Abstract
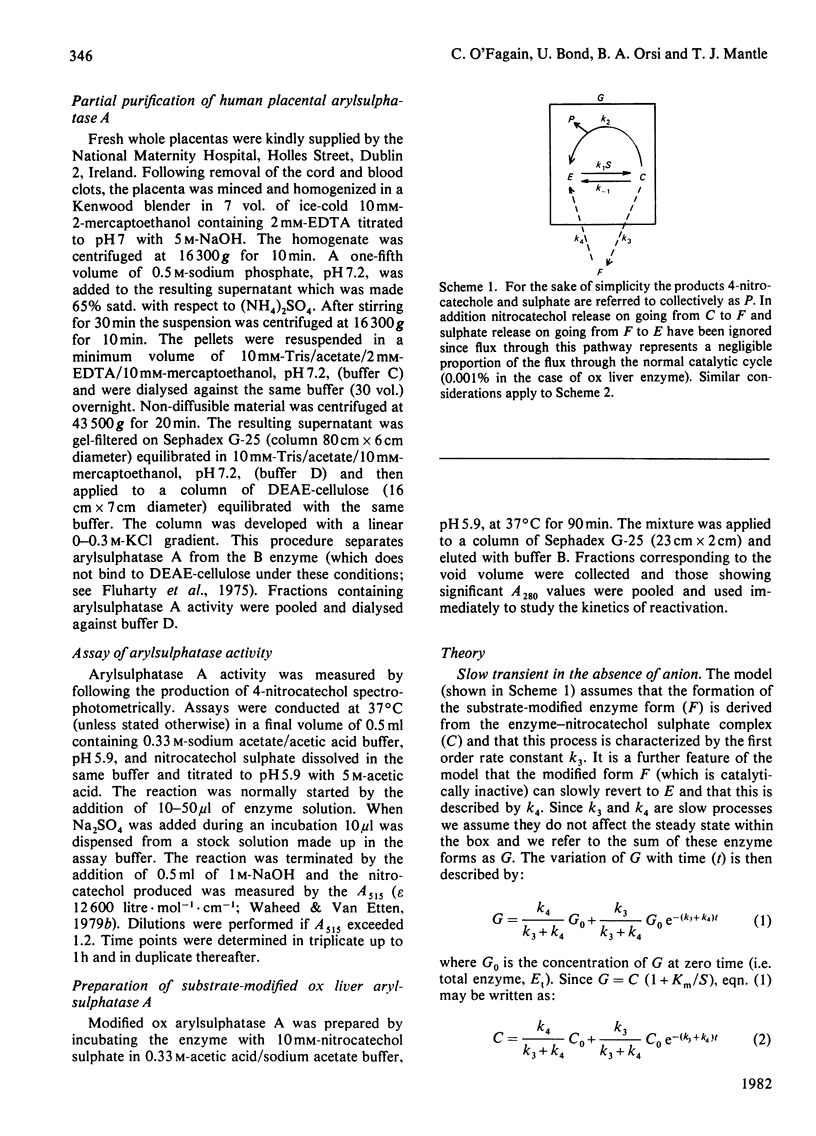
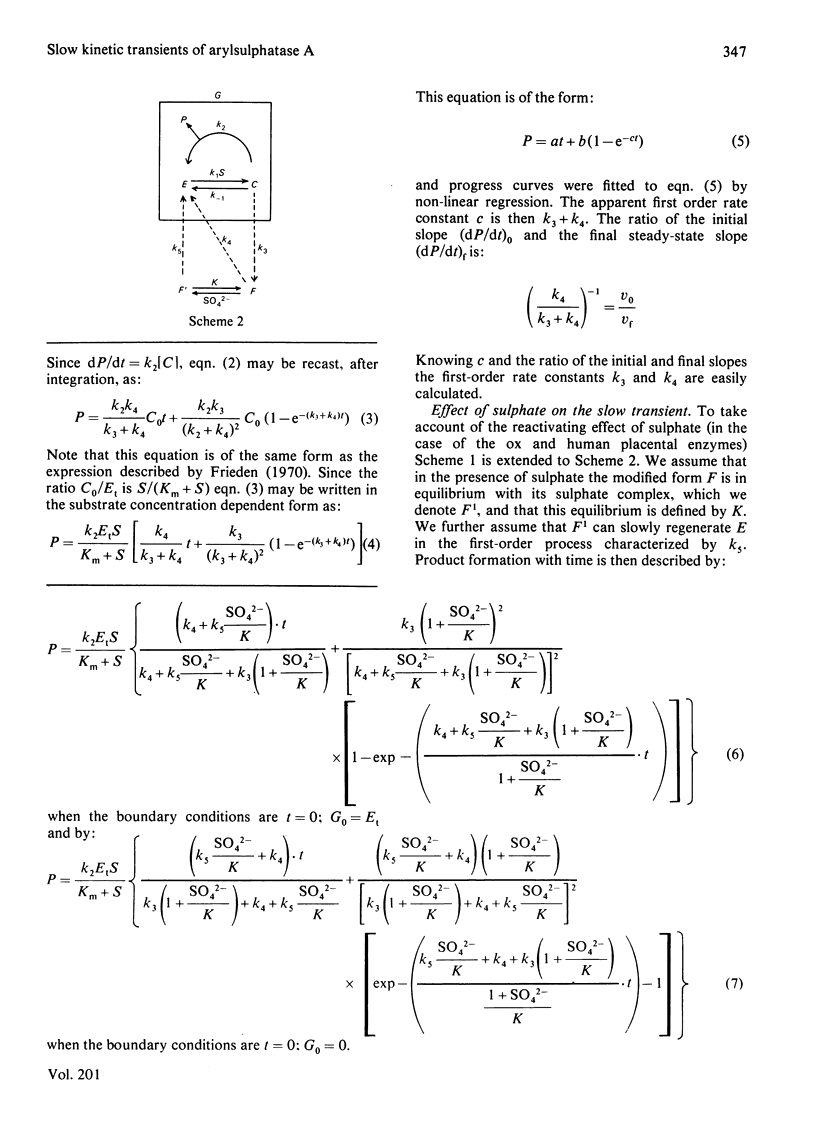
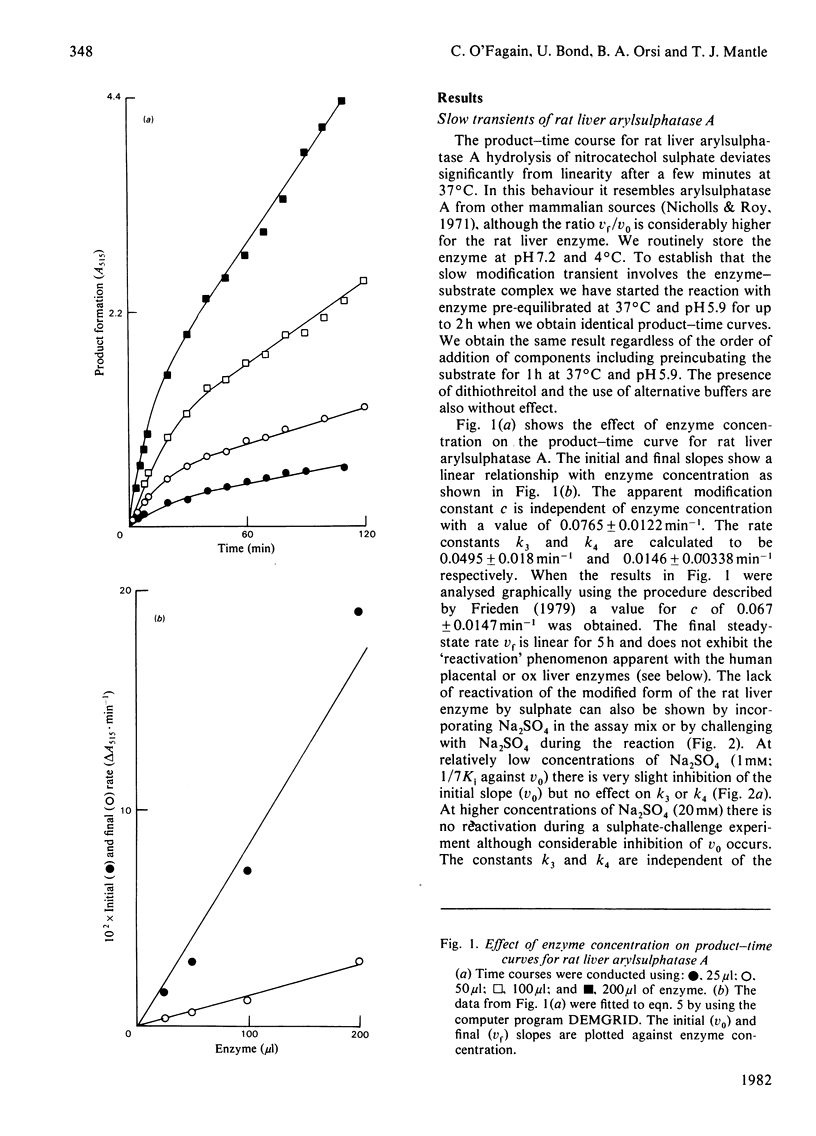
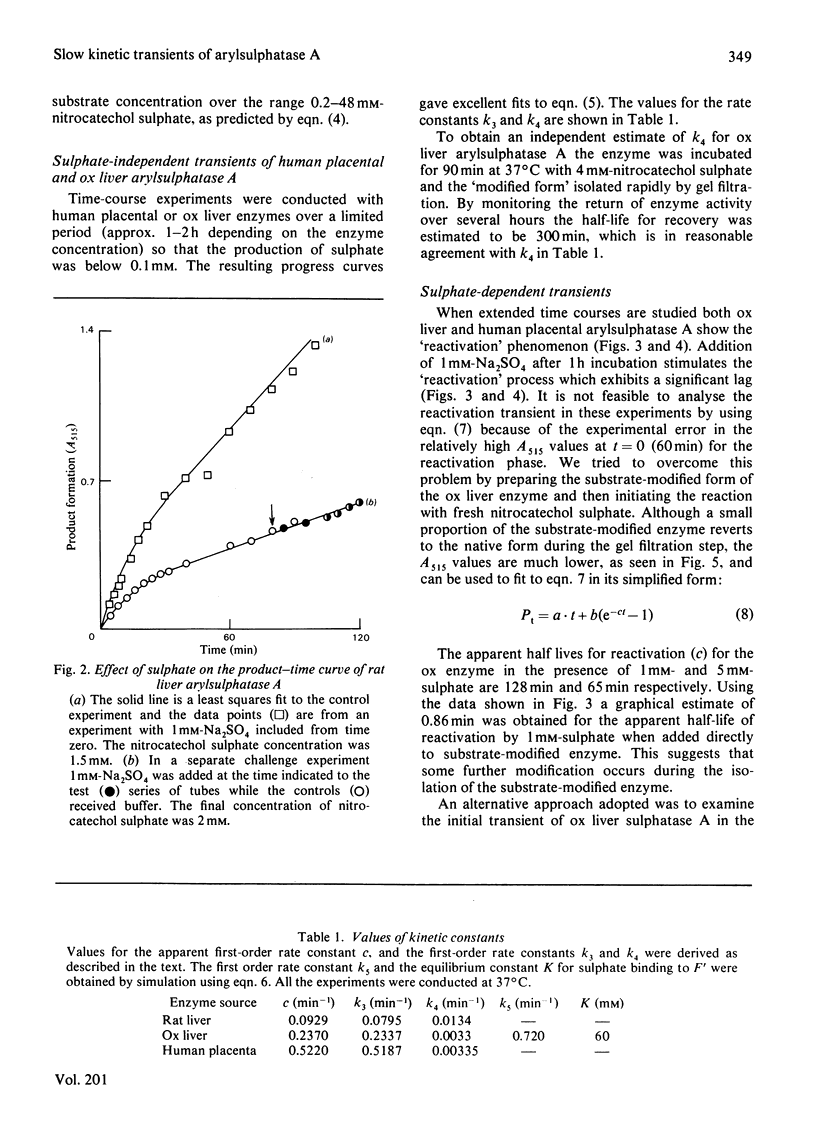
A simple model is described to account for the anomalous time course of arylsulphatase A. In the case of the ox liver and human placental enzymes the enzyme-nitrocatechol sulphate complex can, in addition to forming products, slowly break down to form an inactive species which can turn in slowly regenerate active enzyme. When the inactive form binds sulphate the rate of reactivation is enhanced, 218-fold in the case of the ox enzyme. Rat liver arylsulphatase A is refractory to reactivation by sulphate.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen E., Roy A. B. The sulphatase of ox liver. XI. The isoelectric focussing of a purified preparation of sulphatase B. Biochim Biophys Acta. 1968 Oct 21;168(2):243–251. doi: 10.1016/0005-2795(68)90147-5. [DOI] [PubMed] [Google Scholar]
- BAUM H., DODGSON K. S., SPENCER B. Studies on sulphatases. 21. The anomalous kinetics of arylsulphatase A of human tissues: the anomalies. Biochem J. 1958 Aug;69(4):567–572. doi: 10.1042/bj0690567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAUM H., DODGSON K. S. Studies on sulphatases. 22. The anomalous kinetics of arylsulphatase A of human tissues: interpretation of the anomalies. Biochem J. 1958 Aug;69(4):573–582. doi: 10.1042/bj0690573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fluharty A. L., Stevens R. L., Fung D., Peak S., Kihara H. Uridine diphospho-N-acetylgalactosamine-4-sulfate sulfohydrolase activity of human arylsulfatase B and its deficiency in the Maroteaux-Lamy syndrome. Biochem Biophys Res Commun. 1975 Jan 2;64(3):955–962. doi: 10.1016/0006-291x(75)90140-0. [DOI] [PubMed] [Google Scholar]
- Frieden C. Kinetic aspects of regulation of metabolic processes. The hysteretic enzyme concept. J Biol Chem. 1970 Nov 10;245(21):5788–5799. [PubMed] [Google Scholar]
- Frieden C. Slow transitions and hysteretic behavior in enzymes. Annu Rev Biochem. 1979;48:471–489. doi: 10.1146/annurev.bi.48.070179.002351. [DOI] [PubMed] [Google Scholar]
- Jerfy A., Roy A. B., Tomkins H. J. The sulphatase of ox liver. XIX. On the nature of the polymeric forms of sulphatase A present in dilute solutions. Biochim Biophys Acta. 1976 Feb 13;422(2):335–378. doi: 10.1016/0005-2744(76)90145-5. [DOI] [PubMed] [Google Scholar]
- KITZ R., WILSON I. B. Esters of methanesulfonic acid as irreversible inhibitors of acetylcholinesterase. J Biol Chem. 1962 Oct;237:3245–3249. [PubMed] [Google Scholar]
- Nicholls R. G., Jerfy A., Roy A. B. The determination of the initial velocity of enzyme-catalysed reactions. Anal Biochem. 1974 Sep;61(1):93–100. doi: 10.1016/0003-2697(74)90336-4. [DOI] [PubMed] [Google Scholar]
- Prosser C. I., Roy A. B. The sulphatase of ox liver. XXIII. The nature of substrate-modified sulphatase A. Biochim Biophys Acta. 1980;613(1):130–139. doi: 10.1016/0005-2744(80)90199-0. [DOI] [PubMed] [Google Scholar]
- Roy A. B. Kinetic properties of arylsulphatase A--theoretical treatment. Biochim Biophys Acta. 1972 Aug 28;276(2):488–490. doi: 10.1016/0005-2744(72)91009-1. [DOI] [PubMed] [Google Scholar]
- Roy A. B. The sulphatase of ox liver. XXI: kinetic studies of the substrate-induced inactivation of sulphatase A. Biochim Biophys Acta. 1978 Oct 12;526(2):489–506. doi: 10.1016/0005-2744(78)90140-7. [DOI] [PubMed] [Google Scholar]
- SPENCER B. Studies on sulphatases. 20. Enzymic cleavage of aryl hydrogen sulphates in the presence of H218O. Biochem J. 1958 May;69(1):155–159. doi: 10.1042/bj0690155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soper T. S., Manning J. M. beta-elimination of beta-halo substrates by D-amino acid transaminase associated with inactivation of the enzyme. Trapping of a key intermediate in the reaction. Biochemistry. 1978 Aug 8;17(16):3377–3384. doi: 10.1021/bi00609a031. [DOI] [PubMed] [Google Scholar]
- Stinshoff K. Kinetic properties of the arylsulphatase A from human kidneys. Biochim Biophys Acta. 1972 Aug 28;276(2):475–488. doi: 10.1016/0005-2744(72)91008-x. [DOI] [PubMed] [Google Scholar]
- Waheed A., Van Etten R. L. Covalent modification as the cause of the anomalous kinetics of aryl sulfatase A. Arch Biochem Biophys. 1979 Jun;195(1):248–251. doi: 10.1016/0003-9861(79)90348-5. [DOI] [PubMed] [Google Scholar]
- Waheed A., Van Etten R. L. The monomer -- dimer association of rabbit lver aryl sulfatase A and its relationship to the anomalous kinetics. Arch Biochem Biophys. 1979 Apr 15;194(1):215–225. doi: 10.1016/0003-9861(79)90612-x. [DOI] [PubMed] [Google Scholar]
- Waheed A., Van Etten R. L. The structural basis of anomalous kinetics of rabbit liver aryl sulfatase A. Arch Biochem Biophys. 1980 Aug;203(1):11–24. doi: 10.1016/0003-9861(80)90149-6. [DOI] [PubMed] [Google Scholar]
- Waley S. G. Kinetics of suicide substrates. Biochem J. 1980 Mar 1;185(3):771–773. doi: 10.1042/bj1850771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang E., Walsh C. Suicide substrates for the alanine racemase of Escherichia coli B. Biochemistry. 1978 Apr 4;17(7):1313–1321. doi: 10.1021/bi00600a028. [DOI] [PubMed] [Google Scholar]
- Worwood M., Dodgson K. S., Hook G. E., Rose F. A. Problems associated with the assay of arylsulphatases A and B of rat tissues. Biochem J. 1973 May;134(1):183–190. doi: 10.1042/bj1340183. [DOI] [PMC free article] [PubMed] [Google Scholar]


