Abstract
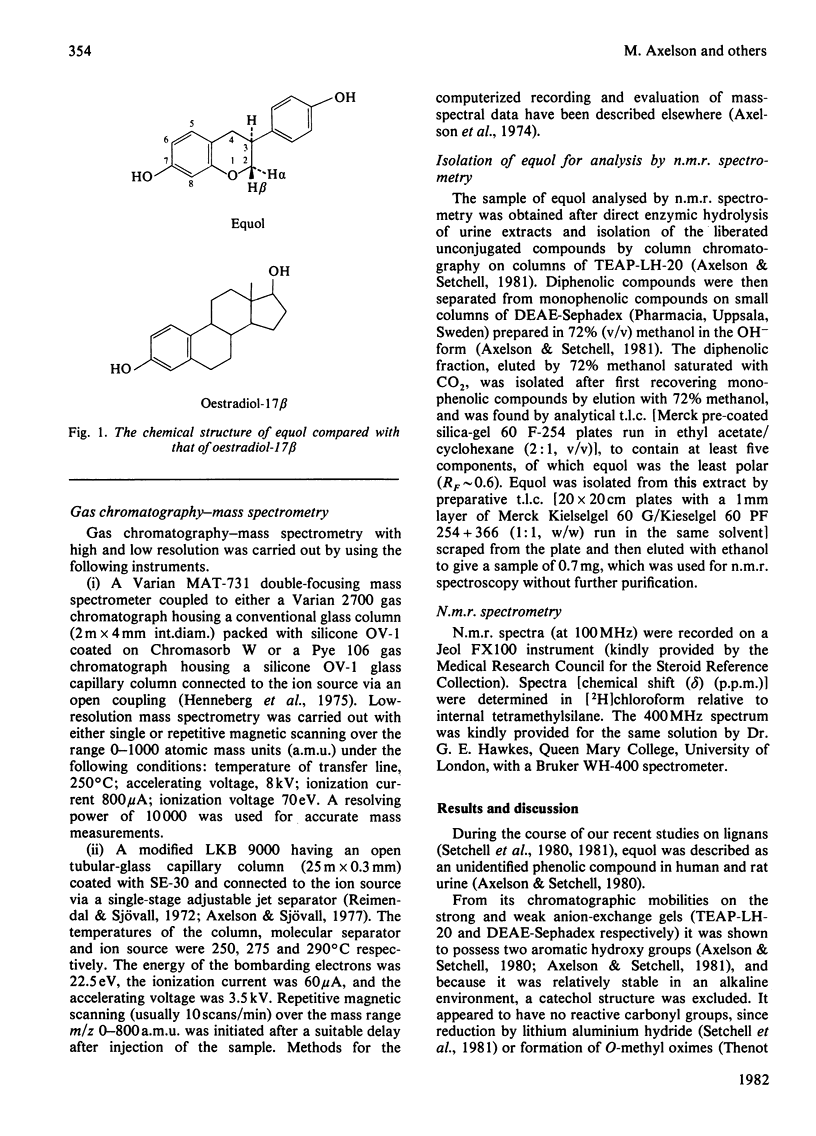
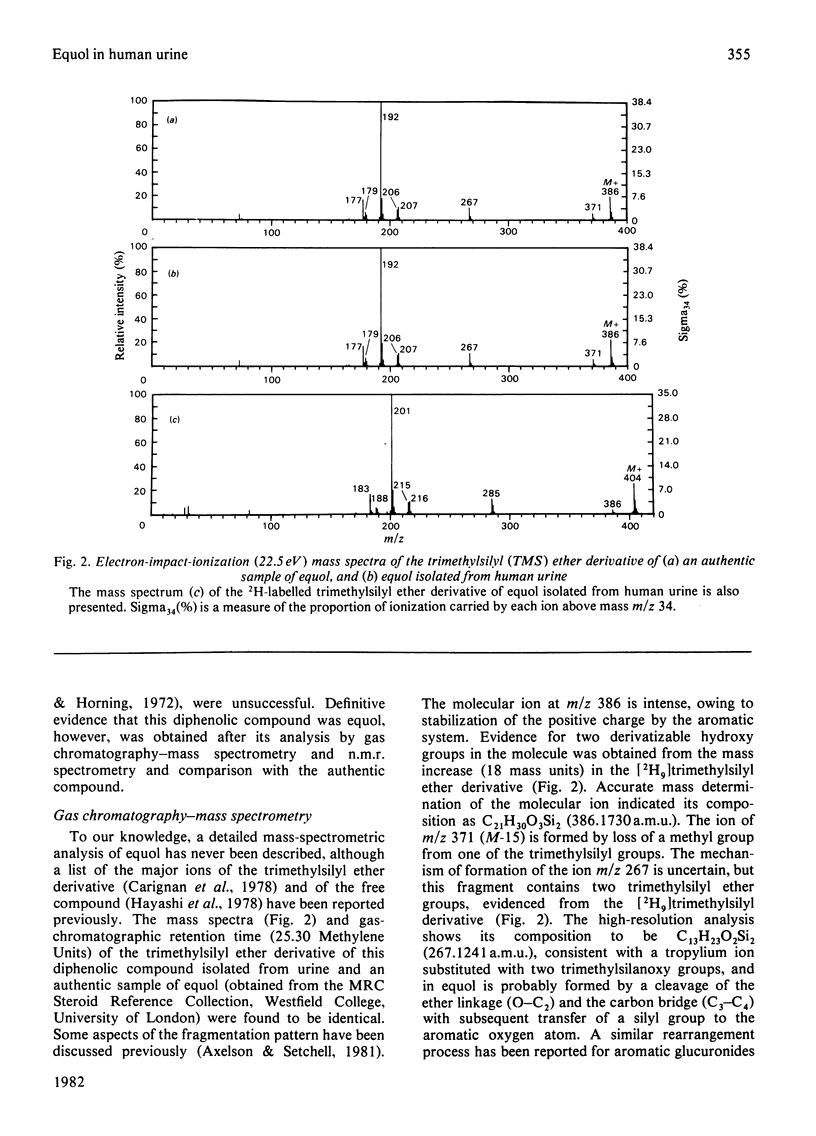
The identification (by gas chromatography–mass spectrometry and n.m.r.) for the first time of the weak oestrogen equol [7-hydroxy-3-(4′-hydroxyphenyl)chroman] in human urine is described. Preliminary results of its quantitative excretion in urine are reported and the potential significance of the occurrence of this compound is discussed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axelson M., Setchell K. D. Conjugation of lignans in human urine. FEBS Lett. 1980 Dec 15;122(1):49–53. doi: 10.1016/0014-5793(80)80399-1. [DOI] [PubMed] [Google Scholar]
- Axelson M., Setchell K. D. The excretion of lignans in rats -- evidence for an intestinal bacterial source for this new group of compounds. FEBS Lett. 1981 Jan 26;123(2):337–342. doi: 10.1016/0014-5793(81)80322-5. [DOI] [PubMed] [Google Scholar]
- Axelson M., Sjövall J. Analysis of unconjugated steroids in plasma by liquid-gel chromatography and glass capillary gas chromatography mass spectrometry. J Steroid Biochem. 1977 Jun;8(6):683–692. doi: 10.1016/0022-4731(77)90297-7. [DOI] [PubMed] [Google Scholar]
- BRADBURY R. B., WHITE D. E. Estrogens and related substances in plants. Vitam Horm. 1954;12:207–233. doi: 10.1016/s0083-6729(08)61013-4. [DOI] [PubMed] [Google Scholar]
- Batterham T. J., Hart N. K., Lamberton J. A. Metabolism of oestrogenic isoflavones in sheep. Nature. 1965 May 1;206(983):509–509. doi: 10.1038/206509a0. [DOI] [PubMed] [Google Scholar]
- Billets S., Lietman P. S., Fenselau C. Mass spectral analysis of glucuronides. J Med Chem. 1973 Jan;16(1):30–33. doi: 10.1021/jm00259a009. [DOI] [PubMed] [Google Scholar]
- COMMON R. H., AINSWORTH L. Identification of equol in the urine of the domestic fowl. Biochim Biophys Acta. 1961 Oct 28;53:403–404. doi: 10.1016/0006-3002(61)90452-8. [DOI] [PubMed] [Google Scholar]
- KLYNE W., WRIGHT A. A. Steroids and other lipids of pregnant cow's urine. J Endocrinol. 1959 Jan;18(1):32–45. doi: 10.1677/joe.0.0180032. [DOI] [PubMed] [Google Scholar]
- KLYNE W., WRIGHT A. A. Steroids and other lipids of pregnant goat's urine. Biochem J. 1957 May;66(1):92–101. doi: 10.1042/bj0660092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrian G. F., Haslewood G. A. Equol, a new inactive phenol isolated from the ketohydroxyoestrin fraction of mares' urine. Biochem J. 1932;26(4):1227–1232. doi: 10.1042/bj0261227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson A., Hill J. L., Davids H. L. An in vitro study of formononetin and biochanin A metabolism in rumen fluid from sheep. Biochim Biophys Acta. 1967 Oct 9;148(1):92–98. doi: 10.1016/0304-4165(67)90282-6. [DOI] [PubMed] [Google Scholar]
- Reimendal R., Sjövall J. Analysis of steroids by off-line computerized gas chromatography-mass spectrometry. Anal Chem. 1972 Jan;44(1):21–29. doi: 10.1021/ac60309a014. [DOI] [PubMed] [Google Scholar]
- Rutten G. A., Luyten J. A. Analysis of steroids by high resolution gas-liquid chromatography. I. Preparation of apolar columns. J Chromatogr. 1972 Dec 20;74(2):177–193. doi: 10.1016/s0021-9673(01)86148-3. [DOI] [PubMed] [Google Scholar]
- Setchell K. D., Lawson A. M., Conway E., Taylor N. F., Kirk D. N., Cooley G., Farrant R. D., Wynn S., Axelson M. The definitive identification of the lignans trans-2,3-bis(3-hydroxybenzyl)-gamma-butyrolactone and 2,3-bis(3-hydroxybenzyl)butane-1,4-diol in human and animal urine. Biochem J. 1981 Aug 1;197(2):447–458. doi: 10.1042/bj1970447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setchell K. D., Lawson A. M., Mitchell F. L., Adlercreutz H., Kirk D. N., Axelson M. Lignans in man and in animal species. Nature. 1980 Oct 23;287(5784):740–742. doi: 10.1038/287740a0. [DOI] [PubMed] [Google Scholar]
- Shutt D. A., Cox R. I. Steroid and phyto-oestrogen binding to sheep uterine receptors in vitro. J Endocrinol. 1972 Feb;52(2):299–310. doi: 10.1677/joe.0.0520299. [DOI] [PubMed] [Google Scholar]
- Shutt D. A. The effects of plant oestrogens on animal reproduction. Endeavour. 1976 Sep;35(126):110–113. doi: 10.1016/0160-9327(76)90004-1. [DOI] [PubMed] [Google Scholar]
- Spiegelhalder B., Röhle G., Siekmann L., Breuer H. Mass-spectrometry of steroid glucuronides. J Steroid Biochem. 1976 Oct;7(10):749–756. doi: 10.1016/0022-4731(76)90175-8. [DOI] [PubMed] [Google Scholar]
- Tang B. Y., Adams N. R. Effect of equol on oestrogen receptors and on synthesis of DNA and protein in the immature rat uterus. J Endocrinol. 1980 May;85(2):291–297. doi: 10.1677/joe.0.0850291. [DOI] [PubMed] [Google Scholar]


