Table 9.
Anticipated effects of Medications used in the treatment of Covid-19 on non-vitamin K antagonist oral anticoagulants plasma levels
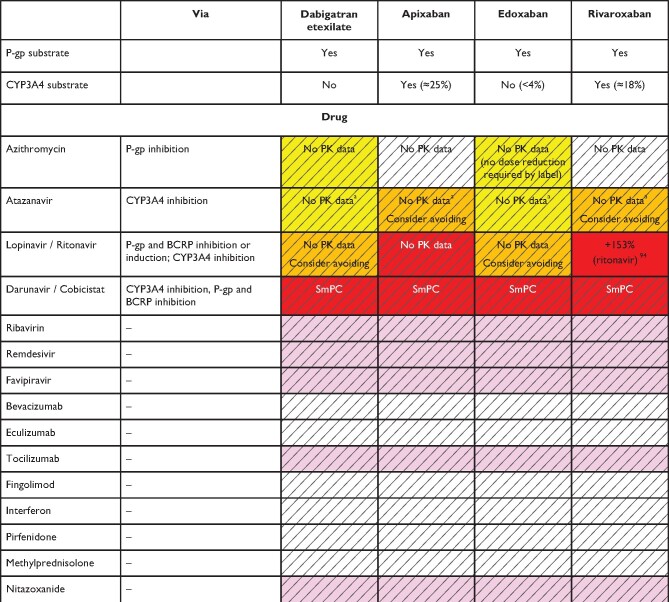
|
Colour coding is based on the respective NOAC SmPC, drug interaction databases, or expert opinion. The hatched colour coding indicates no clinical or PK data available. Some of the colour codes will likely require adaptation as more data become available over time.
White: No relevant drug–drug interaction anticipated.
Yellow: Caution required, especially in case of polypharmacy or in the presence of ≥2 yellow/bleeding risk factors (see Figure 6).
Orange: Consider avoiding concomitant use, careful monitoring required if combined. See Figure 6.
Red: Contraindicated/not advisable due to increased NOAC plasma levels.
Pink: No information retrievable.
Where no data or SmPC instructions were available, expert opinion was generally based on the following principles:
• Strong CYP3A4 and/or inhibitor—should not be used (red).
• Moderate CYP3A4 and/or P-gp inhibitor—use with caution or avoid (orange)
• Mild CYP3A4 and/or P-gp inducers or inhibitors—caution is needed especially with polypharmacy or in the presence of ≥2 bleeding risk factors (yellow).
The use of NOACs is not advisable when atazanavir is given in combination with its enhancers ritonavir or cobicistat.
