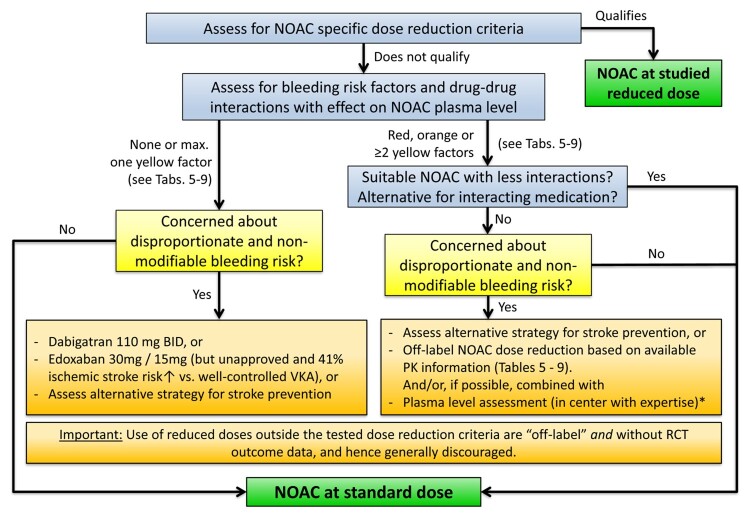
Figure 6.
NOAC selection based on drug–drug interactions and/or risk of bleeding. Dose reduction of all NOACs is primarily recommended along the published dose reduction criteria (see ‘NOAC eligibility and dosing' section, Table 2). Whenever possible, the tested and approved dosing regimen of NOACs should be used. See text for details. *Use of plasma level measurements to guide dosing is generally discouraged and should only be used in rare cases of potentially substantial interactions or special situations, and only in centers with great experience in the performance and interpretation of such assays as well as the care of NOAC-treated patients (see ‘NOAC plasma level measurements: technical approach, indications, pitfalls' section). BID, twice daily; NOAC, non-vitamin K antagonist oral anticoagulant; PK, pharmacokinetic; RCT, randomized clinical trial; VKA, vitamin K antagonist.