Graphical Abstract
Graphical Abstract.
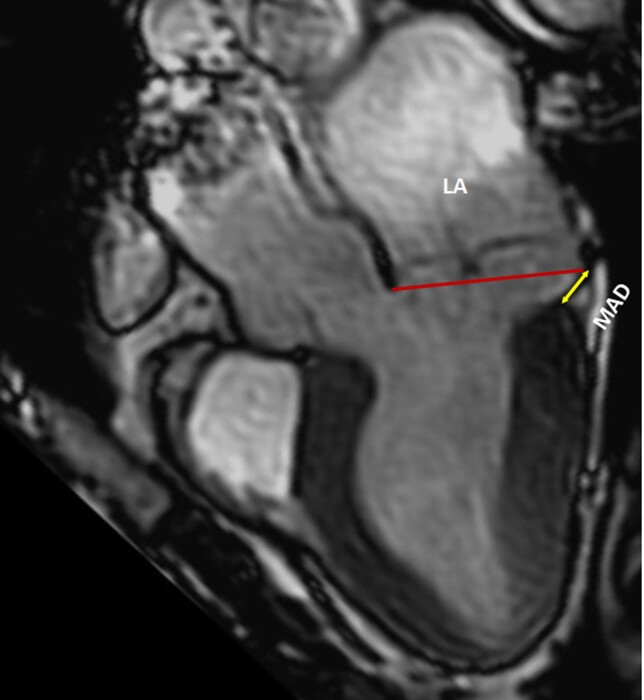
Risk stratification scheme. Risk stratification aiming at assessing the risk of VAs and SCD in patients with MVP, involving two phases based on the clinical and imaging context and the uncovered arrhythmia. In the absence of ventricular tachycardia, phenotypic risk features will trigger the intensity of screening for arrhythmia. Green boxes indicate green heart consensus statements and yellow boxes indicate yellow heart consensus statements. High risk - sustained VT, polymorphic NSVT, fast (>180 bpm) NSVT, VT/NSVT resulting in syncope. ICD = implantable cardioverter defibrillator; LA = left atrium; LGE = late gadolinium enhancement; LV-EF = left ventricular ejection fraction; MAD = mitral annular disjunction; MV = mitral valve; PVCs = premature ventricular contractions; TWI = T-wave inversion; VT = ventricular tachycardia. #Additional ECG monitoring method may be used such as loop recorders.
Keywords: Mitral valve prolapse, Mitral annular disjunction, Sudden cardiac death, Ventricular arrhythmia, Risk stratification
Introduction
Mitral valve prolapse (MVP) is the most common valvular heart disease,1 affecting about 2–3% of the general population2–4 and is well characterized by echocardiography.5 While the outcome in MVP is mostly benign in the absence of mitral regurgitation (MR) and its left-ventricular (LV) consequences,6,7 a small yet poorly defined subset of individuals remain at higher risk of malignant ventricular arrhythmias (VAs) and sudden cardiac death (SCD). This link between MVP and SCD is reported with an annual incidence <1% in unselected individuals with MVP.8,9 However, at autopsy, the prevalence of MVP among young patients with sudden arrhythmic death is reported between 4%10 to up to 7%.11 Due to the low event-rate and the lack of very large cohorts, assessing the precise incidence of SCD in MVP in general and in specific subsets of patients remains challenging.
Mitral annular disjunction (MAD, discussed in detail in section 4) is often observed concomitantly with MVP.12 MAD results in an abnormal motion of the mitral annulus, termed curling.13 It is associated with increased risk of arrhythmias and is therefore and integral component of the arrhythmic MVP (AMVP) complex.14,15
Over the last decade, a multidisciplinary approach has been employed to identify specific MVP subsets with high arrhythmic risk by careful examination of electrocardiograms (ECG), Doppler Eechocardiography, cardiac magnetic resonance (CMR) imaging, cardiac computed tomography imaging (CT) and confirmed by autopsy findings.16
Scope of the document
This consensus statement reviews and summarizes current literature regarding the AMVP complex based on an international multidisciplinary collaboration to collect evidence from experts in clinical cardiology, echocardiography, CMR, cardiac CT, electrophysiology and cardiothoracic surgery. It summarizes the main gaps of knowledge regarding identification, risk stratification and management of AMVP and provides practical suggestions for diagnosis and management of patients with AMVP. All consensus statements were subjected to voting and required at least 75% agreement to reach consensus. The consensus statements are ranked using the coloured heart system (Table 1).
Table 1.
Categories of consensus statements
| Consensus statement | Definition | Symbol |
|---|---|---|
| Recommended/indicated or ‘should do this’ | Scientific evidence that a treatment or procedure is beneficial and effective or, is strongly supported by authors’ consensus. |

|
| May be used or recommended | General agreement and/or scientific evidence favour the usefulness/efficacy of a treatment or procedure. |

|
| Should NOT be used and is NOT recommended | Scientific evidence or general agreement not to use or recommend a treatment of procedure. |

|
Aims of the document
This document aims, using the most recent advances in knowledge regarding SCD and AMVP, to
Define the phenotype of the AMVP complex.
Propose approaches for screening and diagnosing the AMVP complex.
Propose approaches for risk stratification.
Propose comprehensive imaging protocols using transthoracic Doppler-Echocardiography and CMR to assess the AMVP complex.
Propose management approaches.
Highlight critical gaps of knowledge to guide future clinical trials and collaborative research.
Mitral valve prolapse
Definition and diagnostic criteria
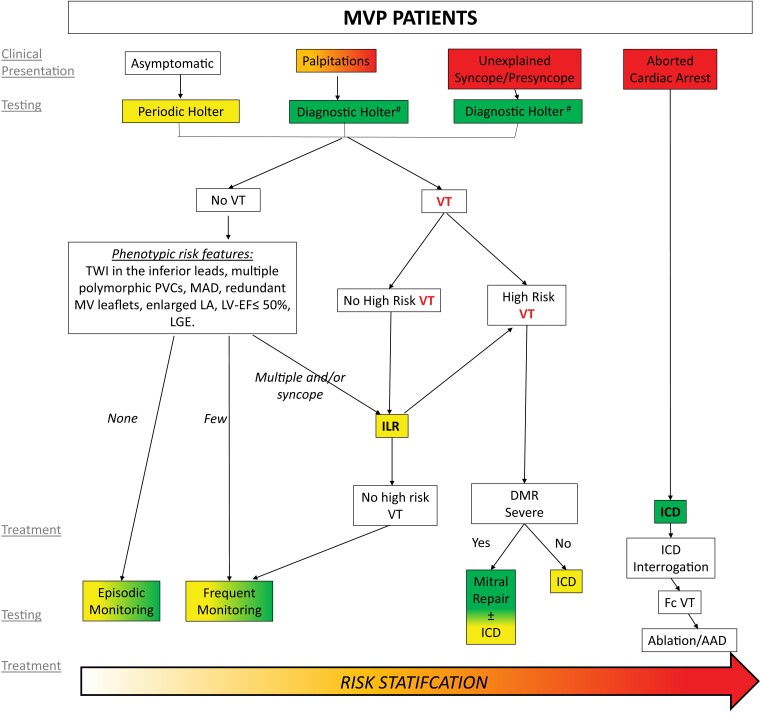
MVP is defined as a systolic displacement of one or both mitral leaflets ≥ 2 mm above the plane of the mitral annulus in the sagittal view of the mitral valve (Figure 1).2,17,18 Two main aetiologies of MVP are described:19
Figure 1.
The mitral valve with and without mitral valve prolapse. Transthoracic echocardiographic long-axis view in end-systole zoomed in on the mitral valve with (A) the location of both mitral leaflets within <2 mm under the plane of the mitral annulus (red line) refutes the diagnosis of mitral valve prolapse. (B) A displacement of both leaflets >2 mm (red arrow) above the plane of the annulus (red line) defines mitral valve prolapse. Note the absence of detachment of the posterior leaflet from the left ventricular myocardium (no mitral annular disjunction).
Myxomatous MVP—also known as Barlow’s disease. Characterized by excess tissue including chordal thickening/elongation, annular dilation and calcification with low probability of chordal rupture.
Fibroelastic deficiency—characterized by chordal thinning, elongation, and/or high probability of rupture, with classic findings of prolapse and MR of varying severity. This is the most common form of MVP.20,21
Epidemiology
MVP is the most prevalent valve abnormality in Western countries.2 Using the current definition, its prevalence varies from 0.6 to 3.1% depending on the age of the population examined (increasing with age) with slight predominance of female sex2–4 but consistent across ethnic groups.4 The prevalence of MVP among athletes is similar to that of the general population.22 While the vast majority of MVP cases are sporadic, familial clusters have been reported.23 Parental MVP was associated with a ∼5 fold increase in the risk of MVP, and first degree relatives have a 30–50% likelihood of being affected.24 Taken as a whole, long term survival of subjects with MVP is not different from the general population, with its prognosis determined mainly by MR severity.25
Mitral valve prolapse in the context of connective tissue diseases (syndromic mitral valve prolapse)
Heritable connective tissue diseases with abnormal synthesis and processing of collagen proteins are often characterized by myxomatous MVP. The incidence of MVP in Marfan syndrome patients is linked with age, with up to 75% affected at >60 years.26–29 The reported prevalence of MVP is up to 45% in patients with Loeys-Dietz syndrome. On the other hand, the prevalence of MVP is approximately 6% in patients with Ehlers-Danlos syndrome.29 Patients with osteogenesis imperfecta and adult polycystic kidney disease are also frequently reported to have MVP.26
Long term outcomes and association with sudden cardiac death
Clinical outcome in MVP is primarily determined by both the presence of MR and its severity, as well as its consequences on LV function and size.6,7 Indeed, in the absence of significant changes in MR or LV size/function, MVP is considered a benign condition with excellent prognosis.30,31
However, reports of SCD25,32 and VAs33 in MVP patients, with or without MR, raised the concern that a small subset may incur higher risk of VAs and SCD, independent of MR severity or LV dysfunction.34 SCD in patients with isolated MVP (with or without MAD) was initially described in case reports;11,35–37. Later, the link between SCD and MVP was corroborated by a meta-analysis.38 Importantly, it appeared that MVP patients who suffered from SCD showed a characteristic MVP-phenotype.8,37,39
The prevalence of MVP in published juvenile SCD series ranges from 0 to 24%.8 In young SCD victims, prevalence of myxomatous MVP was between 4%10 and 7%, reaching 13% when considering only the female subgroup.11 The annual risk of SCD in populations with MVP is difficult to estimate due to low incidence. Some estimates range between 0.2% and 0.4% per year,9,15,25 but SCD rates were reported at 1.8% per year with severe MR due to a flail leaflet,34 lower (0.8% per year) in asymptomatic patients with flail and normal ejection fraction, and as low as 0.14% in a community population.38 The excess risk of SCD is even more difficult to assess as the rates of SCD in the general population are highly dependent on age. 40 While some estimates place the risk of SCD in patients with MVP at 3-fold higher than in the general population,16 it is probably only slightly higher than normal in MVP without MR, doubled in MVP with severe degenerative MR and markedly higher with signs of heart failure or LV dysfunction (up to 7.8%/year in patients with NYHA III-IV compared to 1%/year in a symptomatic patients).34
Recently, large cohorts of consecutive patients with isolated MVP, comprehensively characterized with 24-hour-Holter-monitoring, clinical and echocardiographic assessment, demonstrated that VAs are frequent but are rarely severe.14 However, severe arrhythmias are independently linked to excess mortality accumulating over time, irrespective of the severity of degenerative MR.14 Thus it appears that there is a phenotype of MVP prone to developing VAs over time with increased risk of mortality and SCD when VAs become severe.
Mitral annular disjunction
Definition
MAD is characterized by a systolic separation between the ventricular myocardium and the mitral annulus supporting the posterior mitral leaflet,41 Conversely, without MAD the mitral annulus remains attached to the atrial and ventricular myocardium/endocardium. Hence, MAD is associated with the loss of mechanical annular function linked to its normal ventricular myocardial attachment but with maintained electrical function, isolating the left atrium and ventricle electrophysiologically.13,15 The circumferential extension of MAD is limited anteriorly by the mitro-aortic fibrous continuity, between the aortic cusps and the anterior leaflet of the mitral valve. As a consequence, MAD has been observed only at the insertion of the posterior leaflet. It can extend laterally variably under all scallops of the posterior leaflet but preferentially at the central posterior scallop.
Diagnostic criteria
The MAD trench is relatively easy to recognize in long-axis views by transthoracic-Echocardiography42–45 or CMR.45–47 Cardiac CT Scan can also diagnose MAD.48,49 MAD and MVP are defined based on the mitral annulus position,50 yet with MAD, the annulus is particularly difficult to identify precisely throughout systole as it falls briskly in mid-systole, posteriorly to the basal ventricular myocardium. Conversely, the ventricular myocardium, having lost its basal attachment, bulges more apically than normal, forming the apical margin of the MAD trench. This posterior movement of the mitral annulus is also known as ‘curling’ and refers more broadly to the abnormal/excessive systolic upward motion of the posterior mitral annulus and of the adjacent postero-basal myocardium, with prominent basal myocardial wall thickening and hypertrophy.13 The mitral annulus position is best recognized in the long axis view zoomed on the mitral valve using the highest frame rate possible and by reviewing the images frame by frame. In this way, the thin structure of the annulus can be observed from early to late systole (Figure 2).15 In turn, the precise location of the mitral annular position allows measurement of the MAD trench width and of MVP depth. Accordingly, the upper limit of MAD is defined at the level of posterior leaflet insertion on the annulus/left-atrial-wall, whereas the lower limit is defined at the level of the LV myocardium.47 Without dynamic examination, excessive posterior leaflet tissue arising from a normally implanted annulus could falsely be interpreted as MAD. Such dynamic analysis is required both on echocardiographic and CMR examinations (Box 1).
Figure 2.
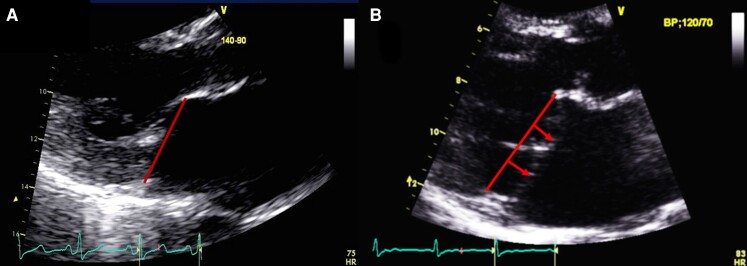
Mitral valve prolapse with and without mitral annular disjunction (MAD) on transthoracic echocardiography (TTE). TTE long-axis view in end-systole displaying bileaflet mitral valve prolapse with (A) MAD (yellow line) of 11 mm length vs. (B) without MAD. The red line indicates the plane of the mitral annulus.
MAD length is measured in the parasternal long axis view (or equivalent sagittal views on CMR), from the insertion of the posterior leaflet on the detached mitral annulus to the border of the bulging LV myocardium.44,46,47,51 Repetitive 2D-transthoracic echocardiography over the years are of interest. MAD is described as a continuum around the annular circumference and is interspersed with normal tissue.46 Annulus disjunction can also be present on the right side of the heart.52
Also, MAD can be present in early childhood in syndromic MVP but whether MAD develops over time remains to be further explored.46,53 MAD could additionally be assessed on 2D/3D transesophageal echocardiography54 when indicated for another reason (e.g. pre-operative examination) or in cases of inconclusive Transthoracic Echocardiography (Figure 3). Finally, MAD lateral extension should be determined in additional apical four-chamber and two-chamber views, by 2D-transthoracic echocardiography and CMR (Figures 3 and 4).46 The latter may be more sensitive for diagnosis.
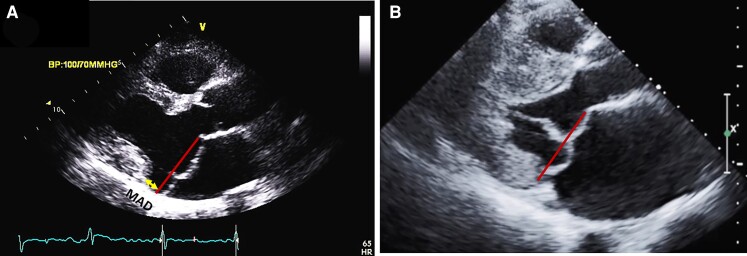
Figure 3.
Mitral valve prolapse and mitral annular disjunction (MAD) on transthoracic echocardiography (TTE) and transoesophageal echocardiography (TEE). (A) TEE 3D view, displaying a large prolapse of P2 precisely assessed by ‘photo-realistic’ tools now available. Severe mitral regurgitation is associated as shown on 120° TEE (B) and TTE apical four-chamber views (C), with severe left atrial dilatation. A 10 mm MAD is diagnosed both on these TEE (D) and TTE (E) views.
Figure 4.
Mitral valve prolapse and mitral annulus disjunction (MAD) on cardiac magnetic resonance (CMR). CMR long-axis view in end-systole displaying mitral valve prolapse with MAD (yellow arrow). The red line indicates the plane of the mitral annulus.
Epidemiology
MAD was first described in autopsy studies to be present in approximately 6% of human hearts,44,46,55–58. The reported prevalence of MAD varies partly due to the different imaging modalities, various cut-offs, heterogeneous subpopulations, and various MR-severity grades.42,43,45,59–61 Amongst patients with MVP, the prevalence of MAD varies between 20 and 58% (reported pooled rate of 32.6% from three studies).14,42,48,61–64 In the specific subset of patients with myxomatous MVP, MAD prevalence varies between 21.8% and 98% (pooled rate of 50.8% from 3 studies).14,43,44,61,64,65 In patients with MAD, reported presence of MVP was 78%.46
In syndromic MVP, MAD prevalence is reported between 34%66 in patients with Marfan syndrome and 40% in carriers of Loeys-Dietz syndrome.67 In these cases, MAD is a marker of severe disease including more arrhythmic events, a higher need for mitral valve intervention, and among patients with extensive MAD, more arrhythmic events.66,67 The first large prospective cohort of isolated MVP with systematic MAD assessment reported a prevalence of 30% of MAD, generally in younger patients.15 Advanced myxomatous-degeneration, denoted by marked leaflet-redundancy and bileaflet-MVP, was the strongest MAD-associated MVP feature. The association was independent of all baseline characteristics, whereas MR-severity was not independently associated with MAD.15
The arrhythmic mitral valve complex/phenotype
Arrhythmic MVP phenotypes: proposed consensus definition and classification
Arrhythmic mitral valve—definition
The arrhythmic mitral valve complex is defined by presence of MVP (with or without MAD), combined with frequent and/or complex VA in the absence of any other well-defined arrhythmic substrate (e.g. active ischaemia, ventricular scar due to another defined ethology, primary cardiomyopathy or channelopathy, Box 2).
Arrhythmic mitral valve—phenotypes
The writing committee recognizes two main phenotypes within the spectrum of this complex:
Severe degenerative mitral regurgitation
Patients with MVP and severe MR irrespective of valvular morphology are at increased risk of excess-mortality68 including excess SCD, compared to the general population.34 The risk of SCD is higher in patients with severe symptoms, atrial arrhythmias and reduced LV-systolic function. However, even without these risk factors, MVP patients with at least moderate to severe MR incur excess-mortality69 and SCD, with double the incidence compared to the general population.34 Surgical MR correction appears to normalize overall mortality69 and SCD incidence.34
Severe Myxomatous MVP independent of MR severity
As a group, the overall survival of subjects with MVP without severe MR or LV dysfunction, is equivalent to that of the general population.30 However, several reports of SCD in patients with MVP and in the absence of another relevant pathology,11,25,32,33 have indicated the existence of a small subgroup of patients with a malignant course. Morphologically, this phenotype most often involves MAD, severe myxomatous degeneration with marked leaflet redundancy, excess leaflet length and thickness, and most often but not always, bileaflet MVP. MR may be absent to severe.14 This phenotype is not limited to young patients14 and is linked to more frequent occurrence of VAs. MAD appears as an important component of this phenotype. Importantly, the arrhythmic outcome of these patients is independent of gender, MR severity, LVEF or bileaflet-MVP.14
Atrial arrhythmia phenotype
Patients with MVP and left atrial (LA) dilatation, in excess of that expected from MR, incur a higher risk of atrial arrhythmias.70–72 The combination of LA enlargement 72 and atrial fibrillation73 is associated with excess mortality, independent of all baseline characteristics including MR severity and LV parameters. Importantly, surgery is followed by improved survival, 72,73 suggesting that both severe LA enlargement and atrial arrhythmias, even when paroxysmal, should be accounted for in clinical decision making.
MAD in the absence of MVP
While MAD associated with MVP is frequent and associated with the occurrence of ventricular arrhythmic events,15 isolated MAD without MVP has been described on static CMR examination.46 However, due to the problems associated with precise determination of the mitral annular position, its exact nature and whether MVP may have been under-detected remains uncertain. Even more unsubstantiated is its association with outcome. Accordingly, large cohorts with long-term follow-up are required to better define MAD without MVP and its outcome and consequences.
Determinants of arrhythmia
Clinical risk factors
Data derived form case reports and autopsies of SCD victims with MVP suggest excess female prevalence.37,57,74 This is likely the result of selection bias, as a nation-wide autopsy study found a similar proportion of both sexes58 and a large cohort of consecutive MVP patients did not find any association between sex and arrhythmia.14
Several non-specific symptoms such as chest pain, palpitations, dyspnoea on exercise and syncope have been attributed to MVP. Yet, multiple large cohort studies (e.g. Framingham offspring study75) have shown that the incidence of these symptoms is comparable in subjects with and without MVP.2,75,76 Nevertheless, syncope is uncommon in non-selected subjects with MVP (2–3.6%)2,75 and was reported in 35% of MVP patients with malignant arrhythmias or SCD.57 Furthermore, in a large cohort of consecutive MVP patients, syncope was more frequently reported in patients with documented severe VAs.14 Therefore, syncope and particularly unexplained syncope, may have a high discriminative value in identifying MVP patients at risk of malignant arrhythmias. Palpitations and chest pain are frequent but are reported in similar proportions in subjects with MVP with and without history of VAs.14
Electrocardiography
T Wave inversion
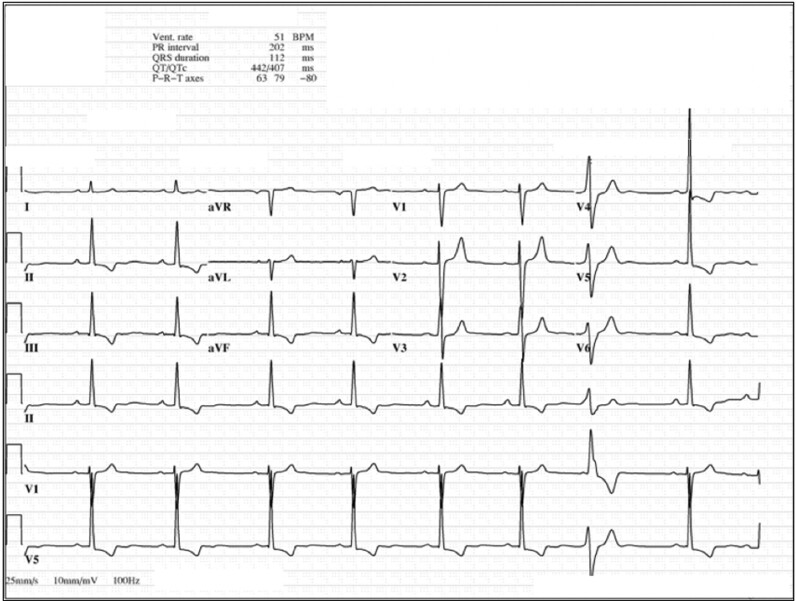
Repolarization abnormalities have long been recognized as markers of arrhythmic risk in subjects with MVP. The most consistent feature is T wave inversion (TWI), most often in the inferior and lateral leads (Figure 5). TWI or biphasic T waves, in the presence of normal QRS, have been observed in the majority (65%) of patients with MVP who present with malignant arrhythmias38 and more importantly, have been independently associated with VAs in a large unselected cohort of MVP patients.14 This is thought to be the result of abnormal stretch of the papillary muscles and adjacent myocardium,77 and/or locally disturbed repolarization of the same regions.78 However, the specificity of this finding, as a marker for high arrhythmic risk, is questionable since the reported rates of TWI range as high as 40% among subjects with MVP.79
Figure 5.
Twelve-lead electrocardiogram (ECG) showing T wave inversion in infero-lateral leads. An ECG of a 44-year-old female who presented with ventricular fibrillation and was found to have mitral valve prolapse. The tracing is remarkable for T wave inversion in leads II, III, AVF, V4-V6.
QT prolongation
Subjects with MVP have been found to have longer QT intervals than control populations in some80 but not all reports.11,81 Furthermore, QT prolongation was found to correlate with more severe leaflet prolapse and thickening of anterior leaflet82 and to be independently associated with VAs among MVP patients.14 An animal model simulating papillary muscle traction found alteration in the repolarization properties of the involved regions providing an explanation for the observed QT prolongation and arrhythmic potential.83 Importantly, while prolonged QT has been consistently shown to be associated with SCD in several large population studies,84,85 to date, its link to mortality in subjects with MVP or MAD remains uncertain.
Fragmented QRS
Fragmented QRS (fQRS) is defined as the presence of additional R waves (R′) or notching in the R wave or the S wave, or the presence of >1 R′ in two contiguous leads.86 It has been shown to be a marker of localized scar86 and to be associated with increased risk of arrhythmic mortality and SCD 87 in a variety of populations.88–90 A recent study has shown that fQRS was associated with complex VA in patients with MVP (Table 2).91
Table 2.
Ventricular ectopy—definitions
| Ectopy type | Definition |
|---|---|
| Polymorphic PVCs | Three or more distinct PVC morphologies (not slight variations of the same morphology) suggestive of multiple origins rather than multiple exits of a single site. |
| Short coupled PVC | A PVC with coupling interval less than 350 ms.92 |
| NSVT | Three or more consecutive ventricular beats at a rate of ≥100 beats per minute lasting 30 s or less. |
| Monomorphic NSVT | NSVT with a single QRS morphology. |
| Polymorphic NSVT | NSVT with a constant beat to beat change in QRS morphology. |
| Sustained ventricular tachycardia | VT lasting more the 30 s or requiring immediate termination by external means. |
| Frequent ventricular ectopy | Premature ventricular beat constituting at least 5% of the total complexes. |
| Complex ventricular arrhythmia | NSVT, VT, and VF. |
NSVT, non-sustained ventricular tachycardia; PVC, premature ventricular contractions.
Further research is needed to determine the sensitivity and specificity of this feature in MVP patients as well as its role in clinical practice.
Premature ventricular contractions
The observation of premature ventricular contraction (PVCs) on repeated standard ECG tracings, usually indicates a significant burden of ventricular ectopy.93 While the total burden of ventricular ectopy may not be inferred, captured tracings of non-sustained ventricular tachycardia (NSVT) is a marker of higher risk94 (Table 2). PVC morphologies observed are often compatible with papillary muscle or mitral annular/LV basal origins. In many cases, a fascicular origin may be inferred, but these are by no means consistent or exclusive findings.14,74 Ectopy arising from the outflow tracts are also common.95 A unique pattern of NSVTs with morphologies alternating between papillary and right ventricular outflow tract has been described in a small cohort of MVP patients that survived out-of-hospital SCD.74 The prognostic significance of the various morphologies remains speculative.
Holter monitoring
VAs on 24 h Holter monitoring are common in MVP patients, reported in 43–85% of cases.14,75 Most patients present with mild to moderate VAs, while severe VAs are rare (<10%) but were associated with a 3-fold increased risk of mortality in one large cohort study (Table 3).14 We acknowledge the limitation of using specific cutoff values derived from a single centre, albeit from a large cohort. While these data are consistent with previous studies from other patient populations showing increased mortality with higher burden of ectopy96,97 and with NSVT,98,99 additional validation by other cohorts is needed.
Table 3.
Arrhythmia severity classification
| Severity | Arrhythmia burden/rate | Risk of mortality HR [95% CI] | References |
|---|---|---|---|
| Mild ventricular arrhythmia | PVC≥5% and/or VT runs <120 bpm |
1.20 [0.68–2.14], P = 0.5 | 14 |
| Moderate ventricular arrhythmia | VT runs 120–179 bpm | ||
| Severe ventricular arrhythmia | VT runs ≥180 bpm and/or history of sustained VT/VF | 2.94 [1.36–6.36] P = 0.006 |
HR, hazard ratio; PVC, premature ventricular contractions; VT, ventricular tachycardia.
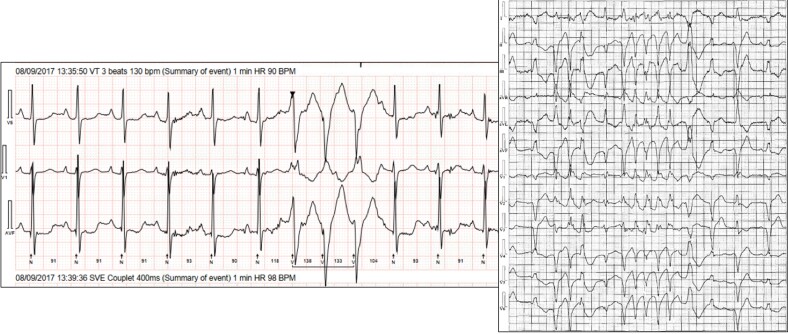
24 h Holter monitoring is superior to a standard ECG as a screening modality in all patients with MVP (including asymptomatic) to assess arrhythmia burden and disease prognosis. In most asymptomatic MVP patients, detection of simple and infrequent (total burden < 5% of single PVCs) VAs on 24 h Holter monitoring may not warrant further evaluation. Symptomatic patients with mild VAs detected on 24 h Holter monitoring would require a longer period of rhythm monitoring (∼7 days) to accurately detect overall PVC burden.93 However, patients with multifocal ventricular ectopy, short coupled PVCs (coupling interval <350 ms)100 or fast NSVT are at risk of SCD and warrant further investigation (Figure 6).11,101 Unexplained presyncope or syncope with frequent or complex VAs (Table 2) on Holter monitoring should lead to strong consideration of long-term rhythm monitoring (e.g.repeated Holter monitoring, external or implantable loop recorders),102 especially if other phenotypic risk features are present.
Figure 6.
24 h Holter monitor showing complex ventricular ectopy and non-sustained ventricular tachycardia (NSVT). 24 h Holter monitoring of two patients with mitral valve prolapse, mitral annular disjunction, and palpitations. It shows a right bundle branch block with non-sustained ventricular tachycardia, likely from the posteromedial papillary muscle.
Echocardiographic predictors of arrhythmias
Pathophysiological predictors of arrhythmias
MR quantification is essential for evaluating the risk of VAs,11 as several studies have demonstrated excess mortality associated with increasing MR severity,12,13 as well as a specific increase in the risk of SCD in patients with severe degenerative MR.34 MR quantification is performed by Doppler-Echocardiography based on stroke volumes measurement by Doppler or by a combination of Doppler and LV volume measurements. The most commonly used method is however the flow convergence (proximal isovelocity surface area) method based on shifting of colour baseline to define the radius of the flow convergence103. By these methods, the variables measured are the regurgitant volume and the effective regurgitant orifice area. The thresholds for severe degenerative MR are ≥60 mL/beat for regurgitant volume and ≥40 mm2 for effective regurgitant orifice area but mortality risk increases already at 20–30 mm2 with a linear increase with larger values.20,104
degenerative MR with reduced LV function in itself is also associated with higher risk of SCD34 and excess-mortality105,106 although its causal link to VAs has not been proven. LV ejection fraction <60% and end-systolic diameters ≥40 mm are the accepted thresholds for reduced LV function and accepted indication for mitral valve surgery.20
Morphologic predictors of arrhythmias
Morphologic features associated with arrhythmia consist of MAD11,13,37 and severe myxomatous degeneration, defined by thick and redundant leaflets with multi-segment bileaflet MVP. Their combined presence characterizes the typical phenotype of the AMVP complex.14 Patients presenting with the AMVP complex are at excess risk of developing VAs, independent of MR severity.14 Importantly, bileaflet prolapse is quite prevalent among patients with MVP, and while small series have suggested an association with higher mortality107,108 this was not confirmed in larger cohorts or population based studies.30 Of note, MAD in subjects with MVP is in itself linked with VAs during follow-up.15 However, MAD within the first 10 years of follow-up is not associated with excess mortality15 because arrhythmia occurrence is often delayed long after MVP and MAD diagnosis. SCD and excess mortality are even more delayed after detection of severe arrhythmias.14 Whether MAD depth is a predictor of more frequent arrhythmias is unclear as is the mechanism of MAD-associated arrhythmia, although myocardial fibrosis is hypothesized as causative.
Strain predictors of arrhythmias
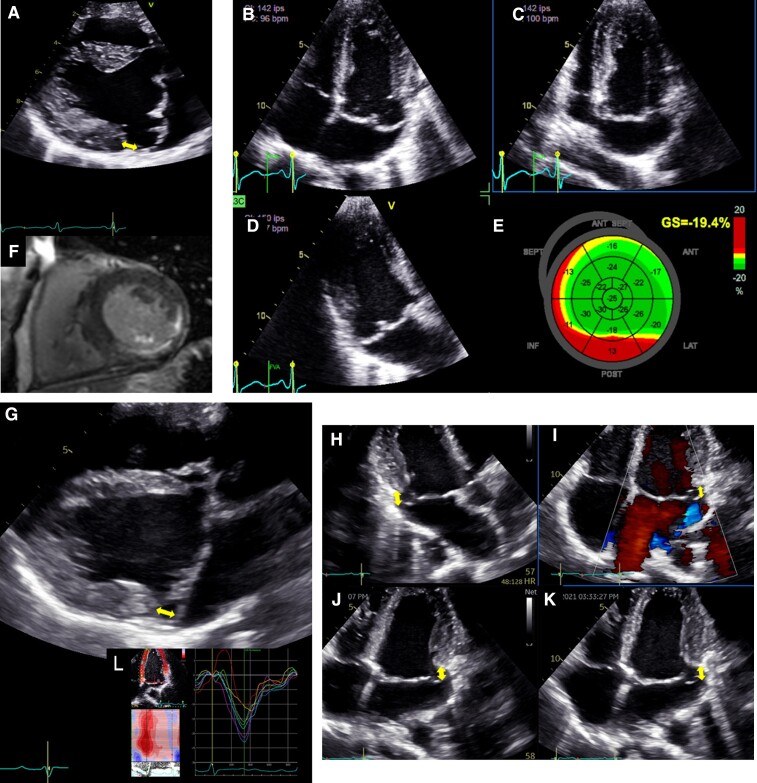
Patients presenting with the AMVP complex exhibit more frequent myxomatous disease (40 vs. 60%)14 with left ventricular mechanical dispersion.46 While the global longitudinal strain value can be preserved (slightly altered as compared to patients without arrhythmias), some degree of dispersion in longitudinal strain peaks may be observed.109 Post-systolic deformations are often observed with marked MAD and MVP (Figure 7) attributed to increased interaction between wall deformation and mitral valve motion. Longitudinal strain is useful in assessing interplay between mitral leaflets and myocardium, and in demonstrating changes in temporal pattern of myocardial deformation.109 It has been suggested that providing these relevant automatically measured parameters could identify higher risk of VAs. However, in light of the current scarcity of data, further research is need to verify the clinical utility and predictive value of this.
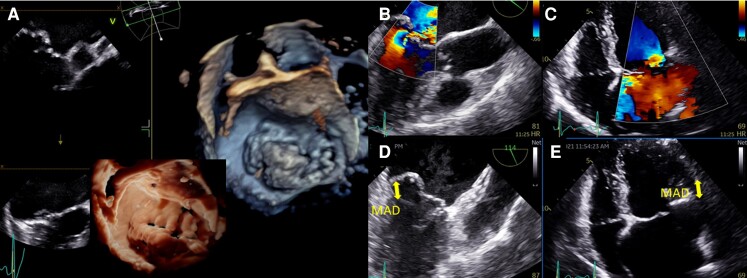
Figure 7.
Left ventricular mechanical dispersion associated with mitral annular disjunction (MAD). First panel: Transthoracic echocardiographic long-axis view in end-systole (A) displaying mitral valve prolapse with MAD (yellow arrow). Transthoracic echocardiographic apical four-chamber (B) three-chamber (C) and two-chamber (D) views for the measurement of global longitudinal strain (E). Note the disjunction inducing mechanical dispersion and post-systolic shortening of the posterior-basal segment of the left ventricle (in red). The patient has been seen for ventricular premature complexes. (F) The disjunction is associated with fibrosis in the basal inferolateral segments of the left ventricle and mild mitral regurgitation. Second panel: Transthoracic echocardiographic long-axis view in end-systole (G) displaying mitral valve prolapse with MAD (yellow arrow). Note here the disjunction involving all the insertion of the posterior leaflet on the annulus on transthoracic echocardiographic apical three-chamber (H) and four-chamber views (I, J, K). The depth of the disjunction is only mild yet the circumferential extension is extensive. (L) It leads to mechanical dispersion, post-systolic shortening of the posterior-basal segment of the left ventricle. The patient has been seen for palpitations and > 10% ventricular premature complexes.
Cardiac magnetic resonance imaging
CMR can also identify prolapsed scallops using a stack of cine images and is ideally suited for risk stratification of VAs in patients with MVP, due to its unique ability to non-invasively identify focal myocardial fibrosis using late gadolinium enhancement (LGE). Several studies suggested an association between LGE at the mid-wall of the papillary muscles or patchy myocardial fibrosis (non-ischaemic pattern) in the LV infero-basal region, and complex VAs.110–112 Diffuse LV fibrosis determined by T1 mapping is also associated with increased risk of complex VAs among patients with MVP (significant shorter post-contrast T1 time).112 Overall, fibrosis is common in MVP (28–37%),110,113 usually located close to the annulus in the basal left ventricular wall including papillary muscles and inferior wall.39 Importantly, only LGE within the mitral apparatus (papillary muscles and peri-annular region) has a clear pathophysiological association with arrhythmia. The significance of LGE in other regions remains unclear in this context.
Any CMR standard protocol can identify MAD,47 but, complete assessment of MAD extent over the mitral annulus circumference warrants six LV long-axis cine sequences with 30° interslice rotation.46 Comprehensive MAD assessment should include careful description of the mitral valve, MAD severity, LV remodelling and fibrosis. Important mitral characteristics include bileaflet MVP, myxomatous MVP, mitral annulus enlargement in systole, prolapse depth, increased ratio of basal to midventricular wall thickness laterally, and presence of curling.37,47 Assessment of MAD severity comprises extent around the mitral annulus and width of MAD. The link between MAD extent and VAs remains ill-defined.44,47
Genetics
The evidence for a potential role of systematic genetic analysis in these patients is weak. Some data exist for familial clustering of non-syndromic MVP with X-linked inheritance pattern as first reported in 1969.114 Later studies reported autosomal dominant transmission suggested MVP as the most common Mendelian cardiovascular abnormality with various phenotypic expressions.115 Filamin A (FLNA) mutations have been described recently in a large family. The FLNA-MVP phenotype is characterized by both congenital and degenerative alterations of the MV apparatus with more severe disease in men due to the X-linked inheritance.27 A truncating FLNA variant has been linked to the AMVP proposing a proarrhythmic effect of this variant.116
MVP has been related to the Dachsous1 gene (DCHS1), a member of the cadherin superfamily.117 In clinical practice, genetic testing is suggested in patients with syndromic disease (Marfan and Loeys Dietz syndrome, etc.) due to the implications on follow up for aortic disease. Routine genetic testing in non-syndromic MVP is currently not justified.
Biomarkers
There is no established circulating biomarker associated with the AMVP. Short, noncoding micro RNAs (miRNA)118 and proteomics have been employed to identify patients with MVP.119,120 A recent study explored stretch-related and fibrosis-related circulating biomarkers in 72 patients with MVP/MAD.121 Circulating soluble suppression of tumourigenicity-2 (sST2) was associated with VAs, supporting the theory of stretch induced arrhythmia mechanisms. Although tissue TGF-β1 signalling contributes to fibrosis and matrix remodelling,122,123 circulating TGF-β1 level was not related to VAs.
Currently, the assessment of biomarkers in these patients is not justified.
Pathological evidence
MVP has been an underappreciated cause of SCD for a long time. MVP has been frequently overlooked in pathological examinations due to the absence of a uniform criteria and lack of awareness of its potential role as a cause of SCD. This resulted in uncertainty regarding the true burden of MVP.124 Careful mitral valve structural analysis of SCD patients with MVP suggested an association with increased annular circumference, leaflet length and thickness, and presence/extent of endocardial fibrous plaques (friction lesions) on the LV myocardium.11,125 Among victims of SCD with MVP, bileaflet MVP was present in 70% and endocardial fibrous plaques in the posterolateral wall in >50%. Moreover, histological analysis revealed elongated MAD and LV fibrosis in SCD cases with MVP vs. normal control hearts.13 In particular, patchy fibrosis was localized at the level of PMs with adjacent free wall in all and of the inferobasal wall in 88% of young SCD victims.13
Mechanism of arrhythmia
The uneven distribution of major arrhythmias in MVP remains unexplained but may be linked to the anatomical substrates and triggers.
Substrate
Histological evidence of myocardial substrate is consistent with patchy fibrosis between the mitral valve, papillary muscles (PM) and adjacent infero-basal and LV myocardium in MVP patients who sustained SCD.11,13 These findings correlate with CMR LGE distribution in the papillary muscles and infero-basal LV wall. These regionalized fibrotic, molecular and cellular changes suggest a reactive response to increased mechanical traction from a prolapsing mitral valve.
The areas of patchy myocardial fibrosis in the sub-valvular apparatus leading to conduction delays serve as the substrate.126
Triggers
Mechanical stretch of papillary muscles may also shorten action potential duration and decrease resting diastolic potential leading to stretch-activated early afterdepolarizations resulting in triggered activity.127 Furthermore, endocardial and mid-myocardial fibrotic changes on the papillary muscles and adjacent LV can create abnormal repolarization resulting in inverted T-waves frequently observed on the inferolateral leads on 12-lead ECG. These abnormalities in ventricular repolarization with QT prolongation, and ST-T changes may give rise to polymorphic VT.16,81
Detailed invasive voltage mapping correlates PVC sites of origin with the papillary muscles, fascicular system, LV outflow tract and mitral annulus with predominant right bundle-branch block morphology (Figure 8). Subtle PVC morphology variations are highly suggestive of multiple exits from a single source.128
Figure 8.
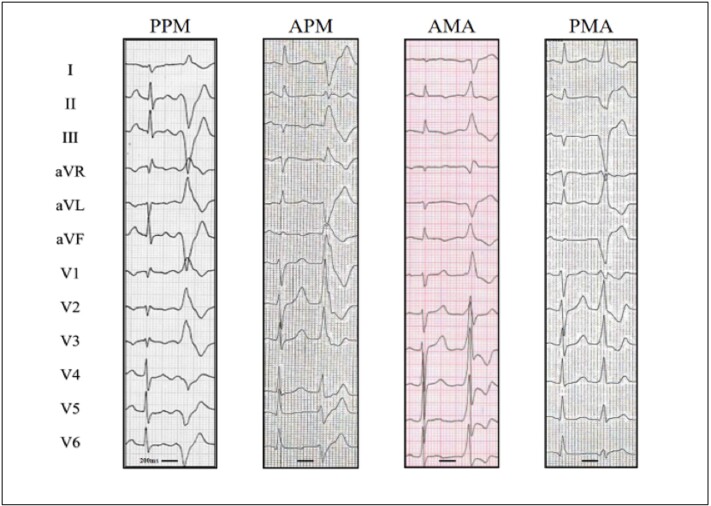
Typical examples of premature ventricular contractions arising from the mitral apparatus. Premature ventricular complexes (PVCs) arising from the posterior papillary muscle (PPM) are characterized by right bundle branch block (RBBB) morphology, a predominantly negative QRS in V5, QRS >130 ms and superior axis with both leads II and III negative. Foci in the anterior papillary muscle (APM) also yield a RBBB morphology, typically with a late R/S transition (V3 to V5), QRS >130 ms and lead II discordantly negative while lead III is positive. Ectopy originating from the mitral annulus result in RBBB morphology with positive precordial concordance. PVCs form the anterior mitral annulus (AMA) are defined by an inferior QRS axis while those originating from the posterior mitral annulus (PMA) result in a superior axis. Image courtesy of Jérôme Hourdain, MD, Hôpital la Timone, Marseilles, France.
Ventricular ectopy arising from Purkinje tissue may trigger ventricular fibrillation in patients with bileaflet MVP and SCD (8,9). Invasive voltage mapping may discover fractionated, split and delayed Purkinje potentials suggesting the presence of abnormal tissue. The Purkinje system is a critical component in arrhythmogenesis and risk of SCD.95,129 It has been suggested that mechanical snap of redundant leaflets on ventricular myocardium sets this cascade into motion,78 but this concept is not measurable and its link to the heterogeneous occurrence of VAs and SCD remains putative.
Risk stratification
Advised screening
Risk stratification aiming at assessing the risk of VAs and SCD is challenging even for the most common and well-studied conditions.130,131 Approaches for screening for AMVP are limited by the lack of prospective outcome data and absent recommendations for rhythm monitoring in current valvular guidelines.7,20
In this novel endeavour, the present expert group was guided by three pillars: (i) all conventional indications for ICDs based on current guidelines apply to MVP,102,132,133 (ii) AMVP, particularly in the presence of LGE on CMR should not be regarded as a ‘structurally normal heart’ and any VAs complicating MVP should not be considered idiopathic or benign, (iii) several clinical, electrocardiographic, echocardiographic and other imaging parameters (detailed in section 6.1) have been shown to be associated with increased risk of death and/or VA in AMVP patients and should be screened for in patients with MVP.
All patients with MVP should be stratified for SCD risk (Green Heart, Table 4). Risk stratification of patients with MVP includes focused history, 12-lead ECG, extended ECG monitoring and detailed echocardiography (Green heart, see statement tables). The use of CMR and implantation of a loop recorder are more selective depending on the probability of VAs. Risk stratification involves two phases based on the clinical and imaging context and the detected arrhythmia (Graphical Abstract).
Table 4.
Risk stratification
| Consensus statement on risk stratification | Symbol | Ref |
|---|---|---|
| Patients with MVP should undergo a directed and structured risk stratification process aimed at identifying AMVP and assessing the risk of SCD. |

|
Expert consensus |
AMVP, arrhythmic mitral valve prolapse; MVP, mitral valve prolapse; SCD, sudden cardiac death.
Clinical and imaging context
In patients with MVP, the risk of VAs is not uniform and is higher in certain contexts. While patients who have recovered from sudden cardiac arrest have overt severe arrhythmias, the other patients are at risk of serious arrhythmias. The risk is higher when they present with unexplained syncope or even presyncope than when they present with isolated palpitations and lower when asymptomatic. Similarly features such as negative T waves, severe myxomatous degeneration with redundant leaflets, MAD and generally bileaflet prolapse, or LGE on CMR represent a higher risk of VAs.
Therefore, the clinical and imaging context strongly influences the intensity of the search for the presence of serious VAs. For example, patients presenting with unexplained syncope, even without VT on Holter monitoring are prime candidates to undergo extended ECG monitoring by longer ECG monitoring or ILR, if necessary, to uncover with the highest sensitivity the existence of VAs. Conversely, asymptomatic patients without complex VA on the initial screening Holter would only require episodic Holter assessment, more frequently if they present with phenotypic risk features. Because VAs may develop secondarily, a plan of reassessment over time is necessary, the frequency and intensity of which depends on the presence and number of phenotypic risk features. The presence of multiple phenotypic risk features suggests a higher arrhythmic risk and justifies more intense monitoring.
Observed arrhythmia
Few studies have addressed the potential for SCD or even for excess-mortality based on the arrhythmia detected, prospectively or retrospectively and the grading of arrhythmia below is mostly based on extension of the general grading of VAs.
High risk: The committee considered the following VAs as high-risk, detected at rest, by ECG, Holter or ILR. Whether their detection during exercise carries a similar high-risk is probable but uncertain.
Sustained VT not originating from the right or left ventricular outflow tract (Box 3).
Spontaneous polymorphic NSVT.
Rapid NSVT monomorphic (>180 bpm) has been associated with subsequent excess-mortality.
Box 3. Localization of origin of ventricular ectopy.
The likely origin of ventricular arrhythmias may be ascertained with accuracy from the QRS morphology on a standard ECG.134–136 Several sets of criteria and algorithms, focusing on the mitral annulus and the papillary muscles have been published and validated.135,137–139 Figure 8 summarizes the most relevant ECG characteristics of ventricular arrhythmias arising from the mitral annulus or the papillary muscles and shows typical examples of PVCs originating from these structures.
A likely mitral apparatus origin (papillary muscles and mitral annular region) may even be suspected based on a 3-lead Holter provided that those include 2 precordial leads (usually V1/2 and V5/6) and an inferior lead (usually aVF) thus allowing the estimation of the maximal QRS width, axis, precordial transition and type of bundle branch like morphology. Nevertheless, 12-lead Holter monitoring is more accurate for ventricular arrhythmia localization and should be preferentially used when available.
The likely origin of ventricular arrhythmia may also be inferred by the distribution of LGE in CMR.
Box 1. Summary of diagnostic criteria for MAD.
MAD is the anomalous attachment of the posterior leaflet, directly on atrial wall.
The slippage of the mitral annulus with MAD in systole is known as curling.
Sagittal views are advised for identifying MAD and assessing its length.
Transthoracic echocardiography is preferentially used as the first line imaging test for MAD diagnosis due to its wide availability with CMR reserved for patients with poor echocardiographic windows or image quality.
MAD diagnosis requires dynamic evaluation through the entire cardiac cycle.
MAD width extent should be evaluated in apical views.
Repeat 2D-transthoracic echocardiography over the years may be of interest to explore MAD development over time.
Box 2. The diagnosis of arrhythmic MVP requires:
The presence of MVP (with or without MAD)
-
The presence of ventricular arrhythmia that is
Frequent (≥5% total PVC burden)or
Complex (NSVT, VT, VF)
The absence of any other well-defined arrhythmic substrate
Intermediate risk: This category is even less well defined. The following VAs were considered by the committee as probable intermediate risk:
Polymorphic PVCs.
NSVT monomorphic, of lower rate (<180 bpm).
Highly frequent or complex PVCs (bigamy and couplets).
Low risk: Patients with frequent PVCs but not complex VAs (and no morphological higher risk features) are considered low risk.
The frequency of repetitive evaluation for risk reclassification is not well defined but regular Holter monitoring may prove helpful for this purpose.
In the absence of structural cardiac abnormalities, outflow-tract VAs are considered benign. Their clinical significance in patients with AMVP remains unclear but concerns have been raised regarding a possible mechanism where outflow-tract ectopy triggers a more malignant arrhythmia arising from the abnormal tissue in papillary muscles.78,129
Clinical evaluation
A comprehensive clinical evaluation is mandatory to identify MVP patients with higher risk of arrhythmias. Family history of SCD is relevant to suggest the possibility of inherited arrhythmia syndromes (long-QT syndrome, arrhythmogenic right ventricular cardiomyopathy) as alternative diagnoses. Family history of MVP should call attention to heritability, as either sporadic or familial forms.23,27 While autopsy studies have focused on patients without any other cause of SCD (young, often female) no demographic characteristic (age or sex) is particularly associated with SCD.30,57 However, with the current state of knowledge it is not yet possible to affirm whether a particular sex or age range is associated with excess risk of sudden death compared with that occurring in the community. Patients may be completely asymptomatic or experience mild nonspecific symptoms (atypical chest pain, anxiety). Common symptoms associated with AMVP are palpitations, chest pain and dyspnoea.37 However, presentation with syncope or presyncope may be a harbinger of SCD and warrants prompt thorough investigation.37 History of ventricular tachycardia (VT) or ventricular fibrillation (VF) should be retrieved from all available ECG tracings. The typical MVP auscultatory feature is an apical high-pitched, late systolic murmur with or without mid-systolic click,140,141 but does not allow inference of SCD risk. Holosystolic apical murmurs generally suggest more significant MR. Although no isolated clinical characteristic is independently predictive of malignant arrhythmias or SCD, clinical presentation with MVP and unexplained syncope, or palpitations should alert the clinician to further risk stratification (Table 5).
Table 5.
Clinical evaluation
| Consensus statement on clinical evaluation | Symbol | Ref |
|---|---|---|
| A careful clinical evaluation, including family history of SCD, previous syncope, comorbidities and physical examination, should be performed in patients with MVP. |

|
Expert consensus |
| Due to possibility of disease progression, clinical re-evaluation is advised periodically or when clinical circumstances have changed (e.g. occurrence of syncope or palpitations). |

|
Expert consensus |
MVP, mitral valve prolapse; SCD, sudden cardiac death.
ECG monitoring
Holter monitoring
Holter monitoring is a crucial tool to establish the diagnosis of AMVP even in the absence of palpitations. The yield of Holter monitoring depending on symptomatic vs. asymptomatic status remains to be defined. Frequent and sometimes polymorphic PVCs may be detected.46 Less frequently, complex VAs may be detected and when ‘severe’ (NSVT > 180 bpm or more severe VAs) are associated with subsequent excess mortality.14 Data regarding the prognostic significance of PVC is not as robust64, yet several studies demonstrated an increased risk of mortality14,96 and heart failure.97,142
In addition, Holter monitoring provides information on the total burden of ventricular ectopy, its morphology, coupling interval and may capture complex ectopy.143 The clinical importance of these markers remain to be established (Table 6).
Table 6.
Extended ECG monitoring
| Consensus statement on extended ECG monitoring | Symbol | Ref |
|---|---|---|
| A standard 24 h Holter monitoring is warranted in all patients with MVP. |

|
46,143 |
| Longer ECG recording (up to 7 days) may be beneficial in selected cases to allow more accurate quantification of PVC burden and/or correlation with symptoms. |

|
93 |
| MVP patients with complex ventricular ectopy (e.g. fast NSVTs) should be followed closely. |

|
Expert consensus |
| Due to the possibility of disease progression, periodic Holter may be advised as part of the routine follow-up of AMVPa patients. |

|
Expert consensus |
| Periodic Holter monitoring of PVC burden and periodic echocardiographic evaluation of LV function may be particularly helpful in patients with frequent PVC, even when asymptomatic and with normal LV function (to allow for a timely diagnosis of PVC induced cardiomyopathy). |

|
Expert Consensus144,145 |
| Longer ECG recording may be advised in patients with MVP and doubtful symptoms (e.g. recurrent pre-syncope or palpitations) in whom 24 h Holter monitoring was not revealing. |

|
Expert consensus |
| ILR may be advised in patients with MVP and unexplained syncope in whom non-invasive ECG monitoring was not revealing or inconclusive. |

|
102,146 |
| ILR may be advised in patients with AMVP, high risk featuresb and negative CMR. |

|
Expert consensus |
| ILR may be advised in patients with AMVP, at least 1 phenotypic risk featurec and positive LGE on CMRd. |

|
Expert consensus |
AMVP—The presence of MVP with or without MAD, frequent ventricular ectopy (≥5% of total beats), complex ectopy or sustained VAs in the absence of any other well-defined arrhythmic substrate (e.g. active ischaemia, ventricular scar due to another defined ethology, primary cardiomyopathy or channelopathy; ventricular scar due to other defined aetiology refers to ischaemic cardiomyopathy, dilated cardiomyopathy, post myocarditis, cardiac sarcoidosis etc.).
High risk features—sustained VT (haemodynamically tolerated), NSVT, unexplained syncope.
Phenotypic risk features—T-wave inversion in the inferior leads, repetitive documented polymorphic PVCs, mitral annular disjunction (MAD) phenotype, redundant MV leaflets, enlarged left atrium or ejection fraction ≤ 50%.
For this purpose, only LGE within the mitral apparatus (papillary muscles and peri-annular region) has a clear pathophysiological relevance. The significance of LGE in other regions remains unclear in this context.
AMVP, arrhythmic mitral valve prolapse; CMR, cardiac magnetic resonance; ECG, electrocardiogram; ILR, implantable loop recorder; LGE, late gadolinium enhancement; MAD, mitral annular disjunction; MVP, mitral valve prolapse; MV, mitral valve; NSVT, non-sustained ventricular tachycardia; PVC, premature ventricular contraction; VA, ventricular arrhythmia; VT, ventricular tachycardia.
Implantable loop recorder
The short duration of a Holter monitor favours implantable loop recorders (ILRs) as an option to improve arrhythmia detection and risk stratification in patients with AMVP.102,146,147 While there are no systematic studies on ILR utility in patients with MVP (only case reports),148,149 it appears to be an useful option to improve sensitivity in detecting AMVP. Thus, ILR use appears reasonable in patients with high risk features such as unexplained syncope with inconclusive Holter monitoring, or intermediate risk cases such as patients with multiple phenotypical risk features including positive LGE on CMR (Table 6).
Echocardiographic protocol
In addition to screening echocardiography, comprehensive Doppler-echocardiography following guideline-based standard imaging protocol is required.150
Mitral morphology should be analyzed and MVP phenotype noted (myxomatous MVP or fibroelastic deficiency with/without flail leaflet) as well as which leaflet(s) is involved.
Regarding the LV, accounting for the volume load of the prolapsing volume (e.g. the volume above the LV muscle but below the prolapsing mitral leaflets in the ventricularized portion of the left atrium) reconciles the disproportionate LV enlargement that is noted in some MVP patients.151 In the presence of MAD, the prolapsing volume should be measured between the mitral annulus and the prolapsing leaflets in end-systole.
The protocol should include:
Integrative grading of MR severity using specific, supportive and quantitative measures to classify degenerative MR from absent to severe according to guidelines.6,7
Leaflets length and thickness measurements, performed in the parasternal long axis view, in mid-diastole. Leaflet-redundancy can be assessed using M-mode over the mitral valve in the parasternal short-axis view and can be graded by evaluating excess valve tissue thickening, according to guidelines.25,152 The use of 3D and global longitudinal strain are of added value to better characterize the valve and the consequences at the level of the LV. Additionally, mechanical dispersion based on longitudinal strain in the apical 4C view has shown potential added value to predict the risk of VAs in MVP patients.
MAD length, measured in parasternal long-axis view at end-systole and defined as the distance between mitral-annulus and systolic bulge of the ventricular myocardium. A range of 5–10 mm has been measured in long axis views.44 MAD can be observed at different locations on the mitral annulus. However, MAD is associated with increased risk of VAs when observed at the posterior LV wall.
Additionally, a distinctive spike during mid-systole to late-systole of the lateral mitral annulus using Doppler has been associated with MAD153 (Table 7).
Table 7.
Detailed echocardiography
| Consensus statement on detailed echocardiography | Symbol | Ref |
|---|---|---|
| In patients with suspected AMVP, comprehensive echocardiography assessment should include evaluation of leaflet length and thickness measurement, annular dimension, MAD characterization, degenerative MR grading and possibly advanced assessmenta of LV function. |

|
14 |
| Due to the possibility of disease progression, periodic complete transthoracic echocardiography may be advised as part of the routine follow up of AMVPb patients. |

|
Expert consensus |
| In the work up of patients with frequent PVCs, syncope or aborted cardiac arrest with no other obvious aetiology, the comprehensive echocardiographic study should include the careful assessment of the mitral valve and the mitral annulus to diagnose AMVP. |

|
Expert consensus |
Advanced assessment of LV function may be based on the combination of Simpson’s bi-plane methods, assessed by 3D echo and Global Longitudinal Strain by speckle tracking imaging.
AMVP—The presence of MVP with or without MAD, frequent ventricular ectopy (≥5% of total beats), complex ectopy or sustained VAs in the absence of any other well-defined arrhythmic substrate (e.g. active ischaemia, ventricular scar due to another defined ethology, primary cardiomyopathy or channelopathy).
AMVP, arrhythmic mitral valve prolapse; MAD, mitral annular disjunction; MR, mitral regurgitation; MVP, mitral valve prolapse; LV, left ventricle; VA, ventricular arrhythmia.
CMR protocol
CMR can refine the echocardiographic findings and provide additional unique information on myocardial structure, both important to improve risk-stratification of AMVP patients.110,113 Particularly, CMR should be performed in all patients who survived a SCD or experienced sustained VAs before implanting an ICD.154 CMR has been shown to increase the rate of aetiological diagnosis in patients with resuscitated SCD or sustained VT.155 Conversely, ruling out alternative aetiologies will increase the certainty in the diagnosis of AMVP. Due to associated risk of SCD, we also suggest performing a CMR in patients with a history of unexplained syncope or documented NSVT.14 It may also be useful in patients with AMVP and at least one phenotypical risk feature.
Furthermore, performing CMR in patients in whom echocardiography does not provide accurate assessment of left and right ventricular function, of structural changes or of mitral characteristics may be of value.154 CMR allows assessment of the presence and severity/extent of MAD or LV dilatation and dysfunction. Most importantly, CMR identifies myocardial fibrosis/scar, considered strongly associated with severe VAs.
CMR should be performed in centres with sufficient expertise and on a 1.5 or 3 T scanner. The CMR protocol should be comprehensive, including the following morphofunctional parameters according to definitions previously mentioned: 2–8,13,45–47,63,64,113,156
Assessment of LV volumes and function (ejection fraction and regional wall motion abnormalities); measurement of LV mass and end-diastolic thickness, including the ratio of basal-to-midventricular wall thickness.
Assessment of left atrium and right ventricular size and function.
Identification of MAD (presence or absence) and its quantification, as longitudinal length at least from the long axis view, and possibly as circumferential extent expressed in degrees.
Identification of the systolic curling (presence or absence) and possibly its quantification.
Measurement of mitral valve annulus diameter (at end-systole and end-diastole in both anteroposterior and inter-commissural aspects), leaflet diastolic thickness, leaflet length and prolapsed distance.
Assessment of myocardial fibrosis/scar with description of the localization (particularly at the level of the papillary muscles or adjacent myocardium, and next to the mitral valve annulus) and its quantification.
In cases where this is uncertainty about the severity of mitral regurgitation on echocardiography, the regurgitant volume and fraction should be calculated on CMR. The former is obtained from the difference between the total LV volume (using planimetry of short-axis cine images) and the forward stroke volume across the aortic valve (using phase-contrast velocity mapping).
In centres with the expertise, assessment of interstitial/reactive fibrosis by T1 mapping (Table 8).
Table 8.
Evaluation by CMR
| Consensus statement on CMR | Symbol | Ref |
|---|---|---|
| CMR should be performed in all AMVP patients who survived a cardiac arrest or experienced sustained VA, beforea implanting an ICD for secondary prevention. |

|
155,157 |
| CMR should be performed in all patients when echocardiography does not provide accurate assessment of LV and RV function and/or evaluation of structural changes. |

|
Expert consensus |
| CMR should be performed in all MVP patients with a history of unexplained syncope and/or NSVT. |

|
Expert consensus |
| CMR should include assessment of LV size and function, assessment of MR severity, leaflet length/thickness measurement, MAD characterization and curling, and LGE assessment. |

|
Expert consensus |
| CMR may be useful in patients with AMVP and at least 1 phenotypic risk featureb. |

|
CMR should not unduly delay the implantation of a defibrillator.
Phenotypic risk features—palpitations, T-wave inversion in the inferior leads, repetitive documented polymorphic PVCs, MAD phenotype, redundant MV leaflets, enlarged left atrium or ejection fraction ≤ 50%.
AMVP, arrhythmic mitral valve prolapse; CMR, cardiac magnetic resonance; ICD, implantable cardioverter defibrillator; LGE, late gadolinium enhancement; LV, left ventricular; MAD, mitral annular disjunction; MR, mitral regurgitation; RV, right ventricle; VA, ventricular arrhythmia.
Electrophysiological study
As there are no robust data specific to this population, we consider the standard programmed stimulation protocol that includes up to three extra-stimuli protocols from at least two sites (RV apex and RVOT) down to ERP or 200 ms. Induction of polymorphic VT and VF by delivery of three extra-stimuli is a nonspecific finding and does not predict future risk of SCD.158–160 However, induction of monomorphic VT may be more specific and linked to MVP if localized to the mitral apparatus. A recent systematic review reported on the outcome of EPS with programmed ventricular stimulation (PVS) in 22 patients with MVP and documented SCD.57 The findings were disappointing with sustained monomorphic VT (5%), NSVT (23%), VF (18%), and non-inducibility of VAs (55%). The fact that sustained monomorphic VT is only rarely induced may imply, that in most cases, the arrhythmia leading to SCD is non-reentrant. Thus, the role of PVS in identifying high-risk MVP is limited. This scarcity of data does not provide conclusive evidence regarding positive or negative predictive value of the test. While the committee does not endorse the use of EPS with PVS, if EPS is used, the induction of sustained monomorphic VT should be viewed as more specific than polymorphic VT or VF.
Exercise stress testing
Exercise stress testing may be an important tool to assess suspected exercise-induced VAs.154 Although there are no data on the significance of these arrhythmias in patients with AMVP, several reports have established exercise-induced VAs as a predictor of cardiovascular mortality even in asymptomatic patients.161,162 Furthermore, exercise stress testing may be useful in assessing exercise tolerance, particularly in AMVP patients who want to engage in sports.163 The value of exercise stress testing for the identification of obstructive coronary artery disease is limited compared to other modalities164 and may be even less reliable in the presence of repolarization abnormalities at baseline. Nevertheless it may be reasonably included, alongside cardiovascular risk factors, in the preliminary assessment of the likelihood of coronary artery disease in patients with AMVP (Table 9).
Table 9.
Exercise stress testing
| Consensus statement on exercise stress testing | Symbol | Ref |
|---|---|---|
| In patients with AMVP, exercise stress testing should be used to assess for adrenergic-dependent rhythm disturbances. |

|
Expert consensus |
| The use of exercise stress testing to assess exercise tolerance in AMVP patients who want to engage in sports is reasonable. |

|
163 |
| Exercise stress testing (plus echocardiography), alongside other risk factors may be used to assess the clinical likelihood of obstructive coronary artery disease. |

|
Expert consensus |
AMVP, arrhythmic mitral valve prolapse.
Management
Medical therapy
Current literature regarding medical management of AMVP patients is sparse. However, some general concepts may be inferred from associated better studied case scenarios. The medical management should be tailored to the specific presentation and aimed at symptomatic relief and improved survival (if and when evidence supporting this effect will be available).
Multiple PVCs
Presence and burden of ventricular ectopy is associated with increased mortality96 even in the absence of structural heart disease.97 However there is no evidence that prophylactic treatment aimed at suppressing PVCs in asymptomatic patients is beneficial.
While impact on long term outcomes remains unclear,144 suspected PVC-induced cardiomyopathy warrants treatment aiming at reducing PVC frequency. Treatment is also advised in symptomatic patients regardless of LV function.145 Beta blockers and verapamil improve symptoms and result in modest reduction of PVC burden.165,166 Flecainide, Propafenone and Amiodarone yield more potent PVC burden reduction with frequent improvement in LV function.167–169 The potential benefit from amiodarone must be balanced against risk of significant adverse effects associated with long term treatment. Sotalol can reduce the PVC burden effectively but failed to improve LV function.170
PVC-induced polymorphic VT/VF
Cases of patients with PVC-induced VF have been described,128,171,172 with triggers arising from Purkinje fibres within papillary muscles or the fascicles. A few cases have been described as drug refractory,171 including in patients with MVP.37,173 Quinidine may have an important role in the treatment of short-coupled PVCs triggering polymorphic VTs due its effect on the Purkinje system although this was not tested prospectively nor specifically in patients with MVP.174
Prevention of SCD
Despite the link between PVC and mortality, there is no evidence that medical therapy alone may lower the risk of SCD.168 Therefore, medical treatment should focus on the improvement of symptoms. The need for an ICD should be assessed in all cases (Table 10).
Table 10.
Medical therapy
| Consensus statement on Medical Therapy | Symbol | Ref |
|---|---|---|
| AMVP with symptomatic severe MR not eligible for surgery should be treated with optimal HF medication. |

|
133 |
| Suspected PVC induced cardiomyopathy in the presence of MVP should be treated. Reasonable options include beta blockers, sotalol, and amiodarone. |

|
133,165,166,168,170 |
AMVP, arrhythmic mitral valve prolapse; HF, heart failure; MVP, mitral valve prolapse; PVC, premature ventricular contraction.
Implantable cardioverter defibrillator
Established primary and secondary prevention ICD indications also apply to patients with MVP.
Primary prevention ICD is indicated by guidelines in symptomatic heart failure with EF≤ 35% despite 3 months of optimal medical therapy.20 It is uncertain whether patients with MVP/degenerative MR fulfill this type of indication as EF in that range is rarely observed in this context.106
Secondary prevention ICD is indicated by guidelines in patients with MVP and documented history of sudden cardiac arrest with ventricular fibrillation (VF) or VT without reversible causes.132
Primary prevention implantation of ICD is more complex as no randomized trial has demonstrated benefit in any MVP subset. In the absence of conclusive data, the present committee concludes that ICD should be a strong consideration in patients presenting with unexplained syncope and high-risk VAs detected by ECG, Holter or ILR and possibly by exercise testing.
In the absence of any published data specific to AMVP, the choice of type of defibrillator (subcutaneous vs. trans-venous) should follow the same considerations used in other arrhythmic aetiologies.132 A careful evaluation of the presenting arrhythmia, likelihood of response to anti-tachycardia pacing or a need for bradycardia pacing are warranted. The potential risk of inappropriate shocks in the specific patients should be balanced against the risk of endovascular infection and lead failure (Table 11).
Table 11.
SCD prevention
| Consensus statement on SCD prevention | Symbol | Ref |
|---|---|---|
| Secondary prevention | ||
| Patients with AMVP and a documented history of VF or hemodynamically not tolerated VT, in the absence of reversible causes should receive an ICD. |

|
133,154 |
| Primary prevention | ||
| MVP with LVEF <35% and symptomatic HF despite ≥3 months of OMT should receive an ICD. |

|
133 |
| In patients with AMVP, history of unexplained syncope and sustained ventricular tachycardia, likely arising from the mitral apparatus, the implantation of a defibrillator is reasonable. |

|
Expert Consensus |
| In patients with AMVP, history of unexplained syncope and NSVT, likely arising from the mitral apparatus, an implantation of a defibrillator may be reasonable. |

|
Expert Consensus |
| In patients with AMVP, 1 high risk featurea and 2 or more phenotypic risk featuresb, the option of an ICD maybe be reasonable. |

|
Expert Consensus |
High risk features—sustained VT (haemodynamically tolerated), NSVT, unexplained syncope.
Phenotypic risk features—T-wave inversion in the inferior leads, repetitive documented polymorphic PVCs, MAD phenotype, redundant MV leaflets, enlarged left atrium or ejection fraction ≤ 50%, LGE. For this purpose, only LGE within the mitral apparatus (papillary muscles and peri-annular region) has a clear pathophysiological relevance. The significance of LGE in other regions remains unclear in this context.
AMVP, arrhythmic mitral valve prolapse; HF, heart failure; ICD, implantable cardioverter defibrillator; LGE, late gadolinium enhancement; MAD, mitral annular disjunction; LVEF, left ventricular ejection fraction; NSVT, non-sustained ventricular tachycardia; OMT, optimal medical therapy; SCD, sudden cardiac death; VF, ventricular fibrillation; VT, ventricular tachycardia.
PVC/VT ablation
VAs in MVP most commonly manifest as focal PVCs but may also involve PVC-triggered VT/VF or scar-related reentrant VT. All of these can be effectively treated by catheter ablation.95,128,173,175–178 Indication for ablation of VAs is independent of MVP phenotype and may include PVC ablation in patients with symptoms refractory to therapy,154,179 to drug treatment, when drug treatment is not tolerated, or when ablation is preferred over long-term drug therapy by patients.
In addition, ablation of PVC/VT may be advised in patients with PVC-induced cardiomyopathy, PVC-induced VF or in patients with frequent ICD therapies.154,179
PVCs in MVP are usually related to the papillary muscles or the Purkinje system,95,171 but ablation of foci within the papillary muscles is challenging, often requiring intracardiac echocardiography, contact force sensing technology and cryoablation to improve catheter contact and effective energy delivery.176,177 Ablation at the papillary muscle should be performed with caution as this may rarely lead to LV dysfunction and may impede mitral valve function.180 Scar-related monomorphic reentrant VTs are uncommon and typically located in the inferobasal or inferolateral left ventricle.95,126
Long-term success rates for PVC ablation in MVP range between 60 and 84%.95,128,171,175,176 The outcome of ablation does not appear to be adversely impacted by presence of MVP.173 Inducibility of sustained VAs and multifocal ectopy may indicate higher risk of VA recurrence during follow-up and may relate to a more diffuse myopathic process (higher degree of LGE on CMR).95
Systolic function and MR severity may improve after effective PVC elimination.128 Ablation of VAs in MVP patients should only be performed in centres with appropriate experience in ablation of left-sided VAs and in interventional and surgical treatment of mitral valve disease (Table 12).
Table 12.
Catheter ablation of VAs in AMVP
| Consensus statement on catheter ablation of VAs | Symbol | References |
|---|---|---|
| Ablation of PVCs in patients with frequent PVCs who are symptomatic or have decreased LV function is advised. |

|
95,128,154,173,175–179 |
| Ablation of VA in MVP patients should be performed in experienced centres with expertise in VA ablation and interventional and surgical treatment of MV regurgitation. |

|
Expert consensus |
| Ablation of papillary muscle PVCs/VA is challenging and use of intracardiac echocardiography, contact force sensing catheters or cryoablation may be helpful to improve catheter contact and effective manipulation. |

|
176,177 |
| Ablation of PVCs is reasonable if triggering VF, particularly if not controlled by medications. |

|
128,129 |
| Ablation of sustained monomorphic VT despite antiarrhythmic treatment or if antiarrhythmic treatment is not desired, or contraindicated should be performed in MVP patients with recurrent ICD therapies. |

|
154,179 |
AMVP, arrhythmic mitral valve prolapse; ICD, implantable cardioverter defibrillator; MVP, mitral valve prolapse; MV, mitral valve; PVC, premature ventricular contraction; VA, ventricular arrhythmia; VF, ventricular fibrillation; VT, ventricular tachycardia.
Role of mitral valve surgery
Surgical intervention for AMVP remains controversial and limited to small case series and reports.181–188 While surgical correction of severe MR tends to reduce VA burden in patients with MVP and degenerative MR,14,189 results are inconsistent.171,190 The respective role of various surgical procedures (repair/replacement, valve tissue resection etc.) remains undefined. Recently surgical cryoablation of VAs during mitral valve surgery has been reported,191,192 but the impact on medium to long-term outcomes remains to be defined. Whether successful mitral surgery for the treatment of severe MR as stand-alone treatment is sufficient to prevent VAs in patients with AMVP and high risk VAs remains uncertain.The need for an ICD may remain. Surgical indications in patients with MR in the moderate range are yet to be determined. Early surgery in patients with MVP and severe MR without VAs restores life-expectancy, by eliminating excess mortality. Thus, mitral surgery, through suppressing the progression of valve prolapse and its consequences, may have a role in preventing SCD, giving credence to the mechanical theory for VAs in MVP.
Well-designed clinical trials are warranted to better define the role of surgery in the treatment of AMVP (Table 13).
Table 13.
MV surgery in AMVP
| Consensus statement on mitral surgery | Symbol | Ref |
|---|---|---|
| Mitral valve surgery may reduce the burden of malignant VAsa in MVP patients and severe MR. |

|
Expert consensus |
| Surgical cryoablation during mitral valve surgery with history of complex VA may be reasonable. |

|
Expert consensus |
Malignant VA—sustained VA, highly symptomatic NSVT.
MR, mitral regurgitation; MVP, mitral valve prolapse; MV, mitral valve; NSVT, non-sustained ventricular tachycardia; VA, ventricular arrhythmia.
Evidence gaps
The association between MVP and SCD has long been debated, suggested by case reports but questioned based on the generally good prognosis of MVP without significant degenerative MR or LV dysfunction. Only recently has the entity of AMVP been better described and its presence recognized based on a prominent phenotype and the increased incidence of VAs detected in patients with the AMVP phenotype. However, while we are starting to modify our clinical algorithms for detection and treatment of AMVP based on current knowledge, much more needs to be learned:
The incidences and mechanisms of the various VAs in patients with MVP in general and in various subsets remains ill defined. The endeavour of defining incidence rates of VAs and SCD will require large samples with systematic rhythm monitoring using both Holter and ILR detection of VA and long-term follow-up. While prospective studies may offer definite conclusions, a more pragmatic approach is needed in order to produce timely results. Therefore, it is crucial that a consortia sharing retrospective data be formed in order to provide initial estimates that may guide management in the current patient population.
Determinants of VAs and SCD are only partially defined. This warrants the formation of large well designed study cohorts that will collect and assess clinical characteristics, imaging data and other biological features such as proteomics, genomics and metabolomics as potential predictors. Here again both prospective cohorts and retrospective consortia are indispensable to resolve this conundrum.
Progression of VAs in parallel with progression of MVP, degenerative MR and their consequences is unknown and will require cohorts with repeat rhythm monitoring.
Intense arrhythmia detection may potentially lead to excess, unnecessary and even harmful interventions. This risk should not be underestimated, mandating a careful design of clinical trials involving patients with MVP.
Clinical outcome associated with VAs is based on relatively limited cohorts and the refined prognostic implications of various VAs should be assessed in large cohorts with long-term follow-up to redefine high, medium and low risk subsets.
Benefits of therapeutic interventions in MVP with VAs can only be affirmed in clinical trials. These may clarify the potential benefit of ICDs in patients with high-risk VAs, of mitral surgery in MVP with degenerative MR and VAs, of surgical cryoablation vs. transcatheter ablation and of antiarrhythmic drugs vs. ablation for frequent PVCs, among the many therapeutic considerations.
Therefore, one of the main goals of this document is to highlight the important and more urgent questions that require our collective attention. By suggesting clearer diagnostic criteria, we aim to enable standardized approaches to patient inclusion in research protocols and pave the way for multicentre international collaborations that are required to tackle these clinical conundrums.
Supplementary material
Supplementary material is available at Europace online.
Supplementary Material
Acknowledgements
The authors thank the EHRA Scientific Document Committee: Dr. Nikolaos Dagres, Prof. Thomas Deneke, Prof. Arthur Wilde, Prof. Frank R. Heinzel, Prof. Christian Meyer, Prof. Lucas Boersma, Prof. Radoslaw Lenarczyk, Prof. Luigi di Biase, Dr. Elena Arbelo, Dr. Avi Sabbag, Prof. Pierre Jais, Prof. Milos Taborsky, Asso. Prof. Markus Stühlinger.
Contributor Information
Avi Sabbag, The Davidai Center for Rhythm Disturbances and Pacing, Chaim Sheba Medical Center, Tel Hashomer 52621, Israel.
Benjamin Essayagh, Department of Cardiovascular Medicine, Simone Veil Hospital, Cannes 06400, France; Department of Cardiovascular Medicine, Mayo Clinic, Rochester 55905, Minnesota.
Juan David Ramírez Barrera, Cardiologist Electrophysiologist, Cardiovid Clinic, Medellin, Colombia 050034, South America.
Cristina Basso, Dipartimento di Scienze Cardio-Toraco-Vascolari e Sanità Pubblica, Università degli Studi di Padova, Padova 35128, Italy.
Ana Berni, Cardiology and Cardiac Electrophysiology, EP Lab. Hospital Angeles Pedregal. Mexico City 10700, Board member, Mexican Society of Cardiology.
Bernard Cosyns, Cardiology Department, Centrum voor hart en vaatziekten, Universitair Ziekenhuis Brussel, Free University of Brussels, Brussels 1090, Belgium.
Jean-Claude Deharo, Department of Cardiology, L’hôpital de la Timone, Marseille, 13005, France.
Thomas Deneke, Clinic for Interventional Electrophysiology, Heart Center RHÖN-KLINIKUM Campus Bad Neustadt, 97616, Germany.
Luigi Di Biase, Albert Einstein College of Medicine at Montefiore Hospital, New York, NY 10467, USA.
Maurice Enriquez-Sarano, Minneapolis Heart Institute, Minneapolis, MN 55407, USA.
Erwan Donal, Service de Cardiologie, CCP-CHU Pontchaillou, Rennes 35033, France.
Katsuhiko Imai, Department of Cardiovascular Surgery, National Hospital Organization Kure Medical Center and Chugoku Cancer Center, Hiroshima 737-0023, Japan.
Han S Lim, Department of Cardiology, Austin and Northern Health, University of Melbourne, Melbourne 3010, Australia.
Nina Ajmone Marsan, Leiden University Medical Center, Cardiology, Leiden 2333, Netherlands.
Mohit K Turagam, Icahn School of Medicine at Mount Sinai, New York, NY 10029, USA.
Petr Peichl, Department of Cardiology, Institute for Clinical and Experimental Medicine (IKEM), Prague 73117, Czech Republic.
Sunny S Po, Heart Rhythm Institute and Section of Cardiovascular Diseases, University of Oklahoma Health Sciences Center, Oklahoma City, OK 0372, USA.
Kristina Hermann Haugaa, ProCardio Center for Innovation, Department of Cardiology, Oslo University Hospital, Rikshospitalet, Oslo, Norway.
Dipen Shah, Cantonal Hospital, Cardiology Department, CH-1211 Geneva, Switzerland.
Marta de Riva Silva, Department of Cardiology, Leiden University Medical Center, Leiden 2333, The Netherlands.
Philippe Bertrand, Ziekenhuis Oost-Limburg, Hasselt University, Genk, Hasselt 3600, Belgium.
Magdi Saba, Consultant and Reader in Cardiac Electrophysiology, Director, Advanced Ventricular Arrhythmia Training and Research Program, St. George’s Hospital NHS Foundation Trust, St. George’s, University of London, SW17 0QT, UK.
Marc Dweck, Centre for cardiovascular science, University of Edinburgh, EH16 4TJ, UK.
Santiago Nava Townsend, Instituto Nacional De Cardiologia Ich, Electrophysiology Department, Mexico Df 14080, Mexico.
Tachapong Ngarmukos, Faculty of Medicine Ramathibodi Hospital, Mahidol University, Bangkok 73170, Thailand.
Guilherme Fenelon, Coordenador - Centro de Arritmia, Hospital Israelita Albert Einstein, São Paulo - SP, 05652-900, Brazil.
Pasquale Santangeli, Hospital of the University of Pennsylvania, Philadelphia, PA 19104, USA.
Leyla Elif Sade, University of Pittsburgh, UPMC, Heart and Vascular Institute, ittsburgh, PA 15219, USA; C.H.U. du Sart-Tilman, Universite de Liege, Liege 4000, Belgium.
Domenico Corrado, Full Professor of Cardiovascular Medicine, Director, Inherited Arrhythmogenic Cardiomyopathies and Sports Cardiology Unit, Dept. of Cardiac, Thoracic and Vascular Sciences, University of Padua Medical School, Padova 35122, Italy.
Pier Lambiase, UCL & Barts Heart Centre, Co-Director of Cardiovascular Research Barts NHS Trust, Inherited Arrhythmia Clinical Lead, UCL MRC DTP Theme Lead, BHRS Committee Research Lead, Institute of Cardiovascular Science, UCL, Department of Cardiology, Barts Heart Centre E1 1BB, UK.
Prashanthan Sanders, Centre for Heart Rhythm Disorders, University of Adelaide, South Australia 5000, Australia.
Etienne Delacrétaz, Clinique Cecil Hirslanden Lausanne & University Hospital Fribourg, Cardiology 1003, Switzerland.
Arshad Jahangir, University of Wisconsin School of Medicine and Public Health, Milwaukee, MI 53705, USA.
Elizabeth S Kaufman, Clinical Electrophysiologist, MetroHealth Medical Center, Professor, Case Western Reserve University 44106, USA.
Daljeet Kaur Saggu, Consultant Cardiologist and Electrophysiologist, AIG HOSPITAL, Hyderabad 500032, India.
Luc Pierard, C.H.U. du Sart-Tilman, Universite de Liege, Liege 4000, Belgium.
Victoria Delgado, Heart Institute, Hospital University Germans Trias i Pujol, Badalona 08916, Spain.
Patrizio Lancellotti, C.H.U. du Sart-Tilman, Universite de Liege, Liege 4000, Belgium.
References
- 1. Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez-Sarano M. Burden of valvular heart diseases: a population-based study. Lancet 2006;368:1005–11. [DOI] [PubMed] [Google Scholar]
- 2. Freed LA, Levy D, Levine RA, Larson MG, Evans JC, Fuller DL, et al. Prevalence and clinical outcome of mitral-valve prolapse. N Engl J Med 1999;341:1–7. [DOI] [PubMed] [Google Scholar]
- 3. Flack JM, Kvasnicka JH, Gardin JM, Gidding SS, Manolio TA, Jacobs DR Jr. Anthropometric and physiologic correlates of mitral valve prolapse in a biethnic cohort of young adults: the CARDIA study. Am Heart J 1999;138:486–92. [DOI] [PubMed] [Google Scholar]
- 4. Theal M, Sleik K, Anand S, Yi Q, Yusuf S, Lonn E. Prevalence of mitral valve prolapse in ethnic groups. Can J Cardiol 2004;20:511–5. [PubMed] [Google Scholar]
- 5. Levine RA, Triulzi MO, Harrigan P, Weyman AE. The relationship of mitral annular shape to the diagnosis of mitral valve prolapse. Circulation 1987;75:756–67. [DOI] [PubMed] [Google Scholar]
- 6. Baumgartner H, Falk V, Bax JJ, De Bonis M, Hamm C, Holm PJ, et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J 2017;38:2739–91. [DOI] [PubMed] [Google Scholar]
- 7. Otto CM, Nishimura RA, Bonow RO, Carabello BA, Erwin JP III, Gentile F, et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. Circulation 2020:CIR0000000000000923. [DOI] [PubMed] [Google Scholar]
- 8. Basso C, Iliceto S, Thiene G, Perazzolo Marra M. Mitral valve prolapse, ventricular arrhythmias, and sudden death. Circulation 2019;140:952–64. [DOI] [PubMed] [Google Scholar]
- 9. Duren D, Becker A, Dunning A. Long-term follow-up of idiopathic mitral valve prolapse in 300 patients: a prospective study. J Am Coll Cardiol 1988;11:42–7. [DOI] [PubMed] [Google Scholar]
- 10. Delling FN, Aung S, Vittinghoff E, Dave S, Lim LJ, Olgin JE, et al. Antemortem and post-mortem characteristics of lethal mitral valve prolapse among all countywide sudden deaths. JACC Clin Electrophysiol 2021;7:1025–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Basso C, Perazzolo Marra M, Rizzo S, De Lazzari M, Giorgi B, Cipriani A, et al. Arrhythmic mitral valve prolapse and sudden cardiac death. Circulation 2015;132:556–66. [DOI] [PubMed] [Google Scholar]
- 12. Bennett S, Tafuro J, Duckett S, Appaji A, Khan JN, Heatlie G, et al. Definition, prevalence, and clinical significance of mitral annular disjunction in different patient cohorts: a systematic review. Echocardiography 2022;39:514–23. [DOI] [PubMed] [Google Scholar]
- 13. Marra M P, Basso C, De Lazzari M, Rizzo S, Cipriani A, Giorgi B, et al. Morphofunctional abnormalities of mitral annulus and arrhythmic mitral valve prolapse. Circ Cardiovasc Imaging 2016;9:e005030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Essayagh B, Sabbag A, Antoine C, Benfari G, Yang LT, Maalouf J, et al. Presentation and outcome of arrhythmic mitral valve prolapse. J Am Coll Cardiol 2020;76:637–49. [DOI] [PubMed] [Google Scholar]
- 15. Essayagh B, Sabbag A, Antoine C, Benfari G, Batista R, Yang LT, et al. The mitral annular disjunction of mitral valve prolapse: presentation and outcome. JACC Cardiovasc Imaging 2021;14:2073–87. [DOI] [PubMed] [Google Scholar]
- 16. Muthukumar L, Jahangir A, Jan MF, Perez Moreno AC, Khandheria BK, Tajik AJ. Association between malignant mitral valve prolapse and sudden cardiac death: a review. JAMA Cardiol 2020;5:1053–61. [DOI] [PubMed] [Google Scholar]
- 17. Levine R, Stathogiannis E, Newell J, Harrigan P, Weyman A. Reconsideration of echocardiographic standards for mitral valve prolapse: lack of association between leaflet displacement isolated to the apical four chamber view and independent echocardiographic evidence of abnormality. J Am Coll Cardiol 1988;11:1010–19. [DOI] [PubMed] [Google Scholar]
- 18. Fulton BL, Liang JJ, Enriquez A, Garcia FC, Supple GE, Riley MP, et al. Imaging characteristics of papillary muscle site of origin of ventricular arrhythmias in patients with mitral valve prolapse. J Cardiovasc Electrophysiol 2018;29:146–53. [DOI] [PubMed] [Google Scholar]
- 19. Adams DH, Rosenhek R, Falk V. Degenerative mitral valve regurgitation: best practice revolution. Eur Heart J 2010;31:1958–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vahanian A, Beyersdorf F, Praz F, Milojevic M, Baldus S, Bauersachs J, et al. 2021 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J 2021;43:561–632. [DOI] [PubMed] [Google Scholar]
- 21. Hayek E, Gring CN, Griffin BP. Mitral valve prolapse. Lancet 2005;365:507–18. [DOI] [PubMed] [Google Scholar]
- 22. Caselli S, Mango F, Clark J, Pandian NG, Corrado D, Autore C, et al. Prevalence and clinical outcome of athletes with mitral valve prolapse. Circulation 2018;137:2080–2. [DOI] [PubMed] [Google Scholar]
- 23. Delling FN, Rong J, Larson MG, Lehman B, Osypiuk E, Stantchev P, et al. Familial clustering of mitral valve prolapse in the community. Circulation 2015;131:263–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Strahan NV, Murphy EA, Fortuin NJ, Come PC, Humphries JO. Inheritance of the mitral valve prolapse syndrome. Discussion of a three-dimensional penetrance model. Am J Med 1983;74:967–72. [DOI] [PubMed] [Google Scholar]
- 25. Nishimura R, McGoon M, Shub C, Miller F, Ilstrup D, Tajik A. Echocardiographically documented mitral-valve prolapse. Long-term follow-up of 237 patients. N Engl J Med 1985;313:1305–9. [DOI] [PubMed] [Google Scholar]
- 26. Boudoulas KD, Pitsis AA, Mazzaferri EL, Gumina RJ, Triposkiadis F, Boudoulas H. Floppy mitral valve/mitral valve prolapse: a complex entity with multiple genotypes and phenotypes. Prog Cardiovasc Dis 2020;63:308–26. [DOI] [PubMed] [Google Scholar]
- 27. Le Tourneau T, Le Scouarnec S, Cueff C, Bernstein D, Aalberts JJJ, Lecointe S, et al. New insights into mitral valve dystrophy: a Filamin-A genotype-phenotype and outcome study. Eur Heart J 2018;39:1269–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yu M, Georges A, Tucker NR, Kyryachenko S, Toomer K, Schott JJ, et al. Genome-wide association study-driven gene-set analyses, genetic, and functional follow-up suggest glis1 as a susceptibility gene for mitral valve prolapse. Circ Genom Precis Med 2019;12:e002497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dina C, Bouatia-Naji N, Tucker N, Delling FN, Toomer K, Durst R, et al. Genetic association analyses highlight biological pathways underlying mitral valve prolapse. Nat Genet 2015;47:1206–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Avierinos JF, Gersh BJ, Melton LJ, III, Bailey KR, Shub C, Nishimura RA, et al. Natural history of asymptomatic mitral valve prolapse in the community. Circulation 2002; 106: 1355–61. [DOI] [PubMed] [Google Scholar]
- 31. Freed LA, Benjamin EJ, Levy D, Larson MG, Evans JC, Fuller DL, et al. Mitral valve prolapse in the general population: the benign nature of echocardiographic features in the Framingham Heart Study. J Am Coll Cardiol 2002;40:1298–04. [DOI] [PubMed] [Google Scholar]
- 32. Corrado D, Basso C, Nava A, Rossi L, Thiene G. Sudden death in young people with apparently isolated mitral valve prolapse [comment] [see comments]. G Ital Cardiol 1997;27:1097–05. [PubMed] [Google Scholar]
- 33. Kligfield P, Levy D, Devereux RB, Savage DD. Arrhythmias and sudden death in mitral valve prolapse. Am Heart J 1987; 113: 1298. [DOI] [PubMed] [Google Scholar]
- 34. Grigioni F, Enriquez-Sarano M, Ling L, Bailey K, Seward J, Tajik A, et al. Sudden death in mitral regurgitation due to flail leaflet. J Am Coll Cardiol 1999;34:2078–85. [DOI] [PubMed] [Google Scholar]
- 35. Farb A, Tang AL, Atkinson JB, McCarthy WF, Virmani R. Comparison of cardiac findings in patients with mitral valve prolapse who die suddenly to those who have congestive heart failure from mitral regurgitation and to those with non-fatal cardiac conditions. Am J Cardiol 1992;70:234–9. [DOI] [PubMed] [Google Scholar]
- 36. Narayanan K, Uy-Evanado A, Teodorescu C, Reinier K, Nichols GA, Gunson K, et al. Mitral valve prolapse and sudden cardiac arrest in the community. Heart Rhythm 2016;13:498–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hourdain J, Clavel MA, Deharo JC, Asirvatham S, Avierinos JF, Habib G, et al. Common phenotype in patients with mitral valve prolapse who experienced sudden cardiac death. Circulation 2018;138:1067–69. [DOI] [PubMed] [Google Scholar]
- 38. Nalliah CJ, Mahajan R, Elliott AD, Haqqani H, Lau DH, Vohra JK, et al. Mitral valve prolapse and sudden cardiac death: a systematic review and meta-analysis. Heart 2019;105:144–51. [DOI] [PubMed] [Google Scholar]
- 39. Marra M P, Basso C. Mechanical dispersion and arrhythmic mitral valve prolapse: substrate and trigger in electrical instability. Heart 2019;105:1053–54. [DOI] [PubMed] [Google Scholar]
- 40. Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS, Callaway CW, et al. Heart disease and stroke statistics-2021 update: a report from the American Heart Association. Circulation 2021;143:e254–743. [DOI] [PubMed] [Google Scholar]
- 41. Faletra FF, Leo LA, Paiocchi VL, Caretta A, Viani GM, Schlossbauer SA, et al. Anatomy of mitral annulus insights from non-invasive imaging techniques. Eur Heart J Cardiovasc Imaging 2019;20:843–57. [DOI] [PubMed] [Google Scholar]
- 42. Konda T, Tani T, Suganuma N, Nakamura H, Sumida T, Fujii Y, et al. The analysis of mitral annular disjunction detected by echocardiography and comparison with previously reported pathological data. J Echocardiogr 2017;15:176–85. [DOI] [PubMed] [Google Scholar]
- 43. Eriksson MJ, Bitkover CY, Omran AS, David TE, Ivanov J, Ali MJ, et al. Mitral annular disjunction in advanced myxomatous mitral valve disease: echocardiographic detection and surgical correction. J Am Soc Echocardiogr 2005;18:1014–22. [DOI] [PubMed] [Google Scholar]
- 44. Carmo P, Andrade MJ, Aguiar C, Rodrigues R, Gouveia R, Silva JA. Mitral annular disjunction in myxomatous mitral valve disease: a relevant abnormality recognizable by transthoracic echocardiography. Cardiovasc Ultrasound 2010;8:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mantegazza V, Volpato V, Gripari P, Ghulam Ali S, Fusini L, Italiano G, et al. Multimodality imaging assessment of mitral annular disjunction in mitral valve prolapse. Heart 2020; ;107:25–32. . [DOI] [PubMed] [Google Scholar]
- 46. Dejgaard LA, Skjolsvik ET, Lie OH, Ribe M, Stokke MK, Hegbom F, et al. The mitral annulus disjunction arrhythmic syndrome. J Am Coll Cardiol 2018;72:1600–9. [DOI] [PubMed] [Google Scholar]
- 47. Essayagh B, Iacuzio L, Civaia F, Avierinos JF, Tribouilloy C, Levy F. Usefulness of 3-tesla cardiac magnetic resonance to detect mitral annular disjunction in patients with mitral valve prolapse. Am J Cardiol 2019;124:1725–30. [DOI] [PubMed] [Google Scholar]
- 48. Putnam AJ, Kebed K, Mor-Avi V, Rashedi N, Sun D, Patel B, et al. Prevalence of mitral annular disjunction in patients with mitral valve prolapse and severe regurgitation. Int J Cardiovasc Imaging 2020;36:1363–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Toh H, Mori S, Izawa Y, Fujita H, Miwa K, Suzuki M, et al. Prevalence and extent of mitral annular disjunction in structurally normal hearts: comprehensive 3D analysis using cardiac computed tomography. Eur Heart J Cardiovasc Imaging 2021;22:614–22. [DOI] [PubMed] [Google Scholar]
- 50. Antoine C, Mantovani F, Benfari G, Mankad SV, Maalouf JF, Michelena HI, et al. Pathophysiology of degenerative mitral regurgitation: new 3-dimensional imaging insights. Circ Cardiovasc Imaging 2018;11:e005971. [DOI] [PubMed] [Google Scholar]
- 51. Enriquez-Sarano M. Mitral annular disjunction: the forgotten component of myxomatous mitral valve disease. JACC Cardiovasc Imaging 2017;10:1434–6. [DOI] [PubMed] [Google Scholar]
- 52. Aabel EW, Chivulescu M, Dejgaard LA, Ribe M, Gjertsen E, Hopp E, et al. Tricuspid annulus disjunction: novel findings by cardiac magnetic resonance in patients with mitral annulus disjunction. JACC Cardiovasc Imaging 2021. [DOI] [PubMed] [Google Scholar]
- 53. Hiemstra YL, Tomsic A, Gripari P, van Wijngaarden AL, van der Pas SL, Palmen M, et al. Evolution from mitral annular dysfunction to severe mitral regurgitation in Barlow’s disease. Interact Cardiovasc Thorac Surg 2021;32:506–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Essayagh B, Mantovani F, Benfari G, Maalouf JF, Mankad S, Thapa P, et al. Mitral annular disjunction of degenerative mitral regurgitation: 3D evaluation and implications for mitral repair. J Am Soc Echocardiogr 2021. [DOI] [PubMed] [Google Scholar]
- 55. Hutchins GM, Moore GW, Skoog DK. The association of floppy mitral valve with disjunction of the mitral annulus fibrosus. N Engl J Med 1986;314:535–40. [DOI] [PubMed] [Google Scholar]
- 56. Bharati S, Granston AS, Liebson PR, Loeb HS, Rosen KM, Lev M. The conduction system in mitral valve prolapse syndrome with sudden death. Am Heart J 1981;101:667–70. [DOI] [PubMed] [Google Scholar]
- 57. Han HC, Ha FJ, Teh AW, Calafiore P, Jones EF, Johns J, et al. Mitral valve prolapse and sudden cardiac death: a systematic review. J Am Heart Assoc 2018;7:e010584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Han HC, Parsons SA, Teh AW, Sanders P, Neil C, Leong T, et al. Characteristic histopathological findings and cardiac arrest rhythm in isolated mitral valve prolapse and sudden cardiac death. J Am Heart Assoc 2020;9:e015587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lee AP, Hsiung MC, Salgo IS, Fang F, Xie JM, Zhang YC, et al. Quantitative analysis of mitral valve morphology in mitral valve prolapse with real-time 3-dimensional echocardiography: importance of annular saddle shape in the pathogenesis of mitral regurgitation. Circulation 2013;127:832–41. [DOI] [PubMed] [Google Scholar]
- 60. Angelini A, Ho SY, Anderson RH, Davies MJ, Becker AE. A histological study of the atrioventricular junction in hearts with normal and prolapsed leaflets of the mitral valve. Br Heart J 1988;59:712–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Mantegazza V, Tamborini G, Muratori M, Gripari P, Fusini L, Italiano G, et al. Mitral annular disjunction in a large cohort of patients with mitral valve prolapse and significant regurgitation. JACC Cardiovasc Imaging 2019. [DOI] [PubMed] [Google Scholar]
- 62. Lee AP, Jin CN, Fan Y, Wong RHL, Underwood MJ, Wan S. Functional implication of mitral annular disjunction in mitral valve prolapse: a quantitative dynamic 3d echocardiographic study. JACC Cardiovasc Imaging 2017;10:1424–33. [DOI] [PubMed] [Google Scholar]
- 63. Christiansen JP EC, Myerson S. Assessment of mitral annular disjunction by cardiac MRI in patients with mitral valve prolapse. Heart Lung Circ 2010:19S. [Google Scholar]
- 64. Bennett S, Thamman R, Griffiths T, Oxley C, Khan JN, Phan T, et al. Mitral annular disjunction: a systematic review of the literature. Echocardiography 2019;36:1549–58. [DOI] [PubMed] [Google Scholar]
- 65. Konda T, Tani T, Suganuma N, Fujii Y, Ota M, Kitai T, et al. Mitral annular disjunction in patients with primary severe mitral regurgitation and mitral valve prolapse. Echocardiography 2020;37:1716–22. [DOI] [PubMed] [Google Scholar]
- 66. Demolder A, Timmermans F, Duytschaever M, Muino-Mosquera L, De Backer J. Association of mitral annular disjunction with cardiovascular outcomes among patients with marfan syndrome. JAMA Cardiol 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Chivulescu M, Krohg-Sorensen K, Scheirlynck E, Lindberg BR, Dejgaard LA, Lie OH, et al. Mitral annulus disjunction is associated with adverse outcome in Marfan and Loeys-Dietz syndromes. Eur Heart J Cardiovasc Imaging 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Antoine C, Benfari G, Michelena HI, Maalouf JF, Nkomo VT, Thapa P, et al. Clinical outcome of degenerative mitral regurgitation: critical importance of echocardiographic quantitative assessment in routine practice. Circulation 2018;138:1317–26. [DOI] [PubMed] [Google Scholar]
- 69. Enriquez-Sarano M, Avierinos JF, Messika-Zeitoun D, Detaint D, Capps M, Nkomo V, et al. Quantitative determinants of the outcome of asymptomatic mitral regurgitation. N Engl J Med 2005;352:875–83. [DOI] [PubMed] [Google Scholar]
- 70. Messika-Zeitoun D, Bellamy M, Avierinos JF, Breen J, Eusemann C, Rossi A, et al. Left atrial remodelling in mitral regurgitation–methodologic approach, physiological determinants, and outcome implications: a prospective quantitative Doppler-echocardiographic and electron beam-computed tomographic study. Eur Heart J 2007;28:1773–81. [DOI] [PubMed] [Google Scholar]
- 71. Le Tourneau T, Messika-Zeitoun D, Russo A, Detaint D, Topilsky Y, Mahoney DW, et al. Impact of left atrial volume on clinical outcome in organic mitral regurgitation. J Am Coll Cardiol 2010; 56: 570–8. [DOI] [PubMed] [Google Scholar]
- 72. Essayagh B, Antoine C, Benfari G, Messika-Zeitoun D, Michelena H, Le Tourneau T, et al. Prognostic implications of left atrial enlargement in degenerative mitral regurgitation. J Am Coll Cardiol 2019;74:858–70. [DOI] [PubMed] [Google Scholar]
- 73. Grigioni F, Benfari G, Vanoverschelde JL, Tribouilloy C, Avierinos JF, Bursi F, et al. Long-term implications of atrial fibrillation in patients with degenerative mitral regurgitation. J Am Coll Cardiol 2019;73:264–74. [DOI] [PubMed] [Google Scholar]
- 74. Sriram CS, Syed FF, Ferguson ME, Johnson JN, Enriquez-Sarano M, Cetta F, et al. Malignant bileaflet mitral valve prolapse syndrome in patients with otherwise idiopathic out-of-hospital cardiac arrest. J Am Coll Cardiol 2013;62:222–30. [DOI] [PubMed] [Google Scholar]
- 75. Savage DD, Devereux RB, Garrison RJ, Castelli WP, Anderson SJ, Levy D, et al. Mitral valve prolapse in the general population. 2. Clinical features: the Framingham study. Am Heart J 1983;106:577–81. [DOI] [PubMed] [Google Scholar]
- 76. Devereux RB, Kramer-Fox R, Kligfield P. Mitral valve prolapse: causes, clinical manifestations, and management. Ann Intern Med 1989;111:305–17. [DOI] [PubMed] [Google Scholar]
- 77. Gibson DG, Brown DJ. Abnormal left ventricular wall movement in patients with chest pain and normal coronary arteriograms. Relation to inferior T wave changes and mitral prolapse. Br Heart J 1979;41:385–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Noseworthy PA, Asirvatham SJ. The knot that binds mitral valve prolapse and sudden cardiac death. Circulation 2015;132:551–2. [DOI] [PubMed] [Google Scholar]
- 79. Bhutto ZR, Barron JT, Liebson PR, Uretz EF, Parrillo JE. Electrocardiographic abnormalities in mitral valve prolapse. Am J Cardiol 1992;70:265–6. [DOI] [PubMed] [Google Scholar]
- 80. Bekheit SG, Ali AA, Deglin SM, Jain AC. Analysis of QT interval in patients with idiopathic mitral valve prolapse. Chest 1982;81:620–5. [DOI] [PubMed] [Google Scholar]
- 81. Levy D, Savage D. Prevalence and clinical features of mitral valve prolapse. Am Heart J 1987;113:1281–90. [DOI] [PubMed] [Google Scholar]
- 82. Zouridakis EG, Parthenakis FI, Kochiadakis GE, Kanoupakis EM, Vardas PE. QT dispersion in patients with mitral valve prolapse is related to the echocardiographic degree of the prolapse and mitral leaflet thickness. Europace 2001;3:292–8. [DOI] [PubMed] [Google Scholar]
- 83. Gornick CC, Tobler HG, Pritzker MC, Tuna IC, Almquist A, Benditt DG. Electrophysiologic effects of papillary muscle traction in the intact heart. Circulation 1986;73:1013–21. [DOI] [PubMed] [Google Scholar]
- 84. Algra A, Tijssen JG, Roelandt JR, Pool J, Lubsen J. QTc prolongation measured by standard 12-lead electrocardiography is an independent risk factor for sudden death due to cardiac arrest. Circulation 1991;83:1888–94. [DOI] [PubMed] [Google Scholar]
- 85. O’Neal WT, Singleton MJ, Roberts JD, Tereshchenko LG, Sotoodehnia N, Chen LY, et al. Association between QT-interval components and sudden cardiac death. Circ Arrhythm Electrophysiol 2017;10:e005485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Das MK, Khan B, Jacob S, Kumar A, Mahenthiran J. Significance of a fragmented QRS complex versus a Q wave in patients with coronary artery disease. Circulation 2006;113:2495–501. [DOI] [PubMed] [Google Scholar]
- 87. Das MK, Saha C, El Masry H, Peng J, Dandamudi G, Mahenthiran J, et al. Fragmented QRS on a 12-lead ECG: a predictor of mortality and cardiac events in patients with coronary artery disease. Heart Rhythm 2007;4:1385–92. [DOI] [PubMed] [Google Scholar]
- 88. Ogura S, Nakamura K, Morita H, Nakagawa K, Nishii N, Akagi S, et al. Fragmented QRS as a predictor of cardiac events in patients with cardiac sarcoidosis. J Cardiol 2022;79:446–52. [DOI] [PubMed] [Google Scholar]
- 89. Rattanawong P, Riangwiwat T, Prasitlumkum N, Limpruttidham N, Kanjanahattakij N, Chongsathidkiet P, et al. Baseline fragmented QRS increases the risk of major arrhythmic events in Brugada syndrome: systematic review and meta-analysis. Ann Noninvasive Electrocardiol 2018; 23:e12507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Vogels RJ, Teuwen CP, Ramdjan TT, Evertz R, Knops P, Witsenburg M, et al. Usefulness of fragmented QRS complexes in patients with congenital heart disease to predict ventricular tachyarrhythmias. Am J Cardiol 2017;119:126–31. [DOI] [PubMed] [Google Scholar]
- 91. Kaya Ü, Eren H. Fragmented QRS may be associated with complex ventricular arrhythmias in mitral valve prolapse. Minerva Cardioangiol 2020;68:577–85. [DOI] [PubMed] [Google Scholar]
- 92. von Alvensleben JC, Etheridge SP, Viskin S, Collins KK. Short-coupled premature ventricular beats leading to ventricular fibrillation in a young patient: a sudden arrhythmia death syndrome case report and literature review. HeartRhythm Case Rep 2020;6:815–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Hsia BC, Greige N, Patel SK, Clark RM, Ferrick KJ, Fisher JD, et al. Determining the optimal duration for premature ventricular contraction monitoring. Heart Rhythm 2020;17:2119–25. [DOI] [PubMed] [Google Scholar]
- 94. Engel G, Cho S, Ghayoumi A, Yamazaki T, Chun S, Fearon WF, et al. Prognostic significance of PVCs and resting heart rate. Ann Noninvasive Electrocardiol 2007;12:121–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Marano PJ, Lim LJ, Sanchez JM, Alvi R, Nah G, Badhwar N, et al. Long-term outcomes of ablation for ventricular arrhythmias in mitral valve prolapse. J Interv Card Electrophysiol 2021;61:145–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Ataklte F, Erqou S, Laukkanen J, Kaptoge S. Meta-analysis of ventricular premature complexes and their relation to cardiac mortality in general populations. Am J Cardiol 2013;112:1263–70. [DOI] [PubMed] [Google Scholar]
- 97. Dukes JW, Dewland TA, Vittinghoff E, Mandyam MC, Heckbert SR, Siscovick DS, et al. Ventricular ectopy as a predictor of heart failure and death. J Am Coll Cardiol 2015;66:101–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Bikkina M, Larson MG, Levy D. Prognostic implications of asymptomatic ventricular arrhythmias: the Framingham Heart Study. Ann Intern Med 1992;117:990–6. [DOI] [PubMed] [Google Scholar]
- 99. Lin CY, Chang SL, Chung FP, Chen YY, Lin YJ, Lo LW, et al. Long-term outcome of non-sustained ventricular tachycardia in structurally normal hearts. PLoS One 2016;11:e0160181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Steinberg C, Davies B, Mellor G, Tadros R, Laksman ZW, Roberts JD, et al. Short-coupled ventricular fibrillation represents a distinct phenotype among latent causes of unexplained cardiac arrest: a report from the CASPER registry. Eur Heart J 2021;42:2827–38. [DOI] [PubMed] [Google Scholar]
- 101. Miller MA, Dukkipati SR, Turagam M, Liao SL, Adams DH, Reddy VY. Arrhythmic mitral valve prolapse: JACC review topic of the week. J Am Coll Cardiol 2018;72:2904–14. [DOI] [PubMed] [Google Scholar]
- 102. Brignole M, Moya A, de Lange FJ, Deharo JC, Elliott PM, Fanciulli A, et al. 2018 ESC guidelines for the diagnosis and management of syncope. Eur Heart J 2018;39:1883–948. [DOI] [PubMed] [Google Scholar]
- 103. Lancellotti P, Tribouilloy C, Hagendorff A, Popescu BA, Edvardsen T, Pierard LA, et al. Recommendations for the echocardiographic assessment of native valvular regurgitation: an executive summary from the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2013;14:611–44. [DOI] [PubMed] [Google Scholar]
- 104. Lancellotti P, Pibarot P, Chambers J, La Canna G, Pepi M, Dulgheru R, et al. Multi-modality imaging assessment of native valvular regurgitation: an EACVI and ESC council of valvular heart disease position paper. Eur Heart J Cardiovasc Imaging 2022;23:e171–232. [DOI] [PubMed] [Google Scholar]
- 105. Tribouilloy C, Grigioni F, Avierinos JF, Barbieri A, Rusinaru D, Szymanski C, et al. Survival implication of left ventricular end-systolic diameter in mitral regurgitation due to flail leaflets: a long-term follow-up multicenter study. J Am Coll Cardiol 2009;54:1961–68. [DOI] [PubMed] [Google Scholar]
- 106. Tribouilloy C, Rusinaru D, Grigioni F, Michelena HI, Vanoverschelde JL, Avierinos JF, et al. Long-term mortality associated with left ventricular dysfunction in mitral regurgitation due to flail leaflets: a multicenter analysis. Circ Cardiovasc Imaging 2014;7:363–70. [DOI] [PubMed] [Google Scholar]
- 107. Nordhues BD, Siontis KC, Scott CG, Nkomo VT, Ackerman MJ, Asirvatham SJ, et al. Bileaflet mitral valve prolapse and risk of ventricular dysrhythmias and death. J Cardiovasc Electrophysiol 2016;27:463–8. [DOI] [PubMed] [Google Scholar]
- 108. Avierinos JF, Detaint D, Messika-Zeitoun D, Mohty D, Enriquez-Sarano M. Risk, determinants, and outcome implications of progression of mitral regurgitation after diagnosis of mitral valve prolapse in a single community. Am J Cardiol 2008;101:662–7. [DOI] [PubMed] [Google Scholar]
- 109. Huttin O, Pierre S, Venner C, Voilliot D, Sellal JM, Aliot E, et al. Interactions between mitral valve and left ventricle analysed by 2D speckle tracking in patients with mitral valve prolapse: one more piece to the puzzle. Eur Heart J Cardiovasc Imaging 2017;18:323–31. [DOI] [PubMed] [Google Scholar]
- 110. Kitkungvan D, Nabi F, Kim RJ, Bonow RO, Khan MA, Xu J, et al. Myocardial fibrosis in patients with primary mitral regurgitation with and without prolapse. J Am Coll Cardiol 2018;72:823–34. [DOI] [PubMed] [Google Scholar]
- 111. Han Y, Peters DC, Salton CJ, Bzymek D, Nezafat R, Goddu B, et al. Cardiovascular magnetic resonance characterization of mitral valve prolapse. JACC Cardiovasc Imaging 2008;1:294–303. [DOI] [PubMed] [Google Scholar]
- 112. Bui AH, Roujol S, Foppa M, Kissinger KV, Goddu B, Hauser TH, et al. Diffuse myocardial fibrosis in patients with mitral valve prolapse and ventricular arrhythmia. Heart 2017;103:204–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Constant Dit Beaufils AL, Huttin O, Jobbe-Duval A, Senage T, Filippetti L, Piriou N, et al. Replacement myocardial fibrosis in patients with mitral valve prolapse: relation to mitral regurgitation, ventricular remodeling, and arrhythmia. Circulation 2021;143:1763–74. [DOI] [PubMed] [Google Scholar]
- 114. Monteleone PL, Fagan LF. Possible X-linked congenital heart disease. Circulation 1969;39:611–4. [DOI] [PubMed] [Google Scholar]
- 115. Devereux RB, Brown WT, Kramer-Fox R, Sachs I. Inheritance of mitral valve prolapse: effect of age and sex on gene expression. Ann Intern Med 1982;97:826–32. [DOI] [PubMed] [Google Scholar]
- 116. Bains S, Tester DJ, Asirvatham SJ, Noseworthy PA, Ackerman MJ, Giudicessi JR. A novel truncating variant in FLNC-encoded filamin C may serve as a proarrhythmic genetic substrate for arrhythmogenic bileaflet mitral valve prolapse syndrome. Mayo Clin Proc 2019;94:906–13. [DOI] [PubMed] [Google Scholar]
- 117. Durst R, Sauls K, Peal DS, deVlaming A, Toomer K, Leyne M, et al. Mutations in DCHS1 cause mitral valve prolapse. Nature 2015;525:109–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Songia P, Chiesa M, Alfieri V, Massaiu I, Moschetta D, Myasoedova V, et al. Putative circulating MicroRNAs are able to identify patients with mitral valve prolapse and severe regurgitation. Int J Mol Sci 2021;22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Tan HT, Ling LH, Dolor-Torres MC, Yip JW, Richards AM, Chung MC. Proteomics discovery of biomarkers for mitral regurgitation caused by mitral valve prolapse. J Proteomics 2013;94:337–45. [DOI] [PubMed] [Google Scholar]
- 120. Tan HT, Lim TK, Richards AM, Kofidis T, Teoh KL, Ling LH, et al. Unravelling the proteome of degenerative human mitral valves. Proteomics 2015;15:2934–44. [DOI] [PubMed] [Google Scholar]
- 121. Scheirlynck E, Dejgaard LA, Skjolsvik E, Lie OH, Motoc A, Hopp E, et al. Increased levels of sST2 in patients with mitral annulus disjunction and ventricular arrhythmias. Open Heart 2019;6:e001016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Hagler MA, Hadley TM, Zhang H, Mehra K, Roos CM, Schaff HV, et al. TGF-beta signalling and reactive oxygen species drive fibrosis and matrix remodelling in myxomatous mitral valves. Cardiovasc Res 2013;99:175–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Rizzo S, Basso C, Lazzarini E, Celeghin R, Paolin A, Gerosa G, et al. TGF-beta1 pathway activation and adherens junction molecular pattern in nonsyndromic mitral valve prolapse. Cardiovasc Pathol 2015; 24: 359–67. [DOI] [PubMed] [Google Scholar]
- 124. Basso C, Aguilera B, Banner J, Cohle S, d’Amati G, de Gouveia RH, et al. Guidelines for autopsy investigation of sudden cardiac death: 2017 update from the Association for European Cardiovascular Pathology. Virchows Archiv Int J Pathol 2017; 471: 691–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Chesler E, King RA, Edwards JE. The myxomatous mitral valve and sudden death. Circulation 1983;67:632–39. [DOI] [PubMed] [Google Scholar]
- 126. Vergara P, Scarfo I, Esposito A, Colantoni C, Palmisano A, Altizio S, et al. Characterization of the electrophysiological substrate in patients with Barlow’s disease. J Cardiovasc Electrophysiol 2021;32:3179–86. [DOI] [PubMed] [Google Scholar]
- 127. Franz MR. Mechano-electrical feedback. Cardiovasc Res 2000;45:263–6. [DOI] [PubMed] [Google Scholar]
- 128. Enriquez A, Shirai Y, Huang J, Liang J, Briceno D, Hayashi T, et al. Papillary muscle ventricular arrhythmias in patients with arrhythmic mitral valve prolapse: electrophysiologic substrate and catheter ablation outcomes. J Cardiovasc Electrophysiol 2019;30:827–35. [DOI] [PubMed] [Google Scholar]
- 129. Syed FF, Ackerman MJ, McLeod CJ, Kapa S, Mulpuru SK, Sriram CS, et al. Sites of successful ventricular fibrillation ablation in bileaflet mitral valve prolapse syndrome. Circ Arrhythm Electrophysiol 2016;9. [DOI] [PubMed] [Google Scholar]
- 130. Sabbag A, Suleiman M, Laish-Farkash A, Samania N, Kazatsker M, Goldenberg I, et al. Contemporary rates of appropriate shock therapy in patients who receive implantable device therapy in a real-world setting: from the Israeli ICD registry. Heart Rhythm 2015;12:2426–33. [DOI] [PubMed] [Google Scholar]
- 131. Dagres N, Peek N, Leclercq C, Hindricks G. The PROFID project. Eur Heart J 2020;41:3781–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Priori SG, Blomstrom-Lundqvist C, Mazzanti A, Blom N, Borggrefe M, Camm J, et al. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: the task force for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death of the European Society of Cardiology (ESC) endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Europace 2015;17:1601–87. [DOI] [PubMed] [Google Scholar]
- 133. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Bohm M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2021. [DOI] [PubMed] [Google Scholar]
- 134. Josephson ME, Callans DJ. Using the twelve-lead electrocardiogram to localize the site of origin of ventricular tachycardia. Heart Rhythm 2005;2:443–6. [DOI] [PubMed] [Google Scholar]
- 135. Enriquez A, Baranchuk A, Briceno D, Saenz L, Garcia F. How to use the 12-lead ECG to predict the site of origin of idiopathic ventricular arrhythmias. Heart Rhythm 2019;16:1538–44. [DOI] [PubMed] [Google Scholar]
- 136. de Riva M, Watanabe M, Zeppenfeld K. Twelve-lead ECG of ventricular tachycardia in structural heart disease. Circ Arrhythm Electrophysiol 2015;8:951–62. [DOI] [PubMed] [Google Scholar]
- 137. Al’Aref SJ, Ip JE, Markowitz SM, Liu CF, Thomas G, Frenkel D, et al. Differentiation of papillary muscle from fascicular and mitral annular ventricular arrhythmias in patients with and without structural heart disease. Circ Arrhythm Electrophysiol 2015;8:616–24. [DOI] [PubMed] [Google Scholar]
- 138. Kumagai K, Yamauchi Y, Takahashi A, Yokoyama Y, Sekiguchi Y, Watanabe J, et al. Idiopathic left ventricular tachycardia originating from the mitral annulus. J Cardiovasc Electrophysiol 2005;16:1029–36. [DOI] [PubMed] [Google Scholar]
- 139. Tada H, Ito S, Naito S, Kurosaki K, Kubota S, Sugiyasu A, et al. Idiopathic ventricular arrhythmia arising from the mitral annulus: a distinct subgroup of idiopathic ventricular arrhythmias. J Am Coll Cardiol 2005;45:877–86. [DOI] [PubMed] [Google Scholar]
- 140. Jeresaty RM. The syndrome associated with mid-systolic click and-or late systolic murmur. Analysis of 32 cases. Chest 1971;59:643–7. [DOI] [PubMed] [Google Scholar]
- 141. Barlow JB, Bosman CK. Aneurysmal protrusion of the posterior leaflet of the mitral valve. An auscultatory-electrocardiographic syndrome. Am Heart J 1966;71:166–78. [DOI] [PubMed] [Google Scholar]
- 142. Kim YG, Choi YY, Han K-D, Min KJ, Choi HY, Shim J, et al. Premature ventricular contraction increases the risk of heart failure and ventricular tachyarrhythmia. Sci Rep 2021;11:12698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Tani T, Konda T, Kitai T, Ota M, Furukawa Y. Mitral annular disjunction-a new disease spectrum. Cardiol Clin 2021;39:289–94. [DOI] [PubMed] [Google Scholar]
- 144. Latchamsetty R, Bogun F. Premature ventricular complex-induced cardiomyopathy. JACC Clin Electrophysiol 2019;5:537–50. [DOI] [PubMed] [Google Scholar]
- 145. Arnar DO, Mairesse GH, Boriani G, Calkins H, Chin A, Coats A, et al. Management of asymptomatic arrhythmias: a European Heart Rhythm Association (EHRA) consensus document, endorsed by the Heart Failure Association (HFA), Heart Rhythm Society (HRS), Asia Pacific Heart Rhythm Society (APHRS), Cardiac Arrhythmia Society of Southern Africa (CASSA), and Latin America Heart Rhythm Society (LAHRS). Europace 2019;21:844–5. [DOI] [PubMed] [Google Scholar]
- 146. Bisignani A, De Bonis S, Mancuso L, Ceravolo G, Bisignani G. Implantable loop recorder in clinical practice. J Arrhythm 2019;35:25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Padmanabhan D, Kancharla K, El-Harasis MA, Isath A, Makkar N, Noseworthy PA, et al. Diagnostic and therapeutic value of implantable loop recorder: a tertiary care center experience. Pacing Clin Electrophysiol 2019;42:38–45. [DOI] [PubMed] [Google Scholar]
- 148. Freeman WK, Thibodeau J, Abuissa H. Unrecognized ventricular tachycardia in a patient with mitral annulus disjunction and syncope. HeartRhythm Case Rep 2020;6:646–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Joyce T, Ferns S. Malignant mitral valve prolapse: an uncommon variation of a common condition. BMJ Case Rep 2020;13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s guidelines and standards committee and the chamber quantification writing group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr 2005;18:1440–63. [DOI] [PubMed] [Google Scholar]
- 151. Wolff R, Uretsky S. Defining the left ventricular base in mitral valve prolapse: impact on systolic function and regurgitation. Int J Cardiovasc Imaging 2020;36:2221–27. [DOI] [PubMed] [Google Scholar]
- 152. Davies M, Moore B, Braimbridge M. The floppy mitral valve: study of incidence, pathology and complications in surgical, necropsy and forensic material. Br Heart J 1978;40:468–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Muthukumar L, Rahman F, Jan MF, Shaikh A, Kalvin L, Dhala A, et al. The pickelhaube sign: novel echocardiographic risk marker for malignant mitral valve prolapse syndrome. JACC Cardiovasc Imaging 2017;10:1078–80. [DOI] [PubMed] [Google Scholar]
- 154. Priori SG, Blomström-Lundqvist C, Mazzanti A, Blom N, Borggrefe M, Camm J, et al. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: the task force for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Eur Heart J 2015;36:2793–867. [DOI] [PubMed] [Google Scholar]
- 155. White JA, Fine NM, Gula L, Yee R, Skanes A, Klein G, et al. Utility of cardiovascular magnetic resonance in identifying substrate for malignant ventricular arrhythmias. Circ Cardiovasc Imaging 2012;5:12–20. [DOI] [PubMed] [Google Scholar]
- 156. Guglielmo M, Fusini L, Muscogiuri G, Baessato F, Loffreno A, Cavaliere A, et al. T1 mapping and cardiac magnetic resonance feature tracking in mitral valve prolapse. Eur Radiol 2021;31:1100–09. [DOI] [PubMed] [Google Scholar]
- 157. Stiles MK, Wilde AAM, Abrams DJ, Ackerman MJ, Albert CM, Behr ER, et al. 2020 APHRS/HRS expert consensus statement on the investigation of decedents with sudden unexplained death and patients with sudden cardiac arrest, and of their families. Heart Rhythm 2021;18:e1–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Babuty D, Cosnay P, Breuillac JC, Charniot JC, Delhomme C, Fauchier L, et al. Ventricular arrhythmia factors in mitral valve prolapse. Pacing Clin Electrophysiol 1994;17:1090–99. [DOI] [PubMed] [Google Scholar]
- 159. Morady F, Shen E, Bhandari A, Schwartz A, Scheinman MM. Programmed ventricular stimulation in mitral valve prolapse: analysis of 36 patients. Am J Cardiol 1984;53:135–8. [DOI] [PubMed] [Google Scholar]
- 160. Rosenthal ME, Hamer A, Gang ES, Oseran DS, Mandel WJ, Peter T. The yield of programmed ventricular stimulation in mitral valve prolapse patients with ventricular arrhythmias. Am Heart J 1985; 110: 970–76. [DOI] [PubMed] [Google Scholar]
- 161. Frolkis JP, Pothier CE, Blackstone EH, Lauer MS. Frequent ventricular ectopy after exercise as a predictor of death. N Engl J Med 2003;348:781–90. [DOI] [PubMed] [Google Scholar]
- 162. Refaat MM, Gharios C, Moorthy MV, Abdulhai F, Blumenthal RS, Jaffa MA, et al. Exercise-induced ventricular ectopy and cardiovascular mortality in asymptomatic individuals. J Am Coll Cardiol 2021; 78: 2267–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Pelliccia A, Sharma S, Gati S, Back M, Borjesson M, Caselli S, et al. 2020 ESC Guidelines on sports cardiology and exercise in patients with cardiovascular disease. Eur Heart J 2021;42:17–96. [DOI] [PubMed] [Google Scholar]
- 164. Knuuti J, Wijns W, Saraste A, Capodanno D, Barbato E, Funck-Brentano C, et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J 2020;41:407–77. [DOI] [PubMed] [Google Scholar]
- 165. Ling Z, Liu Z, Su L, Zipunnikov V, Wu J, Du H, et al. Radiofrequency ablation versus antiarrhythmic medication for treatment of ventricular premature beats from the right ventricular outflow tract. Circ Arrhythm Electrophysiol 2014;7:237–43. [DOI] [PubMed] [Google Scholar]
- 166. Krittayaphong R, Bhuripanyo K, Punlee K, Kangkagate C, Chaithiraphan S. Effect of atenolol on symptomatic ventricular arrhythmia without structural heart disease: a randomized placebo-controlled study. Am Heart J 2002;144:e10. [DOI] [PubMed] [Google Scholar]
- 167. Stec S, Sikorska A, Zaborska B, Krynski T, Szymot J, Kulakowski P. Benign symptomatic premature ventricular complexes: short- and long-term efficacy of antiarrhythmic drugs and radiofrequency ablation. Kardiol Pol 2012;70:351–8. [PubMed] [Google Scholar]
- 168. Singh SN, Fletcher RD, Fisher SG, Singh BN, Lewis HD, Deedwania PC, et al. Amiodarone in patients with congestive heart failure and asymptomatic ventricular arrhythmia. Survival trial of antiarrhythmic therapy in congestive heart failure. N Engl J Med 1995;333:77–82. [DOI] [PubMed] [Google Scholar]
- 169. Hyman MC, Mustin D, Supple G, Schaller RD, Santangeli P, Arkles J, et al. Class IC antiarrhythmic drugs for suspected premature ventricular contraction-induced cardiomyopathy. Heart Rhythm 2018;15:159–63. [DOI] [PubMed] [Google Scholar]
- 170. Anderson JL, Askins JC, Gilbert EM, Miller RH, Keefe DL, Somberg JC, et al. Multicenter trial of sotalol for suppression of frequent, complex ventricular arrhythmias: a double-blind, randomized, placebo-controlled evaluation of two doses. J Am Coll Cardiol 1986;8:752–62. [DOI] [PubMed] [Google Scholar]
- 171. Vaidya VR, DeSimone CV, Damle N, Naksuk N, Syed FF, Ackerman MJ, et al. Reduction in malignant ventricular arrhythmia and appropriate shocks following surgical correction of bileaflet mitral valve prolapse. J Interv Card Electrophysiol 2016;46:137–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Saha T, Norris R, Luebbert J. Recurrent premature ventricular contraction-induced ventricular fibrillation and resuscitated sudden death in a 26-year-old pregnant woman with bileaflet mitral valve prolapse. HeartRhythm Case Rep 2018;4:58–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Hong T, Yang M, Zhong L, Lee YH, Vaidya VR, Asirvatham SJ, et al. Ventricular premature contraction associated with mitral valve prolapse. Int J Cardiol 2016;221:1144–9. [DOI] [PubMed] [Google Scholar]
- 174. Viskin S, Chorin E, Viskin D, Hochstadt A, Halkin A, Tovia-Brodie O, et al. Quinidine-responsive polymorphic ventricular tachycardia in patients with coronary heart disease. Circulation 2019;139:2304–14. [DOI] [PubMed] [Google Scholar]
- 175. Bumgarner JM, Patel D, Kumar A, Clevenger JR, Trulock KM, Popovic Z, et al. Management and outcomes in mitral valve prolapse with ventricular arrhythmias undergoing ablation and/or implantation of ICDs. Pacing Clin Electrophysiol 2019;42:447–52. [DOI] [PubMed] [Google Scholar]
- 176. Peichl P, Baran J, Wichterle D, Cihak R, Skala T, Aldhoon B, et al. The tip of the muscle is a dominant location of ventricular ectopy originating from papillary muscles in the left ventricle. J Cardiovasc Electrophysiol 2018;29:64–70. [DOI] [PubMed] [Google Scholar]
- 177. Rivera S, Tomas L, Ricapito MP, Nicolas V, Reinoso M, Caro M, et al. Updated results on catheter ablation of ventricular arrhythmias arising from the papillary muscles of the left ventricle. J Arrhythm 2019;35:99–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Lee A, Hamilton-Craig C, Denman R, Haqqani HM. Catheter ablation of papillary muscle arrhythmias: Implications of mitral valve prolapse and systolic dysfunction. Pacing Clin Electrophysiol 2018;41:750–8. [DOI] [PubMed] [Google Scholar]
- 179. Cronin EM, Bogun FM, Maury P, Peichl P, Chen M, Namboodiri N, et al. 2019 HRS/EHRA/APHRS/LAHRS expert consensus statement on catheter ablation of ventricular arrhythmias. Europace 2019; 21:1143–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Mochizuki A, Nagahara D, Takahashi H, Saito R, Fujito T, Miura T. Worsening of mitral valve regurgitation after radiofrequency catheter ablation of ventricular arrhythmia originating from a left ventricular papillary muscle. HeartRhythm Case Rep 2017;3:215–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Pocock WA, Barlow JB, Marcus RH, Barlow CW. Mitral valvuloplasty for life-threatening ventricular arrhythmias in mitral valve prolapse. Am Heart J 1991;121:199–202. [DOI] [PubMed] [Google Scholar]
- 182. Kay JH, Krohn BG, Zubiate P, Hoffman RL. Surgical correction of severe mitral prolapse without mitral insufficiency but with pronounced cardiac arrhythmias. J Thorac Cardiovasc Surg 1979;78:259–68. [PubMed] [Google Scholar]
- 183. Al-Bassam MS, Cooley DA. Arrhythmia with mitral valve prolapse: results of annuloplasty in two patients. Cardiovasc Dis 1978;5:397–405. [PMC free article] [PubMed] [Google Scholar]
- 184. Ross A, DeWeese JA, Yu PN. Refractory ventricular arrhythmias in a patient with mitral valve prolapse. Successful control with mitral valve replacement. J Electrocardiol 1978;11:289–95. [DOI] [PubMed] [Google Scholar]
- 185. Missotten A, Dotremont G, Goddeeris P, Piessens J, De Geest H. Mitral valve replacement in a patient with mitral valve prolapse complicated by severe ventricular arrhythmias. Acta Cardiol 1980;35:391–9. [PubMed] [Google Scholar]
- 186. Beroukhim RS, Reed JH, Schaffer MS, Yetman AT. Surgical correction of mitral valve prolapse: a cure for recurrent ventricular tachycardia in Marfan syndrome? Pediatr Cardiol 2006;27:755–8. [DOI] [PubMed] [Google Scholar]
- 187. Abbadi DR, Purbey R, Poornima IG. Mitral valve repair is an effective treatment for ventricular arrhythmias in mitral valve prolapse syndrome. Int J Cardiol 2014;177:e16–8. [DOI] [PubMed] [Google Scholar]
- 188. Alqarawi W, Birnie DH, Burwash IG. Mitral valve repair results in suppression of ventricular arrhythmias and normalization of repolarization abnormalities in mitral valve prolapse. HeartRhythm Case Rep 2018;4:191–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Naksuk N, Syed FF, Krittanawong C, Anderson MJ, Ebrille E, DeSimone CV, et al. The effect of mitral valve surgery on ventricular arrhythmia in patients with bileaflet mitral valve prolapse. Indian Pacing Electrophysiol J 2016;16:187–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Vohra J, Sathe S, Warren R, Totalis J, Hunt D. Malignant ventricular arrhythmias in patients with mitral valve prolapse and mild mitral regurgitation. PACE 1993;16:387–93. [DOI] [PubMed] [Google Scholar]
- 191. Van Dessel PF, Van Hemel NM, Van Swieten HA, De Bakker JM, Jessurun ER. Successful surgical ablation of sustained ventricular tachycardia associated with mitral valve prolapse guided by a multielectrode basket catheter. Pacing Clin Electrophysiol 2001;24:1029–31. [DOI] [PubMed] [Google Scholar]
- 192. El-Eshmawi A, Pandis D, Miller MA, Boateng P, Dukkipati SR, Reddy VY, et al. Surgical cryoablation of papillary muscle PVCs during mitral valve surgery: therapeutic consideration for malignant MVP. J Am Coll Cardiol 2020;76:3061–62. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.