson I., Wilkinson C. E., Jackson J. F., de Pomerai D. I., Clayton R. M., Truman D. E., Williamson R. Isolation and cell-free translation of chick lens crystallin mRNA during normal development and transdifferentiation of neural retina. Dev Biol. 1978 Aug;65(2):372–382. doi: 10.1016/0012-1606(78)90033-7. [DOI] [PubMed] [Google Scholar]
- Truman D. E., Brown A. G., Campbell J. C. The relationship between the ontogeny of antigens and of the polypeptide chains of the crystallins during chick lens development. Exp Eye Res. 1972 Jan;13(1):58–69. doi: 10.1016/0014-4835(72)90125-x. [DOI] [PubMed] [Google Scholar]
- Vogt V. M. Purification and further properties of single-strand-specific nuclease from Aspergillus oryzae. Eur J Biochem. 1973 Feb 15;33(1):192–200. doi: 10.1111/j.1432-1033.1973.tb02669.x. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. A., Hagenbüchle O., Schibler U. A single mouse alpha-amylase gene specifies two different tissue-specific mRNAs. Cell. 1981 Feb;23(2):451–458. doi: 10.1016/0092-8674(81)90140-9. [DOI] [PubMed] [Google Scholar]