Abstract
Cardiac arrhythmias are a major cause of death and disability. A large number of experimental cell and animal models have been developed to study arrhythmogenic diseases. These models have provided important insights into the underlying arrhythmia mechanisms and translational options for their therapeutic management. This position paper from the ESC Working Group on Cardiac Cellular Electrophysiology provides an overview of (i) currently available in vitro, ex vivo, and in vivo electrophysiological research methodologies, (ii) the most commonly used experimental (cellular and animal) models for cardiac arrhythmias including relevant species differences, (iii) the use of human cardiac tissue, induced pluripotent stem cell (hiPSC)-derived and in silico models to study cardiac arrhythmias, and (iv) the availability, relevance, limitations, and opportunities of these cellular and animal models to recapitulate specific acquired and inherited arrhythmogenic diseases, including atrial fibrillation, heart failure, cardiomyopathy, myocarditis, sinus node, and conduction disorders and channelopathies. By promoting a better understanding of these models and their limitations, this position paper aims to improve the quality of basic research in cardiac electrophysiology, with the ultimate goal to facilitate the clinical translation and application of basic electrophysiological research findings on arrhythmia mechanisms and therapies.
Keywords: Animal models, Experimental models, Arrhythmias, Atrial fibrillation, Mechanisms, Cardiac electrophysiology, Cellular electrophysiology, Ion channels, Position paper
Introduction
Cardiac arrhythmias are a major cause of death and disability. Despite important advances in arrhythmia management, numerous knowledge gaps remain.1 During the past few decades, a large number of experimental cell and animal models have been developed to study different arrhythmogenic diseases. These models have provided important insights into the underlying arrhythmia mechanisms and translational options for their therapeutic management. Each experimental model has specific advantages and limitations, making it more or less suitable to study specific arrhythmogenic diseases, but a comprehensive overview of the different experimental models, electrophysiological techniques, and their optimal use is currently lacking.
This position paper from the ESC Working Group on Cardiac Cellular Electrophysiology addresses this knowledge gap and provides an overview of currently available electrophysiological research methodologies, the most common experimental (cellular and animal) models for cardiac arrhythmias, and their relevance and suitability for certain research questions. Additionally, intrinsic model limitations, as well as opportunities to advance the current state-of-the-art, are discussed. By promoting a better understanding of these opportunities and their limitations, this position paper aims to improve the quality of basic research in cardiac electrophysiology, with the ultimate goal to facilitate the clinical translation and application of basic electrophysiological research findings on arrhythmia mechanisms and therapies, thereby contributing to the overall goal of the ESC to disseminate evidence-based scientific knowledge to cardiovascular professionals so they can provide better care to patients.
Cellular and whole heart electrophysiology techniques
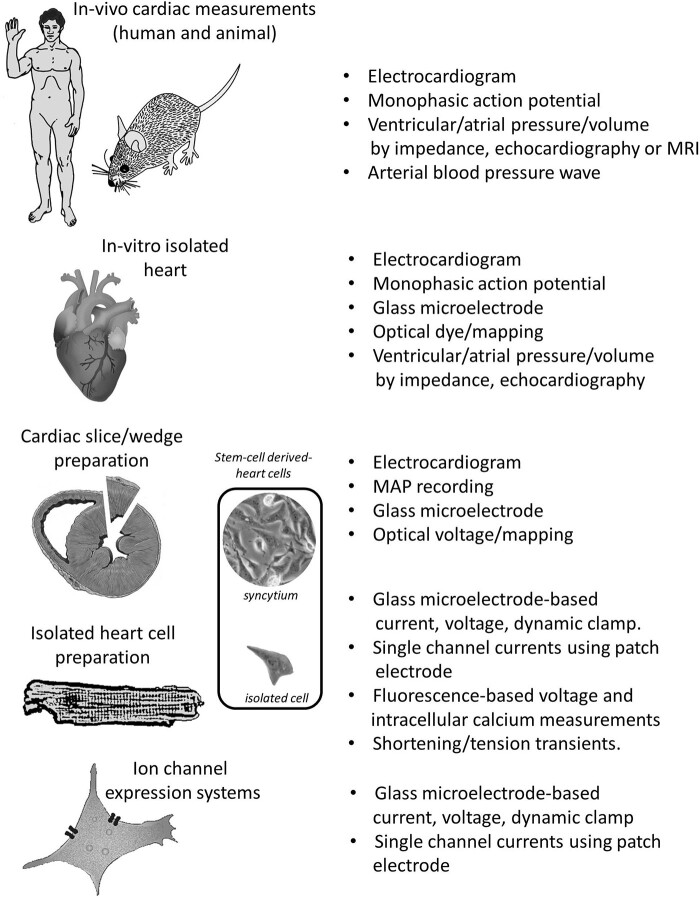
A variety of different techniques allow for a multi-scale investigation of electrophysiological features from in vivo, ex vivo whole heart, to the cellular level (Figure 1), providing insights into physiological and pathophysiological electrical activity and arrhythmogenesis on different levels.
Figure 1.
Hierarchy of preparations and techniques in cardiac electrophysiology. Preparations range from organism (mouse-to-human), isolated heart, multicellular preparations (e.g. slice/wedge or papillary/trabeculae preparations), isolated cardiac cells (e.g. atrial, ventricular, nodal or Purkinje cells) to ion-channel expression systems (e.g. HEK). The associated techniques for measuring cardiac function are shown on the right. Comparable preparations (syncytium and single cell) for stem-cell-derived cardiomyocytes are indicated in a separate panel.
Whole heart electrophysiology (in vivo)
Electrocardiography (ECG) has been successfully employed for monitoring and analysing cardiac electrical activity and arrhythmia in different species ranging from mice to horses.2 In ECGs of anaesthetized animals complex effects of anaesthesia on the electro-mechanical function of the heart and on the autonomic nervous system must be taken into account. Advances in ECG telemetry have enabled reliable recordings in awake conscious animals.3 Moreover, these ECG techniques allow investigation of both spontaneous and induced arrhythmia formation, e.g. after AV-block induction or during transvenous or transoesophageal arrhythmia induction.4,5 Although ECG morphology is similar in different species, a number of inter-species differences exist that may hinder clinical translation (see ‘Species differences in cardiac electrophysiology’ section and Figure 2). Higher resolution non-invasive ECG imaging is possible in large animal models.6,7 Monophasic action potentials (MAP) can be recorded in vivo invasively from endocardial and epicardial surfaces of the heart in anaesthetized animals using a contact electrode catheter technique. Monophasic APs reproduce the repolarization time course of transmembrane APs, providing information on AP duration (APD) and configuration (including proarrhythmic early afterdepolarizations) but not on AP amplitude or upstroke velocity.8 Advances in optical mapping in combination with a novel ratiometric voltage-sensor and a high-speed camera above the epicardial surface have more recently allowed high-resolution cardiac electrophysiology in large animals in vivo.9
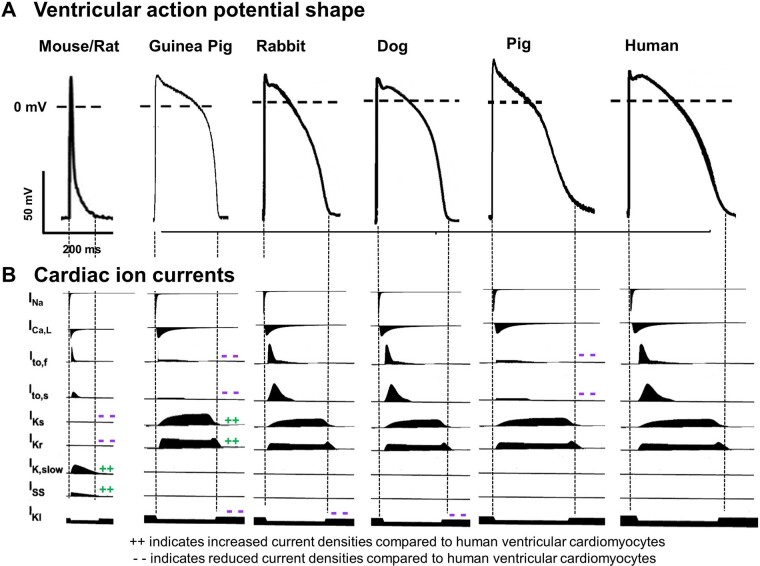
Figure 2.
Species differences in cellular electrophysiology. Schematic representation of action potentials (A) and major ionic currents (B) in commonly used species for cardiac electrophysiology research.
Whole-heart and cardiac tissue electrophysiology (ex vivo)
In 1895, Oscar Langendorff showed that an isolated mammalian heart can be kept alive for hours by blood perfusion through a cannula attached to the ascending aorta.10 Nowadays, retrograde Langendorff perfusion11,12 and its variants like the working heart setup13 are considered invaluable analytic tools to assess many important aspects of cardiac physiology. In the Langendorff-perfused heart electrophysiological properties (such as MAP and conduction velocities), arrhythmia inducibility, contractility and metabolics can be quantified at the whole-heart level at baseline and at pro-arrhythmic conditions such as ischaemia–reperfusion injury, and cell-based regeneration. The Langendorff-perfused heart also allows to manipulate electrical activation and propagation at the whole heart level without systemic circulatory effects or haemodynamic instability that may be encountered in vivo. In addition to MAP measurements (see above), the conventional sharp microelectrode technique has been used in numerous studies to investigate cardiac action potential characteristics, applied for the first time by Coraboeuf and Weidman in isolated heart tissue.14 This approach is however limited by the fact that only localized information is obtained. More recently, optical mapping has been emerged as a powerful tool to study electrical activity of the whole heart or intact parts of the cardiac conduction system: sinoatrial node (SAN),15 atrioventricular node or the Purkinje-fibres network.16 The principle of optical mapping is to irradiate the sample and detect the fluorescence emitted from fluorescent indicators (e.g. voltage and/or Ca2+ sensors). It is based on wide-field illumination of the whole heart or cardiac tissue, while an objective lens is used to collect the fluorescence signal. A high-speed imaging system (at kHz frame rate) is used to capture and visualize small variations in fluorescence intensity associated with voltage or Ca2+ transients. Multimodal (simultaneous) acquisition of voltage and Ca2+ signals is also possible by using spectrally distinct fluorescence dyes.17 Many electrophysiological properties can be assessed by mapping the heart on a single view, including detailed assessment of epicardial conduction velocities. In addition, multi-view panoramic mapping is particularly useful to study self-propagating rotors on the heart surface during arrhythmias. Excitation-contraction uncoupling agents, such as blebbistatin, have traditionally been used to avoid artefacts due to tissue movement. This is particularly useful in optical mapping of the pacemaker impulse in isolated atrio/sinus preparations. On the other hand, uncoupling molecules may affect arrhythmia inducibility due to mechano-electric feedback mechanisms18 and recent work has investigated mechanical phase singularities during arrhythmia.19 With novel optogenetics tools20 it is possible to achieve full optical control of cardiac electrical activity or sinus node pacemaking.15 To assess transmural cardiac electrophysiological heterogeneity and its consequences for arrhythmogenesis, canine isolated coronary-perfused ventricular wedge preparation and floating microelectrodes have been employed.21
Cellular electrophysiological techniques (in vitro)
In isolated cardiomyocytes, impaling with a sharp micro-electrode can accurately measure membrane voltage while minimally disturbing the intracellular environment, but the resistance associated with the small size of the electrode tip poses technical challenges for the injection of current and accurate voltage-clamp recordings of some currents. The patch-clamp methodology consecutively developed by Neher and Sakmann uses larger glass electrodes and allows single-channel recordings from the patch underneath the electrode (cell-attached patch), as well as whole-cell recordings after opening the membrane within the patch. In general, two different patch-clamp modes are used: current clamp in which currents are applied and resulting changes in membrane potentials are assessed (e.g. to measure APs), and voltage clamp which applies predefined membrane voltage protocols together with pipette/bath solutions and blockers to measure specific ion currents. With the conventional whole-cell approach, the opening of the membrane allows the pipette solution to dialyse the intracellular environment, thus providing experimental control over, e.g. intracellular ion concentrations. Alternatively, the whole-cell configuration can be established using pharmacological agents in the patch pipette that form pores in the cell membrane. This perforated patch-clamp methodology aims to maintain a more physiological intracellular milieu. Recent developments have also led to the dynamic-clamp technique, in which the current injected into a cell via a low-resistance electrode is a mathematical function of the instantaneous Em of the cell.22 This can be used to mimic the coupling of other cells (myocytes/non-myocytes) to an isolated heart cell23 or modulation of the biophysical properties of one or more currents.24 There are several limitations to this technique including the fact that dynamic clamp can simulate the electrical but not the ionic aspects of ion-channel activity, e.g. Ca2+ current without the Ca2+ ions. In addition to the more traditional patch-clamp approach, fluorescence-based techniques are increasingly used to measure membrane potential, as well as cellular Ca2+ transients and pro-arrhythmic Ca2+-release events.15,25,26 While such approaches allow for more high-throughput, non-invasive analyses, they have certain limitations: in particular, voltage-sensitive dyes do not provide information on actual membrane voltage (only relative values), which may potentially impact on AP results. Overall, single-cell cardiac electrophysiology continues to provide electrophysiologists with key information and technical challenges now and for decades to come. For a detailed overview of cardiac ion channels and APs, the interested reader is referred to the comprehensive recent review by Varro et al.27
Species differences in cardiac electrophysiology
Depolarizing cardiac ion channels/currents are highly conserved among species, with INa currents conducted by voltage-gated SCN5A/Nav1.5 channels as main depolarizing ion currents in atrial and ventricular cardiomyocytes and an important role for ICa, L currents (Cav1.2) as depolarizing ion current in the conduction system. Despite these similarities in depolarizing ion currents, pronounced species differences exist in heart rate, ranging from 600 b.p.m. resting heart rate in small animals such as mice, 300 b.p.m. in rats, and 150–200 b.p.m. in rabbits, to similar heart rates as in humans in bigger animals such as pigs and dogs (∼60–80 b.p.m.). Accordingly, AP duration is also different between species, largely due to important species-specific differences in repolarizing ion currents/channels (Figure 2).28
Mouse/rat
In rodents, the AP shape is markedly different than in human and other bigger species: the plateau phase of the action potential is missing, resulting in a more triangular AP shape with a very short APD. In contrast to humans, repolarization in mice and rats is mainly driven by the rapidly activating, slowly inactivating delayed rectifier potassium currents IK, slow1 and IK, slow2 and the fast and slow components of the transient outward potassium current Ito, f and Ito, s28; while the rapid and slow delayed rectifier K+ currents (IKr and IKs)—the main repolarizing ion currents in human cardiomyocytes—are functionally irrelevant. In addition, other repolarizing ion currents such as the atrial IKur are differentially expressed in rodents.29
Rabbit
Pronounced similarities exist in AP shape, as well as function and gating kinetics of various cardiac potassium channels between rabbits and humans. In both species, the rapid and slow delayed rectifier K+ currents (IKr and IKs) conducted by KCNQ1/KCNE1 and KCNH2 are the main repolarizing ion currents.28–31 In other potassium currents, however, some inter-species differences exist: In humans, Ito is formed of two distinct subtypes named as Ito, fast and Ito, slow—with fast and slow recovery from inactivation, determined by Kv3.4 and Kv1.4, respectively.32 In contrast, in rabbits Ito, slow is the primary transient Kv current in the left ventricle (LV),33 while in the right ventricle (RV) Ito, fast and its role in LQT1-related arrhythmogenesis has recently been confirmed.34
Zebrafish
Zebrafish also share pronounced similarities with human cardiac electrophysiology in terms of ventricular AP shape, AP/QT duration, and repolarizing ion currents, with an important role for IKr as main repolarizing current.35 While zebrafish have provided ground-breaking insights into (early) principles of heart development, the adult cardiac structure differs significantly between mammals and fish, with fish having only one atrium and one ventricle. Moreover, the body temperature is much lower in zebrafish, which affects biophysical ion channel properties, potentially decreasing its translational relevance regarding cardiac conduction and arrhythmia mechanisms.36
Pig
Porcine ventricular APs resemble those of humans in many aspects: configuration with dominant plateau phase, duration, rate dependence and transmural heterogeneity with populations of M-like cells.37 Major contributing ionic currents (fast Na+ current, INa; L-type Ca2+ current, ICaL; and IKr, IKs and inward-rectifier K+ current, IK1) similar to those of human have been reported,38,39 but atrial and ventricular ion currents and calcium handling are less well-characterized than for other species. One major difference is the lack of voltage-dependent, 4-aminopyridine-sensitive Ito in porcine myocytes.40 A similar ventricular AP pattern was also shown in minipigs.41,42
Dog
From all experimental species, canine ventricular action potential probably shows the highest level of similarity to human.43–46 Various aspects of cardiac electrophysiology and arrhythmogenesis have been addressed in canine preparations with potential translational relevance.47–49 However, although contributions of major ionic currents in canine cardiac cells seem very similar to human, also significant quantitative differences exist—for example in the sensitivity to IKr-block—which have to be considered when translating to human conditions.30,50
Animal models used in cardiac electrophysiology research
Historically, large animals have been preferentially used for cardiac electrophysiological studies, given their relative similarity to human hearts and their heart size allowing for the use of multiple electrodes and (transmural) needles. Dogs are arguably the most often used large animal model, followed by pigs, rabbit, guinea pigs, sheep, cats, and goats. Dogs are easily accustomed to experimental conditions (e.g. to chronic and conscious instrumentation). Pigs have a heart anatomy closely resembling human, and are therefore increasingly used to refine novel (catheter-based) arrhythmia mapping, ablation, and device-based pacing techniques. Porcine preparations are generally more susceptible to (ventricular) arrhythmias and SCD than human hearts,51 whereas sheep and goats are more resilient (stable). In a proof-of-concept study in chronically instrumented conscious goats, transition from paroxysmal atrial fibrillation (AF) to sustained AF due to chronic atrial pacing was documented (‘AF begets AF’52) allowing to elucidate underlying electrophysiological, contractile and structural remodelling in detail.53,54 Mechanisms of ventricular ‘torsades-de-pointes’ arrhythmias, both spontaneous and drug-induced, have been addressed in hypertrophied hearts following chronic atrio-ventricular block in dogs55,56 and in genetically modified rabbit models of inherited arrhythmia syndromes.57 In a multi-scaled effort, contributions of remodelled ionic currents,58 calcium handling,59 spatial and temporal electrophysiological heterogeneity,60,61 and autonomic modulation62 to triggering and maintenance of ventricular arrhythmia and sudden cardiac death have been established.
Due to increasing costs and ethical restrictions for large-animal research, and promoted by the miniaturization of in vivo equipment, rats and mice are increasingly used. Mice carry certain limitations due to intrinsic differences compared to humans, including ion current characteristics (see ‘Species differences in cardiac electrophysiology’ section), heart rate, and (basic) sympathetic tone. To their advantage, mice are easy to breed, relatively cheap to house, and can be genetically modified, e.g. by overexpression of genes of interest using a (cardiac-specific) promotor, by deletion through knock-out strategies or CRISPR-Cas technology, by tamoxifen-induced conditional targeting, or AAV-based gene transfer to specific cardiac regions of interest.63 Mice are typically bred by inbreeding, resulting in identical genetic backgrounds. However, mice can also be outbred to enable identification of potential genetic modifiers.64 Similar approaches include the use of outbred mice, recombinant inbred rodents, or randomly mutagenized mice to identify novel genes modulating cardiac traits including electrical function.65,66
Wild-type or genetically modified mice are easily subjected to well-established interventions simulating clinical triggers of arrhythmogenic cardiac remodelling, e.g. LV hypertrophy (by aortic banding, nephrectomy/volume overload or chronic administration of isoproterenol or angiotensin-II) or ischaemia/infarction (coronary artery ligation), ultimately resulting in heart failure (HF). These models have been very useful in elucidating pro-arrhythmic mechanisms through detailed in vivo, ex vivo, whole heart, and cellular electrophysiological studies (as described in detail in ‘Cellular and whole heart electrophysiology techniques’ section), and molecular investigations, often performed in the same hearts or in distinct cardiac regions, including conduction system, atria, LV vs. RV, transmural, etc. Ageing studies are more feasible given the short murine life-span of around 18–24 months. Interestingly, despite their small heart size, mice are able to develop sustained complex arrhythmias such as AF and ventricular fibrillation (VF).67,68
Thanks to novel developments in animal transgenesis, rabbits—that more closely resemble humans in terms of cardiac electrophysiology28—have also entered the range of species in whom genetic manipulation can successfully replicate certain (genetic) human cardiac diseases such as hypertrophic cardiomyopathy (HCM) or channelopathies.57,69–71 In addition, pigs, which also closely resemble humans, have recently been successfully modified genetically to mimic Brugada syndrome (BrS).72
In summary, despite their intrinsic limitations indicated above, animal models allow to examine, modulate and dissect different components of arrhythmogenicity, i.e. increased triggered activity, alterations in the myocardial substrate (conduction, repolarization) and neurohumoral/systemic modulation. Of note, ECG and cardiac phenotype of the chosen animal model should mimic clinical features to facilitate translation of experimental results into clinical concepts.
Cellular models used in electrophysiology research
Various cellular models can be used for electrophysiological studies. To study characteristics and pharmacology of specific ion channels, these can be transiently expressed in heterologous expression systems such as Chinese Hamster Ovary (CHO) cells, Human Embryonic Kidney (HEK293) cells, and Xenopus oocytes. These cells are inexpensive, can be kept in culture for a long period of time, and are relatively easy to transfect and patch. By co-transfecting cDNA of the ion channel in question with accessory subunits, interacting proteins, and/or other genes of interest, their modulatory effect can be investigated. Furthermore, expression systems allow for functional investigation of the consequences and putative pathogenicity of mutations identified in patients with inherited cardiac arrhythmias. However, these heterologous cell systems do not fully recapitulate the cardiomyocyte environment and may have distinct differences in intracellular pathways and ion channel trafficking systems and/or lack certain interacting proteins. This can be (partly) overcome by using for instance HL-1 cells, which originate from the AT-1 mouse atrial cardiomyocyte tumour lineage, and partly retain cardiac morphological and functional properties of atrial cardiomyocytes. HL-1 cells can be cultured, transfected and transduced, and studied by (electro)physiological analyses. Similarly, neonatal cells from rat, mouse or rabbit cardiomyocytes can be kept in culture for days and are relatively easy to transfect or transduce, allowing overexpression or knock-down of genes followed by electrophysiological assessment. However, the immature nature of these cells results in certain differences in, e.g. cardiac ion channel isoform expression, t-tubule structure, and post-translational modification compared to adult cardiomyocytes.
For electrophysiological assessments under more physiological conditions, adult cardiomyocytes isolated from animal models or human heart samples are generally considered most appropriate for investigating ion channel (dys)function and AP characteristics by patch-clamp analysis, as well as fluorescence-based quantification of intracellular calcium homeostasis. Freshly isolated cardiomyocytes of the working myocardium, the SAN or of the atrioventricular conduction system retain most of their anatomical and functional features, including (stable) resting membrane potentials, contractile properties, and subcellular distribution of ion channels, although dissociation-induced changes have been described. They furthermore allow for isolation and investigation of cardiomyocytes from various regions of the heart, including right and left atria,73 LV vs. RV, (sub)epicardium vs. (sub)endocardium vs. (mid)myocardium, and Purkinje fibres from large mammals74 or mice expressing EGFP-labelled connexin-40.75 Nevertheless, the disruption of cardiomyocytes from adjacent cells and the extracellular matrix likely does have functional consequences: for instance, Na+-current density is higher at the intercalated disc region of coupled cells than in isolated cells.76 Primary adult cardiomyocytes isolated from some animal models77 and human samples78 can also be kept in culture during a few days for gene modification studies. However, in both neonatal and adult cardiomyocytes, the culturing process may itself induce structural and functional remodelling. On the other hand, studies in human cardiomyocytes critically depend on availability of patient tissues and there is often a limited availability of appropriate controls. Electrophysiological properties of the intact SAN region can be characterized using the sharp intracellular electrode technique on SAN tissue strips,79,80 using surface electrograms in combination with optical mapping of membrane potential in isolated SAN to record impulse conduction81,82 or confocal live imaging of intracellular Ca2+ release.83
Human-induced pluripotent stem-cell-derived cardiomyocytes in electrophysiology research
The use of human-induced pluripotent stem-cell-derived cardiomyocytes (hiPSC-CMs) has recently gained prominence to screen novel drugs for potential pro-arrhythmic effects as a consequence of drug-induced block of IKr84 in the comprehensive in vitro pro-arrhythmia assessment (CiPA) initiative, as well as to generate experimental models for basic research into human genetic diseases that predispose to lethal cardiac arrhythmias. In addition to conventional patch-clamp and microelectrodes approaches, a variety of invasive and non-invasive methods exist for the electrophysiological analysis of hiPSC-CMs, which have both advantages and limitations depending on the research question to be investigated. While a promising tool for (compound) screening purposes, automated patch clamp requires high density, homogeneous single-cell suspensions which can be challenging when working with hiPSC-CMs multi-electrode arrays (MEAs) allow long-term measurements from clusters and monolayers of hiPSC-CMs, with field-potential duration used as measure of QT and AP duration, and activation maps for conduction velocity measurements. Similarly, fluorescence-based measurements using voltage- and calcium-sensitive dyes allow for more high-throughput, non-invasive analyses.
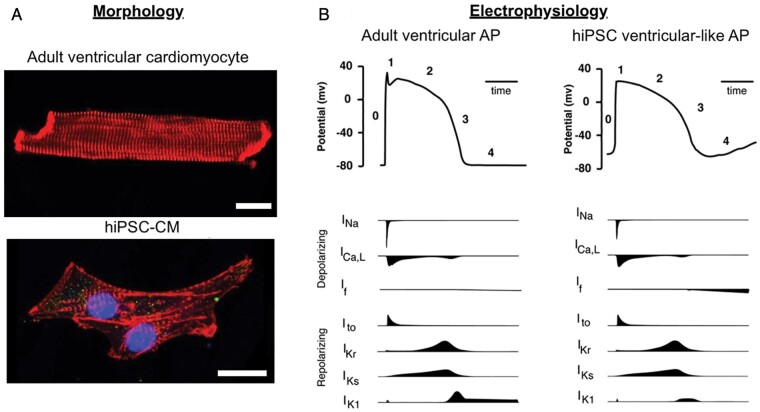
A frequent criticism of iPSC-CMs is their embryonic electrophysiological phenotype (Figure 3). Human-induced pluripotent stem-cell-derived cardiomyocytes lack T-tubules [where the Ca2+ released by ryanodine receptors (RyR2) locally affects sarcolemmal ion channels and transporters], hampering analyses on the mechanisms linking intracellular Ca2+ handling abnormalities and triggered activity.26 This problem can be partly solved by obtaining more mature hiPSC-CMs with hormonal treatment85 or using nanostructured/biomimetic substrates.86,87 APs in hiPSC-CMs are generated by a sequential activation of INa, ICa, L, and repolarizing K+ currents similar to adult ventricular cardiomyocytes, and accordingly have demonstrated similar mutation-induced electrophysiological consequences as adult mutant mouse cardiomyocytes,88 as well as clear similarities between in vitro effects of pharmacological interventions in hiPSC-CMs and their reported clinical efficacy.89,90 However, hiPSC-CMs display an unstable diastolic potential caused by insufficient expression of IK1 and significant expression of the funny current (If).25 To overcome this, IK1 density can be artificially enhanced by cells transduction with Kir2.191 or injection of an in silico IK1, inducing a more physiological and stable resting membrane potential, in addition to a ventricular-like, more ‘mature’ action potential morphology.92,93,94 IKs density and APD-prolongation in response to IKs blockade are generally small and highly variable among hiPSC-CMs Although IKs with proper kinetics can be recorded in hiPSC-CMs,94,95 in some reports the current pharmacologically identified as IKs has kinetics sharply diverging from that observed in mature myocytes. IKr can be more consistently recorded in hiPSC-CMs, whose APD has been proposed as a reporter of drugs’ arrhythmogenic risk.96 It also remains unclear whether stem-cell-derived cardiomyocytes possess one general phenotype or whether the process results in a mixture of nodal, atrial and ventricular cells.97 Certainly, it appears that the differentiation conditions can be manipulated to generate cells with a predominately atrial or ventricular phenotype.98,99
Figure 3.
Comparison of morphological and electrophysiological features of adult ventricular cardiomyocytes and human-induced pluripotent stem cell (hiPSC)-derived cardiomyocytes. (A) Microscopic image of an adult cardiomyocyte from mouse stained with anti-alpha-actinin (red) and a hiPSC-derived cardiomyocyte stained with anti-alpha-actinin (red), DAPI (blue), and Nav1.5 (green). Scale bars are 20 µm. (B) Schematic of ventricular action potentials (APs) during rapid upstroke (Phase 0), early repolarization (1), plateau (2), late repolarization (3), and diastole (4). The ionic currents involved are shown below the AP traces.
Human-induced pluripotent stem-cell-derived cardiomyocytes can be cultured as a monoculture or mixed with other cell types, typically cardiac fibroblasts. While isolated hiPSC-CMs have a variable electrophysiological phenotype, monolayers of electrically coupled cells generally have a more stable spontaneous rate and electrophysiology. Three-dimensional tissue preparations, referred to as engineered heart tissue, can be created from hiPSC-CMs held within a matrix (e.g. fibrinogen) to create microspheres, trabeculae or sheets of tissue.100 These 3D preparations are commonly stable in beating rate and electrophysiology over many weeks.101
Integration of experimental electrophysiology data into computational models
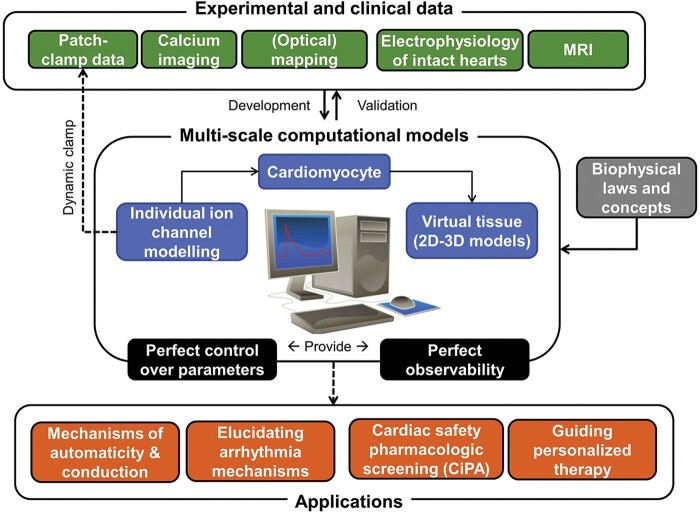
Since the first computational models of the cardiac AP were developed in the 1960s, in silico modelling of cardiac electrophysiology has advanced significantly.102 Ion channel and AP models are available for most cell types and many species.103,104 Tissue and organ-level models have been developed and can be used to simulate atrial and ventricular arrhythmias in the presence of different structural and functional substrates.105 Computer models provide perfect control over individual parameters, overcoming limited selectivity of most pharmacological tools or compensatory effects in genetic animal models (Figure 4). Computer models also offer perfect observability, enabling detailed analyses of multiple components (e.g. ion currents, membrane potential, intracellular concentrations) at the same moment in time, which is not possible during experiments. Accordingly, computer models have been used extensively to study the mechanisms of heart automaticity106,107 and arrhythmia.103,104 In addition, computer models have been used to improve our knowledge of the contribution of individual ionic currents in combination with dynamic clamp recording systems.22,108 Recently, the first applications of in silico models with important clinical implications have emerged. Initial proof-of-concept studies with prospective, simulation-guided ablation of atrial and ventricular arrhythmias have been conducted.109–111 Cardiac cellular electrophysiology models play a central role in predicting the proarrhythmic risk of new drugs as part of the CiPA initiative.112,113
Figure 4.
Summary of the role of computational modelling in cardiac electrophysiology. Experimental and clinical data are combined with biophysical laws and concepts for the development and validation of multi-scale (channel, cardiomyocyte, two-dimensional, and three-dimensional tissue) computational models. These models provide perfect control over parameters and perfect observability. Applications of these models are indicated with dashed arrows and include dynamic clamp (direct application of ion-channel models in patch-clamp experiments), as well as mechanistic, regulatory and clinical applications.
The role of computational models in clinical arrhythmia management and drug development is expected to increase, raising important questions regarding model complexity, validation, and uncertainty quantification.113,114 Traditionally, the distinction between model calibration (parameter estimation) and validation using independent data has been largely ignored for cardiomyocyte models, due to the paucity of experimental data. Moreover, only a single ‘representative’ model was generated and analysed. However, it has become increasingly common to study populations of models that reflect cell-to-cell variability.115 In addition, several studies have employed true validation data sets in the development of new cardiomyocyte models,116 as well as in their application (e.g. predicting the proarrhythmic risk of drugs that were not used to calibrate the model in the CiPA initiative112). As our understanding of cardiac cellular electrophysiology expands, models are becoming increasingly complex, e.g. integrating molecular ion-channel dynamics, localized changes in calcium handling and post-translational regulation through signalling cascades,117,118 making extensive validation and establishing the context of use114 increasingly challenging. At the same time, this level of complexity can currently not be simulated at the organ level, so that new approaches are needed to span the different spatial and temporal scales involved in cardiac electrophysiology. For example, recent approaches have made it possible to investigate the interaction between re-entry-induced Ca2+ loading, subsequent triggered activity and re-induction of re-entry in tissue-level models with spontaneous Ca2+-release events based on detailed subcellular models.119 Above all, close collaboration between experimental cardiac electrophysiologists, computational modellers, and clinicians is needed for the development of well-validated models and their clinical applications.
Suitability of individual models for specific research questions
Simplification vs. integration
Although pro-arrhythmic mechanisms may occur on the single cell-level, arrhythmogenesis in vivo is strongly affected by factors at the tissue/organ level and by neurohumoral activity. The challenging task is integration from molecular function (artificial membranes → heterologous expression systems) through the subsequent levels of complexity (myocyte → tissue → organ). A crucial question in this process is whether the detected molecular abnormality is ‘biologically relevant’. This depends on its magnitude, but also on system responsiveness. The latter may vary, for example, according to the AP phase on which the abnormality impacts,120 the presence of buffering mechanisms,121 connectivity to neighbouring cells,122 etc. In silico modelling may provide a powerful tool to this end, but there is room for improvement also at the experimental level. One aspect that may deserve attention is system behaviour under realistic (‘dynamic’) conditions, not considered in classical biophysical evaluation. At cardiomyocyte level, ‘action-potential clamp’ and ‘dynamic-clamp’ are simple and powerful tools; they can provide stringent optimization of numerical models123 and experimentally test AP response to channel dysfunction/modulation.120 At the tissue level, integrated information on dynamic response can be obtained from ‘electrical restitution’ of propagation and repolarization, which have been directly linked to arrhythmogenesis.124
Disease-modelling of common (acquired) arrhythmogenic disorders
Beside the biological relevance of an abnormal finding in the whole body context, the relevance of the animal model is critical for clinical translation. Key features of the clinical phenotype that are relevant for diagnosis, therapy, and/or prognosis must be reproduced by the animal model (Table 1). Fortunately, this seems to be the case in many instances, surprisingly even when species differences in electrical activity are substantial (e.g. for murine models).125,126
Table 1.
Disease-specific experimental models for cardiac electrophysiology research
| Species | Experimental model | Important facts | References |
|---|---|---|---|
| Common (acquired) arrhythmogenic disorders | |||
| Atrial fibrillation | |||
| Mouse | Genetic models with mutations in ion channels or fibrosis-related genes |
|
127–130 |
| Goat, dog, pig, rabbit | Atrial tachy-pacing-induced AF |
|
52 , 127 , 128 |
| Rat, rabbit, dog, sheep | Myocardial-infarction/ischaemia-induced AF |
|
133–135 |
| Dog, sheep | Vagal-induced AF |
|
127 |
| Dog, sheep | Sterile pericarditis |
|
132 |
| Dog | Ventricular tachypacing-induced heart failure |
|
127 , 128 |
| Horse | Spontaneous AF (no alterations necessary) |
|
128 |
| Cell lines | Fibroblasts, HEK-cells, HL-1 cells |
|
136 |
| Human | hiPSC-CM |
|
136 |
| Human | Isolated human atrial CM |
|
136 |
| In silico | Cellular, tissue, and organ models |
|
104 , 109 , 111 , 137 , 138 |
| Heart failure with reduced ejection fraction | |||
| Rat | Spontaneous hypertensive rats or salt-sensitive rats |
|
141 |
| Mouse, rat, rabbit, pig, sheep | Coronary-artery ligation |
|
141 , 142 , 144 , 146 |
| Mouse, rat | Aortic constriction |
|
141 |
| Rabbit, dog, pig | Ventricular tachypacing |
|
143 , 146 |
| Mouse | Chemical anticancer therapy |
|
141 |
| Human | LV samples, explanted hearts |
|
147 , 148 |
| Heart failure with preserved ejection fraction | |||
| Mouse, rat | Disease models for hypertensive heart disease + metabolic syndrome, chronic kidney failure |
|
151 , 154 , 155 |
| Rat | Chronic volume-overload (high-salt diet) model for HFpEF |
|
152 , 153 , 156 |
| Myocarditis | |||
| Mouse | Coxsackie B-virus |
|
161 |
| Mouse | Immunization with cardiac myosin |
|
162 , 163 |
| Dog | Canine parvovirus or non-viral pathogens (Chagas) |
|
164 |
| Sinus and AV node diseases | |||
| Mouse | Genetic mouse models with mutations in ion channels (and ankyrin B) involved in SAN pacemaking |
|
167–173 |
| Mouse, rat, rabbit, dog | Heart failure, tachypacing-induced AF, diabetes-models |
|
177–183 |
| Inherited arrhythmogenic disorders | |||
| Ion channel diseases with altered Na+-channel function | |||
| HEK/CHO cells | Transfected HEK/CHO-cells expressing various SCN5A mutations |
|
187–190 |
| Dog | Arterially perfused RV wedges with Na+/Ca2+ blockade or activation of INa, late |
|
184 , 186 , 187 |
| Mouse | Genetic mouse models with various scn5a mutations |
|
168 , 176 , 189 , 191–197 |
| Pig | Genetic pig model with SCN5A(E558X/+) mutation |
|
72 |
| Human | SCN5A mutant hiPSC-CM lines |
|
88–90 , 198 |
| In silico | Incorporation of various SCN5A mutations in computational models |
|
199 , 200 |
| Ion channel diseases with altered K+-channel function | |||
| HEK/CHO cells | Transfected HEK/CHO-cells expressing various K+ channel mutations |
|
|
| Mouse | Transgenic mouse models expressing human K+ channel mutations (KCNQ1, KCNH2) |
|
204–206 |
| Mouse | Mouse models with altered murine K+ channels (Kv1.4, Kv4.2) |
|
207 |
| Zebrafish | Zebrafish models with loss-of-function in KCNH2 |
|
208 , 209 |
| Zebrafish | Zebrafish models with gain-of-function in KCNH2 |
|
210 |
| Rabbit | Transgenic rabbit models expressing loss-of-function mutations in K+ channels (KCNQ1-Y315S; KCNH2-G628S; KCNE1-G52R) |
|
57 , 70 , 211–213 |
| Rabbit | Transgenic rabbit models expressing gain-of-function mutations in K+ channels (KCNH2-N588K) |
|
71 |
| Human | LQT1/LQT2/SQT1/SQT2 hiPSC-CM lines with mutant KCNQ1/KCNH2 |
|
93–95 , 214–216 |
| In silico | Incorporation of various K+ channel mutations in computational models |
|
|
| Catecholaminergic polymorphic ventricular tachycardia | |||
| HEK/CHO cells | Transfected HEK/CHO-cells expressing various RyR2 or Casq1 mutations |
|
|
| Mouse | Heterozygous knock-in mouse models with various RyR2 or Casq1 mutations |
|
218–221 |
| Human | hiPSC-CM lines with mutant RyR2 or Casq1 |
|
26 |
| In silico | Incorporation of various RyR2 or Casq1 mutations in computational models |
|
|
| Genetic cardiomyopathies | |||
| Mouse | Mouse models with sarcomeric mutations |
|
222 , 223 , 225–228 |
| Mouse | Mouse models with desmosomal mutations |
|
231–233 |
| Rabbit | Rabbit models with sarcomeric mutations (β-MHC-Q403, ELC1v, or cTnI) |
|
229 |
AF, atrial fibrillation; APD, action potential duration; AV, atrio-ventricular; CHO, Chinese Hamster Ovary; CM, cardiomyocyte; HCM, hypertrophic cardiomyopathy; hiPSC-CM, human-induced pluripotent stem-cell-derived cardiomyocyte; HFpEF, heart failure with preserved ejection fraction; LV, left ventricle; RV, right ventricle; SAN, sinoatrial node; SND, sinoatrial node dysfunction; VT/VF, ventricular tachycardia/ventricular fibrillation.
Atrial fibrillation
As the most prevalent clinically relevant arrhythmia with significant impact on morbidity and mortality, numerous studies have attempted to model AF.127,128 However, all models used to study AF have limitations in terms of predictability, reliability or transferability. Due to the multifactorial nature of AF, to date even the most sophisticated model is unable to fully recapitulate the diversity of aetiologies and pathological mechanisms of human AF. Thus, it is important to keep in mind the question being addressed and the limitations of each model.
Rodents are widely used models due to their easy handling, low costs, and easy genetic manipulation, despite the aforementioned electrophysiological differences to humans limiting transferability. Nonetheless, a large number of transgenic mouse models with either spontaneous AF or increased vulnerability to burst-pacing induced AF have been developed.129 In addition, rodents have been used to study AF promotion due to a large number of risk factors, including endurance exercise and sleep-disordered breathing.130 Rodent models are suitable to perform in vivo and ex vivo experiments in isolated hearts and to study new therapeutic interventions early in the development pipeline.129
Large animal models are commonly used in preclinical AF studies.127,128 AF is easily induced and relatively stable in goats, making this model suitable to study the progressive nature of AF.52 Goat, dog, pig, and rabbit models have also been used to study atrial tachycardia-related electrical and structural remodelling using rapid atrial pacing.127,131 The atrial burst-pacing model can be used for simulating paroxysmal AF but is also commonly used to study AF inducibility in the presence of disease-related background remodelling. For example, atrio-ventricular (AV) block in goats leads to progressive atrial dilatation and prolonged AF.127 Dogs and sheep are typically used for vagal or ischaemic AF promotion and in sterile pericarditis as a model of post-operative AF.127,132 Furthermore, the dog ventricular tachypacing model of congestive HF has provided important insight in the relation between AF and HF.127,128 Although this model does not present big changes in refractoriness, it has pronounced structural remodelling and impaired conduction. Models investigating AF promotion after myocardial infarction are available in several species and have revealed complex changes in atrial electrophysiology, which depend on the presence of atrial ischaemia and timing of the experiments (acute vs. chronic setting).133–135 Recently, horses have emerged as an interesting model for AF because, like humans, they spontaneously develop AF.128
Many cell lines are available for AF research, including fibroblasts, stem cells, HEK-293T, and HL-1 cells.136 Human iPSC-derived cardiomyocytes can model familial AF, but atrial-like cells are required and their similarity to adult human atrial cardiomyocytes is limited. Importantly, none of these cellular models can capture the decades of atrial remodelling present in most AF patients. Isolated human atrial cardiomyocytes may therefore represent the most clinically relevant cellular AF model for pharmacological testing or adenovirus-based gene-therapy studies. Nevertheless, tissue is generally only available from patients undergoing open heart surgery and is restricted to parts of the atria (e.g. appendages). In addition, these models lack organ-dependent environmental factors.136
In silico models are powerful tools for testing hypotheses, predicting effects of new therapeutic targets or capturing dynamic systems at different scales (cells-tissue-organ-person),104 and are increasingly used in AF research, e.g. to study antiarrhythmic drug137,138 and ablation therapy.109,111 Combined, these experimental and computational models can increase our understanding of AF mechanisms and facilitate the development of novel therapeutic approaches.
Arrhythmias in heart failure with reduced ejection fraction
Heart failure often develops secondary to other pathologies such as myocardial infarction, hypertension, diabetes, anticancer therapy, kidney failure, infection, or genetic cardiomyopathies. About half of the patients diagnosed with HF die within 5 years, around 50% due to by pump failure and 50% by sudden cardiac death due to fatal arrhythmias.139
Several animal experimental models mimicking the various aetiologies have been developed to explore the mechanisms involved both in cardiac failure and arrhythmogenesis. Cardiac remodelling in HF is often characterized by ventricular hypertrophy of the remaining viable tissue. It has to be kept in mind, however, that only a minority of patients with LV hypertrophy develop HF.140 Most HF animal models employ rodents and concern heart failure with reduced ejection fraction (HFrEF).141 To reproduce hypertension-related HF, genetically selected rats, e.g. spontaneous hypertensive rats or salt-sensitive rats (Dahl), are used. However, in most animal models, enhanced afterload is obtained by surgery via aortic constriction. Other surgical techniques include coronary artery ligation, which can be permanent to induce a myocardial infarction, or temporary to induce ischaemia reperfusion and mimic reperfusion in the hospital in patients suffering an infarction. Chemical treatment with anticancer therapies, including monocrotalin to induce RV HF and pancreatic toxic compounds to induce type-1 diabetes are also commonly used, together with dietary interventions and genetic models to induce glucose intolerance and type 2 diabetes. Rodent models of HF are reviewed in Gomes et al.141
Besides small rodents, other experimental animal models of HF are used in an attempt to get closer to human heart function, including rabbits with combined pressure and volume overload or coronary artery ligation,142 cats with pressure overload, ventricular tachypacing in dogs143 and pigs, pigs with myocardial infarction,144 and sheep.145 More details, as well as advantages and disadvantages of these bigger animal experimental models have recently been reviewed.146
Finally, human LV samples or complete explanted hearts are used by some groups to investigate HF-related electrophysiological remodelling,144,147,148 but these studies are often limited by the limited availability of appropriate healthy heart tissue as control.
Arrhythmias in Heart Failure with Preserved Ejection Fraction
Incidence of arrhythmias (in particular AF) and sudden cardiac death is also increased in Arrhythmias in heart failure with preserved ejection fraction (HFpEF) patients,149 although the true prevalence of arrhythmogenic sudden death requires further study.150 Developing appropriate animal models for HFpEF has been complicated by the heterogeneity of clinical phenotypes. However, in the past decade models of hypertensive heart disease, metabolic syndrome, chronic kidney failure, and ageing have been developed that mimic relevant clinical features of HFpEF.151 Yet, to date only few studies, mainly in rodents, have investigated pathomechanisms of arrhythmias in these models. In rats with HFpEF due to chronic volume overload (high salt diet), spontaneous ventricular tachycardia (VTs) were documented and related to delayed repolarization.152,153 Increased intracellular Ca2+ load and altered activity of the Na+/Ca2+ exchanger have been implicated in cellular arrhythmogenesis in a cardiorenal HFpEF model.154,155 In rats predisposed to HFpEF, age-related atrial remodelling (fibrosis, enlargement, conduction abnormalities) facilitates AF.156 Clearly, the complex interaction between cardiac remodelling and arrhythmogenesis in HFpEF warrants further research in clinically relevant animal models.
Myocarditis
Arrhythmias are a common manifestation of myocarditis (incidence up to 45%157). Most common arrhythmias are VT, VF, and AV block.158 Arrhythmogenic mechanisms are mainly triggered activity (due to inflammatory modulation of ion-channel properties in acute myocarditis) and re-entry (scar-related in chronic myocarditis).159 Transition to dilated cardiomyopathy occurs in up to 50% of cases.160 In mice, infection with a Coxsackie Virus B strain (Nancy strain) is the most commonly used model of acute infectious myocarditis, but a variety of other rodent models exist.161 Non-infectious autoimmune myocarditis is often induced by immunization of cardiac myosin.162,163 Of note, time course and extent of remodelling in myocarditis in mice are strongly strain dependent.161,163 Dogs are the most common large animal model for investigating arrhythmias in myocarditis, facilitating ambulatory monitoring.164 Canine parvovirus is the best studied natural pathogen associated with myocarditis, whereas experimental canine models have focused on non-viral pathogens (e.g. Chagas disease). Future studies may profit from more standardized experimental protocols as a basis for pooled data analyses.161
Sinus and atrioventricular node disease
Sinoatrial node dysfunction (SND) and AV block together account for over half of the permanent electronic pacemaker implantations worldwide. Sinoatrial node dysfunction can be distinguished into primary familial or secondary forms, which are associated with cardiovascular or systemic disease,165 and age-related SND.166 During the last two decades, models of primary and secondary SND forms have been developed. Genetically modified mouse models of inherited SND due to mutations in ion channels involved in SAN pacemaking have been created by global or conditional gene knockout,167–170 or by heart specific and time controlled expression of mutant ion channel proteins.171 These include If (HCN4),171,172 L-type Cav1.3,167 Nav1.5 channels,168 and RyR2.83 All these models except RyR2 also present with variable degrees of AV block, thus providing mechanistic insights about the origin of primary heart block. In addition, mouse models of primary SND due to lack of the scaffolding protein Ankyrin-B have been developed.173 These mouse models have provided insights about the mechanisms of primary familial SND forms, such as SND associated with ventricular non-compaction,174 the sinus node dysfunction and deafness syndrome,169 autoimmune congenital heart block,175 and SND associated with Lev-Lenègre syndrome.176
Numerous models of secondary SND exist. Models of SND secondary to HF have been developed using dogs,177 rabbits,178 and mice.179 A canine AF model induced by rapid atrial pacing also presents with SND180 and has provided insights into the relationship between AF and SND. Animal models of diabetes also often present with SND,181 enabling studies into the mechanistic link between these pathologies. Recently, models of bradycardia182 and AF130 secondary to long-term intensive exercise have been developed. Finally, age-related SND has been studied using old mice and rats.183
Disease-modelling of inherited arrhythmogenic disorders
Ion channel diseases with altered Na+-channel function
Mutations in SCN5A encoding NaV1.5 lead to disease entities associated with reduced peak INa (cardiac conduction/Lev-Lenègre disease; BrS), increased late INa (LQTS type 3, LQT3), or a combination of these (overlap syndrome).
Pharmacological interventions that interfere with the inactivation of Na+ channel (sea anemone toxin II, veratridine, anthopleurin) have been exploited for understanding the contribution of late INa to AP repolarization and arrhythmogenesis on cellular, multicellular and organ levels. Such experiments in canine arterially perfused LV wedges184 and rabbit Langendorff-perfused hearts185 have revealed mechanistic insights into LQT3 arrhythmogenesis. Pharmacological modulation of canine arterially perfused RV wedges with a combination of Na+ and Ca2+-channel blockers and K+-channel openers has also been employed to model BrS.186,187
Expressing SCN5A mutant channels in expression systems (e.g. HEK293/CHO) allows detailed assessment of the biophysical consequences,187,188 but may not fully recapitulate the cardiomyocyte environment.189,190 Scn5a transgenic mice may overcome such shortcomings, with Na+-channel contribution to APs being relatively comparable between mouse and human. Indeed, cardiomyocytes from Scn5a-1798insD mice showed biophysical properties in line with a clinical overlap syndrome, whereas previous studies in expression systems did not.189 Scn5a mouse models also allow assessment of the functional impact in distinct regions of the myocardium and conduction system.168,189,191,192 Furthermore, their use has provided insight into pro-arrhythmic intracellular Na+ and Ca2+ dysregulation and (age-dependent) electrical and structural remodelling.176,192,193 Indeed, Na+-channel gain of function (late INa enhancement as in LQT3) is arrhythmogenic mainly because it perturbs intracellular Na+ homeostasis, leading to Ca2+-store instability and energy imbalance.194 Finally, they allow exploration of the modulatory role of the autonomic nervous system, co-morbidities and genetic modifiers, and enable chronic pharmacological studies.195–197 More recently, a pig model carrying a BrS-associated SCN5A mutation was successfully generated, displaying conduction slowing and increased susceptibility to ventricular arrhythmias.72 While such large animal models may have some benefits over mice in terms of clinical transferability, their generation is time-consuming and costly, thus limiting their availability for research.
In recent years, a number of SCN5A mutant hiPSC-CM lines have been generated, which recapitulate nicely the LQT3, BrS, and/or overlap syndrome phenotypes.88,89 These hiPSC-CMs have so far been predominantly used for pharmacological studies90,198 but may also be useful for prediction of mutation pathogenicity and patient-specific arrhythmia risk.89 Sophisticated computational modelling could theoretically also predict pathogenicity,199 or at least INa abnormalities,200 directly from genetic variants. Unfortunately, the accuracy of such predictions is still inadequate for SCN5A; thus, variant’s biophysical descriptors must still be obtained experimentally. Whereas interpreting loss of Na+-channel function in terms of conduction disturbance (Lenegre’s syndrome) is straightforward, modelling BrS is far more challenging. The BrS phenotype can be reproduced as a ‘repolarization’201 or ‘propagation’202 disorder, further underscoring the increasingly recognized complexity of the disorder.
Ion channel diseases with altered K+-channel function
Mutations in genes encoding for cardiac repolarizing K+ currents lead to disease entities associated with altered cardiac repolarization (LQTS and SQTS).
Mice were the first species utilized to generate genetic models of K+ channelopathies. They, however, do not represent an optimal model organism for modelling cardiac K+ channel-related diseases (LQTS, SQTS) due to the aforementioned differences in cardiac repolarization patterns and responsible K+ currents between mice and humans (‘Species differences in cellular electrophysiology’ section and Figure 2).203,204 Consequently, mouse models with genetic manipulation of human channel subunits usually do not show a proarrhythmic phenotype,204,205 unless significant remodelling of other channels relevant for murine cardiac repolarization occurs.206 Genetic manipulations of K+ channels contributing to murine cardiac repolarization (Kv 4.2, Kv.1.4), in contrast, may result in arrhythmias when associated with increased dispersion of repolarization.207
Another animal model frequently used in LQTS and SQTS-related research is the zebrafish. Spontaneous LQTS and SQTS zebrafish mutants exist and can be found by screening-approaches. The zebrafish breakdance, which carries the trafficking-deficient KCNH2-I59S mutation, recapitulates severe forms of human LQT2 with 2 : 1 atrioventricular block due to prolonged ventricular APD.208,209 The zebrafish reggae expresses the missense mutation KCNH2-L499P, producing similar IKr dysfunction and shortened QT as observed in human SQT1.210 But these models do have limitations due to their differences in cardiac morphology and structure as highlighted in ‘Species differences in cellular electrophysiology’ section.
Several transgenic rabbit models for K+ channel-related LQTS have been generated by over-expression of human loss-of-function mutated K+ channels: LQT1 (KCNQ1/KvLQT1-Y315S), LQT2 (KCNH2/HERG-G628S),57 and LQT5 (KCNE1/minK-G52R).70 Similarly, a short-QT syndrome (SQTS) rabbit model has been engineered based on over-expression of gain-of-function mutated KCNH2/HERG-N588K (SQT1).71 In LQT1 and LQT2 rabbits, IKs (LQT1) or IKr (LQT2) were completely eliminated due to a dominant-negative effect, resulting in APD/QT prolongation in both, and the development of spontaneous VT, sudden cardiac death, and sex differences in arrhythmogenic risk with pro-arrhythmic effects of oestradiol in LQT2.57,211 In transgenic LQT5 rabbits, in contrast, IKs was altered with accelerated deactivation kinetics70 but not reduced, leading to a partial phenotype with only slightly prolonged QT-intervals and no spontaneous arrhythmias. In SQT1 rabbits, steady-state IKr was increased due to impaired channel inactivation,71 leading to shortened atrial and ventricular APD and QT, and increased VT/VF and AF inducibility; thus mimicking the human disease phenotype on atrial and ventricular levels. These transgenic rabbit models have been used to investigate mechanisms of arrhythmogenesis and pro- and anti-arrhythmic effects of various drugs, hormones, and metabolites.212,213
Human-induced pluripotent stem-cell-derived cardiomyocytes from LQT1 and LQT2 patients have been shown to recapitulate clinical phenotypes,214 disclose ‘modifier genes’,93,94 and allowed to devise and test therapeutic approaches.95,215,216 Nonetheless, because of hiPS-CMs immaturity and variability, some caveats should be considered, as discussed in ‘Human-induced pluripotent stem-cell-derived cardiomyocytes in electrophysiology research’ section.
Apart from these genetic models for K+ channelopathies, several species such as dogs and guinea pigs are often employed as ‘drug-induced’ long-QT models, particularly for safety pharmacology research. Due to space limitations, we cannot comprehensively cover all these models in this position paper.217
Ion channel diseases with altered susceptibility to sympathetic stimulation (CPVT)
CPVT is caused by mutations in RyR2 or other genes that code for proteins within the RyR2 macromolecular complex.
Different experimental models have been used to determine the mechanisms involved in catecholaminergic polymorphic ventricular tachycardia (CPVT) by different mutations. Plasmids for RyR2 carrying mutations identified in CPVT patients have been transfected into heterologous expression systems. Given that the RyR2 is an intracellular channel, its biophysical properties have also been analysed by incorporating single channels into lipid bilayers of cell lines. However, as these cells lack all the components that make up the RyR2 complex in cardiomyocytes, the link to arrhythmia generation is difficult to assess.
Heterozygous knock-in mouse models have been shown to be a valuable tool for exploring the underlying cause of VT and replicate many features associated with CPVT, including disease progression, and drug response.218–221 However, there are significant differences in Ca2+ handling between mice and larger mammals, with a much smaller contribution of the electrogenic Na+/Ca2+ exchanger in mice. Rabbits have more similar ion-channel and Ca2+-handling patterns to humans, but no heterozygous knock-in CPVT rabbit model has been reported so far. Human-induced pluripotent stem-cell-derived cardiomyocyte from CPVT patients can generate cells with typical nodal/pacemaker, atrial, and ventricular electrical properties, expressing channels and transporters involved in Ca2+ handling (see ‘Human-induced pluripotent stem-cell-derived cardiomyocyte’ section). Although they have an immature phenotype compared to adult cardiomyocytes, they retain channels and transporters from human cardiomyocytes, making them a valuable tool to study mechanisms underlying CPVT.26
Genetic arrhythmogenic cardiomyopathies
Mutations in genes encoding sarcomeric proteins such as cardiac β-myosin heavy-chain, alpha-tropomyosin, cardiac troponin and others can cause familial HCM characterized by myocyte disarray, interstitial fibrosis, ventricular dysfunction, and increased risk of VT and SCD.
The first genetic cardiomyopathy animal models were transgenic mice expressing mutations in various sarcomeric proteins. These showed a broad spectrum of disease phenotypes, however, several did not develop LV hypertrophy, the key element of HCM in humans,222,223 likely due to pronounced differences in the composition of cardiac sarcomeric proteins between humans and mice with β-MHC as predominant form in human ventricles and α-MHC in mice.224 Nevertheless, recent studies in HCM mouse models have implicated intracellular Ca2+ dysregulation and enhanced late Na+ current in the generation of pro-arrhythmic early and delayed after-depolarizations.225 Moreover, these preclinical models have been instrumental in developing novel therapeutic strategies,226 including the cardiac myosin inhibitor mavacamten.227,228 In 1999, the first transgenic rabbit model for HCM was generated by targeted cardiac-specific expression of the mutant β-MHC-Q403. These HCM rabbits showed cardiac hypertrophy, interstitial fibrosis, myocyte disarray, and premature arrhythmic death,229 but thus far no electrophysiological studies have been performed in these models to better understand arrhythmogenic mechanisms in HCM.
Arrhythmogenic cardiomyopathy (ACM), caused by mutations in predominantly desmosomal genes is an inherited, familial disorder characterized by progressive replacement of cardiomyocytes by fibrofatty tissue, ultimately resulting in ventricular dilation, cardiac dysfunction, life-threatening arrhythmias and sudden cardiac death.230 Disturbed desmosomal organization in the setting of ACM leads to myocardial fibrosis formation, fibro-fatty replacement and cardiac dilation, setting the stage for arrhythmias. While most mouse models of ACM do not fully recapitulate the human disease phenotype (such as for instance fibro-fatty replacement),231 they have been vital for identifying Ca2+ dysregulation as a contributing factor and for establishing reduced Na+ current as a pro-arrhythmic feature during early disease stages prior to the development of overt cardiomyopathy.233,233
Outlook
Current in silico, in vitro, and in vivo models offer a wide variety of electrophysiological research techniques. Strategies that combine different methodological approaches are expected to offer the most comprehensive assessment of cardiac electrophysiology, at the same time reducing animal experiments as much as possible. Personalized disease understanding and individualized, mechanism-based therapy planning is one major goal of next-generation electrophysiological research. While conventional cellular electrophysiological techniques will remain essential tools for detailed analyses, future scientific efforts in cardiac electrophysiology will certainly require additional novel methods and technologies that allow for (i) the identification of mechanisms underlying physiology and disease at system scale (e.g. ‘-omics’ approaches combined with computational simulations, with particular focus on epigenetic effects); (ii) in vitro validation employing advanced cellular models; (iii) pre-clinical translation of interventions derived from (i) and (ii) in animals that model the disease of interest as closely as possible; and (iv) optimization of (i)–(iii) using artificial intelligence and machine learning where appropriate. The present and future armamentarium of techniques and models will allow basic, translational, and clinical electrophysiological researchers to employ optimized approaches tailored to individual, ‘personalized’ scientific needs.
Acknowledgements
This manuscript was written by nucleus members of the ESC Working Group on Cardiac Cellular Electrophysiology 2018–2020.
Conflict of interest: none declared.
Contributor Information
Katja E Odening, Translational Cardiology, Department of Cardiology, Inselspital, Bern University Hospital, Bern, Switzerland; Institute of Physiology, University of Bern, Bern, Switzerland.
Ana-Maria Gomez, Signaling and cardiovascular pathophysiology—UMR-S 1180, Inserm, Université Paris-Saclay, 92296 Châtenay-Malabry, France.
Dobromir Dobrev, Institute of Pharmacology, West German Heart and Vascular Center, University Duisburg-Essen, Essen, Germany.
Larissa Fabritz, Institute of Cardiovascular Sciences, University of Birmingham, Birmingham, UK; Department of Cardiology, University Hospital Birmingham NHS Trust, Birmingham, UK.
Frank R Heinzel, Department of Internal Medicine and Cardiology, Charité - Universitätsmedizin Berlin, Campus Virchow-Klinikum, Berlin, Germany; DZHK (German Centre for Cardiovascular Research), Partner Site, Berlin, Germany.
Matteo E Mangoni, Institut de Génomique Fonctionnelle, Université de Montpellier, CNRS, INSERM, Montpellier, France.
Cristina E Molina, Institute of Experimental Cardiovascular Research, University Medical Center Hamburg-Eppendorf, Hamburg, Germany; German Centre for Cardiovascular Research (DZHK), Partner Site, Hamburg/Kiel/Lübeck, Germany.
Leonardo Sacconi, National Institute of Optics and European Laboratory for Non Linear Spectroscopy, Italy; Institute for Experimental Cardiovascular Medicine, University Freiburg, Germany.
Godfrey Smith, Institute of Cardiovascular and Medical Sciences, University of Glasgow, UK.
Milan Stengl, Department of Physiology, Faculty of Medicine in Pilsen, Charles University, Pilsen, Czech Republic.
Dierk Thomas, Department of Cardiology, University Hospital Heidelberg, Heidelberg, Germany; Heidelberg Center for Heart Rhythm Disorders (HCR), University Hospital Heidelberg, Heidelberg, Germany; German Centre for Cardiovascular Research (DZHK), Partner Site, Heidelberg/Mannheim, Germany.
Antonio Zaza, Department of Biotechnology and Bioscience, University of Milano-Bicocca, Milano, Italy.
Carol Ann Remme, Department of Experimental Cardiology, Amsterdam UMC, location AMC, Amsterdam, The Netherlands.
Jordi Heijman, Department of Cardiology, CARIM School for Cardiovascular Diseases, Maastricht University, Maastricht, The Netherlands.
References
- 1. Goette A, Auricchio A, Boriani G, Braunschweig F, Terradellas JB, Burri H et al. ; ESC Scientific Document Group. EHRA White Paper: knowledge gaps in arrhythmia management-status 2019. Europace 2019;21:993–4. [DOI] [PubMed] [Google Scholar]
- 2. Kaese S, Frommeyer G, Verheule S, van Loon G, Gehrmann J, Breithardt G et al. The ECG in cardiovascular-relevant animal models of electrophysiology. Herzschrittmacherther Elektrophysiol 2013;24:84–91. [DOI] [PubMed] [Google Scholar]
- 3. Killingsworth CR, Ritscher DE, Walcott GP, Rollins DL, Ideker RE, Smith WM. Continuous telemetry from a chronic canine model of sudden cardiac death. J Cardiovasc Electrophysiol 2000;11:1333–41. [DOI] [PubMed] [Google Scholar]
- 4. Verheule S, Sato T, Everett T, Engle SK, Otten D, Rubart-von der Lohe M et al. Increased vulnerability to atrial fibrillation in transgenic mice with selective atrial fibrosis caused by overexpression of TGF-beta1. Circ Res 2004;94:1458–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Odening KE, Kirk M, Brunner M, Ziv O, Lorvidhaya P, Liu GX et al. Electrophysiological studies of transgenic long QT type 1 and type 2 rabbits reveal genotype-specific differences in ventricular refractoriness and His conduction. Am J Physiol Heart Circ Physiol 2010;299:H643–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cluitmans MJM, Bonizzi P, Karel JMH, Das M, Kietselaer B, de Jong MMJ et al. In vivo validation of electrocardiographic imaging. JACC Clin Electrophysiol 2017;3:232–42. [DOI] [PubMed] [Google Scholar]
- 7. Hohmann S, Rettmann ME, Konishi H, Borenstein A, Wang S, Suzuki A et al. Spatial accuracy of a clinically established noninvasive electrocardiographic imaging system for the detection of focal activation in an intact porcine model. Circ Arrhythm Electrophysiol 2019;12:e007570. [DOI] [PubMed] [Google Scholar]
- 8. Franz MR. Current status of monophasic action potential recording: theories, measurements and interpretations. Cardiovasc Res 1999;41:25–40. [DOI] [PubMed] [Google Scholar]
- 9. Lee P, Quintanilla JG, Alfonso-Almazan JM, Galan-Arriola C, Yan P, Sanchez-Gonzalez J et al. In vivo ratiometric optical mapping enables high-resolution cardiac electrophysiology in pig models. Cardiovasc Res 2019;115:1659–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Langendorff O. Untersuchungen am überlebenden Säugethierherzen. Pflügers Arch 1895;61:291–332. [Google Scholar]
- 11. Skrzypiec-Spring M, Grotthus B, Szelag A, Schulz R. Isolated heart perfusion according to Langendorff—still viable in the new millennium. J Pharmacol Toxicol Methods 2007;55:113–26. [DOI] [PubMed] [Google Scholar]
- 12. Bell RM, Mocanu MM, Yellon DM. Retrograde heart perfusion: the Langendorff technique of isolated heart perfusion. J Mol Cell Cardiol 2011;50:940–50. [DOI] [PubMed] [Google Scholar]
- 13. Neely JR, Liebermeister H, Battersby EJ, Morgan HE. Effect of pressure development on oxygen consumption by isolated rat heart. Am J Physiol 1967;212:804–14. [DOI] [PubMed] [Google Scholar]
- 14. Coraboeuf E, Weidmann S. Potentiel de repos et potentiels d'action du muscle cardiaque, mesures a l'aide d'electrodes internes. Comptes Rendus des Seances de la Societe de Biologie et de. Ses Filiales 1949;143:1329–31. [Google Scholar]
- 15. Dong R, Mu UMR, Reith AJM, O'Shea C, He S, Duan K et al. A protocol for dual calcium-voltage optical mapping in murine sinoatrial preparation with optogenetic pacing. Front Physiol 2019;10:954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Martinez ME, Walton RD, Bayer JD, Haissaguerre M, Vigmond EJ, Hocini M et al. Role of the Purkinje-muscle junction on the ventricular repolarization heterogeneity in the healthy and ischemic ovine ventricular myocardium. Front Physiol 2018;9:718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lee P, Bollensdorff C, Quinn TA, Wuskell JP, Loew LM, Kohl P. Single-sensor system for spatially resolved, continuous, and multiparametric optical mapping of cardiac tissue. Heart Rhythm 2011;8:1482–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Quinn TA, Kohl P. Cardiac mechano-electric coupling: acute effects of mechanical stimulation on heart rate and rhythm. Physiol Rev 2021;101:37–92. [DOI] [PubMed] [Google Scholar]
- 19. Christoph J, Chebbok M, Richter C, Schroder-Schetelig J, Bittihn P, Stein S et al. Electromechanical vortex filaments during cardiac fibrillation. Nature 2018;555:667–72. [DOI] [PubMed] [Google Scholar]
- 20. Bruegmann T, Malan D, Hesse M, Beiert T, Fuegemann CJ, Fleischmann BK et al. Optogenetic control of heart muscle in vitro and in vivo. Nat Methods 2010;7:897–900. [DOI] [PubMed] [Google Scholar]
- 21. Yan GX, Shimizu W, Antzelevitch C. Characteristics and distribution of M cells in arterially perfused canine left ventricular wedge preparations. Circulation 1998;98:1921–7. [DOI] [PubMed] [Google Scholar]
- 22. Wilders R. Dynamic clamp: a powerful tool in cardiac electrophysiology. J Physiol 2006;576:349–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Berecki G, Wilders R, de Jonge B, van Ginneken AC, Verkerk AO. Re-evaluation of the action potential upstroke velocity as a measure of the Na+ current in cardiac myocytes at physiological conditions. PLoS One 2010;5:e15772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kettlewell S, Saxena P, Dempster J, Colman MA, Myles RC, Smith GL et al. Dynamic clamping human and rabbit atrial calcium current: narrowing ICaL window abolishes early afterdepolarizations. J Physiol 2019;597:3619–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lu HR, Hortigon-Vinagre MP, Zamora V, Kopljar I, De Bondt A, Gallacher DJ et al. Application of optical action potentials in human induced pluripotent stem cells-derived cardiomyocytes to predict drug-induced cardiac arrhythmias. J Pharmacol Toxicol Methods 2017;87:53–67. [DOI] [PubMed] [Google Scholar]
- 26. Zhang XH, Morad M. Calcium signaling in human stem cell-derived cardiomyocytes: evidence from normal subjects and CPVT afflicted patients. Cell Calcium 2016;59:98–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Varro A, Tomek J, Nagy N, Virag L, Passini E, Rodriguez B et al. Cardiac transmembrane ion channels and action potentials: cellular physiology and arrhythmogenic behavior. Physiol Rev 2021;101:1083–1176. [DOI] [PubMed] [Google Scholar]
- 28. Nerbonne JM. Molecular basis of functional voltage-gated K+ channel diversity in the mammalian myocardium. J Physiol 2000;525: 285–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nerbonne JM, Kass RS. Molecular physiology of cardiac repolarization. Physiol Rev 2005;85:1205–53. [DOI] [PubMed] [Google Scholar]
- 30. Jost N, Virag L, Comtois P, Ordog B, Szuts V, Seprenyi G et al. Ionic mechanisms limiting cardiac repolarization reserve in humans compared to dogs. J Physiol 2013;591:4189–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Varro A, Lathrop DA, Hester SB, Nanasi PP, Papp JG. Ionic currents and action potentials in rabbit, rat, and guinea pig ventricular myocytes. Basic Res Cardiol 1993;88:93–102. [DOI] [PubMed] [Google Scholar]
- 32. Patel SP, Campbell DL. Transient outward potassium current, ‘Ito’, phenotypes in the mammalian left ventricle: underlying molecular, cellular and biophysical mechanisms. J Physiol 2005;569:7–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fedida D, Giles WR. Regional variations in action potentials and transient outward current in myocytes isolated from rabbit left ventricle. J Physiol 1991;442:191–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Choi BR, Li W, Terentyev D, Kabakov AY, Zhong M, Rees CM et al. Transient outward K+ current (Ito) underlies the right ventricular initiation of polymorphic ventricular tachycardia in a transgenic rabbit model of long-QT syndrome type 1. Circ Arrhythm Electrophysiol 2018;11:e005414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ravens U. Ionic basis of cardiac electrophysiology in zebrafish compared to human hearts. Prog Biophys Mol Biol 2018;138:38–44. [DOI] [PubMed] [Google Scholar]
- 36. Verkerk AO, Remme CA. Zebrafish: a novel research tool for cardiac (patho)electrophysiology and ion channel disorders. Front Physiol 2012;3:255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Stankovicova T, Szilard M, De Scheerder I, Sipido KR. M cells and transmural heterogeneity of action potential configuration in myocytes from the left ventricular wall of the pig heart. Cardiovasc Res 2000;45:952–60. [DOI] [PubMed] [Google Scholar]
- 38. Verkerk AO, van Ginneken AC, Berecki G, den Ruijter HM, Schumacher CA, Veldkamp MW et al. Incorporated sarcolemmal fish oil fatty acids shorten pig ventricular action potentials. Cardiovasc Res 2006;70:509–20. [DOI] [PubMed] [Google Scholar]
- 39. Hegyi B, Bossuyt J, Griffiths LG, Shimkunas R, Coulibaly Z, Jian Z et al. Complex electrophysiological remodeling in postinfarction ischemic heart failure. Proc Natl Acad Sci U S A 2018;115:E3036–E3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Li GR, Du XL, Siow YL, O K, Tse HF, Lau CP. Calcium-activated transient outward chloride current and phase 1 repolarization of swine ventricular action potential. Cardiovasc Res 2003;58:89–98. [DOI] [PubMed] [Google Scholar]
- 41. Arlock P, Mow T, Sjoberg T, Arner A, Steen S, Laursen M. Ion currents of cardiomyocytes in different regions of the Gottingen minipig heart. J Pharmacol Toxicol Methods 2017;86:12–8. [DOI] [PubMed] [Google Scholar]
- 42. Laursen M, Olesen SP, Grunnet M, Mow T, Jespersen T. Characterization of cardiac repolarization in the Gottingen minipig. J Pharmacol Toxicol Methods 2011;63:186–95. [DOI] [PubMed] [Google Scholar]
- 43. Dixon JE, Shi W, Wang HS, McDonald C, Yu H, Wymore RS et al. Role of the Kv4.3 K+ channel in ventricular muscle. A molecular correlate for the transient outward current. Circ Res 1996;79:659–68. [DOI] [PubMed] [Google Scholar]
- 44. Szentadrassy N, Banyasz T, Biro T, Szabo G, Toth BI, Magyar J et al. Apico-basal inhomogeneity in distribution of ion channels in canine and human ventricular myocardium. Cardiovasc Res 2005;65:851–60. [DOI] [PubMed] [Google Scholar]
- 45. Szabo G, Szentandrassy N, Biro T, Toth BI, Czifra G, Magyar J et al. Asymmetrical distribution of ion channels in canine and human left-ventricular wall: epicardium versus midmyocardium. Pflugers Arch 2005;450:307–16. [DOI] [PubMed] [Google Scholar]
- 46. Nagy N, Acsai K, Kormos A, Sebők Z, Farkas AS, Jost N et al. [Ca2+]i-induced augmentation of the inward rectifier potassium current (IK1) in canine and human ventricular myocardium. Pflugers Arch 2013;465:1621–35. [DOI] [PubMed] [Google Scholar]
- 47. Liu DW, Gintant GA, Antzelevitch C. Ionic bases for electrophysiological distinctions among epicardial, midmyocardial, and endocardial myocytes from the free wall of the canine left ventricle. Circ Res 1993;72:671–87. [DOI] [PubMed] [Google Scholar]
- 48. Liu DW, Antzelevitch C. Characteristics of the delayed rectifier current (IKr and IKs) in canine ventricular epicardial, midmyocardial, and endocardial myocytes. A weaker IKs contributes to the longer action potential of the M cell. Circ Res 1995;76:351–65. [DOI] [PubMed] [Google Scholar]
- 49. Volders PG, Sipido KR, Carmeliet E, Spatjens RL, Wellens HJ, Vos MA. Repolarizing K+ currents ITO1 and IKs are larger in right than left canine ventricular midmyocardium. Circulation 1999;99:206–10. [DOI] [PubMed] [Google Scholar]
- 50. O'Hara T, Rudy Y. Quantitative comparison of cardiac ventricular myocyte electrophysiology and response to drugs in human and nonhuman species. Am J Physiol Heart Circ Physiol 2012;302:H1023–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Caluori G, Wojtaszczyk A, Yasin O, Pesl M, Wolf J, Belaskova S et al. Comparing the incidence of ventricular arrhythmias during epicardial ablation in swine versus canine models. Pacing Clin Electrophysiol 2019;42:862–7. [DOI] [PubMed] [Google Scholar]
- 52. Wijffels MC, Kirchhof CJ, Dorland R, Allessie MA. Atrial fibrillation begets atrial fibrillation. A study in awake chronically instrumented goats. Circulation 1995;92:1954–68. [DOI] [PubMed] [Google Scholar]
- 53. Ausma J, Wijffels M, Thone F, Wouters L, Allessie M, Borgers M. Structural changes of atrial myocardium due to sustained atrial fibrillation in the goat. Circulation 1997;96:3157–63. [DOI] [PubMed] [Google Scholar]
- 54. Allessie M, Ausma J, Schotten U. Electrical, contractile and structural remodeling during atrial fibrillation. Cardiovasc Res 2002;54:230–46. [DOI] [PubMed] [Google Scholar]
- 55. Vos MA, Verduyn SC, Gorgels AP, Lipcsei GC, Wellens HJ. Reproducible induction of early afterdepolarizations and torsade de pointes arrhythmias by d-sotalol and pacing in dogs with chronic atrioventricular block. Circulation 1995;91:864–72. [DOI] [PubMed] [Google Scholar]
- 56. Vos MA, de Groot SH, Verduyn SC, van der Zande J, Leunissen HD, Cleutjens JP et al. Enhanced susceptibility for acquired torsade de pointes arrhythmias in the dog with chronic, complete AV block is related to cardiac hypertrophy and electrical remodeling. Circulation 1998;98:1125–35. [DOI] [PubMed] [Google Scholar]
- 57. Brunner M, Peng X, Liu GX, Ren XQ, Ziv O, Choi BR et al. Mechanisms of cardiac arrhythmias and sudden death in transgenic rabbits with long QT syndrome. J Clin Invest 2008;118:2246–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Volders PG, Sipido KR, Vos MA, Spatjens RL, Leunissen JD, Carmeliet E et al. Downregulation of delayed rectifier K+ currents in dogs with chronic complete atrioventricular block and acquired torsades de pointes. Circulation 1999;100:2455–61. [DOI] [PubMed] [Google Scholar]
- 59. Sipido KR, Volders PG, de Groot SH, Verdonck F, Van de Werf F, Wellens HJ et al. Enhanced Ca2+ release and Na/Ca exchange activity in hypertrophied canine ventricular myocytes: potential link between contractile adaptation and arrhythmogenesis. Circulation 2000;102:2137–44. [DOI] [PubMed] [Google Scholar]
- 60. Verduyn SC, Vos MA, van der Zande J, van der Hulst FF, Wellens HJ. Role of interventricular dispersion of repolarization in acquired torsade-de-pointes arrhythmias: reversal by magnesium. Cardiovasc Res 1997;34:453–63. [DOI] [PubMed] [Google Scholar]
- 61. Thomsen MB, Verduyn SC, Stengl M, Beekman JD, de Pater G, van Opstal J et al. Increased short-term variability of repolarization predicts d-sotalol-induced torsades de pointes in dogs. Circulation 2004;110:2453–9. [DOI] [PubMed] [Google Scholar]
- 62. Sprenkeler DJ, Beekman JDM, Bossu A, Dunnink A, Vos MA. Pro-arrhythmic ventricular remodeling is associated with increased respiratory and low-frequency oscillations of monophasic action potential duration in the chronic atrioventricular block dog model. Front Physiol 2019;10:1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ni L, Scott L Jr., Campbell HM, Pan X, Alsina KM, Reynolds J et al. Atrial-specific gene delivery using an adeno-associated viral vector. Circ Res 2019;124:256–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Podliesna S, Bezzina CR, Lodder EM. Complex genetics of cardiovascular traits in mice: F2-mapping of QTLs and their underlying genes. Methods Mol Biol 2017;1488:431–54. [DOI] [PubMed] [Google Scholar]
- 65. Nicod J, Davies RW, Cai N, Hassett C, Goodstadt L, Cosgrove C et al. Genome-wide association of multiple complex traits in outbred mice by ultra-low-coverage sequencing. Nat Genet 2016;48:912–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Adriaens ME, Lodder EM, Moreno-Moral A, Silhavy J, Heinig M, Glinge C et al. Systems genetics approaches in rat identify novel genes and gene networks associated with cardiac conduction. J Am Heart Assoc 2018;7:e009243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kovoor P, Wickman K, Maguire CT, Pu W, Gehrmann J, Berul CI et al. Evaluation of the role of IKACh in atrial fibrillation using a mouse knockout model. J Am Coll Cardiol 2001;37:2136–43. [DOI] [PubMed] [Google Scholar]
- 68. Cerrone M, Colombi B, Santoro M, di Barletta MR, Scelsi M, Villani L et al. Bidirectional ventricular tachycardia and fibrillation elicited in a knock-in mouse model carrier of a mutation in the cardiac ryanodine receptor. Circ Res 2005;96:e77-82. [DOI] [PubMed] [Google Scholar]
- 69. Sanbe A, James J, Tuzcu V, Nas S, Martin L, Gulick J et al. Transgenic rabbit model for human troponin I-based hypertrophic cardiomyopathy. Circulation 2005;111:2330–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Major P, Baczko I, Hiripi L, Odening KE, Juhasz V, Kohajda Z et al. A novel transgenic rabbit model with reduced repolarization reserve: long QT syndrome caused by a dominant-negative mutation of the KCNE1 gene. Br J Pharmacol 2016;173:2046–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Odening KE, Bodi I, Franke G, Rieke R, Ryan de Medeiros A, Perez-Feliz S et al. Transgenic short-QT syndrome 1 rabbits mimic the human disease phenotype with QT/action potential duration shortening in the atria and ventricles and increased ventricular tachycardia/ventricular fibrillation inducibility. Eur Heart J 2019;40:842–53. [DOI] [PubMed] [Google Scholar]
- 72. Park DS, Cerrone M, Morley G, Vasquez C, Fowler S, Liu N et al. Genetically engineered SCN5A mutant pig hearts exhibit conduction defects and arrhythmias. J Clin Invest 2015;125:403–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Jin G, Manninger M, Adelsmayr G, Schwarzl M, Alogna A, Schonleitner P et al. Cellular contribution to left and right atrial dysfunction in chronic arterial hypertension in pigs. ESC Heart Fail 2021;8:151–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Pinto JM, Sosunov EA, Gainullin RZ, Rosen MR, Boyden PA. Effects of mibefradil, a T-type calcium current antagonist, on electrophysiology of Purkinje fibers that survived in the infarcted canine heart. J Cardiovasc Electrophysiol 1999;10:1224–35. [DOI] [PubMed] [Google Scholar]
- 75. Meysen S, Marger L, Hewett KW, Jarry-Guichard T, Agarkova I, Chauvin JP et al. Nkx2.5 cell-autonomous gene function is required for the postnatal formation of the peripheral ventricular conduction system. Dev Biol 2007;303:740–53. [DOI] [PubMed] [Google Scholar]
- 76. Lin X, Liu N, Lu J, Zhang J, Anumonwo JM, Isom LL et al. Subcellular heterogeneity of sodium current properties in adult cardiac ventricular myocytes. Heart Rhythm 2011;8:1923–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Schluter KD, Schreiber D. Adult ventricular cardiomyocytes: isolation and culture. Methods Mol Biol 2005;290:305–14. [DOI] [PubMed] [Google Scholar]
- 78. Molina CE, Leroy J, Richter W, Xie M, Scheitrum C, Lee IO et al. Cyclic adenosine monophosphate phosphodiesterase type 4 protects against atrial arrhythmias. J Am Coll Cardiol 2012;59:2182–90. [DOI] [PubMed] [Google Scholar]
- 79. Brown H, Difrancesco D. Voltage-clamp investigations of membrane currents underlying pace-maker activity in rabbit sino-atrial node. J Physiol 1980;308:331–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Verheijck EE, van Kempen MJ, Veereschild M, Lurvink J, Jongsma HJ, Bouman LN. Electrophysiological features of the mouse sinoatrial node in relation to connexin distribution. Cardiovasc Res 2001;52:40–50. [DOI] [PubMed] [Google Scholar]
- 81. Maier SK, Westenbroek RE, Yamanushi TT, Dobrzynski H, Boyett MR, Catterall WA et al. An unexpected requirement for brain-type sodium channels for control of heart rate in the mouse sinoatrial node. Proc Natl Acad Sci U S A 2003;100:3507–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Efimov IR, Nikolski VP, Rothenberg F, Greener ID, Li J, Dobrzynski H et al. Structure-function relationship in the AV junction. Anat Rec A Discov Mol Cell Evol Biol 2004;280:952–65. [DOI] [PubMed] [Google Scholar]
- 83. Neco P, Torrente AG, Mesirca P, Zorio E, Liu N, Priori SG et al. Paradoxical effect of increased diastolic Ca2+ release and decreased sinoatrial node activity in a mouse model of catecholaminergic polymorphic ventricular tachycardia. Circulation 2012;126:392–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Blinova K, Dang Q, Millard D, Smith G, Pierson J, Guo L et al. International multisite study of human-induced pluripotent stem cell-derived cardiomyocytes for drug proarrhythmic potential assessment. Cell Rep 2018;24:3582–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Parikh SS, Blackwell DJ, Gomez-Hurtado N, Frisk M, Wang L, Kim K et al. Thyroid and glucocorticoid hormones promote functional T-tubule development in human-induced pluripotent stem cell-derived cardiomyocytes. Circ Res 2017;121:1323–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Macadangdang J, Guan X, Smith AS, Lucero R, Czerniecki S, Childers MK et al. Nanopatterned human iPSC-based model of a dystrophin-null cardiomyopathic phenotype. Cell Mol Bioeng 2015;8:320–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Martella D, Paoli P, Pioner JM, Sacconi L, Coppini R, Santini L et al. Liquid crystalline networks toward regenerative medicine and tissue repair. Small 2017;13:1702677. [DOI] [PubMed] [Google Scholar]
- 88. Davis RP, Casini S, van den Berg CW, Hoekstra M, Remme CA, Dambrot C et al. Cardiomyocytes derived from pluripotent stem cells recapitulate electrophysiological characteristics of an overlap syndrome of cardiac sodium channel disease. Circulation 2012;125:3079–91. [DOI] [PubMed] [Google Scholar]
- 89. Casini S, Verkerk AO, Remme CA. Human iPSC-derived cardiomyocytes for investigation of disease mechanisms and therapeutic strategies in inherited arrhythmia syndromes: strengths and limitations. Cardiovasc Drugs Ther 2017;31:325–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Portero V, Casini S, Hoekstra M, Verkerk AO, Mengarelli I, Belardinelli L et al. Anti-arrhythmic potential of the late sodium current inhibitor GS-458967 in murine Scn5a-1798insD+/- and human SCN5A-1795insD+/- iPSC-derived cardiomyocytes. Cardiovasc Res 2017;113:829–38. [DOI] [PubMed] [Google Scholar]
- 91. Lieu DK, Fu JD, Chiamvimonvat N, Tung KC, McNerney GP, Huser T et al. Mechanism-based facilitated maturation of human pluripotent stem cell-derived cardiomyocytes. Circ Arrhythm Electrophysiol 2013;6:191–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Verkerk AO, Veerman CC, Zegers JG, Mengarelli I, Bezzina CR, Wilders R. Patch-clamp recording from human induced pluripotent stem cell-derived cardiomyocytes: improving action potential characteristics through dynamic clamp. Int J Mol Sci 2017;18:1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Lee YK, Sala L, Mura M, Rocchetti M, Pedrazzini M, Ran X et al. MTMR4 SNVs modulate ion channel degradation and clinical severity in congenital long QT syndrome: insights in the mechanism of action of protective modifier genes. Cardiovasc Res 2021;117:767–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Ronchi C, Bernardi J, Mura M, Stefanello M, Badone B, Rocchetti M et al. NOS1AP polymorphisms reduce NOS1 activity and interact with prolonged repolarization in arrhythmogenesis. Cardiovasc Res 2021;117:472–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Ma D, Wei H, Lu J, Huang D, Liu Z, Loh LJ et al. Characterization of a novel KCNQ1 mutation for type 1 long QT syndrome and assessment of the therapeutic potential of a novel IKs activator using patient-specific induced pluripotent stem cell-derived cardiomyocytes. Stem Cell Res Ther 2015;6:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Braam SR, Tertoolen L, van de Stolpe A, Meyer T, Passier R, Mummery CL. Prediction of drug-induced cardiotoxicity using human embryonic stem cell-derived cardiomyocytes. Stem Cell Res 2010;4:107–16. [DOI] [PubMed] [Google Scholar]
- 97. Giles WR, Noble D. Rigorous phenotyping of cardiac iPSC preparations requires knowledge of their resting potential(s). Biophys J 2016;110:278–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Devalla HD, Schwach V, Ford JW, Milnes JT, El-Haou S, Jackson C et al. Atrial-like cardiomyocytes from human pluripotent stem cells are a robust preclinical model for assessing atrial-selective pharmacology. EMBO Mol Med 2015;7:394–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Cyganek L, Tiburcy M, Sekeres K, Gerstenberg K, Bohnenberger H, Lenz C et al. Deep phenotyping of human induced pluripotent stem cell-derived atrial and ventricular cardiomyocytes. JCI Insight 2018;3:e99941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Schaaf S, Eder A, Vollert I, Stohr A, Hansen A, Eschenhagen T. Generation of strip-format fibrin-based engineered heart tissue (EHT). Methods Mol Biol 2014;1181:121–9. [DOI] [PubMed] [Google Scholar]
- 101. Beauchamp P, Moritz W, Kelm JM, Ullrich ND, Agarkova I, Anson BD et al. Development and characterization of a scaffold-free 3D spheroid model of induced pluripotent stem cell-derived human cardiomyocytes. Tissue Eng Part C Methods 2015;21:852–61. [DOI] [PubMed] [Google Scholar]
- 102. Noble D, Garny A, Noble PJ. How the Hodgkin-Huxley equations inspired the Cardiac Physiome Project. J Physiol 2012;590:2613–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Winslow RL, Cortassa S, O'Rourke B, Hashambhoy YL, Rice JJ, Greenstein JL. Integrative modeling of the cardiac ventricular myocyte. Wiley Interdiscip Rev Syst Biol Med 2011;3:392–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Heijman J, Erfanian Abdoust P, Voigt N, Nattel S, Dobrev D. Computational models of atrial cellular electrophysiology and calcium handling, and their role in atrial fibrillation. J Physiol 2016;594:537–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Benson AP, Stevenson-Cocks HJ, Whittaker DG, White E, Colman MA. Multi-scale approaches for the simulation of cardiac electrophysiology: II - Tissue-level structure and function. Methods 2021;185:60–81. [DOI] [PubMed] [Google Scholar]
- 106. Lyashkov AE, Behar J, Lakatta EG, Yaniv Y, Maltsev VA. Positive feedback mechanisms among local Ca releases, NCX, and ICaL ignite pacemaker action potentials. Biophys J 2018;114:1176–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Trovato C, Passini E, Nagy N, Varro A, Abi-Gerges N, Severi S et al. Human Purkinje in silico model enables mechanistic investigations into automaticity and pro-arrhythmic abnormalities. J Mol Cell Cardiol 2020;142:24–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Ravagli E, Bucchi A, Bartolucci C, Paina M, Baruscotti M, DiFrancesco D et al. Cell-specific dynamic clamp analysis of the role of funny if current in cardiac pacemaking. Prog Biophys Mol Biol 2016;120:50–66. [DOI] [PubMed] [Google Scholar]
- 109. Shim J, Hwang M, Song JS, Lim B, Kim TH, Joung B et al. Virtual in-silico modeling guided catheter ablation predicts effective linear ablation lesion set for longstanding persistent atrial fibrillation: multicenter prospective randomized study. Front Physiol 2017;8:792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Prakosa A, Arevalo HJ, Deng D, Boyle PM, Nikolov PP, Ashikaga H et al. Personalized virtual-heart technology for guiding the ablation of infarct-related ventricular tachycardia. Nat Biomed Eng 2018;2:732–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Boyle PM, Zghaib T, Zahid S, Ali RL, Deng D, Franceschi WH et al. Computationally guided personalized targeted ablation of persistent atrial fibrillation. Nat Biomed Eng 2019;3:870–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Li Z, Ridder BJ, Han X, Wu WW, Sheng J, Tran PN et al. Assessment of an in silico mechanistic model for proarrhythmia risk prediction under the CiPA initiative. Clin Pharmacol Ther 2019;105:466–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Li Z, Mirams GR, Yoshinaga T, Ridder BJ, Han X, Chen JE et al. General principles for the validation of proarrhythmia risk prediction models: an extension of the CiPA in silico strategy. Clin Pharmacol Ther 2020;107:102–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Whittaker DG, Clerx M, Lei CL, Christini DJ, Mirams GR. Calibration of ionic and cellular cardiac electrophysiology models. Wiley Interdiscip Rev Syst Biol Med 2020;12:e1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Ni H, Morotti S, Grandi E. A heart for diversity: simulating variability in cardiac arrhythmia research. Front Physiol 2018;9:958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Tomek J, Bueno-Orovio A, Passini E, Zhou X, Minchole A, Britton O et al. Development, calibration, and validation of a novel human ventricular myocyte model in health, disease, and drug block. Elife 2019;8:e48890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Yang PC, DeMarco KR, Aghasafari P, Jeng MT, Dawson JRD, Bekker S et al. A computational pipeline to predict cardiotoxicity: from the atom to the rhythm. Circ Res 2020;126:947–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Sutanto H, Lyon A, Lumens J, Schotten U, Dobrev D, Heijman J. Cardiomyocyte calcium handling in health and disease: Insights from in vitro and in silico studies. Prog Biophys Mol Biol 2020;157:54–75. [DOI] [PubMed] [Google Scholar]
- 119. Colman MA. Arrhythmia mechanisms and spontaneous calcium release: bi-directional coupling between re-entrant and focal excitation. PLoS Comput Biol 2019;15:e1007260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Altomare C, Bartolucci C, Sala L, Bernardi J, Mostacciuolo G, Rocchetti M et al. IKr impact on repolarization and its variability assessed by dynamic clamp. Circ Arrhythm Electrophysiol 2015;8:1265–75. [DOI] [PubMed] [Google Scholar]
- 121. Biliczki P, Virag L, Iost N, Papp JG, Varro A. Interaction of different potassium channels in cardiac repolarization in dog ventricular preparations: role of repolarization reserve. Br J Pharmacol 2002;137:361–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Pumir A, Arutunyan A, Krinsky V, Sarvazyan N. Genesis of ectopic waves: role of coupling, automaticity, and heterogeneity. Biophys J 2005;89:2332–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Bartolucci C, Altomare C, Bennati M, Furini S, Zaza A, Severi S. Combined action potential- and dynamic-clamp for accurate computational modelling of the cardiac IKr current. J Mol Cell Cardiol 2015;79:187–94. [DOI] [PubMed] [Google Scholar]
- 124. Qu Z, Weiss JN. Dynamics and cardiac arrhythmias. J Cardiovasc Electrophysiol 2006;17:1042–9. [DOI] [PubMed] [Google Scholar]
- 125. Jeron A, Mitchell GF, Zhou J, Murata M, London B, Buckett P et al. Inducible polymorphic ventricular tachyarrhythmias in a transgenic mouse model with a long Q-T phenotype. Am J Physiol Heart Circ Physiol 2000;278:H1891–1898. [DOI] [PubMed] [Google Scholar]
- 126. Liu N, Rizzi N, Boveri L, Priori SG. Ryanodine receptor and calsequestrin in arrhythmogenesis: what we have learnt from genetic diseases and transgenic mice. J Mol Cell Cardiol 2009;46:149–59. [DOI] [PubMed] [Google Scholar]
- 127. Nishida K, Michael G, Dobrev D, Nattel S. Animal models for atrial fibrillation: clinical insights and scientific opportunities. Europace 2010;12:160–72. [DOI] [PubMed] [Google Scholar]
- 128. Schüttler D, Bapat A, Kääb S, Lee K, Tomsits P, Clauss S et al. Animal models of atrial fibrillation. Circ Res 2020;127:91–110. [DOI] [PubMed] [Google Scholar]
- 129. Dobrev D, Wehrens XHT. Mouse models of cardiac arrhythmias. Circ Res 2018;123:332–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Aschar-Sobbi R, Izaddoustdar F, Korogyi AS, Wang Q, Farman GP, Yang F et al. Increased atrial arrhythmia susceptibility induced by intense endurance exercise in mice requires TNFalpha. Nat Commun 2015;6:6018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Manninger M, Zweiker D, van Hunnik A, Alogna A, Prassl AJ, Schipke J et al. Arterial hypertension drives arrhythmia progression via specific structural remodeling in a porcine model of atrial fibrillation. Heart Rhythm 2018;15:1328–36. [DOI] [PubMed] [Google Scholar]
- 132. Dobrev D, Aguilar M, Heijman J, Guichard JB, Nattel S. Postoperative atrial fibrillation: mechanisms, manifestations and management. Nat Rev Cardiol 2019;16:417–36. [DOI] [PubMed] [Google Scholar]
- 133. Miyauchi Y, Zhou S, Okuyama Y, Miyauchi M, Hayashi H, Hamabe A et al. Altered atrial electrical restitution and heterogeneous sympathetic hyperinnervation in hearts with chronic left ventricular myocardial infarction: implications for atrial fibrillation. Circulation 2003;108:360–6. [DOI] [PubMed] [Google Scholar]
- 134. Alasady M, Shipp NJ, Brooks AG, Lim HS, Lau DH, Barlow D et al. Myocardial infarction and atrial fibrillation: importance of atrial ischemia. Circ Arrhythm Electrophysiol 2013;6:738–45. [DOI] [PubMed] [Google Scholar]
- 135. Kettlewell S, Burton FL, Smith GL, Workman AJ. Chronic myocardial infarction promotes atrial action potential alternans, afterdepolarizations, and fibrillation. Cardiovasc Res 2013;99:215–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. van Gorp PRR, Trines SA, Pijnappels DA, de Vries AAF. Multicellular in vitro models of cardiac arrhythmias: focus on atrial fibrillation. Front Cardiovasc Med 2020;7:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Ellinwood N, Dobrev D, Morotti S, Grandi E. In silico assessment of efficacy and safety of IKur inhibitors in chronic atrial fibrillation: role of kinetics and state-dependence of drug binding. Front Pharmacol 2017;8:799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Schmidt C, Wiedmann F, Zhou XB, Heijman J, Voigt N, Ratte A et al. Inverse remodelling of K2P3.1 K+ channel expression and action potential duration in left ventricular dysfunction and atrial fibrillation: implications for patient-specific antiarrhythmic drug therapy. Eur Heart J 2017;38:1764–74. [DOI] [PubMed] [Google Scholar]
- 139. Saour B, Smith B, Yancy CW. Heart failure and sudden cardiac death. Card Electrophysiol Clin 2017;9:709–23. [DOI] [PubMed] [Google Scholar]
- 140. Desai RV, Ahmed MI, Mujib M, Aban IB, Zile MR, Ahmed A. Natural history of concentric left ventricular geometry in community-dwelling older adults without heart failure during seven years of follow-up. Am J Cardiol 2011;107:321–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Gomes AC, Falcao-Pires I, Pires AL, Bras-Silva C, Leite-Moreira AF. Rodent models of heart failure: an updated review. Heart Fail Rev 2013;18:219–49. [DOI] [PubMed] [Google Scholar]
- 142. Shannon TR, Pogwizd SM, Bers DM. Elevated sarcoplasmic reticulum Ca2+ leak in intact ventricular myocytes from rabbits in heart failure. Circ Res 2003;93:592–4. [DOI] [PubMed] [Google Scholar]
- 143. O’Rourke B, Kass DA, Tomaselli GF, KäÄB S, Tunin R, Marbán E, Mechanisms of altered excitation-contraction coupling in canine tachycardia-induced heart failure, I: experimental studies. Circ Res 1999;84:562–70. [DOI] [PubMed] [Google Scholar]
- 144. Dries E, Santiago DJ, Gilbert G, Lenaerts I, Vandenberk B, Nagaraju CK et al. Hyperactive ryanodine receptors in human heart failure and ischaemic cardiomyopathy reside outside of couplons. Cardiovasc Res 2018;114:1512–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Pinali C, Bennett H, Davenport JB, Trafford AW, Kitmitto A. Three-dimensional reconstruction of cardiac sarcoplasmic reticulum reveals a continuous network linking transverse-tubules: this organization is perturbed in heart failure. Circ Res 2013;113:1219–30. [DOI] [PubMed] [Google Scholar]
- 146. Milani-Nejad N, Janssen PM. Small and large animal models in cardiac contraction research: advantages and disadvantages. Pharmacol Ther 2014;141:235–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Ambrosi CM, Yamada KA, Nerbonne JM, Efimov IR. Gender differences in electrophysiological gene expression in failing and non-failing human hearts. PLoS One 2013;8:e54635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Lang D, Holzem K, Kang C, Xiao M, Hwang HJ, Ewald GA et al. Arrhythmogenic remodeling of beta2 versus beta1 adrenergic signaling in the human failing heart. Circ Arrhythm Electrophysiol 2015;8:409–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Patel RB, Vaduganathan M, Shah SJ, Butler J. Atrial fibrillation in heart failure with preserved ejection fraction: insights into mechanisms and therapeutics. Pharmacol Ther 2017;176:32–9. [DOI] [PubMed] [Google Scholar]
- 150. Vaduganathan M, Patel RB, Michel A, Shah SJ, Senni M, Gheorghiade M et al. Mode of death in heart failure with preserved ejection fraction. J Am Coll Cardiol 2017;69:556–69. [DOI] [PubMed] [Google Scholar]
- 151. Lourenco AP, Leite-Moreira AF, Balligand JL, Bauersachs J, Dawson D, de Boer RA et al. An integrative translational approach to study heart failure with preserved ejection fraction: a position paper from the Working Group on Myocardial Function of the European Society of Cardiology. Eur J Heart Fail 2018;20:216–27. [DOI] [PubMed] [Google Scholar]
- 152. Cho JH, Zhang R, Kilfoil PJ, Gallet R, de Couto G, Bresee C et al. Delayed repolarization underlies ventricular arrhythmias in rats with heart failure and preserved ejection fraction. Circulation 2017;136:2037–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Cho JH, Zhang R, Aynaszyan S, Holm K, Goldhaber JI, Marban E et al. Ventricular arrhythmias underlie sudden death in rats with heart failure and preserved ejection fraction. Circ Arrhythm Electrophysiol 2018;11:e006452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Primessnig U, Schonleitner P, Holl A, Pfeiffer S, Bracic T, Rau T et al. Novel pathomechanisms of cardiomyocyte dysfunction in a model of heart failure with preserved ejection fraction. Eur J Heart Fail 2016;18:987–97. [DOI] [PubMed] [Google Scholar]
- 155. Primessnig U, Bracic T, Levijoki J, Otsomaa L, Pollesello P, Falcke M et al. Long-term effects of Na+/Ca2+ exchanger inhibition with ORM-11035 improves cardiac function and remodelling without lowering blood pressure in a model of heart failure with preserved ejection fraction. Eur J Heart Fail 2019;21:1543–52. [DOI] [PubMed] [Google Scholar]
- 156. Mesquita TRR, Zhang R, de Couto G, Valle J, Sanchez L, Rogers RG et al. Mechanisms of atrial fibrillation in aged rats with heart failure with preserved ejection fraction. Heart Rhythm 2020;17:1025–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Miyake CY, Teele SA, Chen L, Motonaga KS, Dubin AM, Balasubramanian S et al. In-hospital arrhythmia development and outcomes in pediatric patients with acute myocarditis. Am J Cardiol 2014;113:535–40. [DOI] [PubMed] [Google Scholar]
- 158. Sagar S, Liu PP, Cooper LT Jr. Myocarditis. Lancet 2012;379:738–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Ali-Ahmed F, Dalgaard F, Al-Khatib SM. Sudden cardiac death in patients with myocarditis: evaluation, risk stratification, and management. Am Heart J 2020;220:29–40. [DOI] [PubMed] [Google Scholar]
- 160. D'Ambrosio A, Patti G, Manzoli A, Sinagra G, Di Lenarda A, Silvestri F et al. The fate of acute myocarditis between spontaneous improvement and evolution to dilated cardiomyopathy: a review. Heart 2001;85:499–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Blyszczuk P. Myocarditis in humans and in experimental animal models. Front Cardiovasc Med 2019;6:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Neu N, Rose NR, Beisel KW, Herskowitz A, Gurri-Glass G, Craig SW. Cardiac myosin induces myocarditis in genetically predisposed mice. J Immunol 1987;139:3630–6. [PubMed] [Google Scholar]
- 163. Hua X, Hu G, Hu Q, Chang Y, Hu Y, Gao L et al. Single-cell RNA sequencing to dissect the immunological network of autoimmune myocarditis. Circulation 2020;142:384–400. [DOI] [PubMed] [Google Scholar]
- 164. Gianfranchesco Filippi M, de Castro Ferreira Lima M, Paes AC, Sarita Cruz Aleixo A, Oba E, Ferreira de Souza F et al. Evaluation of heart rate variability and behavior of electrocardiographic parameters in dogs affected by chronic Monocytic Ehrlichiosis. PLoS One 2019;14:e0216552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Mesirca P, Fedorov VV, Hund TJ, Torrente AG, Bidaud I, Mohler PJ et al. Pharmacologic approach to sinoatrial node dysfunction. Annu Rev Pharmacol Toxicol 2021;61:757–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Peters CH, Sharpe EJ, Proenza C. Cardiac pacemaker activity and aging. Annu Rev Physiol 2020;82:21–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Mangoni ME, Couette B, Bourinet E, Platzer J, Reimer D, Striessnig J et al. Functional role of L-type Cav1.3 Ca2+ channels in cardiac pacemaker activity. Proc Natl Acad Sci U S A 2003;100:5543–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Lei M, Goddard C, Liu J, Leoni AL, Royer A, Fung SS et al. Sinus node dysfunction following targeted disruption of the murine cardiac sodium channel gene Scn5a. J Physiol 2005;567:387–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Baig SM, Koschak A, Lieb A, Gebhart M, Dafinger C, Nurnberg G et al. Loss of Ca(v)1.3 (CACNA1D) function in a human channelopathy with bradycardia and congenital deafness. Nat Neurosci 2011;14:77–84. [DOI] [PubMed] [Google Scholar]
- 170. Hoesl E, Stieber J, Herrmann S, Feil S, Tybl E, Hofmann F et al. Tamoxifen-inducible gene deletion in the cardiac conduction system. J Mol Cell Cardiol 2008;45:62–9. [DOI] [PubMed] [Google Scholar]
- 171. Mesirca P, Alig J, Torrente AG, Muller JC, Marger L, Rollin A et al. Cardiac arrhythmia induced by genetic silencing of ‘funny’ (f) channels is rescued by GIRK4 inactivation. Nat Commun 2014;5:4664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Alig J, Marger L, Mesirca P, Ehmke H, Mangoni ME, Isbrandt D. Control of heart rate by cAMP sensitivity of HCN channels. Proc Natl Acad Sci U S A 2009;106:12189–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Le Scouarnec S, Bhasin N, Vieyres C, Hund TJ, Cunha SR, Koval O et al. Dysfunction in ankyrin-B-dependent ion channel and transporter targeting causes human sinus node disease. Proc Natl Acad Sci U S A 2008;105:15617–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Milano A, Vermeer AM, Lodder EM, Barc J, Verkerk AO, Postma AV et al. HCN4 mutations in multiple families with bradycardia and left ventricular noncompaction cardiomyopathy. J Am Coll Cardiol 2014;64:745–56. [DOI] [PubMed] [Google Scholar]
- 175. Karnabi E, Qu Y, Mancarella S, Boutjdir M. Rescue and worsening of congenital heart block-associated electrocardiographic abnormalities in two transgenic mice. J Cardiovasc Electrophysiol 2011;22:922–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Royer A, van Veen TA, Le Bouter S, Marionneau C, Griol-Charhbili V, Leoni AL et al. Mouse model of SCN5A-linked hereditary Lenegre's disease: age-related conduction slowing and myocardial fibrosis. Circulation 2005;111:1738–46. [DOI] [PubMed] [Google Scholar]
- 177. Zicha S, Fernandezvelasco M, Lonardo G, Lheureux N, Nattel S. Sinus node dysfunction and hyperpolarization-activated (HCN) channel subunit remodeling in a canine heart failure model. Cardiovasc Res 2005;66:472–81. [DOI] [PubMed] [Google Scholar]
- 178. Verkerk AO, Wilders R, Coronel R, Ravesloot JH, Verheijck EE. Ionic remodeling of sinoatrial node cells by heart failure. Circulation 2003;108:760–6. [DOI] [PubMed] [Google Scholar]
- 179. Mackasey M, Egom EE, Jansen HJ, Hua R, Moghtadaei M, Liu Y et al. Natriuretic peptide receptor-C protects against angiotensin II-mediated sinoatrial node disease in mice. JACC Basic Transl Sci 2018;3:824–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Yeh YH, Burstein B, Qi XY, Sakabe M, Chartier D, Comtois P et al. Funny current downregulation and sinus node dysfunction associated with atrial tachyarrhythmia: a molecular basis for tachycardia-bradycardia syndrome. Circulation 2009;119:1576–85. [DOI] [PubMed] [Google Scholar]
- 181. Howarth FC, Al-Sharhan R, Al-Hammadi A, Qureshi MA. Effects of streptozotocin-induced diabetes on action potentials in the sinoatrial node compared with other regions of the rat heart. Mol Cell Biochem 2007;300:39–46. [DOI] [PubMed] [Google Scholar]
- 182. D’Souza A, Bucchi A, Johnsen AB, Logantha SJRJ, Monfredi O, Yanni J et al. Exercise training reduces resting heart rate via downregulation of the funny channel HCN4. Nat Commun 2014;5:3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Larson ED, St Clair JR, Sumner WA, Bannister RA, Proenza C. Depressed paceJmaker activity of sinoatrial node myocytes contributes to the age-dependent decline in maximum heart rate. Proc Natl Acad Sci U S A 2013;110:18011–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Shimizu W, Antzelevitch C. Sodium channel block with mexiletine is effective in reducing dispersion of repolarization and preventing torsade des pointes in LQT2 and LQT3 models of the long-QT syndrome. Circulation 1997;96:2038–47. [DOI] [PubMed] [Google Scholar]
- 185. Milberg P, Reinsch N, Wasmer K, Monnig G, Stypmann J, Osada N et al. Transmural dispersion of repolarization as a key factor of arrhythmogenicity in a novel intact heart model of LQT3. Cardiovasc Res 2005;65:397–404. [DOI] [PubMed] [Google Scholar]
- 186. Patocskai B, Yoon N, Antzelevitch C. Mechanisms underlying epicardial radiofrequency ablation to suppress arrhythmogenesis in experimental models of Brugada syndrome. JACC Clin Electrophysiol 2017;3:353–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Sendfeld F, Selga E, Scornik FS, Perez GJ, Mills NL, Brugada R. Experimental models of Brugada syndrome. Int J Mol Sci 2019;20:2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Rivolta I, Abriel H, Tateyama M, Liu H, Memmi M, Vardas P et al. Inherited Brugada and long QT-3 syndrome mutations of a single residue of the cardiac sodium channel confer distinct channel and clinical phenotypes. J Biol Chem 2001;276:30623–30. [DOI] [PubMed] [Google Scholar]
- 189. Remme CA, Verkerk AO, Nuyens D, van Ginneken AC, van Brunschot S, Belterman CN et al. Overlap syndrome of cardiac sodium channel disease in mice carrying the equivalent mutation of human SCN5A-1795insD. Circulation 2006;114:2584–94. [DOI] [PubMed] [Google Scholar]
- 190. Casini S, Albesa M, Wang Z, Portero V, Ross-Kaschitza D, Rougier JS et al. Functional consequences of the SCN5A-p.Y1977N mutation within the PY ubiquitylation motif: discrepancy between HEK293 cells and transgenic mice. Int J Mol Sci 2019;20:5033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Boukens BJ, Sylva M, de Gier-de Vries C, Remme CA, Bezzina CR, Christoffels VM et al. Reduced sodium channel function unmasks residual embryonic slow conduction in the adult right ventricular outflow tract. Circ Res 2013;113:137–41. [DOI] [PubMed] [Google Scholar]
- 192. Kelly A, Salerno S, Connolly A, Bishop M, Charpentier F, Stolen T et al. Normal interventricular differences in tissue architecture underlie right ventricular susceptibility to conduction abnormalities in a mouse model of Brugada syndrome. Cardiovasc Res 2018;114:724–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Rivaud MR, Baartscheer A, Verkerk AO, Beekman L, Rajamani S, Belardinelli L et al. Enhanced late sodium current underlies pro-arrhythmic intracellular sodium and calcium dysregulation in murine sodium channelopathy. Int J Cardiol 2018;263:54–62. [DOI] [PubMed] [Google Scholar]
- 194. Zaza A, Rocchetti M. The late Na+ current - origin and pathophysiological relevance. Cardiovasc Drugs Ther 2013;27:61–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Remme CA, Scicluna BP, Verkerk AO, Amin AS, van Brunschot S, Beekman L et al. Genetically determined differences in sodium current characteristics modulate conduction disease severity in mice with cardiac sodium channelopathy. Circ Res 2009;104:1283–92. [DOI] [PubMed] [Google Scholar]
- 196. Fabritz L, Damke D, Emmerich M, Kaufmann SG, Theis K, Blana A et al. Autonomic modulation and antiarrhythmic therapy in a model of long QT syndrome type 3. Cardiovasc Res 2010;87:60–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Rivaud MR, Jansen JA, Postema PG, Nannenberg EA, Mizusawa Y, van der Nagel R et al. A common co-morbidity modulates disease expression and treatment efficacy in inherited cardiac sodium channelopathy. Eur Heart J 2018;39:2898–907. [DOI] [PubMed] [Google Scholar]
- 198. Ma D, Wei H, Zhao Y, Lu J, Li G, Sahib NB et al. Modeling type 3 long QT syndrome with cardiomyocytes derived from patient-specific induced pluripotent stem cells. Int J Cardiol 2013;168:5277–86. [DOI] [PubMed] [Google Scholar]
- 199. Leong IU, Stuckey A, Lai D, Skinner JR, Love DR. Assessment of the predictive accuracy of five in silico prediction tools, alone or in combination, and two metaservers to classify long QT syndrome gene mutations. BMC Med Genet 2015;16:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Clerx M, Heijman J, Collins P, Volders PGA. Predicting changes to INa from missense mutations in human SCN5A. Sci Rep 2018;8:12797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Xia L, Zhang Y, Zhang H, Wei Q, Liu F, Crozier S. Simulation of Brugada syndrome using cellular and three-dimensional whole-heart modeling approaches. Physiol Meas 2006;27:1125–42. [DOI] [PubMed] [Google Scholar]
- 202. Hoogendijk MG, Potse M, Vinet A, de Bakker JM, Coronel R. ST segment elevation by current-to-load mismatch: an experimental and computational study. Heart Rhythm 2011;8:111–8. [DOI] [PubMed] [Google Scholar]
- 203. Xu H, Guo W, Nerbonne JM. Four kinetically distinct depolarization-activated K+ currents in adult mouse ventricular myocytes. J Gen Physiol 1999;113:661–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Nerbonne JM, Nichols CG, Schwarz TL, Escande D. Genetic manipulation of cardiac K+ channel function in mice: what have we learned, and where do we go from here? Circ Res 2001;89:944–56. [DOI] [PubMed] [Google Scholar]
- 205. Charpentier F, Demolombe S, Escande D. Cardiac channelopathies: from men to mice. Ann Med 2004;36: 28–34. [DOI] [PubMed] [Google Scholar]
- 206. Demolombe S, Lande G, Charpentier F, van Roon MA, van den Hoff MJ, Toumaniantz G et al. Transgenic mice overexpressing human KvLQT1 dominant-negative isoform. Part I: phenotypic characterisation. Cardiovasc Res 2001;50:314–27. [DOI] [PubMed] [Google Scholar]
- 207. London B, Baker LC, Petkova-Kirova P, Nerbonne JM, Choi BR, Salama G. Dispersion of repolarization and refractoriness are determinants of arrhythmia phenotype in transgenic mice with long QT. J Physiol 2007;578:115–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Arnaout R, Ferrer T, Huisken J, Spitzer K, Stainier DY, Tristani-Firouzi M et al. Zebrafish model for human long QT syndrome. Proc Natl Acad Sci U S A 2007;104:11316–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Peal DS, Mills RW, Lynch SN, Mosley JM, Lim E, Ellinor PT et al. Novel chemical suppressors of long QT syndrome identified by an in vivo functional screen. Circulation 2011;123:23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Hassel D, Scholz EP, Trano N, Friedrich O, Just S, Meder B et al. Deficient zebrafish ether-a-go-go-related gene channel gating causes short-QT syndrome in zebrafish reggae mutants. Circulation 2008;117:866–75. [DOI] [PubMed] [Google Scholar]
- 211. Odening KE, Choi BR, Liu GX, Hartmann K, Ziv O, Chaves L et al. Estradiol promotes sudden cardiac death in transgenic long QT type 2 rabbits while progesterone is protective. Heart Rhythm 2012;9:823–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Lang CN, Koren G, Odening KE. Transgenic rabbit models to investigate the cardiac ion channel disease long QT syndrome. Prog Biophys Mol Biol 2016;121:142–56. [DOI] [PubMed] [Google Scholar]
- 213. Baczko I, Hornyik T, Brunner M, Koren G, Odening KE. Transgenic rabbit models in proarrhythmia research. Front Pharmacol 2020;11:853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Moretti A, Bellin M, Welling A, Jung CB, Lam JT, Bott-Flugel L et al. Patient-specific induced pluripotent stem-cell models for long-QT syndrome. N Engl J Med 2010;363:1397–409. [DOI] [PubMed] [Google Scholar]
- 215. Sala L, Yu Z, Ward-van Oostwaard D, van Veldhoven JP, Moretti A, Laugwitz KL et al. A new hERG allosteric modulator rescues genetic and drug-induced long-QT syndrome phenotypes in cardiomyocytes from isogenic pairs of patient induced pluripotent stem cells. EMBO Mol Med 2016;8:1065–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Mehta A, Ramachandra CJA, Singh P, Chitre A, Lua CH, Mura M et al. Identification of a targeted and testable antiarrhythmic therapy for long-QT syndrome type 2 using a patient-specific cellular model. Eur Heart J 2018;39:1446–55. [DOI] [PubMed] [Google Scholar]
- 217. Lawrence CL, Pollard CE, Hammond TG, Valentin JP. Nonclinical proarrhythmia models: predicting Torsades de Pointes. J Pharmacol Toxicol Methods 2005;52:46–59. [DOI] [PubMed] [Google Scholar]
- 218. Fernández-Velasco M, Rueda A, Rizzi N, Benitah J-P, Colombi B, Napolitano C et al. Increased Ca2+ sensitivity of the ryanodine receptor mutant RyR2-R4496C underlies catecholaminergic polymorphic ventricular tachycardia. Circ Res 2009;104:201–9, 212p following 209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Uchinoumi H, Yano M, Suetomi T, Ono M, Xu X, Tateishi H et al. Catecholaminergic polymorphic ventricular tachycardia is caused by mutation-linked defective conformational regulation of the ryanodine receptor. Circ Res 2010;106:1413–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Zhao YT, Valdivia CR, Gurrola GB, Powers PP, Willis BC, Moss RL et al. Arrhythmogenesis in a catecholaminergic polymorphic ventricular tachycardia mutation that depresses ryanodine receptor function. Proc Natl Acad Sci U S A 2015;112:E1669–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Wang YY, Mesirca P, Marques-Sule E, Zahradnikova A Jr., Villejoubert O, D'Ocon P et al. RyR2R420Q catecholaminergic polymorphic ventricular tachycardia mutation induces bradycardia by disturbing the coupled clock pacemaker mechanism. JCI Insight 2017;2:e91872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Oberst L, Zhao G, Park JT, Brugada R, Michael LH, Entman ML et al. Dominant-negative effect of a mutant cardiac troponin T on cardiac structure and function in transgenic mice. J Clin Invest 1998;102:1498–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Muthuchamy M, Pieples K, Rethinasamy P, Hoit B, Grupp IL, Boivin GP et al. Mouse model of a familial hypertrophic cardiomyopathy mutation in alpha-tropomyosin manifests cardiac dysfunction. Circ Res 1999;85:47–56. [DOI] [PubMed] [Google Scholar]
- 224. Hasenfuss G. Animal models of human cardiovascular disease, heart failure and hypertrophy. Cardiovasc Res 1998;39:60–76. [DOI] [PubMed] [Google Scholar]
- 225. Coppini R, Santini L, Olivotto I, Ackerman MJ, Cerbai E. Abnormalities in sodium current and calcium homoeostasis as drivers of arrhythmogenesis in hypertrophic cardiomyopathy. Cardiovasc Res 2020;116:1585–99. [DOI] [PubMed] [Google Scholar]
- 226. Santini L, Palandri C, Nediani C, Cerbai E, Coppini R. Modelling genetic diseases for drug development: hypertrophic cardiomyopathy. Pharmacol Res 2020;160:105176. [DOI] [PubMed] [Google Scholar]
- 227. Green EM, Wakimoto H, Anderson RL, Evanchik MJ, Gorham JM, Harrison BC et al. A small-molecule inhibitor of sarcomere contractility suppresses hypertrophic cardiomyopathy in mice. Science 2016;351:617–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Olivotto I, Oreziak A, Barriales-Villa R, Abraham TP, Masri A, Garcia-Pavia P et al. Mavacamten for treatment of symptomatic obstructive hypertrophic cardiomyopathy (EXPLORER-HCM): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2020;396:759–69. [DOI] [PubMed] [Google Scholar]
- 229. Marian AJ, Wu Y, Lim DS, McCluggage M, Youker K, Yu QT et al. A transgenic rabbit model for human hypertrophic cardiomyopathy. J Clin Invest 1999;104:1683–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. van der Voorn SM, Te Riele A, Basso C, Calkins H, Remme CA, van Veen TAB. Arrhythmogenic cardiomyopathy: pathogenesis, pro-arrhythmic remodelling, and novel approaches for risk stratification and therapy. Cardiovasc Res 2020;116:1571–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Lodder EM, Rizzo S. Mouse models in arrhythmogenic right ventricular cardiomyopathy. Front Physiol 2012;3:221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Rizzo S, Lodder EM, Verkerk AO, Wolswinkel R, Beekman L, Pilichou K et al. Intercalated disc abnormalities, reduced Na+ current density, and conduction slowing in desmoglein-2 mutant mice prior to cardiomyopathic changes. Cardiovasc Res 2012;95:409–18. [DOI] [PubMed] [Google Scholar]
- 233. Kim JC, Perez-Hernandez M, Alvarado FJ, Maurya SR, Montnach J, Yin Y et al. Disruption of homeostasis and connexin 43 hemichannel function in the right ventricle precedes overt arrhythmogenic cardiomyopathy in plakophilin-2-deficient mice. Circulation 2019;140:1015–30. [DOI] [PMC free article] [PubMed] [Google Scholar]