Abstract
BACKGROUND:
Women with elevated body mass index are encouraged to lose weight before pregnancy, but no trials have tested the effects of prepregnancy weight loss on health outcomes.
OBJECTIVE:
This study aimed to determine whether prepregnancy weight loss reduces gestational weight gain and improves pregnancy outcomes.
STUDY DESIGN:
Pragmatic randomized clinical trial was conducted between May 2015 and October 2019 at Kaiser Permanente Northwest, an integrated health system. Data collection was blind to condition assignment. Eligible participants were women aged 18 to 40 years with a body mass index of ≥27 kg/m2 who were planning pregnancy within 2 years. Recruitment contacts were sent to 27,665 health system members who met age and body mass index criteria; 329 women attended screening visits, and 326 were randomized. They were randomized to either a behavioral weight loss intervention or usual care control. The intervention consisted of health coaching phone sessions weekly for 6 months and then monthly for 18 months or until end of pregnancy. We used logistic regression to examine the a priori primary hypothesis that participants in the intervention would be less likely to exceed National Academy of Medicine guidelines for gestational weight gain during each trimester and overall. Secondary and exploratory outcomes included absolute weight gain before and during pregnancy and perinatal and newborn outcomes.
RESULTS:
Of the 326 participants, 169 had singleton pregnancies lasting ≥14 weeks (analytical cohort: intervention, 89; control, 80). At baseline, mean age was 31.3±3.5 years, and body mass index was 34.8±5.8 kg/m2. Participants in the intervention group lost more weight before pregnancy than those in the control group (−0.25±0.51 vs −0.03±0.21 kg/wk; P<.001). However, participants in the intervention group gained more weight than those in the control group in the second trimester (0.42±0.26 vs 0.33±0.28 kg/wk; P=.04) and third trimester (0.56±0.37 vs 0.43±0.33 kg/wk; P=.02) and overall (13.2±8.20 vs 10.3±7.41 kg; P=.03). Nevertheless, arms did not differ in rates of exceeding gestational weight gain guidelines at any time point. Spontaneous pregnancy loss was less common in the intervention arm than in the control arm (8 [4.9%] vs 19 [11.8%]; odds ratio, 0.39 [0.16–0.92]), but we found no other differences in the secondary or exploratory outcomes.
CONCLUSION:
Participation in the prepregnancy weight loss intervention had no effect on women’s likelihood of exceeding gestational weight gain guidelines. Although the intervention group successfully lost weight before conception, the intervention group was associated with greater weight gain in late pregnancy. To effectively reduce weight throughout pregnancy and improve maternal and child outcomes, prepregnancy weight loss interventions may need to be combined with intensive weight management that continues throughout delivery.
Keywords: behavioral weight loss intervention, gestational weight guidelines, obesity, overweight, prepregnancy, prenatal counseling, weight management
Both starting pregnancy with an elevated body mass index (BMI) and gaining an excessive amount of weight during pregnancy are associated with adverse pregnancy outcomes.1,2 Because of this, guidelines encourage women with elevated BMIs (about half of reproductive-aged women)3 to lose weight before conception and avoid excessive gestational weight gain (GWG).4 However, no studies have prospectively evaluated how weight management started before pregnancy affects GWG or birth outcomes.5
We conducted a pragmatic randomized clinical trial to test the efficacy of a behavioral weight management intervention initiated before pregnancy for women who were overweight or obese and were planning pregnancy.6 The intervention consisted of individualized health coaching phone sessions. We hypothesized that women assigned to the intervention would lose more weight before pregnancy and gain less weight during pregnancy than women assigned to a usual care control arm, resulting in a lower likelihood of exceeding National Academy of Medicine (NAM) guidelines for GWG,4,7 lower infant birthweight, and better perinatal outcomes.
Materials and Methods
Study setting
We recruited participants from Kaiser Permanente Northwest (KPNW), a nonprofit integrated healthcare system serving individuals in Oregon and Southwest Washington. The study was conducted and reported in accordance with a previously published protocol6 that was approved by the KPNW institutional review board. A Data and Safety Monitoring Board provided independent study monitoring.
Participants
We used electronic medical records (EMR) to identify potentially eligible participants. Between May 2015 and September 2016, we contacted all female KPNW members aged 18 to 40 years with BMIs ≥27 kg/m2 via letters, emails, and text messages. Interested women attended information sessions outlining eligibility criteria including that they be planning pregnancy in the next 2 years, not currently pregnant, and not have conditions or be on medications that would affect weight loss or participation.6 Eligibility of interested women was confirmed at a screening visit through questionnaires and interviews with research staff.
Baseline visit and initial session
At a baseline visit, participant’s height and weight were recorded in light indoor clothing with their shoes removed; weight was measured with a regularly calibrated digital scale. Participants were then randomized to the intervention or control arm (1:1 ratio). Randomization was stratified by age (<30, ≥30), BMI (27–30, 31–35, ≥36 kg/m2), and parity (0, ≥1). Allocation was concealed until the randomization button was pressed.
Women assigned to the intervention group attended an introductory session, reviewing the study goals and website (~30–40 minutes). Women in the control group were given information on having a healthy pregnancy (~5–10 minutes); participants in the intervention group received this information in later sessions.6 All women received routine prenatal care through their obstetrical provider. To foster retention, the study team sent yearly birthday and holiday cards.
Intervention
The intervention was designed to be implementable in a wide variety of settings and consisted of individualized 20-to 30-minute telephone counseling sessions with the health coach, a trained behavioral interventionist, and access to a personalized intervention website.6 Sessions occurred weekly for 6 months and then monthly for 18 months or until end of pregnancy (mean, 42 sessions).
Participants were encouraged to lose weight before pregnancy (0.2–0.4 kg/wk) by following the dietary approaches to stop hypertension dietary plan without sodium restriction8 at a customized caloric target set using the Harris-Benedict equation9 and to exercise, working toward 2 daily goals: 60 minutes of moderate-intensity physical activity and walking at least 10,000 steps. Intervention goals are shown in Table 1. When a participant reported becoming pregnant, they continued participating in the intervention with the primary goal changed to keeping GWG within NAM guidelines (Table 2) and with modifications to their dietary plan and caloric target.
TABLE 1.
Overall Prepare intervention goals
| Goal | Specific instructions |
|---|---|
| Be an active and engaged participant |
|
| Manage calories to be within your customized calorie targeta |
|
| Follow the DASH dietary eating pattern for a healthy diet every day |
|
| Increase your daily physical activity |
|
| Keep records |
|
DASH, dietary approaches to stop hypertension.
Initial calorie needs were set using the Harris-Benedict equation.9 Coaches modified calorie targets as needed to meet weight loss and maintenance goals.
TABLE 2.
The 2009 NAM guidelines for GWG
| Prepregnancy BMI category | Prepregnancy BMI range (kg/m2) | Rates of weight gain in first trimester (kg) | Rates of weight gain in second and third trimester (kg/wk) | Total weight gain (kg) |
|---|---|---|---|---|
| Underweight | <18.5 | 0.5–2.0 | 0.44–0.50 | 12.5–18.0 |
| Normal weight | 18.5–24.9 | 0.5–2.0 | 0.35–0.50 | 11.5–16.0 |
| Overweight | 25.0–29.9 | 0.5–2.0 | 0.23–0.33 | 7.0–11.5 |
| Obese (includes all classes) | ≥30.0 | 0.5–2.0 | 0.17–0.27 | 5.0–9.0 |
BMI, body mass index; GWG, gestational weight gain; NAM, National Academy of Medicine.
Intervention fidelity
Mean duration in the intervention was 17.9 months. Completion rates were 70.9% and 73.3% for weekly and monthly phone calls, with average lengths of 21.8 and 20.1 minutes per completed call, respectively. Among women who became pregnant during the intervention, weight, food, and exercise were logged on the study website with a mean of 4.5, 4.3, and 4.8 times per week during the weekly phase and 2.3, 1.9, and 2.5 days per week during the monthly phase, respectively; among those who did not experience pregnancy during the intervention, weight, food, and exercise were logged on the website with a mean of 3.1, 3.4, and 3.5 times per week during the weekly phase and 0.9, 1.0, and 1.1 days per week during the monthly phase, respectively. One participant dropped out of the intervention.
Outcome measures
Our primary outcome variable was whether women exceeded NAM guidelines for GWG in each trimester (in the full analytical cohort) and overall (for pregnancies lasting ≥37 weeks). GWG was chosen because it is associated with increased risk of infants who are large for gestational age (LGA), cesarean delivery, gestational hypertension (HTN-P), and gestational diabetes mellitus (GDM).10–17 We examined trimester-specific GWG because the rate and causes of weight gain vary across pregnancies4,16,18 and early and late GWGs may have different associations with outcomes.16,19,20 Secondary outcomes included mean weight change before and during pregnancies, offspring birthweight, and birthweight relative to gestational age z-score.21 In exploratory analyses, we examined perinatal outcomes and adverse events.
We assessed outcome variables through EMR follow-up. EMR data were collected by clinical staff during the course of routine medical care, and the staff were unaware of the study. Past research has documented the accuracy of EMR weight, including in populations of pregnant women.22–26 We were unable to obtain data from 4 participants (2.3%) who left KPNW before giving birth (Figure 1).
FIGURE 1. CONSORT diagram.
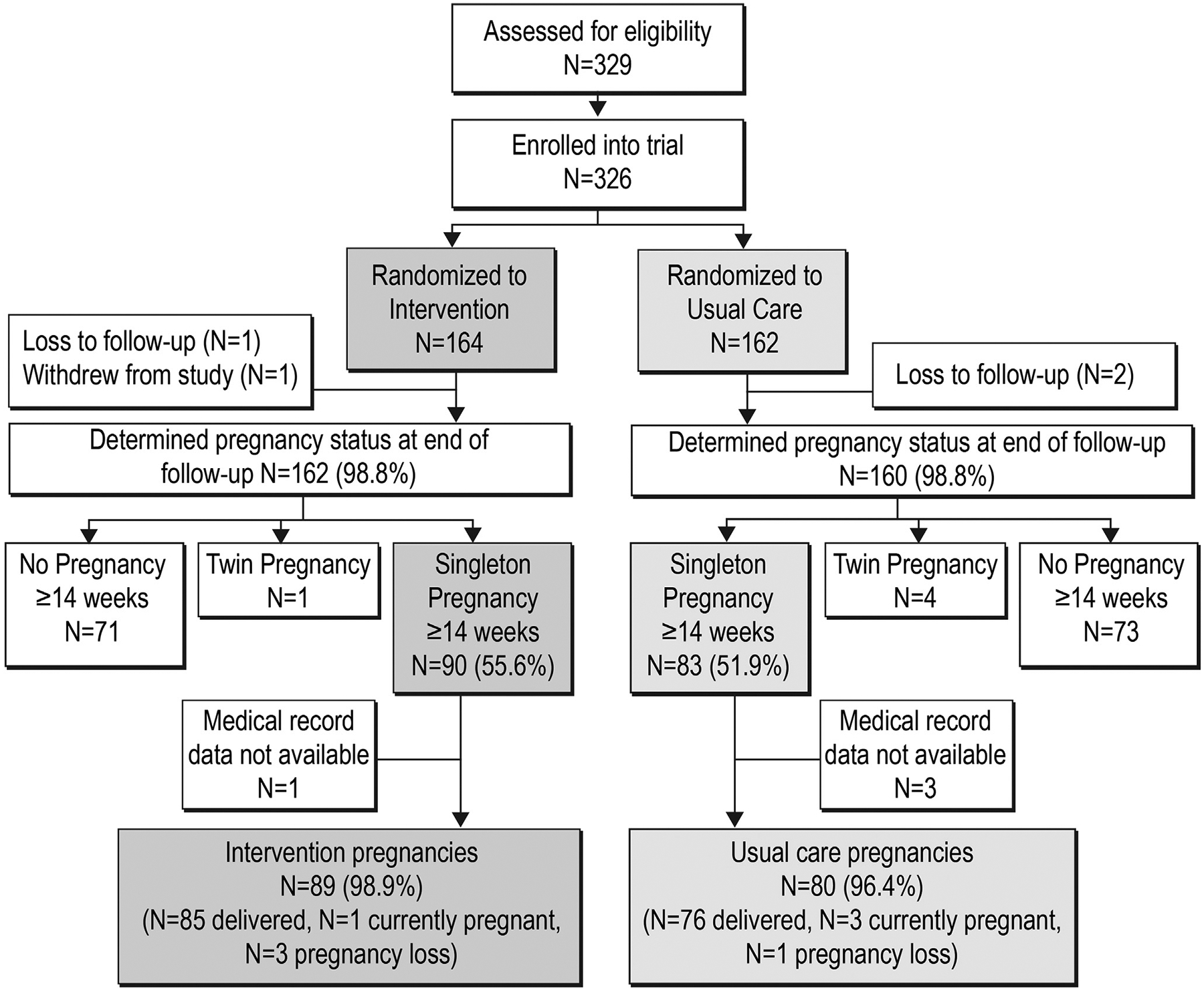
CONSORT, Consolidated Standards of Reporting Trials.
Maternal weight outcomes
We captured the following maternal weights in the EMR: self-reported prepregnancy weight (reported to obstetrical providers at first prenatal visit), weight at end of first trimester (14 weeks’ gestation±20 days) and second trimester (28 weeks’ gestation±20 days), and last recorded weight before delivery. Self-reported prepregnancy weight was used as a surrogate for weight at conception because this measurement method has been validated in research contexts.11,27–32 However, if self-reported prepregnancy weight was unavailable, we used measured weight at first prenatal visit if before 11 weeks’ gestation (8 [4.7%]; correlation, 0.99). Preconception weight change was calculated as weight change from randomization to self-reported prepregnancy weight (or measured weight at first prenatal visit) in both kilograms per week and total kilograms. Trimester-specific GWG was calculated as kilograms per week; total GWG in kilograms was calculated for those who delivered at or after 37 weeks’ gestation (141 women). Each GWG measurement was categorized as exceeding or not exceeding NAM guidelines on the basis of prepregnancy BMI.
Offspring weight outcomes
We collected newborn birthweight, estimated date of delivery (EDD), and date of birth (DOB) from the EMR. We calculated date of conception (DOC=EDD–40 weeks) and gestational age at birth (DOB–DOC).
Perinatal outcomes and adverse events
The variables below were identified by EMR codes and then adjudicated by blinded medical chart review (E.S.L., K.V.):
GDM was based on clinical diagnosis by obstetrician, oral glucose tolerance test results,33 and/or referral to diabetes case management.
Preterm birth was defined as delivery before 37 weeks’ gestation.
HTN-P included hypertension with onset during pregnancy, preeclampsia, and eclampsia.
Cesarean delivery was based on delivery notes.
LGA was defined as birthweight ≥90th percentile for gestational age.
Maternal adverse events included spontaneous pregnancy loss before 20 weeks’ gestation, bone fracture at any site, and hospitalization unrelated to delivery.
Infant adverse events included fetal death (loss after 20 weeks’ gestation); small for gestational age (birthweight <10th percentile for gestational age); hypoglycemia requiring treatment; respiratory distress requiring oxygen, continuous positive airway pressure, or mechanical ventilation; and congenital anomalies.
Demographics
Self-reported age, race, ethnicity, income, education level, and information about previous pregnancies were collected at baseline and supplemented by EMR review.
Other variables
We determined whether participants received fertility treatment after randomization, a potential confounding variable, on the basis of EMRs of fertility medication dispensing and/or procedures. Diet and exercise levels at 20 weeks and satisfaction with the intervention were collected via electronic questionnaires and will be the subject of future analyses.
Power calculations
We conducted sample size calculations for our primary outcome, exceeding NAM guidelines for GWG, to detect a 20% difference between the 2 arms, which translates into a clinically meaningful difference.11 To detect a 20.5% difference at 80% power with an alpha level of 0.05, we needed 75 pregnancies in each arm. Historic EMR data suggested a 50% pregnancy rate in our population, yielding a recruitment target of 300 women.
Statistical analyses
Analyses were based on modified intention-to-treat principles.34,35 We included pregnancies lasting at least 14 weeks, including pregnancies that did not result in live births or were ongoing at study end; pregnancies were included in analyses for trimesters for which they could contribute data (Table 3). We only included singleton pregnancies because multiple gestation pregnancies have different weight gain recommendations.4 Pregnancy rate did not differ between the 2 arms (54% in the intervention group vs 49% in the control group). All randomized participants were included in adverse event analyses, regardless of pregnancy status.
TABLE 3.
Reasons for ineligibility in analyses and missing data
| Weight variable | Number of participants | Reasons for ineligibility | Number of participants with missing weights at each time point | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Eligible, n (% of total cohort [N=169]) | Analyzed, n (% eligible) | Pregnant at randomizationa | Late pregnancy loss (14–20 wk) | Preterm birth (28–37 wk) | Pregnancy ongoing at end of follow-up | Total | Pregnancyonsetb | End of first trimesterc | End of second trimesterd | ≥37 wke | |
| Prepregnancy weight loss | 157 (92.9) | 156 (99.4) | 12 | — | — | — | 1 | 1 | — | — | — |
| First-trimester GWG | 169 (100.0) | 165 (97.6) | — | — | — | — | 4 | 1 | 3 | — | — |
| Second trimester GWG | 165 (97.6) | 160 (97.0) | — | 4 | — | — | 5f | — | 3 | 3 | — |
| Third trimester GWG | 160 (94.7) | 157 (98.1) | — | 4 | 1 | 4 | 3 | — | — | 3 | — |
| Total GWGg | 149 (88.2) | 141 (94.6) | — | 4 | 12 | 4 | 8 | 1 | — | — | 7 |
GWG, gestational weight gain.
Although participants self-reported not being pregnant at the baseline visit, it was later determined that some were already pregnant;
Self-reported prepregnancy weight or, if not available, weight at first prenatal visit (must have been measured before 11 weeks’ gestation);
Weight had to be measured at 14 weeks’ gestation±20 days;
Weight had to be measured at 28 weeks’ gestation±20 days;
Weight had to be measured at or after 37 weeks’ gestation to be included in the total GWG analysis;
Total does not equal sum of row because 1 person was missing at both end of the first trimester and end of the second trimester weight so only counted once in total;
Participants had to be at full term (≥37 weeks’ gestation) to be included in the total GWG analysis.
We adjusted for multiple comparisons in primary analyses using Bonferroni correction. For exploratory analyses, no correction was applied. Given the small amount of missing data (Table 3), no missing data imputation methods were applied. All analysis assumptions were verified.
For the primary analysis, we compared rates of exceeding NAM guidelines for GWG for each trimester and overall between the intervention and control arms using logistic regression. For the secondary outcome, we used linear regression models to compare continuous measures of trimester-specific GWG and total GWG across arms. We controlled for weight at randomization in both models. Baseline demographic factors were not included in models as they did not differ between arms. Because the intervention was delivered in a 6-month intensive phase (weekly contact) followed by a maintenance phase (monthly contact) for up to 18 additional months and because pregnancy onset could occur at any time, women had varying amounts of intervention exposure. We therefore further stratified analyses on the basis of time from randomization to becoming pregnant using the following 3 strata: intensive phase (0–6 months), maintenance phase (6–24 months), and after maintenance phase (>24 months).
In additional secondary analyses, we compared offspring birthweight between the 2 arms using 2-sample t tests. In exploratory analyses, we used unadjusted logistic regression models to compare rates of each binary perinatal outcome and adverse events between the 2 arms. In addition, for maternal events, we adjusted for whether outcome had occurred in a prior pregnancy.
Sensitivity analyses examined results stratified by BMI status at pregnancy onset, excluded women who received fertility treatment, and used measured weight at first prenatal visit instead of self-reported prepregnancy weight. In exploratory analyses, we stratified results by GDM status.
Results
Participants
Of 329 women assessed for eligibility, 326 were eligible and randomized to the intervention group (n=164) or control group (n=162). The analytical cohort comprised 169 participants (89 in the intervention group and 80 in the control group) who had singleton pregnancies lasting ≥14 weeks (Figure 1). Demographics at randomization did not differ between the 2 arms in the analytical cohort (Table 4). In the intervention arm, 90% of participants were still participating in health coaching visits at the onset of pregnancy: 34% transitioned from weekly to monthly sessions during pregnancy, whereas 56% had only monthly sessions throughout pregnancy. Women in the analytical cohort were younger, had lower weights, were less likely to be nulliparous, and had higher incomes than women who were not in the cohort (Table 5).
TABLE 4.
Demographics at randomization and pregnancy onset
| Variable | Overall (N=169) | Intervention group (n=89) | Usual care control group (n=80) |
|---|---|---|---|
| Randomization | |||
| Age, y (mean [SD]) | 31.3 (3.5) | 31.6 (3.5) | 30.9 (3.5) |
| Weight, kg (mean [SD]) | 95.2 (17.2) | 95.6 (17.8) | 94.9 (16.6) |
| BMI, kg/m2 (mean [SD]) | 34.8 (5.8) | 34.9 (6.0) | 34.7 (5.5) |
| Patients with a BMI of 27.0–29.9 | 37 (21.9) | 19 (21.4) | 18 (22.5) |
| Patients with a BMI of 30.0–34.9 | 65 (38.5) | 34 (38.2) | 31 (38.8) |
| Patients with a BMI of ≥35.0 | 67 (39.6) | 36 (40.5) | 31 (38.8) |
| Race | |||
| White | 142 (84.0) | 72 (80.9) | 70 (87.5) |
| Asian | 2 (1.2) | 0 (0.0) | 2 (2.5) |
| Black | 7 (4.2) | 5 (5.6) | 2 (2.5) |
| >1 race | 14 (8.3) | 11 (12.4) | 3 (3.8) |
| Did not report | 4 (2.4) | 1 (1.1) | 3 (3.8) |
| Hispanic ethnicity | 16 (9.5) | 6 (6.7) | 10 (12.5) |
| Education | |||
| High school graduate or GED certificate | 24 (14.2) | 10 (11.2) | 14 (17.5) |
| Technical school graduate | 10 (6.0) | 8 (9.0) | 2 (2.5) |
| College graduate or higher | 135 (79.9) | 71 (79.8) | 64 (80.0) |
| Income | |||
| <$45,000 | 20 (11.8) | 12 (13.5) | 8 (10.0) |
| $45,000–$89,999 | 76 (45.0) | 47 (52.8) | 29 (36.3) |
| ≥$90,000 | 69 (40.8) | 28 (31.5) | 41 (51.3) |
| Not reported | 4 (2.4) | 2 (2.3) | 2 (2.5) |
| Previous live birth | 70 (41.4) | 35 (39.3) | 35 (43.8) |
| Pregnancy onset | |||
| Time from randomization to pregnancy onset | |||
| <6 mo | 56 (33.1) | 30 (33.7)a | 26 (32.5) |
| 6–24 mo | 86 (50.9) | 50 (56.2)b | 36 (45.0) |
| >24 mo | 27 (16.0) | 9 (10.1)c | 18 (22.5) |
| Infertility treatment between randomization and pregnancy onset | 27 (16.0) | 13 (14.6) | 14 (17.5) |
| BMI category at pregnancy onsetd | |||
| Overweight (25.0–29.9 kg/m2) | 48 (28.6) | 27 (30.7) | 21 (26.2) |
| Obese (≥30.0 kg/m2) | 120 (71.4) | 61 (69.3) | 59 (73.8) |
Data are presented as number (percentage), unless otherwise specified.
BMI, body mass index; GED, General Educational Development; SD, standard deviation.
Pregnancy onset occurred during weekly intervention phase; intervention continued during pregnancy (weekly sessions in early pregnancy transitioned to monthly sessions in later pregnancy);
Pregnancy onset occurred during monthly intervention phase; intervention continued during pregnancy (only monthly sessions during pregnancy);
Pregnancy onset occurred after the intervention ended; there was no intervention administered during pregnancy;
One participant in the intervention group was missing BMI information at pregnancy onset.
TABLE 5.
Demographics of women who experienced pregnancy lasting at least 14 weeks (analytical cohort) vs those who did not experience pregnancy lasting at least 14 weeks (not included in analyses)
| Variable | Overall (N=326) | Pregnancy at ≥14 wk (included in analyses) (n=169) | No pregnancy at ≥14 wk (not included in analyses) (n=157) | P value |
|---|---|---|---|---|
| Age at randomization, y (mean [SD]) | 31.7 (3.8) | 31.3 (3.5) | 32.1 (4.1) | .04 |
| Weight, kg (mean [SD]) | 100.7 (21.7) | 95.3 (17.2) | 106.4 (24.4) | <.001 |
| BMI, kg/m2 (mean [SD]) | 36.8 (7.3) | 34.8 (5.8) | 38.8 (8.2) | |
| Patients with a BMI of 27.0−29.9 | 59 (18.1) | 37 (21.9) | 22 (14.0) | <.001 |
| Patients with a BMI of 30.0−34.9 | 104 (31.9) | 65 (38.5) | 39 (24.8) | |
| Patients with a BMI of ≥35.0 | 163 (50.0) | 67 (39.6) | 96 (60.2) | |
| Race | ||||
| White | 264 (81.0) | 142 (84.0) | 122 (77.7) | |
| Asian | 5 (1.5) | 2 (1.2) | 3 (1.9) | |
| American Indian or Alaska Native | 1 (0.3) | 0 (0.0) | 1 (0.6) | |
| Native Hawaiian or Other Pacific Islander | 1 (0.3) | 0 (0.0) | 1 (0.6) | |
| Black | 13 (4.0) | 7 (4.1) | 6 (3.8) | |
| >1 race | 35 (10.7) | 14 (8.3) | 21 (13.4) | |
| Did not wish to report | 7 (2.2) | 4 (2.4) | 3 (1.9) | |
| Hispanic ethnicity | ||||
| No | 302 (92.6) | 153 (90.5) | 149 (94.9) | |
| Yes | 23 (7.1) | 16 (9.5) | 7 (4.5) | |
| Do not wish to report | 1 (0.3) | 0 (0.0) | 1 (0.6) | |
| Education | ||||
| High school graduate or GED certificate | 55 (16.9) | 24 (14.2) | 31 (19.8) | |
| Technical school graduate | 23 (7.1) | 10 (5.9) | 13 (8.3) | |
| College graduate or higher | 248 (76.1) | 135 (79.9) | 113 (72.0) | |
| Income | ||||
| <$45,000 | 43 (13.2) | 20 (11.8) | 23 (14.7) | |
| $45,000–$89,999 | 162 (49.7) | 76 (45.0) | 86 (54.8) | |
| ≥$90,000 | 110 (33.7) | 69 (40.8) | 41 (26.1) | |
| Did not wish to report | 11 (3.4) | 4 (2.4) | 7 (4.5) | |
| Previous live birth | 109 (33.4) | 71 (42.0) | 38 (24.1) |
Data are presented as number (percentage), unless otherwise specified.
BMI, body mass index; GED, General Educational Development; SD, standard deviation.
Outcomes
Weight loss before pregnancy onset
Women lost 3.7±8.3 kg (3.5% of randomization weight) in the intervention arm and 0.6±8.1 kg (0.5%) in the control arm before pregnancy (0.25±0.51 vs 0.03±0.21 kg/wk; P<.001) (Figure 2). Overall, BMI decreased by 1.32±2.86 kg/m2 in the intervention arm and 0.25±2.93 kg/m2 in the control arm (P=.02). A total of 13 participants (15.5%) in the intervention group dropped from the obese to overweight category between randomization and pregnancy onset, compared with 4 participants (5.3%) in the usual care group (P=.04), although all women remained in the overweight or obese category (Table 4).
FIGURE 2. Gestational weight gain between intervention and usual care groups.
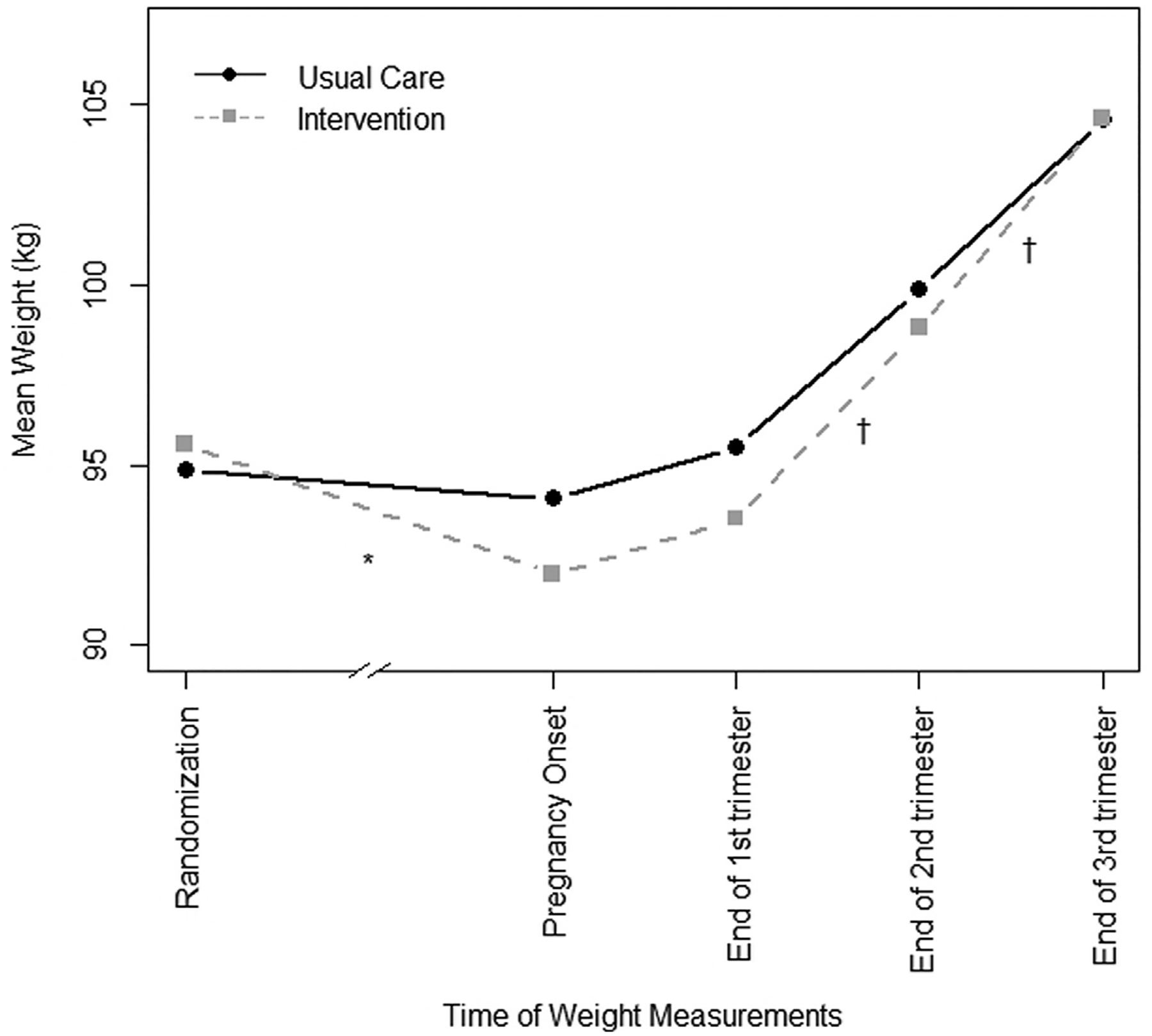
The numbers in analyses decrease in later time points in pregnancy as shown in Table 6. *P<.001; †P<.05.
Gestational weight gain
There was no significant difference in percentage of participants exceeding NAM guidelines for GWG between the 2 arms in any trimester or overall (Table 6). Mean weight gain was actually higher in the intervention arm in the second (0.42±0.26 vs 0.33±0.28 kg/wk; P=.04) and third (0.56±0.37 vs 0.43±0.33 kg/wk; P=.02) trimesters, leading to higher overall weight gain among the cohort with term pregnancies ≥37 weeks (141 women; 13.24±8.20 vs 10.32±7.41 kg; P=.03). Results were the same in sensitivity analyses in which we (1) examined effects of the intervention separately for those who were overweight or obese at pregnancy onset, (2) used measured weight at first prenatal visit for all participants rather than self-reported prepregnancy weight, (3) excluded women who underwent fertility treatments, and (4) examined effects on women with and without GDM diagnoses separately.
TABLE 6.
Odds of exceeding NAM gestational weight gain (GWG) guidelines during pregnancy in intervention group vs usual care control group
| Stage of pregnancy | Intervention Group N=89a | Usual Care Control Group N=80a | Odds Ratio (OR) (95% CI) | ||
|---|---|---|---|---|---|
| N exceeding/total N | Percentage exceeding | N exceeding/total N | Percentage exceeding | ||
| First trimester | 36/86 | 41.9% | 32/79 | 40.5% | 1.06 (0.57, 1.97) |
| Second trimester | 59/82 | 72.0% | 53/78 | 67.9% | 1.21 (0.62, 2.38) |
| Third trimester | 65/82 | 79.3% | 54/75 | 72.0% | 1.49 (0.71, 3.10) |
| Total GWGb | 50/75 | 66.7% | 36/66 | 54.5% | 1.67 (0.84, 3.30) |
Total Ns are lower at later time points due to pregnancy loss, pregnancies that were ongoing at time of final data collection, and missing data (see Table 3 for reasons for missing data);
Total GWG analyses only included those who had full term delivery (≥37 weeks).
Weight outcomes stratified by time in intervention arm before pregnancy onset
In prespecified stratified analyses, among those who became pregnant within 6 months and between 6 and 24 months of randomization (ie, those still receiving the intervention at the time of conception), participants in the intervention group lost significantly more weight before pregnancy (<6 months [30], −0.64±0.75 kg/wk; 6–24 months [47], −0.09±0.16 kg/wk) than participants in the control group (<6 months [26], −0.11±0.36 kg/wk; P<.001; 6–24 months [35], 0.00±0.15 kg/wk; P=.005) (Figure 3). Among those who became pregnant after the intervention’s completion (>24 months), there was no difference between the 2 arms in weight change before pregnancy between intervention (9) and control arms (18) (0.04±0.06 vs −0.03±0.10 kg/wk; P=.09).
FIGURE 3. Gestational weight gain stratified by time from randomization to pregnancy onset.
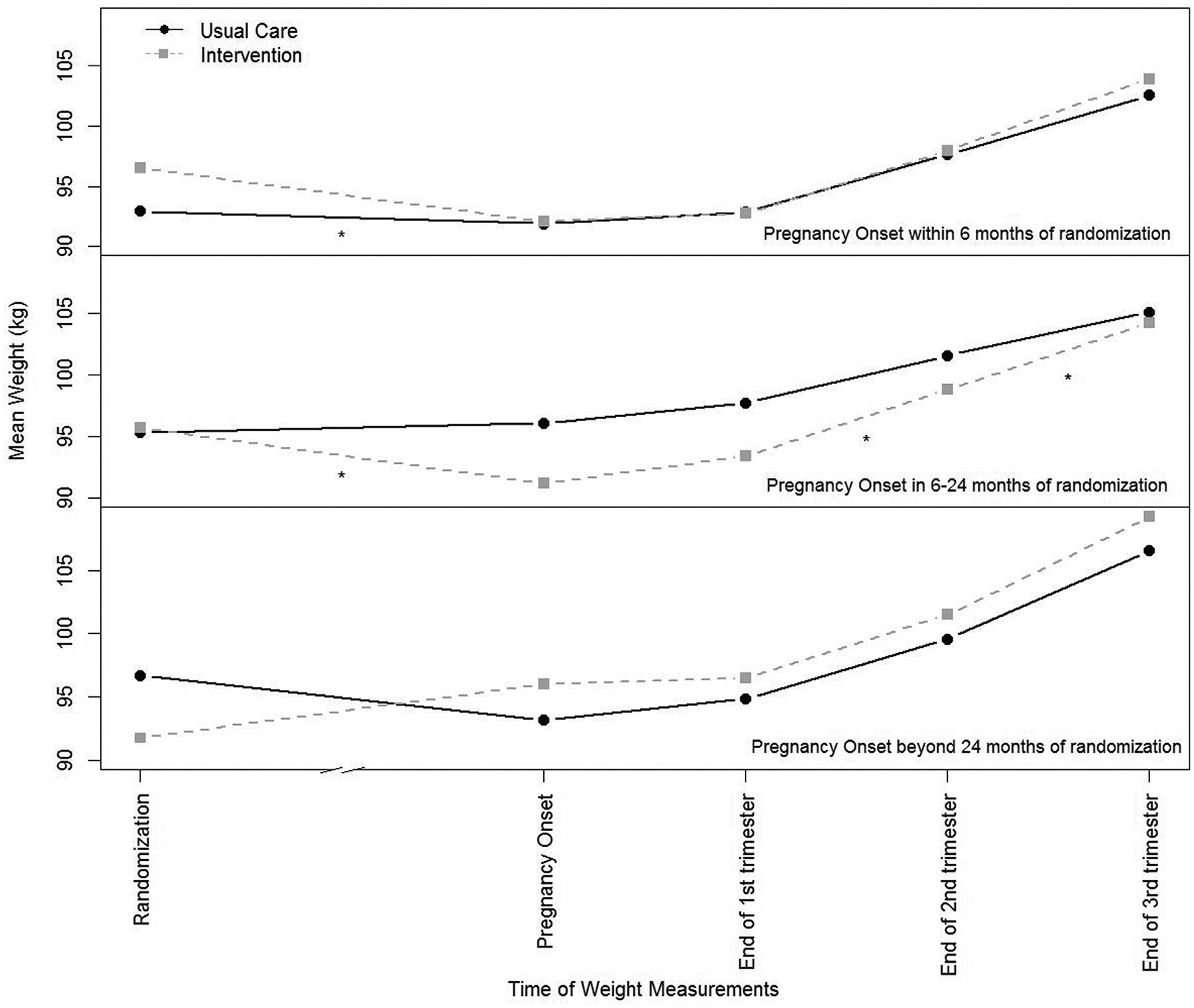
The numbers in analyses: 30 participants in the intervention group and 26 in the control group had pregnancy onset within 6 months of randomization; 47 participants in the intervention group and 35 in the control group had pregnancy onset in 6 to 24 months of randomization; and 9 participants in the intervention group and 18 in the control group had pregnancy onset beyond 24 months of randomization. The numbers in analyses decrease in later time points in pregnancy as shown in Table 7. *P<.05.
Participants who became pregnant during the maintenance phase of the intervention (6–24 months) gained significantly more weight in the second and third trimesters than participants in the control group who became pregnant 6 to 24 months after baseline. Although participants in the intervention group (40) with term pregnancies in this stratum gained more weight overall (14.1±7.7 kg) than those in the control group (30) (10.0±7·9; P=.04), they were not significantly more likely to exceed overall GWG guidelines (72.5% vs 50.0%; odds ratio, 2.64; 95% confidence interval [CI], 0.97–7.14) (Table 7). There were no significant differences between the 2 arms in GWG or likelihood of exceeding GWG guidelines for those who became pregnant less than 6 months or more than 24 months from randomization.
TABLE 7.
Odds of exceeding NAM guidelines for GWG between the intervention group and the usual care control group stratified by time from randomization to pregnancy onset
| Stage of pregnancy | <6 mo (n=56a) | 6–24 mo (n=86a) | >24 mo (n=27a) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Number exceeding/total number (%) | Odds ratio (95% CI) | Number exceeding/total number (%) | Odds ratio (95% CI) | Number exceeding/total number (%) | Odds ratio (95% CI) | ||||
| Intervention | Usual care | Intervention | Usual care | Intervention | Usual care | ||||
| First trimester | 11/30 (36.7) | 10/26 (38.5) | 0.93 (0.31–2.74) | 23/47 (48.9) | 16/35 (45.7) | 1.14 (0.47–2.74) | 2/9 (22.2) | 6/18 (33.3) | 0.57 (0.09–3.64) |
| Second trimester | 19/29 (65.5) | 19/25 (76.0) | 0.60 (0.18–1.98) | 35/44 (79.6) | 22/35 (62.9) | 2.30 (0.84–6.27) | 5/9 (55.6) | 12/18 (66.7) | 0.63 (0.12–3.22) |
| Third trimester | 25/29 (86.2) | 20/25 (80.0) | 1.56 (0.37–6.60) | 35/45 (77.8) | 21/35 (60.0) | 2.33 (0.88–6.19) | 5/8 (62.5) | 13/15 (86.7) | 0.26 (0.03–2.02) |
| Totalb | 16/27 (59.3) | 13/24 (54.2) | 1.23 (0.41–3.74) | 29/40 (72.5) | 15/30 (50.0) | 2.64 (0.97–7.14) | 5/8 (62.5) | 8/12 (66.7) | 0.83 (0.13–4.40) |
CI, confidence interval; GWG, gestational weight gain; NAM, National Academy of Medicine.
Total numbers decrease at later time points in pregnancy due to pregnancy loss, pregnancies that were ongoing at time of data collection, and missing data (Table 3 explains the reasons for the missing data);
Only included those who had full-term delivery (≥37 weeks’ gestation).
Newborn weight, perinatal outcomes, and adverse events
We found no significant differences between the 2 arms in preplanned analyses of newborn birthweight or perinatal outcomes (Table 8). Across the entire cohort, fewer in the intervention arm experienced spontaneous pregnancy loss than in the control arm, but other adverse events did not differ between the 2 arms.
TABLE 8.
Maternal and offspring perinatal outcomes and adverse events between the intervention group and the usual care control group
| Participants who experienced pregnancy at ≥14 wk (analytical cohort) | Intervention (n=89) | Usual care (n=80) | Unadjusted OR (95% CI) | Adjusted ORa (95% CI) |
|---|---|---|---|---|
| GDMb | 22 (24.7) | 28 (35.4) | 0.60 (0.31–1.17) | 0.67 (0.33–1.36) |
| HTN-Pc | 16 (18.0) | 13 (16.3) | 1.13 (0.51–2.52) | 1.00 (0.43–2.36) |
| Congenital anomaliesd | 6 (6.7) | 5 (6.4) | 1.06 (0.31–3.60) | — |
| Participants who delivered offspring | Intervention (n=85) | Usual care (n=76) | Unadjusted OR (95% CI) | Adjusted ORa (95% CI) |
| Cesarean delivery | 31 (36.5) | 26 (34.2) | 1.10 (0.58–2.11) | 0.88 (0.43, 1.83) |
| Preterm birth (<37 wk) | 7 (8.2) | 5 (6.6) | 1.27 (0.39–4.20) | 1.41 (0.42–4.81) |
| All participants randomized into study | Intervention (n=163) | Usual care (n=162) | Unadjusted OR (95% CI) | Adjusted ORa (95% CI) |
| Pregnancy losse | 8 (4.9) | 19 (11.8) | 0.39 (0.17–0.92) | 0.39 (0.16–0.92) |
| Fracturef | 2 (1.2) | 3 (1.9) | 0.65 (0.11–3.97) | — |
| Hospitalization unrelated to deliveryf | 14 (8.6) | 6 (3.7) | 2.43 (0.91–6.48) | — |
| Offspring | Intervention (n=85) | Usual care (n=76) | Unadjusted OR (95% CI) | Standardized differenceg (95% CI) |
| Birthweight, g (mean [SD]) | 3465.7 (585.6) | 3493.4 (594.4) | — | −0.05 (−0.36 to 0.26) |
| Birthweight for gestational age, z-score,h (mean [SD]) | 0.2 (0.9) | 0.3 (1.0) | — | −0.06 (−0.37 to 0.25) |
| LGAi | 14 (16.5) | 13 (17.1) | 0.96 (0.42–2.19) | — |
| SGAj | 6 (7.1) | 1 (1.3) | 5.70 (0.67–48.44) | — |
| Hypoglycemiad | 14 (16.5) | 16 (21.6) | 0.71 (0.32–1.59) | — |
| Respiratory morbidityd | 11 (12.9) | 8 (10.8) | 1.23 (0.47–3.23) | — |
Data are presented as number (percentage), unless otherwise specified.
CI, confidence interval; HTN-P, gestation hypertension; GDM, gestational diabetes mellitus; LGA, large for gestational age; OR, odds ratio; SD, standard deviation; SGA, small for gestational age.
Adjusted for whether participant had the condition in a previous pregnancy;
GDM analysis excludes 1 person who had type 2 diabetes at randomization;
Includes hypertension with onset during pregnancy and preeclampsia or eclampsia;
Data on congenital anomalies, hypoglycemia, and respiratory morbidity are missing for 2 mother-offspring pairs;
Includes miscarriage and ectopic pregnancy before 20 weeks’ gestation; women with more than 1 episode of pregnancy loss were only counted once;
Women with more than 1 hospitalization or fracture were only counted once;
Measure of association is different because newborn weights are continuous;
Adjusted for gestational age and sex using methods of Oken et al21;
Birthweight >90th percentile for age;
Birthweight <10th percentile for age.
Comment
Principal findings
Participation in a successful prepregnancy behavioral weight loss intervention had no significant effect on women’s likelihood of exceeding NAM guidelines for GWG compared with usual care. However, participants in the intervention group—especially those in the maintenance phase during pregnancy—gained significantly more weight during the second half of pregnancy than those in the control group, becoming indistinguishable from controls by the end of pregnancy.
Results
Previous research has identified effective interventions to limit GWG.36 However, those interventions usually do not start until after critical periods of fetal programming and placental development. As a result, multiple organizations recommend women who are overweight or obese to lose weight before becoming pregnant.4,37–39 To our knowledge, this is the first trial to test this recommendation by examining the impact of a prepregnancy behavioral weight loss intervention. Our results are consistent with a recent observational study finding that among women with BMIs over 25 kg/m2 at conception, those with weight loss in the year before pregnancy experienced 2.8 kg more GWG than women with stable weight before pregnancy.40 In contrast, the Finnish RADIEL randomized control trial (RCT) found no differences in GWG between those who received a health education intervention before and during pregnancies and those who did not.41 The RADIEL intervention was less intense than in the Prepare intervention (quarterly contacts, with 38% having only 1 visit before pregnancy) and did not contain behavior change components. Furthermore, it is difficult to compare their findings with ours because weight change before pregnancy was not reported and some women had BMIs <25 kg/m2 and therefore did not need to lose weight.
Clinical implications
These data are consistent with findings that risk of weight regain in nonpregnant adults increases after an intensive weight loss phase.42–44 Although frequent intervention contact has been shown to be key to successful weight loss and maintenance,45 contact rates were low (none to monthly) for participants by mid to late pregnancy, as nearly all participants had moved beyond the intensive phase by the last trimester. Because weight loss induces changes in energy regulation that promote subsequent weight regain and weight cycling,44,46 pregnancy could exacerbate the tendency to regain lost weight through hormonal changes that elevate appetite,47 concurrent with reduced social pressure to control weight.48,49
Although remote interventions are not typically as powerful as in person, the prepregnancy weight loss in the Prepare intervention arm (−3.7 kg) exceeded the average weight loss in the systematic evidence review on obesity management in nonpregnant adults (−2.4 kg; 95% CI, −2.8 to −1.9) that was the basis for the US Preventive Services Task Force’s B-level recommendation to “offer or refer adults with obesity to intensive, multicomponent behavior-based weight loss and weight loss maintenance interventions.”50,51 The degree of prepregnancy weight loss in the Prepare study has been shown to have considerable metabolic benefits in nonpregnant populations, including improved insulin sensitivity, β-cell function, blood pressure, triglyceride concentrations, and lean muscle mass.52 Consistent with an improved metabolic environment, participants in the intervention arm had a 10% lower absolute rate of GDM than in the control arm (25% vs 35%, respectively), but we had insufficient power to evaluate whether or not a difference of this size was due to chance.
In addition, the intervention arm of the full cohort had a 50% reduced risk of spontaneous pregnancy loss than the control arm (5% vs 12%). This difference was significant, further indicating that prepregnancy weight loss may have had favorable effects on the early intrauterine environment.53
Research implications
We examined GWG by trimester because of emerging data on the importance of the first-trimester intrauterine environment for long-term outcomes for mothers and offspring.17,19,54 Because the first-trimester environment is influenced by prepregnancy weight, prepregnancy weight loss could affect postpartum health of mothers and children regardless of GWG in later pregnancy.17,19,54 Although our data show similar weights at delivery, we do not know how lower weights at conception and the first trimester will impact postpartum weight retention or childhood growth. To best inform clinical guidelines, more long-term data are needed.
Strengths and limitations
A challenge with conducting prepregnancy RCTs is uncertainty about number of participants that will experience pregnancy. We met our recruitment goals, exceeding our target pregnancies by 12%. We had high retention (98%), which we attribute to EMR outcome collection and our low-burden intervention. Pregnancy is an ideal time to utilize EMR weights for research because women have frequent weight measurements; these weight data are also used during frontline clinical decisions. Previous work has demonstrated research weights are comparable with EMR weights; furthermore, any measurement error would be distributed equally between the 2 arms.
Our pragmatic design was responsive to variable pregnancy onset, which is consistent with what would occur when prepregnancy weight loss programs are implemented in clinical settings. Although this led to variability in intervention intensity before and during pregnancies, we accounted for this in our stratification analyses. However, the number of women in some of the categories (particularly the number of women in each arm who became pregnant after the intervention) was quite small, leading to large CIs and reducing the robustness of conclusions about these groups. Small sample sizes also precluded testing for intervention effects on secondary and exploratory outcomes within each stratum.
Our study sample was limited in that most participants were white and highly educated relative to the broader population. Furthermore, because the trial was conducted within the KPNW integrated healthcare system, the mix of insurance types does not reflect the broader population. We chose a phone-based prepregnancy weight loss intervention so that it would be widely implementable among many different settings and populations, including those with limited access to attend frequent sessions in person. It is not known, however, whether the effects of implementing the intervention would be similar in a more diverse sample. As such, future research would need to examine effects in a variety of settings as the impact of any intervention likely depends on social, environmental, and individual factors.50
Conclusion
Although the Prepare intervention resulted in prepregnancy weight loss, which may have improved the early intrauterine environment, it also had the unintended consequence of greater GWG in later pregnancy. Efforts aimed at improving weight in the earliest stages of pregnancy may need to be combined with intensive weight management that continues through delivery.
AJOG at a Glance.
Why was this study conducted?
This study aimed to determine the effect of intentional weight loss before pregnancy on gestational weight gain and pregnancy outcomes.
Key findings
A prepregnancy behavioral weight loss intervention had no effect on adherence to gestational weight guidelines. However, participants in the intervention group gained significantly more weight during pregnancy than those in the control group (13.2 vs 10.3 kg).
What does this add to what is known?
Although practice guidelines recommend that women who are overweight or obese lose weight before pregnancy, no previous trials have tested the effects of prepregnancy weight loss on gestational weight gain or pregnancy outcomes. This trial showed that intentional weight loss before pregnancy did not impact adherence to weight gain guidelines during pregnancy and, instead, resulted in increased gestational weight gain.
Acknowledgments
We thank the participants and staff of the Prepare study for their dedication to study. We also thank the following people at the Kaiser Center for Health Research (paid for by direct and indirect funding from a grant from the National Institute of Diabetes and Digestive and Kidney Diseases [R01/R56 KD099882]): Mi H. Lee, MPH, who assisted in data collection and management; Neon Brooks, PhD, who assisted with editing; and Cassandra Angus, who contributed to paper formatting.
This study was supported, in part, by a grant (R01/R56 DK099882) from the National Institute of Diabetes and Digestive and Kidney Diseases. The sponsor had no role in any of the following: design or conduct of the study; collection management, analysis, or interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Footnotes
The authors report no conflict of interest.
This study was registered on January 26, 2015, on ClinicalTrails.gov (identifier number: NCT02346162) (https://clinicaltrials.gov/ct2/show/NCT02346162), before initial participant enrollment in May 2015.
References
- 1.Giannakou K, Evangelou E, Yiallouros P, et al. Risk factors for gestational diabetes: an umbrella review of meta-analyses of observational studies. PLoS One 2019;14:e0215372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.LifeCycle Project-Maternal Obesity and Childhood Outcomes Study Group, Voerman E, Santos S, et al. Association of gestational weight gain with adverse maternal and infant outcomes. JAMA 2019;321:1702–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deputy NP, Dub B, Sharma AJ. Prevalence and trends in prepregnancy normal weight - 48 states, New York City, and District of Columbia, 2011e2015. MMWR Morb Mortal Wkly Rep 2018;66:1402–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Institute of Medicine (US) and National Research Council (US) Committee to Reexamine IOM Pregnancy Weight Guidelines. Setting the stage for revising pregnancy weight guidelines: conceptual framework. In: Rasmussen KM, Yaktine AL, eds. Weight gain during pregnancy: reexamining the guidelines. Washington, DC: National Academies Press; 2009. p. 1–13. [PubMed] [Google Scholar]
- 5.Opray N, Grivell RM, Deussen AR, Dodd JM. Directed preconception health programs and interventions for improving pregnancy outcomes for women who are overweight or obese. Cochrane Database Syst Rev 2015;7: CD010932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.LeBlanc ES, Vesco KK, Funk KL, Karanja N, Smith N, Stevens VJ. Prepare, a randomized trial to promote and evaluate weight loss among overweight and obese women planning pregnancy: study design and rationale. Contemp Clin Trials 2016;49:174–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Siega-Riz AM, Bodnar LM, Stotland NE, Stang J. The current understanding of gestational weight gain among women with obesity and the need for future research. 2019. Available at: 10.31478/202001a. Accessed Aug. 31, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fung TT, Chiuve SE, McCullough ML, Rexrode KM, Logroscino G, Hu FB. Adherence to a DASH-style diet and risk of coronary heart disease and stroke in women. Arch Intern Med 2008;168:713–20. [DOI] [PubMed] [Google Scholar]
- 9.Roza AM, Shizgal HM. The Harris Benedict equation reevaluated: resting energy requirements and the body cell mass. Am J Clin Nutr 1984;40:168–82. [DOI] [PubMed] [Google Scholar]
- 10.Goldstein RF, Abell SK, Ranasinha S, et al. Association of gestational weight gain with maternal and infant outcomes: a systematic review and meta-analysis. JAMA 2017;317: 2207–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson J, Clifton RG, Roberts JM, et al. Pregnancy outcomes with weight gain above or below the 2009 Institute of Medicine guidelines. Obstet Gynecol 2013;121:969–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de la Torre L, Flick AA, Istwan N, et al. The effect of new antepartum weight gain guidelines and prepregnancy body mass index on the development of pregnancy-related hypertension. Am J Perinatol 2011;28:285–92. [DOI] [PubMed] [Google Scholar]
- 13.Macdonald-Wallis C, Tilling K, Fraser A, Nelson SM, Lawlor DA. Gestational weight gain as a risk factor for hypertensive disorders of pregnancy. Am J Obstet Gynecol 2013;209: 327.e1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Truong YN, Yee LM, Caughey AB, Cheng YW. Weight gain in pregnancy: does the Institute of Medicine have it right? Am J Obstet Gynecol 2015;212:362.e1–8. [DOI] [PubMed] [Google Scholar]
- 15.Kominiarek MA, Saade G, Mele L, et al. Association between gestational weight gain and perinatal outcomes. Obstet Gynecol 2018;132: 875–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hedderson MM, Gunderson EP, Ferrara A. Gestational weight gain and risk of gestational diabetes mellitus. Obstet Gynecol 2010;115: 597–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gibson KS, Waters TP, Catalano PM. Maternal weight gain in women who develop gestational diabetes mellitus. Obstet Gynecol 2012;119:560–5. [DOI] [PubMed] [Google Scholar]
- 18.van Raaij JM, Peek ME, Vermaat-Miedema SH, Schonk CM, Hautvast JG. New equations for estimating body fat mass in pregnancy from body density or total body water. Am J Clin Nutr 1988;48:24–9. [DOI] [PubMed] [Google Scholar]
- 19.Walter JR, Perng W, Kleinman KP, Rifas-Shiman SL, Rich-Edwards JW, Oken E. Associations of trimester-specific gestational weight gain with maternal adiposity and systolic blood pressure at 3 and 7 years postpartum. Am J Obstet Gynecol 2015;212:499.e1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sridhar SB, Xu F, Hedderson MM. Trimester-specific gestational weight gain and infant size for gestational age. PLoS One 2016;11:e0159500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oken E, Kleinman KP, Rich-Edwards J, Gillman MW. A nearly continuous measure of birth weight for gestational age using a United States national reference. BMC Pediatr 2003;3:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stevens VJ, Wagner EL, Rossner J, Craddick S, Greenlick MR. Validity and usefulness of medical chart weights in the long-term evaluation of weight loss programs. Addict Behav 1988;13:171–5. [DOI] [PubMed] [Google Scholar]
- 23.Arterburn D, Ichikawa L, Ludman EJ, et al. Validity of clinical body weight measures as substitutes for missing data in a randomized trial. Obes Res Clin Pract 2008;2:277–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DiMaria-Ghalili RA. Medical record versus researcher measures of height and weight. Biol Res Nurs 2006;8:15–23. [DOI] [PubMed] [Google Scholar]
- 25.Howe LD, Tilling K, Lawlor DA. Accuracy of height and weight data from child health records. Arch Dis Child 2009;94:950–4. [DOI] [PubMed] [Google Scholar]
- 26.Leo MC, Lindberg NM, Vesco KK, Stevens VJ. Validity of medical chart weights and heights for obese pregnant women. EGEMS (Wash DC) 2014;2:1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Headen I, Cohen AK, Mujahid M, Abrams B. The accuracy of self-reported pregnancy-related weight: a systematic review. Obes Rev 2017;18:350–69. [DOI] [PubMed] [Google Scholar]
- 28.Bannon AL, Waring ME, Leung K, et al. Comparison of self-reported and measured prepregnancy weight: implications for gestational weight gain counseling. Matern Child Health J 2017;21:1469–78. [DOI] [PubMed] [Google Scholar]
- 29.Han E, Abrams B, Sridhar S, Xu F, Hedderson M. Validity of self-reported prepregnancy weight and body mass index classification in an integrated health care delivery system. Paediatr Perinat Epidemiol 2016;30: 314–9. [DOI] [PubMed] [Google Scholar]
- 30.Cnattingius S, Bergström R, Lipworth L, Kramer MS. Prepregnancy weight and the risk of adverse pregnancy outcomes. N Engl J Med 1998;338:147–52. [DOI] [PubMed] [Google Scholar]
- 31.Hauger MS, Gibbons L, Vik T, Belizán JM. Prepregnancy weight status and the risk of adverse pregnancy outcome. Acta Obstet Gynecol Scand 2008;87:953–9. [DOI] [PubMed] [Google Scholar]
- 32.Ovesen P, Rasmussen S, Kesmodel U. Effect of prepregnancy maternal overweight and obesity on pregnancy outcome. Obstet Gynecol 2011;118:305–12. [DOI] [PubMed] [Google Scholar]
- 33.American Diabetes Association position paper. Gestational Diabetes Mellitus. Diabetes Care 2003;26:s103–5. [DOI] [PubMed] [Google Scholar]
- 34.Abraha I, Montedori A. Modified intention to treat reporting in randomised controlled trials: systematic review. BMJ 2010;340:c2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fergusson D, Aaron SD, Guyatt G, Hébert P. Post-randomisation exclusions: the intention to treat principle and excluding patients from analysis. BMJ 2002;325:652–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.International Weight Management in Pregnancy (i-WIP) Collaborative Group. Effect of diet and physical activity based interventions in pregnancy on gestational weight gain and pregnancy outcomes: meta-analysis of individual participant data from randomised trials. BMJ 2017;358:j3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hanson MA, Bardsley A, De-Regil LM, et al. The International Federation of Gynecology and Obstetrics (FIGO) recommendations on adolescent, preconception, and maternal nutrition: “Think Nutrition First”. Int J Gynaecol Obstet. The International Federation of Gynecology and Obstetrics 2015;131(Suppl4):S213–53. [DOI] [PubMed] [Google Scholar]
- 38.Davies GA, Maxwell C, McLeod L, et al. SOGC clinical practice guidelines: obesity in pregnancy. No. 239, February 2010. Int J Gynaecol Obstet 2010;110:167–73. [DOI] [PubMed] [Google Scholar]
- 39.National Institute of Health and Care Excellence. Weight management before, during and after pregnancy. 2010. Available at: https://www.nice.org.uk/Guidance/PH27. Accessed Aug. 31, 2020.
- 40.Lecorguillé M, Jacota M, de Lauzon-Guillain B, et al. An association between maternal weight change in the year before pregnancy and infant birth weight: ELFE, a French national birth cohort study. PLoS Med 2019;16:e1002871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rönö K, Stach-Lempinen B, Eriksson JG, et al. Prevention of gestational diabetes with a prepregnancy lifestyle intervention—findings from a randomized controlled trial. Int J Womens Health 2018;10:493–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Svetkey LP, Stevens VJ, Brantley PJ, et al. Comparison of strategies for sustaining weight loss: the weight loss maintenance randomized controlled trial. JAMA 2008;299:1139–48. [DOI] [PubMed] [Google Scholar]
- 43.Ross KM, Qiu P, You L, Wing RR. Characterizing the pattern of weight loss and regain in adults enrolled in a 12-week Internet-based weight management program. Obesity (Silver Spring) 2018;26:318–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.MacLean PS, Wing RR, Davidson T, et al. NIH working group report: innovative research to improve maintenance of weight loss. Obesity (Silver Spring) 2015;23:7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.LeBlanc EL PC, Webber EM, Redmond N, Rushkin M, O’Connor EA. Behavioral and pharmacotherapy weight loss interventions to prevent obesity-related morbidity and mortality in adults: an updated systematic review for the U.S. Preventive Services Task Force. Rockville, MD: Agency for Healthcare Research and Quality (US); 2018. [PubMed] [Google Scholar]
- 46.Sumithran P, Proietto J. The defence of body weight: a physiological basis for weight regain after weight loss. Clin Sci (Lond) 2013;124:231–41. [DOI] [PubMed] [Google Scholar]
- 47.Brunton PJ, Russell JA. The expectant brain: adapting for motherhood. Nat Rev Neurosci 2008;9:11–25. [DOI] [PubMed] [Google Scholar]
- 48.Groth SW, Morrison-Beedy D, Meng Y. How pregnant African American women view pregnancy weight gain. J Obstet Gynecol Neonatal Nurs 2012;41:798–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Keely A, Cunningham-Burley S, Elliott L, Sandall J, Whittaker A. “If she wants to eat…and eat and eat…fine! It’s gonna feed the baby”: pregnant women and partners’ perceptions and experiences of pregnancy with a BMI >40kg/m2. Midwifery 2017;49:87–94. [DOI] [PubMed] [Google Scholar]
- 50.US Preventive Services Task Force, Curry SJ, Krist AH, et al. Behavioral weight loss interventions to prevent obesity-related morbidity and mortality in adults: US Preventive Services Task Force recommendation statement. JAMA 2018;320:1163–71. [DOI] [PubMed] [Google Scholar]
- 51.LeBlanc ES, Patnode CD, Webber EM, Redmond N, Rushkin M, O’Connor EA. Behavioral and pharmacotherapy weight loss interventions to prevent obesity-related morbidity and mortality in adults: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA 2018;320:1172–91. [DOI] [PubMed] [Google Scholar]
- 52.Ashtary-Larky D, Ghanavati M, Lamuchi-Deli N, et al. Rapid weight loss vs. slow weight loss: which is more effective on body composition and metabolic risk factors? Int J Endocrinol Metab 2017;15:e13249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Boots C, Stephenson MD. Does obesity increase the risk of miscarriage in spontaneous conception: a systematic review. Semin Reprod Med 2011;29:507–13. [DOI] [PubMed] [Google Scholar]
- 54.Catalano PM, Farrell K, Thomas A, et al. Perinatal risk factors for childhood obesity and metabolic dysregulation. Am J Clin Nutr 2009;90:1303–13. [DOI] [PMC free article] [PubMed] [Google Scholar]


