ABSTRACT
Background and Aims
Underwater endoscopic mucosal resection (UEMR) has emerged as a promising alternative to conventional endoscopic mucosal resection (CEMR) for the treatment of colorectal laterally spreading tumors (LSTs). This study aimed to compare the efficacy and safety of UEMR and CEMR in managing LSTs measuring 10–30 mm.
Methods
A post hoc analysis was performed on 88 patients with 88 colorectal LSTs, who were randomly assigned to two treatment groups: 42 with CEMR and 46 with UEMR. The primary outcome was the rate of R0 resection, defined as the absence of neoplastic cells at the resection margin. The secondary outcomes included en bloc resection rates, procedure times, and postprocedural complications. The data were analyzed via chi‐square tests, t tests, and the Mann–Whitney U test where appropriate.
Results
No significant difference was found in the R0 resection rate between UEMR and CEMR. However, UEMR achieved a significantly higher en bloc resection rate, particularly for LSTs ranging from 20 to 30 mm (42.9% for CEMR vs. 100% for UEMR; p = 0.009). Additionally, UEMR resulted in a shorter median procedure time (85.0 s for UEMR vs. 207.5 s for CEMR; p < 0.001). There was no significant difference in bleeding complications or the number of clips used between the two groups.
Conclusions
Compared with CEMR, UEMR offers a higher en bloc resection rate and a shorter procedure time, particularly for larger lesions, without increasing the risk of complications. UEMR should be considered a preferred option for managing colorectal LSTs, especially those measuring 20–30 mm.
Keywords: conventional endoscopic mucosal resection, curative resection, en bloc resection, laterally spreading tumors, underwater endoscopic mucosal resection
1. Introduction
Conventional endoscopic mucosal resection (CEMR) is recommended for managing colorectal laterally spreading tumours (LSTs) ≥ 10 mm in size with no signs of submucosal invasion [1, 2]. This technique has been shown to reduce the need for surgical intervention as well as the treatment costs for colorectal LSTs [3]. However, data have indicated that lesions greater than 20 mm in size treated with CEMR have a high rate of local recurrence [4, 5, 6]. To address the limitations of CEMR, endoscopic submucosal dissection (ESD) was developed, which results in higher rates of en bloc resection and lower recurrence rates than CEMR [7]. However, ESD is a challenging technique that requires specialized instruments, incurs higher costs, involves longer procedure times, and has more complications than CEMR does [8]. In contrast, underwater endoscopic mucosal resection (UEMR), first introduced in 2012, has shown potential for achieving en bloc resection of LSTs [9, 10].
By definition, colorectal LSTs are flat lesions measuring 10 mm or more [11]. The rate of high‐grade dysplasia of LSTs ranges from 20.9% to 33.8% [12, 13]. Therefore, early detection and complete resection of these lesions are crucial for preventing mortality from colorectal cancer [14]. A significant limitation of the CEMR technique is that submucosal injection causes flat lesions to spread out, increasing surface tension and making it difficult to capture the entire lesion with a snare. In contrast, UEMR facilitates the capture of the lesion completely because of the water effect, which transforms flat lesions into the 0–Is type, thereby increasing the likelihood of complete resection. To date, numerous studies have evaluated the efficacy of endoscopic mucosal resection (mainly CEMR) for managing nonpedunculated colorectal lesions, but few studies have focused specifically on LSTs. Additionally, comparative studies on the efficacy of UEMR and CEMR in managing LSTs are lacking. This study aimed to compare the efficacy and safety of UEMR and CEMR in managing LSTs measuring 10–30 mm.
2. Methods
2.1. Study Design
This is a post hoc analysis of an RCT comparing the efficacy and safety of UEMR with those of CEMR in managing nonpedunculated lesions measuring 10–30 mm (https://doi.org/10.21203/rs.3.rs‐5124107/v1). This study utilized data from a previous randomized controlled trial (RCT), with a total sample size of 88 cases distributed across both groups. On the basis of sample size calculations using conventional parameters (significance level of 0.05, statistical power of 0.8, and anticipated effect size), a larger cohort would ideally be needed to maximize statistical power and reliably detect smaller effect sizes. However, as this post hoc analysis is exploratory, the results aim to provide initial insights to guide future research. While the power to detect smaller associations may be limited, the findings from this analysis will serve as a foundation for further studies with larger samples to confirm these results.
The study protocol adhered to the guidelines of the Helsinki Declaration 1975 and was approved by the ethics committee of the University of Medicine and Pharmacy at Ho Chi Minh City in October 2022 (No. 722/HĐĐĐ‐ĐHYD). The study has been registered with ClinicalTrials.gov Identifier: NCT05825664. All patients provided written informed consent to participate in the study.
2.2. Study Population
The inclusion criterion consisted of patients aged over 18 years with one LST measuring 10–30 mm and classified as NICE type 1 or type 2. The size of the lesion was estimated via a snare that can adjust the loop size according to the markings on the handle or the cap attached to the endoscope (D‐201‐14304), which has an outer diameter of 15 mm.
The exclusion criteria included the presence of any of the following factors: (i) LST with signs of invasion (ulceration, hardening, friable tissue, poor mobility, positive “nonlifting” signs); (ii) NICE type 3; (iii) advanced colorectal cancer; (iv) two or more LSTs measuring ≥ 10 mm; (v) unstable chronic diseases (diabetes, hypertension, heart failure, renal failure, liver failure, chronic lung disease); or (vi) uncontrolled coagulopathy (INR > 1.5; platelet count < 100.000/mm3).
2.3. Randomization
Randomization was carried out via SPSS software, and patients were assigned to two groups at a 1:1 ratio. The outcomes of this random allocation for the intervention method were stored in sealed, numbered envelopes. Upon identifying a patient with a target lesion, the appropriate envelope was opened to determine whether to use the CEMR or UEMR technique on the basis of the previous random assignment. Both patients and pathologists were unaware of the assigned intervention method.
2.4. Experiences of Endoscopists
The procedures were performed by endoscopists with over 10 years of experience in colonoscopy and proficiency in CEMR techniques. Prior to the study, these endoscopists had performed UEMR on at least 30 nonpedunculated colorectal lesions.
2.5. Endoscopic Procedure
All procedures were conducted via CF‐HQ190I colonoscopes (Olympus Medical Systems, Tokyo, Japan). Additional equipment included a flushing and CO2 insufflation system (Sun Technology Joint Stock Company, Ho Chi Minh City, Vietnam), D‐201‐14304 endoscope attachments (Olympus Medical Systems, Tokyo, Japan), and a VIO300D electrosurgical unit (ERBE, Germany), which was set to Endocut Q mode (Effect 3, Duration 1, Interval 6) and Force Coagulation (Effect 2, 30 W) for both the UEMR and CEMR techniques.
Patients were administered intravenous sedation and positioned on their left side. A complete colonoscopy was performed. Upon identifying the target lesion, an assessment was conducted via white light and NBI. The morphology of the lesions was assessed according to the Paris classification [15]. Histopathologic prediction and depth of invasion were evaluated on the basis of the NICE classification [16]. The corresponding envelope was then opened to determine the endoscopic resection method for LSTs as per the previous random allocation.
The steps for CEMR include (1) injecting distilled water into the submucosa; (2) using a snare to capture the lesion; and (3) tightening the snare while performing electrosurgery (Figure 1). For UEMR, the steps are as follows: (1) completely suction air from the colon; (2) insufflating the segment containing the lesion with water; (3) using a snare to capture the lesion; and (4) tightening the snare with electrosurgery, similar to the CEMR technique (Figure 2).
FIGURE 1.
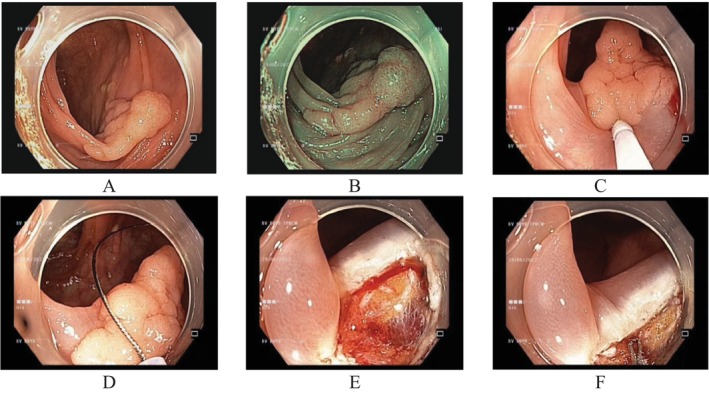
CEMR for en bloc resection of a granular LST 20 mm in size. (A) LST at the transverse colon under white light endoscopy; (B) under NBI; (C) submucosal injection with distilled water; (D) complete capture of the LST; (E) immediate bleeding after en bloc resection of the LST; and (F) coagulation with tip snare.
FIGURE 2.
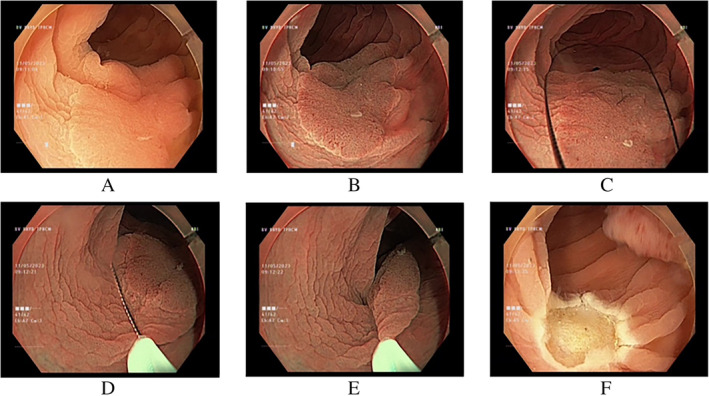
UEMR for en bloc resection of nongranular LSTs 20 mm in size. (A) LST at the sigmoid colon under white light endoscopy; (B) under NBI; (C–E) capturing the LST completely underwater; and (F) en bloc resection of the LST.
After mucosal resection, the site was evaluated again under NBI mode. If any residual lesions were detected, additional resection was performed via the same method. Intraprocedural bleeding was addressed endoscopically through the injection of adrenaline (1:20.000), clip application, or cauterization. The defect site was then closed with endoclips.
2.6. Monitoring for Postprocedure Complications
The endoscopists who performed the intervention contacted patients for follow‐up complications within 2 weeks after the endoscopic procedures. Patients were instructed to self‐monitor for symptoms, including rectal bleeding, abdominal pain, and fever, and could immediately contact the endoscopists if symptoms of complications were suspected.
2.7. Histopathological Evaluation
All the tissue samples were fixed on foam, spread with pins, and then immersed in 10% formalin before being sent to the pathology department of the University of Medicine and Pharmacy at Ho Chi Minh City. The samples were sliced into thin sections with a thicknesses of 2–3 mm parallel to the long axis (not less than 2 mm). H&E staining was subsequently performed. The specimens were evaluated according to the WHO 2019 classification [17] and the revised Vienna classification [18]. The specimens were reviewed by an experienced gastrointestinal pathologist who was blinded to the resection techniques of all patients.
2.8. Study Outcomes
The primary outcome of the study was the rate of curative resection (R0). Curative resection was defined as the absence of neoplastic cells present at the resection margin. Non‐R0 occurs when neoplastic cells are present at the margin or when the margin is unclear (RX).
The secondary outcomes of the study include the following:
The rate of en bloc resection is defined as when the lesion is completely removed after a single resection.
The procedure time, which was calculated from the start of the intervention until complete resection of the lesion was confirmed by NBI, indicated that no residual tissue remained. For CEMR, the time is measured from the beginning of needle insertion into the biopsy channel. For UEMR, the time is measured from the beginning of water insufflation into the bowel. The procedure time does not include the duration of interventions for managing bleeding complications.
Bleeding complications include intraprocedural bleeding (considered a complication if hemostatic intervention is required), early bleeding occurring within ≤ 24 h, and late bleeding occurring after > 24 h. Bleeding complications are identified by symptoms of hematochezia, signs of blood loss, or a decrease in hemoglobin (Hb) of > 2 g/dL following the procedure. Perforation complications are identified by the presence of omentum and/or evidence of air or fluid from the gastrointestinal tract in the abdominal cavity, as observed by imaging studies. All complications were monitored for 2 weeks after the intervention.
The number of clips used refers to the total number of clips applied to close the resection site.
2.9. Statistical Analysis
Data analysis was performed via IBM SPSS Statistics for Windows (Version 25.0, Armonk, NY: IBM Corp). Descriptive statistics were used to calculate means for continuous variables and proportions for categorical variables. Categorical variables are shown as counts and percentages, and comparisons were conducted via Pearson's chi‐square test. Continuous variables were assessed for normality via the Kolmogorov–Smirnov test. Those that exhibited a normal distribution were reported as the mean and standard deviation (SD) and were compared via a t test. In contrast, variables with a nonnormal distribution are presented as the median, along with the upper and lower quartiles, and comparisons were made via the Mann–Whitney U test. A p value of < 0.05 was considered statistically significant. The significance threshold was adjusted via the Bonferroni correction method in the post hoc analysis to reduce type I error.
2.10. Study Results
Between October 2022 and July 2024, we collected 88 patients with 88 colorectal LSTs, of which 42 lesions were resected via CEMR and 46 lesions were resected via UEMR (Figure 3).
FIGURE 3.
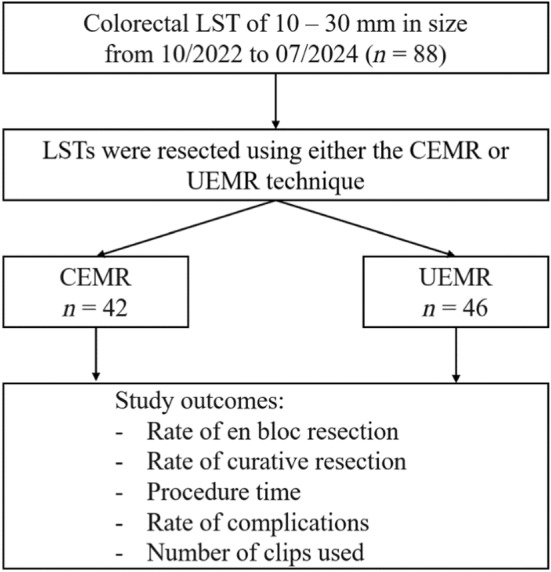
Study flowchart. CEMR: Conventional endoscopic mucosal resection; LST: Laterally spreading tumor; UEMR: Underwater endoscopic mucosal resection.
The clinical characteristics of the patients in the two groups are presented in Table 1. There were no differences between the two groups regarding age, sex, indications for endoscopy, clinical manifestations, or personal and family medical history. The endoscopic and histopathological characteristics of the lesions are detailed in Table 2. However, there were no differences between the two groups in terms of location, size, macroscopic appearance, NICE classification, or histopathology.
TABLE 1.
Patient characteristics in the study.
| Characteristics | Total (n = 88) | CEMR (n = 42) | UEMR (n = 46) | p |
|---|---|---|---|---|
| Age, median, (min—max) | 58 (27–85) | 61 (27–83) | 59 (35–85) | 0.384** |
| Sex, n (%) | 0.157 | |||
| Male | 54 (61.4) | 29 (69.0) | 25 (54.3) | |
| Female | 34 (38.6) | 13 (31.0) | 21 (45.7) | |
| Indication for colonoscopy, n (%) | ||||
| Abdominal pain | 24 (27.3) | 13 (31.0) | 11 (23.9) | 0.459 |
| Screening | 23 (26.1) | 8 (19.0) | 15 (32.6) | 0.148 |
| Blood in stool | 5 (5.7) | 2 (4.8) | 3 (6.5) | 1* |
| Diarrhea | 9 (10.2) | 3 (7.1) | 6 (13.0) | 0.489* |
| Constipation | 17 (19.3) | 9 (21.4) | 8 (17.4) | 0.632 |
| Postoperation CRC | 9 (10.2) | 7 (16.7) | 2 (4.3) | 0.080* |
| Stool change | 1 (1.1) | 0 (0.0) | 1 (2.2) | 1* |
| Clinical symptoms, n (%) | ||||
| No symptom | 33 (37.5) | 15 (35.7) | 18 (39.1) | 0.741 |
| Abdominal pain | 22 (25.0) | 11 (26.2) | 11 (23.9) | 0.805 |
| Diarrhea | 11 (12.5) | 6 (14.3) | 5 (10.9) | 0.628 |
| Blood in stool | 5 (5.7) | 1 (2.4) | 4 (8.7) | 0.363* |
| Constipation | 17 (19.3) | 9 (21.4) | 8 (17.4) | 0.632 |
| Hypertension, n (%) | 45 (51.1) | 20 (47.6) | 25 (54.3) | 0.528 |
| Diabetes, n (%) | 8 (9.1) | 5 (11.9) | 3 (6.5) | 0.471* |
| Kidney failure, n (%) | 2 (2.3) | 0 (0.0) | 2 (4.3) | 0.495* |
| Personal history of CRC, n (%) | 3 (3.4) | 2 (4.8) | 1 (2.2) | 0.604* |
| Family history of CRC, n (%) | 2 (2.3) | 1 (2.4) | 1 (2.2) | 1* |
Abbreviations: CEMR, conventional endoscopic mucosal resection; CRC, colorectal cancer; UEMR, underwater endoscopic mucosal resection.
Fisher's exact test.
Mann–Whitney U test.
TABLE 2.
Endoscopic and pathological characteristics of the colorectal LSTs.
| Characteristics | Total (n = 88) | CEMR (n = 42) | UEMR (n = 46) | p |
|---|---|---|---|---|
| Location of lesion, n (%) | ||||
| Rectum | 10 (11.4) | 2 (4.8) | 8 (17.4) | 0.093* |
| Sigmoid colon | 24 (27.3) | 12 (28.6) | 12 (26.1) | 0.794 |
| Descending colon | 9 (10.2) | 4 (9.5) | 5 (10.9) | 1* |
| Tranverse colon | 16 (18.2) | 9 (21.4) | 7 (15.2) | 0.451 |
| Ascending colon | 23 (26.1) | 13 (31.0) | 10 (21.7) | 0.362 |
| Cecum | 6 (6.8) | 2 (4.8) | 4 (8.7) | 0.678* |
| Size, median (IQR), (mm) | 20 (10–30) | 12 (10–30) | 15 (10–25) | 0.124** |
| 10–19 mm | 69 (78.4) | 35 (83.3) | 34 (73.9) | |
| 20–30 mm | 19 (21.6) | 7 (16.7) | 12 (26.1) | |
| NICE classification, n (%) | 0.429 | |||
| Type 1 | 12 (13.6) | 7 (16.7) | 5 (10.9) | |
| Type 2 | 76 (86.4) | 35 (83.3) | 41 (89.1) | |
| LST classification, n (%) | 0.431 | |||
| Granular type | 20 (22.7) | 8 (19.0) | 12 (26.1) | |
| Nongranular type | 68 (77.3) | 34 (81.0) | 34 (73.9) | |
| WHO classification, n (%) | ||||
| Nonneoplastic polyp | 4 (4.5) | 2 (4.8) | 2 (4.3) | 1* |
| Tubular adenoma | 47 (53.4) | 19 (45.2) | 28 (60.9) | 0.142 |
| Tubulovillous adenoma | 10 (11.4) | 6 (14.3) | 4 (8.7) | 0.509* |
| Serrated lesion | 27 (30.7) | 15 (35.7) | 12 (26.1) | 0.328 |
| Vienna classification, n (%) | ||||
| No dysplasia | 10 (11.4) | 4 (9.5) | 6 (13.0) | 0.742* |
| Low‐grade dysplasia | 68 (77.3) | 36 (85.7) | 32 (69.6) | 0.071 |
| High‐grade dysplasia | 10 (11.4) | 2 (4.8) | 8 (17.4) | 0.093* |
Abbreviations: CEMR, conventional endoscopic mucosal resection; UEMR, underwater endoscopic mucosal resection.
Fisher's exact test.
Mann–Whitney U test.
Overall, the results of this analysis indicated that UEMR was associated with a greater rate of en bloc resection with a significantly shorter procedure time, and there was no difference in the rate of R0 resection (Table 3). In the subgroup analysis according to the size of the lesion, UEMR used fewer clips for closure in the 10–19 mm group and had a higher rate of R0 resection in the 20–30 mm group than CEMR did (Tables 4 and 5).
TABLE 3.
Study outcomes in the whole group (n = 88).
| Outcomes | CEMR (n = 42) | UEMR (n = 46) | p | Adjusted p *** |
|---|---|---|---|---|
| En bloc resection, n (%) | 0.022* | p of < 0.01 is considered statistically significant | ||
| Yes | 37 (88.1) | 46 (100.0) | ||
| No | 5 (11.9) | 0 (0.0) | ||
| R0, n (%) | — | |||
| Yes | 35 (100.0) | 44 (100.0) | ||
| No | 0 (0.0) | 0 (0.0) | ||
| Procedure time, median (IQR), (s) | 207.5 (55–900) | 85 (15–610) | < 0.001** | |
| Bleeding, n (%) | 0.105* | |||
| Yes | 3 (7.1) | 0 (0.0) | ||
| No | 39 (92.9) | 46 (100.0) | ||
| Hemoclip, median (IQR) | 3 (2–9) | 3 (1–9) | 0.332** |
Abbreviations: CEMR, conventional endoscopic mucosal resection; UEMR, underwater endoscopic mucosal resection.
Fisher's exact test.
Mann–Whitney U test.
Bonferroni correction.
TABLE 4.
Study outcomes in the 10–19 mm LST group (n = 69).
| Outcomes | CEMR (n = 35) | UEMR (n = 34) | p | Adjusted p *** |
|---|---|---|---|---|
| En bloc resection, n (%) | 1* | p of < 0.01 is considered statistically significant | ||
| Yes | 34 (97.1) | 34 (100.0) | ||
| No | 1 (2.9) | 0 (0.0) | ||
| R0, n (%) | 1* | |||
| Yes | 32 (94.1) | 32 (94.1) | ||
| No | 2 (5.9) | 2 (5.9) | ||
| Procedure time, median (IQR), (s) | 185 (55–505) | 72.5 (15–300) | < 0.001** | |
| Bleeding, n (%) | 1* | |||
| Yes | 1 (2.9) | 0 (0.0) | ||
| No | 34 (97.1) | 34 (100.0) | ||
| Hemoclip, median (IQR) | 3 (2–5) | 2 (1–5) | 0.042** |
Abbreviations: CEMR, conventional endoscopic mucosal resection; UEMR, underwater endoscopic mucosal resection.
Fisher's exact test.
Mann–Whitney U test.
Bonferroni correction.
TABLE 5.
Study outcomes in the 20–30 mm LST group (n = 19).
| Outcomes | CEMR (n = 7) | UEMR (n = 12) | p | Adjusted p *** |
|---|---|---|---|---|
| En bloc resection, n (%) | 0.009* | p of < 0.01 is considered statistically significant | ||
| Yes | 3 (42.9) | 12 (100.0) | ||
| No | 4 (57.1) | 0 (0.0) | ||
| R0, n (%) | — | |||
| Yes | 3 (100.0) | 12 (100.0) | ||
| No | 0 (0.0) | 0 (0.0) | ||
| Procedure time, median (IQR), (s) | 455 (205–900) | 140 (65–610) | < 0.001** | |
| Bleeding, n (%) | 0.123* | |||
| Yes | 2 (28.6) | 0 (0.0) | ||
| No | 5 (71.4) | 12 (100.0) | ||
| Hemoclip, median (IQR) | 6 (3–9) | 5.5 (2–9) | 0.536** |
Abbreviations: CEMR, conventional endoscopic mucosal resection; UEMR, underwater endoscopic mucosal resection.
Fisher's exact test.
Mann–Whitney U test.
Bonferroni correction.
3. Discussion
Our study revealed that, compared with CEMR, UEMR had a greater rate of en bloc resection in the 20–30 mm group, with a shorter procedure time. There were no differences in bleeding complications or the number of clips used for closure between the two groups. The rate of R0 resection was similar for both groups.
Most studies have evaluated the efficacy of CEMR in managing LSTs [19]. For lesions of < 20 mm, complete resection can often be achieved in one cut, whereas lesions of ≥ 20 mm typically require piecemeal resection [1]. Compared with EMR, ESD was more effective, with a higher rate of R0 resection (93.6% vs. 84%) and a lower recurrence rate (1.1% vs. 12.6%) but an increased risk of perforation complications (5.9% vs. 1.2%) [19]. Recently, UEMR has been introduced and has demonstrated a high rate of en bloc resection, even for lesions measuring 20–30 mm [9, 10, 20]. These findings suggest that UEMRs have the potential to replace CEMRs in managing LSTs.
We found no difference in the rate of R0 resection between the two groups. R0 resection is a crucial factor that needs to be assessed, as achieving R0 resection may help reduce the risk of local recurrence and colorectal cancer in the future [21]. Data from a retrospective study conducted in the United States comparing CEMR (from 2007 to 2012) with UEMR (from 2012 to 2015) in managing colorectal polyps revealed that UEMR had a lower local recurrence rate than CEMR in the subgroup of LSTs measuring ≥ 15 mm, specifically 3 of 42 (7.1%) for UEMR compared with 9 of 30 (30%) for CEMR, with an OR of 5.6 (95% CI 1.4–22.8) [22]. Compared with CEMR, follow‐up after intervention is necessary to assess the efficacy of UEMR better than that of CEMR in managing LSTs.
We noted that the rate of en bloc resection for UEMR was greater than that for CEMR, particularly in the 20–30 mm group (42.9% vs. 100%; p = 0.009). This result is consistent with a multicenter RCT by Hamerski et al. [23], which gathered data from three centers in the United States and one center in Italy, showing that UEMR had a significantly higher rate of en bloc resection than CEMR did. In the CEMR technique, a needle is used to inject fluid into the submucosa, which can cause flat lesions to spread out and increase surface tension, thereby reducing the chances of capturing the lesion completely. In contrast, UEMR demonstrates the ability to capture LSTs completely under water due to foam effects, allowing the lesion to transform from type 0‐IIa to 0‐Is. This facilitates a higher rate of complete resection in one cut [24]. Our study revealed that the number of cuts required to completely resect lesions of 20–30 mm was lower for UEMR than for CEMR (1 vs. 1.8; p = 0.004). Similar results were reported in another study, which indicated that UEMR required an average of 1 cut compared with 1.3 cuts in the CEMR group (p = 0.002) [22]. Furthermore, research data indicate that UEMR has a strong ability for en bloc resection of nonpedunculated lesions and is considered a bridge therapy between CEMR and ESD for lesions ranging from 20 to 30 mm in size [20, 25, 26]. Therefore, UEMR may be considered a preferred option for en bloc resection of these lesions.
Our study revealed that UEMR required significantly less time than CEMR did. This aligns with results from a multicenter RCT conducted in the United States and Italy [23]. Reducing the procedure time may help decrease the duration of anesthesia, lower the dosage of anesthetic agents, and reduce the risks associated with prolonged anesthesia.
We did not observe differences in bleeding complications or the number of clips used for closure between the UEMR and CEMR groups. This may be attributed to the fact that all lesions in our study were resected by endoscopists with over 10 years of experience in interventional endoscopy and proficiency in CEMR techniques. Data from a cohort study also suggested that UEMR could be safely performed by endoscopists experienced in CEMR without prior UEMR training [27]. A multicenter retrospective study in Italy comparing 83 UEMRs and 86 CEMRs in community hospitals reported no differences in the rates of complete resection and complications [28]. These findings indicate that UEMR has the potential for widespread implementation in clinical practice. Further data on the experience of endoscopists with these two techniques for resecting colorectal LSTs are needed.
Our study has several limitations. First, the study was conducted at a single endoscopy center with experienced endoscopists. Second, although the study demonstrated that UEMR has a higher rate of R0 resection than CEMR does, it did not assess the recurrence rate of lesions after resection. Compared with CEMR, follow‐up and re‐evaluating these patients will provide clearer insights into the efficacy of UEMR. Third, as this was a post hoc analysis, unavoidable biases may have occurred. Therefore, further RCTs comparing the efficacy of UEMR and CEMR, specifically for LSTs, are necessary.
In conclusion, compared with CEMR, UEMR has a greater rate of en bloc resection with a significantly shorter procedure time, and there are no differences in complications or the number of clips used for closure. Thus, UEMR should be considered a preferred option for managing colorectal LSTs, particularly for lesions of 20–30 mm.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding: This study was supported by the University Medical Center Ho Chi Minh City, Ho Chi Minh City, Vietnam.
References
- 1. Kaltenbach T., Anderson J. C., Burke C. A., et al., “Endoscopic Removal of Colorectal Lesions‐Recommendations by the US Multi‐Society Task Force on Colorectal Cancer,” Gastroenterology 158, no. 4 (2020): 1095–1129, 10.1053/j.gastro.2019.12.018. [DOI] [PubMed] [Google Scholar]
- 2. Ferlitsch M., Moss A., Hassan C., et al., “Colorectal Polypectomy and Endoscopic Mucosal Resection (EMR): European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline,” Endoscopy 49, no. 3 (2017): 270–297, 10.1055/s-0043-102569. [DOI] [PubMed] [Google Scholar]
- 3. Jayanna M., Burgess N. G., Singh R., et al., “Cost Analysis of Endoscopic Mucosal Resection vs Surgery for Large Laterally Spreading Colorectal Lesions,” Clinical Gastroenterology and Hepatology 14, no. 2 (2016): 271–278.e1‐2, 10.1016/j.cgh.2015.08.037. [DOI] [PubMed] [Google Scholar]
- 4. Garg R., Singh A., Aggarwal M., et al., “Underwater Endoscopic Mucosal Resection for 10 mm or Larger Nonpedunculated Colorectal Polyps: A Systematic Review and Meta‐Analysis,” Clinical Endoscopy 54, no. 3 (2021): 379–389, 10.5946/ce.2020.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fukami N. and Lee J. H., “Endoscopic Treatment of Large Sessile and Flat Colorectal Lesions,” Current Opinion in Gastroenterology 22, no. 1 (2006): 54–59, 10.1097/01.mog.0000198075.59910.1f. [DOI] [PubMed] [Google Scholar]
- 6. Knabe M., Pohl J., Gerges C., Ell C., Neuhaus H., and Schumacher B., “Standardized Long‐Term Follow‐Up After Endoscopic Resection of Large, Nonpedunculated Colorectal Lesions: A Prospective Two‐Center Study,” American Journal of Gastroenterology 109, no. 2 (2014): 183–189, 10.1038/ajg.2013.419. [DOI] [PubMed] [Google Scholar]
- 7. Fujiya M., Tanaka K., Dokoshi T., et al., “Efficacy and Adverse Events of EMR and Endoscopic Submucosal Dissection for the Treatment of Colon Neoplasms: A Meta‐Analysis of Studies Comparing EMR and Endoscopic Submucosal Dissection,” Gastrointestinal Endoscopy 81, no. 3 (2015): 583–595, 10.1016/j.gie.2014.07.034. [DOI] [PubMed] [Google Scholar]
- 8. Tanaka S., Kashida H., Saito Y., et al., “Japan Gastroenterological Endoscopy Society guidelines for colorectal endoscopic submucosal dissection/endoscopic mucosal resection,” Digestive Endoscopy 32, no. 2 (2020): 219–239, 10.1111/den.13545. [DOI] [PubMed] [Google Scholar]
- 9. Binmoeller K. F., Weilert F., Shah J., Bhat Y., and Kane S., “"Underwater" EMR Without Submucosal Injection for Large Sessile Colorectal Polyps (With Video),” Gastrointestinal Endoscopy 75, no. 5 (2012): 1086–1091, 10.1016/j.gie.2011.12.022. [DOI] [PubMed] [Google Scholar]
- 10. Binmoeller K. F., Hamerski C. M., Shah J. N., Bhat Y. M., Kane S. D., and Garcia‐Kennedy R., “Attempted Underwater en Bloc Resection for Large (2‐4 Cm) Colorectal Laterally Spreading Tumors (With Video),” Gastrointestinal Endoscopy 81, no. 3 (2015): 713–718, 10.1016/j.gie.2014.10.044. [DOI] [PubMed] [Google Scholar]
- 11. Kudo S., Kashida H., Tamura T., et al., “Colonoscopic Diagnosis and Management of Nonpolypoid Early Colorectal Cancer,” World Journal of Surgery 24, no. 9 (2000): 1081–1090, 10.1007/s002680010154. [DOI] [PubMed] [Google Scholar]
- 12. Kim B. C., Chang H. J., Han K. S., et al., “Clinicopathological Differences of Laterally Spreading Tumors of the Colorectum According to Gross Appearance,” Endoscopy 43, no. 2 (2011): 100–107, 10.1055/s-0030-1256027. [DOI] [PubMed] [Google Scholar]
- 13. Rotondano G., Bianco M., Buffoli F., Gizzi G., Tessari F., and Cipolletta L., “The Cooperative Italian FLIN Study Group: Prevalence and Clinico‐Pathological Features of Colorectal Laterally Spreading Tumors,” Endoscopy 43, no. 10 (2011): 856–861, 10.1055/s-0030-1256639. [DOI] [PubMed] [Google Scholar]
- 14. Zauber A. G., Winawer S. J., O'Brien M. J., et al., “Colonoscopic Polypectomy and Long‐Term Prevention of Colorectal‐Cancer Deaths,” New England Journal of Medicine 366, no. 8 (2012): 687–696, 10.1056/NEJMoa1100370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. The Paris endoscopic classification of superficial neoplastic lesions , “Esophagus, Stomach, and Colon: November 30 to December 1, 2002,” Gastrointestinal Endoscopy 58, no. 6 Suppl (2003): S3–S43, 10.1016/s0016-5107(03)02159-x. [DOI] [PubMed] [Google Scholar]
- 16. Hewett D. G., Kaltenbach T., Sano Y., et al., “Validation of a Simple Classification System for Endoscopic Diagnosis of Small Colorectal Polyps Using Narrow‐Band Imaging,” Gastroenterology 143, no. 3 (2012): 599–607 e1, 10.1053/j.gastro.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 17. Nagtegaal I. D., Odze R. D., Klimstra D., et al., “The 2019 WHO Classification of Tumours of the Digestive System,” Histopathology 76, no. 2 (2020): 182–188, 10.1111/his.13975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Choi H. J., Lee B. I., Choi H., et al., “Diagnostic Accuracy and Interobserver Agreement in Predicting the Submucosal Invasion of Colorectal Tumors Using Gross Findings, Pit Patterns, and Microvasculatures,” Clinical Endoscopy 46, no. 2 (2013): 168–171, 10.5946/ce.2013.46.2.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Russo P., Barbeiro S., Awadie H., Libanio D., Dinis‐Ribeiro M., and Bourke M., “Management of Colorectal Laterally Spreading Tumors: A Systematic Review and Meta‐Analysis,” Endoscopy International Open 7, no. 2 (2019): E239–E259, 10.1055/a-0732-487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Saito Y. and Ono A., “Underwater Endoscopic Mucosal Resection for Colorectal Lesions: A Bridge Between Conventional Endoscopic Mucosal Resection and Endoscopic Submucosal Dissection,” Gastroenterology 161, no. 5 (2021): 1369–1371, 10.1053/j.gastro.2021.08.039. [DOI] [PubMed] [Google Scholar]
- 21. Parihar V., Sopena‐Falco J., Leung E., et al., “R0 Resection Margin—A New Quality Measure in the Era of National Bowel Screening,” Irish Medical Journal 113, no. 1 (2020): 7. [PubMed] [Google Scholar]
- 22. Schenck R. J., Jahann D. A., Patrie J. T., et al., “Underwater Endoscopic Mucosal Resection Is Associated With Fewer Recurrences and Earlier Curative Resections Compared to Conventional Endoscopic Mucosal Resection for Large Colorectal Polyps,” Surgical Endoscopy 31, no. 10 (2017): 4174–4183, 10.1007/s00464-017-5474-4. [DOI] [PubMed] [Google Scholar]
- 23. Hamerski C., Samarasena J., Lee D. P., et al., “Underwater Versus Conventional Endoscopic Mucosal Resection for the Treatment of Colorectal Laterally Spreading Tumors—Results From an International, Multicenter, Randomized Controlled Trial,” American Journal of Gastroenterology 114 (2019): S75. [Google Scholar]
- 24. Uchima H., Colan‐Hernandez J., and Binmoeller K. F., “Peristaltic Contractions Help Snaring During Underwater Endoscopic Mucosal Resection of Colonic Non‐granular Pseudodepressed Laterally Spreading Tumor,” Digestive Endoscopy 33, no. 4 (2021): e74–e76, 10.1111/den.13952. [DOI] [PubMed] [Google Scholar]
- 25. Nagl S., Ebigbo A., Goelder S. K., et al., “Underwater vs Conventional Endoscopic Mucosal Resection of Large Sessile or Flat Colorectal Polyps: A Prospective Randomized Controlled Trial,” Gastroenterology 161, no. 5 (2021): 1460–1474.e1, 10.1053/j.gastro.2021.07.044. [DOI] [PubMed] [Google Scholar]
- 26. Rodríguez Sánchez J., Alvarez‐Gonzalez M. A., Pellisé M., et al., “Underwater Versus Conventional EMR of Large Nonpedunculated Colorectal Lesions: A Multicenter Randomized Controlled Trial,” Gastrointestinal Endoscopy 97, no. 5 (2023): 941–951.e2, 10.1016/j.gie.2022.12.013. [DOI] [PubMed] [Google Scholar]
- 27. Curcio G., Granata A., Ligresti D., et al., “Underwater Colorectal EMR: Remodeling Endoscopic Mucosal Resection,” Gastrointestinal Endoscopy 81, no. 5 (2015): 1238–1242, 10.1016/j.gie.2014.12.055. [DOI] [PubMed] [Google Scholar]
- 28. Cadoni S., Liggi M., Gallittu P., et al., “Underwater Endoscopic Colorectal Polyp Resection: Feasibility in Everyday Clinical Practice,” United European Gastroenterology Journal 6, no. 3 (2018): 454–462, 10.1177/2050640617733923. [DOI] [PMC free article] [PubMed] [Google Scholar]


