Abstract
Human immunodeficiency virus type 1 (HIV-1)-specific cytotoxic T-lymphocyte (CTL) responses play a major role in the antiviral immune response, but the relative contribution of CTL responses restricted by different HLA class I molecules is less well defined. HLA-B60 or the related allele B61 is expressed in 10 to 20% of Caucasoid populations and is even more highly prevalent in Asian populations, but yet no CTL epitopes restricted by these alleles have been defined. Here we report the definition of five novel HLA-B60-restricted HIV-1-specific CTL epitopes, using peripheral blood mononuclear cells in enzyme-linked immunospot (Elispot) assays and using CTL clones and lines in cytolytic assays. The dominant HLA-B60-restricted epitope, Nef peptide KEKGGLEGL, was targeted by all eight subjects with B60 and also by both subjects with B61 studied. This study additionally establishes the utility of the Elispot assay as a more rapid and efficient method of defining novel CTL epitopes. This approach will help to define new CTL epitopes that may play an important role in the immune control of HIV-1.
Human immunodeficiency virus type 1 (HIV-1)-specific cytotoxic T lymphocytes (CTL) are considered to play a central role in the immune response against HIV-1 (7, 21). During untreated acute HIV-1 infection, virus-specific CTL activity is associated with the initial decrease of viremia (4, 33). High levels of HIV-1-specific CTL are detectable in subjects with asymptomatic chronic infection (42) but generally decline with disease progression (31). Also during chronic HIV-1 infection, HLA-A2-restricted Gag-specific CTL responses are inversely associated with HIV-1 viral load, when quantified using peptide-major histocompatibility complex class I tetramers (37). In vitro studies have demonstrated potent inhibition of viral replication by HIV-1-specific CTL, mediated by both lytic and nonlytic mechanisms (45), and in vivo there is strong evidence that AIDS viruses evolve to escape CTL recognition by epitope-specific mutations (5, 14, 18, 20, 32, 38). The critical role of virus-specific CTL responses for the control of viremia has recently been directly demonstrated by CD8+ T-cell depletion studies in simian immunodeficiency virus infection in macaques showing that CD8+ T cells effectively suppress viral replication (24, 40).
However, differences in the effectiveness of HIV-1-specific CTL responses of different specificities are increasingly apparent. Certain HLA class I molecules have been associated with more rapid or slower progression of HIV-1 disease (9, 13, 17, 23, 29, 30). The possible mechanism by which HLA class I molecules might influence the speed of disease progression is unclear. A better understanding of these interactions depends upon the precise fine mapping of the optimal CTL epitopes and the definition of HLA class I restriction of these responses. Potentially an important value of well-defined optimal CTL epitopes is that these could be incorporated in future vaccines aimed at inducing HIV-1-specific CTL responses.
HLA-B60 and HLA-B61 are closely related major histocompatibility complex class I molecules (1) for which HIV-1-specific CTL epitopes have not been defined to date (6). Their high prevalence in certain populations that are severely affected by the global HIV epidemic, such as those in India and Thailand, makes the need to define the dominant CTL epitopes presented by these restriction elements more urgent. For example, 30% of Thai-Chinese express HLA-B60 and 38% of Indian populations express HLA-B61 (10, 22). HLA-B60 and -B61 indeed are the most prevalent of the HLA-B alleles in each of these respective populations. In Caucasoids of North America and Europe also, these alleles are not uncommon, B60 and B61 being expressed in approximately 10 to 20% of such populations. In African population studies, however, these alleles are extremely rare (10, 22).
In these studies, we applied the sensitive and rapid enzyme-linked immunospot (Elispot) technique to define CTL responses in persons who express HLA-B60 or -B61. Five novel HLA-B60-restricted CTL epitopes were characterized in p17Gag, p24Gag, gp41Env, reverse transcriptase (RT), and Nef. Responses to two of these epitopes were also detected in two of two subjects studied with HLA-B61. The hierarchy of these B60-restricted responses was defined in eight subjects with HLA-B60. By comparing Elispot assay to traditional techniques, we demonstrate that the Elispot assay is a rapid, inexpensive, and less labor-intensive method of defining novel CTL epitopes and characterizing the breadth of CTL responses.
MATERIALS AND METHODS
Patients.
HIV-1-specific CTL responses were analyzed in detail in two patients. Individual 166j was infected with HIV-1 in May 1997 and diagnosed and treated during symptomatic acute-phase HIV-1 infection, prior to seroconversion. At the time of the analysis of CTL responses, the subject had been treated for 2 years with highly active antiretroviral therapy but had undergone structured therapy interruption twice for 3 weeks and 17 weeks, respectively. Viral load during the time of CTL analysis was below the level of detection (<50 copies of HIV-1 RNA/ml of plasma), and CD4+ T-cell counts were 600 to 800 cells/μl.
Subject 161j is an individual with long-term nonprogressive HIV-1 infection, who has been described previously (26). Briefly, he has been HIV-1 infected since 1978 and has never received any antiretroviral therapy, and his viral load consistently has remained below the limit of detection (<50 RNA copies/ml of plasma). CD4+ T-cell count at the time of CTL analysis was 670 cells/μl.
An additional six HIV-1-infected individuals with HLA-B60 were screened for HIV-1-specific CTL responses against the newly defined HLA-B60-restricted CTL epitopes, as were two HIV-1-infected individuals with HLA-B61 (Table 1). All these subjects were adults except subject 019-TCH, who was a child, aged 6 years and 11 months, infected via mother-to-child transmission. All individuals studied, including subjects 166j and 161j, were Caucasian, apart from subject 3134f, who was Hispanic.
TABLE 1.
HLA types of study subjectsa
| Subject | HLA type
|
||
|---|---|---|---|
| A | B | Cw | |
| 166j | A3/− | B14/60 | Cw3/8 |
| 161j | A2/3 | B7/60 | Cw3/7 |
| 321d | A3/24 | B18/60 | Cw3/7 |
| 3220a | A2/68 | B60/63 | Cw3/7 |
| 3549j | A32/68 | B14/60 | Cw3/8 |
| PSL002 | A2/3 | B49/60 | Cw3/7 |
| 019-TCH | A2/32 | B51/60 | Cw3/8 |
| 013-57i | A3/38 | B51/60 | Cw15/16 |
| 3134f | A2/31 | B61/− | Cw3/− |
| 3572i | A25/32 | B18/61 | Cw2/12 |
HLA class I types of eight subjects with HLA-B60 and two subjects with HLA-B61 who were studied.
HLA typing.
HLA class I typing was performed at the Massachusetts General Hospital Tissue Typing Laboratory using sequence-specific primer PCR (8). HLA class I types of the subjects studied are shown in Table 1. HLA-B60 is encoded by B*4001, and HLA-B61 is most commonly encoded by B*4002 (39). The sequence-specific primer PCR used did not distinguish B*4002 and the less frequent subtypes, including HLA-B*4003, -B*4004, -B*4006, and -B*4008 (39).
Cell lines and media.
Epstein-Barr virus (EBV)-transformed B-lymphoblastoid cell lines were established and maintained in R20 medium (RPMI 1640 medium [Sigma, St. Louis, Mo.] supplemented with 2 mM l-glutamine, 50 U of penicillin per ml, 50 μg of streptomycin per ml, 10 mM HEPES, and 20% heat-inactivated fetal calf serum [Sigma]) as previously described (42). For culture of CTL clones, medium containing 10% fetal calf serum (R10) supplemented with 50 U of recombinant interleukin-2 (kindly provided by M. Gately, Hoffmann-La Roche, Nutley, N.J.) per ml was used.
Generation of CTL clones.
CTL clones were isolated by limiting dilution as previously described (25, 43), using the anti-CD3-specific monoclonal antibody (MAb) 12F6 as stimulus for T-cell proliferation. Developing clones were screened for HIV-1-specific CTL activity by 51Cr release assay (42) against autologous B-cell lines pulsed with the peptides recognized in the Elispot assays. HIV-1-specific clones were maintained by stimulation every 14 to 21 days with an anti-CD3 MAb and irradiated allogeneic peripheral blood mononuclear cells (PBMC). Fine mapping of the novel CD8+ T-cell responses identified in the Elispot assay was achieved in a 51Cr release assay using truncations of the recognized 15- to 20-mer peptide (43). HLA restriction of CTL epitopes was determined using a panel of target cells matched through only one of the HLA-A, HLA-B, or HLA-C class I alleles expressed by the effector cells (43).
Synthetic HIV-1 peptides.
Peptides corresponding to previously described optimal HIV-1 CTL epitopes (6) were synthesized on an automated peptide synthesizer (Model 432A; Applied Biosystems, Foster City, Calif.). In addition, a panel of 259 overlapping peptides, 15 to 20 amino acids in length and overlapping by 10 to 11 amino acids, spanning the entire p17Gag, p24Gag, gp41Env, gp120Env, RT, and Nef B-clade SF2 sequence, were used. These peptides were provided in part by the National Institute for Biological Standards and Control Centralized Facility for AIDS Reagents, supported by European Union Program EVA and the United Kingdom Medical Research Council.
Elispot assay.
Fresh PBMC were separated from whole blood by Ficoll-Hypaque (Sigma) density gradient centrifugation and plated in 96-well polyvinylidene difluoride-backed plates (MAIP S45; Millipore, Bedford, Mass.) that had been previously coated with 100 μl of anti-gamma-interferon (IFN-γ) MAb 1-D1k (0.5 μg/ml; Mabtech, Stockholm, Sweden) overnight at 4°C. Peptides were added directly to the wells at a final 10−5 M concentration. Cells were added to the wells at 25,000 to 100,000 cells per well in a final volume of 130 μl of R10. For negative controls, 100,000 PBMC were incubated with R10 alone, without adding peptides. The plates were incubated at 37°C with 5% CO2 overnight (14 to 16 h), then washed six times with phosphate-buffered saline (PBS) before 100 μl of biotinylated anti-IFN-γ MAb 7-B6-1 (1 μg/ml; Mabtech) was added, and incubated at room temperature for 90 min. After the plates were washed again with PBS, 100 μl of 1:20,000-diluted streptavidin-alkaline phosphatase conjugate (Mabtech) was added per well. The plates were incubated at room temperature for 45 min. Wells were again washed with PBS, and individual IFN-γ-producing cells were detected as dark spots after a 20- to 30-min color reaction with 5-bromo-4-chloro-3-indolylphosphate and nitroblue tetrazolium using an alkaline phosphatase-conjugated substrate (Bio-Rad Laboratories, Hercules, Calif.). Spots were counted by direct visualization and are expressed as spot-forming cells (SFC) per 106 PBMC or per 106 input cells. The number of specific IFN-γ-secreting T cells was calculated by subtracting the negative control value from the established SFC count. Results of 60 or more SFC/106 input cells were considered positive. Negative controls were always <60 SFC/106 input cells. CD8+ T-cell dependence of all responses to synthetic peptides was confirmed by CD8 and CD4 depletion and enrichment studies using magnetic beads (MACS; Miltenyi Biotech, Hamburg, Germany), according to the manufacturer's protocol.
Fine mapping and HLA restriction of immunodominant CTL epitopes using Elispot assay.
For the fine mapping of the epitope by Elispot assay, the same peptide truncations were used as for the chromium release assay. A total of 500 to 1,000 clonal T cells per well or 50,000 PBMC were incubated with concentrations from 10−4 to 10−11 M peptide overnight on the Elispot plate. All assays were run in duplicate. The optimal peptide was defined as the peptide that induced 50% maximal specific IFN-γ production by T cells at the lowest peptide concentration.
HLA restriction of the immunodominant epitope by Elispot assay was performed using the same panel of target B cells as described for the chromium release assay. The B cells were incubated for 1 h with 100 μM optimal epitope and then washed four times in R10. Some 2,000 to 5,000 clonal target cells or 50,000 fresh PBMC were added to 500 B cells per well and incubated overnight. Target cells without peptide and pulsed with non-HLA-matched peptides were used as negative controls.
RESULTS
HIV-1-specific CTL responses can be rapidly identified using Elispot assays.
In order to define CTL responses restricted through HLA-B60, two subjects (166j and 161j) expressing this allele were evaluated. These studies employed a panel of overlapping peptides spanning p17Gag, p24Gag, gp41Env, gp120Env, RT, and Nef, as well as previously described optimal peptides (5) predicted to be targeted through other expressed alleles in these subjects. Table 2 summarizes the recognized 15- to 20-mers, as well as the recognized optimal HLA-matched CTL epitopes that are included in these larger peptides. For peptide-specific T-cell responses against five of the overlapping peptides (Nef-10, p17-9A, p24-5, RT-40, and gp41-31), no optimal HIV-1 CTL epitopes had been described previously (6), and these responses were further investigated, as illustrated for the overlapping Nef-10 peptide in subject 166j (Fig. 1).
TABLE 2.
Responses detected in Elispot assays of PBMC from subjects 166j and 161j
| Patient | Overlapping peptide | Amino acid sequence of overlapping peptide | Dependence of response
|
Amino acid sequence of optimal CTL epitope | HLA restriction of optimal CTL epitope | |
|---|---|---|---|---|---|---|
| CD8 | CD4 | |||||
| 166j (A3/− B14/60 Cw3/8) | p17-9a | YCVHQRIEIKDTKEAL | + | − | Not described | |
| p24-17 | FRDYVDRFYKTLRAEQASQD | + | − | DRFYKTLRA | B14 | |
| RT-40 | HRTKIEELRQHLLRW | + | − | Not described | ||
| gp41-8 | LQARILAVERYLKDQQL | + | − | ERYLKDQQL | B14 | |
| Nef-8 | PQVLRPMTYKAAVDLSHFL | + | − | QVPLRPMTYK | A3 | |
| KAAVDLSHFL | Cw8 | |||||
| Nef-9 | KAAVDLSHFLKEKGGLEGLI | + | − | KAAVDLSHFL | Cw8 | |
| Nef-10 | KEKGGLEGLIHSQRRQDILDL | + | − | Not described | ||
| 161j (A2/3 B7/60 Cw3/7) | p17-8 | GSEELRSLYNTVATL | + | − | SLYNTVATL | A2 |
| p17-9a | YCVHQRIEIKDTKEAL | + | − | Not described | ||
| p24-4 | AFSPEVIPMFSALSEGATPQ | − | + | Not described | ||
| p24-5 | SALSEGATPQDLNTMLNTVG | + | − | Not described | ||
| RT-40 | HRTKIEELRQHLLRW | + | − | Not described | ||
| gp41-31 | KYCWNLLQYWSQELKNSAVSL | + | − | Not described | ||
| gp41-36 | HIPRRIRQGLERALL | + | − | IPRRIRQGL | B7 | |
| Nef-10 | KEKGGLEGLIHSQRRQDILDL | + | − | Not described | ||
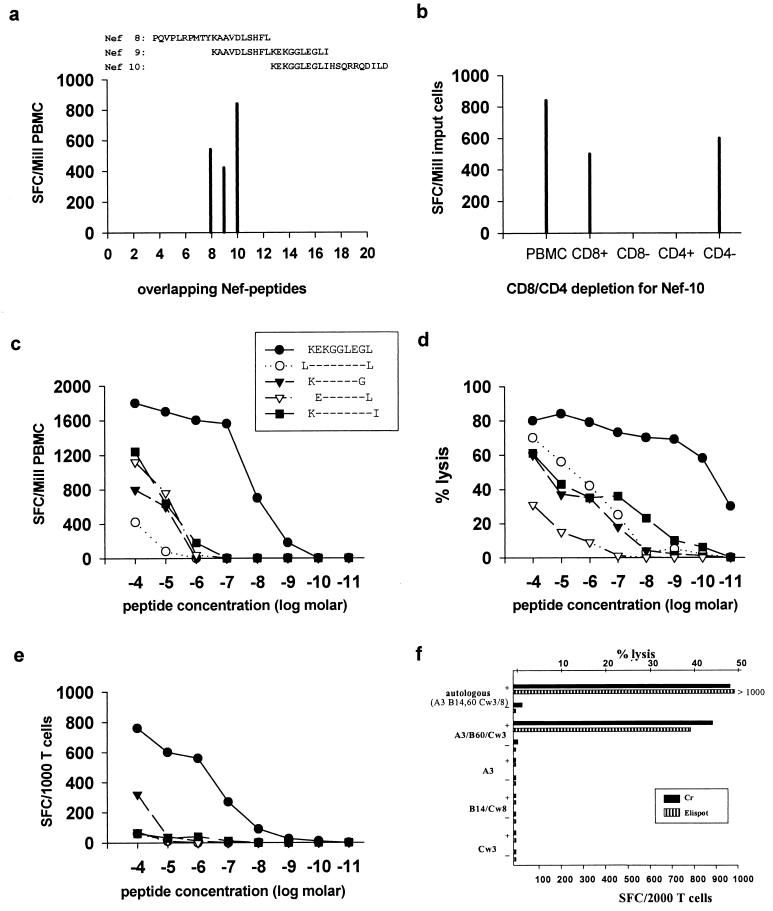
FIG. 1.
Definition of an HLA-B60-restricted novel epitope in Nef. The subject studied was 166j (HLA A3/− B14/60 Cw3/8). (a) Recognition of 3 of 20 20-mer overlapping peptides spanning Nef in the Elispot assay. Frequency of responses is expressed as SFC per million PBMC. (b) CD8+ T-cell dependence of the response toward Nef-10 demonstrated in the Elispot assay following CD4-CD8 enrichment or depletion. Frequency of IFN-γ-producing cells is expressed as SFC per million input cells. (c) Titration curves using PBMC in an Elispot assay mixture incubated with serial dilutions of peptides as shown. (d) Titration curves using CTL specific clones in a standard chromium release assay; the peptides (and symbols) are the same as shown in panel c. (e) Titration curves using the CTL clones employed in panel d but in an Elispot assay, incubated with the same peptides as shown in panels c and d. (f) Determination of HLA restriction using CTL clones in an Elispot assay (hatched bars) and in a chromium release assay (solid bars). Targets either were pulsed with no peptide (−) or were pulsed with peptide KEKGGLEGL (+). The HLA class I types of the targets used were A2/3, B7/60, and Cw3/7; A3/−, B7/−, and Cw7/−; A28/29, B14/44, and Cw5/8; and A34/68, B57/71, and Cw3/7 (matching HLA class I alleles are shown in boldface).
PBMC from subject 166j showed recognition of three overlapping Nef peptides (Nef-8, -9, and -10) (Fig. 1a). The responses to Nef-8 and Nef-9 were due to the recognition of the A3-restricted epitope QVPLRPMTYK (31) and the Cw8-restricted epitope KAAVDLSHFL (reference 34 and data not shown). CD8+ T-cell dependence of responses to these overlapping peptides was confirmed by CD4 and CD8 depletion and enrichment studies as shown for peptide Nef-10 in Fig. 1b. Using two 15-mer peptides that overlapped by 10 amino acids and together spanned the 20-mer Nef-10, it was determined that the optimal epitope was within the 15-mer KEKGGLEGLIWSQRR (data not shown). By incubating PBMC in the Elispot assay with serial dilutions of the 9-mer KEKGGLEGL (KL9) and serial dilutions of four additional peptides which had 1 amino acid added to or deleted from the N- or C-terminal residues of the KL9 sequence, respectively, the 9-mer KEKGGLEGL was demonstrated as the optimal CTL epitope (Fig. 1c).
The Elispot assay measures the ability of T cells to produce IFN-γ but not the ability to kill target cells. To determine if the optimal Elispot-identified epitope represented a true CTL epitope, the Nef-10 peptide was used to isolate peptide-specific CTL clones. The optimal CTL epitope was then defined by the classical approach using the 51Cr release assay (43). Serial dilutions of the same peptide truncations used in the Elispot assay were also used in this assay. The optimal epitope was defined as the peptide that could sensitize targets for 50% of maximal recognition by CD8+ T cells at the lowest peptide concentration. The 9-mer KEKGGLEGL was confirmed as the optimal HIV-1 CTL epitope recognized by this subject in the standard 51Cr release assay (Fig. 1d). Furthermore, the Elispot assay was repeated using the peptide-specific CD8+ T-cell clones instead of uncultured PBMC, leading to the same result (Fig. 1e). Peptide-specific responses to the optimal epitope were lost at a higher peptide concentration in the Elispot assay than in the 51Cr release assay. However, the peptide concentration at which targets were sensitized for 50% of maximal recognition by CD8+ T cells could be lowered in the Elispot assay by increasing the number of cells used per well, but not in the 51Cr release assay (data not shown), suggesting methodical differences between the assays and not functional differences between IFN-γ production and cytotoxicity. The advantage of the Elispot assay was that a significantly lower number of CD8+ T cells was required (500 to 1,000 T cells per well compared to 100,000 T cells per well in the 51Cr release assay). Furthermore, no B-cell lines were required as targets in the Elispot assay, even when CTL clones alone were incubated with peptide.
After fine mapping of the optimal CTL epitope, the HLA restriction of this response was determined on peptide-specific T-cell clones using both the 51Cr release assay and, independently, the Elispot assay. Both assays showed that the CTL response against the Nef epitope KEKGGLEGL is HLA-B60 restricted (Fig. 1f). In contrast, definition of the HLA restriction for KEKGGLEGL was not possible using PBMC, as a high, non-peptide-specific release of IFN-γ was induced when PBMC were incubated either with allogeneic, partially HLA-matched B cells or even with autologous EBV-transformed B cells, irrespective of whether these cells were pulsed with peptide (data not shown).
Characterization of two additional HLA-B60-restricted optimal HIV-1 CTL epitopes by both Elispot and 51Cr release assay.
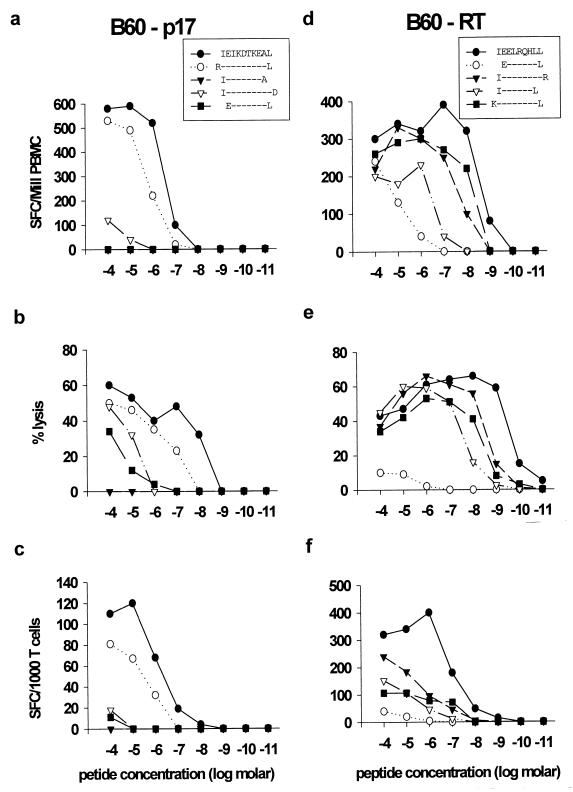
Using the approach described above, two further novel HLA-B60-restricted CTL epitopes in p17 (IEIKDTKEAL) and RT (IEELRQHLL) were identified in subject 166j (Fig. 2). As before, the use of peptide truncations in the Elispot assay allowed the prediction of the two optimal epitopes using PBMC, without any prior in vitro expansion or cloning by limiting dilution (Fig. 2a and b). The optimization of these two epitopes was confirmed with CD8+ T-cell clones in both the 51Cr release assay (Fig. 2c and d) and the Elispot assay (Fig. 2e and f). Again, in the 51Cr release assay the optimal peptide was recognized at a lower concentration than that in the Elispot assay; however, a much higher number of clonal CD8+ T cells were required. Only target cells expressing HLA-B60 could present these peptides for recognition by the CTL clones, and hence both responses were HLA-B60 restricted (data not shown).
FIG. 2.
Definition of novel HLA-B60-restricted CTL epitopes in p17Gag (a to c) and RT (d to f). The subject studied was 166j (HLA A3/− B14/60 Cw3/8). (a and d) Titration curves of truncated peptides using PBMC in an Elispot assay. (b and e) Titration curves using peptides in panels a and d, respectively, in chromium release assays using peptide-specific CTL clones and autologous B-cell line targets. (c and f) Titration curves using peptides in panels a and d, respectively, incubated in Elispot assays with the respective CTL clones.
Identification of two further novel HLA-B60-restricted CTL epitopes in subject 161j.
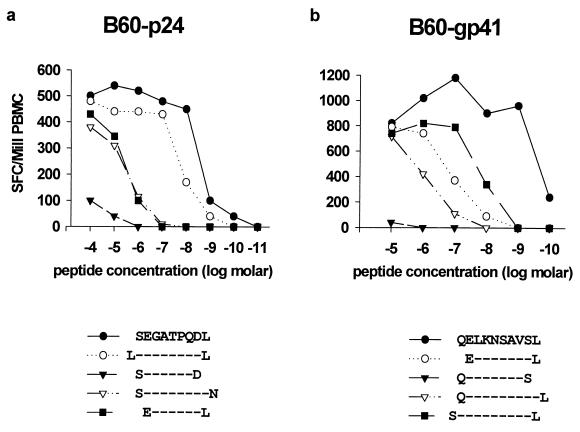
The HIV-1-specific CTL responses in a second subject, 161j, were analyzed in the same way. CTL from this subject recognized the novel HLA-B60-restricted epitopes in p17Gag, RT, and Nef that were described above for subject 166j (data not shown), as well as additional novel HLA-B60-restricted CTL epitopes in p24Gag (SEGATPQDL) and gp41Env (QELKNSAVSL) (Fig. 3). Once again, the optimal sequences of these epitopes were all reliably predictable using PBMC incubated with truncations of the optimal epitope in an Elispot assay (Fig. 3a and b), and the HLA restriction was subsequently determined using peptide-specific T-cell lines (data not shown).
FIG. 3.
Recognition of novel HLA-B60-restricted CTL epitopes in p24Gag and gp41Env. The subject studied was 161j (HLA A2/3 B7/60 Cw3/7). (a) Incubation of PBMC with peptide truncations of p24Gag peptide SEGATPQDL in an Elispot assay as shown. (b) Incubation of PBMC with peptide truncations of gp41 peptide QELKNSAVSL in an Elispot assay as shown.
Recognition of HLA-B60-restricted epitope peptide by HLA-B61-positive subjects.
Previous studies have shown that similar HLA class I molecules may present the identical peptides as CTL epitopes (16, 17, 41). Since the common forms of HLA-B60 (B*4001) and HLA-B61 (B*4002) differ by only 8 amino acids in total (1, 39) and the established peptide binding motifs of these two closely related alleles are also similar (15), two subjects with HLA-B61 (3134f and 3572i) were also analyzed for their responses to the peptides that had been established above as HLA-B60-restricted CTL epitopes. In both subjects, strong responses against the p24Gag epitope (SEGATPQDL) and the p24Nef epitope (KEKGGLEGL) were observed (Table 3).
TABLE 3.
Frequency of responses to HLA-B60-restricted epitope peptides in eight subjects with HLA-B60 and two subjects with HLA-B61a
| Subject | Viral load | Antiviral treatment | CTL response (SFC/million PBMC)
|
||||
|---|---|---|---|---|---|---|---|
| B60-p17 | B60-p24 | B60-RT | B60-gp41 | B60-Nef | |||
| 166j | <50 | 1 PI, 2 NRTI | 680 | 0 | 500 | 0 | 940 |
| 161j | <50 | No treatment | 420 | 540 | 120 | 980 | 520 |
| PSL002 | 240,965 | No treatment | 320 | 0 | 0 | 0 | 600 |
| 3220a | <50 | 1 PI, 2 NRTI | 0 | 0 | 0 | 0 | 100 |
| 3549j | <50 | 1 PI, 2 NRTI | 180 | 0 | 0 | 0 | 250 |
| 321d | <50 | 1 PI, 2 NRTI | >1,000 | 0 | 190 | 0 | 360 |
| 019-TCH | 3,540 | 1 NRTI | 0 | 0 | 0 | 75 | 1,150 |
| 013-57i | 2,000 | No treatment | 140 | 0 | 0 | 0 | 740 |
| 3134f | <50 | 1 PI, 2 NRTI | 0 | 70 | 0 | 0 | >1,500 |
| 3572i | 850 | No treatment | 0 | 520 | 0 | 0 | 620 |
For each subject, viral load (copies of HIV-1 RNA per milliliter) and treatment (protease inhibitor [PI] and nucleoside RT inhibitor[s] [NRTI]) at the time of study are shown.
Frequency of recognition of HLA-B60-restricted HIV-1 CTL epitopes.
In order to determine the frequency of recognition of these novel HLA-B60-restricted CTL epitopes, PBMC from six additional HIV-1-infected subjects with HLA-B60 were screened by Elispot assay for recognition of these epitopes (Table 3). In all individuals, responses against at least one of these epitopes were detectable (median, two; range, one to three). The Nef epitope KEKGGLEGL was recognized by all subjects and was the immunodominant HLA-B60-restricted epitope overall in six of eight of the HLA-B60-positive subjects tested. For two subjects, 166j and PSL002, longitudinal samples beginning from the time of primary HIV-1 infection were analyzed. Interestingly, in both subjects CTL responses directed against the HLA-B60-restricted Nef epitope were recognized prior to (subject 166j) or at the time of (subject PSL002) HIV-1 seroconversion and earlier than the responses against other HLA-B60-restricted epitopes, which were developed later during the course of HIV-1 infection (data not shown). In subject PSL002, the HLA-B60 epitopes were the only recognized CTL epitopes described for his HLA type, and in subject 166j, two more B14-restricted epitopes in p24Gag and gp41Env were recognized prior to seroconversion. These findings provide further evidence for the dominant role of the HLA-B60-restricted Nef epitope within the newly defined HLA-B60-restricted CTL epitopes.
DISCUSSION
This study focuses on previously undescribed HLA-B60-restricted HIV-1-specific CTL responses. Five novel epitopes, located in p17Gag, p24Gag, RT, gp41Env, and Nef, were defined. The hierarchy of these responses was determined in eight subjects, showing that the B60-Nef peptide was the immunodominant HLA-B60-restricted epitope in six of these eight persons. Two subjects who did not express HLA-B60 but who expressed the closely related allele HLA-B61 also showed recognition of epitopes that were defined in the HLA-B60-positive subjects. Finally, the Elispot assay was established as a rapid and efficient method of fine mapping CTL epitopes that has considerable advantages over limiting-dilution assay-based methods.
A median of two (range, one to five) of the five new HLA-B60-restricted CTL epitopes was recognized by eight HIV-1-infected subjects expressing the corresponding HLA class I molecules. Remarkably, the HLA-B60-restricted Nef epitope was recognized by PBMC of all eight individuals and was the strongest HLA-B60-restricted CTL response in six of them. CTL responses against the HLA-B60-restricted Nef epitope developed early during primary HIV-1 infection, as indicated by longitudinal studies with two subjects from the time of HIV-1 seroconversion, and contributed strongly to the total HIV-1-specific CTL responses in these two subjects (data not shown). Taken together, this indicates a dominant role of the Nef epitope within the HLA-B60-restricted CTL response in chronic infection and at least in the two subjects studied longitudinally during acute infection.
Presentation of identical epitopes by closely similar HLA class I molecules has been well described previously (3, 11, 12, 17, 41). In view of the close sequence similarity between the common forms of HLA-B60 (B*4001) and HLA-B61 (B*4002) (1, 39) and between the peptide binding motifs of these two closely related alleles (15), the epitopes that were defined in the HLA-B60-positive subjects were also tested for recognition using PBMC from two subjects expressing HLA-B61. Responses to two of the five HLA-B60-restricted epitopes in p24Gag and Nef were also observed at high levels in these HLA-B61-positive subjects. It is noteworthy that the commonest HLA-B61 subtype in Caucasians is HLA-B*4002, as it is in the Southeast Asian populations where HLA-B61 is highly prevalent (2, 34, 36).
It is well established that single amino acid changes within epitopes can abrogate CTL recognition (5, 14, 18, 20, 32, 38). A comparison of the novel HLA-B60-restricted B-clade CTL epitope sequences described above was therefore made with the corresponding C- and E-clade sequences that would be relevant in India and Southeast Asia where B60 and B61 are highly prevalent (Table 4). These epitope sequences showed some cross-clade variations, but significantly, the primary anchor residues in positions 2 (P2) and at the C terminus (PC) were highly conserved. This implies that in C-clade or E-clade HIV-1 infection HLA-B60- and -B61-restricted CTL responses were likely to be generated towards these specificities.
TABLE 4.
Comparison of the novel B60- and B61-restricted B-clade CTL epitope sequences with the corresponding C- and E-clade sequences
| Protein | Clade B
|
Clade C
|
Clade E
|
|||
|---|---|---|---|---|---|---|
| Sequence | % Conservationa | Sequence | % Conservationa | Sequence | % Conservationa | |
| p17 | IEVKDTKEAL | 65 (71) | IEVRDTKEAL | 72 (89) | IEVKDTKEAL | 100 (100) |
| p24 | SEGATPQDL | 100 (100) | SEGATPQDL | 100 (100) | SEGATPQDL | 100 (100) |
| RT | IEELRQHLL | 86 (100) | IEELRAHLL | 100 (100) | IEELREHLL | 59 (100) |
| gp41 | QELKNSAVSL | 59 (98) | LELKKSAISL | 60 (100) | QELKISAISL | 75 (100) |
| Nef | KEKGGLEGL | 82 (100) | KEKGGLEGL | 76 (91) | KEKGGLDGL | 54 (96) |
Conservation within the anchor positions P2 and PC is indicated in parentheses.
The alignment of the novel HLA-B60-restricted HIV-1-specific CTL epitopes with previously described hepatitis C virus (HCV)-specific and EBV-specific CTL epitopes (28, 35, 44) showed a very restricted usage of amino acids at the primary anchor position 2 (P2) and at the C terminus (PC) (Table 5). In 10 of 10 described HLA-B60-restricted CTL epitopes, the medium-sized hydrophobic residue Leu was present at PC, and in 10 of 10 epitopes the negatively charged Glu residue was situated at P2. The single exception was the HCV core epitope (28), which presented an uncharged polar Gln in this position. The anchor residues were consistent with the peptide binding motif of HLA-B60 that has been defined by peptide elution from HLA-B60 (15). The association between the peptide binding motif determined by peptide elution and the amino acid sequence of the actual CTL epitopes is not always as strong (19), suggesting that very few residues other than Glu at P2 and Leu at PC can be tolerated in HLA-B60 binding motifs. Similarly, B61 binding peptides appear to require Glu at P2 (14). In contrast, while the HLA-B61 binding motif (15) suggests a strong preference for Val in the PC anchor position, from the sequencing of particular eluted peptides (14) and from the recognition of B60 binding peptides shown above, evidently Ala, Leu, and Ile can also be accommodated in addition to Val at this position in peptides binding effectively to HLA-B61.
TABLE 5.
HLA-B60 and HLA-B61 peptide binding motifs and alignment of 10 defined HLA-B60-restricted virus-specific CTL epitopes
| HLA-B60 | Sequencea | Reference |
|---|---|---|
| Peptide binding motif | 12345678C | 14 |
| -E------L | ||
| V | ||
| I | ||
| HCV core | GQIVGGVY L | 26 |
| HCV E2 (530–539) | GENDTDVFVL | 43 |
| HCV E2 (654–662) | LEDRDRSE L | 43 |
| HCV NS5A (2152–2160) | HEYPVGSQ L | 43 |
| EBV LMP2 (200–208) | IEDPPFNS L | 33 |
| HIV-1 p17 (92–101) | IEIKDTKEAL | |
| HIV-1 p24 (176–184) | SEGATPQD L | |
| HIV-1 RT (369–377) | IEELRQHL L | |
| HIV-1 gp41 (810–819) | QELKNSAVSL | |
| HIV-1 Nef (92–101) | KEKGGLEG L |
Primary anchors are indicated by boldface. Secondary anchors are indicated by underlining.
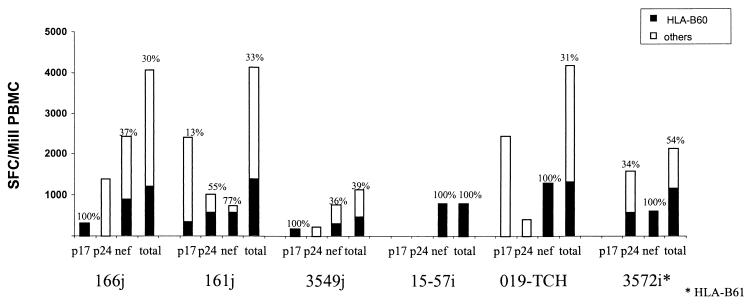
The newly defined HLA-B60-restricted epitopes appeared to play a major role in the total HIV-1-specific CTL responses in the studied individuals. In six subjects for whom sufficient cells enabled the analysis to be undertaken, CTL responses directed against the B60-restricted epitopes contributed on average 48% (range, 30 to 100%) of the total CTL responses directed against p17Gag, p24Gag, and Nef, representing in several cases the only detectable CTL response directed against some of these regions of HIV-1 (Fig. 4). How far antiretroviral treatment may have affected the hierarchy of detected immune responses could not be addressed from this study, but previous studies of the kinetics of HIV-1-specific CTL responses after the initiation of antiretroviral treatment showed that CTL responses decline after initiation of treatment but that the hierarchy of the CTL responses is maintained (27; M. A. Altfeld, E. S. Rosenberg, R. L. Eldridge, P. J. R. Goulder, and B. D. Walker, unpublished data). For reasons that remain unclear, important differences exist between different HLA alleles in their contribution to the total HLA-restricted CTL response against HIV-1 (5). This may be important in the association of slow and rapid disease progression with different HLA class I molecules (9, 13, 17, 23, 29, 30). It will be useful to extend these comparisons to cover the total HIV-specific CTL response for each of the commonly occurring HLA class I molecules. An approach based on the Elispot assay now makes such an undertaking quite feasible.
FIG. 4.
Contribution of responses toward HLA-B60-restricted CTL epitope peptides in p17Gag (IEIKTDKEAL), p24Gag (SEGATPQDL), and p24Nef (KEKGGLEGL) compared to total CTL activity detected toward epitopes within these proteins for six subjects. The method used was as illustrated in Fig. 1.
The value of the Elispot assay in enabling novel CTL epitopes to be rapidly fine mapped and their contribution to the total antiviral CTL response to be estimated quickly and efficiently is illustrated in these studies. The chief advantages of the Elispot assay over limiting-dilution assay-based methods to define CTL epitopes are the reduction of time taken, the more restricted need for work with radioactive material, and the smaller amount of cells necessary to define novel responses. This approach is thus much more rapid and much less labor- and cost-intensive. The only difficulty that could not be overcome in the definition of the novel CTL epitopes in the Elispot assay using PBMC was the failure to determine HLA restriction. The generation of CTL clones or peptide-specific lines enabled the HLA restriction to be determined, but this remains a laborious and time-consuming process. Recent studies using PBMC incubated with peptide-pulsed HLA-matched B-cell lines and stained for intracellular IFN-γ production prior to flow cytometric analysis have overcome the problem of high background that obscures determination of the HLA restriction in corresponding Elispot assays (P. J. R. Goulder, M. Addo, Y. Tang, K. Annamalai, M. G. Hammond, M. Bunce, E. S. Rosenberg, and B. D. Walker, unpublished data). Thus, a combination of the Elispot assay to fine map the epitope and intracellular cytokine staining assays to determine the HLA restriction and CD8 dependence of the response can now potentially enable novel epitopes to be defined within 48 h of receiving fresh blood from a previously unstudied subject. Although these assays do not address the cytotoxic functionality of the antigen-specific CD8+ T cells whose epitope specificities are defined, the advantage of these methods in their accessibility to laboratories in developing countries at the center of the global epidemic is self-evident.
In conclusion, this study describes the rapid identification of five novel HLA-B60-restricted CTL epitopes in HIV-1. These CTL epitopes were frequently recognized in HIV-1-infected individuals expressing HLA-B60 or the closely related allele HLA-B61 and contributed importantly to the total antiviral Gag- and Nef-specific CTL response in these subjects. The relevance of these responses to vaccine research will be dependent on future studies analyzing the impact of these responses on HIV-1 disease. The more rapid and cost-effective characterization of CTL epitopes by Elispot assay significantly eases the identification of new CTL epitopes and will help to provide further insights into the dynamic interaction between HIV-1 and the cellular immune system.
ACKNOWLEDGMENTS
We are greatly indebted to Nancy Karthas, Lynne Lewis, Rosemary Galvin, and Catherine Kneut for the collection of blood samples and provision of clinical data in order to study the CTL responses described above, and their input is gratefully acknowledged.
This work was supported by grants to M.A.A. from the Deutscher Akademischer Austauschdienst (DAAD) (grant D/99/08826); to P.J.R.G. from the Elizabeth Glaser Pediatric AIDS Foundation, the Medical Research Foundation (UK) (grant G108/274), and the National Institutes of Health (AI46995); to M.M.A. from the Deutsche Forschungsgemeinschaft (DFG); to S.A.K. from the National Institutes of Health (AI39966); and to B.D.W. through the National Institutes of Health (AI28568, AI30914, and U01-AI48023) and the Doris Duke Charitable Foundation. P.J.R.G. is an Elizabeth Glaser Scientist of the Elizabeth Glaser Pediatric AIDS Foundation. B.D.W. is a Doris Duke Distinguished Clinical Science Professor.
REFERENCES
- 1.Arnett K L, Parham P. HLA class I nucleotide sequences. Tissue Antigens. 1995;46:217–257. doi: 10.1111/j.1399-0039.1995.tb03124.x. [DOI] [PubMed] [Google Scholar]
- 2.Bannai M, Tokunaga K, Lin L, Ogawa A, Fujisawa K, Juji T. HLA-B40, B18, B27, and B37 allele discrimination using group-specific amplification and SSCP method. Hum Immunol. 1996;46:107–113. doi: 10.1016/0198-8859(96)00016-x. [DOI] [PubMed] [Google Scholar]
- 3.Bertoni R, Sidney J, Fowler P, Chesnut R W, Chisari F V, Sette A. Human histocompatibility leukocyte antigen-binding supermotifs predict broadly cross-reactive cytotoxic T lymphocyte responses in patients with acute hepatitis. J Clin Investig. 1997;100:503–513. doi: 10.1172/JCI119559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borrow P, Lewicki H, Hahn B H, Shaw G M, Oldstone M B. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J Virol. 1994;68:6103–6110. doi: 10.1128/jvi.68.9.6103-6110.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borrow P, Lewicki H, Wei X, Horwitz M S, Peffer N, Meyers H, Nelson J A, Gairin J E, Hahn B H, Oldstone M B, Shaw G M. Antiviral pressure exerted by HIV-1-specific cytotoxic T lymphocytes (CTLs) during primary infection demonstrated by rapid selection of CTL escape virus. Nat Med. 1997;3:205–211. doi: 10.1038/nm0297-205. [DOI] [PubMed] [Google Scholar]
- 6.Brander C, Goulder P J R. Recent advances in HIV-1 CTL epitope characterization, p. IV-1–IV-17. In: Korber B T M, Brander C, Walker B D, Koup R A, Moore J, Haynes B, Meyer G, editors. HIV molecular database. Los Alamos, N.Mex: Los Alamos National Laboratory; 1999. [Google Scholar]
- 7.Brander C, Walker B D. T lymphocyte responses in HIV-1 infection: implications for vaccine development. Curr Opin Immunol. 1999;11:451–459. doi: 10.1016/S0952-7915(99)80076-4. [DOI] [PubMed] [Google Scholar]
- 8.Bunce M, Fanning G C, Welsh K I. Comprehensive, serologically equivalent DNA typing for HLA-B by PCR using sequence-specific primers (PCR-SSP) Tissue Antigens. 1995;45:81–90. doi: 10.1111/j.1399-0039.1995.tb02422.x. [DOI] [PubMed] [Google Scholar]
- 9.Carrington M, Nelson G W, Martin M P, Kissner T, Vlahov D, Goedert J J, Kaslow R, Buchbinder S, Hoots K, O'Brien S J. HLA and HIV-1: heterozygote advantage and B*35-Cw*04 disadvantage. Science. 1999;283:1748–1752. doi: 10.1126/science.283.5408.1748. [DOI] [PubMed] [Google Scholar]
- 10.Clayton J, Lonjou C, Whittle D. Allele and haplotype frequencies for HLA loci in various ethnic groups. In: Charron D, editor. Proceedings of the XIIth International Histocompatibility Workshop. Vol. 1. Paris, France: EDK; 1997. pp. 665–820. [Google Scholar]
- 11.Culmann B, Gomard E, Kieny M P, Guy B, Dreyfus F, Saimot A G, Sereni D, Levy J P. An antigenic peptide of the HIV-1 NEF protein recognized by cytotoxic T lymphocytes of seropositive individuals in association with different HLA-B molecules. Eur J Immunol. 1989;19:2383–2386. doi: 10.1002/eji.1830191231. [DOI] [PubMed] [Google Scholar]
- 12.del Guercio M F, Sidney J, Hermanson G, Perez C, Grey H M, Kubo R T, Sette A. Binding of a peptide antigen to multiple HLA alleles allows definition of an A2-like supertype. J Immunol. 1995;154:685–693. [PubMed] [Google Scholar]
- 13.Evans D T, Knapp L A, Jing P, Mitchen J L, Dykhuizen M, Montefiori D C, Pauza C D, Watkins D I. Rapid and slow progressors differ by a single MHC class I haplotype in a family of MHC-defined rhesus macaques infected with SIV. Immunol Lett. 1999;66:53–59. doi: 10.1016/s0165-2478(98)00151-5. [DOI] [PubMed] [Google Scholar]
- 14.Evans D T, O'Connor D H, Jing P, Dzuris J L, Sidney J, da Silva J, Allen T M, Horton H, Venham J E, Rudersdorf R A, Vogel T, Pauza C D, Bontrop R E, DeMars R, Sette A, Hughes A L, Watkins D I. Virus-specific cytotoxic T-lymphocyte responses select for amino-acid variation in simian immunodeficiency virus Env and Nef. Nat Med. 1999;5:1270–1276. doi: 10.1038/15224. [DOI] [PubMed] [Google Scholar]
- 15.Falk K, Rotzschke O, Takiguchi M, Gnau V, Stevanovic S, Jung G, Rammensee H G. Peptide motifs of HLA-B58, B60, B61, and B62 molecules. Immunogenetics. 1995;41:165–168. doi: 10.1007/BF00182333. [DOI] [PubMed] [Google Scholar]
- 16.Goulder P J, Brander C, Annamalai K, Mngqundaniso N, Govender U, Tang Y, He S, Hartman K E, O'Callaghan C A, Ogg G S, Altfeld M A, Rosenberg E S, Cao H, Kalams S A, Hammond M, Bunce M, Pelton S I, Burchett S A, McIntosh K, Coovadia H M, Walker B D. Differential narrow focusing of immunodominant human immunodeficiency virus Gag-specific cytotoxic T-lymphocyte responses in infected African and Caucasoid adults and children. J Virol. 2000;74:5679–5690. doi: 10.1128/jvi.74.12.5679-5690.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goulder P J, Bunce M, Krausa P, McIntyre K, Crowley S, Morgan B, Edwards A, Giangrande P, Phillips R E, McMichael A J. Novel, cross-restricted, conserved, and immunodominant cytotoxic T lymphocyte epitopes in slow progressors in HIV type 1 infection. AIDS Res Hum Retrovir. 1996;12:1691–1698. doi: 10.1089/aid.1996.12.1691. [DOI] [PubMed] [Google Scholar]
- 18.Goulder P J, Phillips R E, Colbert R A, McAdam S, Ogg G, Nowak M A, Giangrande P, Luzzi G, Morgan B, Edwards A, McMichael A J, Rowland-Jones S. Late escape from an immunodominant cytotoxic T-lymphocyte response associated with progression to AIDS. Nat Med. 1997;3:212–217. doi: 10.1038/nm0297-212. [DOI] [PubMed] [Google Scholar]
- 19.Goulder P J, Reid S W, Price D A, O'Callaghan C A, McMichael A J, Phillips R E, Jones E Y. Combined structural and immunological refinement of HIV-1 HLA-B8-restricted cytotoxic T lymphocyte epitopes. Eur J Immunol. 1997;27:1515–1521. doi: 10.1002/eji.1830270630. [DOI] [PubMed] [Google Scholar]
- 20.Goulder P J, Walker B D. The great escape—AIDS viruses and immune control. Nat Med. 1999;5:1233–1235. doi: 10.1038/15184. [DOI] [PubMed] [Google Scholar]
- 21.Goulder P J R. Anti-HIV cellular immunity: recent advances towards vaccine designe. AIDS. 1999;13(Suppl. A):S121–136. [PubMed] [Google Scholar]
- 22.Imanishi T, Akaza T, Kimura A, Tokunaga K, Gojobori T. Allele and haplotype frequencies for HLA and complement loci in various ethnic groups. In: Tsuji K, Aizawa M, Sasazuki T, editors. HLA 1991—Proceedings of the Xth International Histocompatibility Workshop and Conference. Vol. 1. Paris, France: EDK; 1992. pp. 1065–1220. [Google Scholar]
- 23.Jeffery K J, Usuku K, Hall S E, Matsumoto W, Taylor G P, Procter J, Bunce M, Ogg G S, Welsh K I, Weber J N, Lloyd A L, Nowak M A, Nagai M, Kodama D, Izumo S, Osame M, Bangham C R. HLA alleles determine human T-lymphotropic virus-I (HTLV-I) proviral load and the risk of HTLV-I-associated myelopathy. Proc Natl Acad Sci USA. 1999;96:3848–3853. doi: 10.1073/pnas.96.7.3848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jin X, Bauer D E, Tuttleton S E, Lewin S, Gettie A, Blanchard J, Irwin C E, Safrit J T, Mittler J, Weinberger L, Kostrikis L G, Zhang L, Perelson A S, Ho D D. Dramatic rise in plasma viremia after CD8(+) T cell depletion in simian immunodeficiency virus-infected macaques. J Exp Med. 1999;189:991–998. doi: 10.1084/jem.189.6.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnson R P, Trocha A, Yang L, Mazzara G P, Panicali D L, Buchanan T M, Walker B D. HIV-1 gag-specific cytotoxic T lymphocytes recognize multiple highly conserved epitopes. Fine specificity of the gag-specific response defined by using unstimulated peripheral blood mononuclear cells and cloned effector cells. J Immunol. 1991;147:1512–1521. [PubMed] [Google Scholar]
- 26.Kalams S A, Buchbinder S P, Rosenberg E S, Billingsley J M, Colbert D S, Jones N G, Shea A K, Trocha A K, Walker B D. Association between virus-specific cytotoxic T-lymphocyte and helper responses in human immunodeficiency virus type 1 infection. J Virol. 1999;73:6715–6720. doi: 10.1128/jvi.73.8.6715-6720.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kalams S A, Goulder P J, Shea A K, Jones N G, Trocha A K, Ogg G S, Walker B D. Levels of human immunodeficiency virus type 1-specific cytotoxic T-lymphocyte effector and memory responses decline after suppression of viremia with highly active antiretroviral therapy. J Virol. 1999;73:6721–6728. doi: 10.1128/jvi.73.8.6721-6728.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaneko T, Nakamura I, Kita H, Hiroishi K, Moriyama T, Imawari M. Three new cytotoxic T cell epitopes identified within the hepatitis C virus nucleoprotein. J Gen Virol. 1996;77:1305–1309. doi: 10.1099/0022-1317-77-6-1305. [DOI] [PubMed] [Google Scholar]
- 29.Kaslow R A, Carrington M, Apple R, Park L, Munoz A, Saah A J, Goedert J J, Winkler C, O'Brien S J, Rinaldo C, Detels R, Blattner W, Phair J, Erlich H, Mann D L. Influence of combinations of human major histocompatibility complex genes on the course of HIV-1 infection. Nat Med. 1996;2:405–411. doi: 10.1038/nm0496-405. [DOI] [PubMed] [Google Scholar]
- 30.Keet I P, Tang J, Klein M R, LeBlanc S, Enger C, Rivers C, Apple R J, Mann D, Goedert J J, Miedema F, Kaslow R A. Consistent associations of HLA class I and II and transporter gene products with progression of human immunodeficiency virus type 1 infection in homosexual men. J Infect Dis. 1999;180:299–309. doi: 10.1086/314862. [DOI] [PubMed] [Google Scholar]
- 31.Klein M R, van Baalen C A, Holwerda A M, Kerkhof Garde S R, Bende R J, Keet I P, Eeftinck-Schattenkerk J K, Osterhaus A D, Schuitemaker H, Miedema F. Kinetics of Gag-specific cytotoxic T lymphocyte responses during the clinical course of HIV-1 infection: a longitudinal analysis of rapid progressors and long-term asymptomatics. J Exp Med. 1995;181:1365–1372. doi: 10.1084/jem.181.4.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koenig S, Conley A J, Brewah Y A, Jones G M, Leath S, Boots L J, Davey V, Pantaleo G, Demarest J F, Carter C, et al. Transfer of HIV-1-specific cytotoxic T lymphocytes to an AIDS patient leads to selection for mutant HIV variants and subsequent disease progression. Nat Med. 1995;1:330–336. doi: 10.1038/nm0495-330. [DOI] [PubMed] [Google Scholar]
- 33.Koup R A, Safrit J T, Cao Y, Andrews C A, McLeod G, Borkowsky W, Farthing C, Ho D D. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J Virol. 1994;68:4650–4655. doi: 10.1128/jvi.68.7.4650-4655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee K W, Kim Y S. Serologic ambiguity and allelic frequency of the HLA-B40 family in the Korean population. Tissue Antigens. 1997;49:383–388. doi: 10.1111/j.1399-0039.1997.tb02766.x. [DOI] [PubMed] [Google Scholar]
- 35.Lee S P, Tierney R J, Thomas W A, Brooks J M, Rickinson A B. Conserved CTL epitopes within EBV latent membrane protein 2: a potential target for CTL-based tumor therapy. J Immunol. 1997;158:3325–3334. [PubMed] [Google Scholar]
- 36.Ogawa A, Tokunaga K, Lin L, Kashiwase K, Tanaka H, Herrero M J, Vilches C, Park M H, Jia G J, Chimge N O, Sideltseva E W, Ishikawa Y, Akaza T, Tadokoro K, Juji T. Diversity of HLA-B61 alleles and haplotypes in East Asians and Spanish Gypsies. Tissue Antigens. 1998;51:356–366. doi: 10.1111/j.1399-0039.1998.tb02974.x. [DOI] [PubMed] [Google Scholar]
- 37.Ogg G S, Jin X, Bonhoeffer S, Dunbar P R, Nowak M A, Monard S, Segal J P, Cao Y, Rowland-Jones S L, Cerundolo V, Hurley A, Markowitz M, Ho D D, Nixon D F, McMichael A J. Quantitation of HIV-1-specific cytotoxic T lymphocytes and plasma load of viral RNA. Science. 1998;279:2103–2106. doi: 10.1126/science.279.5359.2103. [DOI] [PubMed] [Google Scholar]
- 38.Price D A, Goulder P J, Klenerman P, Sewell A K, Easterbrook P J, Troop M, Bangham C R, Phillips R E. Positive selection of HIV-1 cytotoxic T lymphocyte escape variants during primary infection. Proc Natl Acad Sci USA. 1997;94:1890–1895. doi: 10.1073/pnas.94.5.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Raffoux C, Williams R C, Gorodezky C, Albert E D, Gyodi E, Hammond M G, Layrisse Z, Mariani M, de la Rosa G, Tiercy J M, Tokunaga K, Yang E. HLA-B12, B13, B21, B37, B40, B41, B48, B81, B4005. In: Charron D, editor. Proceedings of the XIIth International Histocompatibility Workshop. Vol. 1. Paris, France: EDK; 1997. pp. 69–72. [Google Scholar]
- 40.Schmitz J E, Kuroda M J, Santra S, Sasseville V G, Simon M A, Lifton M A, Racz P, Tenner-Racz K, Dalesandro M, Scallon B J, Ghrayeb J, Forman M A, Montefiori D C, Rieber E P, Letvin N L, Reimann K A. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science. 1999;283:857–860. doi: 10.1126/science.283.5403.857. [DOI] [PubMed] [Google Scholar]
- 41.Threlkeld S C, Wentworth P A, Kalams S A, Wilkes B M, Ruhl D J, Keogh E, Sidney J, Southwood S, Walker B D, Sette A. Degenerate and promiscuous recognition by CTL of peptides presented by the MHC class I A3-like superfamily: implications for vaccine development. J Immunol. 1997;159:1648–1657. [PubMed] [Google Scholar]
- 42.Walker B D, Chakrabarti S, Moss B, Paradis T J, Flynn T, Durno A G, Blumberg R S, Kaplan J C, Hirsch M S, Schooley R T. HIV-specific cytotoxic T lymphocytes in seropositive individuals. Nature. 1987;328:345–348. doi: 10.1038/328345a0. [DOI] [PubMed] [Google Scholar]
- 43.Walker B D, Flexner C, Birch-Limberger K, Fisher L, Paradis T J, Aldovini A, Young R, Moss B, Schooley R T. Long-term culture and fine specificity of human cytotoxic T-lymphocyte clones reactive with human immunodeficiency virus type 1. Proc Natl Acad Sci USA. 1989;86:9514–9518. doi: 10.1073/pnas.86.23.9514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wong D K, Dudley D D, Afdhal N H, Dienstag J, Rice C M, Wang L, Houghton M, Walker B D, Koziel M J. Liver-derived CTL in hepatitis C virus infection: breadth and specificity of responses in a cohort of persons with chronic infection. J Immunol. 1998;160:1479–1488. [PubMed] [Google Scholar]
- 45.Yang O O, Walker B D. CD8+ cells in human immunodeficiency virus type I pathogenesis: cytolytic and noncytolytic inhibition of viral replication. Adv Immunol. 1997;66:273–311. doi: 10.1016/s0065-2776(08)60600-8. [DOI] [PubMed] [Google Scholar]