Abstract
Whilst there is a clear clinical benefit of oral anticoagulation (OAC) in patients with atrial fibrillation (AF) and venous thromboembolism (VTE) in reducing the risks of thromboembolism, major bleeding events (especially intracranial bleeds) may still occur and be devastating. The decision to initiate and continue anticoagulation is often based on a careful assessment of both the thromboembolism and bleeding risk. The more common and validated bleeding risk factors have been used to formulate bleeding risk stratification scores, but thromboembolism and bleeding risk factors often overlap. Also, many factors that increase bleeding risk are transient and modifiable, such as variable international normalized ratio values, surgical procedures, vascular procedures, or drug–drug and food–drug interactions. Bleeding risk is also not a static ‘one off’ assessment based on baseline factors but is dynamic, being influenced by ageing, incident comorbidities, and drug therapies. In this Consensus Document, we comprehensively review the published evidence and propose a consensus on bleeding risk assessments in patients with AF and VTE, with the view to summarizing ‘best practice’ when approaching antithrombotic therapy in these patients. We address the epidemiology and size of the problem of bleeding risk in AF and VTE, review established bleeding risk factors, and summarize definitions of bleeding. Patient values and preferences, balancing the risk of bleeding against thromboembolism are reviewed, and the prognostic implications of bleeding are discussed. We propose consensus statements that may help to define evidence gaps and assist in everyday clinical practice.
Keywords: Bleeding, Oral anticoagulation, Atrial fibrillation, Venous thromboembolism, Risk assessment
Introduction and scope
Whilst there is a clear clinical benefit of oral anticoagulation (OAC) in patients with atrial fibrillation (AF) and venous thromboembolism (VTE) in preventing future thromboembolic events, major bleeding events [especially intracranial haemorrhage (ICH)] may still occur and be devastating.1 The decision to initiate and continue anticoagulation is often based on a careful assessment of the risks of both thromboembolism and bleeding. It is well recognized that the net clinical benefit of OAC generally outweigh the risks of bleeding, especially in AF patients at high ischaemic risk.2
The more common and validated bleeding risk factors have been used to formulate bleeding risk stratification scores, but many of these are also risk factors for thromboembolism. Many factors that increase bleeding are transient and modifiable. Bleeding risk is not static, with a ‘one off’ assessment based on baseline factors, but dynamic, influenced by ageing, incident comorbidities, and drug therapies. Another factor is ethnicity, where East Asians appear more sensitive to antithrombotic therapy-related bleeding.3
In 2011, the European Heart Rhythm Association (EHRA) and European Society of Cardiology (ESC) Working Group on Thrombosis published a position document on Bleeding Risk Assessment and Management in AF Patients.4 Over the last decade, there have been advances in our understanding of the epidemiology, risks, and clinical prediction of bleeding, in patients with AF as well as VTE. We also have seen a major growth in the efforts to improve thromboprophylaxis, with increasing use of the non-vitamin K antagonist oral anticoagulants (NOACs) for AF and VTE,5,6 comprising of direct thrombin inhibitors (dabigatran) and direct factor Xa inhibitors (rivaroxaban, apixaban, and edoxaban).
Non-vitamin K antagonist oral anticoagulants offer improved effectiveness, safety, and convenience compared with vitamin K antagonists (VKA, e.g. warfarin, acenocoumarol, or phenprocoumon). The risks of thromboembolism and bleeding from VKAs are highly dependent on the quality of anticoagulation control, as reflected by the average time in therapeutic range (TTR), with the target international normalized ratio (INR) being 2.0–3.0.7 Whilst a lower INR range may reduce bleeding risk, especially in East Asian populations, it greatly increases the risk of thromboembolism.8 However, when using warfarin as part of triple antithrombotic therapy, a lower INR of 2.0–2.5 was associated with reduced bleeding risk compared with higher INRs.9
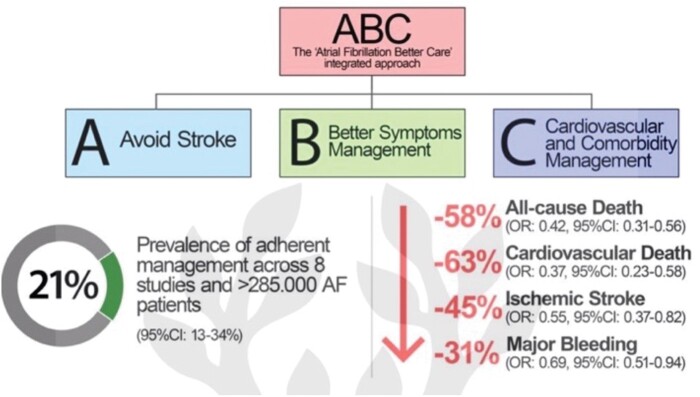
Furthermore, AF management has evolved towards a more integrated and holistic approach, summed up as the ABC (Atrial fibrillation Better Care) pathway: ‘A’ Avoid stroke (with Anticoagulants); ‘B’ Better symptom management; ‘C’ Cardiovascular and Comorbidity management10 and is recommended in several guidelines, including the recent ESC Guidelines for the diagnosis and management of AF,11 and the 2021 Asia Pacific Heart Rhythm Society guidelines.12 In a systematic review, AF patients who were managed adherent to the ABC pathway had a lower risk of all-cause death [odds ratio (OR): 0.42, 95% confidence interval (CI) 0.31–0.56], cardiovascular death (OR: 0.37, 95% CI 0.23–0.58), stroke (OR: 0.55, 95% CI 0.37–0.82), and major bleeding (OR: 0.69, 95% CI 0.51–0.94)13 (Figure 1).
Figure 1.
ABC pathway and improved outcomes in patients with AF. ABC, Atrial fibrillation Better Care; AF, atrial fibrillation.
Given the advances over the last decade, including the development and approval of reversal agents for NOACs, the ESC Working Group on Thrombosis, in collaboration with the EHRA, Acute CardioVascular Care Association, and Asia-Pacific Heart Rhythm Society convened a Task Force, with the remit to review the published evidence and to propose a consensus on bleeding risk assessment in patients with AF and VTE, with a view to facilitating ‘best practice’. This position paper summarizes the available evidence and puts forwards consensus statements that may help to define evidence gaps and simple practical approaches to assist in everyday clinical practice.
The ultimate judgement regarding the care of each individual patient must be made by the healthcare provider and the patient together, considering all the circumstances presented by that patient.
Literature searches were performed on the following databases: PubMed/MEDLINE and the Cochrane Library (including the Cochrane Database of Systematic Reviews and the Cochrane Controlled Trials Registry), restricted to human subjects and English language sources. Articles related to animal experimentation were only cited when the information was important to understanding pathophysiological concepts pertinent to patient management and comparable data were not available from human studies.
Systematic review
Epidemiology of bleeding with oral anticoagulant in atrial fibrillation
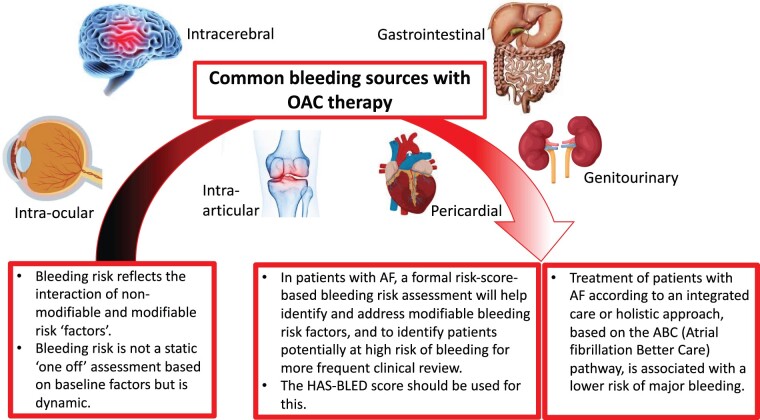
Current guidelines suggest that most patients with AF will require OAC to reduce the risk of stroke,11,12,14 although OAC increases the risk of bleeding. Randomized controlled trials (RCTs) in AF patients treated with VKA reported annual rates of major bleeding of 1.4–3.4%,15 with much lower rates with NOACs.2 The most serious bleed, ICH, is rare, occurring in 0.1–2.5% patients per year,16 with more recent studies reporting a lower rate of 0.7–0.8%.2 Importantly, OAC-related ICH leads to poorer clinical outcomes, greater disability, and higher mortality than ICH that is non-OAC related17 (Figure 2). The risk of bleeding (and stroke) is highest when AF is newly diagnosed and during the initiation of OAC.18
Figure 2.
Common bleeding sources with oral anticoagulant therapy.
Different variables have been observed to predict the risk of anticoagulation-related bleeding in patients with AF (Figure 3). Individual TTR and INR variability were associated with bleeding complications, in particular ICH.19 Non-vitamin K antagonist oral anticoagulants showed a lower incidence of major bleeding (−14%) and ICH (−52%) compared to warfarin.2,20 However, the risk of gastrointestinal bleeding is not reduced with higher dose NOACs compared to warfarin.2
Figure 3.
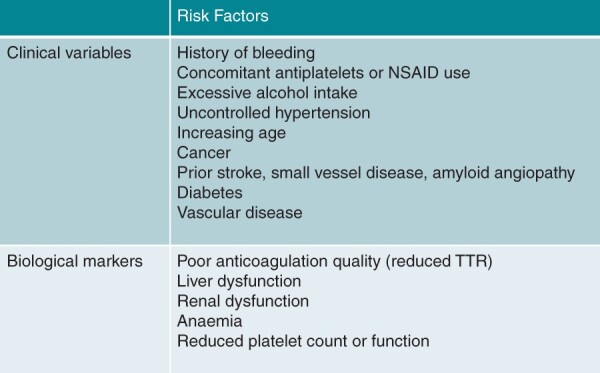
Risk factors for anticoagulation-related bleeding.
Epidemiology of bleeding with oral anticoagulant in venous thromboembolism
Venous thromboembolism, whether deep vein thrombosis (DVT) or pulmonary embolism (PE), requires anticoagulation to prevent complications or disease progression. Current guidelines recommend a minimum of 3 months’ treatment for patients with a transient or reversible risk factor, whereas longer term treatment is needed for patients with an unprovoked event or due to a persistent risk factor.21,22 Prediction of bleeding risk is crucial for patients at high risk of recurrent thrombosis.
A systematic review and meta-analysis comprising of 33 studies reported a 2.06% rate of VKA-related major bleeding (95% CI 2.04–2.08%) during the initial 3 months of anticoagulation, and a fatal bleeding rate of 0.37% (95% CI 0.36–0.38%),23 similar to the 2.2% major and 0.55% fatal bleeding reported in the RIETE registry.24 During the extended phase beyond the first 3 months, the rate of major bleeding associated with VKA treatment was 2.74% (95% CI 2.71–2.77).23,25
In general, NOACs are at least as effective as LMWH/VKA but are associated with less bleeding. A systematic review and meta-analysis of 10 trials showed that in patients with VTE, NOACs were associated with a lower risk of major bleeding [1.08% vs. 1.73%, risk ratio (RR) 0.63, 95% CI 0.51–0.77],26 as well as fatal bleeding (RR 0.36%, 95% CI 0.15–0.87), compared to VKA. During the extended phase, there was a non-significant increase in major bleeding in patients receiving NOACs against placebo. Reduced-dose apixaban27 and rivaroxaban28 have been compared against standard-dose, aspirin, or placebo. Data from a meta-analysis showed that major or clinically relevant non-major bleeding events were similar with reduced-dose NOACs as with aspirin or placebo (RR 1.19, 95% CI 0.81–1.77), whereas there was no significant difference compared to full-dose NOAC, with a trend towards less bleeding with the reduced dose (RR 0.74, 95% CI 0.52–1.05).29
Definitions of bleeding
Defining bleeding events during OAC therapy is important to both quantify its prognostic impact and address the related diagnostic and therapeutic measures, and several definitions are in use (Table 1), including either qualitative definitions or objective quantitative data, such as drop in haemoglobin, or frequently both. The most widely used are the Thrombolysis in Myocardial Infarction (TIMI),30 Global Use of Strategies To Open occluded arteries (GUSTO),31 International Society of Thrombosis and Haemostasis (ISTH),32,33 and the Bleeding Academic Research Consortium (BARC)34 classifications, and all have been shown to predict mortality.35,36 Heterogeneity in bleeding definitions may, at least partly, account for the variability in the reported rate of haemorrhagic complications with OAC.16
Table 1.
Most frequently used bleeding definitions
| TIMI30 | GUSTO31 | ISTH32,33 | BARC34 |
|---|---|---|---|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|||
|
|||
|
Clinical bleeding risk factors with oral anticoagulant for atrial fibrillation or venous thromboembolism
Studies reporting risk factors associated with bleeding are similar whether OAC is taken for VTE or AF21,22,37 and are summarized in Tables 2–9, including age (Table 2), hypertension (Table 3), renal impairment (Table 4), abnormal liver function (Table 5), prior stroke (Table 6), prior bleeding (Table 7), anaemia (Table 8), and malignancy (Table 9).
Table 2.
Summary of ‘age’ as a risk factor for bleeding in AF patients receiving OACs
| Study | Subjects (n) | Type of OACs | Age groups | Main findings | RR/OR/HR (95% CI) | P value |
|---|---|---|---|---|---|---|
| SPAF Investigators, 1996 | 555 | VKA | Age >75 vs. ≤75 years | Major bleeding (per year): 4.2% vs. 1.7% | RR 2.6 | 0.009 |
| Pengo et al., 2001 | 433 | VKA | Age >75 vs. ≤75 years | Major bleeding (per year): 5.1% vs. 1.0% | RR 6.6 (1.2–3.7) | 0.032 |
| Fang et al., 2004 | 1190 | VKA | Incremental risk per 5 years | The risk for intracranial haemorrhage increased at ≥85 years of age. | adjusted OR 2.5 (1.3–4.7) compared to age 70–74 years | NR |
| Pisters et al., 2010 | 5333 | VKA | Age >65 vs. ≤65 years | 1-year event rate of major bleeding: 2.3% vs. 0.7% | OR 2.66 (1.33–5.32) | <0.001 |
| Hankey et al., 2014 | 14 264 | VKA/rivaroxaban | Per decade increase in age | Age is an important risk factor of ICH | HR 1.35 (1.13–1.63) | 0.001 |
| O’Brien et al., 2015 | 7411 | VKA/dabigatran | Age >75 vs. ≤75 years | Older age had good ability to identify those who bled vs. not. | HR 1.38 (1.17–1.61) | NR |
| Chao et al., 2020 | 64 169 | VKA/NOACs | Age >90, 75–89 and 65–74 years |
|
NR | NR |
AF, atrial fibrillation; HR, hazard ratio; ICH , intra-cranial haemorrhage; NR, not reported; OACs, oral anticoagulants; OR, odds ratio; NOAC, non-vitamin K antagonist oral anticoagulant; RR, relative risk; SPAF, Stroke Prevention in Atrial Fibrillation; VKA, vitamin K antagonists.
Table 3.
Summary of ‘hypertension’ as a risk factor for bleeding in AF patients receiving OACs
| Study | Subject (n) | Type of OACs | Definition of hypertension | Main findings | RR/HR (95% CI) | P value |
|---|---|---|---|---|---|---|
| SPAF Investigators, 1996 | 555 | VKA |
|
Increase risk of ICH in patients with poor controlled hypertension |
|
|
| Fang et al., 2011 | 9186 | VKA | Diagnosed hypertension as per guideline | Prevalence of hypertension in patients with or without major bleeding: 64.7% vs. 61.9% | HR 1.5 (1.2–1.9) | 0.001 |
| Hankey et al., 2014 | 14 264 | VKA/rivaroxaban | Each 10 mmHg increase of diastolic BP | Increased diastolic BP is independently associated with ICH | HR 1.17 (1.01–1.36) | 0.042 |
| Park et al., 2019 | 19 679 | VKA/edoxaban |
|
|
|
|
| Böhm et al., 2020 | 18 107 |
|
|
Any bleeding rate (per year): 24.99% vs. 17.30% vs. 14.71% vs. 14.61% |
|
NR |
AF, atrial fibrillation; BP, blood pressure; HR, hazard ratio; ICH , intra-cranial haemorrhage; NR, not reported; OACs, oral anticoagulants; RR, relative risk; SPAF, Stroke Prevention in Atrial Fibrillation; VKA, vitamin K antagonists.
Table 4.
Summary of ‘abnormal renal function’ as a risk factor for bleeding in AF patients receiving OACs
| Study | Subjects (n) | Type of OACs | Definition | Main findings | OR/HR (95% CI) | P value |
|---|---|---|---|---|---|---|
| Pisters et al., 2010 | 5333 | VKA | Presence of chronic dialysis, renal transplantation, or serum creatinine >200 mmol/L | The rate of major haemorrhage was 1.3% in patients without kidney failure vs. 5.4% in those with kidney failure. | OR 2.86 (1.33–6.18) | <0.001 |
| Fang et al., 2011 | 9186 | VKA | eGFR <30 mL/min | Prevalence of renal impairment in patients with or without major bleeding: 5.9% vs. 2.7% | HR 4.3 (3.2–5.8) | <0.001 |
| Fox et al., 2011 | 14 264 | VKA/rivaroxaban |
|
|
NR | NR |
| Hohnloser et al., 2012 | 18 122 | VKA/apixaban |
|
|
NR | NR |
| O'Brien et al., 2015 | 7411 | VKA/dabigatran | eGFR <60 mL/min/1.73 m2 | Prevalence of renal impairment in patients with or without major bleeding: 48.4% vs. 34.0% | HR 1.44 (1.21–1.72) | NR |
AF, atrial fibrillation; HR, hazard ratio; eGFR, estimated glomerular filtration rate; NR, not reported; OACs, oral anticoagulants; OR, odds ratio; VKA, vitamin K antagonists.
Table 5.
Summary of ‘abnormal liver function’ as a risk factor for bleeding in AF patients receiving OACs
| Study | Subjects (n) | Type of OACs | Study population | Main findings | HR (95% CI) | P value |
|---|---|---|---|---|---|---|
| Fang et al., 2011 | 9186 | VKA | Diagnosed cirrhosis | Prevalence of liver cirrhosis in patients with or without major bleeding: 1.2% vs. 0.5% | HR 2.6 (1.1–6.1) | 0.03 |
| Efird et al., 2014 | 103 897 | VKA | Patients were defined as having liver disease if there was record ≥1 of the ICD9 codes for chronic liver disease, recorded either in the inpatient or outpatient setting, during the study period. | Patients with liver disease had more haemorrhages when compared with patients without. | HR 2.02 (1.69–2.42) | <0.001 |
| Hylek et al., 2014 | 18 122 | Apixaban/VKA | Patients with AF randomized to apixaban/VKA. Liver dysfunction not defined in paper | Only 8 patients with liver dysfunction experienced a major haemorrhage, precluding any definitive conclusion regarding this subgroup | HR 0.44 (0.22–0.88) | 0.020 |
AF, atrial fibrillation; HR, hazard ratio; ICD9, International Classification of Diseases-Ninth Revision; OACs, oral anticoagulants; VKA, vitamin K antagonists.
Table 6.
Summary of ‘stroke history’ as a risk factor for bleeding in AF patients receiving OACs
| Study | Subjects (n) | Type of OACs | Definition | Main findings | RR/HR (95% CI) | P value |
|---|---|---|---|---|---|---|
| Pengo et al., 2001 | 433 | VKA | History of thromboembolism | A higher frequency of major primary bleeding in patients who had suffered a previous thromboembolic event | NR | 0.03 |
| Fang et al., 2004 | 1190 | VKA | History of cerebrovascular disease | Prevalence of cerebrovascular disease in patients with or without ICH: 37% vs. 20% | NR | NR |
| Fang et al., 2011 | 9186 | VKA | Prior stroke | Prevalence of prior stroke in patients with or without major bleeding: 17.4% vs. 12.4% | HR 1.4 (1.1–1.9) | 0.01 |
| Hankey et al., 2014 | 14 264 | VKA/rivaroxaban | Previous stroke or TIA | Previous stroke or TIA is an independent factor associated with ICH | HR 1.42 (1.02–1.96) | 0.036 |
| Hylek et al., 2014 | 18 122 | Apixaban/VKA | Prior stroke/TIA/SE | Rate of ISTH major haemorrhage was 18.9% in patients without history vs. 24.5% in those with history (apixaban) and 19.5% vs. 23.4% (warfarin). | HR 1.23 (1.038–1.45) | 0.016 |
| O'Brien et al., 2015 | 7411 | VKA/dabigatran | Prior stroke | Prevalence of prior stroke in patients with or without major bleeding: 13.1% vs. 9.2% | NR | NR |
AF, atrial fibrillation; HR, hazard ratio; ICH , intra-cranial haemorrhage; NR, not reported; OACs, oral anticoagulants; OR, odds ratio; RR, relative risk; TIA, transient ischaemic attack; VKA, vitamin K antagonists.
Table 7.
Summary of ‘bleeding history’ as a risk factor for bleeding in AF patients receiving OACs
| Study | n | Type of OACs | Definition | Main findings | OR/HR (95% CI) | P value |
|---|---|---|---|---|---|---|
| Pisters et al., 2010 | 5333 | VKA | Prior major bleeding (ICH, hospitalization, haemoglobin decrease >2 g/L, and/or blood transfusion) | The rate of major haemorrhage was 1.3% in patients without prior major bleeding vs. 14.8% in those with prior major bleeding. | OR 7.51 (3.00–18.78) | <0.001 |
| Fang et al., 2011 | 9186 | VKA | Prior GI haemorrhage | Prevalence of prior GI bleeding in patients with or without major bleeding: 12.1% vs. 6.8% | HR 2.1 (1.5–2.9) | <0.001 |
| Hylek et al., 2014 | 18 122 | Apixaban/VKA | Bleeding history | Rate of ISTH major haemorrhage was 16.5% in patients without bleeding history vs. 25.2% in those with prior bleeding history (apixaban) and 16.4% vs. 22.5% (warfarin). | HR 1.38 (1.17–1.63) | 0.002 |
| O'Brien et al., 2015 | 7411 | VKA/dabigatran | Bleeding history | Bleeding history had good ability to identify those who bled vs. not. | HR 1.73 (1.34–2.23) | NR |
| Šinigoj et al., 2020a | 2260 |
|
Bleeding history | History of bleeding was a significant predictor of major bleeding. | HR 3.32 (1.87–5.90) | <0.001 |
AF, atrial fibrillation; GI, gastrointestinal; HR, hazard ratio; ICH , intra-cranial haemorrhage; NR, not reported; OACs, oral anticoagulants; OR, odds ratio; VKA, vitamin K antagonists.
Šinigoj et al. is restricted to individuals aged 85 and older.
Table 8.
Summary of ‘anaemia’ as a risk factor for bleeding in AF patients receiving OACs
| Study | Subjects (n) | Type of OACs | Definition | Main findings | HR (95% CI) | P value |
|---|---|---|---|---|---|---|
| Fang et al., 2011 | 9186 | VKA | Hb <13 g/dL in men and <12 g/dL in women | The rate of major haemorrhage was 12.1% in patients without anaemia vs. 18.8% in those with anaemia. | HR 4.2 (3.4–5.3) | <0.001 |
| O'Brien et al., 2015 | 7411 |
|
Reduced Hb/haematocrit/history of anaemia | Reduced haemoglobin/haematocrit/history of anaemia had good ability to identify those who bled vs. not. | HR 2.07 (1.74–2.47) | NR |
| Bonde et al., 2019 | 18 734 |
|
|
OAC was associated with a 5.3% (95% CI 2.1–8.7%) increased standardized absolute risk of major bleeding among AF patients with moderate/severe anaemia. | HR 1.78 (1.30–2.48) | NR |
| Krittayaphong et al., 2021 | 1562 |
|
Hb <13 g/dL for male and <12 g/dL for female | Anaemia was found to be an independent risk factor for major bleeding. | HR 2.96 (1.81–4.84) | NR |
AF, atrial fibrillation; Hb, haemoglobin; HR, hazard ratio; NR, not reported; OACs, oral anticoagulants; NOAC, non-vitamin K antagonist oral anticoagulant; VKA, vitamin K antagonists.
Table 9.
Summary of ‘malignancy’ as a risk factor for bleeding in AF patients receiving OACs
| Study | Subjects (n) | Type of OACs | Definition | Main findings | HR (95% CI) | P value |
|---|---|---|---|---|---|---|
| Fang et al., 2011 | 9186 | VKA | Any diagnosis of cancer | Prevalence of diagnosed cancer in patients with or without major bleeding: 18.0% vs. 15.1% | HR 1.7 (1.3–2.2) | <0.001 |
| O'Brien et al., 2015 | 7411 | VKA/dabigatran | History of cancer | The rate of major bleeding was 23.3% in patients without cancer vs. 30.8% in those with cancer. | NR | <0.0001 |
| Melloni et al., 2017 | 9749 | VKA/dabigatran | Any diagnosis of cancer | The rate of major bleeding was 3.45 per 100 patient-years in patients without cancer vs. 5.13 per 100 patient-years in those with cancer. | HR 1.21 (1.04–1.40) | 0.02 |
| Vedovati et al., 2018 | 2288 |
|
Patients with active cancer, at time of inclusion in the study, in presence of a diagnosis of cancer or any anti-cancer treatment within 6 months before the study inclusion, or recurrent locally advanced or metastatic cancer; patients with history of cancer | The higher bleeding risk found in cancer compared to non-cancer patients was mainly due to an excess of bleeding at GI and at genitourinary sites. | HR 2.58 (1.08–6.16) | 0.033 |
AF, atrial fibrillation; GI, gastrointestinal; HR, hazard ratio; NR, not reported; OACs, oral anticoagulants; VKA, vitamin K antagonists.
Dynamic and modifiable nature of bleeding risk
Some bleeding risk factors are non-modifiable, such as age, sex, prior bleeding, or stroke, whereas other risks may be correctable, such as uncontrolled blood pressure (BP), transient renal or liver impairment, labile INR, excessive alcohol intake, or concomitant use of aspirin or non-steroidal anti-inflammatory drugs (NSAIDs) in an anticoagulated patient.
It is crucial to recognize that bleeding risk is not a static ‘one-off’ assessment based on baseline factors but dynamic, being influenced by ageing, incident comorbidities, and drug therapies.38–40 Therefore, bleeding risk assessment needs to be performed and repeated frequently over the course of the patient journey, in response to change in clinical characteristics and treatments.
Increasing age is associated with increasing risk of bleeding on OAC (Table 2).41–43 The risk of ICH is higher with VKAs than with NOACs, and the benefit of NOAC over VKA in reducing ICH is consistent irrespective of advanced age.42,44,45
Most studies show systolic hypertension to be a risk factor for bleeding in patients on OAC, especially ICH,46,47 although others did not show a relationship between BP at trial entry and subsequent bleeding.48,49 In the sub-analysis of the ENGAGE-AF trial, patients with a systolic BP above 140 mmHg experienced a higher risk of major bleeding compared to those with a systolic BP between 130 and 140 mmHg.47 Importantly, although the efficacy and safety of edoxaban were consistent across the full range of systolic BPs, the superior safety profile of edoxaban compared to VKA was most pronounced among patients with elevated diastolic BP.47 In a nationwide Korean population registry, the risk of ICH was found to be lowest with BP <130/80 mmHg.50 Based on these associations, it appears prudent to maintain good control of BP in patients on OAC.
In an analysis of 19 566 anticoagulated AF patients, 76.6% of the 3032 patients who experienced major bleeding (ICH or bleeding requiring hospitalization and blood transfusion) had acquired new bleeding risk factors, compared with only 59.0% of those patients without major bleeding (P < 0.001).38 A recent study from Taiwan enrolling 24 990 AF patients with low bleeding, showed that ∼21% of patients acquired at least one new bleeding risk factor at 1 year, including hypertension (5.84%), stroke (5.33%), bleeding (5.06%), concomitant use of antiplatelet agents or NSAIDs (4.34%), abnormal renal function (3.08%), and abnormal liver function (2.22%).40 In the data from ORBIT AF, about a quarter of patients had >20% decline in estimated glomerular filtration rate (eGFR) during 2 years of follow-up, and 3.7% of patients receiving NOACs had eGFR decline sufficient to warrant recommended dose reductions.51 Real-world data from the PREFER in AF registry suggests that each single point decrease on a modifiable bleeding risk scale was associated with a 30% lower risk of major bleeding.43
Laboratory-, biomarker-, and imaging-based risk factors for bleeding in patients with atrial fibrillation or venous thromboembolism
Many blood, urine, and imaging biomarkers have been shown to improve the accuracy of bleeding risk stratification in AF52–54 but their clinical applicability remains limited.
The blood biomarker-based ABC-bleeding risk score [including growth differentiation factor-15 (GDF-15), troponin T, and haemoglobin] has been shown to perform better at bleeding prediction than clinical factor-based bleeding risk scores in patients with AF receiving OAC or both OAC and APT, and in different geographic regions,55–58 but this finding was not confirmed in another study.59 Only marginal enhancement in predictive ability of the HAS-BLED score for major bleeding was observed, after consecutively adding different blood-based biomarkers.60 Blood (e.g. eGFR) and urine (e.g. proteinuria) based biomarkers of renal dysfunction have been used to improve clinical risk stratification for bleeding (as well as stroke) in AF.61,62
In patients with VTE, information on biomarkers and bleeding risk is sparse.63 Bleeding risk scores evaluated in VTE patients receiving OAC treatment, including biomarkers, such as haemoglobin and/or creatinine (or creatinine clearance), generally have modest predictive performance.64,65
There are limitations to using laboratory-based biomarkers at any one time point, to assess bleeding risk, due to the dynamic nature of bleeding risk such that regular re-evaluation of bleeding risk is of utmost importance. Also, some biomarkers are non-specific and predictive of various non-bleeding outcomes.66–69 Furthermore, some biomarkers exhibit diurnal variation and inter-/intra-assay variability and may be expensive.70 Some, such as GDF-15, are not routinely available. Although every effort should be made to improve current risk prediction tools and inclusion of laboratory-based variables is of upmost importance, especially when these are widely available, incorporation of these should not lead to loss of simplicity that ultimately detracts from regular or easy bleeding risk estimation.71
In patients with AF on OAC, small vessel disease on magnetic resonance imaging cerebral imaging is an independent risk factor for ischaemic stroke72 and the presence of cerebral microbleed(s) was independently associated with ICH.73 The addition of cerebral microbleeds to the HAS-BLED score (c-index 0.66, 95% CI 0.53–0.80) significantly improved the prediction of ICH significantly over the HAS-BLED score alone (c-index 0.41, 95% CI 0.29–0.53).73
Current published bleeding risk schema in atrial fibrillation and venous thromboembolism
The purpose of a bleeding risk score is three-fold: (i) to identify risk factors that are modifiable, that can be addressed to reduce bleeding risk; (ii) to identify people who require more regular monitoring and follow-up; and (iii) to estimate an individual’s risk of bleeding on antithrombotic/OAC therapy. Bleeding risk assessment using only modifiable bleeding risk factors alone is an inferior strategy to formal bleeding risk scores.74–76
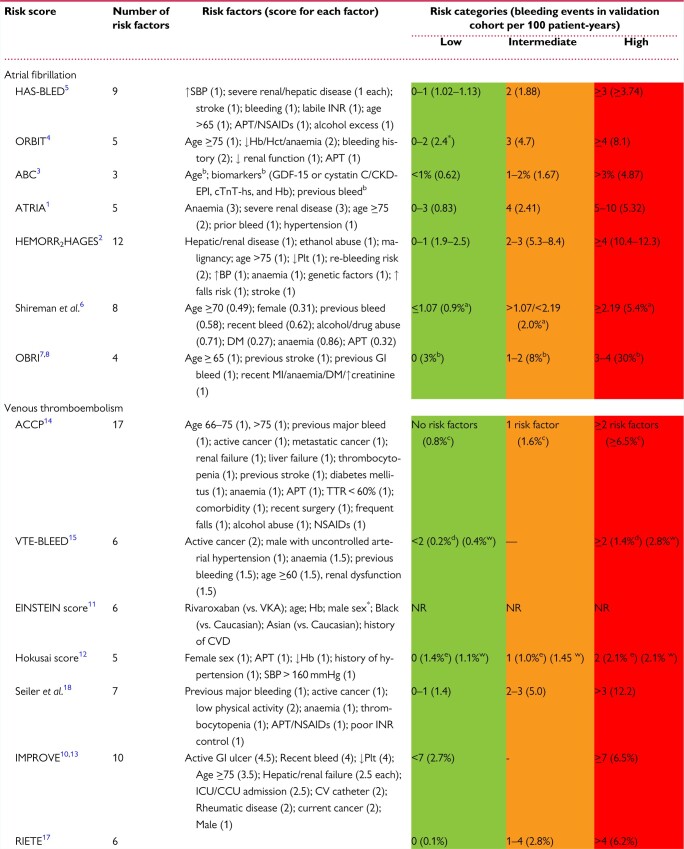
Numerous bleeding risk scores (Table 10) are available for patients with AF55,62,77–83 and VTE.37,84–92 These incorporate numerous risk factors, including demographic and clinical information plus biomarkers, ranging from 355,89 to 1737 factors, with age included in most scores.48,52,60,71–76,78,79,81–84 The scores vary in the definitions of common risk factors and in their complexity and ease of calculation, which can hinder clinical utility. Most scores stratify patients into low, intermediate, and high risk, demonstrating major bleeding rates ranging from <1%55 to 30%80 and 0.1%90 to 12.2 per 100 patient-years91 in the low- and high-risk groups for AF and VTE bleeding risk scores, respectively, in validation cohorts (Table 10).
Table 10.
Bleeding risk scores for atrial fibrillation and venous thromboembolism—risk factors and scoring, risk categories, and bleeding events in the validation cohorts
Among the seven bleeding risk scores for AF,55,62,77–82 the HAS-BLED score79 has been most widely validated across the spectrum of the AF patient pathway, from OAC/antithrombotic-naïve newly-diagnosed patients to those established on OAC93 (both VKA and NOAC),94,95 and is predictive of ICH.96 In a recent contemporary cohort of AF patients from the ESC EHRA EORP-AF registry who were treated with NOACs, the ORBIT score did not provide reclassification improvement, showing even poorer calibration compared to HAS-BLED.97 These findings do not support the preferential use of ORBIT in NOAC-treated AF patients.
The HAS-BLED score has also been validated in non-AF populations, including those with VTE, acute coronary syndrome (ACS), or percutaneous coronary interventions (PCI), or those undergoing bridging therapy.98–101 A Patient Centred Outcomes Research Institute (PCORI) systematic review of 38 studies102 evaluated the prognostic precision of HAS-BLED,79 HEMORR2HAGES,77 ATRIA,62 and ABC-Bleeding,55 concluded that HAS-BLED was the best score for predicting major bleeding but with a modest strength of evidence.102 In a prospective cluster randomized (mAFA-II) trial using App-based mHealth intervention, using the HAS-BLED score, dynamic bleeding risk monitoring and scheduling of high bleeding risk (HBR) patients for review and follow-up reduced major bleeding events (mAFA 2.1% vs. usual care 4.3%, P = 0.004), addressed modifiable bleeding risk and increased OAC uptake, compared to a decrease of 25% amongst those receiving usual care.103
Eight37,84–91 clinical risk scores for predicting major bleeding in patients with VTE (Table 10) have been developed, some focusing on the acute phase,84,87,90 long-term treatment,88,89 specific sub-groups of VTE, for example, cancer-associated thromboembolism,104,105 and the elderly,91 with three85,86,88 derived from cohorts treated with NOACs. A number of prediction rules attempting to quantify the bleeding risk of an individual by adding weighted88–90 or unweighted37,79,81,99 risk factors have been derived from and/or tested in VTE patient cohorts (Table 10).
The bleeding risk scores for VTE have been less extensively validated than those for AF.92 The main weakness of these scores remains the lack of prospective independent validation in large, real-world contemporary populations treated with NOACs. Trials have not prospectively tested the efficacy and safety of coagulation regimens tailored to bleeding risk. De Winter et al.92 critically appraised the prognostic ability of seven of the bleeding risk scores developed for VTE (ACCP,37 EINSTEIN,85 Hokusai,86 Kuijer,89 RIETE,90 Seiler,91 VTE-BLEED88) and seven validated in VTE cohorts but derived in AF or mixed-indication cohorts (ATRIA,62 HAS-BLED,79 HEMORR2HAGES,77 mOBRI,81 OBRI,82 ORBIT,78 Shireman80) The predictive ability, evidenced by the c-statistic, in the derivation and internal validation studies ranged from 0.65 to 0.75 (median 0.68) but was lower in the external validation studies (range 0.52–0.71, median 0.59).92 Bleeding risk scores derived in non-VTE populations have poor discriminative ability (c-statistic 0.52–0.71; median 0.57); the only exception was the recalibrated HAS-BLED score (c-statistic 0.69).99 They concluded that the current evidence does not support the implementation of existing bleeding risk scores to assist in treatment decisions to cease or extend OAC after the initial 3-month period.92 External validation of the VTE-BLEED score,88 derived from a population treated with dabigatran or warfarin, demonstrated predictive ability across patient groups,106–108 and for ICH and/or fatal bleeding.109 External validation of the EINSTEIN or Hokusai scores has not been undertaken.
More recently, the prognostic precision of six bleeding risk scores (HAS-BLED,79 ORBIT,78 ATRIA-Bleeding,62 Kuijer,89 RIETE,90 and VTE-BLEED88) for predicting major bleeding was compared in a prospective multicentre cohort of 1034 people receiving a NOAC for VTE and found to be modest, with c-statistics for VTE-BLEED 0.674 (95% CI 0.593–0.755), ORBIT 0.645 (95% CI 0.523–0.767), and RIETE 0.604 (95% CI 0.510–0.697), with no significant difference between bleeding scores in predicting major bleeding.64 Another study65 compared the predictive ability of 10 clinical bleeding risk scores (VTE-BLEED,88 RIETE,90 ACCP,37 Seiler,91 Kuijer,89 Kearon, OBRI,81,82 ATRIA,62 HAS-BLED,79 and HEMORR2HAGES77) for major and clinically relevant bleeding, in 743 patients ≥65 years receiving extended (≥3 months) VKA therapy following VTE. The c-statistics ranged from 0.47 (OBRI81,82) to 0.70 (Seiler91) for major bleeding and 0.52 (OBRI81,82) to 0.67 (HEMORR2HAGES77) for clinically relevant bleeding. A recent review of bleeding risk assessment in patients with VTE110 concluded that the HAS-BLED or RIETE scores are beneficial in identifying patients at HBR during early phase OAC treatment, with VTE-BLEED advantageous in identifying low-risk patients who could benefit from extended OAC for secondary prophylaxis.
In summary, simple bleeding risk scores based on clinical factors generally have modest predictive value and calibration for bleeding events (c-indexes ∼0.6). More complicated clinical bleeding risk scores modestly improve prediction (perhaps to 0.65) and the addition of biomarkers will always statistically improve on clinical factor-based scores (with c-indexes ∼0.7). All these approaches offer far from perfect prediction (c-indexes <0.9) but ultimately, bleeding risk scores need to balance statistical prediction against simplicity and practicality (incorporating both modifiable and non-modifiable bleeding risks), for use in everyday busy clinical scenarios. In contrast to ischaemic risk prediction tools, a limitation of current bleeding prediction tools is an unclear immediate actionability for treatment decisions, which may explain lower implementation in clinical practice. However, as illustrated in the mAFA-II trial,103 where appropriate use of the HAS-BLED score is associated with lowered major bleeds and increased OAC uptake, the increasing recognition of the importance of bleeding on prognosis should inform decision-making based on bleeding risk assessment in clinical practice.
Patient values and preferences
Clinical guidelines advocate inclusion of patient preferences in treatment decisions, particularly for OAC.11,14,111 A 2017 systematic review of OAC preferences among AF patients found 27 studies conducted across 12 countries.112 Sixteen studies (106–121) examined patients’ general perceptions of OAC, predominantly in those already receiving OAC, utilizing standard trade-off scenarios or conjoint or discrete choice analysis, or preference questionnaires.112 Most patients would accept a higher risk of bleeding for a corresponding reduction in stroke risk, but there was considerable variability in the number of bleeds that would be accepted.113–117 This contrasted with the perception of physicians, who generally worried more about the harm from bleeding.115,118,119 Eleven studies114,120–129 assessed patient preferences towards VKAs vs. NOACs. Where efficacy and safety were similar, patients commonly favoured simpler, more convenient treatment regimens, preferring less frequent dosing, fixed-dose medication, without need for regular monitoring or bridging, or drug–food interactions.112 These results are supported by two previous systematic reviews130,131 and more recent studies,132–134 including an international survey (USA, Canada, France, Germany, and Japan) of 934 AF patients receiving OAC for stroke prevention.132 A reduction in major bleeding was second to stroke prevention as the most valued attribute of OAC; preferences were the same regardless of demographic characteristics, stroke knowledge, stroke concern, perception of AF severity, or medication burden.132,133
A recent systematic review of values and preferences amongst VTE patients evaluating 49 studies (34 quantitative and 15 qualitative)135 concluded that patients valued reduction in VTE risk over the potential risks associated with OAC treatment (i.e. bleeding)135–137 and preferred oral medication.135 Most studies indicated that although VTE patients preferred to avoid adverse events, only one-fifth to one-quarter feared bleeding events135 and among those who had experienced deleterious consequences, most ‘were not afraid’ of adverse outcomes.138–140 Among cancer patients, risk of major bleeding was the third most important consideration related to VTE treatment, after ensuring that VTE prophylaxis did not interfere with cancer treatment and OAC efficacy.141,142 As with AF patients, convenience attributes (e.g. OAC monitoring, dosing frequency, and dietary restrictions) were less important than efficacy121,140,143–145 and safety. Venous thromboembolism patients who were made aware of the need for OAC treatment and understood the risks/benefits were more accepting of OAC.146–150
Shared decision-making151 is important to enable healthcare professionals to inform and educate patients and their family/caregivers about the treatment options, risks, benefits, and length of treatment (which may differ depending on the indication, VTE vs. AF), and to allow open dialogue to discuss patients’ concerns and treatment preferences and goals, barriers/enablers to implementation, and how patients will incorporate OAC into their daily routine, to increase the uptake of OAC and long-term adherence.11,135,152–155
Approach to assessment and bleeding risk mitigation
General atrial fibrillation population
After the evaluation of thromboembolic risk, most guidelines suggest paying attention to the evaluation of bleeding risk. Quality indicators for the care and outcomes of adults with AF published by EHRA include the proportion of patients with bleeding risk assessment using a validated method, such as the HAS-BLED score.156
The important aspect is the appropriate use of a validated score, given the limitations of all bleeding risk scores highlighted above, and the dynamic nature of bleeding risk. All clinical guidelines for the management of AF recommend bleeding risk assessment for people prior to, or on OAC, with the HAS-BLED score recommended by the ESC,11 American College of Chest Physicians,14 and Asia-Pacific Heart Rhythm Society,12 given its simplicity and evidence base, including evaluation in a prospective cluster RCT.103 The ACC/AHA/HRS AF guidelines did not propose any specific bleeding risk scheme.157
The 2021 NICE guideline acknowledged low- to very low-quality evidence for its recommended use of the ORBIT risk score, based on better calibration in NOAC users,158 but also further emphasized attention to modifiable risk factors for bleeding, including uncontrolled hypertension; poor INR control; concurrent medication, including antiplatelets, selective serotonin reuptake inhibitors (SSRIs), and NSAIDs; excessive alcohol consumption; and addressing reversible causes of anaemia. Of note, all these modifiable risk factors listed are already included within the HAS-BLED score.
The 2020 ESC AF guideline emphasizes that, irrespectively of the score used, the main aim is to identify patients with modifiable or potentially modifiable bleeding risk factors.11 This may include controlling BP, cessation of non-essential antiplatelet therapy (APT) or NSAIDs, improving TTR, and reduction/cessation of alcohol (Figure 4). Most of the modifiable bleeding risk factors listed in the ESC AF guideline are components of the HAS-BLED score. Often an individual patient’s bleeding risk is based on the interaction of non-modifiable and modifiable bleeding risks. Simply focusing on modifiable bleeding risk factors alone as a measure of predicting bleeding risk is an inferior strategy to formal assessment with a bleeding risk score.74–76
Figure 4.
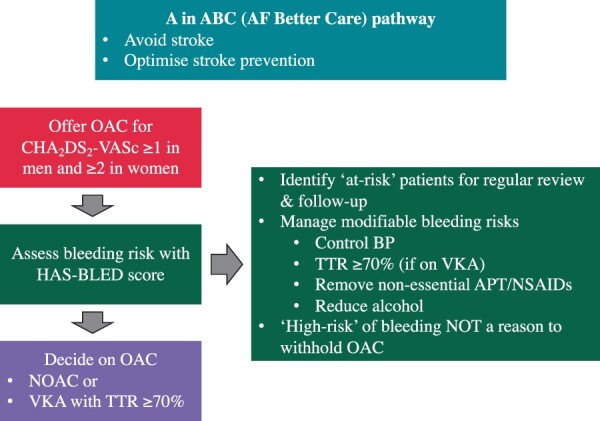
A in the atrial fibrillation better care pathway. ABC, Atrial fibrillation Better Care; APT, antiplatelet therapy; BP, blood pressure; CHA2DS2-VASc, congestive heart failure, hypertension, age 75 years (2 points), diabetes, stroke/TIA/thromboembolism (2 points), vascular disease, age 65–74 years, sex category (female); DM, diabetes mellitus; HAS-BLED, (uncontrolled) hypertension, abnormal renal, or liver function, stroke, bleeding, labile international normalized ratio, elderly, drugs/drink (alcohol); HF, heart failure; NOAC, non-vitamin K antagonist oral anticoagulant; NSAIDs, non-steroidal anti-inflammatory drugs; OAC, oral anticoagulation; OSA, obstructive sleep apnoea; TTR, time in the therapeutic range; VKA, vitamin K antagonist. Adapted from Ref.83
Generally, HBR should not a reason to withhold OAC, except for specific situations in which the risk/benefit ratio excessively favours no antithrombotic treatment.11,157,159–161 Instead, efforts should be made to identify and address all modifiable bleeding risk and provide more regular review, to assess bleeding risk frequently since it is dynamic.11,14,38,162
General venous thromboembolism population
Notwithstanding the limitations of bleeding risk scores for VTE discussed earlier, bleeding risk assessment is recommended both upon initiation of anticoagulation for VTE and at follow-up visits, the frequency of which should increase if the bleeding risk is high.21 Of note, the aim is not to withhold OAC if one or more bleeding risk factors are found, but (like in AF) to identify and address potentially modifiable factors.
Consequently, current VTE guidelines leave the choice of the tool for assessing bleeding risk to the discretion of the clinician, with many guidelines avoiding endorsement of a particular score.21,22 However, the 2020 NICE VTE guideline163 recommends using the HAS-BLED score and advises stopping anticoagulation if the HAS-BLED score is 4 or more and cannot be modified. In case of persistent HBR, the patient’s personalized risk: benefit ratio of anticoagulant treatment should be assessed and if judged to favour extended anticoagulation, a reduced dose of the NOACs apixaban (2.5 mg twice daily) or rivaroxaban (10 mg once daily) should be considered after 6 months of therapeutic anticoagulation. Aspirin is not an alternative to anticoagulation for extended secondary VTE prevention and may be considered only in patients who refuse to take or are unable to tolerate OAC.21
Surgery and endoscopic and endovascular procedures
Peri-ablation of atrial arrhythmias
Catheter ablation, especially left-sided ablation, is associated with a small but relevant ∼0.5% risk of severe bleeding164 related to vascular access and peri-interventional anticoagulation.165 It also carries a risk of thrombotic events, with left-sided procedures carrying a higher risk of thrombosis and stroke.
The incidence of vascular complications depends on type of vascular access (arterial, venous, or both), site and size of vascular access (i.e. femoral vs. subclavian or jugular), number of introduced catheters, length of the procedure, patient profile (i.e. obesity and baseline coagulation parameters), type of anticoagulation used, management of catheterization site during and after the procedure, and operator experience. The stroke and transient ischaemic attack rate are ∼1% in large studies, with reported bleeding rates of 1% for cardiac tamponade and 1–2% for access site bleeds.165 The risk of perforation even with AF ablation is reported to occur in <1% of cases in contemporary series, with use of intracardiac echo shown to reduce the risk.165,166
Continuation of OAC for AF ablation is safe with a trend towards fewer bleeding events and may also help to prevent peri-procedural stroke (Table 11).167 Most guidelines agree on three main points11,14,160,161,168: (i) uninterrupted OAC is recommended for patients undergoing ablation; (ii) after the procedure, OAC is essential for at least 8 weeks in all patients; and (iii) long-term OAC beyond the first 8 weeks, should be considered on the basis of risk profile (CHA2DS2-VASc). Regarding the type of OAC, NOACs, and VKAs are both options, although meta-analyses report a trend favouring NOACs with respect to major bleeding.169
Table 11.
Randomized controlled trial of uninterrupted oral anticoagulation in atrial fibrillation catheter ablation
| COMPARE4 | VENTURE-AF10 | RE-CIRCUIT-AF11 | AXAFA-AFNET 512 | ELIMINATE-AF13 | |
|---|---|---|---|---|---|
| OAC treatment | Heparin bridging vs. warfarin (1:1) | Rivaroxaban vs. warfarin (1:1) | Dabigatran vs. warfarin (1:1) | Apixaban vs. warfarin (1:1) | Edoxaban vs. warfarin (2:1) |
| Number of patient (n) | 790/793 | 124/124 | 317/318 | 318/315 | 411/203 |
| Age (years), mean or median | 61/24 | 58.6/60.5 | 59.1/59.3 | 64.0/64.0 | 60.0/61.0 |
| Male gender (%) | 76/74 | 68.4/72.6 | 72.6/77 | 69/65 | 70.6/73.4 |
| BMI, kg/m2, mean or median | NA | 29.8/28.9 | 28.5/28.8 | 28.4/28.2 | 28.1/27.8 |
| CHA2DS2-VASc score | 1: 29/26 | 1.5/1.7 | 2/2.2 | 2.4/2.2 | 0: 23.4/21.7 |
| 2: 34/36 | 1: 26.5/28.1 | ||||
| ≥3: 37/38 | ≥2: 50.1/50.2 | ||||
| Prior stroke or TIA (%) | 7/8 | 0/2.4 | 3.2/2.8 | 7.5/7.3 | 5.4/3.9 |
| Congestive heart failure (%) | 15/17 | 9.7/7.3 | 9.8/10.7 | 24.5/22.9 | 17.3/19.2 |
| Hypertension (%) | 81/83 | 47.6/46 | 52.4/55.7 | 89/91.4 | 60.8/59.6 |
| Diabetes (%) | 38/40 | 6.5/11.3 | 9.5/10.7 | 12.9/11.1 | 13.4/15.8 |
| Types of AF (%) | |||||
| Paroxysmal AF | 29/25 | 76.6/70.2 | 67.2/68.9 | 59.4/56.5 | 69.1/64.5 |
| Persistent AF | 71/75 | 23.4/29.8 | 32.8/31.2 | 40.6/43.6 | 25.5/30 |
| TEE prior to ablation (%) | NA | NA | 100 | 84.6 | 74.6 |
| Duration of OAC before ablation | 3–4 weeks | 3 weeks | 4–8 weeks | 30 days | 21–28 days |
| Estimated NOAC compliance (%) | NA | 99.9 | 97.6 | 97 | 97 |
| INR, time in therapeutic range (%) | NA | 79.8 | 85.7 | 84 | 84 |
| ACT (s), mean or median | NA | 302/332 | 330/340 | 310/348 | 3014/322.6 |
| Primary outcome | Thromboembolic events (stroke/TIA/systemic thromboembolism) | Major bleeding events (ISTH) | Major bleeding events (ISTH) | All-cause mortality, stroke or major bleeding (BARC ≥ 2) | All-cause mortality, stroke or major bleeding event (ISTH) |
| Follow-up | 48 h | 30 days | 8 weeks | 3 months | 90 days |
| Primary outcome event (%) | 4.9/0.25* | 0/0.8 | 1.6/6.9* | 6.9/7.3 | 2.7/1.7 |
| Death (%) | 0/0 | 0/0.8 | 0/0 | 0.3/0.3 | 0/0 |
| Ischaemic stroke (%) | 3.7/0.25 | 0/0.8 | 0/0.3 | 0.6/0 | 0.3/0 |
| Major bleeding (%) | 0.76/0.38 | 0/0.8 | 1.6/6.9 | 3.1/4.4 | 2.4/1.7 |
| Death/ischaemic stroke/major bleeding (%) | 5.7/0.63 | 0/2.4 | 1.6/7.2 | 4.0/4.7 | 2.7/1.7 |
AF, atrial fibrillation; ACT, activated clotted time; BARC, Bleeding Academic Research Consortium; BMI, body mass index; INR, international normalized ratio; ISTH, International Society on Thrombosis and Haemostasis; NOAC, non-vitamin K antagonist oral anticoagulant; NA, not available; OAC, oral anticoagulant; TEE, transoesophageal echocardiogram; TIA, transient ischaemic attack.
P < 0.01.
Cardiovascular implantable electronic device
In patients without mechanical valves, anticoagulation may be briefly interrupted for cardiovascular implantable electronic device (CIED) implantation, without bridging. In patients with mechanical valves, uninterrupted VKA is preferable to interruption of VKA with heparin bridging (see section on bridging).
In patients on NOACs, the BRUISE-CONTROL 2 trial compared patients with a last intake 2 days before the implantation for rivaroxaban, apixaban, and (based on glomerular filtration rate) dabigatran vs. continued NOAC until the morning of the procedure. The study was prematurely stopped due to futility because of the far lower rate of events than anticipated and similar rates of bleeding and embolic events.170 Therefore, both stopping or continuing NOAC are possible options and supported by subgroup analyses from the pivotal Phase III trials and large observational analyses (Table 12).171–175 For patients on a NOAC, a strategy as for low bleeding risk interventions (i.e. infrequent bleeding or with non-severe clinical impact) with intake of the last dose the day before the procedure is appropriate in most cases,161 with resumption of NOAC intake on the first post-operative day. Procedures with uninterrupted OAC should be carried out by an experienced operator, with close attention paid to achieving good haemostasis.
Table 12.
Prospective and retrospective cohort studies (sample size ≥100) of peri-procedural oral anticoagulation in atrial fibrillation patients undergoing cardiac rhythm device procedures
| Study | Design | Subjects (n) | Age (years), mean | Continued OAC (%) | Interrupted OAC (%) | Timing of OAC Interruption (h), mean or median | Timing of OAC resumption | Antiplatelet therapy (%) | Clinically significant haematoma (%) | Other device-related bleeding (%) | Thromboembolic and other complications (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Birnie et al.16 | BRUISE CONTROL 1 prospective randomized control trial | Warfarin 681 | 72 years | 50.3% |
|
NA | NA |
|
|
|
|
| Black-Meier et al.7,23 |
|
|
|
|
|
NA | NA |
|
|
|
|
| Essebag et al.19 | Post-hoc analysis of RE-LY trial |
|
73 years | 0% | 100% |
|
NOAC: 34 h |
|
|
|
|
| Leef et al.20 | Post-hoc analysis of ROCKET-AF trial | VKA 211 Rivaroxaban 242 | 75 years | 25% | 75% |
|
|
— |
|
|
|
| Ricciardi et al.24 | Prospective randomized pilot trial |
|
76 years | 49.5% | 50.5% |
|
≥24 h |
|
|
|
|
| Birnie et al.18 | BRUISE CONTROL 2 prospective randomized control trial |
|
74 | 49.3% | 50.5% |
|
≥24 h |
|
|
|
|
| Tsai et al.22 | Retrospective analysis |
|
78 years | 100% | 0% | NA | NA |
|
+ve NOAC: 1% |
|
0% |
| Steffel et al.21 | Post-hoc analysis of ENGAGE AF trial |
|
74 years | 26% | 74% | median 7 days (pre + post) | NA |
|
NA |
|
|
−ve , interrupted; +ve, continued; MI, myocardial infarction; NA, not available; NOAC, non-vitamin K antagonist oral anticoagulant; OAC, oral anticoagulant; SE , systemic embolism; TIA, transient ischaemic attack; VKA , vitamin K antagonist.
Surgical procedures
The periprocedural management of patients receiving OAC represents a frequent clinical challenge for physicians. Given the relatively scarce evidence-base on this subject, most available recommendations are based on expert consensus.16,176–179 This section focuses on recommendations regarding patients with AF or VTE with a clinical indication for OAC who require elective surgery or an endoscopic or endovascular procedure. Briefly, the periprocedural strategy to reduce the risk of adverse outcomes is based on careful assessment of two risks: (i) the bleeding risk associated with the procedure, and (ii) the thromboembolic risk associated with the condition that underlies the indication for OAC.
The risk of bleeding with a given procedure must consider both the prevalence of haemorrhagic complications and its consequences. Thus, procedures with low rates of bleeding but relevant associated sequelae (e.g. intracranial or spinal surgery) should be classified as high risk. In addition, it is also pertinent to contemplate comorbid conditions (e.g. older age, kidney or liver dysfunction) that can increase the risk of peri-procedural bleeding. Different professional societies have made several attempts to categorize the risk of bleeding related to different interventional procedures.177–179
The thromboembolic risk associated with the indication for OAC is classified according to the annual risk of arterial or venous thromboembolism: high if the risk is >10%, moderate between 5% and 10%, and low when <5% (Table 13).176,177,179
Table 13.
Stratification of thromboembolic risk according to clinical indication for oral anticoagulation
| Risk | Indication for OAC | |
|---|---|---|
| AF | VTE | |
| High | • CHA2DS2-VASc ≥7 • Recent (within 3 months) stroke/TIA • Rheumatic mitral valve disease | • Recent (within 3 months) VTE • Severe thrombophilia (e.g. homozygous factor V Leiden or prothrombin 20 210 mutation, protein C, protein S, or antithrombin deficiency, antiphospholipid syndrome, multiple defects) |
| Moderate |
|
• VTE within the past 3–12 months • Non-severe thrombophilia (e.g. heterozygous factor • V Leiden or prothrombin gene mutation) • Recurrent VTE • Active cancer + VTE |
| Low |
|
|
Modified from Ref.179
AF, atrial fibrillation; OAC, oral anticoagulation; TIA, transient ischaemic attack; VTE, venous thromboembolism.
Despite general recommendations, an individualized approach by the local physicians (surgeons, anaesthesiologists, etc.) involved in the procedure is mandatory. For some procedures with low haemorrhagic risk (e.g. diagnostic endoscopy without biopsy), uninterrupted OAC is a safe strategy both in patients on VKA (INR ≤3 on the day of the procedure) or NOACs.170,180 The general recommendation is to consider peri-procedural temporary interruption without bridging for patients with low or moderate thromboembolic risk and reserve bridging only for patients at high risk. Bridging is rarely needed in patients on NOACs, given the short half-life of these agents. When a temporary interruption is required, the recommended duration for withholding OAC before the procedure is mostly based on the procedural bleeding risk and the INR values 5–7 days before the procedure in case of VKAs or the renal function in case of NOACs (Table 14).
Table 14.
Recommended duration for withholding OAC prior to a procedure when temporary interruption is needed
| NOAC | ||||||
|---|---|---|---|---|---|---|
| Procedural bleed risk | ||||||
| CrCl (mL/min) | <15 | 15–29 | 30–49 | 50–79 | ≥80 | |
| Dabigatran | Low | ≥96 ha | ≥72 h | ≥48 h | ≥36 h | ≥24 h |
| Intermediate, high or uncertain | No dataa | ≥120 h | ≥96 h | ≥72 h | ≥48 h | |
| CrCl (mL/min) | <15 | 15–29 | ≥30 | |||
| Apixaban, rivaroxaban, or edoxaban | Low | ≥48 h | ≥36 h | ≥24 h | ||
| Intermediate, high, or uncertain | ≥72 hb | ≥72 hb | ≥48 h | |||
| VKA | ||||||
| INR 5–7 days prior to the procedure c | <2 | 2–3 | >3 | |||
| Warfarind | 3–4 days | 5 days | >5 days | |||
CrCl, creatinine clearance; DOAC, direct acting oral anticoagulant; dTT, dilute thrombin time; INR, international normalized ratio; VKA, vitamin K antagonist.
Consider measuring dTT.
Consider measuring agent-specific antiXa level.
INR must be measured again 24 h before the procedure.
If other VKA than warfarin is used, the durations may be adjusted according to the drug half-life.
When treatment on uninterrupted OAC is not feasible, the peri-procedural strategy will depend on the assessment of the patient’s risk of thromboembolism (Figure 5) and is discussed in more detail in the section on ‘Bridging’ later.
Figure 5.
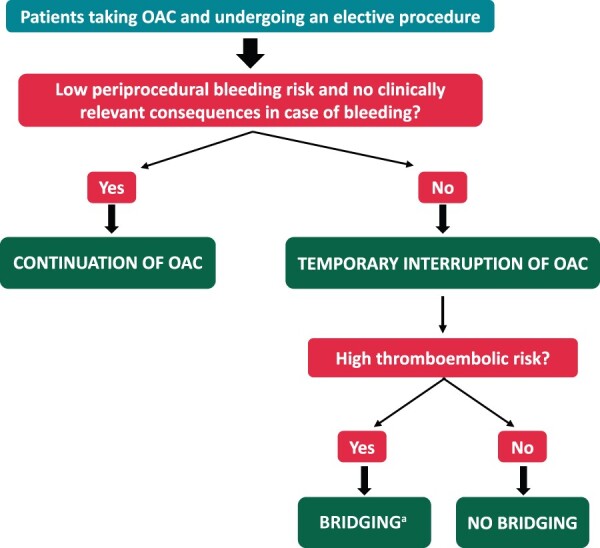
Simplified algorithm for selecting the periprocedural management strategy of OAC in patients undergoing an elective surgery or invasive procedure. aBridging with parenteral heparin is generally not necessary with DOACs. DOAC, direct oral anticoagulant; OAC, oral anticoagulation.
Post-procedure, OAC may be re-initiated once haemostasis is achieved and in the absence of a bleeding complication. In most situations with low post-procedural bleeding risk, OAC can be resumed within 24 h (generally on the day following the procedure), whereas it is reasonable to wait for 48–72 h if the risk of post-procedural bleeding is high.177,179,181
A detailed explanation regarding measures to mitigate bleeding in patients on OAC requiring emergency surgery or invasive procedure is beyond the scope of this manuscript and can be found elsewhere.161,179,182 Notably, depending on the type of procedure and its associated bleeding risk, such patients may require a reversal agent, such as intravenous vitamin K for VKAs (INR reduction in 4–6 h), idarucizumab for dabigatran or andexanet alfa for factor Xa inhibitors,183,184 although it should be noted that idarucizumab was evaluated in patients requiring urgent surgery in only one small study185 and andexanet has not been studied in this setting. If antidotes are not available for an emergency procedure or the patient has active major or life-threatening bleeding, administration of haemostatic agents should be considered, with four-factor prothrombin complex concentrate (PCC) and PCC as first options for VKAs and NOACs, respectively.182,186
Presentation with acute coronary syndrome and/or requiring percutaneous coronary intervention
In patients requiring combined OAC and APT, such as those with AF or VTE presenting with ACS and/or undergoing PCI, the risk of bleeding is increased.187 In this setting, the predictive value of scores is generally poor, with the HAS-BLED score performing best188,189 and shown to predict significant bleeding in AF patients undergoing PCI.190 The Academic Research Consortium (ARC) has defined HBR (BARC 3 or 5 bleeding) for patients undergoing PCI as the presence of one major or two minor characteristics191 (Table 15), which can be found in up to 40% of patients.
Table 15.
ARC major and minor criteria for HBR at time of PCI High bleeding risk defined as at least one major or two minor criteria
| Major | Minor |
|---|---|
| Age ≥75 years | |
| Anticipated use of long-term oral anticoagulationa | |
| Severe or end-stage CKD (eGFR <30 mL/min) | Moderate CKD (eGFR 30–59 mL/min) |
| Haemoglobin <11 g/dL | Haemoglobin 11–12.9 g/dL for men and 11–11.9 g/dL for women |
| Spontaneous bleeding requiring hospitalization and/or transfusion in the past 6 months or at any time, if recurrent | Spontaneous bleeding requiring hospitalization and/or transfusion within the past 12 months not meeting the major criterion |
| Moderate or severe baseline thrombocytopeniab (platelet count <100 × 109 per litre) | |
| Chronic bleeding diathesis | |
| Liver cirrhosis with portal hypertension | |
| Chronic use of oral NSAIDs or steroids | |
| Active malignancyc (excluding non-melanoma skin cancer) within the past 12 months | |
|
Any ischaemic stroke at any time not meeting the major criterion |
| Non-deferrable major surgery on DAPT | |
| Recent major surgery or major trauma within 30 days prior to PCI |
bAVM, brain arterio-venous malformation; CKD, chronic kidney disease; DAPT, dual-antiplatelet therapy; eGFR, estimated glomerular filtration rate; HBR, high bleeding risk; ICH, intracranial haemorrhage; NSAID, non-steroidal anti-inflammatory drug; PCI, percutaneous coronary intervention.
This excludes dual pathway inhibition doses.
Baseline thrombocytopenia defined as thrombocytopenia prior to PCI.
Active malignancy is defined as diagnosis within 12 months and/or ongoing requirement for treatment (including surgery, chemotherapy, or radiotherapy).
National Institutes of Health Stroke Scale (NIHSS) score ≥5.
An increased risk of bleeding is apparent in both the peri-PCI and post-discharge periods and strategies to minimize such risk should therefore be applied before, during, and after PCI.192 Pre-PCI approaches include avoidance of routine pre-treatment with APT, with P2Y12-inhibitor generally given only after coronary angiography has confirmed the decision to proceed to PCI.192,193
Peri-PCI strategies include the preferential use of the radial approach and avoidance of glycoprotein IIb/IIIa inhibitors.
For elective procedures, European guidelines recommend uninterrupted VKA if the INR < 2.5,193 whereas North American guidelines recommend uninterrupted VKA if INR < 2,194 with interruption of VKA considered when INR is above these thresholds. Intra-PCI administration of reduced-dose UFH is recommended.193,194
In patients on NOAC, timely interruption in elective patients may be considered, as indicated in the European guidelines193 and is clearly recommended by North American guidelines194 with both guidelines recommending administration of weight-adjusted dose UFH, owing to the uncertain protection of NOAC against PCI-related ischaemic events.195,196 Because of that, UFH should be also administered to patients on NOAC undergoing PCI in the emergency setting.193
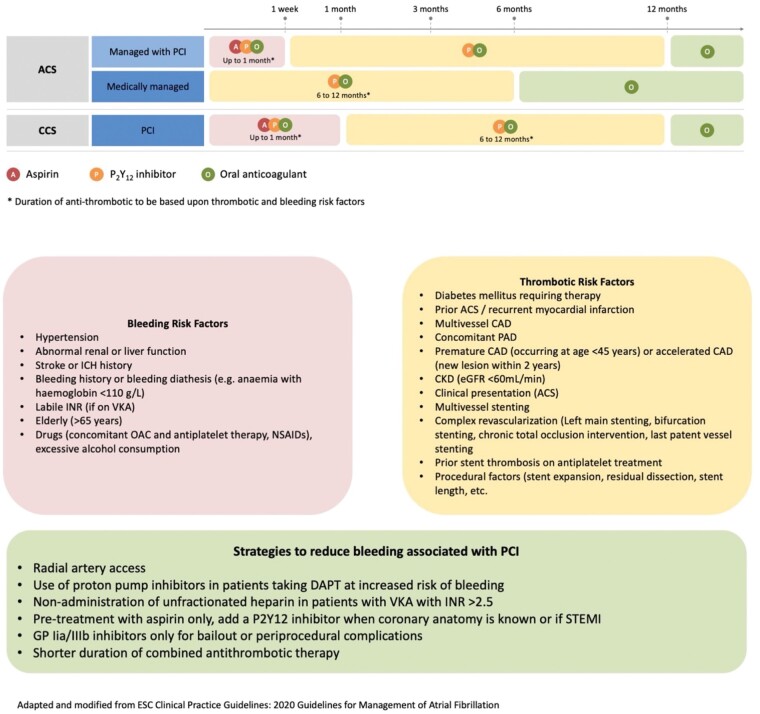
Following PCI, the type and duration of APT should be carefully considered to minimize bleeding.192 An initial short course of triple antithrombotic therapy (TAT) with OAC and dual APT (DAPT) of aspirin and clopidogrel is warranted to limit the early hazard of ischaemic events (Figure 6).11 To mitigate the increased risk of bleeding associated with TAT, the more potent P2Y12-inhibitors prasugrel and ticagrelor should be avoided, with European guidelines indicating that ticagrelor or prasugrel be used as part of TAT only in exceptional circumstances such as stent thrombosis whilst on TAT with clopidogrel, aspirin and OAC,193 and North American guidelines suggesting that ticagrelor can be considered in patients at particularly high stent thrombosis risk although prasugrel should be avoided.194
Figure 6.
Management of antithrombotics in patients presenting with ACS and/or requiring PCI or stents. ACS, acute coronary syndrome; PCS, percutaneous coronary interventions.
The duration of TAT should be minimized, generally ranging from 1 to 4 weeks (Figure 6). Subsequent antithrombotic management is determined by whether long-term OAC is indicated. In most AF and VTE patients for whom indefinite OAC is warranted, double antithrombotic therapy (DAT) with OAC and single APT (SAPT), preferably clopidogrel, should follow initial TAT and be maintained up to 6–12 months, based on the patient’s bleeding and ischaemic risks193,194 (Figure 6), followed by OAC alone indefinitely.193,194,197,198 Prolongation of DAT beyond 1 year may be considered in selected patients with both clinical and/or anatomical features for increased ischaemic cardiac events, including diabetes, multi-vessel disease, incomplete revascularization, and left main or last remaining vessel stenting, and, importantly, low risk of bleeding193,194 (Figure 6). In contrast, in patients with a first episode of VTE, in whom OAC is discontinued after 3 months, DAPT comprising of aspirin and clopidogrel should be resumed upon OAC cessation with duration tailored to type of event and procedural characteristics.194
In addition to limiting the duration of TAT, as well as of DAT, strategies to minimize the risk of bleeding should also aim to reduce the intensity of OAC. A target INR at the lower end of the therapeutic range (2.0–2.5) is recommended with VKA,193 aiming for TTR >65–70%.199 NOACs are preferable to VKA as part of combination therapy and switching from warfarin should be routinely considered.193 To date, no specific NOAC appears preferable since no head-to-head comparisons have been performed and all of them given as part of DAT have shown a favourable safety and efficacy profile compared to TAT including warfarin.200–203 In the AUGUSTUS trial, amongst patients with AF and either ACS or PCI treated with a P2Y12 inhibitor, treatment with apixaban, without aspirin, resulted in less bleeding and fewer hospitalizations than regimens that included a VKA, aspirin, or both.202 Sub-analysis of data from the RE-DUAL PCI trial, which compared DAT (dabigatran 110 or 150 mg bid, clopidogrel or ticagrelor) with TAT (warfarin, clopidogrel or ticagrelor, and aspirin), showed that DAT with dabigatran reduced bleeding both in non-HBR and HBR patients, with a greater magnitude of benefit among non-HBR patients.204 NOACs should be given at the recommended doses, with the possible exceptions of dabigatran and rivaroxaban for which the lower doses of 110 mg twice daily and 15 mg once daily respectively, are preferable when used as part of TAT.193
In patients at HBR not on OAC when presenting for PCI, but developing an indication for OAC later, several bleeding-avoidance strategies should be considered: (i) while in patients with ST-elevation myocardial infarction (STEMI) DAPT (aspirin plus ticagrelor or prasugrel, clopidogrel only if the stronger P2Y12-inhibtors are contraindicated, not available, or in patents at HBR) should be started when the diagnosis is confirmed at first medical contact, ‘pre-treatment’ is not routine strategy in patients with non-ST-elevation MI (NSTEMI) and a planned early invasive strategy. Therefore, in the setting of NSTEMI, avoidance of DAPT pre-treatment in patients at HBR reduce bleeding risk205,206; (ii) radial is preferred over femoral access and is associated with significantly reduced bleeding complications206,207; (iii) in patients not pre-treated with oral APT, during urgent/emergency PCI, intravenous antiplatelet agents may be used, and due to better safety profile, the intravenous P2Y12-inhibitor cangrelor may be preferred over glycoprotein IIb/IIIa inhibitors208; (iv) newer generation drug eluting stents have displaced bare metal stents also in HBR patients as their quick re-endothelialization allows a shorter duration of DAPT after PCI209; and finally, (v) administration of proton-pump inhibitors and avoidance of NSAIDs is recommended to minimize bleeding risk.210
Patients with cancer
Patients with cancer, particularly gastric or urothelial tumours, have an increased risk of bleeding on OAC compared to patients without cancer,211–213 and proton-pump inhibitors should be routinely considered to mitigate this risk.
In patients with AF, registry data214 and Subgroup analyses of pivotal phase 3 trials213,215,216 indicate similar or lower bleeding with NOAC compared to VKA in patients with cancer, with the exception of patients with gastrointestinal cancers or active gastrointestinal mucosal abnormalities.217
In cancer patients in whom OAC is indicated for the treatment or prevention of VTE, NOACs have been shown to significantly reduce bleeding compared with VKA.218 In comparison to LMWH, apixaban and edoxaban appear to have similar safety profile to LMWH,27,219 with excess bleeding mainly observed in patients with gastrointestinal cancer.219,220 A meta-analysis of 23 RCTs including 6980 patients, showed no difference in major bleeding between LMWH and VKA treatment (4.7% vs. 4.8%, RR 0.99, 95% CI 0.67–1.45), whereas NOACs significantly lowered bleeding risk compared to VKA (2.5% vs. 4.2%, RR 0.58, 95% CI 0.35–0.99). Pooled data from the only two RCTs comparing NOACs against LMWH showed significantly higher incidence of major bleeding with NOACs (6.5% vs. 3.7%, RR 1.75, 95% CI 1.10–2.77).221
Bridging therapy
Patients treated with oral anticoagulant undergoing interventional or surgical procedures
There may be specific clinical scenarios, when temporary interruption of OAC may be necessary, such as when an interventional procedure or surgery is planned.
While bridging with either UFH or LMWH, may theoretically reduce the peri-procedural thrombotic risk, this substantially increases peri-procedural bleeding.181
In patients undergoing CIED implantation, randomized data in VKA-treated patients indicate lower thromboembolic and bleeding rates180 and reduced length of stay180,222 if the VKA is uninterrupted, without bridging. Heparin-bridging results in a 4.5-fold increase in postoperative haematoma compared to a continued warfarin strategy.180 A clinically meaningful pocket haematoma after the implantation of a CIED is an independent risk factor (7- to 8-fold risk) for subsequent device infection.223,224 Irrespective of the perioperative anticoagulation strategy used, the incidence of thromboembolic events is 0–1% (Table 12).
In AF patients, the randomized, double-blind, placebo-controlled BRIDGE trial demonstrated no ischaemic benefit but significantly increased bleeding in patients randomized to bridging.181 A meta-analysis of 18 studies (6 randomized and 12 observational studies) including 23 364 patients,225 bridging significantly increased overall bleeding events (RR: 2.83, 95% CI: 2.00–4.01) including major bleeding (RR: 3.00, 95% CI: 1.78–5.06), without significant reduction in ischaemic risk (RR: 1.26, 95% CI: 0.61–2.58).
Post-operatively, bridging with parenteral agents is not required with NOACs, but could be considered in selected high thromboembolic risk patients when resuming VKA. Thus, a routine bridging strategy is not recommended in the current 2020 ESC AF Guideline11 and a recent ESC/EHRA document on the use of NOACs226 which emphasize that this approach should be avoided.
Patients treated with oral anticoagulant with prior stent requiring surgery
In patients with prior coronary stenting, antithrombotic therapy is required to reduce the risk of stent thrombosis. The thrombotic risk falls with time from PCI, being relatively high in the first 3–6 months, intermediate at 6–12 months, and low beyond 12 months.227 Whilst OAC may be discontinued for elective or urgent surgery, there is concern that patients with prior stenting on single or no APT, may be left with insufficient antithrombotic protection to prevent stent thrombosis. In such patients, a bridging APT strategy may be required for those at high ischaemic risk although there are no large clinical trial data in AF patients per se.
The decision on APT bridging requires a careful evaluation of bleeding risk and perioperative ischaemic (stent thrombosis) risk. The risk of perioperative haemorrhage should also be considered, being very high with hepatic resection, and high with many other surgical procedures including splenectomy, gastrectomy, thyroid surgery, nephrectomy and prostatectomy, and among cardiac surgical procedures, relatively high when re-intervention and aortic surgery is performed.227 Additionally, the site of potential bleeding is critical, for example even relatively minor bleeding in patients undergoing neurosurgery or ophthalmic surgery can be catastrophic. Bridging of APT indicates a strategy of usually starting (or continuing with) aspirin, and consideration given to temporary transition with an intravenous antiplatelet agent in patients who would otherwise require DAPT (if they were not on OAC).
There are specific clinical (including ACS as indication for PCI, prior stent thrombosis, diabetes, and CKD) and angiographic (including long stented segment length, bifurcation stenting, small stent diameter, last remaining conduit) risk factors which increase ischaemic risk.227,228
For patients with high ischaemic and HBR, consideration should be given to postponing elective surgery beyond 6 months post-PCI, when SAPT with aspirin may be considered or if this is not possible, every effort should be made to employ bridging strategies that mitigate risk, with use DAPT with clopidogrel rather than more potent P2Y12 inhibitors, or preferably using intravenous cangrelor, which has a short half-life in case of major bleeding.179,227
Consensus statements
Bleeding risk reflects the interaction of non-modifiable and modifiable bleeding risks. Simply focusing on modifiable bleeding risk factors alone as a measure of predicting bleeding risk is an inferior strategy to the use of formal bleeding risk scores.
Bleeding risk is not a static ‘one off’ assessment based on baseline factors but is dynamic, being influenced by ageing, incident comorbidities, surgical/interventional procedures, and use of modifiers (such as proton pump inhibitors) or drug therapies.
Simple bleeding risk scores based on clinical factors generally have modest predictive value and calibration for bleeding events. More complex clinical bleeding risk scores can improve prediction, at least statistically, and the addition of biomarkers improves the performance of clinical factor-based bleeding risk scores. Ultimately, the use of bleeding risk scores needs to balance statistical prediction against simplicity and practicality (incorporating both modifiable and non-modifiable bleeding risks), for use in everyday busy clinical scenarios.
In patients with AF, a formal structured risk-score-based bleeding risk assessment is recommended to help identify non-modifiable and address modifiable bleeding risk factors, and to identify patients potentially at high risk of bleeding who should be scheduled for more frequent clinical review. For a formal risk-score-based assessment of bleeding risk, the HAS-BLED score should be used.
Treatment of patients with AF according to an integrated care or holistic approach, based on the ABC (Atrial fibrillation Better Care) pathway, is associated with a lower risk of major bleeding and this should be applied. Appropriate use of the HAS-BLED score as part of the ABC pathway is associated with less major bleeding and an increase in OAC uptake.
In VTE patients, the choice of the bleeding risk score for assessing the individual’s bleeding risk is at the discretion of the clinician. The 2020 NICE VTE guideline recommends use of the HAS-BLED score.
Contributor Information
Diana A Gorog, School of Life and Medical Sciences, Postgraduate Medical School, University of Hertfordshire, College Lane, Hatfield, UK; Faculty of Medicine, National Heart & Lung Institute, Imperial College, London, UK.
Ying X Gue, Liverpool Centre for Cardiovascular Science, University of Liverpool, Liverpool Heart & Chest Hospital, Liverpool, UK.
Tze-Fan Chao, Division of Cardiology, Department of Medicine, Taipei Veterans General Hospital, Taipei, Taiwan; Institute of Clinical Medicine, Cardiovascular Research Center, National Yang Ming Chiao Tung University, Taipei, Taiwan.
Laurent Fauchier, Faculty of Medicine, University of Tours, Tours, France.
Jose Luis Ferreiro, Department of Cardiology, Hospital Universitario de Bellvitge, Ciber Cardiovascular (CIBERCV), L’Hospitalet de Llobregat, Barcelona, Spain; BIOHEART-Cardiovascular Diseases Group, Cardiovascular, Respiratory and Systemic Diseases and Cellular Aging Program, Institut d’Investigació Biomèdica de Bellvitge—IDIBELL, L’Hospitalet de Llobregat, Barcelona, Spain.
Kurt Huber, 3rd Department of Medicine, Cardiology and Intensive Care Medicine, Wilhelminenhospital and Sigmund Freud University, Medical Faculty, Vienna, Austria.
Stavros V Konstantinidis, Center for Thrombosis and Hemostasis, University Medical Center of the Johannes Gutenberg University, Mainz, Germany.
Deirdre A Lane, Liverpool Centre for Cardiovascular Science, University of Liverpool, Liverpool Heart & Chest Hospital, Liverpool, UK; Aalborg Thrombosis Research Unit, Department of Clinical Medicine, Aalborg University, Aalborg, Denmark.
Francisco Marin, Department of Cardiology, Hospital Clínico Universitario Virgen de la Arrixaca (IMIB-Arrixaca), CIBERCV, Universidad de Murcia, Murcia, Spain.
Jonas Oldgren, Department of Medical Sciences, Uppsala Clinical Research Center, Uppsala University, Uppsala, Sweden.
Tatjana Potpara, School of Medicine, Belgrade University, Belgrade, Serbia.
Vanessa Roldan, Servicio de Hematología, Hospital Universitario Morales Meseguer, Universidad de Murcia, IMIB-Arrixaca, Murcia, España.
Andrea Rubboli, Division of Cardiology, Department of Cardiovascular Diseases—AUSL Romagna, SMaria delle Croci Hospital, Ravenna, Italy.
Dirk Sibbing, Department of Cardiology, Ludwig-Maximilians-Universität München, Munich, Germany; DZHK (German Center for Cardiovascular Research), Partner Site Munich Heart Alliance, Munich, Germany.
Hung-Fat Tse, Division of Cardiology, Department of Medicine, University of Hong Kong, Hong Kong, Hong Kong.
Gemma Vilahur, Research Institute Hospital de la Santa Creu i Sant Pau, IIB-Sant Pau, Barcelona, Spain; CIBERCV Instituto de Salud Carlos III, Barcelona, Spain.
Gregory Y H Lip, Liverpool Centre for Cardiovascular Science, University of Liverpool, Liverpool Heart & Chest Hospital, Liverpool, UK; Aalborg Thrombosis Research Unit, Department of Clinical Medicine, Aalborg University, Aalborg, Denmark.
References
- 1. Lother A, Kaier K, Ahrens I, Bothe W, Wolf D, Zehender M et al. Bleeding complications drive in-hospital mortality of patients with atrial fibrillation after transcatheter aortic valve replacement. Thromb Haemost 2020;120:1580–6. [DOI] [PubMed] [Google Scholar]
- 2. Ruff CT, Giugliano RP, Braunwald E, Hoffman EB, Deenadayalu N, Ezekowitz MD et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet 2014;383:955–62. [DOI] [PubMed] [Google Scholar]
- 3. Kim HK, Tantry US, Smith SCJr., Jeong MH, Park SJ, Kim MH et al. The East Asian Paradox: an updated position statement on the challenges to the current antithrombotic strategy in patients with cardiovascular disease. Thromb Haemost 2021;121:422–32. [DOI] [PubMed] [Google Scholar]
- 4. Lip GY, Andreotti F, Fauchier L, Huber K, Hylek E, Knight E et al. Bleeding risk assessment and management in atrial fibrillation patients. Executive Summary of a Position Document from the European Heart Rhythm Association [EHRA], endorsed by the European Society of Cardiology [ESC] Working Group on Thrombosis. Thromb Haemost 2011;106:997–1011. [DOI] [PubMed] [Google Scholar]
- 5. Pritchett RV, Bem D, Turner GM, Thomas GN, Clarke JL, Fellows R et al. Improving the prescription of oral anticoagulants in atrial fibrillation: a systematic review. Thromb Haemost 2019;119:294–307. [DOI] [PubMed] [Google Scholar]
- 6. Becattini C, Agnelli G. Treatment of venous thromboembolism with new anticoagulant agents. J Am Coll Cardiol 2016;67:1941–55. [DOI] [PubMed] [Google Scholar]
- 7. Chao TF, Guo Y. Should we adopt a standard international normalized ratio range of 2.0 to 3.0 for Asian patients with atrial fibrillation? An appeal for evidence-based management, not eminence-based recommendations. Thromb Haemost 2020;120:366–8. [DOI] [PubMed] [Google Scholar]
- 8. Pandey AK, Xu K, Zhang L, Gupta S, Eikelboom J, Cook O et al. Lower versus standard INR targets in atrial fibrillation: a systematic review and meta-analysis of randomized controlled trials. Thromb Haemost 2020;120:484–94. [DOI] [PubMed] [Google Scholar]
- 9. Rossini R, Musumeci G, Lettieri C, Molfese M, Mihalcsik L, Mantovani P et al. Long-term outcomes in patients undergoing coronary stenting on dual oral antiplatelet treatment requiring oral anticoagulant therapy. Am J Cardiol 2008;102:1618–23. [DOI] [PubMed] [Google Scholar]
- 10. Lip GYH. The ABC pathway: an integrated approach to improve AF management. Nat Rev Cardiol 2017;14:627–8. [DOI] [PubMed] [Google Scholar]
- 11. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C et al. ; ESC Scientific Document Group . 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2021;42:373–498. [DOI] [PubMed] [Google Scholar]
- 12. Chao TF, Joung B, Takahashi Y, Lim TW, Choi EK, Chan YH et al. Focused update consensus guidelines of the Asia Pacific Heart Rhythm Society on stroke prevention in atrial fibrillation: executive Summary. Thromb Haemost 2021;122:20–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Romiti GF, Pastori D, Rivera-Caravaca JM, Ding WY, Gue YX, Menichelli D et al. Adherence to the ‘atrial fibrillation better care’ (ABC) pathway in patients with atrial fibrillation. Thromb Haemost 2022;122:406–14. [DOI] [PubMed] [Google Scholar]
- 14. Lip GYH, Banerjee A, Boriani G, Chiang C. e, Fargo R, Freedman B et al. antithrombotic therapy for atrial fibrillation: CHEST guideline and expert panel report. Chest 2018;154:1121–201. [DOI] [PubMed] [Google Scholar]
- 15. Agarwal S, Hachamovitch R, Menon V. Current trial-associated outcomes with warfarin in prevention of stroke in patients with nonvalvular atrial fibrillation: a meta-analysis. Arch Intern Med 2012;172:623–31; discussion 631–3. [DOI] [PubMed] [Google Scholar]
- 16. Lip GYH, Andreotti F, Fauchier L, Huber K, Hylek E, Knight E et al. ; Document reviewers. Bleeding risk assessment and management in atrial fibrillation patients: a position document from the European Heart Rhythm Association, endorsed by the European Society of Cardiology Working Group on Thrombosis. Europace 2011;13:723–46. [DOI] [PubMed] [Google Scholar]
- 17. Steiner T, Rosand J, Diringer M. Intracerebral hemorrhage associated with oral anticoagulant therapy: current practices and unresolved questions. Stroke 2006;37:256–62. [DOI] [PubMed] [Google Scholar]
- 18. Garcia DA, Lopes RD, Hylek EM. New-onset atrial fibrillation and warfarin initiation: high risk periods and implications for new antithrombotic drugs. Thromb Haemost 2010;104:1099–105. [DOI] [PubMed] [Google Scholar]
- 19. Björck F, Renlund H, Lip GY, Wester P, Svensson PJ, Själander A. Outcomes in a warfarin-treated population with atrial fibrillation. JAMA Cardiol 2016;1:172–80. [DOI] [PubMed] [Google Scholar]
- 20. Lopez-Lopez JA, Sterne JAC, Thom HHZ, Higgins JPT, Hingorani AD, Okoli GN et al. Oral anticoagulants for prevention of stroke in atrial fibrillation: systematic review, network meta-analysis, and cost effectiveness analysis. BMJ 2017;359:j5058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Konstantinides SV, Meyer G, Becattini C, Bueno H, Geersing GJ, Harjola VP et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS): the task force for the diagnosis and management of acute pulmonary embolism of the European Society of Cardiology (ESC). Eur Respir J 2019;54:543–603. [DOI] [PubMed] [Google Scholar]
- 22. Ortel TL, Neumann I, Ageno W, Beyth R, Clark NP, Cuker A et al. American Society of Hematology 2020 guidelines for management of venous thromboembolism: treatment of deep vein thrombosis and pulmonary embolism. Blood Adv 2020;4:4693–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Linkins LA, Choi PT, Douketis JD. Clinical impact of bleeding in patients taking oral anticoagulant therapy for venous thromboembolism: a meta-analysis. Ann Intern Med 2003;139:893–900. [DOI] [PubMed] [Google Scholar]
- 24. Nieto JA, Solano R, Ruiz-Ribó MD, Ruiz-Gimenez N, Prandoni P, Kearon C et al. ; for the RIETE Investigators . Fatal bleeding in patients receiving anticoagulant therapy for venous thromboembolism: findings from the RIETE registry. J Thromb Haemost 2010;8:1216–22. [DOI] [PubMed] [Google Scholar]
- 25. Lecumberri R, Alfonso A, Jiménez D, Fernández Capitán C, Prandoni P, Wells PS et al. ; RIETE investigators . Dynamics of case-fatalilty rates of recurrent thromboembolism and major bleeding in patients treated for venous thromboembolism. Thromb Haemost 2013;110:834–43. [DOI] [PubMed] [Google Scholar]
- 26. Kakkos SK, Kirkilesis GI, Tsolakis IA. Editor's Choice - efficacy and safety of the new oral anticoagulants dabigatran, rivaroxaban, apixaban, and edoxaban in the treatment and secondary prevention of venous thromboembolism: a systematic review and meta-analysis of phase III trials. Eur J Vasc Endovasc Surg 2014;48:565–75. [DOI] [PubMed] [Google Scholar]
- 27. Agnelli G, Buller HR, Cohen A, Curto M, Gallus AS, Johnson M et al. Apixaban for extended treatment of venous thromboembolism. N Engl J Med 2013;368:699–708. [DOI] [PubMed] [Google Scholar]
- 28. Weitz JI, Lensing AWA, Prins MH, Bauersachs R, Beyer-Westendorf J, Bounameaux H et al. Rivaroxaban or aspirin for extended treatment of venous thromboembolism. N Engl J Med 2017;376:1211–22. [DOI] [PubMed] [Google Scholar]
- 29. Vasanthamohan L, Boonyawat K, Chai-Adisaksopha C, Crowther M. Reduced-dose direct oral anticoagulants in the extended treatment of venous thromboembolism: a systematic review and meta-analysis. J Thromb Haemost 2018;16:1288–95. [DOI] [PubMed] [Google Scholar]
- 30. Bovill EG, Terrin ML, Stump DC, Berke AD, Frederick M, Collen D et al. Hemorrhagic events during therapy with recombinant tissue-type plasminogen activator, heparin, and aspirin for acute myocardial infarction. Results of the Thrombolysis in Myocardial Infarction (TIMI), phase II trial. Ann Intern Med 1991;115:256–65. [DOI] [PubMed] [Google Scholar]
- 31. GUSTO Investigators. An international randomized trial comparing four thrombolytic strategies for acute myocardial infarction. N Engl J Med 1993;329:673–82. [DOI] [PubMed] [Google Scholar]
- 32. Schulman S, Kearon C; Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis . Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost 2005;3:692–4. [DOI] [PubMed] [Google Scholar]
- 33. Schulman S, Angerås U, Bergqvist D, Eriksson B, Lassen MR, Fisher W; Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis . Definition of major bleeding in clinical investigations of antihemostatic medicinal products in surgical patients. J Thromb Haemost 2010;8:202–4. [DOI] [PubMed] [Google Scholar]
- 34. Mehran R, Rao SV, Bhatt DL, Gibson CM, Caixeta A, Eikelboom J et al. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation 2011;123:2736–47. [DOI] [PubMed] [Google Scholar]
- 35. Kikkert WJ, van Geloven N, van der Laan MH, Vis MM, Baan J, Koch KT et al. The prognostic value of bleeding academic research consortium (BARC)-defined bleeding complications in ST-segment elevation myocardial infarction: a comparison with the TIMI (Thrombolysis In Myocardial Infarction), GUSTO (Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries), and ISTH (International Society on Thrombosis and Haemostasis) bleeding classifications. J Am Coll Cardiol 2014;63:1866–75. [DOI] [PubMed] [Google Scholar]
- 36. Franco L, Becattini C, Beyer‐Westendorf J, Vanni S, Nitti C, Re R et al. Definition of major bleeding: prognostic classification. J Thromb Haemost 2020;18:2852–60. [DOI] [PubMed] [Google Scholar]
- 37. Kearon C, Akl EA, Ornelas J, Blaivas A, Jimenez D, Bounameaux H et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest 2016;149:315–52. [DOI] [PubMed] [Google Scholar]
- 38. Chao T-F, Lip G, Lin Y-J, Chang S-L, Lo L-W, Hu Y-F et al. Incident risk factors and major bleeding in patients with atrial fibrillation treated with oral anticoagulants: a comparison of baseline, follow-up and delta HAS-BLED scores with an approach focused on modifiable bleeding risk factors. Thromb Haemost 2018;47:768–77. [DOI] [PubMed] [Google Scholar]
- 39. Chang TY, Lip GYH, Chen SA, Chao TF. Importance of risk reassessment in patients with atrial fibrillation in guidelines: assessing risk as a dynamic process. Can J Cardiol 2019;35:611–8. [DOI] [PubMed] [Google Scholar]
- 40. Chao T-F, Chan Y-H, Chiang C-E, Tuan T-C, Liao J-N, Chen T-J et al. Continuation or discontinuation of oral anticoagulants after HAS-BLED scores increase in patients with atrial fibrillation. Clin Res Cardiol 2022;111:23–33. [DOI] [PubMed] [Google Scholar]
- 41. Reilly PA, Lehr T, Haertter S, Connolly SJ, Yusuf S, Eikelboom JW et al. The effect of dabigatran plasma concentrations and patient characteristics on the frequency of ischemic stroke and major bleeding in atrial fibrillation patients: the RE-LY Trial (Randomized Evaluation of Long-Term Anticoagulation Therapy). J Am Coll Cardiol 2014;63:321–8. [DOI] [PubMed] [Google Scholar]
- 42. Kato ET, Giugliano RP, Ruff CT, Koretsune Y, Yamashita T, Kiss RG et al. Efficacy and safety of edoxaban in elderly patients with atrial fibrillation in the ENGAGE AF-TIMI 48 Trial. J Am Heart Assoc 2016;5:e003432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rohla M, Weiss TW, Pecen L, Patti G, Siller-Matula JM, Schnabel RB et al. Risk factors for thromboembolic and bleeding events in anticoagulated patients with atrial fibrillation: the prospective, multicentre observational PREvention oF thromboembolic events - European Registry in Atrial Fibrillation (PREFER in AF). BMJ Open 2019;9:e022478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chao TF, Chiang CE, Liao JN, Chen TJ, Lip GYH, Chen SA. Comparing the effectiveness and safety of nonvitamin K antagonist oral anticoagulants and warfarin in elderly Asian patients with atrial fibrillation: a nationwide cohort study. Chest 2020;157:1266–77. [DOI] [PubMed] [Google Scholar]
- 45. Eikelboom JW, Wallentin L, Connolly SJ, Ezekowitz M, Healey JS, Oldgren J et al. Risk of bleeding with 2 doses of dabigatran compared with warfarin in older and younger patients with atrial fibrillation: an analysis of the randomized evaluation of long-term anticoagulant therapy (RE-LY) trial. Circulation 2011;123:2363–72. [DOI] [PubMed] [Google Scholar]
- 46. Goodman SG, Wojdyla DM, Piccini JP, White HD, Paolini JF, Nessel CC et al. Factors associated with major bleeding events: insights from the ROCKET AF trial (rivaroxaban once-daily oral direct factor Xa inhibition compared with vitamin K antagonism for prevention of stroke and embolism trial in atrial fibrillation). J Am Coll Cardiol 2014;63:891–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Park S, Bergmark BA, Shi M, Lanz H-J, Chung N, Ruff CT et al. Edoxaban versus warfarin stratified by average blood pressure in 19 679 patients with atrial fibrillation and a history of hypertension in the ENGAGE AF-TIMI 48 trial. Hypertension 2019;74:597–605. [DOI] [PubMed] [Google Scholar]
- 48. Rao MP, Halvorsen S, Wojdyla D, Thomas L, Alexander JH, Hylek EM et al. Blood pressure control and risk of stroke or systemic embolism in patients with atrial fibrillation: results from the apixaban for reduction in stroke and other thromboembolic events in atrial fibrillation (ARISTOTLE) Trial. J Am Heart Assoc 2015;4:e002015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Vemulapalli S, Hellkamp AS, Jones WS, Piccini JP, Mahaffey KW, Becker RC et al. Blood pressure control and stroke or bleeding risk in anticoagulated patients with atrial fibrillation: results from the ROCKET AF Trial. Am Heart J 2016;178:74–84. [DOI] [PubMed] [Google Scholar]
- 50. Kim D, Yang P-S, Kim T-H, Jang E, Shin H, Kim HY et al. Ideal blood pressure in patients with atrial fibrillation. J Am Coll Cardiol 2018;72:1233–45. [DOI] [PubMed] [Google Scholar]
- 51. Inohara T, Holmes DN, Pieper K, Blanco RG, Allen LA, Fonarow GC et al. ; ORBIT AF Patients and Investigators . Decline in renal function and oral anticoagulation dose reduction among patients with atrial fibrillation. Heart 2020;106:358–64. [DOI] [PubMed] [Google Scholar]
- 52. Vílchez JA, Roldán V, Hernández-Romero D, Valdés M, Lip GY, Marín F. Biomarkers in atrial fibrillation: an overview. Int J Clin Pract 2014;68:434–43. [DOI] [PubMed] [Google Scholar]
- 53. Noubiap JJ, Sanders P, Nattel S, Lau DH. Biomarkers in atrial fibrillation: pathogenesis and clinical implications. Card Electrophysiol Clin 2021;13:221–33. [DOI] [PubMed] [Google Scholar]
- 54. Berg DD, Ruff CT, Morrow DA. Biomarkers for risk assessment in atrial fibrillation. Clin Chem 2021;67:87–95. [DOI] [PubMed] [Google Scholar]
- 55. Hijazi Z, Oldgren J, Lindbäck J, Alexander JH, Connolly SJ, Eikelboom JW et al. The novel biomarker-based ABC (age, biomarkers, clinical history)-bleeding risk score for patients with atrial fibrillation: a derivation and validation study. Lancet 2016;387:2302–11. [DOI] [PubMed] [Google Scholar]
- 56. Berg DD, Ruff CT, Jarolim P, Giugliano RP, Nordio F, Lanz HJ et al. Performance of the ABC scores for assessing the risk of stroke or systemic embolism and bleeding in patients with atrial fibrillation in ENGAGE AF-TIMI 48. Circulation 2019;139:760–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Pol T, Hijazi Z, Lindbäck J, Alexander JH, Bahit MC, De Caterina R et al. Evaluation of the prognostic value of GDF-15, ABC-AF-bleeding score and ABC-AF-death score in patients with atrial fibrillation across different geographical areas. Open Heart 2021;8:e001471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hijazi Z, Oldgren J, Lindbäck J, Alexander JH, Alings M, De Caterina R et al. Evaluation of the age, biomarkers, and clinical history-bleeding risk score in patients with atrial fibrillation with combined aspirin and anticoagulation therapy enrolled in the ARISTOTLE and RE-LY Trials. JAMA Netw Open 2020;3:e2015943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Esteve-Pastor MA, Rivera-Caravaca JM, Roldan V, Vicente V, Valdés M, Marín F et al. Long-term bleeding risk prediction in ‘real world’ patients with atrial fibrillation: Comparison of the HAS-BLED and ABC-Bleeding risk scores. The Murcia Atrial Fibrillation Project. Thromb Haemost 2017;117:1848–58. [DOI] [PubMed] [Google Scholar]
- 60. Rivera-Caravaca JM, Marín F, Vilchez JA, Gálvez J, Esteve-Pastor MA, Vicente V et al. Refining stroke and bleeding prediction in atrial fibrillation by adding consecutive biomarkers to clinical risk scores. Stroke 2019;50:1372–9. [DOI] [PubMed] [Google Scholar]
- 61. Singer DE, Chang Y, Borowsky LH, Fang MC, Pomernacki NK, Udaltsova N et al. A new risk scheme to predict ischemic stroke and other thromboembolism in atrial fibrillation: the ATRIA study stroke risk score. J Am Heart Assoc 2013;2:e000250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Fang MC, Go AS, Chang Y, Borowsky LH, Pomernacki NK, Udaltsova N et al. A new risk scheme to predict warfarin-associated hemorrhage: the ATRIA (Anticoagulation and Risk Factors in Atrial Fibrillation) Study. J Am Coll Cardiol 2011;58:395–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Galeano-Valle F, Ordieres-Ortega L, Oblitas CM, Del-Toro-Cervera J, Alvarez-Sala-Walther L, Demelo-Rodríguez P. Inflammatory biomarkers in the short-term prognosis of venous thromboembolism: a narrative review. Int J Mol Sci 2021;22:2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Vedovati MC, Mancuso A, Pierpaoli L, Paliani U, Conti S, Ascani A et al. Prediction of major bleeding in patients receiving DOACs for venous thromboembolism: a prospective cohort study. Int J Cardiol 2020;301:167–72. [DOI] [PubMed] [Google Scholar]
- 65. Frei AN, Stalder O, Limacher A, Méan M, Baumgartner C, Rodondi N et al. Comparison of bleeding risk scores in elderly patients receiving extended anticoagulation with vitamin K antagonists for venous thromboembolism. Thromb Haemost 2021;121:1512–22. [DOI] [PubMed] [Google Scholar]
- 66. Ban N, Siegfried CJ, Lin JB, Shui Y-B, Sein J, Pita-Thomas W et al. GDF15 is elevated in mice following retinal ganglion cell death and in glaucoma patients. JCI Insight 2017;2:e91455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Camelo-Castillo A, Rivera-Caravaca JM, Marín F, Vicente V, Lip GYH, Roldán V. Predicting adverse events beyond stroke and bleeding with the ABC-stroke and ABC-bleeding scores in patients with atrial fibrillation: the Murcia AF project. Thromb Haemost 2020;120:1200–7. [DOI] [PubMed] [Google Scholar]
- 68. Myhre PL, Prebensen C, Strand H, Røysland R, Jonassen CM, Rangberg A et al. Growth differentiation factor 15 provides prognostic information superior to established cardiovascular and inflammatory biomarkers in unselected patients hospitalized with COVID-19. Circulation 2020;142:2128–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Alserawan L, Peñacoba P, Orozco Echevarría SE, Castillo D, Ortiz E, Martínez-Martínez L et al. Growth differentiation factor 15 (GDF-15): a novel biomarker associated with poorer respiratory function in COVID-19. Diagnostics 2021;11:1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Esteve-Pastor MA, Roldán V, Rivera-Caravaca JM, Ramírez-Macías I, Lip GYH, Marín F. The use of biomarkers in clinical management guidelines: a critical appraisal. Thromb Haemost 2019;119:1901–19. [DOI] [PubMed] [Google Scholar]
- 71. Proietti M, Mujovic N, Potpara TS. Optimizing stroke and bleeding risk assessment in patients with atrial fibrillation: a balance of evidence, practicality and precision. Thromb Haemost 2018;118:2014–7. [DOI] [PubMed] [Google Scholar]
- 72. Du H, Wilson D, Ambler G, Banerjee G, Shakeshaft C, Cohen H et al. ; Clinical Relevance of Microbleeds in Stroke (CROMIS-2) Collaborators . Small vessel disease and ischemic stroke risk during anticoagulation for atrial fibrillation after cerebral ischemia. Stroke 2021;52:91–9. [DOI] [PubMed] [Google Scholar]
- 73. Wilson D, Ambler G, Shakeshaft C, Brown MM, Charidimou A, Al-Shahi Salman R et al. ; CROMIS-2 orators . Cerebral microbleeds and intracranial haemorrhage risk in patients anticoagulated for atrial fibrillation after acute ischaemic stroke or transient ischaemic attack (CROMIS-2): a multicentre observational cohort study. Lancet Neurol 2018;17:539–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Esteve-Pastor MA, Rivera-Caravaca JM, Shantsila A, Roldán V, Lip GYH, Marín F. Assessing bleeding risk in atrial fibrillation patients: comparing a bleeding risk score based only on modifiable bleeding risk factors against the HAS-BLED score. The AMADEUS trial. Thromb Haemost 2017;117:2261–6. [DOI] [PubMed] [Google Scholar]
- 75. Guo Y, Zhu H, Chen Y, Lip GYH. Comparing bleeding risk assessment focused on modifiable risk factors only versus validated bleeding risk scores in atrial fibrillation. Am J Med 2018;131:185–92. [DOI] [PubMed] [Google Scholar]
- 76. Chao T-F, Lip GYH, Lin Y-J, Chang S-L, Lo L-W, Hu Y-F et al. Major bleeding and intracranial hemorrhage risk prediction in patients with atrial fibrillation: attention to modifiable bleeding risk factors or use of a bleeding risk stratification score? A nationwide cohort study. Int J Cardiol 2018;254:157–61. [DOI] [PubMed] [Google Scholar]
- 77. Gage BF, Yan Y, Milligan PE, Waterman AD, Culverhouse R, Rich MW et al. Clinical classification schemes for predicting hemorrhage: results from the National Registry of Atrial Fibrillation (NRAF). Am Heart J 2006;151:713–9. [DOI] [PubMed] [Google Scholar]
- 78. O'Brien EC, Simon DN, Thomas LE, Hylek EM, Gersh BJ, Ansell JE et al. The ORBIT bleeding score: a simple bedside score to assess bleeding risk in atrial fibrillation. Eur Heart J 2015;36:3258–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest 2010;138:1093–100. [DOI] [PubMed] [Google Scholar]
- 80. Shireman TI, Mahnken JD, Howard PA, Kresowik TF, Hou Q, Ellerbeck EF. Development of a contemporary bleeding risk model for elderly warfarin recipients. Chest 2006;130:1390–6. [DOI] [PubMed] [Google Scholar]
- 81. Beyth RJ, Quinn LM, Landefeld CS. Prospective evaluation of an index for predicting the risk of major bleeding in outpatients treated with warfarin. Am J Med 1998;105:91–9. [DOI] [PubMed] [Google Scholar]
- 82. Landefeld CS, Goldman L. Major bleeding in outpatients treated with warfarin: incidence and prediction by factors known at the start of outpatient therapy. Am J Med 1989;87:144–52. [DOI] [PubMed] [Google Scholar]
- 83. Lane DA, Lip GYH. Stroke and bleeding risk stratification in atrial fibrillation: a critical appraisal. Eur Heart J Suppl 2020;22(Suppl O):O14–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Decousus H, Tapson VF, Bergmann J-F, Chong BH, Froehlich JB, Kakkar AK et al. ; IMPROVE Investigators . Factors at admission associated with bleeding risk in medical patients: findings from the IMPROVE investigators. Chest 2011;139:69–79. [DOI] [PubMed] [Google Scholar]
- 85. Di Nisio M, Ageno W, Rutjes AW, Pap AF, Büller HR. Risk of major bleeding in patients with venous thromboembolism treated with rivaroxaban or with heparin and vitamin K antagonists. Thromb Haemost 2016;115:424–32. [DOI] [PubMed] [Google Scholar]
- 86. Di Nisio M, Raskob G, Büller HR, Grosso MA, Zhang G, Winters SM et al. Prediction of major and clinically relevant bleeding in patients with VTE treated with edoxaban or vitamin K antagonists. Thromb Haemost 2017;117:784–93. [DOI] [PubMed] [Google Scholar]
- 87. Hostler DC, Marx ES, Moores LK, Petteys SK, Hostler JM, Mitchell JD et al. Validation of the international medical prevention registry on venous thromboembolism bleeding risk score. Chest 2016;149:372–9. [DOI] [PubMed] [Google Scholar]
- 88. Klok FA, Hösel V, Clemens A, Yollo WD, Tilke C, Schulman S et al. Prediction of bleeding events in patients with venous thromboembolism on stable anticoagulation treatment. Eur Respir J 2016;48:1369–76. [DOI] [PubMed] [Google Scholar]
- 89. Kuijer PM, Hutten BA, Prins MH, Buller HR. Prediction of the risk of bleeding during anticoagulant treatment for venous thromboembolism. Arch Intern Med 1999;159:457–60. [DOI] [PubMed] [Google Scholar]
- 90. Ruíz-Giménez N, Suárez C, González R, Nieto JA, Todolí JA, Samperiz AL et al. ; RIETE Investigators . Predictive variables for major bleeding events in patients presenting with documented acute venous thromboembolism. Findings from the RIETE Registry. Thromb Haemost 2008;100:26–31. [DOI] [PubMed] [Google Scholar]
- 91. Seiler E, Limacher A, Méan M, Beer H-J, Osterwalder J, Frauchiger B et al. Derivation and validation of a novel bleeding risk score for elderly patients with venous thromboembolism on extended anticoagulation. Thromb Haemost 2017;117:1930–6. [DOI] [PubMed] [Google Scholar]
- 92. de Winter MA, van Es N, Büller HR, Visseren FLJ, Nijkeuter M. Prediction models for recurrence and bleeding in patients with venous thromboembolism: a systematic review and critical appraisal. Thromb Res 2021;199:85–96. [DOI] [PubMed] [Google Scholar]
- 93. Apostolakis S, Lane DA, Guo Y, Buller H, Lip GY. Performance of the HEMORR(2)HAGES, ATRIA, and HAS-BLED bleeding risk-prediction scores in patients with atrial fibrillation undergoing anticoagulation: the AMADEUS (evaluating the use of SR34006 compared to warfarin or acenocoumarol in patients with atrial fibrillation) study. J Am Coll Cardiol 2012;60:861–7. [DOI] [PubMed] [Google Scholar]
- 94. Gorman EW, Perkel D, Dennis D, Yates J, Heidel RE, Wortham D. Validation of the HAS-BLED tool in atrial fibrillation patients receiving rivaroxaban. J Atr Fibrillation 2016;9:1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Senoo K, Lip GY. Predictive abilities of the HAS-BLED and ORBIT bleeding risk scores in non-warfarin anticoagulated atrial fibrillation patients: an ancillary analysis from the AMADEUS trial. Int J Cardiol 2016;221:379–82. [DOI] [PubMed] [Google Scholar]
- 96. Lip GYH, Lin H-J, Hsu H-C, Su T-C, Chen M-F, Lee Y-T et al. Comparative assessment of the HAS-BLED score with other published bleeding risk scoring schemes, for intracranial haemorrhage risk in a non-atrial fibrillation population: the Chin-Shan Community Cohort Study. Int J Cardiol 2013;168:1832–6. [DOI] [PubMed] [Google Scholar]
- 97. Proietti M, Romiti GF, Vitolo M, Potpara TS, Boriani G, Lip GYH. Comparison of HAS-BLED and ORBIT bleeding risk scores in AF patients treated with NOACs: a report from the ESC-EHRA EORP-AF general long-term registry. Eur Heart J Qual Care Clin Outcomes 2021. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 98. Lip GY, Lane DA. Bleeding risk assessment in atrial fibrillation: observations on the use and misuse of bleeding risk scores. J Thromb Haemost 2016;14:1711–4. [DOI] [PubMed] [Google Scholar]
- 99. Brown JD, Goodin AJ, Lip GYH, Adams VR. Risk stratification for bleeding complications in patients with venous thromboembolism: application of the HAS-BLED bleeding score during the first 6 months of anticoagulant treatment. JAHA 2018;7:e007901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Kooiman J, van Hagen N, Iglesias del Sol A, Planken EV, Lip GYH, van der Meer FJM et al. The HAS-BLED score identifies patients with acute venous thromboembolism at high risk of major bleeding complications during the first six months of anticoagulant treatment. PLoS One 2015;10:e0122520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Costa F, Tijssen JG, Ariotti S, Giatti S, Moscarella E, Guastaroba P et al. Incremental value of the CRUSADE, ACUITY, and HAS-BLED risk scores for the prediction of hemorrhagic events after coronary stent implantation in patients undergoing long or short duration of dual antiplatelet therapy. J Am Heart Assoc 2015;4:e002524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Borre ED, Goode A, Raitz G, Shah B, Lowenstern A, Chatterjee R et al. Predicting thromboembolic and bleeding event risk in patients with non-valvular atrial fibrillation: a systematic review. Thromb Haemost 2018;118:2171–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Guo Y, Lane DA, Chen Y, Lip GYH. Regular bleeding risk assessment associated with reduction in bleeding outcomes: the mAFA-ii randomized trial. Am J Med 2020;133:1195–1202.e1192. [DOI] [PubMed] [Google Scholar]
- 104. Alatri A, Mazzolai L, Kucher N, Aujesky D, Beer JH, Baldi T et al. The modified ottawa score and clinical events in hospitalized patients with cancer-associated thrombosis from the Swiss VTE registry. Semin Thromb Hemost 2017;43:871–6. [DOI] [PubMed] [Google Scholar]
- 105. Fuentes HE, Tafur AJ, Caprini JA, Alatri A, Trujillo-Santos J, Farge-Bancel D et al. ; RIETE Investigators . Prediction of early mortality in patients with cancer-associated thrombosis in the RIETE Database. Int Angiol 2019;38:173–84. [DOI] [PubMed] [Google Scholar]
- 106. Klok FA, Barco S, Konstantinides SV. External validation of the VTE-BLEED score for predicting major bleeding in stable anticoagulated patients with venous thromboembolism. Thromb Haemost 2017;117:1164–70. [DOI] [PubMed] [Google Scholar]
- 107. Klok FA, Barco S, Turpie AGG, Haas S, Kreutz R, Mantovani LG et al. Predictive value of venous thromboembolism (VTE)-BLEED to predict major bleeding and other adverse events in a practice-based cohort of patients with VTE: results of the XALIA study. Br J Haematol 2018;183:457–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Klok FA, Presles E, Tromeur C, Barco S, Konstantinides SV, Sanchez O et al. ; PADIS‐PE Investigators . Evaluation of the predictive value of the bleeding prediction score VTE-BLEED for recurrent venous thromboembolism. Res Pract Thromb Haemost 2019;3:364–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Klok FA, Barco S, Konstantinides SV. Evaluation of VTE-BLEED for predicting intracranial or fatal bleeding in stable anticoagulated patients with venous thromboembolism. Eur Respir J 2018;51:1800077. [DOI] [PubMed] [Google Scholar]
- 110. Nopp S, Ay C. Bleeding risk assessment in patients with venous thromboembolism. Hamostaseologie 2021;41:267–74. [DOI] [PubMed] [Google Scholar]
- 111. Witt DM, Nieuwlaat R, Clark NP, Ansell J, Holbrook A, Skov J et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: optimal management of anticoagulation therapy. Blood Adv 2018;2:3257–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Wilke T, Bauer S, Mueller S, Kohlmann T, Bauersachs R. Patient preferences for oral anticoagulation therapy in atrial fibrillation: a systematic literature review. Patient 2017;10:17–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Alonso-Coello P, Montori VM, Díaz MG, Devereaux PJ, Mas G, Diez AI et al. Values and preferences for oral antithrombotic therapy in patients with atrial fibrillation: physician and patient perspectives. Health Expect 2015;18:2318–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Bottger B, Thate-Waschke IM, Bauersachs R, Kohlmann T, Wilke T. Preferences for anticoagulation therapy in atrial fibrillation: the patients' view. J Thromb Thrombolysis 2015;40:406–15. [DOI] [PubMed] [Google Scholar]
- 115. Devereaux PJ, Anderson DR, Gardner MJ, Putnam W, Flowerdew GJ, Brownell BF et al. Differences between perspectives of physicians and patients on anticoagulation in patients with atrial fibrillation: observational study. BMJ 2001;323:1218–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Man-Son-Hing M, Laupacis A, O'Connor A, Wells G, Lemelin J, Wood W et al. Warfarin for atrial fibrillation. Arch Intern Med 1996;156:1841–8. [PubMed] [Google Scholar]
- 117. Najafzadeh M, Gagne JJ, Choudhry NK, Polinski JM, Avorn J, Schneeweiss SS. Patients' preferences in anticoagulant therapy: discrete choice experiment. Circ Cardiovasc Qual Outcomes 2014;7:912–9. [DOI] [PubMed] [Google Scholar]
- 118. Levitan BY, Gonzalez JM, Hauber AB, Lees M, Piccini JP et al. Patient and physician preferences in the United States for benefits and risks of anticoagulant use in atrial fibrillation: results from a conjoint-analysis study. Value Health 2013;16. [Google Scholar]
- 119. Okumura KI, Yasaka M, Gonzalez JM, Hauber AB, Iwamoto K et al. Japanese patients and physicians preferences for anticoagulants use in atrial fibrillation: results from a conjoint-analysis study. Value Health 2012;15:A380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Andrade JG, Krahn AD, Skanes AC, Purdham D, Ciaccia A, Connors S. Values and preferences of physicians and patients with nonvalvular atrial fibrillation who receive oral anticoagulation therapy for stroke prevention. Can J Cardiol 2016;32:747–53. [DOI] [PubMed] [Google Scholar]
- 121. Attaya S, Bornstein T, Ronquillo N, Volgman R, Braun LT, Trohman R et al. Study of warfarin patients investigating attitudes toward therapy change (SWITCH Survey). Am J Therap 2012;19:432–5. [DOI] [PubMed] [Google Scholar]
- 122. Barcellona D, Luzza M, Battino N, Fenu L, Marongiu F. The criteria of the Italian Federation of Thrombosis Centres on DOACs: a "real world" application in nonvalvular atrial fibrillation patients already on vitamin K antagonist. Intern Emerg Med 2015;10:157–63. [DOI] [PubMed] [Google Scholar]
- 123. Boom MS, Berghuis EM, Nieuwkerk PT, Pinedo S, Büller HR. When do patients prefer a direct oral anticoagulant over a vitamin K antagonist? Nether J Med 2015;73:368–72. [PubMed] [Google Scholar]
- 124. Gebler-Hughes ES, Kemp L, Bond MJ. Patients' perspectives regarding long-term warfarin therapy and the potential transition to new oral anticoagulant therapy. Therap Adv Drug Safety 2014;5:220–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Ghijben P, Lancsar E, Zavarsek S. Preferences for oral anticoagulants in atrial fibrillation: a best-best discrete choice experiment. Pharmacoeconomics 2014;32:1115–27. [DOI] [PubMed] [Google Scholar]
- 126. Cottrell S, LeReun C, Tilden D, Robinson P. Preference and willingness to pay study to assess the value of an anticoagulation therapy modelled on dabigatran etexilate using discrete choice analysis: a UK pilot study of warfarin-naive atrial fibrillation patients. Value Health 2009;12:A339. [Google Scholar]
- 127. Wang Y, Xie F, Kong MC, Lee LH, Ng HJ, Ko Y. Patient-reported health preferences of anticoagulant-related outcomes. J Thromb Thrombolysis 2015;40:268–73. [DOI] [PubMed] [Google Scholar]
- 128. Wang Y, Kong MC, Ko Y. Utility evaluation of health states related to stroke and stroke prophylaxis. Value Health 2013;16:A292. [Google Scholar]
- 129. Zamorano JL, Greiner W, Campbell D, Sandberg A, Oberdiek AMS, Bakhai A. Healthcare policy and patient satisfaction with chronic treatment for stroke prevention: results from the European Patient Survey in Atrial Fibrillation (EUPS-AF) [poster] European Society of Cardiology. Munich, Germany: European Heart Journal 2012; suppl_1.
- 130. MacLean S, Mulla S, Akl EA, Jankowski M, Vandvik PO, Ebrahim S et al. Patient values and preferences in decision making for antithrombotic therapy: a systematic review: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2012;141:e1S–23S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Man-Son-Hing M, Gage BF, Montgomery AA, Howitt A, Thomson R, Devereaux PJ et al. Preference-based antithrombotic therapy in atrial fibrillation: implications for clinical decision making. Med Decis Making 2005;25:548–59. [DOI] [PubMed] [Google Scholar]
- 132. Lane DA, Meyerhoff J, Rohner U, Lip GYH. Atrial fibrillation patient preferences for oral anticoagulation and stroke knowledge: results of a conjoint analysis. Clin Cardiol 2018;41:855–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Lane DA, Meyerhoff J, Rohner U, Lip GYH. Patients' perceptions of atrial fibrillation, stroke risk, and oral anticoagulation treatment: an international survey. TH Open 2018;2:e233–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Weernink MGM, Vaanholt MCW, Groothuis-Oudshoorn CGM, von Birgelen C, IJzerman MJ, van Til JA. Patients' priorities for oral anticoagulation therapy in non-valvular atrial fibrillation: a multi-criteria decision analysis. Am J Cardiovasc Drugs 2018;18:493–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Etxeandia-Ikobaltzeta I, Zhang Y, Brundisini F, Florez ID, Wiercioch W, Nieuwlaat R et al. Patient values and preferences regarding VTE disease: a systematic review to inform American Society of Hematology guidelines. Blood Advances 2020;4:953–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Haac BE, O'Hara NN, Mullins CD, Stein DM, Manson TT, Johal H et al. Patient preferences for venous thromboembolism prophylaxis after injury: a discrete choice experiment. BMJ Open 2017;7:e016676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Quante M, Thate-Waschke I, Schofer M. [What are the reasons for patient preference? A comparison between oral and subcutaneous administration]. Z Orthop Unfall 2012;150:397–403. [DOI] [PubMed] [Google Scholar]
- 138. Barcellona D, Contu P, Sorano GG, Pengo V, Marongiu F. The management of oral anticoagulant therapy: the patient's point of view. Thromb Haemost 2000;83:49–53. [PubMed] [Google Scholar]
- 139. Keita I, Aubin-Auger I, Lalanne C, Aubert J-P, Chassany O, Duracinsky M et al. Assessment of quality of life, satisfaction with anticoagulation therapy, and adherence to treatment in patients receiving long-course vitamin K antagonists or direct oral anticoagulants for venous thromboembolism. Patient Prefer Adherence 2017;11:1625–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Lutsey PL, Horvath KJ, Fullam L, Moll S, Rooney MR, Cushman M et al. Anticoagulant preferences and concerns among venous thromboembolism patients. Thromb Haemost 2018;118:553–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Cajfinger F, Debourdeau P, Lamblin A, Benatar V, Falvo N, Benhamou Y et al. ; TROPIQUE investigators . Low-molecular-weight heparins for cancer-associated thrombosis: adherence to clinical practice guidelines and patient perception in TROPIQUE, a 409-patient prospective observational study. Thromb Res 2016;144:85–92. [DOI] [PubMed] [Google Scholar]
- 142. Noble S, Matzdorff A, Maraveyas A, Holm MV, Pisa G. Assessing patients' anticoagulation preferences for the treatment of cancer-associated thrombosis using conjoint methodology. Haematologica 2015;100:1486–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Brekelmans MP, Kappelhof M, Nieuwkerk PT, Nierman M, Buller HR, Coppens M. Preference for direct oral anticoagulants in patients treated with vitamin K antagonists for venous thromboembolism. Neth J Med 2017;75:50–5. [PubMed] [Google Scholar]
- 144. Elewa HF, DeRemer CE, Keller K, Gujral J, Joshua TV. Patients satisfaction with warfarin and willingness to switch to dabigatran: a patient survey. J Thromb Thrombolysis 2014;38:115–20. [DOI] [PubMed] [Google Scholar]
- 145. Zolfaghari S, Harenberg J, Frölich L, Weiss C, Wehling M, Wild P et al. Development of recommendations to continue anticoagulation with one of the two types of oral anticoagulants based on the identification of patients' preference. Semin Thromb Hemost 2015;41:166–77. [DOI] [PubMed] [Google Scholar]
- 146. Apenteng PN, Fitzmaurice D, Litchfield I, Harrison S, Heneghan C, Ward A et al. Patients' perceptions and experiences of the prevention of hospital-acquired thrombosis: a qualitative study. BMJ Open 2016;6:e013839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Kuljis J, Money AG, Perry M, Barnett J, Young T. Technology-assisted self-testing and management of oral anticoagulation therapy: a qualitative patient-focused study. Scand J Caring Sci 2017;31:603–17. [DOI] [PubMed] [Google Scholar]
- 148. Najafzadeh M, Kim SC, Patterson C, Schneeweiss S, Katz JN, Brick GW et al. Patients' perception about risks and benefits of antithrombotic treatment for the prevention of venous thromboembolism (VTE) after orthopedic surgery: a qualitative study. BMC Musculoskelet Disord 2015;16:319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Noble SI, Nelson A, Turner C, Finlay IG. Acceptability of low molecular weight heparin thromboprophylaxis for inpatients receiving palliative care: qualitative study. BMJ 2006;332:577–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Seaman S, Nelson A, Noble S. Cancer-associated thrombosis, low-molecular-weight heparin, and the patient experience: a qualitative study. Patient Prefer Adherence 2014;8:453–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Ali-Ahmed F, Pieper K, North R, Allen LA, Chan PS, Ezekowitz MD et al. Shared decision-making in atrial fibrillation: patient-reported involvement in treatment decisions. Eur Heart J Qual Care Clin Outcomes 2020;6:263–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Escobar C, Borrás X, Bover Freire R, González-Juanatey C, Morillas M, Muñoz AV et al. A Delphi consensus on the management of oral anticoagulation in patients with non-valvular atrial fibrillation in Spain: ACOPREFERENCE study. PLoS One 2020;15:e0231565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Lane DA, Aguinaga L, Blomström-Lundqvist C, Boriani G, Dan G-A, Hills MT et al. Cardiac tachyarrhythmias and patient values and preferences for their management: the European Heart Rhythm Association (EHRA) consensus document endorsed by the Heart Rhythm Society (HRS), Asia Pacific Heart Rhythm Society (APHRS), and Sociedad Latinoamericana de Estimulación Cardíaca y Electrofisiología (SOLEACE). Europace 2015;17:1747–69. [DOI] [PubMed] [Google Scholar]
- 154. Lane DA, Lip GY. Patient's values and preferences for stroke prevention in atrial fibrillation: balancing stroke and bleeding risk with oral anticoagulation. Thromb Haemost 2014;111:381–3. [DOI] [PubMed] [Google Scholar]
- 155. Joseph-Williams N, Elwyn G, Edwards A. Knowledge is not power for patients: a systematic review and thematic synthesis of patient-reported barriers and facilitators to shared decision making. Patient Educ Couns 2014;94:291–309. [DOI] [PubMed] [Google Scholar]
- 156. Arbelo E, Aktaa S, Bollmann A, D'Avila A, Drossart I, Dwight J et al. Quality indicators for the care and outcomes of adults with atrial fibrillation. Europace 2021;23:494–5. [DOI] [PubMed] [Google Scholar]
- 157. January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2019;74:104–32. [DOI] [PubMed] [Google Scholar]
- 158. Perry M, Kemmis Betty S, Downes N, Andrews N, Mackenzie S, Guideline Committee . Atrial fibrillation: diagnosis and management—summary of NICE guidance. BMJ 2021;373:n1150. [DOI] [PubMed] [Google Scholar]
- 159. Chiang C-E, Okumura K, Zhang S, Chao T-F, Siu C-W, Wei Lim T et al. 2017 consensus of the Asia Pacific Heart Rhythm Society on stroke prevention in atrial fibrillation. J Arrhythm 2017;33:345–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Andrade JG, Verma A, Mitchell LB, Parkash R, Leblanc K, Atzema C et al. ; CCS Atrial Fibrillation Guidelines Committee . 2018 Focused Update of the Canadian Cardiovascular Society guidelines for the management of atrial fibrillation. Can J Cardiol 2018;34:1371–92. [DOI] [PubMed] [Google Scholar]
- 161. Steffel J, Collins R, Antz M, Cornu P, Desteghe L, Haeusler KG et al. European Heart Rhythm Association practical guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Europace 2021;23:1612–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Joung B, Lee JM, Lee KH, Kim TH, Choi EK, Lim WH et al. ; KHRS Atrial Fibrillation Guideline Working Group . 2018 Korean guideline of atrial fibrillation management. Korean Circ J 2018;48:1033–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. McCormack T, Harrisingh MC, Horner D, Bewley S; Guideline Committee . Venous thromboembolism in adults: summary of updated NICE guidance on diagnosis, management, and thrombophilia testing. BMJ 2020;369:m1565. [DOI] [PubMed] [Google Scholar]
- 164. Calkins H, Yong P, Miller JM, Olshansky B, Carlson M, Saul JP et al. Catheter ablation of accessory pathways, atrioventricular nodal reentrant tachycardia, and the atrioventricular junction: final results of a prospective, multicenter clinical trial. The Atakr Multicenter Investigators Group. Circulation 1999;99:262–70. [DOI] [PubMed] [Google Scholar]
- 165. Cappato R, Calkins H, Chen S-A, Davies W, Iesaka Y, Kalman J et al. Updated worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circ Arrhythm Electrophysiol 2010;3:32–8. [DOI] [PubMed] [Google Scholar]
- 166. Friedman DJ, Pokorney SD, Ghanem A, Marcello S, Kalsekar I, Yadalam S et al. Predictors of cardiac perforation with catheter ablation of atrial fibrillation. JACC Clin Electrophysiol 2020;6:636–45. [DOI] [PubMed] [Google Scholar]
- 167. Di Biase L, Burkhardt JD, Santangeli P, Mohanty P, Sanchez JE, Horton R et al. Periprocedural stroke and bleeding complications in patients undergoing catheter ablation of atrial fibrillation with different anticoagulation management: results from the Role of Coumadin in Preventing Thromboembolism in Atrial Fibrillation (AF) Patients Undergoing Catheter Ablation (COMPARE) randomized trial. Circulation 2014;129:2638–44. [DOI] [PubMed] [Google Scholar]
- 168. Sticherling C, Marin F, Birnie D, Boriani G, Calkins H, Dan G-A et al. Antithrombotic management in patients undergoing electrophysiological procedures: a European Heart Rhythm Association (EHRA) position document endorsed by the ESC Working Group Thrombosis, Heart Rhythm Society (HRS), and Asia Pacific Heart Rhythm Society (APHRS). Europace 2015;17:1197–214. [DOI] [PubMed] [Google Scholar]
- 169. Romero J, Cerrud-Rodriguez RC, Alviz I, Diaz JC, Rodriguez D, Arshad S et al. Significant benefit of uninterrupted DOACs versus VKA during catheter ablation of atrial fibrillation. JACC Clin Electrophysiol 2019;5:1396–405. [DOI] [PubMed] [Google Scholar]
- 170. Birnie DH, Healey JS, Wells GA, Ayala-Paredes F, Coutu B, Sumner GL et al. Continued vs. interrupted direct oral anticoagulants at the time of device surgery, in patients with moderate to high risk of arterial thrombo-embolic events (BRUISE CONTROL-2). Eur Heart J 2018;39:3973–9. [DOI] [PubMed] [Google Scholar]
- 171. Essebag V, Proietti R, Birnie DH, Wang J, Douketis J, Coutu B et al. Short-term dabigatran interruption before cardiac rhythm device implantation: multi-centre experience from the RE-LY trial. Europace 2017;19:1630–6. [DOI] [PubMed] [Google Scholar]
- 172. Leef GC, Hellkamp AS, Patel MR, Becker RC, Berkowitz SD, Breithardt G et al. Safety and efficacy of rivaroxaban in patients with cardiac implantable electronic devices: observations from the ROCKET AF Trial. JAHA 2017;6:e004663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Steffel J, Ruff CT, Braunwald E, Hamershock RA, Murphy SA, Nieminen M et al. Edoxaban and implantable cardiac device interventions: insights from the ENGAGE AF-TIMI 48 trial. Europace 2019;21:306–12. [DOI] [PubMed] [Google Scholar]
- 174. Tsai C-T, Liao J-N, Chao T-F, Lin Y-J, Chang S-L, Lo L-W et al. Uninterrupted non-vitamin K antagonist oral anticoagulants during implantation of cardiac implantable electronic devices in patients with atrial fibrillation. J Chin Med Assoc 2019;82:256–9. [DOI] [PubMed] [Google Scholar]
- 175. Black-Maier E, Kim S, Steinberg BA, Fonarow GC, Freeman JV, Kowey PR et al. ; Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF) Investigators . Oral anticoagulation management in patients with atrial fibrillation undergoing cardiac implantable electronic device implantation. Clin Cardiol 2017;40:746–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Douketis JD, Spyropoulos AC, Spencer FA, Mayr M, Jaffer AK, Eckman MH et al. Perioperative management of antithrombotic therapy: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2012;141:e326S–50S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Doherty JU, Gluckman TJ, Hucker WJ, Januzzi JL, Ortel TL, Saxonhouse SJ et al. 2017 ACC expert consensus decision pathway for periprocedural management of anticoagulation in patients with nonvalvular atrial fibrillation: a report of the American College of Cardiology Clinical Expert Consensus Document Task Force. J Am Coll Cardiol 2017;69:871–98. [DOI] [PubMed] [Google Scholar]
- 178. Raval AN, Cigarroa JE, Chung MK, Diaz-Sandoval LJ, Diercks D, Piccini JP et al. ; American Heart Association Clinical Pharmacology Subcommittee of the Acute Cardiac Care and General Cardiology Committee of the Council on Clinical Cardiology; Council on Cardiovascular Disease in the Young; and Council on Quality of Care and Outcomes Research . Management of patients on non-vitamin K antagonist oral anticoagulants in the acute care and periprocedural setting: a scientific statement from the American Heart Association. Circulation 2017;135:e604–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Vivas D, Roldán I, Ferrandis R, Marín F, Roldán V, Tello-Montoliu A et al. ; Expert reviewers . Perioperative and periprocedural management of antithrombotic therapy: consensus document of SEC, SEDAR, SEACV, SECTCV, AEC, SECPRE, SEPD, SEGO, SEHH, SETH, SEMERGEN, SEMFYC, SEMG, SEMICYUC, SEMI, SEMES, SEPAR, SENEC, SEO, SEPA, SERVEI, SECOT and AEU. Rev Esp Cardiol (Engl Ed) 2018;71:553–64. [DOI] [PubMed] [Google Scholar]
- 180. Birnie DH, Healey JS, Wells GA, Verma A, Tang AS, Krahn AD et al. Pacemaker or defibrillator surgery without interruption of anticoagulation. N Engl J Med 2013;368:2084–93. [DOI] [PubMed] [Google Scholar]
- 181. Douketis JD, Spyropoulos AC, Kaatz S, Becker RC, Caprini JA, Dunn AS et al. ; BRIDGE Investigators . Perioperative bridging anticoagulation in patients with atrial fibrillation. N Engl J Med 2015;373:823–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Tomaselli GF, Mahaffey KW, Cuker A, Dobesh PP, Doherty JU, Eikelboom JW et al. 2020 ACC expert consensus decision pathway on management of bleeding in patients on oral anticoagulants: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol 2020;76:594–622. [DOI] [PubMed] [Google Scholar]
- 183. Pollack CV, Reilly PA, van Ryn J, Eikelboom JW, Glund S, Bernstein RA et al. Idarucizumab for dabigatran reversal - full cohort analysis. N Engl J Med 2017;377:431–41. [DOI] [PubMed] [Google Scholar]
- 184. Connolly SJ, Crowther M, Eikelboom JW, Gibson CM, Curnutte JT, Lawrence JH et al. ; ANNEXA-4 Investigators . Full study report of andexanet alfa for bleeding associated with factor Xa inhibitors. N Engl J Med 2019;380:1326–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Levy JH, van Ryn J, Sellke FW, Reilly PA, Elsaesser A, Glund S et al. Dabigatran reversal with idarucizumab in patients requiring urgent surgery: a subanalysis of the RE-VERSE AD study. Ann Surg 2021;274:e204–11. [DOI] [PubMed] [Google Scholar]
- 186. Niessner A, Tamargo J, Morais J, Koller L, Wassmann S, Husted SE et al. Reversal strategies for non-vitamin K antagonist oral anticoagulants: a critical appraisal of available evidence and recommendations for clinical management-a joint position paper of the European Society of Cardiology Working Group on Cardiovascular Pharmacotherapy and European Society of Cardiology Working Group on Thrombosis. Eur Heart J 2017;38:1710–6. [DOI] [PubMed] [Google Scholar]
- 187. Hansen ML, Sørensen R, Clausen MT, Fog-Petersen ML, Raunsø J, Gadsbøll N et al. Risk of bleeding with single, dual, or triple therapy with warfarin, aspirin, and clopidogrel in patients with atrial fibrillation. Arch Intern Med 2010;170:1433–41. [DOI] [PubMed] [Google Scholar]
- 188. Roldán V, Marín F, Fernández H, Manzano-Fernandez S, Gallego P, Valdés M et al. Predictive value of the HAS-BLED and ATRIA bleeding scores for the risk of serious bleeding in a "real-world" population with atrial fibrillation receiving anticoagulant therapy. Chest 2013;143:179–84. [DOI] [PubMed] [Google Scholar]
- 189. Riva N, Bellesini M, Di Minno MND, Mumoli N, Pomero F, Franchini M et al. Poor predictive value of contemporary bleeding risk scores during long-term treatment of venous thromboembolism. A multicentre retrospective cohort study. Thromb Haemost 2014;112:511–21. [DOI] [PubMed] [Google Scholar]
- 190. Yoshida R, Ishii H, Morishima I, Tanaka A, Morita Y, Takagi K et al. Impact of nutritional and inflammation status on long-term bleeding in patients undergoing percutaneous coronary intervention with an oral anticoagulant. J Atheroscler Thromb 2019;26:728–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Urban P, Mehran R, Colleran R, Angiolillo DJ, Byrne RA, Capodanno D et al. Defining high bleeding risk in patients undergoing percutaneous coronary intervention. Circulation 2019;140:240–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Capodanno D, Bhatt DL, Gibson CM, James S, Kimura T, Mehran R et al. Bleeding avoidance strategies in percutaneous coronary intervention. Nat Rev Cardiol 2021;9:117–32. [DOI] [PubMed] [Google Scholar]
- 193. Lip GYH, Collet JP, Haude M, Byrne R, Chung EH, Fauchier L et al. 2018 Joint European consensus document on the management of antithrombotic therapy in atrial fibrillation patients presenting with acute coronary syndrome and/or undergoing percutaneous cardiovascular interventions: a joint consensus document of the European Heart Rhythm Association (EHRA), European Society of Cardiology Working Group on Thrombosis, European Association of Percutaneous Cardiovascular Interventions (EAPCI), and European Association of Acute Cardiac Care (ACCA) endorsed by the Heart Rhythm Society (HRS), Asia-Pacific Heart Rhythm Society (APHRS), Latin America Heart Rhythm Society (LAHRS), and Cardiac Arrhythmia Society of Southern Africa (CASSA). Europace 2019;21:192–3. [DOI] [PubMed] [Google Scholar]
- 194. Kumbhani DJ, Cannon CP, Beavers CJ, Bhatt DL, Cuker A, Gluckman TJ et al. 2020 ACC expert consensus decision pathway for anticoagulant and antiplatelet therapy in patients with atrial fibrillation or venous thromboembolism undergoing percutaneous coronary intervention or with atherosclerotic cardiovascular disease: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol 2021;77:629–58. [DOI] [PubMed] [Google Scholar]
- 195. Vranckx P, Verheugt FWA, de Maat MP, Ulmans VAWM, Regar E, Smits P et al. A randomised study of dabigatran in elective percutaneous coronary intervention in stable coronary artery disease patients. Eurointervention 2013;8:1052–60. [DOI] [PubMed] [Google Scholar]
- 196. Vranckx P, Leebeek FWG, Tijssen JGP, Koolen J, Stammen F, Herman J-PR et al. Peri-procedural use of rivaroxaban in elective percutaneous coronary intervention to treat stable coronary artery disease. The X-PLORER trial. Thromb Haemost 2015;114:258–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Yasuda S, Kaikita K, Akao M, Ako J, Matoba T, Nakamura M et al. Antithrombotic therapy for atrial fibrillation with stable coronary disease. N Engl J Med 2019;381:1103–13. [DOI] [PubMed] [Google Scholar]
- 198. Matsumura-Nakano Y, Shizuta S, Komasa A, Morimoto T, Masuda H, Shiomi H et al. ; OAC-ALONE Study Investigators . Open-label randomized trial comparing oral anticoagulation with and without single antiplatelet therapy in patients with atrial fibrillation and stable coronary artery disease beyond 1 year after coronary stent implantation. Circulation 2019;139:604–16. [DOI] [PubMed] [Google Scholar]
- 199. Proietti M, Airaksinen KEJ, Rubboli A, Schlitt A, Kiviniemi T, Karjalainen PP et al. Time in therapeutic range and major adverse outcomes in atrial fibrillation patients undergoing percutaneous coronary intervention: the Atrial Fibrillation Undergoing Coronary Artery Stenting (AFCAS) registry. Am Heart J 2017;190:86–93. [DOI] [PubMed] [Google Scholar]
- 200. Gibson CM, Mehran R, Bode C, Halperin J, Verheugt FW, Wildgoose P et al. Prevention of bleeding in patients with atrial fibrillation undergoing PCI. N Engl J Med 2016;375:2423–34. [DOI] [PubMed] [Google Scholar]
- 201. Cannon CP, Bhatt DL, Oldgren J, Lip GYH, Ellis SG, Kimura T et al. Dual antithrombotic therapy with dabigatran after PCI in atrial fibrillation. N Engl J Med 2017;377:1513–24. [DOI] [PubMed] [Google Scholar]
- 202. Lopes RD, Heizer G, Aronson R, Vora AN, Massaro T, Mehran R et al. Antithrombotic therapy after acute coronary syndrome or PCI in atrial fibrillation. N Engl J Med 2019;380:1509–24. [DOI] [PubMed] [Google Scholar]
- 203. Vranckx P, Valgimigli M, Eckardt L, Lewalter T, Unikas R, Marin F et al. Edoxaban in atrial fibrillation patients with percutaneous coronary intervention by acute or chronic coronary syndrome presentation: a pre-specified analysis of the ENTRUST-AF PCI trial. Eur Heart J 2020;41:4497–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Costa F, Valgimigli M, Steg PG, Bhatt DL, Hohnloser SH, Ten Berg JM et al. Antithrombotic therapy according to baseline bleeding risk in patients with atrial fibrillation undergoing percutaneous coronary intervention: applying the PRECISE-DAPT score in RE-DUAL PCI. Eur Heart J Cardiovasc Pharmacother 2020. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 205. Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 2017;39:119–77. [DOI] [PubMed] [Google Scholar]
- 206. Collet J-P, Thiele H, Barbato E, Barthélémy O, Bauersachs J, Bhatt DL et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: The Task Force for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 2020;42:1289–367. [DOI] [PubMed] [Google Scholar]
- 207. Neumann FJ, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U et al. ESC/EACTS Guidelines on myocardial revascularization. EuroIntervention 2018;14:1435–534. [DOI] [PubMed] [Google Scholar]
- 208. Capodanno D, Milluzzo RP, Angiolillo DJ. Intravenous antiplatelet therapies (glycoprotein IIb/IIIa receptor inhibitors and cangrelor) in percutaneous coronary intervention: from pharmacology to indications for clinical use. Ther Adv Cardiovasc Dis 2019;13:1753944719893274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Krucoff MW, Urban P, Tanguay J-F, McAndrew T, Zhang Y, Rao SV et al. Global approach to high bleeding risk patients with polymer-free drug-coated coronary stents. Circ Cardiovasc Interv 2020;13:e008603. [DOI] [PubMed] [Google Scholar]
- 210. Olsen A-MS, McGettigan P, Gerds TA, Fosbøl EL, Olesen JB, Sindet-Pedersen C et al. Risk of gastrointestinal bleeding associated with oral anticoagulation and non-steroidal anti-inflammatory drugs in patients with atrial fibrillation: a nationwide study. Eur Heart J Cardiovasc Pharmacother 2020;6:292–300. [DOI] [PubMed] [Google Scholar]
- 211. Al-Samkari H, Connors JM. Managing the competing risks of thrombosis, bleeding, and anticoagulation in patients with malignancy. Blood Adv 2019;3:3770–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Angelini DE, Radivoyevitch T, McCrae KR, Khorana AA. Bleeding incidence and risk factors among cancer patients treated with anticoagulation. Am J Hematol 2019;94:780–5. [DOI] [PubMed] [Google Scholar]
- 213. Melloni C, Shrader P, Carver J, Piccini JP, Thomas L, Fonarow GC et al. ; on behalf of the ORBIT-AF Steering Committee. Management and outcomes of patients with atrial fibrillation and a history of cancer: the ORBIT-AF registry. Eur Heart J Qual Care Clin Outcomes 2017;3:192–7. [DOI] [PubMed] [Google Scholar]
- 214. Shah S, Norby FL, Datta YH, Lutsey PL, MacLehose RF, Chen LY et al. Comparative effectiveness of direct oral anticoagulants and warfarin in patients with cancer and atrial fibrillation. Blood Adv 2018;2:200–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Fanola CL, Ruff CT, Murphy SA, Jin J, Duggal A, Babilonia NA et al. Efficacy and safety of edoxaban in patients with active malignancy and atrial fibrillation: analysis of the ENGAGE AF - TIMI 48 trial. J Am Heart Assoc 2018;7:e008987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Chen ST, Hellkamp AS, Becker RC, Berkowitz SD, Breithardt G, Fox KAA et al. Efficacy and safety of rivaroxaban vs. warfarin in patients with non-valvular atrial fibrillation and a history of cancer: observations from ROCKET AF. Eur Heart J Qual Care Clin Outcomes 2019;5:145–52. [DOI] [PubMed] [Google Scholar]
- 217. Delluc A, Wang T-F, Yap E-S, Ay C, Schaefer J, Carrier M et al. Anticoagulation of cancer patients with non-valvular atrial fibrillation receiving chemotherapy: guidance from the SSC of the ISTH. J Thromb Haemost 2019;17:1247–52. [DOI] [PubMed] [Google Scholar]
- 218. Sidahmed S, Abdalla A, Kheiri B, Bala A, Salih M, Bachuwa G et al. Anticoagulants for the treatment of venous thromboembolism in patients with cancer: A comprehensive systematic review, pairwise and network meta-analysis. Crit Rev Oncol Hematol 2020;152:103005. [DOI] [PubMed] [Google Scholar]
- 219. Raskob GE, van Es N, Verhamme P, Carrier M, Di Nisio M, Garcia D et al. ; Hokusai VTE Cancer Investigators . Edoxaban for the treatment of cancer-associated venous thromboembolism. N Engl J Med 2018;378:615–24. [DOI] [PubMed] [Google Scholar]
- 220. Young AM, Marshall A, Thirlwall J, Chapman O, Lokare A, Hill C et al. Comparison of an oral factor Xa inhibitor with low molecular weight heparin in patients with cancer with venous thromboembolism: results of a randomized trial (SELECT-D). J Clin Oncol 2018;36:2017–23. [DOI] [PubMed] [Google Scholar]
- 221. Kirkilesis GI, Kakkos SK, Tsolakis IA. Editor's Choice - a systematic review and meta-analysis of the efficacy and safety of anticoagulation in the treatment of venous thromboembolism in patients with cancer. Eur J Vasc Endovasc Surg 2019;57:685–701. [DOI] [PubMed] [Google Scholar]
- 222. Tolosana JM, Berne P, Mont L, Heras M, Berruezo A, Monteagudo J et al. Preparation for pacemaker or implantable cardiac defibrillator implants in patients with high risk of thrombo-embolic events: oral anticoagulation or bridging with intravenous heparin? A prospective randomized trial. Eur Heart J 2009;30:1880–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Polyzos KA, Konstantelias AA, Falagas ME. Risk factors for cardiac implantable electronic device infection: a systematic review and meta-analysis. Europace 2015;17:767–77. [DOI] [PubMed] [Google Scholar]
- 224. Essebag V, Verma A, Healey JS, Krahn AD, Kalfon E, Coutu B et al. Clinically significant pocket hematoma increases long-term risk of device infection: BRUISE CONTROL INFECTION Study. J Am Coll Cardiol 2016;67:1300–8. [DOI] [PubMed] [Google Scholar]
- 225. Kuo HC, Liu FL, Chen JT, Cherng YG, Tam KW, Tai YH. Thromboembolic and bleeding risk of periprocedural bridging anticoagulation: a systematic review and meta-analysis. Clin Cardiol 2020;43:441–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Steffel J, Verhamme P, Potpara TS, Albaladejo P, Antz M, Desteghe L et al. ; ESC Scientific Document Group . The 2018 European Heart Rhythm Association Practical Guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Eur Heart J 2018;39:1330–93. [DOI] [PubMed] [Google Scholar]
- 227. Rossini R, Tarantini G, Musumeci G, Masiero G, Barbato E, Calabrò P et al. ; Association of Obstetricians Gynecologists Italian Hospital (AOGOI) . A multidisciplinary approach on the perioperative antithrombotic management of patients with coronary stents undergoing surgery: surgery after stenting 2. JACC Cardiovasc Interv 2018;11:417–34. [DOI] [PubMed] [Google Scholar]
- 228. Cao D, Chandiramani R, Capodanno D, Berger JS, Levin MA, Hawn MT et al. Non-cardiac surgery in patients with coronary artery disease: risk evaluation and periprocedural management. Nat Rev Cardiol 2021;18:37–57. [DOI] [PubMed] [Google Scholar]