Abstract
Introduction
The meniscus is an important cushioning structure of the knee joint, with the maintenance of its normal structure and function playing a crucial role in protecting the joint from early degeneration. Stem/progenitor cells could be the key to help researchers to have a deeper understanding of the biological process of meniscal injury repair and may be important in the meniscus tissue regeneration processes. To the best of our knowledge, there is currently a lack of comprehensive reviews on existing research about the meniscus progenitor cells (MPCs).
Objectives
By reviewing the existing MPC literature, we aim to provide insights for future research on meniscus regeneration.
Methods
The isolation methods, biological characteristics and the translational application of MPCs were summarized.
Results
MPCs could be isolated according to their colony-forming ability, marker expression, migration ability, and differential adhesion to fibronectin. Most existing studies on surface markers of MPCs have largely followed the paradigm of mesenchymal stromal/stem cell research. Based on the information provided by their surface markers and expression profile, researchers located MPCs in the peripheral surface area of the meniscus. Few researches have investigated the translation and application of MPCs, with most studies being limited to MPCs extraction and subsequent reimplantation in vivo.
Conclusions
MPCs are a group of meniscus-resident cells, which exhibit certain stem/progenitor cell characteristics, such as the ability to undergo multilineage differentiation in in vitro culture.
Keywords: Meniscus, Meniscus progenitor cell, Mesenchymal stromal/stem cell
Introduction
The meniscus is a crescent-shaped fibrocartilaginous tissue which is located between the femoral condyle and tibial plateau.1, 2, 3 It has important roles in load bearing, joint lubrication and preventing the development of osteoarthritis.2 Its functioning relies on the structural integrity and appropriate organization of the tissue.3, 4, 5 The meniscus injury rate varies in populations with different ages, sexes etc.6, 7 Currently, most repair techniques are designed to treat the tears in vascular areas (red zone) which are considered to possess healing potential.8, 9 However, the management of injuries in avascular areas (white zone) is still a challenging clinical issue. Due to its poor blood supply, suturing and other repair techniques often achieve poor results in the white zone, which can lead to meniscectomy, increasing the risk of early onset of osteoarthritis (OA).10 The past decade has witnessed the rapid development in tissue engineering approaches and strategies include cell-based techniques, eg, seeding cells onto scaffolds before implantation or cell-free techniques which intend to recruit endogenous reparative cells.5, 10, 11, 12 Although some exciting preliminary results have been reported in the laboratory, clinical studies are rare.10, 13
Meniscus cells are heterogeneous groups of cells which reside in the meniscus tissue. Mauck et al found that fibrochondrocytes digested from the meniscus possessed multilineage differentiation ability, indicating some potential for self-regeneration.14 Mesenchymal stromal/stem cells (MSCs) have also attracted interest for use in meniscus tissue engineering strategies. MSCs have become increasingly favorable to researchers, not only for their highly proliferative nature but also due to their relative abundance in tissues.15 Various types of MSCs, including bone marrow MSCs (BM-MSCs), synovium MSCs (SMSCs), adipose-derived MSCs and articular cartilage-derived progenitor cells (ACPCs), have been used to facilitate the regeneration of meniscus.4, 12, 16, 17 However, some were frequently prone to undergo hypertrophic differentiation. Besides, no consensus has been reached by different researchers on choosing the best cell source for meniscus regeneration, which may have hampered their further applications.18, 19 In terms of the potential for endogenous meniscus repair, cell migration and neo-tissue formation have been reported at the site of meniscus defects in vitro,20, 21 indicating that the meniscus may harbor its own progenitor populations which contribute to meniscus regeneration.
Segawa et al initially isolated progenitor cells from meniscus tissue in 2008.22 They postulated that MSCs existed in every intraarticular tissue (including synovium, meniscus, anterior cruciate ligament and articular cartilage), with each tissue source having different characteristics according to their origin.23, 24, 25 They successfully obtained meniscus cells which retained colony-forming ability and multilineage differentiation potential. Since then, several other groups have identified and isolated meniscus progenitor cells (MPCs),26, 27, 28, 29 but various terms have been used to describe this cell population, such as meniscus mesenchymal stem cells,30, 31 meniscus-derived stem cells27 or meniscus stem cells.32 For convenience, in this review we refer to this population as MPCs throughout.
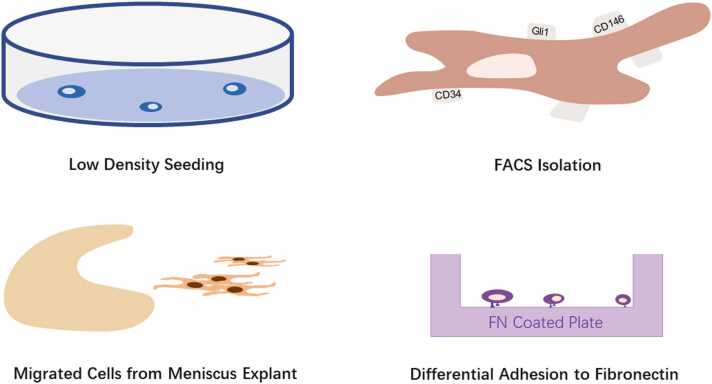
Since different studies used different animal or disease models and isolation methods (Fig. 1), in this review, we have attempted to summarize the existing studies in MPCs identification, characterization, and application. We also highlight some questions which need to be addressed in future studies. The aim is to provide a more comprehensive understanding of MPCs which will hopefully lead to improved clinical translation.
Fig. 1.
Different meniscus progenitor cell isolation methods. FACS, fluorescence-activated cell sorting; FN, fibronectin.
Isolation of MPCs
In earlier studies, researchers isolated MPCs according to their extensive colony-forming ability.22, 27, 29, 32, 33 To be specific, newly harvested meniscus cells were seeded at a very low density,27, 29, 32 ranging from 2 cells/cm227 to 104 cells per 60 cm2 dish.22 The number of colonies positively correlated to the seeding density.22 Cells which were able to form colonies were regarded as MPCs.22, 27, 29, 32 Their clonogenic ability,22, 27, 29, 33 multilineage differentiation potential22, 27, 29, 32, 33 and MSC marker expression22, 29, 32, 33 confirmed the assumption that these colony-forming cells were stem/progenitor cells. Using colony numbers divided by the number of cells seeded into the same culture plate, Huang et al reported the abundance of MPCs in rabbit meniscus to be 0.2% to 0.5% of the total cells present in the meniscus.33
Harvesting cells which migrated out of meniscus explants has been reported to be an efficient method to obtain MPCs.28, 34, 35, 36 Muhammad et al found that menisci from OA patients lacked a continuous superficial layer compared to those from healthy joints.28 In meniscus explant cultures, cells grew out from the uneven and degenerate surfaces of OA menisci while no outgrowth was observed from healthy samples of menisci. This could have been due to the protective effect of the intact surface in the healthy meniscus. Further characterization of the outgrown cells confirmed their stem/progenitor cell properties, as defined by their multilineage differentiation potential and MSC marker expression. Seol et al also reported cell migration in their scratch-injury meniscus explant model,35 these cells also exhibited clonogenic ability and multilineage differentiation potential. However, in their experiments, elongated cells migrated from both noninjured and injured tissue after 10 days of explant culture. The number of cells which migrated out was higher in the red zone compared to the white zone. Gamer et al reported the outgrowth of cells from healthy mouse meniscal explants to be similar to that from human menisci.36 These studies seemed to contrast with each other in one aspect, which was whether the structure of the intact (healthy) or osteoarthritic or injured meniscus surface could affect MPCs' outgrowth. According to Muhammad et al, the incongruent surface which occurs with degeneration in OA was the prerequisite of the cell outgrowth.28 In contrast, the meniscus explants in the animal model used by Seol et al and Gamer et al were all healthy samples.35, 36 We suggest that this could be due to the healthy adult meniscus retaining some capability for repair and regeneration, similar to that found in other tissues of the knee, although the turnover rate is relatively low.15 Excising the meniscus from the knee joint and the explant preparation procedure itself is, of course, a form of injury, which may lead to the change of microenvironment in the tissue and could trigger cell migration. Apart from that, spontaneous healing was observed in rat and rabbit meniscus while this was limited in caprine and human menisci.37, 38, 39 Different biological properties between animal and human meniscus could also contribute to these findings.
Integrins are a group of cell surface proteins which play an important role in extracellular matrix (ECM) adhesion, cell-cell attachment mediation, signal transduction for cell differentiation and proliferation.40 Studies have reported their high expression on MSCs.41 Differential adhesion to fibronectin (DAF) has been used in the isolation of cartilage progenitor cells for decades but has only been used to identify MPCs recently.42, 43, 44, 45 Korpershoek et al found that proliferation ability and multilineage differentiation potential were significantly enhanced in fibronectin adherent-progenitors compared to mixed populations of meniscus cells,42 results which were supported by Wang et al.45 However, Pattappa et al reported, after DAF sorting, that some nonfibronectin adherent cells from the red zone still possessed certain characteristics of MPCs,43 indicating that DAF did not enrich for all MPC subpopulations.
Using stem/progenitor cell markers to directly select MPCs from other cells released on enzymatic digestion of the meniscus is another frequently used isolation method. Based on the fact that the vascular red region of the meniscus manifested a higher success rate in healing than the avascular region, Osawa et al26 hypothesized that cells which were positive for the markers CD34 (vascular endothelial cells) or CD146 (vascular pericytes) may play a vital part in meniscus regeneration. They isolated CD34 and CD146 positive cells from adult and fetus menisci using fluorescence-activated cell sorting (FACS) and proved their multilineage potential. As a crucial transcription factor in hedgehog signaling pathway,46 Gli1 (Glioma-Associated Oncogene Homolog 1) is also considered to be a marker of progenitor cells which contribute to chondrocyte and osteoblast formation during growth and repair in adulthood, for example, in bone fracture repair.47 Wei et al found Gli1 positive cells from mice menisci maintained progenitor characteristics,48 demonstrating better colony forming, migratory and differentiation ability than Gli1 negative cells. They not only played an important role in meniscus development, but were also vital in facilitating meniscus repair in adult mice.
There remain many questions around the use of markers and cell sorting for isolating progenitors for repair of the meniscus and indeed other musculoskeletal tissues. Firstly, the current knowledge on MSCs is lacking definitive markers. Secondly, some MPC markers undergo dynamic changes during in vitro culture49 and it is not yet clear whether changes in marker expression are linked to changes in cells characteristics and so the cells’ progenitor capability. Besides, it was reported in cartilage-derived chondrocytes that they experienced phenotypic change after several passages.50 This may make different cells hard to distinguish after expansion. Thirdly, as there is a difference in marker expression in different species or even strains, it is hard to generalize findings and draw conclusion from studies using various animal models.
Given the absence of comparative studies on all the methods, we cannot provide a definitive conclusion on which is the most efficient method of isolation. According to our own experience, we found that FACS was a rather consuming process, while prolonged explant culture increases the risk of contamination. Enzymatic isolation, on the other hand, may lack specificity and may not effectively distinguish MPCs from other meniscus cells. We tend to use DAF as our preferred choice in isolating MPCs.
Expression profiles of MPCs
It is acknowledged that the term “MSCs” actually represents a group of heterogeneous cell populations. Zha et al categorized the heterogeneity of MSCs into 3 levels: individual level, tissue-origin level, and cell subpopulation level.51 According to this point of view, markers used to precisely label MPCs should ideally comprise of (1) basic markers of MSCs which confirm its progenitor characteristics; (2) meniscus-specific markers; and (3) subpopulation markers which can be used to isolate MPCs with optimal functions. Once the above requirements have been met, surface markers could be a powerful tool for researchers. However, due to the lack of understanding about the development of the meniscus, its pathological and regeneration processes at the molecular level, no tissue-specific markers have been reported until now. Rather, the marker characterization of MPCs is still largely based on those used for MSCs.
The International Society for Cell and Gene Therapy (ISCT) in 2006, recommended that MSCs should be immunopositive for CD105, CD73, and CD9052 but not for the hematopoietic markers (CD45, CD34, CD14 or CD11b, CD79alpha or CD19, and HLA-DR).53 However, it is now recognized that this is only an “entry threshold” and it cannot effectively discriminate MSCs from other cell types.54, 55, 56 Expression of CD90/105 on MPCs was reported to range from 86% of the cells,33 to nearly 100%.22, 29, 32, 42, 45, 49, 57 However, in the study by Garmer et al, CD90 was only expressed by 54% of murine MPCs36, 58 and CD73 expression was reported to be 85% by Korpershoek et al and 95% by Wang et al.42, 45
Analogous to previous work on MSCs derived from other tissues, alternative surface markers used to characterize MPCs include CD29 (integrin subunit beta 1 or ITGB-1), CD44 (hyaluronan receptor), CD106 (vascular cell adhesion molecule 1 or VCAM-1), CD166 (activated-leukocyte cell adhesion molecule or ALCAM). These are all surface markers involved in cell adhesion, and have been proven to play an essential role in MSC transplantation treatment of cartilage defects.59, 60 CD166 was initially found in cancer cells and hematopoietic stem cells61, 62, 63 with a study by Brinkhof et al showing that it can help to distinguish fibroblasts from MSCs.55 All these markers seem to be interdependent, for example, the expression of CD166 was positively correlated with the expression of CD105.64 Depletion of CD90 can cause a reduction in the expression of CD44 and CD166 and lead to an increase in MSCs’ differentiation propensity.52 In the case of MPCs, studies reported that almost all MPCs expressed CD2928, 32, 45, 65 and CD44.22, 29, 32, 36, 45, 49, 57, 65 However, Huang et al found that only 44% of rabbit MPC expressed CD44,33 with this rate increasing to 89% when analyzed by immunocytochemistry.66
Other less investigated markers, including SSEA-4, Nanog, and nucleostemin, are reported to be expressed in approximately 80% of MPCs.30, 66 SSEA-4 and Nanog were previously regarded as markers for human embryonic stem cells,67 but have also been found to be present on MSCs from bone marrow and adipose tissue.68 Their functional significance is, however, not yet fully understood.
CD49b and CD49c are the integrin subunits α2 and α3, respectively, they bind with integrin β1 to form heterodimeric receptors that function to mediate cell-matrix interactions.69 The target ECM proteins of α2β1 are considered to be collagens and laminins, and the ECM ligands of α3β1 are collagens, laminins, fibronectin and entactin.70 Since the DAF isolation method took advantage of MPCs’ rapid adhesion to fibronectin, taking CD49b and CD49c as MPCs markers would be a logical choice. Of the published studies using DAF to isolate MPCs, only one appears to examine the CD49b/49c expression on MPCs. Wang et al found CD49b and CD49c were each present on 65% to 85% and nearly 100% of MPCs, respectively, with a significantly greater number of positive cells for both integrins than mixed meniscus cells.45 Fibronectin was reported as a low-affinity ligand to α3β1 integrin but a relatively specific ligand to α5β1 integrin.71 Research has found that CD49e (α5-integrin) identified a distinct population from chondrocytes, after DAF isolation of ACPCs, with almost all cells expressing CD49e.72 Based on these facts, we infer that CD49e may also represent a potential marker for MPCs, especially when using DAF as the isolation method. However, Kachroo et al found that while 8.9% of freshly isolated chondrocytes expressed CD49e, it was expressed by up to 90% of cells after 120 hours of culture.73 Whether CD49e could be a suitable marker was still under debate.
Another long-standing debate in MSC identification is whether pericytes are the same as or a progenitor of MSCs.74, 75 Discussion of this topic is beyond the content of this review. However, it is noteworthy that some pericyte markers have already been used in MPCs detection, among which CD146 is the most frequently used. Osawa et al found that CD146-positive cells were located in the peripheral meniscus region,26 surrounding α-SMA-positive arterioles. Their numbers here, however, were much lower in adulthood compared to those found in fetal tissue and fetal CD146+ meniscus cells possessed multilineage differentiation potential, with their injection into a rat meniscus defect model facilitating its healing.
Most of the studies of MPC surface markers mentioned above were first investigated in MSCs then empirically used in the identification of MPCs. Single-cell RNA sequencing (scRNA) is a powerful tool in de novo cell clustering and subpopulation recognition. Using scRNA and pseudotime analysis, Sun et al identified endothelial cells (CD146+/CD93+) and fibrochondrocyte progenitors (CD146+/CD93−) as progenitor cells in healthy human menisci.57 In vitro studies also showed FACS-sorted CD146+ meniscus cells had better colony-forming capacity than CD146− cells.57 However, whether CD146 can consistently be used as marker to isolate MPCs from meniscus cells is still under debate.
Using any of the markers described thus far, isolates a heterogeneous cell population from the meniscus, which meets the minimal ISCT standard for MSCs. Though such isolated cell populations may manifest progenitor/stem cell characteristics as a whole, the selection is not be precise enough to completely purify MPCs. Researchers are continuing to strive to find more subpopulation markers, or “stemness” markers,76 which would help to select a subset of cells with superior proliferation, differentiation or whatever targeted ability is deemed necessary.77 STRO-1 is another potential subpopulation marker for MSCs which may be involved in clonogenicity.76 Its expression was reported as being on 75% of human MPCs28 and 86% of rabbit MPCs.30, 66 As previously described, Gli1 was also examined, with Wei et al finding that Gli1+ cells were present in the superficial layer of adolescent mice menisci and correlated with the development of the meniscus.48 In vitro analysis showed that Gli1+ cells have better proliferation, clonogenicity, migration ability, and multilineage differentiation potential than Gli1− cells. They also coexpressed other stem cell markers including Sca-1, CD90, CD200, PDGFR-a, CD248 and PRG4, with injection of Gli1+ cells facilitating healing of meniscal defects.
To summarize, MPCs have similar expression profiles to MSCs, such that commonly used MSC markers are also suitable for MPC characterization. Limited studies have investigated the subpopulation markers, which may not be optimal for identification of MPCs with the best functioning capability. Until now, no tissue-specific marker for MPCs has been reported, since previous studies have confirmed the idea that MSCs from different tissues preserve different expression profiles54, 77, 78, 79; it would seem that further research is needed to fill in the gaps in this field. In undertaking this review, we noticed that there appears to be a species-related or health-status-related difference in marker expression. The human meniscus samples used in these studies were always from OA patients while healthy animal meniscus was used (Table 1). CD166 was reported to be highly expressed by human MPCs, but only moderately expressed by ovine MPCs.65 CD105 was positive in approximately 95% of human and rabbit MPCs but only in 2% of murine meniscal cells.36 CD73, CD44, and CD90 were present on fewer rabbit MPCs than human MPCs.30, 33, 66 There were also some species unique markers. Sca-1 (stem cell antigen-1), a stem cell marker in mice,36, 48, 80 was absent in humans and large animals. STRO-1 was present on human and rabbit MPCs but not on MSCs derived from several canine tissues.81 This difference may hamper the cross-species generalization of research findings in the future. Multiple factors could lead to these differences, including the isolation procedure for MPCs, the age of the sample used to source the cells,36 different culturing conditions,64 the number of passages26, 49 and, especially, the health status of the source tissue. As shown in the single-cell analysis studies by Sun et al and Fu et al, the state and type of meniscus cells as well as their microenvironment endured a drastic change in OA knee joint compared with normal knee joint.57, 82 It is therefore difficult to draw firm conclusions based on current studies. Thus, when reporting marker expression profiles, researchers should clarify all of this background information which can have an impact on the results being described. We hope future studies can provide more useful data and indicate more reliable and specific markers of MPCs.78, 83, 84, 85
Table 1.
Isolation and characterization of MPCs.
| Species | Anatomical location | Disease model/state | Method of progenitor identification | Cell characterization | ||||
|---|---|---|---|---|---|---|---|---|
| Segawa et al22 | Human | Meniscus | OA | Colony forming ability | Clonogenicity; Multilineage differentiation potential | |||
| Shen et al27 | Rabbit | Meniscus | Healthy | Colony forming ability | Morphology (polygonal and star-shaped); Clonogenicity; Multilineage differentiation potential; MHC-II negative; Immunosuppressive | |||
| Shen et al29 | Human | Meniscus | OA | Colony forming ability | Clonogenicity; Multilineage differentiation potential | |||
| Huang et al33 | Rabbit | Avascular meniscus | Healthy | Colony forming ability | Morphology (cobblestone-like); Clonogenicity; Multilineage differentiation potential | |||
| He et al32 | Rat | Comparison between vas and avas | Healthy | Colony forming ability | Morphology (polygonal and star-shaped); Clonogenicity; Multilineage differentiation potential | |||
| Korpershoek et al42 | Human | Meniscus | OA | Differential adhesion to fibronectin (DAF) | Clonogenicity; Multilineage differentiation potential; Fibronectin affinity | |||
| Terpstra et al44 | Human | Meniscus | OA | DAF | - | |||
| Pattappa et al43 | Human | Comparison between vas and avas | OA | DAF | Clonogenicity; Multilineage differentiation potential | |||
| Wang et al45 | Human | Comparison between vas and avas | OA | DAF | Clonogenicity; Fibrochondrogenic differentiation | |||
| Wei et al48 | Mouse | Meniscus | Healthy | Marker expression: Gli1 | Clonogenicity; Migration; Multilineage differentiation potential | |||
| Osawa et al26 | Human | Meniscus | Fetuses/OA | Marker expression: CD34+/146+, CD45− | Multilineage differentiation potential | |||
| Muhammad et al28 | Human | Avascular meniscus | OA | Migration ability | Morphology (spindle-shape); Multilineage differentiation potential; Migration potential | |||
| Seol et al35 | Bovine/goat | Comparison between vas and avas | Healthy | Migration ability | Clonogenicity; Multilineage differentiation potential | |||
| Gamer et al36 | Mouse | Meniscus | Healthy | Migration ability | Morphology (spindle-shape); Clonogenicity; Multilineage differentiation potential | |||
| Schminke et al34 | Human | Avascular meniscus | OA | Migration ability | - | |||
| Gui et al66 | Rabbit | Meniscus | Healthy | No specific | Morphology (cobblestone-like); Clonogenicity; Multilineage differentiation potential | |||
| Ding and Huang30 | Rabbit | Meniscus | Healthy | No specific | Clonogenicity; Multilineage differentiation potential | |||
| Fu et al31 | Human | Meniscus debris | Injured (meniscus tear) | No specific | Morphology (spindle-shaped); Multilineage differentiation potential | |||
| Kisiday et al86 | Ovine | Avascular meniscus | Healthy | No specific | Multilineage differentiation potential | |||
| Linde et al65 | Ovine | Avascular meniscus | Healthy | No specific | Clonogenicity | |||
| Chahla et al49 | Human | Comparison among vas, vas-avas, and avas | Cadaveric | No specific | Clonogenicity; Multilineage differentiation potential | |||
| Sun et al57 | Human | Health/OA | scRNA-seq | Clonogenicity; Multilineage differentiation potential | ||||
Abbreviations: MHC, major histocompatability complex; MPCs, meniscus progenitor cells; OA, osteoarthritis; scRNA, single-cell RNA; vas, vascular; avas, avascular.
Spatial distribution of MPCs
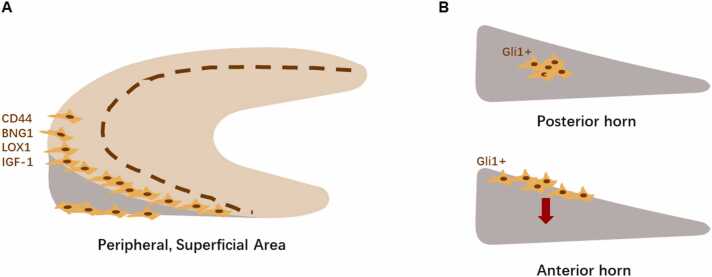
Since studies have proved the existence of MPCs and identified several markers that could be used to help characterize them, researchers have progressed to explore their distribution in vivo. Most studies reported MPCs to be located in the superficial layer of the periphery of the meniscus. Gamer et al located MPCs via immunohistochemical staining for the markers (CD44, Biglycan, Lox, and IGF-1) in the superficial zone of the outer part of the murine meniscus (Fig. 2A).36 In addition, the high synthesis of type I collagen but low synthesis of type II collagen in the in vitro culture of MPCs also supported its origin from the peripheral region. Wei et al investigated the role of Gli1+ MPCs in adolescent murine meniscus and provided a detailed insight of MPCs from a developmental angle.48 They observed murine menisci macroscopically and found that they endured a rapid growth from postnatal at 1 week to 8 weeks of age. This coincided with the finding that the Gli1+ cells’ appearance in the superficial layer of the anterior horn occurred between 2 and 8 weeks of age. Lineage tracing techniques showed that their descendants spread to the central layer of the anterior horn at 6 weeks. As for the posterior horn, few cells in the central area initially expressed Gli1 in 4-week-old mice but they did give rise to many cells after 6 weeks of tracing. This suggested different developmental patterns in different parts of the meniscus structure (Fig. 2B). In adult mice, there were few Gli1+ cells in the superficial layer of the horn area and their density was drastically reduced compared with that seen in adolescent mice. Costaining of MSC markers such as Sca-1, CD90, CD200, and PDGFR-α also confirmed MPCs in the peripheral area of the meniscus, that colabeled with Gli1+. In a study by Seol et al, using a meniscus injury model, cells that had migrated to the defect area were mainly located on the surface, with few occurring in the deeper layers of the tissue.35 The superficial origin of MPCs was similar to the localization of cartilage-resident MSC-like progenitors, which are believed to be retained in the superficial zone of articular cartilage and play an important role in tissue homeostasis.15, 87
Fig. 2.
A, Spatial distribution of MPCs in 8-week mouse meniscus. B, Different development pattern of Gli1+ MPCs in posterior and anterior horns of the meniscus. MPCs, meniscus progenitor cells.
One of the major concerns among researchers is whether the lower regenerative capacity of the white zone of the meniscus can be attributed to the differences in the level of MPCs. Researchers have tried and succeeded in extracting MPCs from the white zone of the meniscus which possessed stem/progenitor characteristics and expressed MSC markers.28, 33, 34, 65, 86 Though the number was less in the white zone, as Seol et al reported, MPCs in the red zone were 8.4 times more abundant than that in the white zone.35 Then the question to address was, if MPCs do exist in the avascular zone of the meniscus, why does the tissue usually fail to repair after injury when the vascular zone enjoys a much higher success rate of repair? Could the stem/progenitor cells from the 2 different areas have different characteristics?
Unfortunately, there remains no clear consensus. Different studies on different characteristics yielded different results. Some studies reported that cells from both areas have similar capabilities, for example, they possessed the same level of migration ability36 and similar levels of proliferation and differentiation potential.43 Others have suggested that there are indeed differences between the 2, for example, a clear diversity in morphology has been reported by different groups.2, 14 Mauck et al first investigated meniscus fibroblasts from the red zone, white zone, and mix area and found that cells which originated from the white zone appeared more chondrocyte-like while red zone cells were more elongated and fibroblast-like.2, 88 This difference in morphology was also reported in a later study on MPCs by Wang et al.45 Some articles highlight the superiority of MPCs from the vascular area, finding that the vascular area-derived MPCs possessed a greater clonogenicity and proliferation ability compared to those from the inner meniscus.42, 45 In multilineage differentiation assays, MPCs from the vascular region were also reported to perform better in adipogenic or osteogenic differentiation assays.14, 43
However, MPCs derived from the avascular region seemed to have higher stem/progenitor marker expression and 2 subpopulations, CD105+/90+/44+/29+ and CD105−/90−/44−/29−, were distinguishable.49 Further, the white zone had the largest proportion of CD105+/90+/44+/29+ cells compared to the red zone. In a murine preclinical model of meniscal injury it was reported that MPCs derived from the inner region of 7-day-aged mice showed increased rates of proliferation and differentiation potential than mix-region-derived MPCs,32 and the injection of inner-derived MPCs resulted in an improved repair score compared to mix-region-derived MPCs in a meniscus injury model.32
To summarize, most studies thus far have shown that MPCs derived from vascular regions possess improved stem/progenitor cell potential than other regional MPCs14, 42, 43, 45; despite this, we have learned that both vascular and avascular regions harbor MPCs with stem/progenitor cell characteristics. Why then does the white zone of the meniscus have such a low endogenous repair capacity? One explanation might be the difference between in vitro and in vivo culture systems used to assess cellular properties. All of the studies mentioned above were based on in vitro observations, that is, to say, the proliferation and differentiation ability of isolated MPCs were investigated in an environment very different from the in vivo situation. The lack of blood vessels in the white zone may result in insufficient circulating nutrients, and contribute to the failure of repair in this region. As suggested by Mauck et al,14 in order to perform better in terms of load-bearing, the inner part of meniscus had to sacrifice vascular and neural invaginations to become a more compact dense tissue, which in itself could also limit the migratory potential of reparative cell types when the tissue in this region is injured.
Comparing MPCs and progenitors from other musculoskeletal tissues
In many of the studies reviewed thus far, isolated MPCs were often compared with unsorted meniscus cells or other tissue-derived stem/progenitor cells in terms of their immunoprofile, gene expression profile, and progenitor cell capacity (Table 2). With regards to stem cell marker expression, CD166 was found to be more highly expressed in BM-MSCs; apart from this no consistent differences were reported between MPCs and other tissue-derived stem/progenitor cells investigated.22, 65 Contradictory results have been presented when comparing mixed meniscus cells, with some studies reporting that MPCs demonstrate a higher expression level of stem cell markers compared to mixed meniscus cells,28, 33 while other studies have reported no significant differences.45 This may be due to the different passage number of the cells used across studies, as surface markers are known to experience dynamic expression level changes during in vitro culture expansion.89 Chahla et al found that while there were 2 distinct subpopulations (CD105+/90+/44+/29+ and CD105−/90−/44−/29−) observed in freshly isolated meniscus cells,49 at passage 2 one of the subpopulations (CD105−/90−/44−/29−) had disappeared. We suggested that “Natural selection” may have taken place after several passages of in vitro culture, whereby subpopulations that proliferate including transient amplifying cells and terminally differentiated cells, may fail to yield daughter cells and finally be eliminated from the culture.90 In addition to this, cells could undergo rapid change in phenotype during in vitro culture, as mentioned before,50 which could also contribute to this.
Table 2.
Marker expression, other cell types cooperation, and application of MPCs.
| Marker expression | Other cell types comparation | Application | |
|---|---|---|---|
| Muhammad et al28 | Positive: STRO1, CD29, CD90, CD105, CD106 Negative: CD45, CD34 |
- | - |
| Ding and Huang30 | Positive: SSEA-4, STRO-1, Nanog, nucleostemin, CD44, CD90 Negative: CD34 |
BM-MSCs | MPCs transplant |
| Gui et al66 | Positive: SSEA-4, Nanog, Nucleostemin, STRO-1, CD44, CD90 Negative: CD34 |
BM-MSCs, TMSCs | - |
| Huang et al33 | Positive: CD90, CD105, CD73, CD44 Negative: CD31, CD34, CD45 |
Fibrochondrocytes, BM-MSCs | MPCs transplant |
| Korpershoek et al42 | Positive: CD90, CD105, CD73 Negative: CD45, CD34, CD79α, CD11b |
- | - |
| Segawa et al22 | Positive: CD90, CD105, CD166, CD44 Negative: CD34, CD45 |
SMSCs, ADMSCs, BM-MSCs, intraarticular ligament-derived MSCs, muscle-MSCs | - |
| Wang et al45 | Positive: CD73, CD90, CD105, CD29, CD44, CD49b, CD49c, CD166 | - | - |
| Gamer et al36 | Positive: CD44, Sca-1 Medium: 54% CD90.2, 35.4% CD73 Negative: CD34 |
- | - |
| Shen et al29 | Positive: CD44, CD90, CD105, CD166 Negative: CD34, CD45 |
BM-MSCs, SMSCs | MPCs transplant |
| Fu et al31 | Positive: CD44, CD90, CD105 Negative: CD34, CD45 |
- | - |
| He et al32 | Positive: CD29, CD44, CD90, CD105 Negative: CD34, CD45 |
- | MPCs transplant |
| Linde et al65 | Positive: CD29, CD44, CD166 | BM-MSCs | - |
| Sun et al57 | Positive: CD146 | - | - |
| Shen et al27 | - | - | MPCs transplant |
| Terpstra et al44 | - | - | MPCs with bioink scaffold |
| Osawa et al26 | - | - | MPCs transplant |
| Seol et al35 | - | ACPCs | - |
| Chahla et al49 | - | BM-MSCs | - |
Abbreviations: ACPCs, articular cartilage-derived progenitor cells; ADMSCs, adipose-derived mesenchymal stromal/stem cells; BM-MSCs, bone marrow-mesenchymal stromal/stem cells; MPCs, meniscus progenitor cells; SMSCs, synovium mesenchymal stromal/stem cells; TMSCs, tendon-derived mesenchymal stromal/stem cells.
Gene expression profiles investigated by most studies can be divided into the following groups: chondrogenic and ECM-related genes, stem cell-related genes and tissue-origin genes. The interaction between cells and the ECM are crucial in promoting tissue homeostasis. While cells in healthy menisci play an important role in ECM metabolism by synthesizing and remodeling its components,91 the ECM itself can also influence and regulate cell fates.92 Meanwhile, the organized healthy ECM structure is crucial for providing the meniscus’s functional role in load bearing. Thus, characterization of ECM-related gene expression profiles is an important part of MPC evaluation. MPCs expressed lower COL2 and higher COL1 compared with unsorted meniscus cells,28, 33, 45 they also less expressed ACAN which is another ECM-related gene at higher levels.42 Though the overall ECM gene expression profile of MPCs was reported to be similar to BM-MSCs and ACPCs,35 lower expression of levels of COL2 and COL1 were shown. In other studies, COL2 expression was reported to be higher in MPCs compared to SMSCs, tendon-derived MSCs, and BM-MSCs.29, 66 Besides, Korpershoek et al found that in contrast to BM-MSCs, MPCs do not undergo in vitro hypertrophic differentiation even when subjected to hypertrophic media.42 Injection of MPCs into the OA knee joint was found to be able to suppress OA and decrease the COLX expression in the tibial plateau.29
The stem cell markers Stro-1, SSEA-4, Nanog, and Nucleostemin were comparably expressed in MPCs and BM-MSCs,33, 66 however, this study reported that MPCs expressed higher gene expression levels of CD44 and Notch1 compared to ACPCs.35 Aiming to investigate whether different stem/progenitor cells from different tissue origins have differing expression profiles, Segawa et al used DNA microarray analysis and found PRELP, OGN, and ECRG4 were more highly expressed in intra-articular tissue-derived cells, especially in MPCs.22 In a meniscus injured model generated by Seol et al, significantly increased expression levels of inflammatory and catabolic genes (IL6, IL8, CXCL2, MMP9) were observed compared with ACPCs and MSCs.35
Compared with other tissue-derived MSCs, MPCs have also shown equivalent progenitor cell capacity. Segawa et al found that meniscus, synovium, intra-articular ligament, and adipose-derived MSCs had higher colony yields compared with muscle and bone marrow-derived MSCs22 and similar results have been presented by Shen et al.29 However, others have reported that both counts and the density of colonies were notably lower in MPCs compared to BM-MSCs.30, 66 MPCs were also found to proliferate faster than unsorted meniscus cells33 and ACPCs,45 with no significant difference being observed in the proliferation ability between MPCs and BM-MSCs.66 As for multilineage differentiation potential, while osteogenic and adipogenic abilities were weaker in MPCs compared to BM-MSC,33, 66 MPCs demonstrated enhanced chondrogenic capacity.33, 66 However, Segawa et al showed that after pellet culture in chondrogenic media, SMSC and BM-MSC pellets had the heaviest wet weights, followed by meniscus MSCs, with adipose-derived MSCs producing the lightest.22 These differences were however minimal and might be owing to the difference in species usage and isolation methodologies.
Current applications of meniscal progenitor cells in meniscus regeneration
In current clinical practice, the use of stem cells derived from extrameniscal tissues (such as BM-MSCs, AD-MSCs, and SMSCs) is the main approach for a stem cell therapy in treating meniscal defects.4, 12 Although these therapies have shown some efficacy, they each have their disadvantages, as reviewed by Jacob et al.4 Alternatively, utilizing autologous meniscal progenitor cells or inducing more meniscal progenitor cell involvement might represent a better choice for repairing meniscal injuries considering their low immunogenicity and outstanding chondrogenic potential.27, 42, 45 Since the discovery of MPCs in the last decade, researchers have conducted few lab-based experiments to evaluate the potential of MPCs in meniscus regeneration. It is noteworthy that most of the experiments mentioned previously in this review paragraphs were in vitro, whereas in vivo experiments in animal models are few, but could provide a more authentic understanding of repair mechanisms. The most frequently used method in applying MPCs to damaged areas in vivo was to directly inject MPCs to the defect area. In one study, different groups of human fetal cells including CD34+ MPCs, CD146+ MPCs, CD34−/CD146− cells, and a PBS control group were transplanted into an athymic rat meniscal injury model26 and repair assessments at 4 weeks post-treatment showed enhanced healing in the CD34+ and CD146+ cell injection groups, whilst CD34−/CD146− cell injection groups displayed incomplete healing and the PBS control group showed no evidence of repair. Interestingly, though the gap was obvious in PBS group, a number of spindle-shaped cells were found on the surface of cut edges of the menisci.26 The efficacy of allogenic MPCs injected into a partial meniscectomy in a rat29 or rabbit model27, 33 was also investigated, in which the MPC-treated groups demonstrated more neo-tissue formation and had morphology and histological staining patterns akin to the normal meniscus, when compared to the control groups.27 The MPC-treated groups also showed more type II collagen deposition via immunohistochemical staining and this was verified by more ordered and larger fibrils via transmission electron microscopy scanning.27 However, no clear divergence was reflected among different groups in ECM-related gene expression.27 Using a small animal fluorescent imaging system, Shen et al further traced the fate of injected labeled MPCs in vivo, reporting that the number of MPCs decreased with time postinjection,27, 29 and that only a few injected MPCs were found after 12 weeks post meniscectomy.27 These studies also described a positive effect on injured meniscal tissues, observed following MPCs injection, which could delay the progression of OA.27, 29, 32 Another study compared the healing potential of MPCs and BM-MSCs treatments in meniscus injuries reported improved results for MPCs, which showed increased cartilage-like tissue formation via histology staining.30, 33 Only one article was found to use MPCs in tissue-engineered scaffolds to treat meniscus defects.44 The authors expressed their preference in utilizing MPCs over mixed meniscus cells for their superior proliferation ability.44 Together this evidence suggests that MPCs show promise as a prospective cell therapy for meniscus repair. Nevertheless, the exploration of MPC applications remains limited. Considering the enhanced chondrogenic ability of MPCs shown in most in vitro studies, these cells hold great promise for avascular area reconstruction and serve as a potential cell source for tissue engineering-based repair strategies. Furthermore, the role and underlying contributions of MPCs in OA pathogenesis are also unclear. We anticipate more studies that delve into the application and translational aspects of MPCs in the future.
Currently, the majority of studies investigating the potential of MPCs in promoting the repair of meniscal injuries have utilized small animal models such as rabbits and rats. However, a limitation of these models is that meniscal tissues in these animals possess a strong intrinsic capacity for repair and regeneration, as noted by Shen et al,27 who reported that meniscal injury models in rabbits and rats were found to almost completely self-heal within 12 weeks postsurgery. This is in stark contrast to the ineffective repair mechanisms observed in human menisci. Therefore, in order to better understand the role of MPCs in promoting the repair of meniscal injuries, it is imperative to conduct studies utilizing larger animal models which more closely resemble human physiology.
Conclusion
Meniscus regeneration is a worldwide challenge and researchers have been seeking progenitor cells to be involved in this process for many years. Studies indicate that a cell population exists in menisci which possess great proliferation, colony-forming ability and multilineage differentiation potential. These are now recognized as MPCs. MPCs can be isolated according to their colony-forming ability, migration ability, marker expression or DAF. In vitro studies show they share similar surface markers with MSCs from other tissues. MPCs mostly locate in the peripheral surface area of the meniscus. In vivo injection of MPCs into animal meniscus injury models enhances meniscus healing, which highlights their potential role in meniscus repair. However, the regulatory mechanism and in vivo evolution of MPCs in injury responses are still not fully characterized and more investigations are needed to help us gain a deeper understanding of their roles in meniscus regeneration and eventually facilitate the clinical translation in the future.
Methods
The electronic databases of EMBASE and PubMed were searched using the following search terms: (meniscus OR menisci OR meniscal) AND (progenitor OR progenitor cell OR multipotent cell OR meniscus-derived OR (stem cell OR MSC OR mesenchymal stem cell OR mesenchymal stromal cell AND (meniscus-derived OR meniscus resident))). A final search was performed on May 5, 2023. Two authors (W-TY and J-SW) screened all selected studies by title, abstract and followed by full-text screening independently. After the removal of duplicates, inconsistencies between the researchers were discussed to achieve a consensus.
Authorship contributions
W.-T.Y.: investigation, writing - original draft; J.-S.W.: writing - review and editing; P.-Z.F.: investigation; S.R.: conceptualization, writing - review and editing; K.W.: conceptualization, writing - review and editing; Z.-Z.Z.: conceptualization, supervision, funding acquisition, writing - review and editing.
Funding
This study was financially supported by grants from the Natural Science Foundation of Guangdong Province for Distinguished Young Scholars (2020B1515020014) and National Outstanding Youth Science Fund Project of National Natural Science Foundation of China (82022046).
Ethics approval
Ethical approval was not necessary for this article as it is a review of other work and was not carried out at the authors’ institutions.
Declaration of Competing Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Disclaimer: None.
Wan-Ting Yan and Jing-Song Wang contributed equally to the article.
Contributor Information
Wan-Ting Yan, Email: yanwtowo@126.com.
Jing-Song Wang, Email: wjsworkmail@163.com.
Sally Roberts, Email: sally.roberts4@nhs.net.
Karina Wright, Email: karina.wright1@nhs.net.
Zheng-Zheng Zhang, Email: zzz1985114@163.com.
References
- 1.Rath E., Richmond J.C. The menisci: basic science and advances in treatment. Br J Sports Med. 2000;34(4):252–257. doi: 10.1136/bjsm.34.4.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Makris E.A., Hadidi P., Athanasiou K.A. The knee meniscus: structure-function, pathophysiology, current repair techniques, and prospects for regeneration. Biomaterials. 2011;32(30):7411–7431. doi: 10.1016/j.biomaterials.2011.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shimomura K., Hamamoto S., Hart D.A., Yoshikawa H., Nakamura N. Meniscal repair and regeneration: current strategies and future perspectives. J Clin Orthop Trauma. 2018;9(3):247–253. doi: 10.1016/j.jcot.2018.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jacob G., Shimomura K., Krych A.J., Nakamura N. The meniscus tear: a review of stem cell therapies. Cells. 2019;9(1) doi: 10.3390/cells9010092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang Z.Z., Chen Y.R., Wang S.J., et al. Orchestrated biomechanical, structural, and biochemical stimuli for engineering anisotropic meniscus. Sci Transl Med. 2019;11(487) doi: 10.1126/scitranslmed.aao0750. [DOI] [PubMed] [Google Scholar]
- 6.Snoeker B.A., Bakker E.W., Kegel C.A., Lucas C. Risk factors for meniscal tears: a systematic review including meta-analysis. J Orthop Sports Phys Ther. 2013;43(6):352–367. doi: 10.2519/jospt.2013.4295. [DOI] [PubMed] [Google Scholar]
- 7.Adams B.G., Houston M.N., Cameron K.L. The epidemiology of meniscus injury. Sports Med Arthrosc Rev. 2021;29(3):e24–e33. doi: 10.1097/JSA.0000000000000329. [DOI] [PubMed] [Google Scholar]
- 8.Woodmass J.M., LaPrade R.F., Sgaglione N.A., Nakamura N., Krych A.J. Meniscal repair: reconsidering indications, techniques, and biologic augmentation. J Bone Jt Surg Am. 2017;99(14):1222–1231. doi: 10.2106/JBJS.17.00297. [DOI] [PubMed] [Google Scholar]
- 9.Kopf S., Beaufils P., Hirschmann M.T., et al. Management of traumatic meniscus tears: the 2019 ESSKA meniscus consensus. Knee Surg Sports Traumatol Arthrosc. 2020;28(4):1177–1194. doi: 10.1007/s00167-020-05847-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bilgen B., Jayasuriya C.T., Owens B.D. Current concepts in meniscus tissue engineering and repair. Adv Healthc Mater. 2018;7(11) doi: 10.1002/adhm.201701407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huey D.J., Hu J.C., Athanasiou K.A. Unlike bone, cartilage regeneration remains elusive. Science. 2012;338(6109):917–921. doi: 10.1126/science.1222454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou Y.F., Zhang D., Yan W.T., Lian K., Zhang Z.Z. Meniscus regeneration with multipotent stromal cell therapies. Front Bioeng Biotechnol. 2022;10 doi: 10.3389/fbioe.2022.796408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moran C.J., Busilacchi A., Lee C.A., Athanasiou K.A., Verdonk P.C. Biological augmentation and tissue engineering approaches in meniscus surgery. Arthroscopy. 2015;31(5):944–955. doi: 10.1016/j.arthro.2014.11.044. [DOI] [PubMed] [Google Scholar]
- 14.Mauck R.L., Martinez-Diaz G.J., Yuan X., Tuan R.S. Regional multilineage differentiation potential of meniscal fibrochondrocytes: implications for meniscus repair. Anat Rec. 2007;290(1):48–58. doi: 10.1002/ar.20419. [DOI] [PubMed] [Google Scholar]
- 15.McGonagle D., Baboolal T.G., Jones E. Native joint-resident mesenchymal stem cells for cartilage repair in osteoarthritis. Nat Rev Rheumatol. 2017;13(12):719–730. doi: 10.1038/nrrheum.2017.182. [DOI] [PubMed] [Google Scholar]
- 16.Rikkers M., Korpershoek J.V., Levato R., Malda J., Vonk L.A. The clinical potential of articular cartilage-derived progenitor cells: a systematic review. NPJ Regen Med. 2022;7(1):2. doi: 10.1038/s41536-021-00203-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Angele P., Docheva D., Pattappa G., Zellner J. Cell-based treatment options facilitate regeneration of cartilage, ligaments and meniscus in demanding conditions of the knee by a whole joint approach. Knee Surg Sports Traumatol Arthrosc. 2022;30(4):1138–1150. doi: 10.1007/s00167-021-06497-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zellner J., Pattappa G., Koch M., et al. Autologous mesenchymal stem cells or meniscal cells: what is the best cell source for regenerative meniscus treatment in an early osteoarthritis situation? Stem Cell Res Ther. 2017;8(1):225. doi: 10.1186/s13287-017-0678-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liang Y., Idrees E., Andrews S.H.J., et al. Plasticity of human meniscus fibrochondrocytes: a study on effects of mitotic divisions and oxygen tension. Sci Rep. 2017;7(1) doi: 10.1038/s41598-017-12096-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kambic H.E., Futani H., McDevitt C.A. Cell, matrix changes and alpha-smooth muscle actin expression in repair of the canine meniscus. Wound Repair Regen. 2000;8(6):554–561. doi: 10.1046/j.1524-475x.2000.00554.x. [DOI] [PubMed] [Google Scholar]
- 21.Hennerbichler A., Moutos F.T., Hennerbichler D., Weinberg J.B., Guilak F. Repair response of the inner and outer regions of the porcine meniscus in vitro. Am J Sports Med. 2007;35(5):754–762. doi: 10.1177/0363546506296416. [DOI] [PubMed] [Google Scholar]
- 22.Segawa Y., Muneta T., Makino H., et al. Mesenchymal stem cells derived from synovium, meniscus, anterior cruciate ligament, and articular chondrocytes share similar gene expression profiles. J Orthop Res. 2009;27(4):435–441. doi: 10.1002/jor.20786. [DOI] [PubMed] [Google Scholar]
- 23.Sakaguchi Y., Sekiya I., Yagishita K., Muneta T. Comparison of human stem cells derived from various mesenchymal tissues: superiority of synovium as a cell source. Arthritis Rheumatol. 2005;52(8):2521–2529. doi: 10.1002/art.21212. [DOI] [PubMed] [Google Scholar]
- 24.Mochizuki T., Muneta T., Sakaguchi Y., et al. Higher chondrogenic potential of fibrous synovium- and adipose synovium-derived cells compared with subcutaneous fat-derived cells: distinguishing properties of mesenchymal stem cells in humans. Arthritis Rheumatol. 2006;54(3):843–853. doi: 10.1002/art.21651. [DOI] [PubMed] [Google Scholar]
- 25.Nimura A., Muneta T., Koga H., et al. Increased proliferation of human synovial mesenchymal stem cells with autologous human serum: comparisons with bone marrow mesenchymal stem cells and with fetal bovine serum. Arthritis Rheumatol. 2008;58(2):501–510. doi: 10.1002/art.23219. [DOI] [PubMed] [Google Scholar]
- 26.Osawa A., Harner C.D., Gharaibeh B., et al. The use of blood vessel-derived stem cells for meniscal regeneration and repair. Med Sci Sports Exerc. 2013;45(5):813–823. doi: 10.1249/MSS.0b013e31827d1e06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shen W., Chen J., Zhu T., et al. Osteoarthritis prevention through meniscal regeneration induced by intra-articular injection of meniscus stem cells. Stem Cells Dev. 2013;22(14):2071–2082. doi: 10.1089/scd.2012.0563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muhammad H., Schminke B., Bode C., et al. Human migratory meniscus progenitor cells are controlled via the TGF-beta pathway. Stem Cell Rep. 2014;3(5):789–803. doi: 10.1016/j.stemcr.2014.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shen W., Chen J., Zhu T., et al. Intra-articular injection of human meniscus stem/progenitor cells promotes meniscus regeneration and ameliorates osteoarthritis through stromal cell-derived factor-1/CXCR4-mediated homing. Stem Cells Transl Med. 2014;3(3):387–394. doi: 10.5966/sctm.2012-0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ding Z., Huang H. Mesenchymal stem cells in rabbit meniscus and bone marrow exhibit a similar feature but a heterogeneous multi-differentiation potential: superiority of meniscus as a cell source for meniscus repair. BMC Musculoskelet Disord. 2015;16:65. doi: 10.1186/s12891-015-0511-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fu W., Xie X., Li Q., et al. Isolation, characterization, and multipotent differentiation of mesenchymal stem cells derived from meniscal debris. Stem Cells Int. 2016;2016 doi: 10.1155/2016/5093725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.He S., Ruan D., Chen Y., et al. Characterization and comparison of postnatal rat meniscus stem cells at different developmental stages. Stem Cells Transl Med. 2019;8(12):1318–1329. doi: 10.1002/sctm.19-0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang H., Wang S., Gui J., Shen H. A study to identify and characterize the stem/progenitor cell in rabbit meniscus. Cytotechnology. 2016;68(5):2083–2103. doi: 10.1007/s10616-016-9949-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schminke B., Kauffmann P., Schubert A., Altherr M., Gelis T., Miosge N. SMURF1 and SMURF2 in progenitor cells from articular cartilage and meniscus during late-stage osteoarthritis. Cartilage. 2021;13(2_suppl):117S–128S. doi: 10.1177/1947603520967069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seol D., Zhou C., Brouillette M.J., et al. Characteristics of meniscus progenitor cells migrated from injured meniscus. J Orthop Res. 2017;35(9):1966–1972. doi: 10.1002/jor.23472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gamer L.W., Shi R.R., Gendelman A., Mathewson D., Gamer J., Rosen V. Identification and characterization of adult mouse meniscus stem/progenitor cells. Connect Tissue Res. 2017;58(3-4):238–245. doi: 10.1080/03008207.2016.1271797. [DOI] [PubMed] [Google Scholar]
- 37.Horie M., Sekiya I., Muneta T., et al. Intra-articular Injected synovial stem cells differentiate into meniscal cells directly and promote meniscal regeneration without mobilization to distant organs in rat massive meniscal defect. Stem Cells. 2009;27(4):878–887. doi: 10.1634/stemcells.2008-0616. [DOI] [PubMed] [Google Scholar]
- 38.Murphy J.M., Fink D.J., Hunziker E.B., Barry F.P. Stem cell therapy in a caprine model of osteoarthritis. Arthritis Rheumatol. 2003;48(12):3464–3474. doi: 10.1002/art.11365. [DOI] [PubMed] [Google Scholar]
- 39.Peretti G.M., Gill T.J., Xu J.W., Randolph M.A., Morse K.R., Zaleske D.J. Cell-based therapy for meniscal repair: a large animal study. Am J Sports Med. 2004;32(1):146–158. doi: 10.1177/0095399703258790. [DOI] [PubMed] [Google Scholar]
- 40.Prowse A.B., Chong F., Gray P.P., Munro T.P. Stem cell integrins: implications for ex-vivo culture and cellular therapies. Stem Cell Res. 2011;6(1):1–12. doi: 10.1016/j.scr.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 41.Shimaya M., Muneta T., Ichinose S., Tsuji K., Sekiya I. Magnesium enhances adherence and cartilage formation of synovial mesenchymal stem cells through integrins. Osteoarthr Cartil. 2010;18(10):1300–1309. doi: 10.1016/j.joca.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 42.Korpershoek J.V., Rikkers M., de Windt T.S., Tryfonidou M.A., Saris D.B.F., Vonk L.A. Selection of highly proliferative and multipotent meniscus progenitors through differential adhesion to fibronectin: a novel approach in meniscus tissue engineering. Int J Mol Sci. 2021;22(16) doi: 10.3390/ijms22168614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pattappa G., Reischl F., Jahns J., et al. Fibronectin adherent cell populations derived from avascular and vascular regions of the meniscus have enhanced clonogenicity and differentiation potential under physioxia. Front Bioeng Biotechnol. 2021;9 doi: 10.3389/fbioe.2021.789621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Terpstra M.L., Li J., Mensinga A., et al. Bioink with cartilage-derived extracellular matrix microfibers enables spatial control of vascular capillary formation in bioprinted constructs. Biofabrication. 2022;14(3) doi: 10.1088/1758-5090/ac6282. [DOI] [PubMed] [Google Scholar]
- 45.Wang J., Roberts S., Li W., Wright K. Phenotypic characterization of regional human meniscus progenitor cells. Front Bioeng Biotechnol. 2022;10 doi: 10.3389/fbioe.2022.1003966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen D., Kang X., Li Z., Chen L., Ma Q., Fan P. Hedgehog/GLI1 signaling pathway regulates the resistance to cisplatin in human osteosarcoma. J Cancer. 2021;12(22):6676–6684. doi: 10.7150/jca.61591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shi Y., He G., Lee W.C., McKenzie J.A., Silva M.J., Long F. Gli1 identifies osteogenic progenitors for bone formation and fracture repair. Nat Commun. 2017;8(1):2043. doi: 10.1038/s41467-017-02171-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wei Y., Sun H., Gui T., et al. The critical role of Hedgehog-responsive mesenchymal progenitors in meniscus development and injury repair. Elife. 2021;10 doi: 10.7554/eLife.62917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chahla J., Papalamprou A., Chan V., et al. Assessing the resident progenitor cell population and the vascularity of the adult human meniscus. Arthroscopy. 2021;37(1):252–265. doi: 10.1016/j.arthro.2020.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Darling E.M., Athanasiou K.A. Rapid phenotypic changes in passaged articular chondrocyte subpopulations. J Orthop Res. 2005;23(2):425–432. doi: 10.1016/j.orthres.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 51.Zha K., Li X., Yang Z., et al. Heterogeneity of mesenchymal stem cells in cartilage regeneration: from characterization to application. NPJ Regen Med. 2021;6(1):14. doi: 10.1038/s41536-021-00122-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moraes D.A., Sibov T.T., Pavon L.F., et al. A reduction in CD90 (THY-1) expression results in increased differentiation of mesenchymal stromal cells. Stem Cell Res Ther. 2016;7(1):97. doi: 10.1186/s13287-016-0359-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dominici M., Le Blanc K., Mueller I., et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 54.Al-Nbaheen M., Vishnubalaji R., Ali D., et al. Human stromal (mesenchymal) stem cells from bone marrow, adipose tissue and skin exhibit differences in molecular phenotype and differentiation potential. Stem Cell Rev Rep. 2013;9(1):32–43. doi: 10.1007/s12015-012-9365-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brinkhof B., Zhang B., Cui Z., Ye H., Wang H. ALCAM (CD166) as a gene expression marker for human mesenchymal stromal cell characterisation. Gene X. 2020;5 doi: 10.1016/j.gene.2020.100031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Roson-Burgo B., Sanchez-Guijo F., Del Canizo C., De Las Rivas J. Insights into the human mesenchymal stromal/stem cell identity through integrative transcriptomic profiling. BMC Genom. 2016;17(1):944. doi: 10.1186/s12864-016-3230-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sun H., Wen X., Li H., et al. Single-cell RNA-seq analysis identifies meniscus progenitors and reveals the progression of meniscus degeneration. Ann Rheum Dis. 2020;79(3):408–417. doi: 10.1136/annrheumdis-2019-215926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sauzay C., Voutetakis K., Chatziioannou A., Chevet E., Avril T. CD90/Thy-1, a cancer-associated cell surface signaling molecule. Front Cell Dev Biol. 2019;7:66. doi: 10.3389/fcell.2019.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Koga H., Shimaya M., Muneta T., et al. Local adherent technique for transplanting mesenchymal stem cells as a potential treatment of cartilage defect. Arthritis Res Ther. 2008;10(4):R84. doi: 10.1186/ar2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kitahashi T., Kogawa R., Nakamura K., Sekiya I. Integrin beta1, PDGFRbeta, and type II collagen are essential for meniscus regeneration by synovial mesenchymal stem cells in rats. Sci Rep. 2022;12(1) doi: 10.1038/s41598-022-18476-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cortes F., Deschaseaux F., Uchida N., et al. HCA, an immunoglobulin-like adhesion molecule present on the earliest human hematopoietic precursor cells, is also expressed by stromal cells in blood-forming tissues. Blood. 1999;93(3):826–837. [PubMed] [Google Scholar]
- 62.Uchida N., Yang Z., Combs J., et al. The characterization, molecular cloning, and expression of a novel hematopoietic cell antigen from CD34+ human bone marrow cells. Blood. 1997;89(8):2706–2716. [PubMed] [Google Scholar]
- 63.Jeannet R., Cai Q., Liu H., Vu H., Kuo Y.H. Alcam regulates long-term hematopoietic stem cell engraftment and self-renewal. Stem Cells. 2013;31(3):560–571. doi: 10.1002/stem.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang B., Kasoju N., Li Q., et al. Effect of substrate topography and chemistry on human mesenchymal stem cell markers: a transcriptome study. Int J Stem Cells. 2019;12(1):84–94. doi: 10.15283/ijsc18102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Linde P.E., Puttlitz C.M., Kisiday J.D. Adult ovine connective tissue cells resemble mesenchymal stromal cells in their propensity for extensive ex vivo expansion. Connect Tissue Res. 2021;62(6):671–680. doi: 10.1080/03008207.2020.1847099. [DOI] [PubMed] [Google Scholar]
- 66.Gui J., Zhang J., Huang H. Isolation and characterization of meniscus derived stem cells from rabbit as a possible treatment for damaged meniscus. Curr Stem Cell Res Ther. 2015;10(4):353–363. [PubMed] [Google Scholar]
- 67.Gang E.J., Bosnakovski D., Figueiredo C.A., Visser J.W., Perlingeiro R.C. SSEA-4 identifies mesenchymal stem cells from bone marrow. Blood. 2007;109(4):1743–1751. doi: 10.1182/blood-2005-11-010504. [DOI] [PubMed] [Google Scholar]
- 68.Pitrone M., Pizzolanti G., Tomasello L., et al. NANOG plays a hierarchical role in the transcription network regulating the pluripotency and plasticity of adipose tissue-derived stem cells. Int J Mol Sci. 2017;18(6) doi: 10.3390/ijms18061107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Docheva D., Popov C., Mutschler W., Schieker M. Human mesenchymal stem cells in contact with their environment: surface characteristics and the integrin system. J Cell Mol Med. 2007;11(1):21–38. doi: 10.1111/j.1582-4934.2007.00001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mathew M.R., McLean S.M., Murray S.B., Bennett H.G., Webb L.A., Esakowitz L. Expression of CD18, CD49b, CD49c and CD49e on lens anterior capsules in human cataracts. Eye. 2003;17(4):473–477. doi: 10.1038/sj.eye.6700380. [DOI] [PubMed] [Google Scholar]
- 71.Tsuji T. Physiological and pathological roles of alpha3beta1 integrin. J Membr Biol. 2004;200(3):115–132. doi: 10.1007/s00232-004-0696-5. [DOI] [PubMed] [Google Scholar]
- 72.Williams R., Khan I.M., Richardson K., et al. Identification and clonal characterisation of a progenitor cell sub-population in normal human articular cartilage. PLoS One. 2010;5(10) doi: 10.1371/journal.pone.0013246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kachroo U., Ramasamy B., Vinod E. Evaluation of CD49e as a distinguishing marker for human articular cartilage derived chondroprogenitors. Knee. 2020;27(3):833–837. doi: 10.1016/j.knee.2020.04.002. [DOI] [PubMed] [Google Scholar]
- 74.Crisan M., Yap S., Casteilla L., et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3(3):301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 75.Caplan A.I. All MSCs are pericytes? Cell Stem Cell. 2008;3(3):229–230. doi: 10.1016/j.stem.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 76.Lv F.J., Tuan R.S., Cheung K.M., Leung V.Y. Concise review: the surface markers and identity of human mesenchymal stem cells. Stem Cells. 2014;32(6):1408–1419. doi: 10.1002/stem.1681. [DOI] [PubMed] [Google Scholar]
- 77.Zha K., Li X., Tian G., et al. Evaluation of CD49f as a novel surface marker to identify functional adipose-derived mesenchymal stem cell subset. Cell Prolif. 2021;54(5) doi: 10.1111/cpr.13017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Uder C., Bruckner S., Winkler S., Tautenhahn H.M., Christ B. Mammalian MSC from selected species: features and applications. Cytometry A. 2018;93(1):32–49. doi: 10.1002/cyto.a.23239. [DOI] [PubMed] [Google Scholar]
- 79.Strioga M., Viswanathan S., Darinskas A., Slaby O., Michalek J. Same or not the same? Comparison of adipose tissue-derived versus bone marrow-derived mesenchymal stem and stromal cells. Stem Cells Dev. 2012;21(14):2724–2752. doi: 10.1089/scd.2011.0722. [DOI] [PubMed] [Google Scholar]
- 80.Holmes C., Stanford W.L. Concise review: stem cell antigen-1: expression, function, and enigma. Stem Cells. 2007;25(6):1339–1347. doi: 10.1634/stemcells.2006-0644. [DOI] [PubMed] [Google Scholar]
- 81.Bearden R.N., Huggins S.S., Cummings K.J., Smith R., Gregory C.A., Saunders W.B. In-vitro characterization of canine multipotent stromal cells isolated from synovium, bone marrow, and adipose tissue: a donor-matched comparative study. Stem Cell Res Ther. 2017;8(1):218. doi: 10.1186/s13287-017-0639-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fu W., Chen S., Yang R., et al. Cellular features of localized microenvironments in human meniscal degeneration: a single-cell transcriptomic study. Elife. 2022;11 doi: 10.7554/eLife.79585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.De Schauwer C., Meyer E., Van de Walle G.R., Van Soom A. Markers of stemness in equine mesenchymal stem cells: a plea for uniformity. Theriogenology. 2011;75(8):1431–1443. doi: 10.1016/j.theriogenology.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 84.Lee T.C., Lee T.H., Huang Y.H., et al. Comparison of surface markers between human and rabbit mesenchymal stem cells. PLoS One. 2014;9(11) doi: 10.1371/journal.pone.0111390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Anjos-Afonso F., Bonnet D. Prospective identification and isolation of murine bone marrow derived multipotent mesenchymal progenitor cells. Best Pract Res Clin Haematol. 2011;24(1):13–24. doi: 10.1016/j.beha.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 86.Kisiday J.D., Liebig B.E., Goodrich L.R. Adult ovine chondrocytes in expansion culture adopt progenitor cell properties that are favorable for cartilage tissue engineering. J Orthop Res. 2020;38(9):1996–2005. doi: 10.1002/jor.24671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dowthwaite G.P., Bishop J.C., Redman S.N., et al. The surface of articular cartilage contains a progenitor cell population. J Cell Sci. 2004;117(Pt 6):889–897. doi: 10.1242/jcs.00912. [DOI] [PubMed] [Google Scholar]
- 88.Nakata K., Shino K., Hamada M., et al. Human meniscus cell: characterization of the primary culture and use for tissue engineering. Clin Orthop Relat Res. 2001;(391 Suppl):S208–S218. [PubMed] [Google Scholar]
- 89.Vinod E., Kachroo U., Rebekah G., Yadav B.K., Ramasamy B. Characterization of human articular chondrocytes and chondroprogenitors derived from non-diseased and osteoarthritic knee joints to assess superiority for cell-based therapy. Acta Histochem. 2020;122(6) doi: 10.1016/j.acthis.2020.151588. [DOI] [PubMed] [Google Scholar]
- 90.Jones P.H., Watt F.M. Separation of human epidermal stem cells from transit amplifying cells on the basis of differences in integrin function and expression. Cell. 1993;73(4):713–724. doi: 10.1016/0092-8674(93)90251-k. [DOI] [PubMed] [Google Scholar]
- 91.Verdonk P.C., Forsyth R.G., Wang J., et al. Characterisation of human knee meniscus cell phenotype. Osteoarthr Cartil. 2005;13(7):548–560. doi: 10.1016/j.joca.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 92.Daley W.P., Peters S.B., Larsen M. Extracellular matrix dynamics in development and regenerative medicine. J Cell Sci. 2008;121(Pt 3):255–264. doi: 10.1242/jcs.006064. [DOI] [PubMed] [Google Scholar]