Introduction
The recent advances in technology combined with the need to manage patients remotely during the coronavirus disease-19 (COVID-19) pandemic, have led to a rapid adaptation of the use of digital devices in clinical practice.1,2 The term digital devices for heart rhythm monitoring in this paper encompasses many of the novel devices, such as patches, various wearable devices, and handheld devices that have been approved by regulatory authorities for medical purposes. Cardiac implantable electronic devices (CIEDs), devices that can deliver therapy (such as life vests) and Holter monitors fall outside the scope of this paper.
Although many perceive the potential benefits from digital workflow, recent surveys show disparities in management with concerns from healthcare professionals of data overload and unsolicited registrations from unfamiliar digital devices.2,3
The aim of the document is to provide up-to-date practical guidance on the use of digital devices for arrhythmias, from early detection through management and implementation, using the categories of consensus (Table 1). To be included, a consensus statement needed at least 80% consensus by the co-authors.
Table 1.
Categories of the consensus statement
| Consensus statement | Definition | Symbol |
|---|---|---|
| Indicated or ‘should do this’ | Scientific evidence that a treatment or procedure is beneficial and effective, or is strongly supported by authors’ consensus |

|
| May be used | General agreement and/or scientific evidence favour the usefulness/ efficacy of a treatment or procedure |

|
| Should NOT be used | Scientific evidence or general agreement not to use or suggest a treatment or procedure |

|
The categorization for our consensus document should not be considered directly similar to the one used for official society guideline recommendations which apply a classification (I–III) and level of evidence (A, B, and C) to recommendations.
Digital heart rhythm devices in clinical practice
Digital devices for heart rhythm monitoring can be divided into two groups based on the technology used to evaluate heart rhythm:
Electrocardiogram (ECG)-based and
Non-ECG based, including photoplethysmography (PPG).
The choice of digital heart rhythm device should be tailored to the patient, considering symptom frequency, expected duration of monitoring, local infrastructure, and patient’s preference (Figures 1 and 4). Regardless of digital device used, clinician overreading of the recordings is necessary.
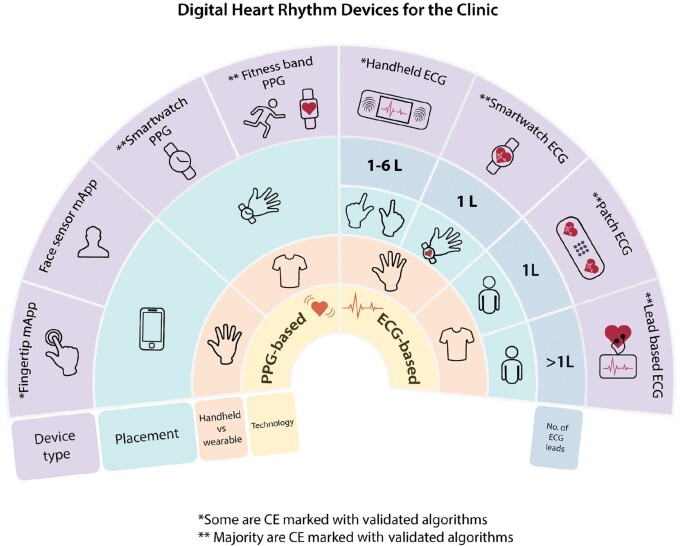
Figure 1.
Overview of digital heart rhythm devices for the clinic. Suggest reading the figure from the inner circle—devices have been divided into devices that provide photoplethysmography (PPG) or electrocardiogram (ECG), followed by the mode of handheld or wearable, and then placement on the body, number of leads, and device type. */**Please see Table 2 for further details. ECG, electrocardiogram; L, lead; mApp, mobile App; PPG, photoplethysmography.
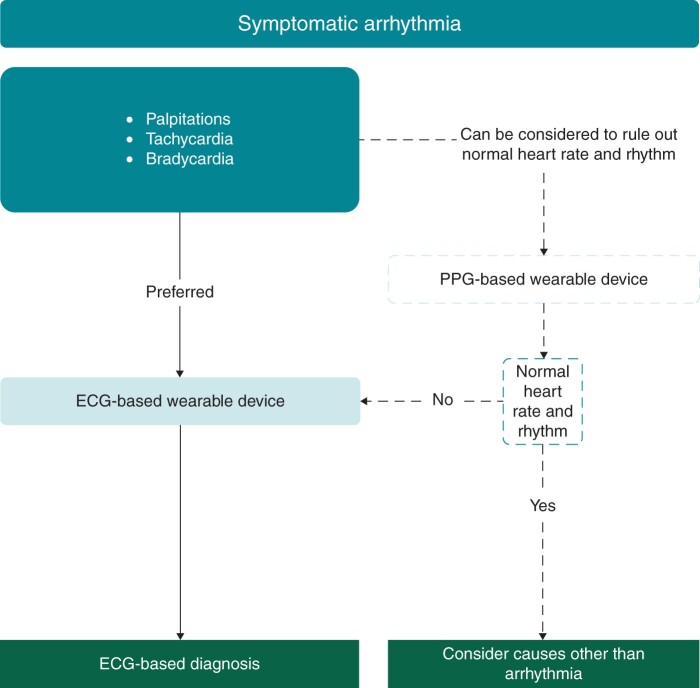
Figure 4.
Suggested workflow for the management of symptomatic arrhythmias.
Electrocardiogram-based digital devices
The currently available digital heart rhythm devices using ECG differ by a number of factors:
Type of device and mode of detection
Area of application
Placement
Number of leads
User feedback
Hardware/software
Battery: rechargeable vs. replaceable
Data storage: in-device vs. cloud-based
Data transfer: direct upload to cloud-based servers vs. paired smartphone/tablet vs. USB connection
ECG display: integrated screen vs. paired device vs. no real-time display
Regulatory
Regulatory clearance: CE/FDA
Validation of use by clinical studies
Handheld electrocardiogram
Single-lead devices usually provide recordings from lead I. Some models can be applied to the chest to record chest-right arm leads that can yield QRS complexes of higher amplitude and with clearer P waves than in lead I.4,5 Leads II and III can be recorded by applying the bipolar device to the left leg (the device can be placed on a dampened trouser to simplify the process), while holding the device with the right and left hand, respectively. A model with three electrodes allows simultaneous recordings of all limb leads by holding the device with both hands and applying the rear electrode against the left leg (Table 2).
Table 2.
Summary of heart rhythm monitoring devices that have been used in the clinical setting for rhythm diagnosis with peer-reviewed publications
| Device | Type | Area of application | Mode of detection | Cardiac sensor | ECG viewing | Battery | Data storage | Data transfer | Regulatory clearance | Validation | References | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Apple Watch | Smartwatch | Wrist-finger | ECG and PPG | 2 electrodes; 1 lead ECG | Integrated and on paired device | Rechargeable | In-mApp and Cloud | Cloud via smartphone/tablet | CE and FDA | Yes | 17–19 |
| Fitbit | Smartwatch | Wrist-finger | ECG and PPG | 2 electrodes; 1 lead ECG | On paired device | Recharegable | In-mApp and Cloud | Cloud via smartphone/tablet | CE and FDA | No | 16 | |
| Huawei | Band | Wrist | PPG | 2 electrodes | On paired device | Recharegable | In-mApp and Cloud | Cloud via smartphone/tablet | Asia | Yes | 37,38 | |
| Samsung | Smartwatch | Wrist-finger | ECG and PPG | 2 electrodes; 1 lead ECG | On paired device | Rechargeable | In-mApp and Cloud | Cloud via smartphone/tablet | CE and FDA | No | NA | |
| Withings | Smartwatch | Wrist-finger | ECG and PPG | 2 electrodes; 1 lead ECG | On paired device | Rechargeable | In-mApp and Cloud | Cloud via smartphone/tablet | CE | No | 21 | |
|
Alivecor Kardia Mobile | Handheld | Fingertips, ±leg or chest | ECG | 2 or 3 electrodes; 1 lead or 6 lead ECG | On paired device | Replaceable | In-mApp and Cloud | Cloud via smartphone/tablet | CE and FDA | Yes | 7 |
| Beurer ME 90 | Handheld | Chest-fingertip or finger-finger | ECG | 2 electrodes; 1 lead ECG | On paired device | Rechargeable | In-device | USB connector | CE and FDA | Yes | 5,8 | |
| Coala Heart Monitor | Handheld | Thumb-chest | ECG | 2 electrodes; 1 lead ECG | On paired device | Rechargeable | In-mApp and Cloud | Cloud via smartphone/tablet | CE and FDA | Yes | 39,40 | |
| ECGCheck | Handheld | Fingertips, ±leg or chest | ECG | 2 electrodes; 1 lead ECG | On paired device | Rechargeable | In-mApp and Cloud | Cloud via smartphone/tablet | CE and FDA | Yes | 41 | |
| Eko DUO | Handheld | Chest | ECG | 2 electrodes; 1 lead ECG | On paired device | Rechargeable | In-mApp and Cloud | Cloud via smartphone/tablet | CE and FDA | Yes | 42 | |
| HeartCheck CardiBeat, ECG Pen, Palm | Handheld | Fingertips, palm/chest/hip | ECG | 2 electrodes; 1 lead ECG | On paired device (CardiBeat); Integrated (ECG Device, ECG Pen, Palm) | Rechargeable (CardiBeat, Palm); Replaceable (ECG Device, ECG Pen) | In-mApp and Cloud (CardiBeat, Palm); In-device (ECG Device, ECG Pen) | Cloud via smartphone/tablet (CardiBeat, Palm); USB (ECG Device, ECG Pen) | CE and FDA | Yes | 43 | |
| MyDiagnostick | Handheld | Hands | ECG | 2 electrodes; 1 lead ECG | Via computer and software program | Rechargeable | In-device | USB connector | CE | Yes | 44 | |
| Omron HCG-801 | Handheld | Finger/chest | ECG | 2 electrodes; 1 lead ECG | Integrated | Replaceable | In-device (SD card) | SD card | FDA | Yes | 45 | |
| SnapECG E-H19 | Handheld | Fingertips | ECG | 2 electrodes; 1 lead ECG | On paired device | Replaceable | Cloud | Unclear | Asia | NA | 46 | |
| Zenicor-ECG | Handheld | Thumbs | ECG | 2 electrodes; 1 lead ECG | Via web-based platform | Replaceable | In-device; transfer to cloud | Cloud | CE | Yes | 47 | |
|
Movesense Medical (Suunto) | Chest strap | Chest | ECG | 2 electrodes; 1 lead ECG | On paired device | Replaceable | In-device, 7 days continuous; in-mApp | Cloud | CE | ? Yes | 35 |
| Zephyr BioHarness 3.0 (Medtronic) | Chest strap | Chest | ECG | 2 electrodes; 1 lead ECG | On paired device | Rechargeable | In-device | Wireless or USB | FDA | Yes | 36 | |
|
Bardy Dx Carnation Ambulatory Monitor (CAM) | Patch | Chest, self-adhesive | ECG | 2 electrodes; 1 lead ECG | Via web-based platform | Single-use | In-device, 14 days continuous | Direct download by company → cloud | CE and FDA | Yes | 48 |
| BioTel Mobile Patient Telemetry (MCOT) | Patch | Chest, self-adhesive | ECG | 3 electrodes; 2 lead ECG | Via web-based platform | Single-use, rechargeable | In-device, 30 days continuous | Wireless near real-time telemetry. Direct download by company → cloud | CE and FDA | Yes | 49,50 | |
| BodyGuardian Mini patches (Preventice) | Patch | Chest, self-adhesive | ECG | 2 electrodes; 1 lead ECG | Via web-based platform | Single-use, rechargeable | In-device, 30 days continuous | Wireless near real-time telemetry. Direct download by company → cloud | CE and FDA | Yes | 51 | |
| Life Signal Biosensor Patch | Patch | Chest, self-adhesive | ECG | 4 electrodes; 2 lead ECG | On paired device or web-based platform | Single-use | In-device, 5 days continuous | Wireless near real-time telemetry and cloud | CE and FDA | Yes | 52 | |
| MyPatch-SL | Patch | Chest, self-adhesive | ECG | 3 electrodes; 2/3 lead ECG | Via web-based platform | Single-use | In-device, 14 days continuous (2 lead), 9 days (3 lead) | USB transfer cable | FDA | No | ||
| S-Patch Cardio (Samsung SDS Wellsis) | Patch | Chest, self-adhesive | ECG | 2 electrodes; 1 lead ECG | Via web-based platform | Single-use | In-device, up to 100 hours continuous | Cloud | CE | Yes | 53 | |
| VitalPatch (VitalConnect) | Patch | Chest, self-adhesive | ECG | 2 electrodes; 1 lead ECG | Via web-based platform | Single-use | In-device, 7 days continuous | Wireless near real-time telemetry and cloud | CE and FDA | Yes | 54 | |
| VivaLink | Patch | Chest, self-adhesive | ECG | 2 electrodes; 1 lead ECG | Via mApp/web-based platform | Mulit-use, rechargeable | In-device, 96 h continuous | Wireless near real-time telemetry. Direct download by company → cloud | CE and FDA | Yes | 55,56 | |
| Zio XT/AT (iRhythm) | Patch | Chest, self-adhesive | ECG | 2 electrodes; 1 lead ECG | Via web-based platform | Single-use | In-device, 14 days continuous | Wireless near real-time telemetry (AT); USB (XT and AT) | CE and FDA | Yes | 10,11,57 | |
|
Cardiio Rhythm | Smartphone mApp | Fingertip or video facial detection | PPG | Smartphone camera | No ECG/HR only | NA | In mApp | NA | none | Yes | 23,58,59 |
| Fibricheck | Smartphone mApp | Fingertip | PPG | Smartphone camera | No ECG. HR + AF detection via algorithms | NA | In mApp | In-mApp and Cloud | CE and FDA | Yes | 29,60 | |
| Preventicus Heartbeats | Smartphone mApp | Fingertip | PPG | Smartphone camera | No ECG. HR + AF detection via algorithms | NA | In mApp | In-mApp | CE | Yes | 26,61,62 |
ECG, electrocardiogram; FDA, American Food and Drug Administration; mApp, mobile application; NA, not applicable; PPG, photoplethysmography.
Importantly, CE-marking as a class IIa-medical device does not ensure that the device’s algorithm for heart rhythm assessment is accurate; clinician oversight is still required for diagnostic interpretation.5,6 A manufacturer may also change the device’s algorithm, thereby impacting its accuracy.4,7 The reported diagnostic accuracy of a device will depend on its algorithm, the patient population using the device, the settings/conditions under which the recording is performed and on the physician interpreting the tracings.7–9 In the USA, the American Food and Drug Administration (FDA) regulates the sale of medical devices. To gain approval a device needs to show evidence that it is safe and effective for a particular use.
Electrocardiogram patches
Electrocardiogram patch monitors are validated, wearable digital devices for heart rhythm monitoring and diagnosis. With their low-profile, water-resistant, wireless, and self-adhesive form-factors, they are easy-to-use, well-tolerated and have high patient adherence.10 Patches have high accuracy and higher diagnostic yields than traditional 24-h Holter monitoring.11 Patch monitoring is cost-effective, with many symptomatic, clinically significant arrhythmias detected within the first week of monitoring.10,12 They are a feasible method for atrial fibrillation (AF) detection even when the observed AF burden is <15%.10 For AF screening in moderate- to high-risk populations, patch monitoring has comparable yield to implanted loop recorders at 2 weeks and 1 month, and 10 times higher yield compared to blood pressure monitoring.13–15 The limitation of these devices has mainly been relatively short battery life, the durability of the adhesive and, in some healthcare systems, lack of reimbursement.
A variety of CE marked/FDA cleared single-use ambulatory ECG patches are available, offering single channel, 5- to 30-day continuous recording with some offering live monitoring using mobile devices or cloud-based technology (Table 2). Several CE/FDA-marked ECG patches (one of which is reusable) offer additional vital signs monitoring and motion tracking via accelerometers. One patch monitor has been FDA cleared for ambulatory QTc monitoring.
Smartwatch electrocardiogram
Smartwatches are direct-to-consumer devices that have increasingly incorporated technology for monitoring health status. Several smartwatches on the market can record a single-lead 30-s ECG tracing by electrodes incorporated in the back of the watch and on the watch crown or case. Electrocardiograms tracings can be viewed in real-time on the watch screen and stored on a smart device mobile application (mApp), and PDFs can be generated and sent wirelessly to the healthcare team. Smartwatches have embedded AF-detection algorithms, but data on algorithm accuracy have until recently been limited.16–19 A recent meta-analysis comparing smartwatch technology (PPG or ECG) showed that smartwatches were non-inferior to routine AF monitoring strategies.20 A limitation with smartwatches has been their limited wear time as they require charging, but newer digital-analogue hybrid watches with single-lead ECG recording capability have extended battery life.21 Importantly, generated ECG tracings still require physician oversight and analysis for rhythm diagnosis.
Photoplethysmograpy recorders
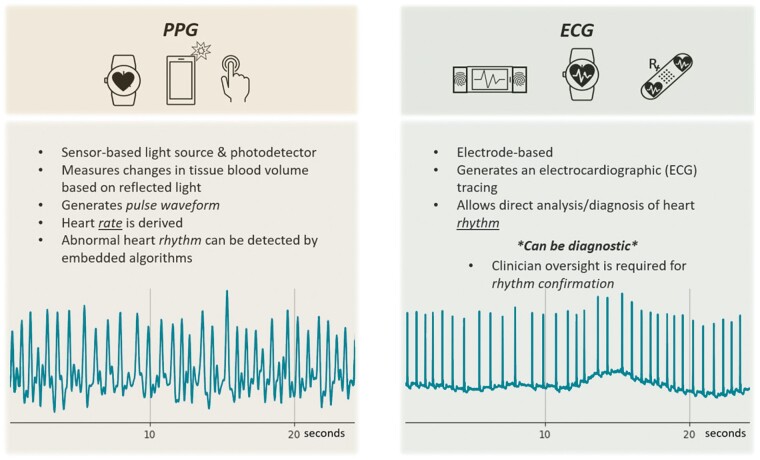
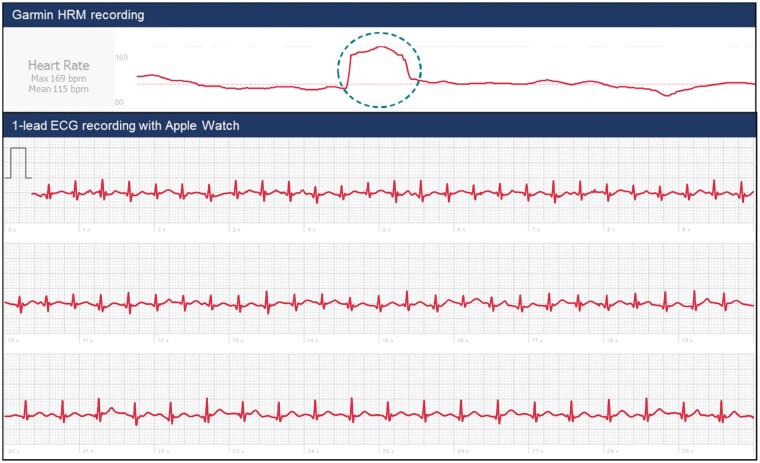
Photoplethysmography is capable of monitoring heart rate and detecting arrhythmias using an optical technique that analyses the peripheral pulse. A light source and a detector are used to measure changes in blood volume within the skin surface, detecting changes in reflected light intensity, generating a peripheral pulse waveform.22 A smartphone camera combined with the LED flashlight has been used for both contact (finger-over-the-camera) and contactless (facial video) PPG.23,24 Photoplethysmography is currently used in clinical routine to measure oxygen saturation and pulse rate.25 The relative ease of PPG technology has allowed its incorporation into various wearable devices to analyse heart rate and rhythm,26 such as chest straps, wristbands, forearm bands, rings, and ear buds.27Automated algorithms in smartwatches have been used to detect AF with high accuracy when measurements were taken in patients in a comfortable sitting position26; however, in ambulatory patients, the accuracy was considerably lower due to artefacts.28 The ubiquity of smartphones and PPG-based apps may allow more convenient and affordable larger scale arrhythmia detection and management. However, AF diagnosis requires confirmation via ECG with clinician oversight (Figure 2).29
Figure 2.
Comparison of photoplethysmography (PPG) vs. electrogram (ECG)-based techniques. In the lower part of the figure an example of a registration from a patient with atrial fibrillation is shown.
Other devices and biotextiles
Some blood pressure monitors can report heart rate. Blood pressure monitors that screen for AF using pulse irregularity have been shown to have a sensitivity of >85.30
Electrode-embedded garments enable wire-free heart rate and rhythm monitoring, often with an active population in mind. Compression garments, such as shirts and sports bras, multi-strap ‘vests’ and single chest straps paired with wristbands, are available aimed at providing wearability and comfort as well as stability to decrease motion artefact.31–33 These and other devices are further discussed extensively in the section on athletes (Figure 10). Few studies using chest straps to detect arrhythmias are published, clinical data regarding the use of electrode-embedded wearables for cardiac rhythm monitoring are limited, and no dedicated algorithms exist.34–36
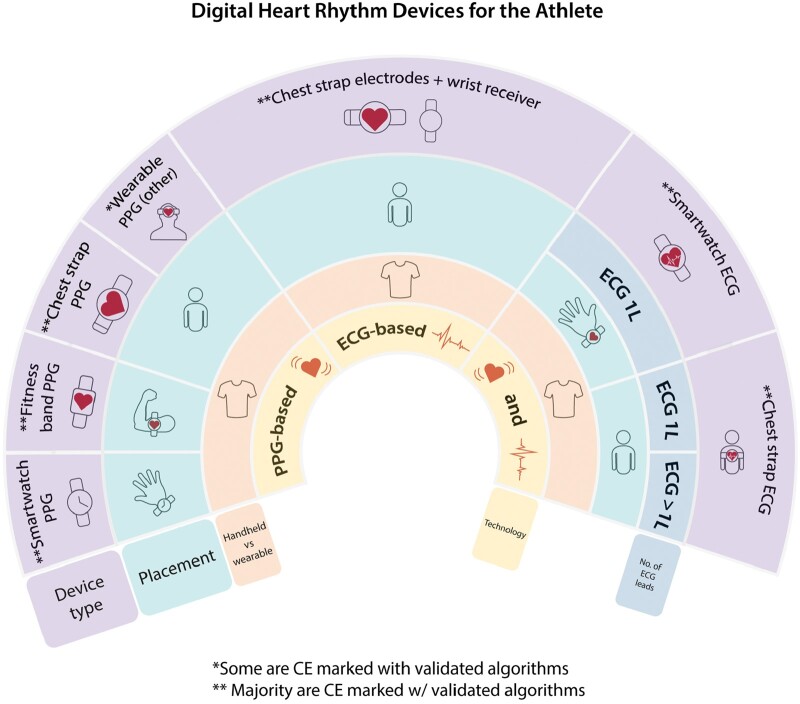
Figure 10.
Overview of digital devices for athletes.
| Consensus statement | |
|---|---|
| Abnormal findings in digital devices should be evaluated in team including a cardiac arrhythmia specialist or a cardiologist |

|
Digital devices in the diagnosis of symptomatic arrhythmias
The 12-lead ECG represents the gold standard for the diagnosis of arrhythmias. However, a 12-lead ECG has limitations of availability and cannot diagnose paroxysmal arrhythmias if the recording is performed during asymptomatic periods. ECG-based digital devices can overcome these limitations of availability. Although most digital devices provide ECGs with fewer than 12 leads, a single-lead ECG may be sufficient to diagnose the type of arrhythmia.
Considerations when using digital devices are
Many digital devices do not continuously record the heart rhythm; in this case, recordings must be user-initiated and in case of haemodynamic compromise, this might not be possible.
Initiating a recording requires several seconds followed by registration for at least 30 s. This delay renders existing digital technologies poorly suited for diagnosing brief arrhythmias.
Before therapeutic decisions are made based on digital device recordings [that is initiating anticoagulation for presumed AF or considering an implantable cardioverter-defibrillator for presumed ventricular tachycardia (VT)], it is imperative to confirm the arrhythmia by carefully ruling out artefact or noise. To minimize the risk of false positives, the quality of the recording is key, and steps to minimize baseline wander and artefacts is of essence.
However, the additional benefit of digital devices is the widespread availability compared to standard ECGs thereby increasing the probability of recording paroxysmal arrhythmias at the right time (Figure 3).
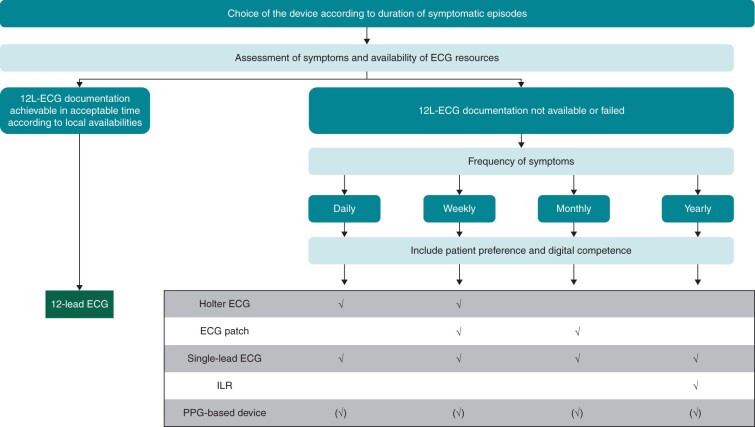
Figure 3.
Choice of ECG device in symptomatic patient. If possible, subject to availability and duration of symptoms a 12-lead ECG should be achieved to evaluate symptomatic arrhythmias. In case of difficulties achieving a 12-lead ECG during symptomatic episodes, assess the frequency of symptoms and patient preference prior to choosing long-term ECG device for heart rhythm monitoring. (✓) possible use; 12 L, 12 lead; ECG, electrocardiogram; ILR, implantable loop recorder.
Photoplethysmography recordings may be of aid in symptomatic patients with a very low probability of symptoms being caused by arrhythmias to document a normal rhythm and normal heart rate. Any arrhythmias detected using PPG recordings should be confirmed by a 12-lead ECG if possible or an ECG-based device when 12-lead ECG is not available, or the duration of arrhythmia does not allow an ECG-based recording. However, even a normal heart rate and rhythm in a PPG recording does not completely exclude atrial arrhythmia (e.g. atrial flutter or focal atrial tachycardia with regular conduction) and should trigger confirmation by an ECG when in doubt (Figure 4).
| Consensus statement | |
|---|---|
| Symptom-rhythm correlation for diagnosis of symptomatic arrhythmias can be achieved with ECG-based digital devices |

|
| For paroxysmal arrhythmias, ECG-based digital devices can be used as an event recorder to document and diagnose arrhythmias |

|
| For establishing a diagnosis, ECG-based wearables are preferred over PPG |

|
Screening for atrial fibrillation
Atrial fibrillation prevalence has been constantly rising and this increase is projected to continue in the years to come.63 Manifestation and characteristics of AF-related symptoms strongly vary among patients and about one-third of patients remain asymptomatic. Asymptomatic, undiagnosed, and undertreated AF contributes to ischaemic strokes and therefore screening for AF bears the potential of preventing stroke and death.64,65 Early diagnosis of AF can also enable early rhythm treatment, which has been shown to reduce mortality, stroke, and cardiovascular hospitalization in clinical AF.66
When considering screening for AF, individuals referred for screening should be informed of the implications of screening and receive information about the next steps in case of positive or ambiguous findings.67
Screening strategies differentiate between opportunistic or systematic screening (Table 3) but other factors are also of importance (Table 4).68 Strategies should be chosen by carefully weighing the risks and benefits of screening.67
Table 3.
Definitions of screening strategies
| Strategy | Definition | Examples |
|---|---|---|
| Opportunistic screening | Screening performed as a part of clinical contacts for any other reason than screening |
|
| Systematic screening | Screening programme performed continuously irrespective of medical contacts or need |
|
| Screening in risk groups | Screening performed in individuals who sustained a prior stroke or transient ischaemic attack |
|
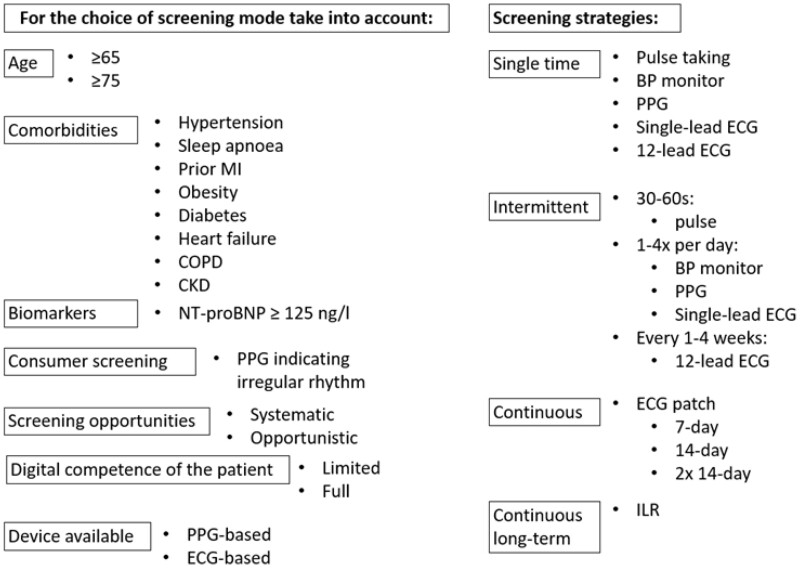
Table 4.
Factors to consider when choosing screening mode
|
BP, blood pressure; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; ECG, electrocardiogram; ILR, implantable loop recorder; MI, myocardial infarction; NT-proBNP, N-terminal-pro hormone brain natriuretic peptide; PPG, photoplethysmography.
Screening for AF can be performed in a variety of settings and in different cohorts ranging from the general population to high-risk patients.17,44,69–96 Detection rates of newly diagnosed AF depend on the screening setting, target population and duration of monitoring and can vary from <1–3.8% in non-selected cohorts of individuals72,73 to as high as to 6.8–7.4% in patients with higher risk.71,73 Increasing the duration and/or the frequency of screening measurements increases the detection rates. Therefore, a screening setting with more than a single measurement should be preferred to increase the screening yield.97
The clinical impact and clinical consequences of AF identified and diagnosed in asymptomatic individuals in the context of screening programmes here termed ‘screening-detected AF’ is not fully elucidated. Based on the current evidence, screening-detected AF should be confirmed by a physician and treated according to current guidelines.67 Two randomized controlled trials (RCTs) on clinical outcomes in screening-detected AF have been published.98,99 In the STROKESTOP study, using a screening intervention of 2 weeks twice daily intermittent single-lead ECGs, a small benefit on the combined endpoint mortality, stroke and major bleeding was seen in the group invited to screening as compared to the control group.98 In the LOOP study, individuals were randomized to be screened for AF using implantable loop recorders, and there was no significant reduction in the primary outcome of stroke and systemic embolism in the screened group.99 These studies raise several topics that need to be investigated further; the difficulties getting the population at highest risk to participate in screening programmes, possible negative aspects of screening such as anxiety, the high background detection of AF in control groups and different subtypes of AF including severity of AF burden and the substrate severity necessitating oral anticoagulant (OAC) therapy.67 Further randomized studies aiming to investigate screening effects on long-term clinical outcomes are currently recruiting, Supplementary material online, Table S2.
Important efforts for evaluation of the effects of systematic screening strategies are currently underway and aim to further clarify strategic pathways, best-suited target cohorts, device selection, screening mode and setting, effect on stroke reduction and more (Supplementary material online, Table S3, Table 4, Figure 5). The additional potential of wearable devices in this context seems evident, but nevertheless requires more evidence to prove a positive risk-benefit ratio.
Figure 5.
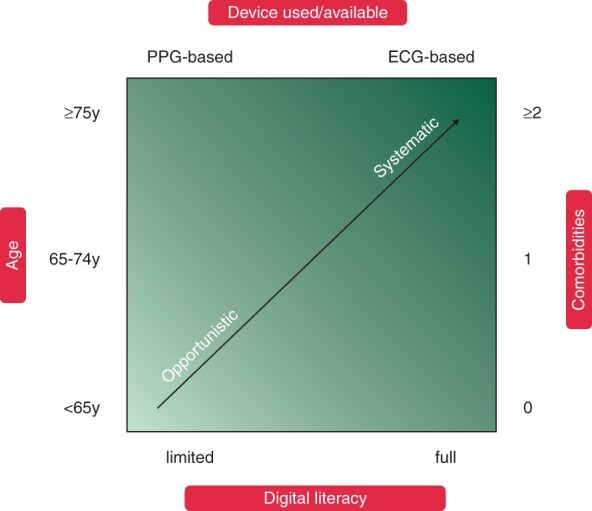
Considerations for atrial fibrillation screening programme (systematic or opportunistic) and digital device based on patient age, comorbidities, and digital literacy.
Figure 6 provides a suggested workflow to assign the most appropriate screening strategy and screening mode to the respective patient.
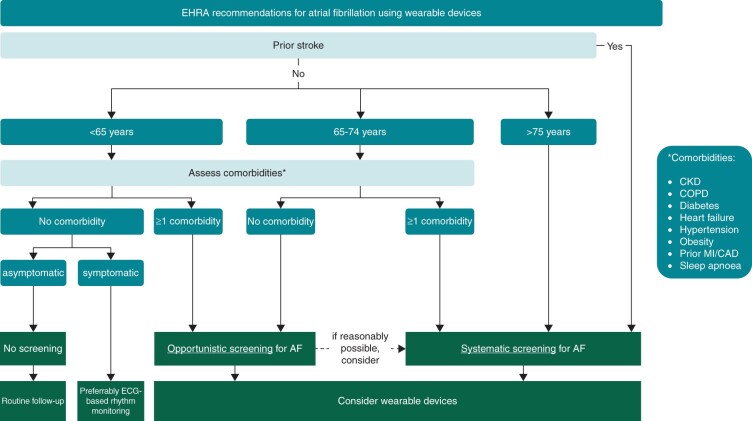
Figure 6.
EHRA suggestions for screening for atrial fibrillation using digital devices. For patients with a prior stroke, a systematic screening approach for AF should always be implemented, preferably immediately after the event. As age is the most important risk factor for stroke, suggestions are based on age, with individuals at or above 75 at highest risk. For younger individuals, screening might still be warranted based on their risk factors as per the CHA2DS2-VASc score, and in addition for individuals at higher risk such as patients with CKD (chronic kidney disease), COPD (chronic obstructive pulmonary disease),100 obesity,101 and sleep apnoea.67,102 AF, atrial fibrillation; CAD, coronary artery disease; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; ECG, electrocardiogram; MI, myocardial infarction.
| Consensus statement | |
|---|---|
| Systematic screening by intermittent ECGa is beneficial to detect AF in individuals aged ≥75 years |

|
| Systematic screening by intermittent ECGa may be beneficial to detect AF in individuals aged ≥65 years with comorbidities increasing the risk of stroke |

|
| Opportunistic screening for AF may be beneficial in patients aged ≥65 years without comorbidities or <65 years with comorbidities |

|
| PPG-based or ECG-based devices are preferred to pulse palpation for AF screening |

|
| In systematic screening for AF, PPG-based or ECG-based devices can be used |

|
| If PPG screening is indicative of AF, an ECG-based method should be used to confirm the diagnosis of AF |

|
| If AF is diagnosed during screening, patients should be informed, appraised for OAC treatment, and AF risk factors managed |

|
| Screening for AF at multiple time points or over a prolonged time should be preferred over single time-point screening to increase the diagnostic yield regardless of symptoms |

|
| The term ‘screening-detected AF’ should be used for AF diagnosed in a screening setting and the diagnosis should be confirmed by a physician |

|
Patient engagement perspective
The majority of available trials of patients’ perspectives in digital devices are small, of short duration, use self-reported outcomes and rarely take into consideration potential harms and financial implications.105 A few studies evaluating the value of digital devices from the patients’ perspective exist, such as studies showing that digital devices can improve patients’ adherence to cardiovascular medications.1,8 In the recent mobile application (app) in AF (mAFA) trial, a randomized trial of mobile health technology in patients with AF used a dedicated app that incorporated patient educational programmes, self-care, and structured follow-up tools. Patients’ satisfaction, drug adherence, anticoagulation satisfaction, and quality of life were significantly improved in the digital devices arm vs. usual care.106
Potential barriers and side effects
Patient engagement might be improved by digital health technology and co-design is a key factor for success of digital devices. In a systematic review of barriers to and facilitators of health technology, patient engagement was highlighted, revealing that acceptability was highly variable, with dropout rates ranging up to 44%. Usability issues were the most cited reasons for dropout. Other barriers included health status, motivation, perceived utility and value, convenience, and accessibility of digital tools.107
Other barriers and side-effects exist
patients may choose a to buy a device over the counter that is not approved as a medical device and hence does not adequately provide optimal diagnostic benefits;
reimbursement for costs related to digital devices vary;
a focus on self-monitoring may increase anxiety;
concerns may exist regarding data protection;
not all patients can or want to engage in their care in the way that is necessary for digital device arrhythmia detection or monitoring; and
for healthcare personnel large amounts of data, unsolicited recordings and recordings sent out of hours can lead to increased workload, and cause legal unclarities.
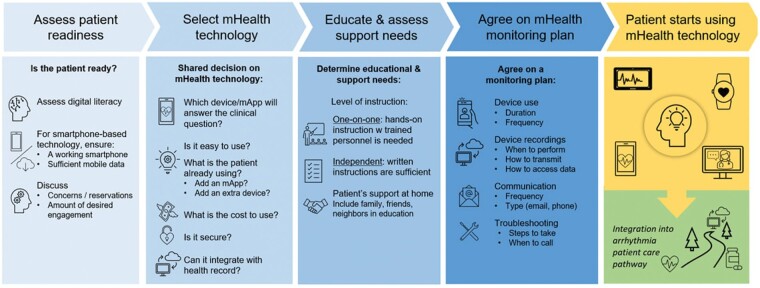
Before engaging a patient in digital health technologies, the pathway outlined in Figure 7 can be consulted. To ensure adoption of and adherence to digital devices, involving patients and caregivers as early as possible in the development process can be beneficial.8,9 Co-design will be essential to create apps and devices with intuitive user interfaces, and which better fulfil patients’ expectations, thereby increasing adherence.
Figure 7.
Patient engagement.
Digital health literacy
Digital literacy, defined as ‘the ability to seek, find, understand, and appraise health information from electronic sources and apply the knowledge gained to addressing or solving a health problem’108 is crucial to ensure digital equity and inclusivity. Digital literacy requires both technical and cognitive skills.109 Digitally health literate patients have the necessary knowledge to use a smartphone-based app or other mobile device, and understand how collected health data or electronic health information can help better manage their health.110–112 Variables such as age, educational background, health, and socioeconomic status can impact the ability to develop digital health literacy.112 Assessing patient digital health literacy, identifying individual needs, and improving both knowledge and skills will be critical to successful patient engagement with, and future adherence to digital health technologies (Figure 7).
| Consensus statement | |
|---|---|
| Following a structured patient pathway is beneficial when engaging patients in the use of digital health technology |

|
Atrial fibrillation care using digital devices
For patients with AF digital devices can be of aid in the guideline-recommended integrated management approach,67 including remote rate and rhythm monitoring. This can be organized as on-demand mobile health prescriptions.113 Self-management can increase patient involvement in the care process and treatment decision-making. Widespread use of digital devices for continuous or on-demand monitoring require new and adapted (digital) infrastructures to accommodate new processes and increased data loads.
Transition from screening to early atrial fibrillation management
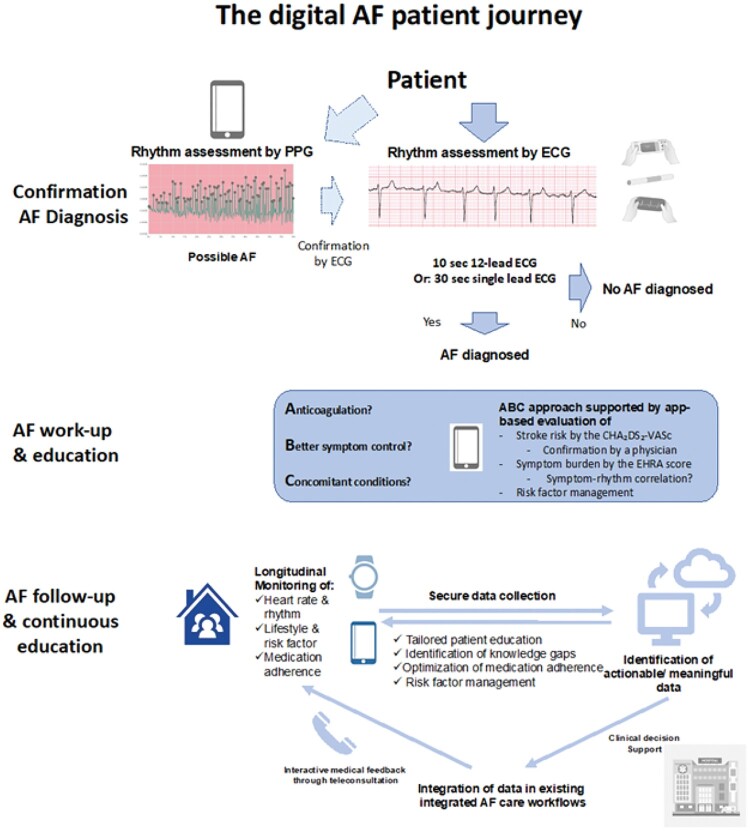
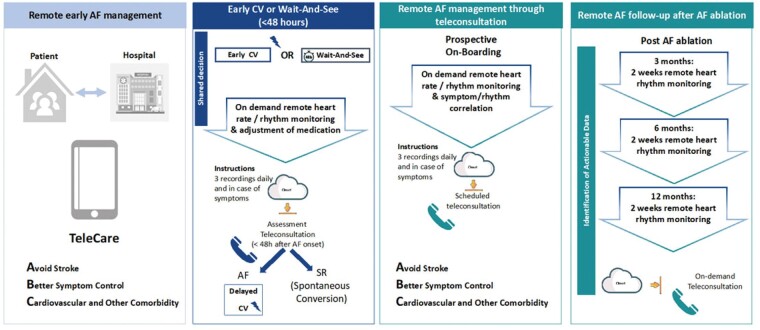
Early detection of AF allows for early initiation of AF management, and early rhythm control therapy lowers the risk of adverse cardiovascular outcomes.66 Strategies for early AF detection should be linked to a comprehensive work-up organized within an integrated management pathway to allow initiation and guidance of AF treatment in newly detected AF patients.114 This transition from AF detection to early AF management can be supported by digital technology (Figure 8).
Figure 8.
The potential uses of digital devices in patients with atrial fibrillation.
Atrial fibrillation work-up and education
Adherence to the ABC-integrated care strategy has been shown to be associated with improved clinical outcomes, and consists of: A, Avoid stroke; B, Better symptom management; and C, Cardiovascular and other comorbidity risk reduction.115,116 Digital devices can be of aid in assessment of stroke risk (A), symptom burden and symptom-rhythm correlation (B), and management of concomitant risk factors (C). Continuous patient education can be provided by a digital infrastructure collecting data longitudinally, which can be managed by intelligent data processing and finally imbedded in an existing multidisciplinary and integrated care approach in an AF clinic.
The mAFA programme included a prospective cluster-randomized clinical trial, which randomized patients to receive usual care, or integrated care based on the ABC Pathway.117 The trial showed that rates of the composite outcome of ischaemic stroke/systemic thromboembolism, death, and rehospitalization were lower with the App-based mAFA intervention. In a long-term extension cohort, the beneficial effects were maintained, with high adherence (conformity to recommendation about day-to-day treatment) at >70% and persistence (continuity) >90% with OACs using the mAFA app-based intervention, and a reduction in bleeding risk (Figure 9).118,119
Figure 9.
Practical examples of digital AF management. Note that for Panels 2–4 randomized controlled trials are still lacking, and these should be views as examples of ongoing practical applications of digital tools. For all on demand remote heart rate/rhythm monitoring, an experienced physician should verify the findings by the device.
Rhythm monitoring of atrial fibrillation
Although PPG technology is not diagnostic of AF according to the 2020 European Society of Cardiology (ESC) Guidelines for the diagnosis and management of AF,67 its widespread accessibility and low cost make it an interesting tool for remote heart rate and rhythm monitoring in patients with known AF. Challenges of PPG recordings include underestimation of the heart rate in AF by up to 10 b.p.m. due to a pulse deficit, inaccurate data in case of for example poor skin contact, activity and variations in skin tone. Precise cut-off values for PPG-based rate control are being determined.120,121
For both PPG-based and single-lead ECG devices diagnosis of regular tachyarrhythmias from the atria can be challenging, based on the lack of (PPG) or difficulty to detect (ECG) p-waves. The distinction between AF, typical atrial flutter, atrial tachycardia, and junctional tachycardia can be difficult to make but is important if considering an ablation strategy. In case of single-lead ECG recordings from a watch placing the watch on an alternative position, such as the ankle or the precordium, can facilitate the detection of P waves.
Peri-cardioversion
Achieving optimal rate control of AF patients waiting for elective cardioversion or patients followed-up using a wait-and-see strategy at the emergency department (ED), can be challenging.67,122 Regular assessment of rate control and the use of a simple preprocedural medication adjustment protocol is effective in optimizing peri-cardioversion rate control.123
The TeleWAS-AF approach supports the management of AF patients peri-cardioversion via remote rate and rhythm monitoring using digital devices, allowing for remote adjustment of rate control medication and detection of spontaneous conversion to sinus rhythm.124 In general, all stable patients who present to the ED with recent-onset symptomatic AF planned for a wait-and-see approach who can use digital solutions for remote heart rate and rhythm monitoring are eligible for this approach. Whether the implementation of digital devices can facilitate the management of AF in the ED and reduce the burden on the ED system is currently investigated in ongoing studies (Figure 9).
Post-ablation
Holter-ECG is frequently used to monitor rhythm at 3, 6, and 12 months after AF ablation to test for AF recurrence. During the COVID-19 pandemic, several centres collected experience on using on-demand digital devices for follow-up after AF ablation.125 In a pilot study from a single-centre patients using digital devices 3 months after AF ablation had similar AF detection rates and a reduced need for additional ECG-monitoring compared to standard-of-care.111 A caveat here is that validation of most devices has not been performed in the post-ablation population, which might be more prone to atrial tachycardias other than AF, which is notably more difficult to diagnose with digital devices using single-lead ECG or PPG. Prior studies have shown that 2 weeks of long-term intermittent monitoring by digital devices more effectively detected AF recurrences and had a higher patients’ usability than short continuous Holter monitoring.126
Atrial fibrillation follow-up
During the COVID-19 pandemic, an on-demand digital approach for the remote management of AF through teleconsultation was used in 40 centres in Europe. The TeleCheck-AF approach implements remote PPG rate and rhythm monitoring in patients managed through teleconsultation.127,128 Patients were instructed to use the PPG app three times daily and in case of symptoms 1 week prior to teleconsultation. This information was then used during teleconsultation (Figure 9). Data indicate a positive centre and patient experience.29 The effect of this intervention on clinical outcomes will be investigated in an RCT.
Mobile platforms and support systems
Despite widespread availability, most AF mobile platforms and support systems are not evaluated for effectiveness and only a minority are CE-approved. The ESC has together with the CATCH-ME Consortium, developed a patient app to enhance patient education, self-management and interaction with healthcare providers and an app for healthcare providers that simplifies treatment choice and optimizes AF guideline adherence.129 Neither app has been studied with regards to clinical outcomes. The Health Buddies application was developed to improve OAC adherence in elderly AF patients, via daily health challenges for them and their grandchildren. This resulted in a small increase in knowledge and continued high adherence to OAC therapy.130 A summary of decision tools and applications available for healthcare professionals is available in the 2020 ESC Guidelines for the diagnosis and management of AF.67
| Consensus statement | |
|---|---|
| Digital AF management workflows should be structured according to an integrated care approach, such as the ABC (Atrial fibrillation Better Care) pathway |

|
| Digital AF management pathways should be integrated in existing AF care workflows provided there is patient engagement |

|
| In structured remote follow-up of patients with already diagnosed AF the use of digital devices may be beneficial |

|
| AF management via teleconsultation supported by digital device-based rate and rhythm monitoring may be an alternative to traditional face-to-face consultations in AF outpatient clinics in accordance with patient preference |

|
| In clinical follow-up after pulmonary vein isolation intermittent rhythm monitoring by digital devices may be suitable |

|
Ventricular arrhythmias and syncope
Digital devices may be an adjunct to conventional arrhythmia monitoring as they can allow ECG documentation during symptomatic episodes and in follow-up after therapy. However, patient-activated digital devices do not replace regular continuous monitoring in case of non-responsive (syncope) or non-tolerated (ventricular arrhythmias) events. In these scenarios, implanted cardiac rhythm monitors have advantages.
Digital devices using ECG can be potentially effective in differentiating VT from supraventricular tachycardia (SVT), whereas PPG cannot distinguish ventricular from supraventricular rhythms. Software algorithms and clinical adjudication is not yet established for ventricular arrhythmias.
Syncope
Implantable or wearable medical ambulatory continuous monitoring for prolonged periods have been used for the evaluation of heart rhythm during syncope. Current direct-to-consumer devices lack patient-activated systems loop recording by post-syncope activation.
A multicentre RCT comparing the use of a handheld ECG device vs. standard care in participants who presented to the ED with palpitations or presyncope showed an increased detection rate of symptomatic arrhythmias in the handheld ECG group.131
Falls associated with syncope lead to accidents that are especially disabling for the elderly. Devices with accelerometers and gyroscopes, such as smart watches, can detect a fall, and if no response is obtained from the wearer, can trigger an emergency response. A recent study suggested its sensitivity needs to be improved.132 Mobile apps that combine analysis of heart rate monitoring together with fall detection, GPS positioning, video recording with a display of patients’ surroundings, and the capability to send alerts either triggered by patients in case of symptoms or automatically in case of detected falls, may become useful.133 Early work has suggested that features extracted from ECG and PPG might aid in predicting neurally mediated syncope.134 Future development of retrospective documentation of the underlying rhythm after triggering an event in haemodynamic compromised or syncopal consumers, and possibilities to combine analysis of continuous rhythm and blood pressure is needed.
Ventricular tachycardia
The use of digital devices for VT detection lags far behind its use for AF. This is due to two issues: (i) sustained VTs may not be haemodynamically tolerated and thus preclude user-initiated recordings, and (ii) tachycardia discriminators need improvement. Sudden increase in pulse rate by digital devices suggests possible paroxysmal tachycardias, but PPGs are not able to discern the origin of the tachyarrhythmias, and most digital devices using ECGs need to be activated through an active process that might not be possible in non-tolerated VT cases. An exception is ECG patches, which provide continuous recording. For other ECG devices, a high burden of premature ventricular contractions or symptom-documented broad complex tachycardias may trigger further cardiology investigations leading to a diagnosis of VT.
There have been case reports of symptomatic VT that patients have recorded with handheld ECG devices or smart watches.135,136 Although it is challenging to diagnose VT without ECG recording, in one case using a wearable smartphone-enabled ‘smart sock’ cardiac monitoring device detected rapid rhythm in an infant and prompted the parents to seek medical attention, which resulted in a diagnosis of fascicular VT.137
Ventricular tachycardia is usually adjudicated only if broad-complex tachycardia is documented in wearable technology and replicated in ECGs or invasive studies. In the future, the 12 leads, bluetooth/smart phone-based ECG acquisition and monitoring system (cvrPhone) with potential to analyse beat-to-beat variability of ECG morphology, detect myocardial ischaemia and lethal arrhythmia susceptibility,138 and 6-lead ECG devices may help to diagnose VT more precisely. In symptomatic patients without structural heart disease wearable technology may be helpful to document arrhythmia ECG in symptomatic VT episodes and can supplement conventional rhythm monitoring.
As there is an increase in the use of digital devices incidental findings of broad complex tachycardias might become more common. Any incidental findings of suspected VT using digital devices it should prompt further diagnostic work-up.
Broad complex tachycardia documented in wearables should prompt cardiology work-up for underlying structural heart disease and trigger further non-invasive and invasive arrhythmia documentation. In the future developments of wearable technologies may help diagnose symptomatic VT and aid in clinical decision-making. Currently, conventional ECG-based continuous rhythm monitoring is still suggested to record episodes of VT.
| Consensus statement | |
|---|---|
| Conventional ECG-based continuous rhythm monitoring is preferrable to record episodes of VT |

|
| Digital devices using ECG may supplement conventional rhythm monitoring in patients with symptoms and without haemodynamic compromise |

|
| The detection of broad complex tachycardia in digital devices should prompt immediate cardiology evaluation |

|
Digital approaches in class I and III antiarrhythmic drug therapy
Predominantly, antiarrhythmic drugs exert their effects by prolonging QRS width (Class 1) or QT intervals (Class III).139 In general, the occurrence of prolongation of the QRS >25% or of the corrected QT above 125% from baseline (or QTc above 500 ms) should lead to termination or dose reduction of antiarrhythmics in most cases.140 Ventricular premature beats and non-sustained VT might be signs of impending proarrhythmic fatal events due to VT or ventricular fibrillation. Due to concerns for QT prolongation and polymorphic VT67 controversy exists regarding the safety of outpatient antiarrhythmic drug initiation.140
Digital devices using ECG tracings can, in some cases, allow a more detailed ECG interpretation incorporating QRS duration and QT interval.141–146
Monitoring QT interval
Few digital devices are FDA-approved for QTc monitoring (KardiaMobile 6L, AliveCor and Biotel Heart MCOT, Philips), but there is a lack of studies on initiation and titration of antiarrhythmic drugs. Therefore, digital devices should be used with caution to monitor drug effects.
Overall, studies of QT intervals in digital devices are small and conflicting. In a small trial comparing a remote wearable monitoring system with manual measurements of QT intervals, there was relatively good accuracy.147 A recent study compared QT intervals in sinus rhythm between a smartphone-ECG with a 12-lead ECG in patients receiving sotalol or dofetilide.148 The smartphone recording was capable of detecting QTc prolongation, with smartphone lead I most accurate in measuring the QTc if <500 ms.148 In contrast, another ECG smartwatch study showed that accurate QT measurements were only achieved in 85% of patients.145 The use of artificial intelligence algorithms in smartwatches to examine the QT intervals in patients treated with macrolide antibiotics, revealed just fair agreement with manual measurements on 12-lead ECGs.21
Studies of single-lead digital devices show variable results, and overall, single-lead ECGs might miss significant information about the QT intervals if the recordings are not validated with a baseline ECG.149 An individual adjustment of the recording vector and comparison to surface 12-lead ECG intervals is necessary at baseline. In case of an observed, potentially clinically relevant, digital device-recorded abnormal ECG finding, a surface 12-lead ECG should be obtained for validation.
In summary, studies on digital devices on initiation of antiarrhythmic drugs are scare, and automatic arrhythmia detection algorithm might miss arrhythmic events, hence more studies are needed before wearable digital devices can be safely used in patients during antiarrhythmic drug initiation, titration and treatment.150
| Consensus statement | |
|---|---|
| Measurements of heart rhythm during initiation of antiarrhythmic drug therapy in outpatients using ECG-based digital devices may be of use |

|
| Measurements of symptomatic/asymptomatic arrhythmic events (supraventricular/VT, ectopic beats) using ECG-based digital devices after initiation of antiarrhythmic drug therapy in outpatients may be of use |

|
| In case a digital device shows an abnormal ECG finding after initiation of antiarrhythmic drug therapy a 12-lead ECG should promptly be taken |

|
Use of digital devices in patients with inherited arrhythmogenic diseases
Inherited arrhythmogenic diseases include genetic disorders (arrhythmia syndromes and cardiomyopathies) presenting with a large spectrum of phenotypes that require non-uniform monitoring intensity.151 The benefit of digital devices in these patients is the ease of use, providing physicians with means to perform ECG monitoring more frequently during everyday activities, but also in specific settings/recognized triggers such as exercise, post-exercise, arousal from sleep, fever, and emotional stress. In addition, digital devices offer the possibility of identifying the arrhythmia during a symptomatic episode, which can aid in obtaining ECG documentation of symptomatic arrhythmias (e.g. malignant ventricular rhythms vs. supraventricular arrhythmias) and to refine patient’s risk stratification (e.g. detection of non-sustained VT in hypertrophic cardiomyopathy or arrhythmogenic cardiomyopathy) but can also to reassure the patient if the cause of their symptoms (e.g. pre-syncope) is not related to a cardiac arrhythmia.152 However, studies of digital devices in this patient group prone to severe arrhythmias are scarce and more studies are needed prior to clinical implementation.
The future clinical application of digital devices in this setting relates to diagnosis, arrhythmia detection and ECG parameter monitoring. There are dynamic features on the ambulatory ECG that may point to certain genetic conditions; the QT interval for the long-QT syndrome (LQTS) or the type 1 ECG in the right precordial chest leads for Brugada syndrome (BrS).151 A 24-h continuous 12-lead ECGs assessment can lead to the detection of a spontaneous type 1 pattern at least once over 24 h in up to 34% initially classified as ‘drug-induced BrS’.152 Specific ECG features of LQTS associated with torsade de pointes (microvolt T-wave alternans) have been detected by using ambulatory ECG monitoring.153
In patients with inherited arrhythmogenic diseases, there are recognized triggers of malignant arrhythmias which require more frequent ECG and rhythm monitoring:151,154
LQTS: electrolyte abnormalities, QT-prolonging drugs, COVID-19 infection
LQTS-2: post-partum
BrS: fever
LQTS-1/arrhythmogenic right ventricular cardiomyopathy/hypertrophic cardiomyopathy/catecholaminergic polymorph VT: sport
Studies of QT intervals in digital devices have shown contradictory results, see section on antiarrhythmic drugs. Certain developments show promise, such as a 6-lead ECG device approved for use in the measurement of a patient’s QTc intervals, and the use of artificial intelligence in digital devices to detect QTc values ≥500 ms.143,145 For patients with LQTS, this may allow early detection of QTc and to assess the response to antiarrhythmic drug therapy. Hence, digital devices have the potential for remote QT monitoring but need to be further assessed in patients with LQTS.145
| Consensus statement | |
|---|---|
| Digital devices may be used in patients with inherited arrhythmogenic diseases to aid diagnosis, arrhythmia detection and monitoring of ECG parameters |

|
| QT measurement by digital devices validated for QT measurement, may be reasonable in patients with LQTS during drug treatment that might prolong QT interval, trigger exposure (e.g. post-partum, exercise, COVID-19 infection) and to assess drug efficacy |

|
Common digital technologies used in athletes
Athletes have been early adopters of digital devices for training guidance with a focus on heart rate monitoring. A plethora of heart rate monitors (HRMs) are commonly worn during athletic training and competition. These use either electrocardiac sensors in chest-worn devices, or PPG technology. The latter is integrated into wrist-, arm- (e.g.), forehead-, and ear-worn devices (Figure 10).
Heart rate chest strap devices consist of two parts: an electrocardiac sensor-embedded chest strap that directly measures cardiac electrical activity, and a wrist-worn receiver displaying heart rate metrics. Heart rate is measured by counting RR intervals without ECG recordings. These devices have high R-wave detection accuracy when compared to Holter as the gold standard.155–157 Key limitations are artefacts due to transmission interference between the strap and the receiver—often caused by inadequate contact, interaction of bras with the strap in female athletes, and general discomfort while wearing.158,159
Wrist-, arm-, forehead-, or ear-worn PPG devices are smaller, more easily worn, and lower cost which make these more widespread, albeit less reliable.160 Algorithms that apply noise filtering and calculate the heart rate using PPG data are a major determinant of heart rate accuracy but are often closed systems. Validation studies using Holter monitor as controls reveal that high-end chest strap devices have superior performance (accuracy of >0.90) compared to PPG-based wrist-worn monitors (highly variable accuracy range, 0.36–0.99).156,157,161–163 None of these devices is designated as a medical-grade HRM during exercise. Nevertheless, some athletes may seek medical attention due to high or (extreme) low heart rate on their monitors, with or without concomitant symptoms. Both athletes and medical professionals should critically evaluate and validate that information, especially when based on PPG during exercise. Abnormal heart rate measurements should be confirmed by simple pulse palpation and ideally ECG recording (Figure 12). The emergence of (single-lead) ECG recording embedded in HRMs is a significant advancement158,164–167; some can provide a three-limb lead ECG.168 Electrocardiogram confirmation is especially important for bradyarrhythmias, to correct for missed pulse detection by the digital device. Other metrics obtained by digital devices such as heart rate variability, acceleration, body position, temperature, and oxygen levels may be of value in athletic monitoring, but will not be discussed in this text.
Figure 12.
Athlete with sudden heart rate accelerations documented on heart rate monitoring device. An athlete (48-year old male) presented to the outpatient clinic with palpitations during cycling exercise with heart rate accelerations from 120 to 180 b.p.m. without any clear triggers. His chest strap band (Garmin Edge 1030) output showed a sudden start and onset of the episodes, which coincided with subjective palpitations. The combination of known chest strap accuracy and symptoms made an arrhythmia likely. Since cardiac evaluation ruled out structural heart disease, and the episodes were of longer duration (see Flowchart A), patient-activated ECG recording was deemed necessary. The patient also happened to have an Apple Watch 4 and was instructed to record an ECG on recurrence of symptoms and/or heart rate accelerations (see Flowchart B, left-sided scenario). He subsequently presented with a recording taken after an heart rate jump and complaints of palpitations (blue dotted circle), which confirmed an SVT (which terminated at the end of recording).
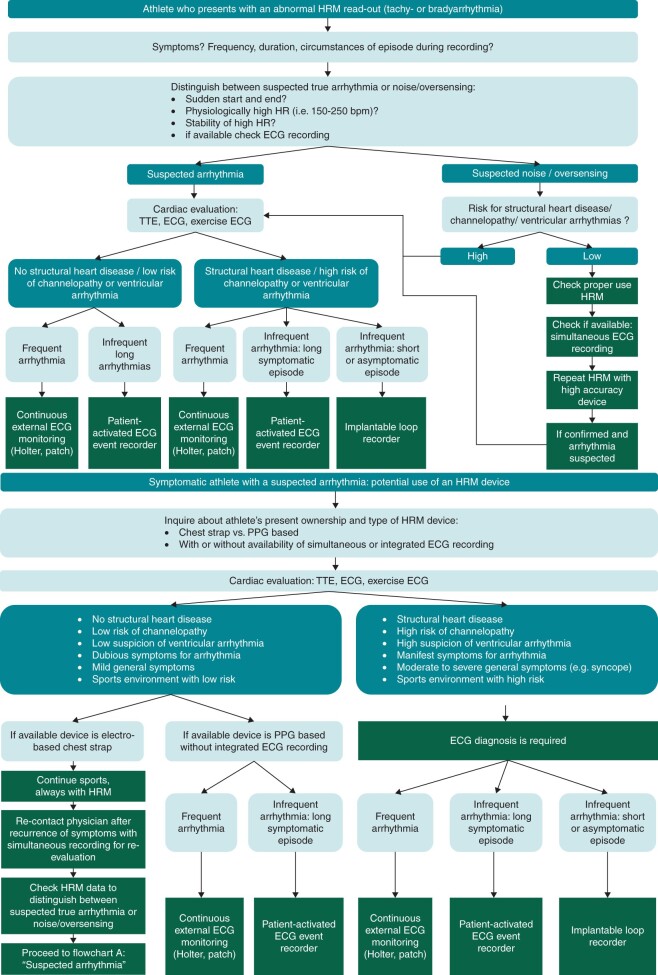
Diagnostic scenarios in athletes with abnormal heart rate readings and/or suspected arrhythmias
There are scenarios in which HRMs with current digital devices may be of value in athletes. We distinguish two base scenarios for which we propose diagnostic evaluation flowcharts (Figure 11): (A) athlete who presents with an abnormal HRM read-out (tachy- or bradyarrhythmia); and (B) symptomatic athlete with a suspected arrhythmia: potential use of an HRM device.
Figure 11.
Flowcharts diagnostic scenarios in athletes with (A) abnormal heart rate monitor (HRM) readings and/or (B) suspected arrhythmias. bpm, beats per minute; ECG, electrocardiogram; HR, heart rate; HRM, heart rate monitor; PPG, photoplethysmography; TTE, transthoracic echocardiogram.
Recent position papers provide guidance, sometimes indicating upper activity levels and/or heart rate, for patients with known arrhythmia syndromes or potentially arrhythmogenic conditions that participate in leisure activities or competitive sports.169,170 For these patients, HRM devices—preferably chest strap devices rather than PPG-based ones—could be used for monitoring maximal heart rate levels as set by their physician (Figure 12).
| Consensus statement | |
|---|---|
| When athletes seek medical attention for abnormal heart rates captured on consumer HRMs, the data should be critically evaluated by an experienced physician (especially when based on PPG technology) to distinguish suspected arrhythmia noise or oversensing |

|
| In athletes using HRMs abnormal readings should be confirmed by ECG recordings |

|
| In case of an abnormal cardiac evaluation, consumer heart rate devices alone do not suffice for diagnosis: an ECG confirmation is mandatory |

|
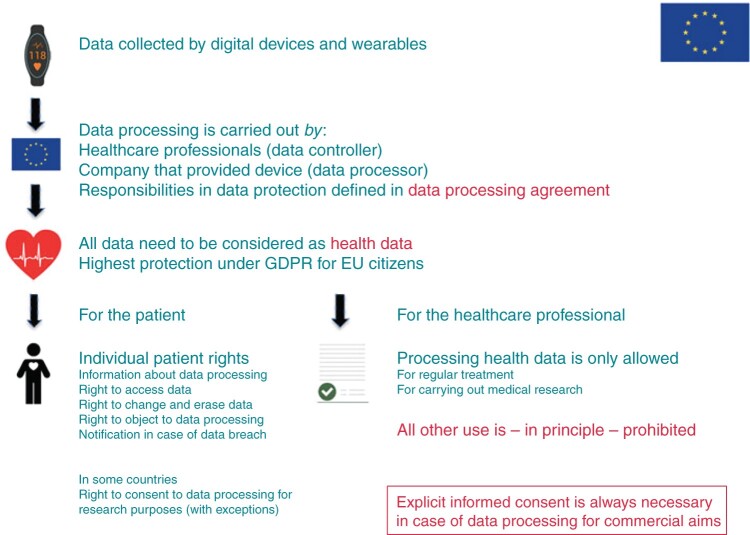
Processing health data—the General Data Protection Regulation
Deployment of digital devices and wearables to monitor and manage arrhythmias implies the processing (for example collection and interpretation) of large amounts of individual data. If using these technologies implies the processing of personal data, and if this is carried out by a data controller or data processor (company or organization) established in the EU, the norms of the General Data Protection Regulation (GDPR) apply.171 Cross-border traffic of large amounts of personal data must be considered, since data are sometimes stored on servers in different countries. If data is transferred within the EU, a high level of data protection is secured. Problems arise when data lands in a country outside the EU; then, a contract (providing the same level of data protection) or explicit consent of the data subject is required.
The GDPR came into force on 25 May 2018 in response to new technological developments that required an updated and stricter European data protection framework. Failure to comply with its requirements, may lead to high financial penalties (imposed by supervisory authorities).6
Data recorded and/or transmitted by digital and wearable technologies are mainly physical or mental health data.172 The GDPR identifies health data as ‘sensitive data’; its processing requires the highest level of protection. The processing of health data is generally prohibited, but circumstances allow the prohibition to be lifted (see Article 9, paragraph 2 GDPR). Processing data from digital devices necessary for the provision of care (detecting and managing arrhythmias) that a patient consented to in the context of a regular treatment relationship is within legal bounds. If a device is employed within the context of a research protocol, the legal ground is that the processing is necessary to carry out the research, provided that specific measures are taken to safeguard the fundamental rights and the interests of the data subject. Would, however, the purpose of the data processing go beyond these goals (commercial aims, pursued by companies), the patient’s free and informed consent is the proper basis; written consent is not required, but the data controller should be able to demonstrate that the person concerned has freely consented to the data processing. If, for instance, a tech company delivering digital devices to hospitals agrees with the care providers that it may collect and use identifiable patient data for its own company purposes, informed consent is required.
Medical professionals, organizations, and companies involved in the application of digital devices and wearables, have in their role of controller or processor important responsibilities regarding data protection; these should be clearly defined in a data processing agreement, as well as the goal and nature of the data processing. The entire ‘cycle of data processing’ should be made transparent and subjected to a data privacy impact assessment which evaluates among other things whether principles of purpose specification (is further processing not incompatible with the defined purpose?) and data minimization (are only data collected that are required for the purpose?) are observed, as well as the involvement of a data protection officer.
An important section of the GDPR is dedicated to data subjects’ rights (chapter III), such as a right to information about the data that are collected, the storage period, who may use them, and so on. Other rights concern e.g. the access to data and the erasure of data. In case of a health data breach the individuals concerned should be notified within 72 h (Figure 13).
Figure 13.
Processing health data.
| Consensus statement | |
|---|---|
| For the collection or processing of individual data of EU citizens when digital devices and wearables are used it is necessary to ensure compliance with the requirements of the General Data Protection Regulation (GDPR) |

|
Future perspectives
Currently, a 30-s single-lead ECG strip is sufficient to diagnose AF.67 Manual interpretation of single-lead tracings using handheld recorders is still recommended by the 2020 ESC Guidelines for the diagnosis and management of AF, but the accuracy of algorithms automated interpretations of single-lead ECG and PPG are improving rapidly.7,67,173 Hence, the accuracy of automated interpretation of handheld ECG and PPG recordings may one day be such that manual interpretation may no longer be mandatory for AF diagnosis.
Artificial intelligence has been applied to predict the risk of dysrhythmias from electronic health records,174 to identify patients with electrographically concealed LQTS,142 to predict the risk of developing AF by analysing an ECG in sinus rhythm,175 or to evaluate clinically meaningful QTc prolongation from ECGs acquired using a handheld recorder.143 Machine learning has promising applications in the field of rhythm diagnosis, but results need to be properly validated across different patient populations and have to be reproducible in different settings.
A field undergoing development is contactless rhythm monitoring. Video plethysmography detects and analyses PPG data collected from the user’s face, using a cell phone camera. Video plethysmography has been demonstrated to correlate with contact PPG, as well as ECG tracings obtained simultaneously on single users,23 and more recently, demonstrated to be feasible for screening multiple persons in the same video.24 These advances raise the prospects of utilizing this technology for mass AF screening in an ambulatory setting. A current limitation is that the subjects need to keep still to stay in focus and yet another is privacy and confidentiality. Moreover, new research has demonstrated that commonly used smart speakers can be turned into short-range active sonars, capable of measuring heart rate and changes in the beat-to-beat intervals in hospitalized patients176 and were shown to accurately detect cardiac arrests.177 Potential applications of this technology include hospital contactless rhythm monitoring for contagious, quarantined patients or burn victims, and contactless home rhythm monitoring for screening and surveillance of common arrhythmias like AF or cardiac arrests.
Rhythm monitoring devices may have sensors able to monitor additional parameters such as daily activity, sleep, oxygen saturation etc., which may contribute to data overload. As with remote monitoring of CIEDs,178 cloud-based algorithms may be developed which integrate different diagnostic parameters to provide scores that facilitate interpretation and allow risk-stratification and triage of these data.
There is growing evidence that systematic screening for AF in high-risk populations (e.g. individuals >75 years old) may reduce the incidence of stroke, which may save costs.98,179 Early detection enables early treatment, which in clinically detected AF been shown to be advantageous.66,180 Telecare services are likely to play an increasing role in logistics, e.g. by implementing low-cost screening of AF by PPG Apps, followed by confirmation with patch ECGs.179 These telecare services unload the diagnostic burden from cardiologists, who can focus on managing patients with confirmed arrhythmias.
The biggest challenge facing widespread utilization of new technologies is the high cost which remains a barrier for a lot of communities across the globe. In addition, improved digital health literacy among patients, and healthcare personnel will be key for successful implementation, and more educational efforts are needed. Clarifications on legal aspects with regards to unsolicited recordings sent to healthcare personnel are needed. Partnerships between health policy makers, industry, and research communities are the key to ensuring accessibility, equity, reimbursement, and inclusion.
Conclusions
Overall digital devices for heart rhythm monitoring are abundant, and with the rapid advancement of technologies likely to increase further. In this practical guide we have shown some examples of possibilities with current devices with regards to early detection, diagnosis, and management of patients with arrhythmias, but also described some of the barriers in implementation. It is also clear that although there is ample data for patients with AF, other arrhythmias have been less well studied.
In the future, a digital workflow will likely be implemented at most cardiology clinics, and the devices available will likely have additional monitoring capabilities and features. We hope that this guide will provide practical guidance for all healthcare professionals interested in heart rhythm monitoring.
Supplementary material
Supplementary material is available at Europace online.
Supplementary Material
Acknowledgements
The authors thank Dr Harald Jorstad (sports cardiologist, Amsterdam UMC, Amsterdam, The Netherlands) who provided us with the case described in the athletes section.
The authors thank the EHRA Scientific Document Committee: Dr Nikolaos Dagres, Prof. Thomas Deneke, Prof. Arthur Wilde, Prof. Frank R. Heinzel, Prof. Christian Meyer, Prof. Lucas Boersma, Prof. Radoslaw Lenarczyk, Prof. Luigi di Biase, Dr Elena Arbelo, Dr Avi Sabbag, Prof. Pierre Jais, Prof. Milos Taborsky, and Assoc. Prof. Markus Stühlinger.
Conflict of interest: Akoum Nazem: Nothing to be declared.
Bordachar Pierre: Nothing to be declared.
Boriani Giuseppe: Has received direct personal payment from healthcare industry: Bayer, Boston Scientific, Medtronic.
Burri Haran: Has received direct personal payment from healthcare industry: Boston Scientific, Abbott, Medtronic, Biotronik. Research funding from: Boston Scientific, Abbott, Medtronic, Biotronik.
Conte Giulio: Payment from healthcare industry to institution: Boston Scientific Research funding from: Swiss National Science Foundation.
Deharo Jean-Claude: Has received direct personal payment from healthcare industry: Abbott, Boston Scientific, Medtronic, Microport, Bayer, Boehringer-Ingelheim, Novartis, Bristol Myers Squibb. Research funding from: Abbott, Boston Scientific, Biotronik, Microport.
Di Biase Luigi: Has received direct personal payment from healthcare industry: Abbott, Boston Scientific, Medtronic, Biotronik, Stereotaxis, Biosense Webster, RMG.
Drossart Inga: Nothing to be declared.
David Duncker: Has received direct personal payment from healthcare industry: Abbott, Astra Zeneca, Bayer, Biotronik, Boehringer Ingelheim, Boston Scientific, Bristol Myers Squibb, CVRx, Medtronic, Microport, Pfizer, Zoll.
Figueiredo Marcio Jansen De Oliveira: Nothing to be declared.
Goette Andreas: Has received direct personal payment from healthcare industry: Abbott, Boston Scientific, Medtronic, Boehringer-Ingelheim, Daiichi Sankyo, Pfizer, Bayer Healthcare, Bristol Myers Squibb, Berlin Chemie AG, OMEICOS, Astra Zeneca.
Han Janet: Has received direct personal payment from healthcare industry: Abbott, Boston Scientific.
Heidbuchel Hein: Has received direct personal payment from healthcare industry: Abbott, Biotronik, Daiichi-Sankyo, Pfizer-BMS, Medscape, and Springer Healthcare Ltd. Research funding from: Biotronik, Abbott, Boston Scientific, Medtronic, Daiichi Sankyo, Pfizer/BMS.
Jais Pierre: Has received direct personal payment from healthcare industry: AFFERA, FARAPULSE. Research funding from: Boston Scientific, Biosense Webster. Direct ownership of shares: Healthcare, InHeart, AFFERA, FARAPULSE.
Leclercq Christophe: Has received direct personal payment from healthcare industry: Biotronik, Abbott, Boston Scientific, Medtronic, Microport.
Linz Dominik: Payment from healthcare industry to institution: Bayer, Medtronic, Microport, Zoll.
Lip Gregory: Consultant and speaker for BMS/Pfizer, Boehringer Ingelheim and Daiichi-Sankyo. No fees are received personally. Research funding from: Boehringer-Ingelheim, BMS.
Malaczynska-Rajpold Katarzyna: Nothing to be declared.
Marquez Murillo Manlio Fabio: Nothing to be declared.
Ploem Corrette: Nothing to be declared.
Soejima Kyoko: Has received direct personal payment from healthcare industry: Abbott Japan, Boehringer-Ingelheim, Daiichi Sankyo, Medtronic Japan.
Stiles Martin: Lecture fees and Consulting from Abbott, Boehringer-Ingelheim, Biosense Webster, Boston Scientific, Ceryx Medical and Medtronic.
Svennberg Emma: Payment from healthcare industry to institution: Bayer, Bristol-Myers Squibb-Pfizer, Boehringer- Ingelheim, Johnson & Johnson, Merck Sharp & Dohme.
Tjong Fleur: Payment from healthcare industry to institution: Boston Scientific, Daiichi Sankyo, Abbotts.
Vernooy Kevin: Has received no direct personal payment from healthcare industry. Payment from healthcare industry to institution: Medtronic, Abbott, Philips, Biosense Webster. Research funding from healthcare industry to institution under direct/personal responsibility: Medtronic, Abbott, Biosense Webster, Biotronik.
Wierda Eric: Nothing to declare.
Contributor Information
Emma Svennberg, Department of Medicine Huddinge, Karolinska Institutet, Stockholm, Sweden.
Fleur Tjong, Heart Center, Amsterdam University Medical Centers, University of Amsterdam, Amsterdam, The Netherlands.
Andreas Goette, St. Vincenz Hospital Paderborn, Paderborn, Germany; MAESTRIA Consortium/AFNET, Münster, Germany.
Nazem Akoum, Heart Institute, University of Washington School of Medicine, Seattle, WA, USA.
Luigi Di Biase, Albert Einstein College of Medicine at Montefiore Hospital, New York, NY, USA.
Pierre Bordachar, Hopital Haut Leveque, LIRYC, Pessac, France.
Giuseppe Boriani, Cardiology Division, Department of Biomedical, Metabolic and Neural Sciences, University of Modena and Reggio Emilia, Policlinico di Modena, Modena, Italy.
Haran Burri, Cardiology Department, University Hospital of Geneva, Geneva, Switzerland.
Giulio Conte, Cardiocentro Ticino Institute, Ente Ospedaliero Cantonale, Lugano, Switzerland.
Jean Claude Deharo, Assistance Publique—Hôpitaux de Marseille, Centre Hospitalier Universitaire La Timone, Service de Cardiologie, Marseille, France; Aix Marseille Université, C2VN, Marseille, France.
Thomas Deneke, Heart Center Bad Neustadt, Bad Neustadt an der Saale, Germany.
Inga Drossart, European Society of Cardiology, Sophia Antipolis, France; ESC Patient Forum, Sophia Antipolis, France.
David Duncker, Hannover Heart Rhythm Center, Department of Cardiology and Angiology, Hannover Medical School, Hannover, Germany.
Janet K Han, Cardiac Arrhythmia Centers, Veterans Affairs Greater Los Angeles Healthcare System and University of California, Los Angeles, CA, USA.
Hein Heidbuchel, Department of Cardiology, Antwerp University Hospital, Antwerp, Belgium; Cardiovascular Research Group, Antwerp University, Antwerp, Belgium.
Pierre Jais, Bordeaux University Hospital, Bordeaux, France.
Marcio Jansen de Oliveira Figueiredo, University of Campinas (UNICAMP), Campinas, Brazil.
Dominik Linz, Department of Cardiology, Maastricht University Medical Centre and Cardiovascular Research Institute Maastricht, Maastricht, the Netherlands.
Gregory Y H Lip, Liverpool Centre for Cardiovascular Science, University of Liverpool and Liverpool Heart & Chest Hospital, Liverpool, UK; Department of Clinical Medicine, Aalborg University, Aalborg, Denmark.
Katarzyna Malaczynska-Rajpold, Heart Division, Royal Brompton and Harefield Hospitals, Guy’s and St. Thomas’ NHS Foundation Trust, London, UK.
Manlio F Márquez, Department of Electrocardiology, Instituto Nacional de Cardiología, Mexico City, Mexico; Cardiology, Electrophysiology Service, American British Cowdray Medical Center, Mexico City, México.
Corrette Ploem, Department of Ethics, Law and Medical Humanities, Amsterdam University Medical Centers, University of Amsterdam, Amsterdam, The Netherlands.
Kyoko Soejima, Kyorin University School of Medicine, Mitaka, Tokyo, Japan.
Martin K Stiles, Waikato Clinical School, University of Auckland, Hamilton, New Zealand.
Eric Wierda, Department of Cardiology, Dijklander Hospital, Hoorn, the Netherlands.
Kevin Vernooy, Department of Cardiology, Cardiovascular Research Institute Maastricht (CARIM), Maastricht University Medical Center, Maastricht, the Netherlands.
Christophe Leclercq, Univ Rennes, CHU Rennes, LTSI-UMR1099, F-35000 Rennes, France.
Christian Meyer, Division of Cardiology/Angiology/Intensive Care, EVK Düsseldorf, Teaching Hospital University of Düsseldorf, Düsseldorf, Germany.
Cristiano Pisani, Arrhythmia Unit, Heart Institute, InCor, University of São Paulo Medical School, São Paulo, Brazil.
Hui Nam Pak, Yonsei University, Severance Cardiovascular Hospital, Yonsei University Health System, Seoul, Republic of Korea.
Dhiraj Gupta, Faculty of Health and Life Sciences, Liverpool Heart and Chest Hospital, University of Liverpool, Liverpool, UK.
Helmut Pürerfellner, Ordensklinikum Linz Elisabethinen, Linz, Austria.
H J G M Crijns, Em. Professor of Cardiology, University of Maastricht, Maastricht, Netherlands.
Edgar Antezana Chavez, Division of Cardiology, Hospital General de Agudos Dr. Cosme Argerich, Pi y Margall 750, C1155AHB Buenos Aires, Argentina; Division of Cardiology, Hospital Belga, Antezana 455, C0000 Cochabamba, Bolivia.
Stephan Willems, Asklepios St. Georg, Cardiology, Hamburg, Germany.
Victor Waldmann, Electrophysiology Unit, European Georges Pompidou Hospital, Paris, France; Adult Congenital Heart Disease Unit, European Georges Pompidou Hospital, Paris, France.
Lukas Dekker, Catharina Ziekenhuis Eindhoven, Eindhoven, Netherlands.
Elaine Wan, Cardiology and Cardiac Electrophysiology, Columbia University, New York, NY, USA.
Pramesh Kavoor, Cardiology Department, Westmead Hospital, Westmead, New South Wales, Australia.
Mohit K Turagam, Icahn School of Medicine at Mount Sinai, New York, NY, USA.
Moritz Sinner, Univ. Hospital Munich, Campus Grosshadern, Munich, Germany.
References
- 1. Han JK, Al-Khatib SM, Albert CM. Changes in the digital health landscape in cardiac electrophysiology: a pre- and peri-pandemic COVID-19 era survey. Cardiovasc Digit Health J 2021;2:55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Manninger M, Zweiker D, Svennberg E, Chatzikyriakou S, Pavlovic N, Zaman JAB et al. Current perspectives on wearable rhythm recordings for clinical decision-making: the wEHRAbles 2 survey. Europace 2021;23:1106–1113. [DOI] [PubMed] [Google Scholar]
- 3. Manninger M, Kosiuk J, Zweiker D, Njeim M, Antolic B, Kircanski B et al. Role of wearable rhythm recordings in clinical decision making—the wEHRAbles project. Clin Cardiol 2020;43:1032–9. DOI: 10.1002/clc.23404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Albert DE. Performance of hand-held electrocardiogram devices to detect atrial fibrillation in a cardiology and geriatric ward setting. Europace 2017;19:1408. [DOI] [PubMed] [Google Scholar]
- 5. Brito R, Mondouagne LP, Stettler C, Combescure C, Burri H. Automatic atrial fibrillation and flutter detection by a handheld ECG recorder, and utility of sequential finger and precordial recordings. J Electrocardiol 2018;51:1135–40. [DOI] [PubMed] [Google Scholar]
- 6. EU . https://ec.europa.eu/info/law/law-topic/data-protection/data-protection-eu_en (last accessed 12 Mar 2022).
- 7. Desteghe L, Raymaekers Z, Lutin M, Vijgen J, Dilling-Boer D, Koopman P et al. Performance of handheld electrocardiogram devices to detect atrial fibrillation in a cardiology and geriatric ward setting. Europace 2017;19:29–39. [DOI] [PubMed] [Google Scholar]
- 8. Nigolian A, Dayal N, Nigolian H, Stettler C, Burri H. Diagnostic accuracy of multi-lead ECGs obtained using a pocket-sized bipolar handheld event recorder. J Electrocardiol 2018;51:278–81. [DOI] [PubMed] [Google Scholar]
- 9. Wong KC, Klimis H, Lowres N, von Huben A, Marschner S, Chow CK. Diagnostic accuracy of handheld electrocardiogram devices in detecting atrial fibrillation in adults in community versus hospital settings: a systematic review and meta-analysis. Heart 2020;106:1211–7. [DOI] [PubMed] [Google Scholar]
- 10. Turakhia MP, Hoang DD, Zimetbaum P, Miller JD, Froelicher VF, Kumar UN et al. Diagnostic utility of a novel leadless arrhythmia monitoring device. Am J Cardiol 2013;112:520–4. [DOI] [PubMed] [Google Scholar]
- 11. Barrett PM, Komatireddy R, Haaser S, Topol S, Sheard J, Encinas J et al. Comparison of 24-hour Holter monitoring with 14-day novel adhesive patch electrocardiographic monitoring. Am J Med 2014;127:95.e11–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Patel UK, Malik P, Patel N, Patel P, Mehta N, Urhoghide E et al. Newer diagnostic and cost-effective ways to identify asymptomatic atrial fibrillation for the prevention of stroke. Cureus 2021;13:e12437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gladstone DJ, Wachter R, Schmalstieg-Bahr K, Quinn FR, Hummers E, Ivers N et al. Screening for atrial fibrillation in the older population: a randomized clinical trial. JAMA Cardiol 2021;6:558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Reiffel JA, Verma A, Kowey PR, Halperin JL, Gersh BJ, Wachter R et al. ; REVEAL AF Investigators . Incidence of previously undiagnosed atrial fibrillation using insertable cardiac monitors in a high-risk population: the REVEAL AF study. JAMA Cardiol 2017;2:1120–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Reiffel JA, Verma A, Kowey PR, Halperin JL, Gersh BJ, Elkind MSV et al. ; REVEAL AF Investigators . Rhythm monitoring strategies in patients at high risk for atrial fibrillation and stroke: a comparative analysis from the REVEAL AF study. Am Heart J 2020;219:128–36. [DOI] [PubMed] [Google Scholar]
- 16. Lubitz SA, Faranesh AZ, Atlas SJ, McManus DD, Singer DE, Pagoto S et al. Rationale and design of a large population study to validate software for the assessment of atrial fibrillation from data acquired by a consumer tracker or smartwatch: the Fitbit Heart Study. Am Heart J 2021;238:16–26. [DOI] [PubMed] [Google Scholar]
- 17. Perez MV, Mahaffey KW, Hedlin H, Rumsfeld JS, Garcia A, Ferris T et al. Large-scale assessment of a smartwatch to identify atrial fibrillation. N Engl J Med 2019;381:1909–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Saghir N, Aggarwal A, Soneji N, Valencia V, Rodgers G, Kurian T. A comparison of manual electrocardiographic interval and waveform analysis in lead 1 of 12-lead ECG and Apple Watch ECG: a validation study. Cardiovasc Digit Health J 2020;1:30–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Seshadri DR, Bittel B, Browsky D, Houghtaling P, Drummond CK, Desai MY et al. Accuracy of Apple Watch for detection of atrial fibrillation. Circulation 2020;141:702–3. [DOI] [PubMed] [Google Scholar]
- 20. Elbey MA, Young D, Kanuri SH, Akella K, Murtaza G, Garg J et al. Diagnostic utility of Smartwatch technology for atrial fibrillation detection—a systematic analysis. J Atr Fibrillation 2021;13:20200446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Maille B, Wilkin M, Million M, Rességuier N, Franceschi F, Koutbi-Franceschi L et al. Smartwatch electrocardiogram and artificial intelligence for assessing cardiac-rhythm safety of drug therapy in the COVID-19 pandemic. The QT-logs study. Int J Cardiol 2021;331:333–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Elgendi M. On the analysis of fingertip photoplethysmogram signals. Curr Cardiol Rev 2012;8:14–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yan BP, Lai WHS, Chan CKY, Chan SC‐H, Chan L‐H, Lam K‐M et al. Contact-free screening of atrial fibrillation by a smartphone using facial pulsatile photoplethysmographic signals. JAHA 2018;7:e008585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yan BP, Lai WHS, Chan CKY, Au ACK, Freedman B, Poh YC et al. High-throughput, contact-free detection of atrial fibrillation from video with deep learning. JAMA Cardiol 2020;5:105–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Friberg L, Engdahl J, Frykman V, Svennberg E, Levin L, Rosenqvist M. Population screening of 75- and 76-year-old men and women for silent atrial fibrillation (STROKESTOP). Europace 2013;15:135–40. [DOI] [PubMed] [Google Scholar]
- 26. Dörr M, Nohturfft V, Brasier N, Bosshard E, Djurdjevic A, Gross S et al. The WATCH AF Trial: smartWATCHes for detection of atrial fibrillation. JACC Clin Electrophysiol 2019;5:199–208. [DOI] [PubMed] [Google Scholar]
- 27. Navalta JW, Montes J, Bodell NG, Salatto RW, Manning JW, DeBeliso M. Concurrent heart rate validity of wearable technology devices during trail running. PLoS One 2020;15:e0238569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tison GH, Sanchez JM, Ballinger B, Singh A, Olgin JE, Pletcher MJ et al. Passive detection of atrial fibrillation using a commercially available smartwatch. JAMA Cardiol 2018;3:409–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gawałko M, Duncker D, Manninger M, van der Velden RMJ, Hermans ANL, Verhaert DVM et al. The European TeleCheck-AF project on remote app-based management of atrial fibrillation during the COVID-19 pandemic: centre and patient experiences. Europace 2021;23:1003–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kane SA, Blake JR, McArdle FJ, Langley P, Sims AJ. Opportunistic detection of atrial fibrillation using blood pressure monitors: a systematic review. Open Heart 2016;3:e000362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fouassier D, Roy X, Blanchard A, Hulot JS. Assessment of signal quality measured with a smart 12-lead ECG acquisition T-shirt. Ann Noninvasive Electrocardiol 2020;25:e12682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Navalta JW, Ramirez GG, Maxwell C, Radzak KN, McGinnis GR. Validity and reliability of three commercially available smart sports bras during treadmill walking and running. Sci Rep 2020;10:7397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pagola J, Juega J, Francisco-Pascual J, Moya A, Sanchis M, Bustamante A et al. Yield of atrial fibrillation detection with Textile Wearable Holter from the acute phase of stroke: pilot study of Crypto-AF registry. Int J Cardiol 2018;251:45–50. [DOI] [PubMed] [Google Scholar]
- 34. Lown M, Yue AM, Shah BN, Corbett SJ, Lewith G, Stuart B et al. Screening for atrial fibrillation using economical and accurate technology (from the SAFETY study). Am J Cardiol 2018;122:1339–44. [DOI] [PubMed] [Google Scholar]
- 35. Hartikainen S, Lipponen JA, Hiltunen P, Rissanen TT, Kolk I, Tarvainen MP et al. Effectiveness of the chest strap electrocardiogram to detect atrial fibrillation. Am J Cardiol 2019;123:1643–8. [DOI] [PubMed] [Google Scholar]
- 36. Ganne C, Talkad SN, Srinivas D, Somanna S. Ruptured blebs and racing hearts: autonomic cardiac changes in neurosurgeons during microsurgical clipping of aneurysms. Br J Neurosurg 2016;30:450–2. [DOI] [PubMed] [Google Scholar]
- 37. Zhang H, Zhang J, Li H-B, Chen Y-X, Yang B, Guo Y-T et al. Validation of single centre pre-mobile atrial fibrillation apps for continuous monitoring of atrial fibrillation in a real-world setting: pilot cohort study. J Med Internet Res 2019;21:e14909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fan Y-Y, Li Y-G, Li J, Cheng W-K, Shan Z-L, Wang Y-T et al. Diagnostic performance of a smart device with photoplethysmography technology for atrial fibrillation detection: pilot study (pre-mAFA II registry). JMIR Mhealth Uhealth 2019;7:e11437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Magnusson P, Lyren A, Mattsson G. Diagnostic yield of chest and thumb ECG after cryptogenic stroke, transient ECG Assessment in Stroke Evaluation (TEASE): an observational trial. BMJ Open 2020;10:e037573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Carnlöf C, Schenck-Gustafsson K, Jensen-Urstad M, Insulander P. Instant electrocardiogram feedback with a new digital technique reduces symptoms caused by palpitations and increases health-related quality of life (the RedHeart study). Eur J Cardiovasc Nurs 2021;20:402–10. [DOI] [PubMed] [Google Scholar]
- 41. Haverkamp HT, Fosse SO, Schuster P. Accuracy and usability of single-lead ECG from smartphones—a clinical study. Indian Pacing Electrophysiol J 2019;19:145–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Attia ZI, Kapa S, Lopez-Jimenez F, McKie PM, Ladewig DJ, Satam G et al. Screening for cardiac contractile dysfunction using an artificial intelligence-enabled electrocardiogram. Nat Med 2019;25:70–4. [DOI] [PubMed] [Google Scholar]
- 43. Bekker CL, Noordergraaf F, Teerenstra S, Pop G, van den Bemt BJF. Diagnostic accuracy of a single-lead portable ECG device for measuring QTc prolongation. Ann Noninvasive Electrocardiol 2020;25:e12683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tieleman RG, Plantinga Y, Rinkes D, Bartels GL, Posma JL, Cator R et al. Validation and clinical use of a novel diagnostic device for screening of atrial fibrillation. Europace 2014;16:1291–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kaleschke G, Hoffmann B, Drewitz I, Steinbeck G, Naebauer M, Goette A et al. Prospective, multicentre validation of a simple, patient-operated electrocardiographic system for the detection of arrhythmias and electrocardiographic changes. Europace 2009;11:1362–8. [DOI] [PubMed] [Google Scholar]
- 46. Guan J, Wang A, Song W, Obore N, He P, Fan S et al. Screening for arrhythmia with the new portable single-lead electrocardiographic device (SnapECG): an application study in community-based elderly population in Nanjing, China. Aging Clin Exp Res 2021;33:133–40. [DOI] [PubMed] [Google Scholar]
- 47. Svennberg E, Stridh M, Engdahl J, Al-Khalili F, Friberg L, Frykman V et al. Safe automatic one-lead electrocardiogram analysis in screening for atrial fibrillation. Europace 2017;19:1449–53. [DOI] [PubMed] [Google Scholar]
- 48. Smith WM, Riddell F, Madon M, Gleva MJ. Comparison of diagnostic value using a small, single channel, P-wave centric sternal ECG monitoring patch with a standard 3-lead Holter system over 24 hours. Am Heart J 2017;185:67–73. [DOI] [PubMed] [Google Scholar]
- 49. Olson JA, Fouts AM, Padanilam BJ, Prystowsky EN. Utility of mobile cardiac outpatient telemetry for the diagnosis of palpitations, presyncope, syncope, and the assessment of therapy efficacy. J Cardiovasc Electrophysiol 2007;18:473–7. [DOI] [PubMed] [Google Scholar]
- 50. Derkac WM, Finkelmeier JR, Horgan DJ, Hutchinson MD. Diagnostic yield of asymptomatic arrhythmias detected by mobile cardiac outpatient telemetry and autotrigger looping event cardiac monitors. J Cardiovasc Electrophysiol 2017;28:1475–8. [DOI] [PubMed] [Google Scholar]
- 51. Teplitzky BA, McRoberts M, Ghanbari H. Deep learning for comprehensive ECG annotation. Heart Rhythm 2020;17:881–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Leff J. Use of Remote Patient Monitoring (RPM) Platform for COVID-19 Patient (NCT04425720). clinicaltrials.gov: Montefiore Medical Center, 2020. https://clinicaltrials.gov/ct2/show/NCT04425720 (last accessed 12 Mar 2022).
- 53. Jeon E, Oh K, Kwon S, Son H, Yun Y, Jung E-S et al. A lightweight deep learning model for fast electrocardiographic beats classification with a wearable cardiac monitor: development and validation study. JMIR Med Inform 2020;8:e17037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Breteler MJM, Huizinga E, van Loon K, Leenen LPH, Dohmen DAJ, Kalkman CJ et al. Reliability of wireless monitoring using a wearable patch sensor in high-risk surgical patients at a step-down unit in the Netherlands: a clinical validation study. BMJ Open 2018;8:e020162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hopkins L, Stacey B, Robinson DBT, James OP, Brown C, Egan RJ et al. Consumer-grade biosensor validation for examining stress in healthcare professionals. Physiol Rep 2020;8:e14454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. VivaLNK Supports Large-Scale-Atrial Fibrillation Study of Wearable ECG Sensors . EP Lab Digest. https://www.eplabdigest.com/vivalnk-supports-large-scale-atrial-fibrillation-study-wearable-ecg-sensors (24 September 2020, date last accessed).
- 57. Steinhubl SR, Waalen J, Edwards AM, Ariniello LM, Mehta RR, Ebner GS et al. Effect of a home-based wearable continuous ecg monitoring patch on detection of undiagnosed atrial fibrillation: the mSToPS randomized clinical trial. JAMA 2018;320:146–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Rozen G, Vaid J, Hosseini SM, Kaadan MI, Rafael A, Roka A et al. Diagnostic accuracy of a novel mobile phone application for the detection and monitoring of atrial fibrillation. Am J Cardiol 2018;121:1187–91. [DOI] [PubMed] [Google Scholar]
- 59. O'Sullivan JW, Grigg S, Crawford W, Turakhia MP, Perez M, Ingelsson E et al. Accuracy of smartphone camera applications for detecting atrial fibrillation: a systematic review and meta-analysis. JAMA Netw Open 2020;3:e202064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Proesmans T, Mortelmans C, Van Haelst R, Verbrugge F, Vandervoort P, Vaes B. Mobile phone-based use of the photoplethysmography technique to detect atrial fibrillation in primary care: diagnostic accuracy study of the FibriCheck app. JMIR Mhealth Uhealth 2019;7:e12284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Koenig N, Seeck A, Eckstein J, Mainka A, Huebner T, Voss A et al. Validation of a new heart rate measurement algorithm for fingertip recording of video signals with smartphones. Telemed J E Health 2016;22:631–6. [DOI] [PubMed] [Google Scholar]
- 62. Krivoshei L, Weber S, Burkard T, Maseli A, Brasier N, Kühne M et al. Smart detection of atrial fibrillation†. Europace 2017;19:753–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Di Carlo A, Bellino L, Consoli D, Mori F, Zaninelli A, Baldereschi M et al. ; National Research Program: Progetto FAI. La Fibrillazione Atriale in Italia . Prevalence of atrial fibrillation in the Italian elderly population and projections from 2020 to 2060 for Italy and the European Union: the FAI Project. Europace 2019;21:1468–75. [DOI] [PubMed] [Google Scholar]
- 64. Friberg L, Rosenqvist M, Lindgren A, Terent A, Norrving B, Asplund K. High prevalence of atrial fibrillation among patients with ischemic stroke. Stroke 2014;45:2599–605. [DOI] [PubMed] [Google Scholar]
- 65. Xiong Q, Proietti M, Senoo K, Lip GY. Asymptomatic versus symptomatic atrial fibrillation: a systematic review of age/gender differences and cardiovascular outcomes. Int J Cardiol 2015;191:172–7. [DOI] [PubMed] [Google Scholar]
- 66. Kirchhof P, Camm AJ, Goette A, Brandes A, Eckardt L, Elvan A et al. Early rhythm-control therapy in patients with atrial fibrillation. N Engl J Med 2020;383:1305–16. [DOI] [PubMed] [Google Scholar]
- 67. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C et al. ; ESC Scientific Document Group . 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio-Thoracic Surgery (EACTS). Eur Heart J 2021;42:373–498. [DOI] [PubMed] [Google Scholar]
- 68. Duncker D, Svennberg E. The catch in atrial fibrillation detection: don't forget to treat. Lancet Healthy Longevity 2021;2:e447–8. [DOI] [PubMed] [Google Scholar]
- 69. Doliwa PS, Frykman V, Rosenqvist M. Short-term ECG for out of hospital detection of silent atrial fibrillation episodes. Scand Cardiovasc J 2009;43:163–8. [DOI] [PubMed] [Google Scholar]
- 70. Claes N, Van Laethem C, Goethals M, Goethals P, Mairesse G, Schwagten B et al. Prevalence of atrial fibrillation in adults participating in a large-scale voluntary screening programme in Belgium. Acta Cardiol 2012;67:273–8. [DOI] [PubMed] [Google Scholar]
- 71. Doliwa Sobocinski P, Anggardh Rooth E, Frykman Kull V, von Arbin M, Wallen H, Rosenqvist M. Improved screening for silent atrial fibrillation after ischaemic stroke. Europace 2012;14:1112–6. [DOI] [PubMed] [Google Scholar]
- 72. Hendrikx T, Hornsten R, Rosenqvist M, Sandstrom H. Screening for atrial fibrillation with baseline and intermittent ECG recording in an out-of-hospital population. BMC Cardiovasc Disord 2013;13:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Engdahl J, Andersson L, Mirskaya M, Rosenqvist M. Stepwise screening of atrial fibrillation in a 75-year-old population: implications for stroke prevention. Circulation 2013;127:930–7. [DOI] [PubMed] [Google Scholar]
- 74. Lowres N, Neubeck L, Salkeld G, Krass I, McLachlan AJ, Redfern J et al. Feasibility and cost-effectiveness of stroke prevention through community screening for atrial fibrillation using iPhone ECG in pharmacies. The SEARCH-AF study. Thromb Haemost 2014;111:1167–76. [DOI] [PubMed] [Google Scholar]
- 75. Kaasenbrood F, Hollander M, Rutten FH, Gerhards LJ, Hoes AW, Tieleman RG. Yield of screening for atrial fibrillation in primary care with a hand-held, single-lead electrocardiogram device during influenza vaccination. Europace 2016;18:1514–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Chan P‐H, Wong C‐K, Poh YC, Pun L, Leung WW‐C, Wong Y‐F et al. Diagnostic performance of a smartphone-based photoplethysmographic application for atrial fibrillation screening in a primary care setting. JAHA 2016;5:e003428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Wiesel J, Salomone TJ. Screening for atrial fibrillation in patients ≥65 years using an automatic blood pressure monitor in a skilled nursing facility. Am J Cardiol 2017;120:1322–4. [DOI] [PubMed] [Google Scholar]
- 78. Chan NY, Choy CC. Screening for atrial fibrillation in 13 122 Hong Kong citizens with smartphone electrocardiogram. Heart 2017;103:24–31. [DOI] [PubMed] [Google Scholar]
- 79. Berge T, Brynildsen J, Larssen HKN, Onarheim S, Jenssen GR, Ihle-Hansen H et al. Systematic screening for atrial fibrillation in a 65-year-old population with risk factors for stroke: data from the Akershus Cardiac Examination 1950 study. Europace 2018;20:f299–305. [DOI] [PubMed] [Google Scholar]
- 80. Tavernier R, Wolf M, Kataria V, Phlips T, Huys R, Taghji P et al. Screening for atrial fibrillation in hospitalised geriatric patients. Heart 2018;104:588–93. [DOI] [PubMed] [Google Scholar]
- 81. Heckbert SR, Austin TR, Jensen PN, Floyd JS, Psaty BM, Soliman EZ et al. Yield and consistency of arrhythmia detection with patch electrocardiographic monitoring: the Multi-Ethnic Study of Atherosclerosis. J Electrocardiol 2018;51:997–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Rooney MR, Soliman EZ, Lutsey PL, Norby FL, Loehr LR, Mosley TH et al. Prevalence and characteristics of subclinical atrial fibrillation in a community-dwelling elderly population: the ARIC study. Circ Arrhythm Electrophysiol 2019;12:e007390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Guo Y, Wang H, Zhang H, Liu T, Liang Z, Xia Y et al. Mobile photoplethysmographic technology to detect atrial fibrillation. J Am Coll Cardiol 2019;74:2365–75. [DOI] [PubMed] [Google Scholar]
- 84. Godin R, Yeung C, Baranchuk A, Guerra P, Healey JS. Screening for atrial fibrillation using a mobile, single-lead electrocardiogram in canadian primary care clinics. Can J Cardiol 2019;35:840–5. [DOI] [PubMed] [Google Scholar]
- 85. Grubb NR, Elder D, Broadhurst P, Reoch A, Tassie E, Neilson A. Atrial fibrillation case finding in over 65 s with cardiovascular risk factors—results of initial Scottish clinical experience. Int J Cardiol 2019;288:94–9. [DOI] [PubMed] [Google Scholar]
- 86. Yan B, Tu H, Lam C, Swift C, Ho MS, Mok VCT et al. Nurse led smartphone electrographic monitoring for atrial fibrillation after ischemic stroke: SPOT-AF. J Stroke 2020;22:387–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Savickas V, Stewart AJ, Rees-Roberts M, Short V, Bhamra SK, Corlett SA et al. Opportunistic screening for atrial fibrillation by clinical pharmacists in UK general practice during the influenza vaccination season: a cross-sectional feasibility study. PLoS Med 2020;17:e1003197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Chen Y, Huang Q-F, Sheng C-S, Zhang W, Shao S, Wang D et al. Detection rate and treatment gap for atrial fibrillation identified through screening in community health centers in China (AF-CATCH): a prospective multicenter study. PLoS Med 2020;17:e1003146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Diamantino AC, Nascimento BR, Beaton AZ, Nunes MCP, Oliveira KKB, Rabelo LC et al. Atrial fibrillation detection with a portable device during cardiovascular screening in primary care. Heart (British Cardiac Society) 2020;106:1261–6. [DOI] [PubMed] [Google Scholar]
- 90. Zwart LA, Jansen RW, Ruiter JH, Germans T, Simsek S, Hemels ME. Opportunistic screening for atrial fibrillation with a single lead device in geriatric patients. J Geriatr Cardiol 2020;17:149–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Zaprutko T, Zaprutko J, Baszko A, Sawicka D, Szałek A, Dymecka M et al. Feasibility of atrial fibrillation screening with mobile health technologies at pharmacies. J Cardiovasc Pharmacol Ther 2020;25:142–51. [DOI] [PubMed] [Google Scholar]
- 92. Langer A, Healey JS, Quinn FR, Honos G, Nault I, Tan M et al. ; AWARE AF Program . Detection of atrial fibrillation in asymptomatic at-risk individuals. Int J Cardiol 2021;334:55–7. [DOI] [PubMed] [Google Scholar]
- 93. Stavrakis S, Elkholey K, Lofgren MM, Asad ZUA, Stephens LD, Freedman B. Screening for atrial fibrillation in American Indian adults in a tribal primary care clinic. JAHA 2021;10:e020069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Zink MD, Mischke KG, Keszei AP, Rummey C, Freedman B, Neumann G et al. Screen-detected atrial fibrillation predicts mortality in elderly subjects. Europace 2021;23:29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Boriani G, Palmisano P, Malavasi VL, Fantecchi E, Vitolo M, Bonini N et al. Clinical factors associated with atrial fibrillation detection on single-time point screening using a hand-held single-lead ECG device. JCM 2021;10:729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Gwynn J, Gwynne K, Rodrigues R, Thompson S, Bolton G, Dimitropoulos Y et al. Atrial fibrillation in Indigenous Australians: a multisite screening study using a single-lead ECG device in aboriginal primary health settings. Heart Lung Circ 2021;30:267–74. [DOI] [PubMed] [Google Scholar]
- 97. Uittenbogaart SB, Verbiest-van Gurp N, Lucassen WAM, Winkens B, Nielen M, Erkens PMG et al. Opportunistic screening versus usual care for detection of atrial fibrillation in primary care: cluster randomised controlled trial. BMJ 2020;370:m3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Svennberg E, Friberg L, Frykman V, Al-Khalili F, Engdahl J, Rosenqvist M. Clinical outcomes in systematic screening for atrial fibrillation (STROKESTOP): a multicentre, parallel group, unmasked, randomised controlled trial. Lancet 2021;398:1498–506. [DOI] [PubMed] [Google Scholar]
- 99. Svendsen JH, Diederichsen SZ, Højberg S, Krieger DW, Graff C, Kronborg C et al. Implantable loop recorder detection of atrial fibrillation to prevent stroke (The LOOP Study): a randomised controlled trial. Lancet 2021;398:1507–16. [DOI] [PubMed] [Google Scholar]
- 100. Simons SO, Elliott A, Sastry M, Hendriks JM, Arzt M, Rienstra M et al. Chronic obstructive pulmonary disease and atrial fibrillation: an interdisciplinary perspective. Eur Heart J 2021;42:532–40. [DOI] [PubMed] [Google Scholar]
- 101. Zia I, Johnson L, Memarian E, Borné Y, Engström G. Anthropometric measures and the risk of developing atrial fibrillation: a Swedish Cohort Study. BMC Cardiovasc Disord 2021;21:602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Freedman B, Camm J, Calkins H, Healey JS, Rosenqvist M, Wang J et al. ; AF-Screen Collaborators . Screening for atrial fibrillation: a report of the AF-SCREEN International Collaboration. Circulation 2017;135:1851–67. [DOI] [PubMed] [Google Scholar]
- 103. Kemp Gudmundsdottir K, Fredriksson T, Svennberg E, Al-Khalili F, Friberg L, Frykman V et al. Stepwise mass screening for atrial fibrillation using N-terminal B-type natriuretic peptide: the STROKESTOP II study. Europace 2020;22:24–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Halcox JPJ, Wareham K, Cardew A, Gilmore M, Barry JP, Phillips C et al. Assessment of remote heart rhythm sampling using the AliveCor heart monitor to screen for atrial fibrillation: the REHEARSE-AF study. Circulation 2017;136:1784–94. [DOI] [PubMed] [Google Scholar]
- 105. Gandapur Y, Kianoush S, Kelli HM, Misra S, Urrea B, Blaha MJ et al. The role of mHealth for improving medication adherence in patients with cardiovascular disease: a systematic review. Eur Heart J Qual Care Clin Outcomes 2016;2:237–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Guo Y, Chen Y, Lane DA, Liu L, Wang Y, Lip GY. Mobile health technology for atrial fibrillation management integrating decision support, education, and patient involvement: mAF App Trial. Am J Med 2017;130:1388–96.e6. [DOI] [PubMed] [Google Scholar]
- 107. Simblett S, Greer B, Matcham F, Curtis H, Polhemus A, Ferrão J et al. Barriers to and facilitators of engagement with remote measurement technology for managing health: systematic review and content analysis of findings. J Med Internet Res 2018;20:e10480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Digital Health Literacy . https://www.who.int/global-coordination-mechanism/working-groups/digital_hl.pdf (last accessed 12 Mar 2022).
- 109. American Library Association . https://literacy.ala.org/digital-literacy (last accessed 12 Mar 2022).
- 110. https://eurohealthnet.eu/publication/digital-health-literacy-how-new-skills-can-help-improve-health-equity-and-sustainability/ (last accessed 12 Mar 2022).
- 111. Lambert CT, Patel D, Bumgarner JM, Kanj M, Cantillon D, Saliba W et al. Atrial fibrillation future clinic. Novel platform to integrate smart device electrocardiogram into clinical practice. Cardiovasc Digit Health J 2021;2:92–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Richtering SS, Hyun K, Neubeck L, Coorey G, Chalmers J, Usherwood T et al. eHealth literacy: predictors in a population with moderate-to-high cardiovascular risk. JMIR Hum Factors 2017;4:e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Hermans ANL, van der Velden RMJ, Gawalko M, Verhaert DVM, Desteghe L, Duncker D et al. ; TeleCheck-AF investigators . On-demand mobile health infrastructures to allow comprehensive remote atrial fibrillation and risk factor management through teleconsultation. Clin Cardiol 2020;43:1232–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Linz D, Hermans A, Tieleman RG. Early atrial fibrillation detection and the transition to comprehensive management. Europace 2021;23:ii46–51. [DOI] [PubMed] [Google Scholar]
- 115. Romiti GF, Pastori D, Rivera-Caravaca JM, Ding WY, Gue YX, Menichelli D et al. Adherence to the ‘Atrial Fibrillation Better Care’ pathway in patients with atrial fibrillation: impact on clinical outcomes—a systematic review and meta-analysis of 285,000 patients. Thromb Haemost 2022;122:406–14. [DOI] [PubMed] [Google Scholar]
- 116. Yoon M, Yang P-S, Jang E, Yu HT, Kim T-H, Uhm J-S et al. Improved population-based clinical outcomes of patients with atrial fibrillation by compliance with the simple ABC (Atrial Fibrillation Better Care) pathway for integrated care management: a nationwide cohort study. Thromb Haemost 2019;119:1695–703. [DOI] [PubMed] [Google Scholar]
- 117. Lip GYH. The ABC pathway: an integrated approach to improve AF management. Nat Rev Cardiol 2017;14:627–8. [DOI] [PubMed] [Google Scholar]
- 118. Guo Y, Guo J, Shi X, Yao Y, Sun Y, Xia Y et al. Mobile health technology-supported atrial fibrillation screening and integrated care: a report from the mAFA-II trial Long-term Extension Cohort. Eur J Intern Med 2020;82:105–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Guo Y, Lane DA, Chen Y, Lip GYH. Regular bleeding risk assessment associated with reduction in bleeding outcomes: the mAFA-II randomized trial. Am J Med 2020;133:1195–202.e2. [DOI] [PubMed] [Google Scholar]
- 120. Harju J, Tarniceriu A, Parak J, Vehkaoja A, Yli-Hankala A, Korhonen I. Monitoring of heart rate and inter-beat intervals with wrist plethysmography in patients with atrial fibrillation. Physiol Meas 2018;39:065007. [DOI] [PubMed] [Google Scholar]
- 121. van der Velden RMJ, Verhaert DVM, Hermans ANL, Duncker D, Manninger M, Betz K et al. ; TeleCheck-AF Investigators . The photoplethysmography dictionary: practical guidance on signal interpretation and clinical scenarios from TeleCheck-AF. Eur Heart J Digit Health 2021;2:363–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Pluymaekers NAHA, Dudink EAMP, Luermans JGLM, Meeder JG, Lenderink T, Widdershoven J et al. Early or delayed cardioversion in recent-onset atrial fibrillation. N Engl J Med 2019;380:1499–508. [DOI] [PubMed] [Google Scholar]
- 123. Lu N, MacGillivray J, Andrade JG, Krahn AD, Hawkins NM, Laksman Z et al. Effectiveness of a simple medication adjustment protocol for optimizing peri-cardioversion rate control: a derivation and validation cohort study. Heart Rhythm O2 2021;2:46–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Pluymaekers NAHA, van der Velden RMJ, Hermans ANL, Gawalko M, Buskes S, Keijenberg JJHMW et al. On-demand mobile health infrastructure for remote rhythm monitoring within a wait-and-see strategy for recent-onset atrial fibrillation: teleWAS-AF. Cardiology 2021;146:392–6. [DOI] [PubMed] [Google Scholar]
- 125. Gawałko M, Duncker D, Manninger M, van der Velden RMJ, Hermans ANL, Verhaert DVM et al. ; TeleCheck-AF investigators . The European TeleCheck-AF project on remote app-based management of atrial fibrillation during the COVID-19 pandemic: centre and patient experiences. Europace 2021;23:1003–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Hermans ANL, Gawalko M, Pluymaekers NAHA, Dinh T, Weijs B, van Mourik MJW et al. Long-term intermittent versus short continuous heart rhythm monitoring for the detection of atrial fibrillation recurrences after catheter ablation. Int J Cardiol 2021;329:105–12. [DOI] [PubMed] [Google Scholar]
- 127. Linz D, Pluymaekers N, Hendriks JM. TeleCheck-AF for COVID-19. Eur Heart J 2020;41:1954–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Pluymaekers NAHA, Hermans ANL, van der Velden RMJ, Gawałko M, den Uijl DW, Buskes S et al. Implementation of an on-demand app-based heart rate and rhythm monitoring infrastructure for the management of atrial fibrillation through teleconsultation: teleCheck-AF. Europace 2021;23:345–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Kotecha D, Chua WWL, Fabritz L, Hendriks J, Casadei B, Schotten U et al. ; European Society of Cardiology (ESC) Atrial Fibrillation Guidelines Taskforce, the CATCH ME consortium and the European Heart Rhythm Association (EHRA) . European Society of Cardiology smartphone and tablet applications for patients with atrial fibrillation and their health care providers. Europace 2018;20:225–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Desteghe L, Kluts K, Vijgen J, Koopman P, Dilling-Boer D, Schurmans J et al. The health buddies app as a novel tool to improve adherence and knowledge in atrial fibrillation patients: a pilot study. JMIR Mhealth Uhealth 2017;5:e98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Reed MJ, Grubb NR, Lang CC, O'Brien R, Simpson K, Padarenga M et al. Multi-centre randomised controlled trial of a smartphone-based event recorder alongside standard care versus standard care for patients presenting to the emergency department with palpitations and pre-syncope: the IPED (Investigation of Palpitations in the ED) study. EClinicalMedicine 2019;8:37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Abou L, Fliflet A, Hawari L, Presti P, Sosnoff JJ, Mahajan HP et al. Sensitivity of Apple Watch fall detection feature among wheelchair users. Assist Technol 2021; doi: 10.1080/10400435.2021.1923087. [DOI] [PubMed] [Google Scholar]
- 133. Cotechini V, Belli A, Palma L, Morettini M, Burattini L, Pierleoni P. A dataset for the development and optimization of fall detection algorithms based on wearable sensors. Data Brief 2019;23:103839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Couceiro R, Carvalho P, Paiva RP, Muehlsteff J, Henriques J, Eickholt C et al. Real-time prediction of neurally mediated syncope. IEEE J Biomed Health Inform 2016;20:508–20. [DOI] [PubMed] [Google Scholar]
- 135. Waks JW, Fein AS, Das S. Wide complex tachycardia recorded with a smartphone cardiac rhythm monitor. JAMA Intern Med 2015;175:437–9. [DOI] [PubMed] [Google Scholar]
- 136. Ringwald M, Crich A, Beysard N. Smart watch recording of ventricular tachycardia: case study. Am J Emerg Med 2020;38:849.e3–5. [DOI] [PubMed] [Google Scholar]
- 137. Young ML, Flores L. Asymptomatic idiopathic belhassen ventricular tachycardia in a neonate detected using ‘smart sock’ wearable smartphone-enabled cardiac monitoring. Am J Case Rep 2020;21:e921092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Sohn K, Dalvin SP, Merchant FM, Kulkarni K, Sana F, Abohashem S et al. Utility of a Smartphone based system (cvrPhone) to predict short-term arrhythmia susceptibility. Sci Rep 2019;9:14497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Nagy N, Márton Z, Kiss L, Varró A, Nánási PP, Tóth A. Role of Ca2+-sensitive K+ currents in controlling ventricular repolarization: possible implications for future antiarrhytmic drug therapy. Curr Med Chem 2011;18:3622–39. [DOI] [PubMed] [Google Scholar]
- 140. Cheung CC, Nattel S, Macle L, Andrade JG. Management of atrial fibrillation in 2021: an updated comparison of the current CCS/CHRS, ESC, and AHA/ACC/HRS guidelines. Can J Cardiol 2021;37:1607–18. [DOI] [PubMed] [Google Scholar]
- 141. Kleiman R, Darpo B, Brown R, Rudo T, Chamoun S, Albert DE et al. Comparison of electrocardiograms (ECG) waveforms and centralized ECG measurements between a simple 6-lead mobile ECG device and a standard 12-lead ECG. Ann Noninvasive Electrocardiol 2021;26:e12872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Bos JM, Attia ZI, Albert DE, Noseworthy PA, Friedman PA, Ackerman MJ. Use of artificial intelligence and deep neural networks in evaluation of patients with electrocardiographically concealed long QT syndrome from the surface 12-lead electrocardiogram. JAMA Cardiol 2021;6:532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Giudicessi JR, Schram M, Bos JM, Galloway CD, Shreibati JB, Johnson PW et al. Artificial intelligence-enabled assessment of the heart rate corrected QT interval using a mobile electrocardiogram device. Circulation 2021;143:1274–86. [DOI] [PubMed] [Google Scholar]
- 144. Caillol T, Strik M, Ramirez FD, Abu-Alrub S, Marchand H, Buliard S et al. Accuracy of a Smartwatch-derived ECG for diagnosing bradyarrhythmias, tachyarrhythmias, and cardiac ischemia. Circ Arrhythm Electrophysiol 2021;14:e009260. [DOI] [PubMed] [Google Scholar]
- 145. Strik M, Caillol T, Ramirez FD, Abu-Alrub S, Marchand H, Welte N et al. Validating QT-interval measurement using the Apple Watch ECG to enable remote monitoring during the COVID-19 pandemic. Circulation 2020;142:416–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Drexler M, Elsner C, Gabelmann V, Gori T, Münzel T. Apple Watch detecting coronary ischaemia during chest pain episodes or an apple a day may keep myocardial infarction away. Eur Heart J 2020;41:2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Castelletti S, Dagradi F, Goulene K, Danza AI, Baldi E, Stramba-Badiale M et al. A wearable remote monitoring system for the identification of subjects with a prolonged QT interval or at risk for drug-induced long QT syndrome. Int J Cardiol 2018;266:89–94. [DOI] [PubMed] [Google Scholar]
- 148. Garabelli PAUL, Stavrakis S, Albert M, Koomson E, Parwani P, Chohan J et al. Comparison of QT interval readings in normal sinus rhythm between a smartphone heart monitor and a 12-lead ECG for healthy volunteers and inpatients receiving sotalol or dofetilide. J Cardiovasc Electrophysiol 2016;27:827–32. [DOI] [PubMed] [Google Scholar]
- 149. López CA, Toro DD, Hadid C, Celano L, Antezana E, Heffner L et al. Usefulness of a single-lead electrocardiographic recording system and wireless transmission during the COVID-19 pandemic. Argent J Cardiol 2020;88:211–5. [Google Scholar]
- 150. Sivakumar S, Bhatti N. Can smartwatch prevent sudden cardiac deaths? A case of smartwatch failure in arrhythmogenic right ventricular dysplasia. Cureus 2021;13:e15904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Priori SG, Blomström-Lundqvist C, Mazzanti A, Blom N, Borggrefe M, Camm J et al. ; Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC) . 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: the Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC)Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Europace 2015;17:1601–87. [DOI] [PubMed] [Google Scholar]
- 152. Gray B, Kirby A, Kabunga P, Freedman SB, Yeates L, Kanthan A et al. Twelve-lead ambulatory electrocardiographic monitoring in Brugada syndrome: potential diagnostic and prognostic implications. Heart Rhythm 2017;14:866–74. [DOI] [PubMed] [Google Scholar]
- 153. Takasugi N, Goto H, Takasugi M, Verrier RL, Kuwahara T, Kubota T et al. Prevalence of microvolt T-wave alternans in patients with long QT syndrome and its association with Torsade de Pointes. Circ Arrhythm Electrophysiol 2016;9:e003206. [DOI] [PubMed] [Google Scholar]
- 154. Vicentini A, Masiello L, D'Amore S, Baldi E, Ghio S, Savastano S et al. ; San Matteo COVID Cardiac Injury Task Force . QTc interval and mortality in a population of SARS-2-CoV infected patients. Circ Arrhythm Electrophysiol 2020;13:e008890. [DOI] [PubMed] [Google Scholar]
- 155. Nunan D, Donovan G, Jakovljevic DG, Hodges LD, Sandercock GR, Brodie DA. Validity and reliability of short-term heart-rate variability from the Polar S810. Med Sci Sports Exerc 2009;41:243–50. [DOI] [PubMed] [Google Scholar]
- 156. Pasadyn SR, Soudan M, Gillinov M, Houghtaling P, Phelan D, Gillinov N et al. Accuracy of commercially available heart rate monitors in athletes: a prospective study. Cardiovasc Diagn Ther 2019;9:379–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Gillinov S, Etiwy M, Wang R, Blackburn G, Phelan D, Gillinov AM et al. Variable accuracy of wearable heart rate monitors during aerobic exercise. Med Sci Sports Exerc 2017;49:1697–703. [DOI] [PubMed] [Google Scholar]
- 158. Gajda R. Is continuous ECG recording on heart rate monitors the most expected function by endurance athletes, coaches, and doctors? Diagnostics (Basel) 2020;10:867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Gajda R, Biernacka EK, Drygas W. Are heart rate monitors valuable tools for diagnosing arrhythmias in endurance athletes? Scand J Med Sci Sports 2018;28:496–516. [DOI] [PubMed] [Google Scholar]
- 160. Alzahrani A, Hu S, Azorin-Peris V, Barrett L, Esliger D, Hayes M et al. A multi-channel opto-electronic sensor to accurately monitor heart rate against motion artefact during exercise. Sensors (Basel) 2015;15:25681–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Boudreaux BD, Hebert EP, Hollander DB, Williams BM, Cormier CL, Naquin MR et al. Validity of wearable activity monitors during cycling and resistance exercise. Med Sci Sports Exerc 2018;50:624–33. [DOI] [PubMed] [Google Scholar]
- 162. Hettiarachchi IT, Hanoun S, Nahavandi D, Nahavandi S. Validation of Polar OH1 optical heart rate sensor for moderate and high intensity physical activities. PLoS One 2019;14:e0217288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Bunn J, Wells E, Manor J, Webster M. Evaluation of earbud and wristwatch heart rate monitors during aerobic and resistance training. Int J Exerc Sci 2019;12:374–84. [PMC free article] [PubMed] [Google Scholar]
- 164. https://www.apple.com/healthcare/apple-watch/ (4 May 2021, date last accessed).
- 165. https://www.fourthfrontier.com (30 August 2021, date last accessed).
- 166. https://www.fitbit.com/global/us/technology/ecg (1 September 2021, date last accessed).
- 167. https://www.samsung.com/us/apps/samsung-health-monitor/ (1 September 2021, date last accessed).
- 168. https://www.getqardio.com/qardiomd-ecg/ (4 May 2021, date last accessed).
- 169. Heidbuchel H, Adami PE, Antz M, Braunschweig F, Delise P, Scherr D et al. Recommendations for participation in leisure-time physical activity and competitive sports in patients with arrhythmias and potentially arrhythmogenic conditions: part 1: supraventricular arrhythmias. A position statement of the Section of Sports Cardiology and Exercise from the European Association of Preventive Cardiology (EAPC) and the European Heart Rhythm Association (EHRA), both associations of the European Society of Cardiology. Eur J Prev Cardiol 2021;28:1539–51. [DOI] [PubMed] [Google Scholar]
- 170. Heidbuchel H, Arbelo E, D'Ascenzi F, Borjesson M, Boveda S, Castelletti S et al. Recommendations for participation in leisure-time physical activity and competitive sports of patients with arrhythmias and potentially arrhythmogenic conditions. Part 2: ventricular arrhythmias, channelopathies, and implantable defibrillators. Europace 2021;23:147–8. [DOI] [PubMed] [Google Scholar]
- 171. Wierda E, Blok S, Somsen GA, van der Velde ET, Tulevski II, Stavrov B et al. Protecting patient privacy in digital health technology: the Dutch m-Health infrastructure of Hartwacht as a learning case. BMJ Innov 2020;6:170–6. [Google Scholar]
- 172. Nielsen JC, Kautzner J, Casado-Arroyo R, Burri H, Callens S, Cowie MR et al. Remote monitoring of cardiac implanted electronic devices: legal requirements and ethical principles—ESC Regulatory Affairs Committee/EHRA joint task force report. Europace 2020;22:1742–58. [DOI] [PubMed] [Google Scholar]
- 173. Brasier N, Raichle CJ, Dörr M, Becke A, Nohturfft V, Weber S et al. Detection of atrial fibrillation with a smartphone camera: first prospective, international, two-centre, clinical validation study (DETECT AF PRO). Europace 2019;21:41–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Zhang Y, Han Y, Gao P, Mo Y, Hao S, Huang J et al. Electronic health record-based prediction of 1-year risk of incident cardiac dysrhythmia: prospective case-finding algorithm development and validation study. JMIR Med Inform 2021;9:e23606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Attia ZI, Noseworthy PA, Lopez-Jimenez F, Asirvatham SJ, Deshmukh AJ, Gersh BJ et al. An artificial intelligence-enabled ECG algorithm for the identification of patients with atrial fibrillation during sinus rhythm: a retrospective analysis of outcome prediction. Lancet 2019;394:861–7. [DOI] [PubMed] [Google Scholar]
- 176. Wang A, Nguyen D, Sridhar AR, Gollakota S. Using smart speakers to contactlessly monitor heart rhythms. Commun Biol 2021;4:319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Chan J, Rea T, Gollakota S, Sunshine JE. Contactless cardiac arrest detection using smart devices. NPJ Digit Med 2019;2:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Burri H, da Costa A, Quesada A, Ricci RP, Favale S, Clementy N et al. ; MORE-CARE Investigators . Risk stratification of cardiovascular and heart failure hospitalizations using integrated device diagnostics in patients with a cardiac resynchronization therapy defibrillator. Europace 2018;20:e69–77. [DOI] [PubMed] [Google Scholar]
- 179. Birkemeyer R, Müller A, Wahler S, von der Schulenburg J-M. A cost-effectiveness analysis model of Preventicus atrial fibrillation screening from the point of view of statutory health insurance in Germany. Health Econ Rev 2020;10:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Willems S, Borof K, Brandes A, Breithardt G, Camm AJ, Crijns HJGM et al. Systematic, early rhythm control strategy for atrial fibrillation in patients with or without symptoms: the EAST-AFNET 4 trial. Eur Heart J 2022;43:1219–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.