Abstract
Human immunodeficiency virus (HIV), the main contributor of the ongoing AIDS epidemic, remains one of the most challenging and complex viruses to target and eradicate due to frequent genome mutation and immune evasion. Despite the development of potent antiretroviral therapies, HIV remains an incurable infection as the virus persists in latent reservoirs throughout the body. To innovate a safe and effective cure strategy for HIV in humans, animal models are needed to better understand viral proliferation, disease progression, and therapeutic response. Nonhuman primates infected with simian immunodeficiency virus (SIV) provide an ideal model to study HIV infection and pathogenesis as they are closely related to humans genetically and express phenotypically similar immune systems. Examining the clinical outcomes of novel treatment strategies within nonhuman primates facilitates our understanding of HIV latency and advances the development of a true cure to HIV.
Keywords: HIV, Viral reservoir, Nonhuman primate, HAND, ART
Introduction
HIV-1 is a contagious and pathogenic virus that affects more than 38 million people worldwide (Global HIV & AIDS statistics—Fact sheet 2023). Although there have been significant advances in the treatment of HIV, there is still no “traditional” cure resulting in total viral genome deletion from all reservoir sites in an infected individual (Kwan et al. 2022). As a result, HIV research continues to be an area of active investigation. The use of nonhuman primates (NHPs) in HIV research has been critical in the study of the virus and has led to numerous discoveries and advances in the development of new treatments and “functional” cures, defined by sustained virus control to low or undetectable levels in the absence of antiretroviral treatment (Bailon et al. 2020). In this review paper, the use of NHPs in HIV research will be outlined, including the benefits, and investigative importance of utilizing the rhesus macaque animal model and the resulting impact in the development of new medicaments for HIV treatment and genome eradication.
NHPs in HIV Research
Animal models, such as the NHP, are essential in biological research as they enable scientists to control variables and obtain samples that would not be practical to realize in humans. Offering the ability to collect longitudinal specimen throughout a course of study, be it blood, urine, cerebrospinal fluid (CSF), bronchioalveolar lavage (BAL), tissue sections, mucosal swabs, etc., NHP models provide invaluable information as a treatment or cure tactic progresses. Yielding sizeable tissue volumes during necropsy, NHPs allow scientists to perform far more biological assays compared to smaller animal models. Due to their strong similarities to humans genetically and immunologically, studies with NHPs have provided valuable insights into the nuances of many viral infections (Stone et al. 1987). Eliciting very similar innate and adaptive immune responses to humans with paralleled susceptibility to most viruses, these animals oftentimes serve as the most appropriate models of viral infections (Estes et al. 2018). Unlike inbred animal models, NHPs display significant outbred genetic variation (Vallender and Miller 2013). While this characteristic closely resembles that of human genomic variance, small cohort sizes in many NHP studies combined with highly controlled environmental conditions reduce statistical power and potentially confound the interpretation of obtained data. Conversely, a more genetically diverse NHP population offers researchers the unique opportunity to investigate the effects of naturally occurring genetic variation on phenotypic presentation not possible in inbred animal models or humans.
Discussed in detail by Estes et al. (2018), Old World monkey species including Macaca nemestrina (pigatail macaque), Macaca fascicularis (cynomolgus macaque), and Macaca mulatta (rhesus macaque) are the most frequently utilized NHPs in viral and immunological studies. While vastly similar genetically, subtle differences in gene expression as it relates to antiviral response confers varied antiviral activity in each NHP species (Wu et al. 2015; Bimber et al. 2017). For example, rhesus macaques express heterogeneous type I interferon-induced, antiviral protein tripartite motif-containing protein 5 (TRIM5), which leads to differential restriction of simian immunodeficiency virus (SIV) (Wu et al. 2015). Pigtail macaques, on the other hand, express only one TRIM5 genotype which does not lead to SIV replication restriction, being more suited for studies investigating HIV progression in principle. In practice, however, the greater availability of the rhesus macaque has bolstered its continued use in research. Macaque models were first utilized in 1984 to demonstrate that acquired immune deficiency syndrome (AIDS) was the result of a type D retroviral infection (Daniel et al. 1984) and are now the most used NHP model for HIV research (Veazey and Lackner 2017). As a physiologically relevant model, the use of NHPs, especially the rhesus macaque, in HIV research has led to numerous advances in the development of new treatments and cure strategies.
Cellular Tropism of HIV
As viral entry of HIV begins with interactions between viral glycoprotein gp120 and the cellular receptor CD4, CD4 + T lymphocytes are susceptible to entry by all strains and phenotypes of HIV (Naif 2013). These cells are the main target of infection and are understood to be the predominant peripheral cell type concealing latent virus (Kim et al. 2018). Divided into groups based on coreceptor usage, HIV utilizes G-protein coupled chemokine receptors CCR5 and/or CXCR4 in addition to CD4 to mediate cellular entry. CCR5, expressed mainly on macrophages, is utilized by R5 strains of HIV and is responsible for primary cell infection and central nervous system (CNS) involvement (Alkhatib et al. 1996; Deng et al. 1996; Dragic et al. 1996). Conversely, X4 strains that preferentially interact with coreceptor CXCR4 mainly expressed on T-lymphocytes are attributed to the viral replication capacity of HIV and commonly emerge in later stages of infection (Scarlatti et al. 1997).
While HIV replication is inefficient in macaques and does not lead to the development of AIDS (Thippeshappa et al. 2020), NHPs closely mimic disease pathogenesis and progression when infected with SIV (Estes et al. 2018). The most common strains of SIV used for immunological challenge, SIVmac251 and SIVmac239, were originally isolated from rhesus macaques of Indian decent and were molecularly cloned to generate viral stocks (Hatziioannou and Evans 2012). Consequently, these strains are well adapted to these animals and result in infections yielding high viral loads with minimal animal-to-animal variation and reproduce the turnover and progressive loss of CD4 + T-cells seen in HIV replication (Veazey et al. 1998). These are undeniably three distinct infectious agents, and the major concerns of using SIV clones to model HIV pathogenesis involve the genetic dissimilarity and unique proteomes making viral genome excision or protein-targeted neutralization strategies difficult to validate in humans. To combat this, a recombinant form of SIVmac239, termed simian-human immunodeficiency virus (SHIV), was engineered to contain env, tat, rev, and vpu genes of HIV-1 (Joag et al. 1996). This chimeric virus includes these genes to imitate envelope protein expression (env), gene upregulation at the 5′ region (tat), gene upregulation at the envelope region (rev), and viral protein U expression (vpu) which aids in CD4 T-cell degradation and virion release (Seitz 2016). Improving the chimera of SHIV to best recapitulate clinical outcomes of HIV by replacing SIV reverse transcriptase with that of HIV-1 to increase infectivity rates, modifying Env amino acid residues to promote CD4 T-cell entry, and performing serial in vivo-passaging results in similar sensitivity to HIV therapeutics (Jiang et al. 2009; Humes et al. 2012; Bar et al. 2019; Ziani et al. 2021). Expressing a clinically relevant antigenic profile and demonstrating comparable neutralization sensitivities are critical for this model to be effective for vaccine studies or research toward a “traditional” cure (Li et al. 2021). These NHP models have been fundamental in studying the impact of HIV on the immune system, the development of drug resistance, and the effects of HIV on the nervous system because of these evolving genetic manipulations.
Latent HIV Reservoirs
HIV and SIV target macrophages and T cells for infection. By binding to surface protein CD4 and engaging chemokine receptors CXCR4 and/or CCR5 as coreceptors, HIV enters the cell (Bleul et al. 1997). Viral reservoirs are formed within the earliest stages of HIV infection within these target cells (Valcour et al. 2012) and persist in long-lived cells commonly found within lymphoid tissues such as peripheral lymph nodes and spleen, the GI tract, and the central nervous system (CNS) (Veazey et al. 1998; Busman-Sahay et al. 2021). Early ART administration relative to infection reduces viral tissue reservoir size and leads to a diminution of unfavorable clinical outcomes for both human patients and simian models (Dragic et al. 1996; Okoye et al. 2018). However, as ART only limits the active replication of virus, latently infected cells are unaffected by therapy, allowing the burden of the viral reservoir to persist. Nonhuman primate models infected with SIV closely recapitulate the development and persistence of these viral reservoirs. While exhibiting the highest densities of viral RNA (vRNA) within lymphoid tissues (especially spleen, GI tract, and lymph nodes), vRNA + cells are detected in every organ of macaques following inoculation with an infectious strain (Estes et al. 2017). In a latent state, characterized by the presence of integrated HIV DNA that is replication competent but transcriptionally silent, these cellular reservoirs evade immune recognition due to absent or limited viral protein expression (Siliciano and Greene 2011).
Treatment of Chronic HIV
Combination antiretroviral therapy (cART) is the current standard of care for HIV infection and currently involves the use of multiple antiretroviral therapy (ART) drugs to suppress the virus via distinctive mechanisms (HIV Clinical Guidelines: Adult and Adolescent ARV—What’s New in the Guidelines | Clinicalinfo.HIV.gov 2023). The five main classes of HIV ARTs include nucleoside/nucleotide reverse transcriptase inhibitors (NRTIs), non-nucleoside reverse transcriptase inhibitors (NNRTIs), protease inhibitors (PI), integrase inhibitors (INIs/INSTIs), and entry inhibitors (Blassel et al. 2021). The inception of the first approved ART, an NRTI monotherapy by the name of zidovudine (AZT), exhibited decreased mortality and opportunistic infection frequency in people living with HIV (PLWH) (Fischl et al. 1987). Despite initial success, these monotherapies proved to be ineffective in slowing the progression to AIDS due to rapid viral mutability leading to drug resistance (Kirschner and Webb 1997). The advent of the first cART regimen, a combination of AZT and another NRTI drug called dideoxycytidine (ddC), came with emerging evidence from a NIAID-funded study demonstrating that this two-drug therapy was more effective than AZT alone in controlling viral load (Meng et al. 1992). Challenges in this first-generation therapy including high pill burden, drug-related toxicity, drug-drug and drug-food interactions, and incomplete viral suppression resulted in poor patient compliance and low therapeutic efficacy (Tseng et al. 2015). Durable and lasting HIV suppression was first achieved through triple-drug therapy consisting of saquinavir, ddC, and AZT (Collier et al. 1996). The success of this three-drug regimen was partially attributed to the addition of a new antiretroviral drug class, the protease inhibitor (Antiretroviral Drug Discovery and Development | NIH: National Institute of Allergy and Infectious Diseases, 2023). By administering drugs from at least two different classes, many patients displayed nearly undetectable viral loads for up to 1 year, hugely outperforming results seen in single-drug therapy. However, prolonged ART of any type can lead to treatment pressure on the virus, causing drug resistant mutations (DRMs) within the genome (Lepri et al. 2000). These DRMS are associated with all of the currently available ART drugs and can lead to treatment failure and viral rebound if alternative therapy is not promptly administered (Blassel et al. 2021).
NHPs have been used to study physiological outcomes of emerging ARTs and combination therapies (Tsai et al. 1988), which has allowed for the further development of targeted and practical treatment plans. Attributed to the minimization of adverse events, advances in cART have led to increased compliance as well as a decrease in therapy switching or drug discontinuation in PLWH (Cicconi et al. 2010). Continuous administration of ART, as opposed to episodic use, has been shown to significantly reduce the risk of opportunistic disease or death from any cause in PLWH (El-Sadr et al. 2006). Prolonged elevation of CD4 + T-cell counts and plasma viral load suppression by cART administration highlights the importance of uninterrupted drug compliance in infected individuals. As antiretroviral drugs have limited penetration into the CSF and viral reservoir sites, discontinuation or interruption of ART inevitably leads to viral rebound by reactivation of latent cells within these tissues and can contribute to the progression of AIDS (Holkmann Olsen et al. 2007). Conversely, long-term cART is associated with an increased occurrence of serious non-AIDS events including osteoporosis, renal and metabolic disorders, cardiovascular disease, liver disease, as well as central nervous system (CNS) complications, necessitating continued research for alternative treatment strategies (Chawla et al. 2018).
HIV and Healthy Aging
As it is estimated that nearly half of PLWH in the USA are now above the age of 50 (Wing 2017) with this figure continuing to rise as longer life expectancies are reached, studying the impact of HIV on normal aging is imperative. Increased risk of noninfectious comorbidities classically appears 10 years earlier in PLWH as compared to the general population, exhibiting evidence of chronic HIV contributing to accelerated aging (Guaraldi et al. 2011). Earlier incidence of chronic diseases such as diabetes, dyslipidemia, osteoporosis, cardiovascular disease, bone fractures, renal failure, and neurocognitive impairment are associated with these patterns of accelerated aging seen in PLWH (Guaraldi et al. 2011; Meir-Shafrir and Pollack 2012). Investigating the long-term effects of ART and the contribution of the viral reservoir to morbidities seen in the older population of PLWH requires longevity studies that closely approximate the human experience of aging. Alongside their 92% genetic homology (Magness et al. 2005) and phenotypic similarity, rhesus macaques have an average lifespan of 25 years with a maximum of 40 years in captivity, resulting in age-related changes that better recapitulate that of humans compared to shorter-lived animal models (Didier et al. 2016; Mattison and Vaughan 2017). Although this extended lifespan equates to lengthy experimental timelines and prohibitive expenditure, associations made from these comparative studies in aging are irreplaceable (Mattison and Vaughan 2017).
Longevity studies within long-lived translational species are also essential in validating the preclinical efficacy of a therapeutic and to ensure that a response is not transient. Studies surveilling the immune landscape of aging models infected with SIV demonstrate marked shifts in CD4 and CD8 T-cell ratios and increased vRNA density within both peripheral blood and lymph nodes compared to young animals (Chang et al. 2017; Zheng et al. 2022). Lymphoid organ architecture is also significantly modified within older infected animals, exhibiting deep CD8 T-cell infiltration of germinal centers not present in young infected animals (Zheng et al. 2022). Consequently, this excessive proliferation of terminally differentiated cytotoxic CD8 T-cells reduces the capacity of the lymphoid organ to generate adaptive immune responses used in the clearance of viral particles and further aggravates chronic inflammation (Dock and Effros 2011; Heigele et al. 2015). This evidence suggests that aging and HIV infection may work cooperatively to induce excessive inflammation within immune organs thereby accelerating age-related comorbidities.
Chronic inflammation, characterized by the prolonged upregulation of proinflammatory cytokines, is exacerbated in HIV infection (Ferrucci and Fabbri 2018) and is referred to as “inflammaging” when contributing to age-related disease pathogenesis. It has been shown in NHP models of HIV that a decrease in CD161 + T-cells and Th17-type effector function dysregulation is more commonly seen in older SIV + rhesus macaques and is accompanied by elevated proinflammatory cytokine levels (Walker et al. 2019). This phenotype is associated with loss of mucosal barrier function and increased gut permeability leading to a pathology referred to as leaky gut, an age-related disease state frequent in PLWH (Alzahrani et al. 2019). These fluctuations in immune landscape contribute directly to the progression of HIV and could affect the interpretation of results when evaluating therapeutic approaches for older populations of PLWH.
HIV within the CNS
Providing substantial insight into the early brain infection pathways, NHP models of HIV enable researchers to examine the intricacies of neuroAIDS in studies that are impractical or unethical with human subjects (Estes et al. 2018). Similarities in brain morphology, cortical development pathways, and brain-to-body mass ratio substantiate the use of NHP models in neuroscientific inquiries (Sun and Hevner 2014). HIV is thought to enter the CNS through the transmigration of infected monocytes across the blood brain barrier (BBB) (MacLean et al. 2004; Eugenin et al. 2006; Valcour et al. 2012; Williams et al. 2012), making myeloid cells, particularly microglia, probable cellular reservoirs for latent virus within the brain (Bell 1998; Perez et al. 2018). Microglial populations are dense within the white matter of the human and primate brain (Mittelbronn et al. 2001; Dos Santos et al. 2020) and are proposed to be integral “conductors” of brain aging by modulating glial activation and phagocytizing white matter-derived debris (Ahn et al. 2022). Reducing or eliminating the viral burden within these cells is imperative to preserve microglial function and in maintaining brain homeostasis pathways (Borrajo et al. 2020). While cART has shown to limit the amount of viral SIV DNA within the grey matter of several brain regions of infected rhesus macaques, levels within the white matter remain high throughout ART administration and contributes to HIV-associated brain pathologies (Perez et al. 2018). As HIV does not productively infect neurons, the effects of HIV on neuronal function is described as indirect and will be expanded upon below (Kovalevich and Langford 2012).
HIV-Associated Neurocognitive Impairment
HIV-associated neurocognitive impairment (HAND) is an umbrella term used to describe an array of neurocognitive dysfunction states originating from HIV infection complications independent of opportunistic infections (Antinori et al. 2007). The subclasses of HAND include asymptomatic neurocognitive impairment (ANI), mild neurocognitive disorder (MND), and HIV associated dementia (HAD). Diagnosed by neuropsychological evaluation and functional status assessments, disease states are assigned by severity of symptomatic presentation degree to which these symptoms interfere with tasks of daily living (Clifford and Ances 2013; Saylor et al. 2016). HIV-associated dementia (HAD), previously referred to as AIDS Dementia Complex (ADC), is a disease state characterized by the progressive decline of subcortical function that generally leads to death less than 1 year following diagnosis (Navia et al. 1986). Accompanied by increasing loss of attention and variable behavioral components, the classical distinguishing symptom of HAD is marked slowing of motor skills. While subcortical HAD incidence dwindled following methods of sustained viral load control, cortical presentation remains in untreated individuals and those with and poor medication compliance (Rumbaugh and Tyor 2015; Eggers et al. 2017).
Although the introduction of cART appreciably decreased the incidence of HAD seen in patients receiving older systemic immunosuppressive therapies, 40% of PLWH today continue to experience neurologic compilations (Ghosh et al. 2017). The current prevalence of HAND is driven by diagnoses of ANI and MND-disease states solely differentiated by functional impairment status (Clifford and Ances 2013). Patients with ANI and MND perform one standard deviation below the mean of the best available population norms within two cognitive domains of neuropsychometric testing (Antinori et al. 2007). The difference between the two diagnoses being that ANI-presenting patients demonstrate no impairment in activities of daily living while those with MND exhibit functional impairments as it relates to the completion of routine tasks. Both ANI and MND are seen in patients living with chronic HIV regardless of sustained cART compliance (Wei et al. 2020). Risk factors for developing HAND include insufficient viral suppression, low CD4 T-cell count, and increasing age (Valcour et al. 2006; Robertson et al. 2007; Heaton et al. 2010). While the current consensus in the field suggests that underlying inflammation leads to non-lethal neurodegeneration ultimately driving HAND (Mamik et al. 2016), more research is warranted to elucidate the precise mechanism of pathology.
As studying the mechanisms behind neuropathogenesis within the CNS of humans is difficult to assess in vivo, NHP models offer a pathway to examine immune pathways of HIV-induced CNS damage (Beck et al. 2018; Mallard and Williams 2018; Moretti et al. 2021). Though further investigation is merited, toxicity induced by chronic cART and latent viral CNS reservoirs have been implicated in the development and progression of HAND by contributing to chronic neuroinflammatory states (Williams et al. 2012; Delery and MacLean 2019; Ash et al. 2021). Increased monocyte turnover was associated with increased severity of encephalitis in NHPs not receiving ART, underscoring the therapeutic potential in eliminating proviral DNA from these cells (Burdo et al. 2010). For these reasons, novel therapeutic approaches that prevent viral replication in reservoirs, or better yet remove viral reservoirs altogether, and allow for discontinuation of cART are under investigation via our most relevant pre-clinical model, the non-human primate.
Reservoir Elimination-Strategies for a Cure
To date, stem cell transplantation is the only cure strategy that resulted in a “traditional” cure in both NHPs and humans. The first documented case of sustained HIV remission, known as the “Berlin patient,” occurred after two allogenic haemopoietic stem-cell transplantation (HSCT) procedures were performed to treat acute myeloid leukemia (Hütter et al. 2009). The stem-cell donor contained a naturally occurring homozygous mutation in HIV coreceptor CCR5 (CCR5Δ32/Δ32), rendering these cells resistant to HIV variants that utilize this receptor for cellular entry (Gupta et al. 2019). Accompanied with chemotherapy and irradiation, the viral myeloid reservoir of the patient was cleared and repopulated with progenitor cells immune to infection by the clade of HIV infecting him. While this has been repeated in similar case studies (Lambros et al. 2014; Verheyen et al. 2019), pre-existing CXCR4 variants not found in the Berlin patient were able to infect the naïve CCR5Δ32/Δ32 donor cells and result in viral rebound. CCR5 and CXCR4 receptor density on the surface of haemopoietic stem cells has been shown to be downregulated by the use of gene editing technology such as CRISPR-Cas9 before transplantation in rhesus monkeys, though expression is limited in vivo (Yu et al. 2020). While these results suggest that stem cell transplantation in combination with gene editing may be a promising approach for treating HIV-1 in humans, the invasiveness of the procedure and expenditure limit its feasibility.
Three central mechanisms behind contemporary HIV-1 cure strategies are aimed at eliminating viral tissue reservoirs and function by (i) administering latency reversing agents (LRAs) to induce HIV transcription within latently infected cells, leading to cytolysis or immune-mediated clearance (“shock and kill”) (Kim et al. 2018; McBrien et al. 2020), (ii) preventing the active transcription of virus within latently infected cells and enhancing the latency of the HIV promoter via epigenetic modifications (“block and lock”) (Ahlenstiel et al. 2020), or (iii) removing the integrated viral genome within latently infected cells by gene editing techniques (Mancuso et al. 2020; Busman-Sahay et al. 2021). These are outlined in the figure.
Protein kinase C agonists, contemporary LRAs, were stringently developed with the use the NHP model (Jiang et al. 2009; Jiang and Dandekar 2015). The “shock and kill” approach via new-generation LRA therapy exhibited successful reactivation of the latent viral genome when tested in clinical trials (Elliott et al. 2015; Archin et al. 2017) but clearance of reactivated cells was shown to be limited. Additionally, it is critical in this line of therapy to minimize the depletion of non-infected cells to reduce adverse outcomes. To encourage the selective clearance of infected cells, researcher prime cells with a pro-apoptotic compound before administering an LRA, driving cells to undergo apoptosis if HIV proteins are expressed (Cummins et al. 2016; Sivanandham et al. 2020). While this method successfully depletes a substantial portion of infected T-cells, in and outside of reservoirs, side effects and signs of drug toxicity when in combination with ART during NHP trials preclude its clinical use (Zerbato et al. 2016; Bricker et al. 2021).
Alternative challenges are faced in the “block and lock” strategy as reservoirs remain within cells and tissues and have the potential to continue exerting genomic influence. By administering latency promoting agents (LPAs) to inhibit or “block” viral transcription and using repressive epigenetic modifications to “lock” the promoter in latency, this tactic is aimed at permanently silencing the expression of the latent viral reservoir (Ahlenstiel et al. 2020). Although the majority of preclinical testing has been performed in humanized mouse models, the first NHP study implementing the “block and lock” strategy demonstrated potent inhibition of SIV reactivation from latently infected CD4 + T-cells ex vivo (Mediouni et al. 2019); however, efficacious delivery to deep tissue reservoirs has yet to progress (Fig. 1).
Fig. 1.
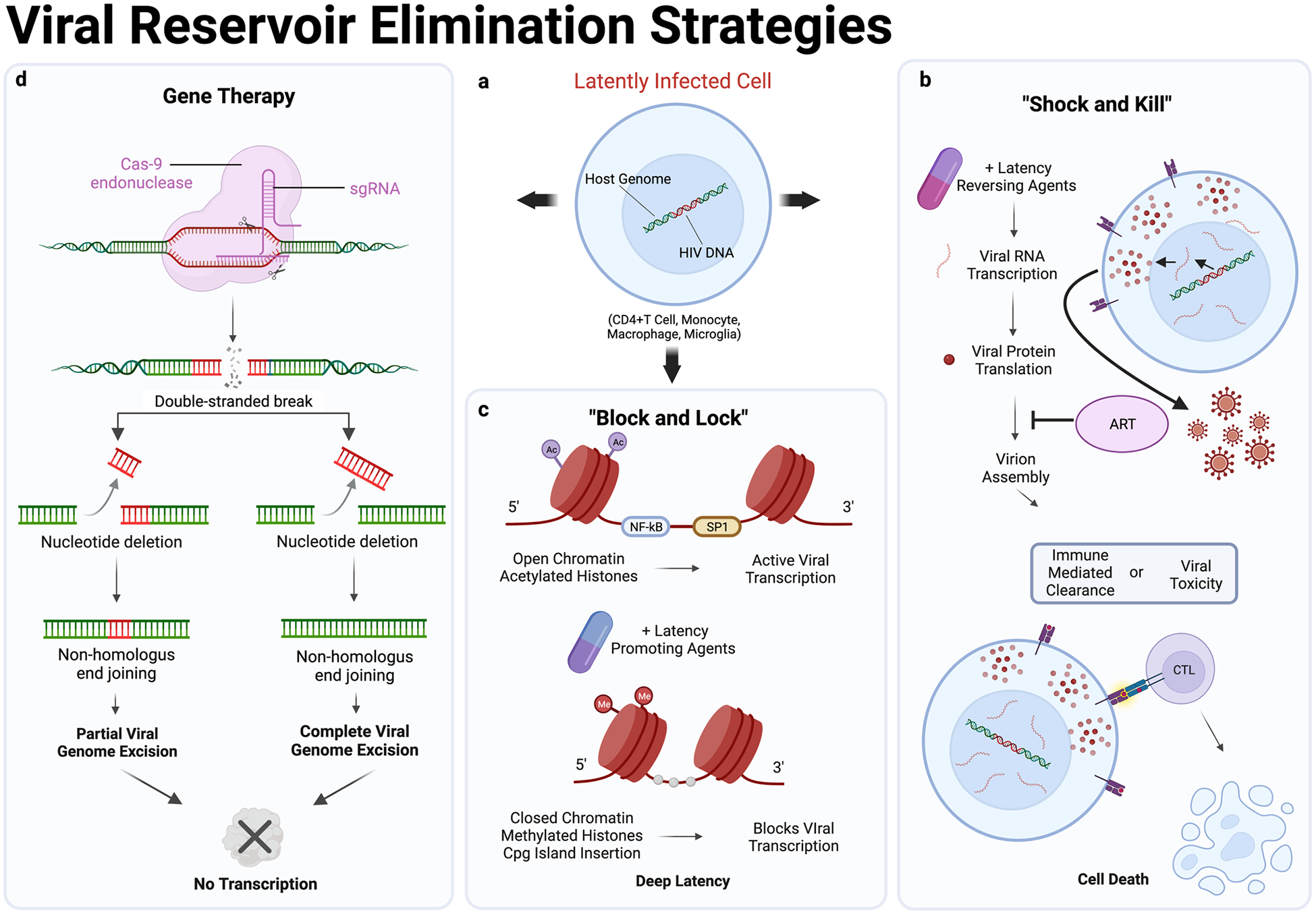
HIV reservoir elimination strategies. a A latently infected CD4 + T cell, monocyte, macrophage, or microglia with integrated HIV DNA within the host genome of the nucleus (Estes et al. 2017). b The “shock and kill” approach of HIV reservoir elimination by which LRAs are administered to encourage viral RNA transcription and viral protein translation within latently infected cells. Virion assembly is blocked by ART which prevents propagating infection to naïve cells. Rampant viral protein expression within the cytosol generates a toxic cellular environment leading to death by apoptosis or major histocompatibility complex I (MHCI) presentation of viral proteins promoting immune mediated clearance of infected cells by cytotoxic lymphocytes (Bricker et al. 2021). c Active HIV transcription occurs when the histone is acetylated and in the open conformation, allowing RNA polymerase II to bind to viral transcription factors such as NFkB and Sp1 (Ilyinskii et al. 1997; Heusinger and Kirchhoff 2017). The “block and lock” approach aims to regulate the transcriptional activity of HIV through histone methylation or cpg island insertion, which acts to close the histone and provide steric hindrance in the binding of RNA polymerase II (Crise et al. 2005). These histone alterations prevent viral transcription and drive the viral genome into a deep state of latency. d CRISPR Cas9, a gene therapy technique to excise the viral genome from latently infected cells, employs single-guide RNAs (sgRNAs) to direct the Cas9 endonuclease to Gag and long-tandem repeat (LTR) regions of the HIV genome. Successful editing of integrated viral DNA by Cas9 results in either the removal of the full-length viral genome or a transcription defective proviral DNA sequence that lacks regions between the 5′LTR to Gag or Gag to 3′LTR (Mancuso et al. 2020)
Gene therapy approaches to silence the transcription of viral DNA or prevent further infection by host cell receptor modification have been tested to elicit a “functional cure” for PLWH. For example, RNA interference through the administration of short hairpin RNAs (shRNA) targeting viral genome segments can effectively inhibit HIV replication in humans (Sugiyama et al. 2009). However, this inhibition through specific shRNA binding often induces escape mutations within the targeted gene, eventually leading to loss of efficacy. Another gene therapy approach utilizing zinc finger nuclease-based disruption of CCR5 of hematopoietic stem/progenitor cells (HSPCs) was tested in NHPs and was shown to reduce the viral load in animals following autologous transplantation (Peterson et al. 2018). This engraftment of SIV-resistant cells significantly reduced the size of the peripheral reservoir and viral circulating RNA levels, but would be a costly and invasive undertaking in the human population.
An intriguing and novel approach utilizes a recombinant adeno-associated virus (rAAV) to deliver gene therapies to prevent HIV infection by blocking cellular entry. Intramuscular injection induces expression and secretion of eCD4-Ig, a fusion antibody with high env specificity. This causes premature structural changes of gp120, effectively neutralizing circulating virus (Gardner et al. 2015).
Arguably, the most progressive system currently under investigation for the complete removal of the HIV viral reservoir is the use of clustered regulatory interspaced short palindromic repeats (CRISPR) in combination with recombinant enzymes to target and excise viral DNA. Delivered to tissues by rAAV serotype 9, this vector expresses Cas9 endonuclease and several guide RNAs (gRNAs) specific to regions of the viral genome to successfully cleave integrated proviral DNA from infected cells of rhesus macaques (Mancuso et al. 2020). While excision efficacy was as high as 95% following the single-dose CRISPR treatment, indicating robust viral particle binding, consistent expression throughout tissues has yet to be accomplished. Concerns regarding the use of recombinant adenovirus vectors as drug delivery vehicles include issues with preexisting vector-specific immunity and a perceived inability to boost by reimmunization. Antibodies generated against the capsid of rAAV9 is an expected outcome following inoculation which could lead to premature neutralization when a secondary immune response is elicited. Phase I clinical trials are currently underway to test this one-time injection therapy in humans which represents an exciting step forward in the realm of HIV therapeutic capability (Excision BioTherapeutics 2022).
The Elusive Vaccine
Though the development of an effective HIV-1 vaccine remains an important goal for researchers, difficulties in eliciting active immunity against the protein of interest, the envelope protein (env), had led scientists to explore passive immunization strategies. Characteristics such as high env mutability (Van Duyne et al. 2019), low env protein trimer density on the surface of the virion (Carlon-Andres et al. 2021), and the protective glycan shield present on env promote viral immune evasion and resist antigenic neutralization (Wei et al. 2003). Additionally, HIV-1 envelope proteins of prominent circulating strains do not typically bind to the inferred germline precursors responsible for broadly neutralizing antibody (bNAb) production (Xiao et al. 2009), necessitating artificial delivery (Welles et al. 2018) or stimulation for assembly. Because of their complex B cell antibody repertoire, NHPs have been used in the development of bNAb vaccination strategies, resulting in several promising candidates aimed at inducing heterologous immunity (Sharma and Thomas 2014). Techniques under investigation such as the stimulation of B cell germlines to produce bNAbs act by binding to multiple sites of the HIV envelope to prevent cellular entry and induce FC-mediated immune response mechanisms leading to infected cell death (Liu et al. 2020). One such vaccine, called ALVAC, was tested in a NHP model and was found to protect macaques from low-dose challenges of highly pathogenic SIV strains, but demonstrated low efficacy overall (44%) in preventing infection (Silva de Castro et al. 2020). Similar results were seen when tested in a randomized human trial of this vaccine, demonstrating a trend toward the prevention of HIV-1 infection (26.4%) with no effect on the viral load or CD4 + T cell count in infected subjects (Rerks-Ngarm et al. 2009). Sequential immunization to induce the production of multiple heterologous bNAbs improved neutralization titers of this vaccine candidate in NHPs but did not prevent SHIV infection when challenged (Barnes and Schoofs 2022). While these studies exhibit potential in continuing the development of bNAb strategies, problems such as off-target antibody production as well as partial clade protection are barriers to realization.
Conclusion
In conclusion, the use of NHPs in HIV-1 research has been critical in the study of the virus and has led to numerous discoveries and advances in the development of new treatments and cure strategies. NHPs offer several advantages over other animal models, including their close genetic similarity to humans and the ability to study the virus in an immunocompetent host. However, the use of NHPs poses several challenges, including their high cost and the difficulty in maintaining and breeding them. Nevertheless, the use of NHPs has enabled researchers to gain valuable insights into the biology of HIV-1 and has allowed for the development of targeted therapies and treatments, as well as novel therapies that could potentially lead to a cure for HIV-1.
Funding
NHP studies in the MacLean Lab are currently supported by R21-MH113517, R01-HL152804, R01-NS104016, R21-MH125716, U42OD024282, U42OD010568, and the TNPRC base grant P51-OD11104.
Footnotes
Conflict of interest The authors declare no conflicts of interest.
References
- Ahlenstiel CL, Symonds G, Kent SJ, Kelleher AD (2020) Block and lock HIV cure strategies to control the latent reservoir. Front Cell Infect Microbiol 10. Available at 10.3389/fcimb.2020.00424 [Accessed December 20, 2022] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn K, Lee SJ, Mook-Jung I (2022) White matter-associated microglia: new players in brain aging and neurodegenerative diseases. Ageing Res Rev 75:101574. 10.1016/j.arr.2022.101574 [DOI] [PubMed] [Google Scholar]
- Alkhatib G, Combadiere C, Broder CC, Feng Y, Kennedy PE, Murphy PM et al. (1996) CC CKR5: a RANTES, MIP-1alpha, MIP-1beta receptor as a fusion cofactor for macrophage-tropic HIV-1. Science 272:1955–1958. 10.1126/science.272.5270.1955 [DOI] [PubMed] [Google Scholar]
- Alzahrani J, Hussain T, Simar D, Palchaudhuri R, Abdel-Mohsen M, Crowe SM et al. (2019) Inflammatory and immunometabolic consequences of gut dysfunction in HIV: parallels with IBD and implications for reservoir persistence and non-AIDS comorbidities. EBioMedicine 46:522–531. 10.1016/j.ebiom.2019.07.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M et al. (2007) Updated research nosology for HIV-associated neurocognitive disorders. Neurology 69:1789–1799. 10.1212/01.WNL.0000287431.88658.8b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antiretroviral Drug Discovery and Development | NIH: National Institute of Allergy and Infectious Diseases (2023). Available at https://www.niaid.nih.gov/diseases-conditions/antiretroviral-drug-development [Accessed May 12, 2023].
- Archin NM, Kirchherr JL, Sung JAM, Clutton G, Sholtis K, Xu Y et al. (2017) Interval dosing with the HDAC inhibitor vorinostat effectively reverses HIV latency. J Clin Invest 127:3126–3135. 10.1172/JCI92684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ash MK, Al-Harthi L, Schneider JR (2021) HIV in the brain: identifying viral reservoirs and addressing the challenges of an HIV cure. Vaccines 9:867. 10.3390/vaccines9080867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailon L, Mothe B, Berman L, Brander C (2020) Novel approaches towards a functional cure of HIV/AIDS. Drugs 80:859–868. 10.1007/s40265-020-01322-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar KJ, Coronado E, Hensley-McBain T, O’Connor MA, Osborn JM, Miller C et al. (2019) Simian-human immunodeficiency virus SHIV.CH505 infection of rhesus macaques results in persistent viral replication and induces intestinal immunopathology. J Virol 93:e00372–e419. 10.1128/JVI.00372-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes CB, Schoofs T (2022) Sequential immunization of macaques elicits heterologous neutralizing antibodies targeting the V3-glycan patch of HIV-1 Env. 10.1126/scitranslmed.abk1533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck SE, Queen SE, Metcalf Pate KA, Mangus LM, Abreu CM, Gama L et al. (2018) An SIV/macaque model targeted to study HIV-associated neurocognitive disorders. J Neurovirol 24:204–212. 10.1007/s13365-017-0582-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell JE (1998) The neuropathology of adult HIV infection. Rev Neurol (paris) 154:816–829 [PubMed] [Google Scholar]
- Bimber BN, Ramakrishnan R, Cervera-Juanes R, Madhira R, Peterson SM, Norgren RB et al. (2017) Whole genome sequencing predicts novel human disease models in rhesus macaques. Genomics 109:214–220. 10.1016/j.ygeno.2017.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blassel L, Zhukova A, Villabona-Arenas CJ, Atkins KE, Hué S, Gascuel O (2021) Drug resistance mutations in HIV: new bioinformatics approaches and challenges. Curr Opin Virol 51. 10.1016/j.coviro.2021.09.009 [DOI] [PubMed] [Google Scholar]
- Bleul CC, Wu L, Hoxie JA, Springer TA, Mackay CR (1997) The HIV coreceptors CXCR4 and CCR5 are differentially expressed and regulated on human T lymphocytes. Proc Natl Acad Sci 94:1925–1930. 10.1073/pnas.94.5.1925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrajo A, Spuch C, Penedo MA, Olivares JM, Agís-Balboa RC (2020) Important role of microglia in HIV-1 associated neurocognitive disorders and the molecular pathways implicated in its pathogenesis. Ann Med 53:43–69. 10.1080/07853890.2020.1814962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bricker KM, Chahroudi A, Mavigner M (2021) New latency reversing agents for HIV-1 cure: insights from nonhuman primate models. Viruses 13:1560. 10.3390/v13081560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdo TH, Soulas C, Orzechowski K, Button J, Krishnan A, Sugimoto C et al. (2010) Increased monocyte turnover from bone marrow correlates with severity of SIV encephalitis and CD163 levels in plasma. PLoS Pathog 6:e1000842. 10.1371/journal.ppat.1000842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busman-Sahay K, Starke CE, Nekorchuk MD, Estes JD (2021) Eliminating HIV reservoirs for a cure: the issue is in the tissue. Curr Opin HIV AIDS 16:200–208. 10.1097/COH.0000000000000688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlon-Andres I, Malinauskas T, Padilla-Parra S (2021) Structure dynamics of HIV-1 Env trimers on native virions engaged with living T cells. Commun Biol 4:1–14. 10.1038/s42003-021-02658-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang WLW, Gonzalez DF, Kieu HT, Castillo LD, Messaoudi I, Shen X et al. (2017) Changes in circulating B cell subsets associated with aging and acute SIV infection in rhesus macaques. PloS One 12:e0170154. 10.1371/journal.pone.0170154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chawla A, Wang C, Patton C, Murray M, Punekar Y, de Ruiter A et al. (2018) A review of long-term toxicity of antiretroviral treatment regimens and implications for an aging population. Infect Dis Ther 7. 10.1007/s40121-018-0201-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicconi P, Cozzi-Lepri A, Castagna A, Trecarichi EM, Antinori A, Gatti F et al. (2010) Insights into reasons for discontinuation according to year of starting first regimen of highly active antiretroviral therapy in a cohort of antiretroviral-naïve patients. HIV Med 11:104–113. 10.1111/j.1468-1293.2009.00750.x [DOI] [PubMed] [Google Scholar]
- Clifford DB, Ances BM (2013) HIV-associated neurocognitive disorder (HAND). Lancet Infect Dis 13:976–986. 10.1016/S1473-3099(13)70269-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier AC, Coombs RW, Schoenfeld DA, Bassett R, Baruch A, Corey L (1996) Combination therapy with zidovudine, didanosine and saquinavir. Antiviral Res 29:99. 10.1016/0166-3542(95)00928-0 [DOI] [PubMed] [Google Scholar]
- Crise B, Li Y, Yuan C, Morcock DR, Whitby D, Munroe DJ et al. (2005) Simian immunodeficiency virus integration preference is similar to that of human immunodeficiency virus type 1. J Virol 79:12199–12204. 10.1128/JVI.79.19.12199-12204.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummins NW, Sainski AM, Dai H, Natesampillai S, Pang Y-P, Bren GD et al. (2016) Prime, shock, and kill: priming CD4 T cells from HIV patients with a BCL-2 antagonist before HIV reactivation reduces HIV reservoir size. J Virol 90:4032–4048. 10.1128/JVI.03179-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel M, King N, Letvin N, Hunt R, Seghal P, Desrosiers R (1984). A New Type D Retrovirus Isolated from Macaques with an Immunodeficiency Syndrome. 10.1126/science.6695172 [DOI] [PubMed] [Google Scholar]
- Delery EC, MacLean AG (2019) Chronic viral neuroinflammation: speculation on underlying mechanisms. Viral Immunol 32:55–62. 10.1089/vim.2018.0093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng H, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M et al. (1996) Identification of a major co-receptor for primary isolates of HIV-1. Nature 381:661–666. 10.1038/381661a0 [DOI] [PubMed] [Google Scholar]
- Didier ES, MacLean AG, Mohan M, Didier PJ, Lackner AA, Kuroda MJ (2016) Contributions of nonhuman primates to research on aging. Vet Pathol 53:277–290. 10.1177/0300985815622974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dock JN, Effros RB (2011) Role of CD8 T cell replicative senescence in human aging and in HIV-mediated immunosenescence. Aging Dis 2:382–397 [PMC free article] [PubMed] [Google Scholar]
- Dos Santos SE, Medeiros M, Porfirio J, Tavares W, Pessôa L, Grinberg L et al. (2020) Similar microglial cell densities across brain structures and mammalian species: implications for brain tissue function. J Neurosci 40:4622–4643. 10.1523/JNEUROSCI.2339-19.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragic T, Litwin V, Allaway GP, Martin SR, Huang Y, Nagashima KA et al. (1996) HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature 381:667–673. 10.1038/381667a0 [DOI] [PubMed] [Google Scholar]
- Eggers C, Arendt G, Hahn K, Husstedt IW, Maschke M, Neuen-Jacob E et al. (2017) HIV-1-associated neurocognitive disorder: epidemiology, pathogenesis, diagnosis, and treatment. J Neurol 264. 10.1007/s00415-017-8503-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott JH, McMahon JH, Chang CC, Lee SA, Hartogensis W, Bumpus N et al. (2015) Short-term administration of disulfiram for reversal of latent HIV infection: a phase 2 dose-escalation study. Lancet HIV 2:e520–e529. 10.1016/S2352-3018(15)00226-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sadr WM, Lundgren JD, Neaton JD, Abrams D, Arduino RC (2006) CD4+ count–guided interruption of antiretroviral treatment. N Engl J Med 355. 10.1056/NEJMoa062360 [DOI] [PubMed] [Google Scholar]
- Estes JD, Kityo C, Ssali F, Swainson L, Makamdop KN, Del Prete GQ et al. (2017) Defining total-body AIDS-virus burden with implications for curative strategies. Nat Med 23:1271–1276. 10.1038/nm.4411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes JD, Wong SW, Brenchley JM (2018) Nonhuman primate models of human viral infections. Nat Rev Immunol 18:390–404. 10.1038/s41577-018-0005-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eugenin EA, Osiecki K, Lopez L, Goldstein H, Calderon TM, Berman JW (2006) CCL2/monocyte chemoattractant protein-1 mediates enhanced transmigration of human immunodeficiency virus (HIV)-infected Leukocytes across the blood–brain barrier: a potential mechanism of HIV–CNS invasion and NeuroAIDS. J Neurosci 26:1098–1106. 10.1523/JNEUROSCI.3863-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Excision BioTherapeutics (2022) A phase 1/2a, sequential cohort, single ascending dose study of the safety, tolerability, biodistribution, and pharmacodynamics of EBT 101 in aviremic HIV-1 infected adults on stable antiretroviral therapy. clinicaltrials.gov Available at https://clinicaltrials.gov/ct2/show/NCT05144386 [Accessed January 11, 2023]. [Google Scholar]
- Ferrucci L, Fabbri E (2018) Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat Rev Cardiol 15:505–522. 10.1038/s41569-018-0064-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl MA, Richman DD, Grieco MH, Gottlieb MS, Volberding PA, Laskin OL et al. (1987) The efficacy of azidothymidine (AZT) in the treatment of patients with AIDS and AIDS-related complex. A double-blind, placebo-controlled trial. N Engl J Med 317:185–191. 10.1056/NEJM198707233170401 [DOI] [PubMed] [Google Scholar]
- Gardner MR, Kattenhorn LM, Kondur HR, von Schaewen M, Dorfman T, Chiang JJ et al. (2015) AAV-expressed eCD4-Ig provides durable protection from multiple SHIV challenges. Nature 519:87–91. 10.1038/nature14264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh AK, Sarkar A, Mitsuya H (2017) HIV-associated neurocognitive disorder (HAND) and the prospect of brain-penetrating protease inhibitors for antiretroviral treatment. Med Res Arch 5:1113. [PMC free article] [PubMed] [Google Scholar]
- Global HIV & AIDS statistics — Fact sheet (2023) Available at https://www.unaids.org/en/resources/fact-sheet [Accessed December 15, 2022]
- Guaraldi G, Orlando G, Zona S, Menozzi M, Carli F, Garlassi E et al. (2011) Premature age-related comorbidities among HIV-infected persons compared with the general population. Clin Infect Dis off Publ Infect Dis Soc Am 53:1120–1126. 10.1093/cid/cir627 [DOI] [PubMed] [Google Scholar]
- Gupta RK, Abdul-Jawad S, McCoy LE, Mok HP, Peppa D, Salgado M et al. (2019) HIV-1 remission following CCR5Δ32/Δ32 haematopoietic stem-cell transplantation. Nature 568:244–248. 10.1038/s41586-019-1027-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatziioannou T, Evans DT (2012) Animal models for HIV/AIDS research. Nat Rev Microbiol 10:852–867. 10.1038/nrmicro2911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Clifford DB, Franklin DR, Woods SP, Ake C, Vaida F et al. (2010) HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER study. Neurology 75:2087–2096. 10.1212/WNL.0b013 e318200d727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heigele A, Joas S, Regensburger K, Kirchhoff F (2015) Increased susceptibility of CD4+ T cells from elderly individuals to HIV-1 infection and apoptosis is associated with reduced CD4 and enhanced CXCR4 and FAS surface expression levels. Retrovirology 12:86. 10.1186/s12977-015-0213-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heusinger E, Kirchhoff F (2017) Primate lentiviruses modulate NF-κB activity by multiple mechanisms to fine-tune viral and cellular gene expression. Front Microbiol 8. Available at 10.3389/fmicb.2017.00198 [Accessed January 17, 2023] [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIV clinical guidelines: Adult and adolescent ARV - what’s new in the guidelines | Clinicalinfo.HIV.gov (2023) Available at https://clinicalinfo.hiv.gov/en/guidelines/hiv-clinical-guidelines-adult-and-adolescent-arv/whats-new [Accessed May 12, 2023].
- Holkmann Olsen C, Mocroft A, Kirk O, Vella S, Blaxhult A, Clumeck N et al. (2007) Interruption of combination antiretroviral therapy and risk of clinical disease progression to AIDS or death. HIV Med 8:96–104. 10.1111/j.1468-1293.2007.00436.x [DOI] [PubMed] [Google Scholar]
- Humes D, Emery S, Laws E, Overbaugh J (2012) A species-specific amino acid difference in the macaque CD4 receptor restricts replication by global circulating HIV-1 variants representing viruses from recent infection. J Virol 86:12472–12483. 10.1128/JVI.02176-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hütter G, Ganepola S, Schneider T, Blau O, Thiel E (2009) Long-term control of HIV by CCR5 Delta32/Delta32 stem-cell transplantation. N Engl J Med [DOI] [PubMed] [Google Scholar]
- Ilyinskii PO, Simon MA, Czajak SC, Lackner AA, Desrosiers RC (1997) Induction of AIDS by simian immunodeficiency virus lacking NF-kappaB and SP1 binding elements. J Virol 71:1880–1887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang G, Dandekar S (2015) Targeting NF-κB signaling with protein kinase C agonists as an emerging strategy for combating HIV latency. AIDS Res Hum Retroviruses 31:4–12. 10.1089/AID.2014.0199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Tian B, Saifuddin M, Agy MB, Emau P, Cairns JS et al. (2009) RT-SHIV, an infectious CCR5-tropic chimeric virus suitable for evaluating HIV reverse transcriptase inhibitors in macaque models. AIDS Res Ther 6:23. 10.1186/1742-6405-6-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joag SV, Li Z, Foresman L, Stephens EB, Zhao LJ, Adany I et al. (1996) Chimeric simian/human immunodeficiency virus that causes progressive loss of CD4+ T cells and AIDS in pig-tailed macaques. J Virol 70. 10.1128/JVI.70.5.3189-3197.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, Anderson JL, Lewin SR (2018) Getting the “kill” into “shock and kill”: strategies to eliminate latent HIV. Cell Host Microbe 23:14–26. 10.1016/j.chom.2017.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschner DE, Webb GF (1997) Understanding drug resistance for monotherapy treatment of HIV infection. Bull Math Biol 59:763–785. 10.1007/BF02458429 [DOI] [PubMed] [Google Scholar]
- Kovalevich J, Langford D (2012) Neuronal toxicity in HIV CNS disease. Future Virol 7:687–698. 10.2217/fvl.12.57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan TH, Chan CP, Wong NS, Lee SS (2022) Awareness of HIV functional cure and willingness in participating in related clinical trials: comparison between antiretroviral naïve and experienced men who have sex with men living with HIV. BMC Infect Dis 22:383. 10.1186/s12879-022-07346-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambros K, Jens V, Stefan E (2014) Shift of HIV tropism in stem-cell transplantation with CCR5 Delta32 mutation. N Engl J Med 371. 10.1056/NEJMc1405805 [DOI] [PubMed] [Google Scholar]
- Lepri AC, Sabin CA, Staszewski S, Hertogs K, Müller A, Rabenau H et al. (2000) Resistance profiles in patients with viral rebound on potent antiretroviral therapy. J Infect Dis 181:1143–1147. 10.1086/315301 [DOI] [PubMed] [Google Scholar]
- Li H, Wang S, Lee F-H, Roark RS, Murphy AI, Smith J et al. (2021) New SHIVs and improved design strategy for modeling HIV-1 transmission, immunopathogenesis, prevention, and cure. J Virol 95:e00071–e121. 10.1128/JVI.00071-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Cao W, Sun M, Li T (2020) Broadly neutralizing antibodies for HIV-1: efficacies, challenges and opportunities. Emerg Microbes Infect 9:194–206. 10.1080/22221751.2020.1713707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean AG, Rasmussen TA, Bieniemy D, Lackner AA (2004) Activation of the blood-brain barrier by SIV (simian immunodeficiency virus) requires cell-associated virus and is not restricted to endothelial cell activation. Biochem Soc Trans 32:750–752. 10.1042/BST0320750 [DOI] [PubMed] [Google Scholar]
- Magness CL, Fellin PC, Thomas MJ, Korth MJ, Agy MB, Proll SC et al. (2005) Analysis of the Macaca mulatta transcriptome and the sequence divergence between Macaca and human. Genome Biol. 6. 10.1186/gb-2005-6-7-r60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallard J, Williams K (2018) An SIV macaque model of SIV and HAND: the need for adjunctive therapies in HIV that target activated monocytes and macrophages. J Neurovirol 24:213–219. 10.1007/s13365-018-0616-6 [DOI] [PubMed] [Google Scholar]
- Mamik MK, Asahchop EL, Chan WF, Zhu Y, Branton WG, McKenzie BA et al. (2016) Insulin treatment prevents neuroinflammation and neuronal injury with restored neurobehavioral function in models of HIV/AIDS neurodegeneration. J Neurosci off J Soc Neurosci 36:10683–10695. 10.1523/JNEUROSCI.1287-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancuso P, Chen C, Kaminski R, Gordon J, Liao S, Robinson JA et al. (2020) CRISPR based editing of SIV proviral DNA in ART treated non-human primates. Nat Commun 11:6065. 10.1038/s41467-020-19821-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattison JA, Vaughan KL (2017) An overview of nonhuman primates in aging research. Exp Gerontol 94:41. 10.1016/j.exger.2016.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBrien JB, Wong AKH, White E, Carnathan DG, Lee JH, Safrit JT et al. (2020) Combination of CD8β depletion and interleukin-15 superagonist N-803 induces virus reactivation in simian-human immunodeficiency virus-infected, long-term ART-treated rhesus macaques. J Virol 94:e00755–e820. 10.1128/JVI.00755-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mediouni S, Kessing CF, Jablonski JA, Thenin-Houssier S, Clementz M, Kovach MD et al. (2019) The Tat inhibitor didehydro-cortistatin A suppresses SIV replication and reactivation. FASEB. J off Publ Fed Am Soc Exp Biol 33:8280–8293. 10.1096/fj.201801165R [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meir-Shafrir K, Pollack S (2012) Accelerated aging in HIV patients. Rambam Maimonides Med J 3:e0025. 10.5041/RMMJ.10089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng TC, Fischl MA, Boota AM, Spector SA, Bennett D, Bassiakos Y et al. (1992) Combination therapy with zidovudine and dideoxycytidine in patients with advanced human immunodeficiency virus infection. A phase I/II study. Ann Intern Med 116:13–20. 10.7326/0003-4819-116-1-13 [DOI] [PubMed] [Google Scholar]
- Mittelbronn M, Dietz K, Schluesener HJ, Meyermann R (2001) Local distribution of microglia in the normal adult human central nervous system differs by up to one order of magnitude. Acta Neuropathol (berl) 101:249–255. 10.1007/s004010000284 [DOI] [PubMed] [Google Scholar]
- Moretti S, Virtuoso S, Sernicola L, Farcomeni S, Maggiorella MT, Borsetti A (2021) Advances in SIV/SHIV non-human primate models of NeuroAIDS. Pathogens 10:1018. 10.3390/pathogens10081018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naif HM (2013) Pathogenesis of HIV infection. Infect Dis Rep 5. 10.4081/idr.2013.s1.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navia BA, Jordan BD, Price RW (1986) The AIDS dementia complex: I. Clinical Features Ann Neurol 19:517–524. 10.1002/ana.410190602 [DOI] [PubMed] [Google Scholar]
- Okoye AA, Hansen SG, Vaidya M, Fukazawa Y, Park H, Duell DM et al. (2018) Early antiretroviral therapy limits SIV reservoir establishment to delay or prevent post-treatment viral rebound. Nat Med 24:1430–1440. 10.1038/s41591-018-0130-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez S, Johnson A-M, Xiang S, Li J, Foley BT, Doyle-Meyers L et al. (2018) Persistence of SIV in the brain of SIV-infected Chinese rhesus macaques with or without antiretroviral therapy. J Neurovirol 24:62–74. 10.1007/s13365-017-0594-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson CW, Wang J, Deleage C, Reddy S, Kaur J, Polacino P et al. (2018) Differential impact of transplantation on peripheral and tissue-associated viral reservoirs: implications for HIV gene therapy. PLoS Pathog 14:e1006956. 10.1371/journal.ppat.1006956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, Paris R et al. (2009) Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med 361:2209–2220. 10.1056/NEJMoa0908492 [DOI] [PubMed] [Google Scholar]
- Robertson KR, Smurzynski M, Parsons TD, Wu K, Bosch RJ, Wu J et al. (2007) The prevalence and incidence of neurocognitive impairment in the HAART era. AIDS Lond Engl 21:1915–1921. 10.1097/QAD.0b013e32828e4e27 [DOI] [PubMed] [Google Scholar]
- Rumbaugh JA, Tyor W (2015) HIV-Associated Neurocognitive Disorders NeurolClin Pract 5:224–231. 10.1212/CPJ.0000000000000117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saylor D, Dickens AM, Sacktor N, Haughey N, Slusher B, Pletnikov M et al. (2016) HIV-associated neurocognitive disorder — pathogenesis and prospects for treatment. Nat Rev Neurol 12:234–248. 10.1038/nrneurol.2016.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarlatti G, Tresoldi E, Björndal A, Fredriksson R, Colognesi C, Deng HK et al. (1997) In vivo evolution of HIV-1 co-receptor usage and sensitivity to chemokine-mediated suppression. Nat Med 3:1259–1265. 10.1038/nm1197-1259 [DOI] [PubMed] [Google Scholar]
- Seitz R (2016) Human immunodeficiency virus (HIV). Transfus Med Hemotherapy 43:203–222. 10.1159/000445852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S, Thomas PG (2014) The two faces of heterologous immunity: protection or immunopathology. J Leukoc Biol 95:405–416. 10.1189/jlb.0713386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siliciano RF, Greene WC (2011) HIV latency. Cold Spring Harb Perspect Med 1:a007096. 10.1101/cshperspect.a007096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva de Castro I, Gordon SN, Liu J, Bissa M, McKinnon K, Trinh HV et al. (2020) Expression of CD40L by the ALVAC-simian immunodeficiency virus vector abrogates T cell responses in macaques. J Virol 94:e01933–e2019. 10.1128/JVI.01933-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivanandham R, Kleinman AJ, Sette P, Brocca-Cofano E, Kilapandal Venkatraman SM, Policicchio BB et al. (2020) Nonhuman primate testing of the impact of different regulatory T cell depletion strategies on reactivation and clearance of latent simian immunodeficiency virus. J Virol 94:e00533–e620. 10.1128/JVI.00533-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone WH, Treichel RC, VandeBerg JL (1987) Genetic significance of some common primate models in biomedical research. Prog Clin Biol Res 229:73–93 [PubMed] [Google Scholar]
- Sugiyama R, Habu Y, Ohnari A, Miyano-Kurosaki N, Takaku H (2009) RNA interference targeted to the conserved dimerization initiation site (DIS) of HIV-1 restricts virus escape mutation. J Biochem (tokyo) 146:481–489. 10.1093/jb/mvp093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun T, Hevner RF (2014) Growth and folding of the mammalian cerebral cortex: from molecules to malformations. Nat Rev Neurosci 15:217–232. 10.1038/nrn3707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thippeshappa R, Kimata JT, Kaushal D (2020) Toward a macaque model of HIV-1 infection: roadblocks, progress, and future strategies. Front Microbiol 11. Available at 10.3389/fmicb.2020.00882 [Accessed January 7, 2023]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai CC, Follis KE, Benveniste RE (1988) Antiviral effects of 3’-azido-3’deoxythymidine, 2’,3’-dideoxycytidine, and 2’,3’-dideoxyadenosine against simian acquired immunodeficiency syndrome-associated type D retrovirus in vitro. AIDS Res Hum Retroviruses 4:359–368. 10.1089/aid.1988.4.359 [DOI] [PubMed] [Google Scholar]
- Tseng A, Seet J, Phillips E (2015) The evolution of three decades of antiretroviral therapy: challenges, triumphs and the promise of the future. Available at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4309625/ [DOI] [PMC free article] [PubMed]
- Valcour V, Chalermchai T, Sailasuta N, Marovich M, Lerdlum S, Suttichom D et al. (2012) Central nervous system viral invasion and inflammation during acute HIV infection. J Infect Dis 206:275–282. 10.1093/infdis/jis326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valcour V, Yee P, Williams AE, Shiramizu B, Watters M, Selnes O et al. (2006) Lowest ever CD4 lymphocyte count (CD4 nadir) as a predictor of current cognitive and neurological status in human immunodeficiency virus type 1 infection–the Hawaii Aging with HIV Cohort. J Neurovirol 12:387–391. 10.1080/13550280600915339 [DOI] [PubMed] [Google Scholar]
- Vallender EJ, Miller GM (2013) Nonhuman primate models in the genomic era: a paradigm shift. ILAR J 54:154–165. 10.1093/ilar/ilt044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Duyne R, Kuo LS, Pham P, Fujii K, Freed EO (2019) Mutations in the HIV-1 envelope glycoprotein can broadly rescue blocks at multiple steps in the virus replication cycle. Proc Natl Acad Sci 116:9040–9049. 10.1073/pnas.1820333116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veazey RS, DeMaria M, Chalifoux LV, Shvetz DE, Pauley DR, Knight HL et al. (1998) Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science 280:427–431. 10.1126/science.280.5362.427 [DOI] [PubMed] [Google Scholar]
- Veazey RS, Lackner AA (2017) Nonhuman primate models and understanding the pathogenesis of HIV infection and AIDS. ILAR J 58:160–171. 10.1093/ilar/ilx032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verheyen J, Thielen A, Lubke N, Dirks M, Widera M, Dittmer U et al. (2019) Rapid rebound of a preexisting CXCR4-tropic human immunodeficiency virus variant after allogeneic transplantation with CCR5 Δ32 homozygous stem cells. 10.1093/cid/ciy565 [DOI] [PubMed] [Google Scholar]
- Walker EM, Slisarenko N, Gerrets GL, Kissinger PJ, Didier ES, Kuroda MJ et al. (2019) Inflammaging phenotype in rhesus macaques is associated with a decline in epithelial barrier-protective functions and increased pro-inflammatory function in CD161expressing cells. GeroScience 41:739–757. 10.1007/s11357-019-00099-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J, Hou J, Su B, Jiang T, Guo C, Wang W et al. (2020) The prevalence of Frascati-criteria-based HIV-associated neurocognitive disorder (HAND) in HIV-infected adults: a systematic review and meta-analysis. Front Neurol 11. 10.3389/fneur.2020.581346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei X, Decker JM, Wang S, Hui H, Kappes JC, Wu X et al. (2003) Antibody neutralization and escape by HIV-1. Nature 422:307–312. 10.1038/nature01470 [DOI] [PubMed] [Google Scholar]
- Welles HC, Jennewein MF, Mason RD, Narpala S, Wang L, Cheng C et al. (2018) Vectored delivery of anti-SIV envelope targeting mAb via AAV8 protects rhesus macaques from repeated limiting dose intrarectal swarm SIVsmE660 challenge. PLoS Pathog 14:e1007395. 10.1371/journal.ppat.1007395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DW, Eugenin EA, Calderon TM, Berman JW (2012) Monocyte maturation, HIV susceptibility, and transmigration across the blood brain barrier are critical in HIV neuropathogenesis. Available at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3289493/ [DOI] [PMC free article] [PubMed]
- Wing EJ (2017) The aging population with HIV infection. Trans Am Clin Climatol Assoc 128:131–144 [Google Scholar]
- Wu F, Ourmanov I, Riddick N, Matsuda K, Whitted S, Plishka RJ et al. (2015) TRIM5α restriction affects clinical outcome and disease progression in simian immunodeficiency virus-infected rhesus macaques. J Virol 89:2233–2240. 10.1128/JVI.02978-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao X, Chen W, Feng Y, Zhu Z, Prabakaran P, Wang Y et al. (2009) Germline-like predecessors of broadly neutralizing antibodies lack measurable binding to HIV-1 envelope glycoproteins: implications for evasion of immune responses and design of vaccine immunogens. Biochem Biophys Res Commun 390:404–409. 10.1016/j.bbrc.2009.09.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu S, Ou Y, Xiao H, Li J, Adah D, Liu S et al. (2020) Experimental Treatment of SIV-infected macaques via autograft of CCR5-disrupted hematopoietic stem and progenitor cells. Mol Ther Methods Clin Dev 17:520–531. 10.1016/j.omtm.2020.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerbato JM, Serrao E, Lenzi G, Kim B, Ambrose Z, Watkins SC et al. (2016) Establishment and reversal of HIV-1 latency in naive and central memory CD4+ T cells in vitro. J Virol 90:8059–8073. 10.1128/JVI.00553-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H-Y, Wang X-H, He X-Y, Chen M, Zhang M-X, Lian X-D et al. (2022) Aging induces severe SIV infection accompanied by an increase in follicular CD8+ T cells with overactive STAT3 signaling. Cell Mol Immunol 19:1042–1053. 10.1038/s41423-022-00899-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziani W, Bauer A, Lu H, Wang X, Wu X, Bar KJ et al. (2021) Immune responses and viral persistence in simian/human immunodeficiency virus SHIV.C.CH848-infected rhesus macaques. J Virol 95:e02198–e2220. 10.1128/JVI.02198-20 [DOI] [PMC free article] [PubMed] [Google Scholar]


