Abstract
Objective:
This article aims to assess the reproducibility of Manufacturer and User Facility Device Experience (MAUDE) data-driven studies by analyzing the data queries used in their research processes.
Methods:
Studies using MAUDE data were sourced from PubMed by searching for “MAUDE” or “Manufacturer and User Facility Device Experience” in titles or abstracts. We manually chose articles with executable queries. The reproducibility of each query was assessed by replicating it in the MAUDE Application Programming Interface. The reproducibility of a query is determined by a reproducibility coefficient that ranges from 0.95 to 1.05. This coefficient is calculated by comparing the number of medical device reports (MDRs) returned by the reproduced queries to the number of reported MDRs in the original studies. We also computed the reproducibility ratio, which is the fraction of reproducible queries in subgroups divided by the query complexity, the device category, and the presence of a data processing flow.
Results:
As of August 8, 2022, we identified 523 articles from which 336 contained queries, and 60 of these were executable. Among these, 14 queries were reproducible. Queries using a single field like product code, product class, or brand name showed higher reproducibility (50%, 33.3%, 31.3%) compared with other fields (8.3%, P = 0.037). Single-category device queries exhibited a higher reproducibility ratio than multicategory ones, but without statistical significance (27.1% versus 8.3%, P = 0.321). Studies including a data processing flow had a higher reproducibility ratio than those without, although this difference was not statistically significant (42.9% versus 17.4%, P = 0.107).
Conclusions:
Our findings indicate that the reproducibility of queries in MAUDE data-driven studies is limited. Enhancing this requires the development of more effective MAUDE data query strategies and improved application programming interfaces.
Keywords: MAUDE, reproducibility, query, patient safety, medical device, FDA, PubMed
To monitor and identify potential safety issues associated with the use of medical devices, the Food and Drug Administration (FDA) collects reports of device malfunctions, adverse events, and deaths and maintains the reports in the Manufacturer and User Facility Device Experience (MAUDE) database. The MAUDE database is a valuable and publicly available resource for patient safety research. The MAUDE data can be leveraged to identify patterns and trends that may indicate a need for further safety investigation or regulatory action.1–3
The use of MAUDE-based studies to investigate patient safety issues has grown rapidly in recent years. These studies have provided valuable insights into the adverse events associated with medical devices and have been used to inform medical device safety training and regulatory decisions.4–6 However, concerns have been raised about the transparency and reproducibility of the data extraction process used in these studies.7–9 To improve the credibility and trustworthiness of MAUDE-based studies, it is important to assess the reproducibility of the queries used to create study cohorts. This is considered the first step in ensuring the reproducibility of MAUDE-based studies.
Query reproducibility could be impacted by various factors, including query design, research topic, or data processing flow. An in-depth investigation of those factors would provide an insightful understanding of query reproduction. This article reports our comprehensive investigation on assessing query reproducibility in MAUDE-based studies. Our study creates measurements for query reproducibility, sheds light on why data queries could not be reproduced, and provides potential solutions for addressing the problems of query reproducibility.
MATERIALS AND METHODS
Search Strategy for MAUDE-Based Studies
The key words connected with Boolean operators expressed as “(MAUDE[Title/Abstract]) OR (Manufacturer and User Facility Device Experience[Title/Abstract])” were used in PubMed to retrieve MAUDE-based articles. Articles containing queries to extract MAUDE data were identified through manual review, followed by a summary statistics analysis.
Selecting Articles With Executable Data Queries
The queries in each of the retrieved articles were reviewed according to the criteria listed in Table 1. Based on the criteria, articles with inexecutable queries were excluded.
TABLE 1.
Criteria to Exclude Inexecutable Queries
| Query Property | Criteria for Inexecutable Queries |
|---|---|
| Timespan | a) Queries did not provide a precise time range for MDR retrieval. b) Queries contained MDRs 10 y earlier than the time the reproduction experiments were conducted. (MAUDE API, see details in Supplementary Materials S1, http://links.lww.com/JPS/A617) c) Queries presented start and end dates with defined days, months, and years, which are not supported by the simply search. (MAUDE API, see details in Supplementary Materials S1, http://links.lww.com/JPS/A617) |
| Query fields | a) Queries did not clearly describe the fields used. b) Queries used simple search and advanced search APIs simultaneously. (MAUDE API, see details in Supplementary Materials S1, http://links.lww.com/JPS/A617) |
| No. MDRs reported | a) Queries did not report the exact number of MDRs reported. b) Queries provided the number of MDRs after postquery data processing and missed the raw number of MDRs returned right after the query was executed. c) The number of returned MDRs shown in the articles was too large to be reproduced (the threshold of the number was set to 2.9 million). |
The review process was performed by 2 authors and finalized through group discussions involving domain experts with expertise in patient safety, health informatics, and medicine. The articles containing executable queries were maintained for further analysis. An example of executable query described in a article10 appears as: “A review of adverse events associated with the Watchman device was performed using the MAUDE database from March 2015 (FDA approval date) through February 2019. Within the database, the search was conducted using the term ‘XXX’ in the ‘brand name’ section to achieve the broadest possible search.”
Of 523 articles retrieved from PubMed, 336 featured a query to the MAUDE database. From 336 articles, 21 that downloaded MAUDE data files were set aside for further analysis.11–31 Upon manual review, 135 articles with unclear queries32–164 and 120 with incomplete queries165–284 were excluded. Ultimately, 60 executable queries were found.5,10,285–342 A comprehensive flow-chart of this selection process is depicted in Figure 1.
FIGURE 1.
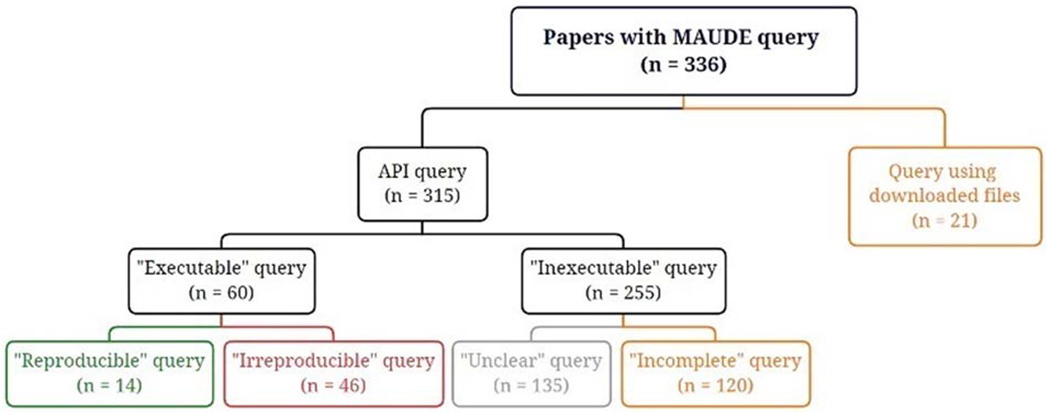
The flowchart shows the process of identifying articles with reproducible queries.
Measuring the Reproducibility of Executable Queries
The executable queries were reproduced by using the MAUDE Application Programming Interface (API) fields with exact key words as described in the original articles. The reproducibility of the queries was measured by comparing the number of medical device reports (MDRs) returned by the reproduced queries with the number reported in the original articles. Specifically, reproducibility is defined by the reproducibility coefficient as follows:
Given the FDA’s routine cleansing of received MDRs, identical queries conducted precleaning and postcleaning may yield varying MDR counts, a ± 5% threshold was applied to determine the query reproducibility. Specifically, a query is considered reproducible if:
An example of our workflow to determine the reproducibility of a query is elaborated in Supplementary S1, http://links.lww.com/JPS/A597.
Evaluating Reproducibility Ratios: Intrasubgroup Assessment and Intersubgroup Comparisons
The reproducibility coefficient of each query could be influenced by factors such as the complexity of the query, the category of the device involved, and whether there is a data processing flow included in the study. Therefore, we divided the articles with executable queries into subgroups based on the query complexity, the device category, and the presence of the data processing flow. The reproducibility ratio, defined as the proportion of reproducible queries in a subgroup, was calculated for each subgroup and compared via χ2 tests.
Query Complexity: API Fields and Estimated API Runs
The complexity of a query, which can influence its reproducibility, is affected by the number of API fields used. Single-field queries use one API field, while multifield queries use 2 or more. The choice of specific fields (like brand name or product code) also adds to the complexity. In addition, the estimated number of runs (ENRs) required to complete a query is another factor, given the limitations of the MAUDE API (detailed in Supplementary S2, http://links.lww.com/JPS/A597). Articles are categorized based on single- versus multifield usage, field selection, and ENR levels.
Device Category
The focus of an article on either a single device category or multiple device categories could affect the reproducibility of its queries. Consequently, the articles are categorized into subgroups of single- and multicategory device focus.
The Presence of a Data Processing Flow
The data processing flow describes how the raw data (returned by queries) are processed in each study step. The presence of a data processing flow can improve the transparency and reproducibility of a study. The articles are divided into subgroups with and without a data processing flow.
RESULTS
Geographic and Expertise Diversity in MAUDE-Based Research Publications
The 336 surveyed articles, categorized by the corresponding authors’ home countries, originated from 18 different countries/international organizations. The majority, with 299 articles, were from the United States. Figure 2 illustrates the nationality distribution and the annual comparison of U.S. and non-U.S. article counts. Despite MAUDE being a U.S. initiative, it has garnered global research interest, evidenced by 28 articles from 14 non-U.S. countries and 8 internationally collaborative publications.
FIGURE 2.
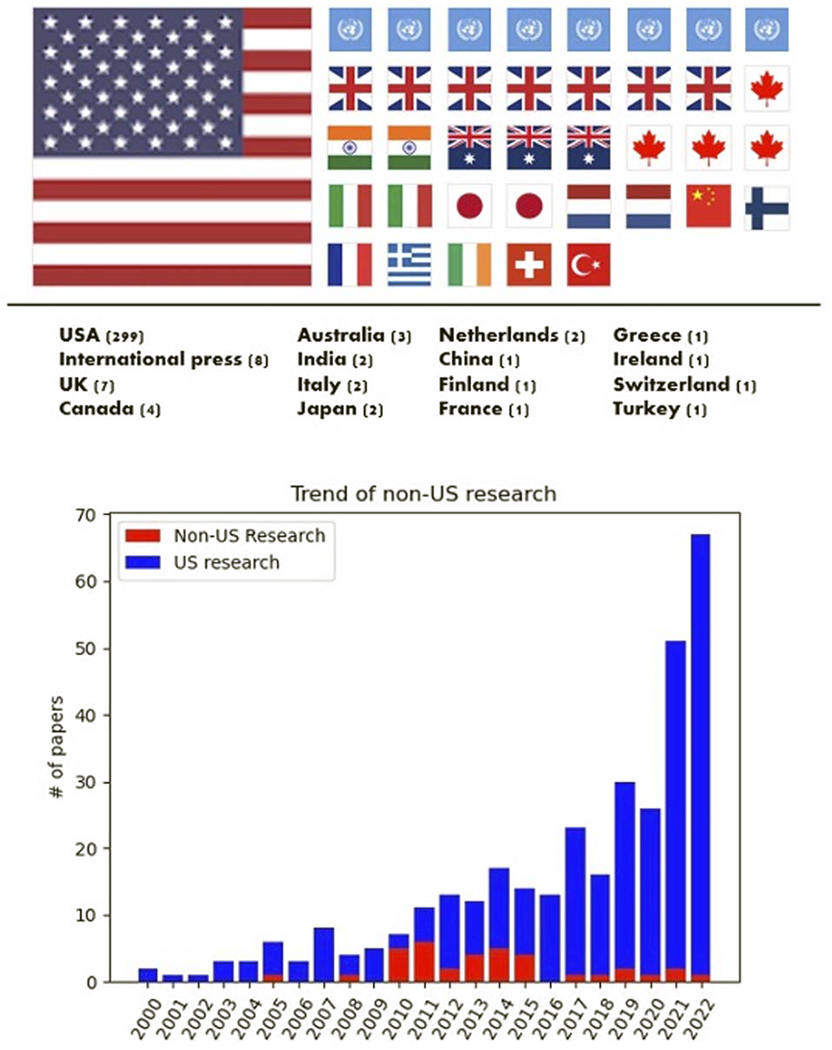
Distribution of nationalities in 336 MAUDE research articles (top) and annual comparison of U.S. and non-U.S. article counts (bottom).
We examined the expertise of the corresponding authors among the 336 articles, discovering that 283 were authored by experts from 18 medical fields (such as dermatology and ophthalmology), while 53 articles had authors from 15 nonmedical disciplines (including informatics, biomedical engineering, and statistics, as well as government and corporate entities). Figure 3 presents the breakdown of these articles across the identified 18 medical and 15 nonmedical fields. Notably, nearly half of the medical-related publications were predominantly contributed by authors specializing in surgery and cardiovascular fields.
FIGURE 3.
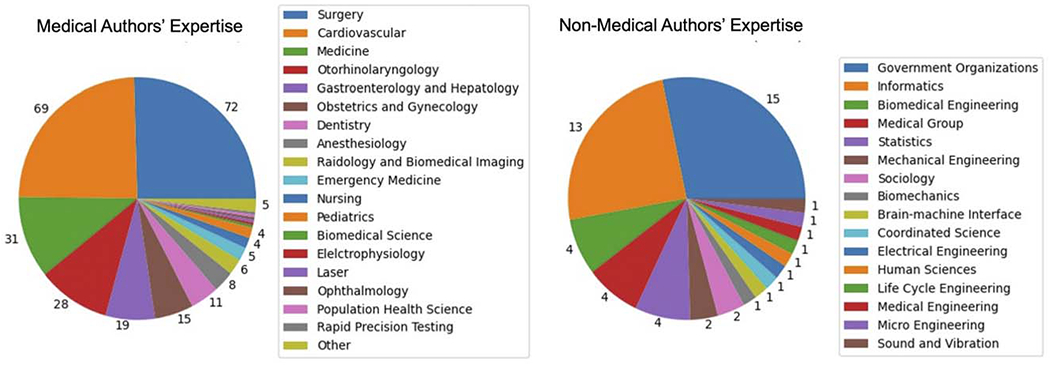
Distribution of 336 corresponding authors’ expertise: 18 medical fields (left) and 15 nonmedical domains (right).
Reproducibility Coefficients and Ratios
The reproducibility coefficients of 14 articles10,285–297 are in the range of 0.95 to 1.05. Results of reproducibility ratios for each subgroup are shown in Table 2.
TABLE 2.
Reproducibility Ratios of Subgroups Divided by Query Complexity, Device Category, and the Presence of Data Processing Flows
| No. Articles With Executable Queries (n = 60) | No. Articles With Reproducible Queries |
|---|---|
| Query complexity | |
| Single-field query (n = 51) | 12 (23.5%) |
| Simple search (n = 23) | 2 (8.7%) |
| Brand name (n = 16) | 5 (31.3%) |
| Product class (n = 3) | 1 (33.3%) |
| Product code (n = 8) | 4 (50.0%) |
| Manufacturer (n = 1) | 0 (0.0%) |
| Multifield query (n = 9) | 2 (22.2%) |
| Brand name + event type (n = 1) | 0 (0.0%) |
| Brand name + manufacturer (n = 1) | 0 (0.0%) |
| Brand name + simple search (n = 1) | 0 (0.0%) |
| Brand name + product code (n = 1) | 0 (0.0%) |
| Event type + product class (n = 1) | 1 (100.0%) |
| Manufacturer + product code (n = 2) | 1 (50.0%) |
| Product class + product code (n = 1) | 0 (0.0%) |
| Brand name + event type + product code (n = 1) | 0 (0.0%) |
| ENRs | |
| 1 (n = 11) | 4 (36.4%) |
| 2 (n = 9) | 2 (22.2%) |
| 3 (n = 4) | 1 (25.0%) |
| 4 (n = 5) | 3 (60.0%) |
| 5 (n = 7) | 1 (14.3%) |
| 6 (n = 2) | 1 (50.0%) |
| 7 (n = 3) | 1 (33.3%) |
| ≥8 (n = 19) | 1 (5.3%) |
| Device category | |
| Single category (n = 48) | 13 (27.1%) |
| Multicategory (n = 12) | 1 (8.3%) |
| Presence of data processing flows | |
| Show (n = 14) | 6 (42.9%) |
| No show (n = 46) | 8 (17.4%) |
Single-field queries use only 1 API field, whereas multifield queries use 2 or more API fields. The ENRs required to complete a query is based on the limitations of the MAUDE API (see Supplementary S2, http://links.lww.com/JPS/A624). A single device category implies that the device under investigation falls into one specific category. In contrast, multiple device categories indicate that the device pertains to several categories. The term “presence of data processing flow” denotes whether the article describes the methods used to handle the MDRs retrieved through these queries.
Single- Versus Multifield Queries
Twelve of the 51 single-field queries (23.5%) were reproducible, while 2 of the 9 multifield queries (22.2%) were reproducible. The difference between the reproducibility ratio of single- and multifield queries (P = 1.000) is insignificant.
Individual API Field
Simple search (23), brand name (16), and product code (8) were the top 3 most frequently used fields in the single-field query. Queries using product code (reproducibility ratio, 50.0%), product class (33.3%), and brand name (31.3%) had the highest reproducibility ratios in the single-field query. Single-field queries using these three fields had significantly higher reproducibility than those using other fields (37.0% versus 8.3%, P = 0.037). For multifield queries, the query using event type + product class was reproducible; however, 1 of the 2 queries using manufacturer + product code was reproducible.
Queries With Different ENRs
Queries with ENR greater than 7 had extremely low reproducibility ratio. Only 1 of the 19 queries was reproducible.
Single- Versus Multicategory Queries
There were 48 single-category and 12 multicategory queries. Thirteen of the 48 single-category queries (27.1%) were reproducible, while 1 of the 12 multicategory queries (8.3%) was reproducible. Single-category queries had higher reproducibility ratio than the multicategory queries without a significant difference (P = 0.321).
Presence of a Data Processing Flow
Fourteen of the 60 articles (25.0%) included a data processing flow. Six of the 14 queries (42.9%) were reproducible, while 8 of the 46 queries (17.4%) without a data processing flow were reproducible. Queries with a data processing flow had higher reproducibility ratio than those without a data processing flow. However, there is no significant difference (P = 0.107).
DISCUSSION
Principal Findings
This article presented an analysis to assess reproducibility of queries used in the MAUDE-based studies. Our study found that the transparency and reproducibility of data extraction from the MAUDE database in MAUDE-based studies is questionable, with only a small percentage of articles and queries being reproducible. Specifically, our study revealed that only 11.5% of 523 identified MAUDE-based articles contained executable queries, and only 23.3% of those executable queries were reproducible. This lack of transparency and reproducibility can negatively impact research, practice, and policy by hindering progress in understanding patient safety issues related to medical devices and making it difficult to use these findings to inform decision making.
Our further analysis results revealed that the single-field query with common search such as product code or brand name, the single device category, and the presence of a data processing flow may enhance the reproducibility of MAUDE data queries. Only 8.3% of the multifield queries were reproducible, compared with 27.1% for the single-field queries. Studies focusing on multiple device categories tended to have more complicated query logics, which required concatenating MDRs retrieved by a larger number of subqueries. Postprocessing procedures like concatenation may affect the query reproducibility in 2 ways: (1) concatenating MDR sets required intensive postquery processing (e.g., deduplication), which was prone to mistakes. (2) the authors were more unlikely to report the number of MDRs both before and after postquery processing. Therefore, it is recommended that MAUDE-based research focus on a single device category. If multiple device categories must be used, it is better to provide the number of MDRs returned by each subquery with a clear description of postquery processing steps.
A large number of authors failed to provide a clear description of queries, which posted barriers to reproduce the results. One hundred thirty-five of the 336 articles (40.2%) exhibited unclear descriptions of queries. Of the 135, the first authors of the 113 (83.7%) had a medical background. One potential reason for unclear query description might be that many authors did not receive formal or sufficient training for data extraction or information retireval. To create a clear query, it is essential to provide query fields, key words in each query field, exact time range for the query, and to report the number of MDRs returned by every single subquery.
Only 14 of the 60 articles (25.0%) with executable queries presented a data flow chart, a standard component of data-driven studies. The data processing flow is indispensable to describe the overall procedure to process the MDRs and the number of MDRs proceeded in each step. One potential reason for studies not providing data processing flows might be due to the lack of expertise of data analysis.
Twenty-three of the 60 articles (38.3%) with executable queries used the simple search function without the selection of specific API fields. The simple search function inclines to yield inconsistent query results. One potential reason for authors to use the simple search function is that they did not fully explore how to use the structured fields in the MAUDE API and the benefits for using these fields. For instance, brand name, manufacturer and product class are the 3 API fields that could be matched with the research topics of 29 articles; however, only 9 articles actually used these structured fields in their queries. Therefore, it is recommended that researchers learn about structured API fields before planning to use simple search. Having an FDA device category at hand also helps decide proper query key words. In addition, a generic name field could be added to the MAUDE API. Generic name is a mandated MDR field where clinicians give phrase-level descriptions of the malfunctioning device. Generic name is characterized by its flexibility: any phrase can be used in this free-text field to describe the reported medical device. This flexibility makes generic name an ideal field to match a large variety of research topics. Nevertheless, generic name cannot be used directly for indexing since there may exist multiple equivalent expressions for the same concept (e.g., ultrasound and ultrasonic, humidification system, and humidifier). An Natural Language Processing-based recommendation system may be needed to retrieve MDRs relevant to the query input via fuzzy matching.
The MAUDE API complicated some of the queries. In situations where the query hit the 500 MDR limitation, the authors had to split the time span used in the query and run extra subqueries for each divided subtime span to obtain the result. The 60 executable queries had 8.63 ENRs on average, while the maximum ENR was 132. A desired query API should be designed to handle queries with a large number of MDRs returned. Another effort made by OpenFDA343 was a URL-based API344 released in June 2014 for MAUDE queries that allows for queries on a total of 121 MDR fields, far more than those included in the original API (shown in Supplementary S3, http://links.lww.com/JPS/A597), and thus can handle various research needs. This API returns up to 1000 MDRs per query run and provides a “skip” parameter that helps decrease the ENR and improve query reproducibility (see Supplementary S4, http://links.lww.com/JPS/A597). However, it requires users to write a long URL with numerous parameters from scratch and returns JSON structured data as the result, which sets a high threshold for researchers without computer science backgrounds. One future direction is to develop a user-friendly version of such an API.
Scope of This Study and Its Limitations
This study has limitations that must be addressed to improve future studies’ understanding and reproducibility of MAUDE queries. First, the size of the executable queries is insufficient to draw statistically significant conclusions. Second, the reproducibility coefficient measurement only considered the numbers of MDRs returned between original and reproduced queries rather than the contents of the MDRs. Investigating the content of the MDRs seems to be very challenging or even impossible because the detailed contents of MDRs are seldom elaborated in articles. Furthermore, reproducibility is subject to numerous influencing factors, and our study serves as a preliminary exploration of these elements. We assessed reproducibility ratios in relation to query complexity, involved device categories, and the presence of data processing flows. Future research ought to broaden this scope, encompassing MAUDE-based articles from more sources, examining reproducibility more comprehensively, and pinpointing additional factors impacting reproducibility. Such efforts will improve the robustness and relevance of studies driven by MAUDE data.
Supplementary Material
Acknowledgments
This research was supported, in part, by the National Library of Medicine of the National Institutes of Health under Award Number R01LM012854.
Footnotes
The authors disclose no conflict of interest.
Clinical Trial Registration: Nonclinical trial study.
Ethical approval. Not applicable.
Consent for publication. Not applicable.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.journalpatientsafety.com).
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Availability of data and materials.
Not applicable.
REFERENCES
- 1.FDA, Medical device reporting regulation history. Available at: https://www.fda.gov/medical-devices/mandatory-reporting-requirements-manufacturers-importers-and-device-user-facilities/medical-device-reporting-regulation-history. Accessed August 1, 2022.
- 2.FDA, About Manufacturer and User Facility Device Experience (MAUDE). Available at: https://www.fda.gov/medical-devices/mandatory-reporting-requirements-manufacturers-importers-and-device-user-facilities/about-manufacturer-and-user-facility-device-experience-maude. Accessed August 1, 2022.
- 3.FDA, MAUDE—Manufacturer and User Facility Device Experience. Available at: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfMAUDE/search.cfm. Accessed August 1, 2022.
- 4.Liebel TC, Daugherty T, Kirsch A, et al. Analysis: using the FDA MAUDE and medical device recall databases to design better devices. Biomed Instrum Technol. 2020;54:178–188. [DOI] [PubMed] [Google Scholar]
- 5.Goel V, Yang Y, Kanwar S, et al. Adverse events and complications associated with intrathecal drug delivery systems: insights from the Manufacturer and User Facility Device Experience (MAUDE) database. Neuromodulation. 2021;24:1181–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Knisely BM, Levine C, Kharod KC, et al. An analysis of FDA adverse event reporting data for trends in medical device use error. Proceedings of the International Symposium on Human Factors and Ergonomics in Health Care. Los Angeles, CA: SAGE Publications, 2020, 9: 130–134. [Google Scholar]
- 7.Piccini JP Sr., Califf RM. Postmarket surveillance and returned product analysis: success but not transparency. Heart Rhythm. 2013;10:1469–1470. [DOI] [PubMed] [Google Scholar]
- 8.Hernandez-Boussard T, Lundgren MP, Shah N. Conflicting information from the Food and Drug Administration: missed opportunity to lead standards for safe and effective medical artificial intelligence solutions [published correction appears in J Am Med Inform Assoc. 2021 May 5]. J Am Med Inform Assoc. 2021;28:1353–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kramer DB, Kesselheim AS. Trust and transparency in medical device regulation. BMJ. 2019;365:14166. [DOI] [PubMed] [Google Scholar]
- 10.Ledesma PA, Uzomah UA, Yu X, et al. MAUDE database analysis of post-approval outcomes following left atrial appendage closure with the Watchman device. Am J Cardiol. 2021;152:78–87. [DOI] [PubMed] [Google Scholar]
- 11.Hankin CS, Schein J, Clark JA, et al. Adverse events involving intravenous patient-controlled analgesia. Am J Health Syst Pharm. 2007;64:1492–1499. [DOI] [PubMed] [Google Scholar]
- 12.Dowdy JC, Sayre RM, Shepherd JG. Indoor tanning injuries: an evaluation of FDA adverse event reporting data [published correction appears in Photodermatol Photoimmunol Photomed. 2009 Dec;25(6):336]. Photodermatol Photoimmunol Photomed. 2009;25:216–220. [DOI] [PubMed] [Google Scholar]
- 13.Magrabi F, Ong MS, Runciman W, et al. Patient safety problems associated with heathcare information technology: an analysis of adverse events reported to the US Food and Drug Administration. AMIA Annu Symp Proc. 2011;2011:853–857. [PMC free article] [PubMed] [Google Scholar]
- 14.DeLuca LA Jr., Simpson A, Beskind D, et al. Analysis of automated external defibrillator device failures reported to the Food and Drug Administration. Ann Emerg Med. 2012;59:103–111. [DOI] [PubMed] [Google Scholar]
- 15.Magrabi F, Ong MS, Runciman W, et al. Using FDA reports to inform a classification for health information technology safety problems. J Am Med Inform Assoc. 2012;19:45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Causon A, Verschuur C, Newman TA. Trends in cochlear implant complications: implications for improving long-term outcomes. Otol Neurotol. 2013;34:259–265. [DOI] [PubMed] [Google Scholar]
- 17.Chai KE, Anthony S, Coiera E, et al. Using statistical text classification to identify health information technology incidents. J Am Med Inform Assoc. 2013;20:980–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thavarajah R, Thennukonda RA. Analysis of adverse events with use of orthodontic sequential aligners as reported in the Manufacturer and User Facility Device Experience database. Indian J Dent Res. 2015;26:582–587. [DOI] [PubMed] [Google Scholar]
- 19.Hebballi NB, Ramoni R, Kalenderian E, et al. The dangers of dental devices as reported in the Food and Drug Administration Manufacturer and User Facility Device Experience database. J Am Dent Assoc. 2015;146:102–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Connor MJ, Marshall DC, Moiseenko V et al. Adverse events involving radiation oncology medical devices: comprehensive analysis of US Food and Drug Administration data, 1991 to 2015. Int J Radiat Oncol Biol Phys. 2017;97:18–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kavanagh KT, Kraman SS, Kavanagh SP. An analysis of the FDA MAUDE database and the search for cobalt toxicity in class 3 Johnson & Johnson/DePuy metal-on-metal hip implants. J Patient Saf. 2018;14:e89–e96. [DOI] [PubMed] [Google Scholar]
- 22.Yao B, Kang H, Wang J, et al. Exploring health information technology events from FDA MAUDE database. Stud Health Technol Inform. 2018;250:187–191. [PubMed] [Google Scholar]
- 23.Kang H, Yu Z, Gong Y. Initializing and growing a database of health information technology (HIT) events by using TF-IDF and Biterm topic modeling. AMIA Annu Symp Proc. 2018;2017:1024–1033. [PMC free article] [PubMed] [Google Scholar]
- 24.Lawal OD, Mohanty M, Elder H, et al. The nature, magnitude, and reporting compliance of device-related events for intravenous patient-controlled analgesia in the FDA Manufacturer and User Facility Device Experience (MAUDE) database. Expert Opin Drug Saf. 2018;17:347–357. [DOI] [PubMed] [Google Scholar]
- 25.Kang H, Wang J, Yao B, et al. Toward safer health care: a review strategy of FDA medical device adverse event database to identify and categorize health information technology related events. JAMIA Open. 2018;2:179–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Delfino JG, Krainak DM, Flesher SA, et al. MRI-related FDA adverse event reports: a 10-yr review. Med Phys. 2019;46:5562–5571. [DOI] [PubMed] [Google Scholar]
- 27.Kavanagh KT, Brown RE Jr., Kraman SS, et al. Reporter’s occupation and source of adverse device event reports contained in the FDA’s MAUDE database. Patient Relat Outcome Meas. 2019;10:205–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pane J, Verhamme KMC, Rebollo I, et al. Descriptive analysis of postmarket surveillance data for hip implants. Pharmacoepidemiol Drug Saf. 2020;29:380–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krouwer JS. An analysis of 2019 FDA adverse events for two insulin pumps and two continuous glucose monitors. J Diabetes Sci Technol. 2022;16:228–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hauser RG, Gornick CC, Abdelhadi RH, et al. Leadless pacemaker perforations: clinical consequences and related device and user problems. J Cardiovasc Electrophysiol. 2022;33:154–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krouwer JS. More focus is needed to reduce adverse events for diabetes devices. J Diabetes Sci Technol. 2022;16:498–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ross MG, Fresquez M, El-Haddad MA. Impact of FDA advisory on reported vacuum-assisted delivery and morbidity. J Matern Fetal Med. 2000;9:321–326. [DOI] [PubMed] [Google Scholar]
- 33.Liu C, Webb CC. Pulmonary artery rupture: serious complication associated with pulmonary artery catheters. Int J Trauma Nurs. 2000;6:19–26. [DOI] [PubMed] [Google Scholar]
- 34.Hauser RG, Hayes DL, Almquist AK, et al. Unexpected ICD pulse generator failure due to electronic circuit damage caused by electrical overstress. Pacing Clin Electrophysiol. 2001;24:1046–1054. [DOI] [PubMed] [Google Scholar]
- 35.Sharp HT, Dodson MK, Draper ML, et al. Complications associated with optical-access laparoscopic trocars. Obstet Gynecol. 2002;99:553–555. [DOI] [PubMed] [Google Scholar]
- 36.Perkins WJ, Davis DH, Huntoon MA, et al. A retained Racz catheter fragment after epidural neurolysis: implications during magnetic resonance imaging. Anesth Analg. 2003;96:1717–1719. [DOI] [PubMed] [Google Scholar]
- 37.Gurtcheff SE, Sharp HT. Complications associated with global endometrial ablation: the utility of the MAUDE database. Obstet Gynecol. 2003;102:1278–1282. [DOI] [PubMed] [Google Scholar]
- 38.Hauser RG, Kallinen L. Deaths associated with implantable cardioverter defibrillator failure and deactivation reported in the United States Food and Drug Administration Manufacturer and User Facility Device Experience database. Heart Rhythm. 2004;1:399–405. [DOI] [PubMed] [Google Scholar]
- 39.Brown SL, Bright RA, Dwyer DE, et al. Breast pump adverse events: reports to the food and drug administration. J Hum Lact. 2005;21:169–174. [DOI] [PubMed] [Google Scholar]
- 40.Nemeth C, Nunnally M, O’Connor M, et al. Making information technology a team player in safety: the case of infusion devices. In: Henriksen K, Battles JB, Marks ES, et al. , eds. Advances in Patient Safety: From Research to Implementation (Volume 1: Research Findings). Rockville, MD: Agency for Healthcare Research and Quality (US); 2005. [PubMed] [Google Scholar]
- 41.Fuller J, Ashar BS, Carey-Corrado J. Trocar-associated injuries and fatalities: an analysis of 1399 reports to the FDA. J Minim Invasive Gynecol. 2005;12:302–307. [DOI] [PubMed] [Google Scholar]
- 42.Madan AK, Ternovits CA, Tichansky DS. Emerging endoluminal therapies for gastroesophageal reflux disease: adverse events. Am J Surg. 2006;192:72–75. [DOI] [PubMed] [Google Scholar]
- 43.Meng MV. Reported failures of the polymer self-locking (Hem-o-lok) clip: review of data from the Food and Drug Administration. J Endourol. 2006;20:1054–1057. [DOI] [PubMed] [Google Scholar]
- 44.Boyles SH, Edwards R, Gregory W, et al. Complications associated with transobturator sling procedures. Int Urogynecol J Pelvic Floor Dysfunct. 2007;18:19–22. [DOI] [PubMed] [Google Scholar]
- 45.Hauser RG, Kallinen LM, Almquist AK, et al. Early failure of a small-diameter high-voltage implantable cardioverter-defibrillator lead. Heart Rhythm. 2007;4:892–896. [DOI] [PubMed] [Google Scholar]
- 46.Della Badia C, Nyirjesy P, Atogho A. Endometrial ablation devices: review of a Manufacturer and User Facility Device Experience database. J Minim Invasive Gynecol. 2007;14:436–441. [DOI] [PubMed] [Google Scholar]
- 47.Erekson EA, Sung VW, Rardin CR, et al. Ethylene vinyl alcohol copolymer erosions after use as a urethral bulking agent. Obstet Gynecol. 2007;109(2 Pt2):490–492. [DOI] [PubMed] [Google Scholar]
- 48.Deng DY, Rutman M, Raz S, et al. Presentation and management of major complications of midurethral slings: are complications under-reported? NeurourolUrodyn. 2007;26:46–52. [DOI] [PubMed] [Google Scholar]
- 49.Blumenthal KB, Sutherland DE, Wagner KR, et al. Bladder neck contractures related to the use of hem-o-lok clips in robot-assisted laparoscopic radical prostatectomy. Urology. 2008;72:158–161. [DOI] [PubMed] [Google Scholar]
- 50.Andonian S, Okeke Z, Okeke DA, et al. Device failures associated with patient injuries during robot-assisted laparoscopic surgeries: a comprehensive review of FDA MAUDE database. Can J Urol. 2008;15:3912–3916. [PubMed] [Google Scholar]
- 51.Connell SS, Balkany TJ, Hodges AV et al. Electrode migration after cochlear implantation. Otol Neurotol. 2008;29:156–159. [DOI] [PubMed] [Google Scholar]
- 52.DiBardino DJ, McElhinney DB, Kaza AK, et al. Analysis of the US Food and Drug Administration Manufacturer and User Facility Device Experience database for adverse events involving Amplatzer septal occluder devices and comparison with the Society of Thoracic Surgery congenital cardiac surgery database. J Thorac Cardiovasc Surg. 2009; 137:1334–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chhatriwalla AK, Cam A, Unzek S, et al. Drug-eluting stent fracture and acute coronary syndrome. Cardiovasc Revasc Med. 2009;10:166–171. [DOI] [PubMed] [Google Scholar]
- 54.ASGE Technology Committee, Parsi MA, Sullivan SA, Goodman A, et al. Automated endoscope reprocessors [published correction appears in Gastrointest Endosc. 2017 Apr;85(4):871–872]. Gastrointest Endosc. 2016;84:885–892. [DOI] [PubMed] [Google Scholar]
- 55.Hauser RG, Katsiyiannis WT, Gornick CC, et al. Deaths and cardiovascular injuries due to device-assisted implantable cardioverter-defibrillator and pacemaker lead extraction. Europace. 2010;12:395–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.ASGE Technology Committee, Kethu SR, Banerjee S, Desilets D, et al. Endoscopic tattooing. Gastrointest Endosc. 2010;72:681–685. [DOI] [PubMed] [Google Scholar]
- 57.ASGE Technology Committee, Kwon RS, Banerjee S, Desilets D, et al. Enteral nutrition access devices. Gastrointest Endosc. 2010;72:236–248. [DOI] [PubMed] [Google Scholar]
- 58.ASGE Technology Committee, Pedrosa MC, Farraye FA, Shergill AK, et al. Minimizing occupational hazards in endoscopy: personal protective equipment, radiation safety, and ergonomics. Gastrointest Endosc. 2010;72:227–235. [DOI] [PubMed] [Google Scholar]
- 59.Frias JP, Lim CG, Ellison JM, et al. Review of adverse events associated with false glucose readings measured by GDH-PQQ-based glucose test strips in the presence of interfering sugars. Diabetes Care. 2010;33:728–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zurawin RK, Zurawin JL. Adverse events due to suspected nickel hypersensitivity in patients with essure micro-inserts [published correction appears in J Minim Invasive Gynecol. 2011 Sep-Oct;18(5): 688]. J Minim Invasive Gynecol. 2011;18:475–482. [DOI] [PubMed] [Google Scholar]
- 61.Lyon ME, Lyon AW. Analysis of the performance of the CONTOUR® TS Blood Glucose Monitoring System: when regulatory performance criteria are met, should we have confidence to use a medical device with all patients? J Diabetes Sci Technol. 2011;5:206–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Clark KK, Sharma DK, Chute CG, et al. Application of a temporal reasoning framework tool in analysis of medical device adverse events. AMIA Annu Symp Proc. 2011;2011:1366–1371. [PMC free article] [PubMed] [Google Scholar]
- 63.ASGE Technology Committee, Banerjee S, Desilets D, Diehl DL, et al. Computer-assisted personalized sedation. Gastrointest Endosc. 2011;73:423–427. [DOI] [PubMed] [Google Scholar]
- 64.ASGE Technology Committee, Varadarajulu S, Banerjee S, Barth B, et al. Enteral stents. Gastrointest Endosc. 2011;74:455–464. [DOI] [PubMed] [Google Scholar]
- 65.ASGE Technology Committee, Varadarajulu S, Banerjee S, Barth BA, et al. GI endoscopes. Gastrointest Endosc. 2011;74:1–6.e6. [DOI] [PubMed] [Google Scholar]
- 66.Levy R, Henderson J, Slavin K, et al. Incidence and avoidance of neurologic complications with paddle type spinal cord stimulation leads. Neuromodulation. 2011;14:412–422. [DOI] [PubMed] [Google Scholar]
- 67.Angel LF, Tapson V Galgon RE, et al. Systematic review of the use of retrievable inferior vena cava filters. J Vasc Interv Radiol. 2011;22:1522–1530.e3. [DOI] [PubMed] [Google Scholar]
- 68.Woo EJ. Adverse events reported after the use of recombinant human bone morphogenetic protein 2. J Oral Maxillofac Surg. 2012;70:765–767. [DOI] [PubMed] [Google Scholar]
- 69.Frantzides CT, Welle SN. Cardiac tamponade as a life-threatening complication in hernia repair. Surgery. 2012;152:133–135. [DOI] [PubMed] [Google Scholar]
- 70.Hauser RG, Abdelhadi R, McGriff D, et al. Deaths caused by the failure of riata and riata ST implantable cardioverter-defibrillator leads. Heart Rhythm. 2012;9:1227–1235. [DOI] [PubMed] [Google Scholar]
- 71.Duggirala HJ, Herz ND, Caños DA, et al. Disproportionality analysis for signal detection of implantable cardioverter-defibrillator-related adverse events in the Food and Drug Administration Medical Device Reporting System. Pharmacoepidemiol Drug Saf. 2012;21:87–93. [DOI] [PubMed] [Google Scholar]
- 72.Durack JC, Thor Johnson D, Fidelman N, et al. Entrapment of the StarClose Vascular Closure System after attempted common femoral artery deployment. Cardiovasc Intervent Radiol. 2012;35:942–944. [DOI] [PubMed] [Google Scholar]
- 73.Lucas SM, Pattison EA, Sundaram CP. Global robotic experience and the type ofsurgical system impact the types of robotic malfunctions and their clinical consequences: an FDA MAUDE review. B J UInt. 2012;109:1222–1227. [DOI] [PubMed] [Google Scholar]
- 74.Shah HN, Badlani GH. Mesh complications in female pelvic floor reconstructive surgery and their management: a systematic review. Indian J Urol. 2012;28:129–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Brown J, Blank K. Minimally invasive endometrial ablation device complications and use outside of the manufacturers’ instructions. Obstet Gynecol. 2012;120:865–870. [DOI] [PubMed] [Google Scholar]
- 76.Cope JU, Samuels-Reid JH, Morrison AE. Pediatric use of insulin pump technology: a retrospective study of adverse events in children ages 1-12 years. J Diabetes Sci Technol. 2012;6:1053–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Woo EJ. Recombinant human bone morphogenetic protein-2: adverse events reported to the Manufacturer and User Facility Device Experience database. Spine J. 2012;12:894–899. [DOI] [PubMed] [Google Scholar]
- 78.Woo EJ. Adverse events after recombinant human BMP2 in nonspinal orthopaedic procedures. Clin Orthop Relat Res. 2013;471:1707–1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pecoraro F, Luzi D. Detecting software failures in the MAUDE database: a preliminary analysis. Stud Health Technol Inform. 2013;192:1098. [PubMed] [Google Scholar]
- 80.Hauser RG, Kallinen Retel LM. Early fatigue fractures in the IS-1 connector leg of a small-diameter ICD lead: value of returned product analysis for improving device safety. Heart Rhythm. 2013;10:1462–1468. [DOI] [PubMed] [Google Scholar]
- 81.Hauser RG, Abdelhadi RH, McGriff DM, et al. Failure of a novel silicone-polyurethane copolymer (Optim™) to prevent implantable cardioverter-defibrillator lead insulation abrasions. Europace. 2013;15: 278–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Koivukangas T, Katisko JP, Koivukangas JP. Technical accuracy of optical and the electromagnetic tracking systems. Springerplus. 2013;2:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ellington DR, Richter HE. The role of vaginal mesh procedures in pelvic organ prolapse surgery in view of complication risk. Obstet Gynecol Int. 2013;2013:356960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Clark K, Sharma D, Qin R, et al. A use case study on late stent thrombosis for ontology-based temporal reasoning and analysis. J Biomed Semantics. 2014;5:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Althunayan AM, Elkoushy MA, Elhilali MM, et al. Adverse events resulting from lasers used in urology. J Endourol. 2014;28:256–260. [DOI] [PubMed] [Google Scholar]
- 86.Latuska RF, Carlson ML, Neff BA, et al. Auricular burns associated with operating microscope use during otologic surgery. Otol Neurotol. 2014;35:227–233. [DOI] [PubMed] [Google Scholar]
- 87.Driessen SR, Arkenbout EA, Thurkow AL, et al. Electromechanical morcellators in minimally invasive gynecologic surgery: an update. J Minim Invasive Gynecol. 2014;21:377–383. [DOI] [PubMed] [Google Scholar]
- 88.Milad MP, Milad EA. Laparoscopic morcellator-related complications. J Minim Invasive Gynecol. 2014;21:486–491. [DOI] [PubMed] [Google Scholar]
- 89.Morshedi MM, Kinney TB. Nickel hypersensitivity in patients with inferior vena cava filters: case report and literature and MAUDE database review. J Vasc Interv Radiol. 2014;25:1187–1191. [DOI] [PubMed] [Google Scholar]
- 90.Englum BR, Pavlisko EN, Mack MC, et al. Pseudoaneurysm formation after medtronic freestyle porcine aortic bioprosthesis implantation: a word of caution. Ann Thorac Surg. 2014;98:2061–2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kuhlmann-Capek MJ, Kilic GS, Shah AB, et al. Enmeshed in controversy: use of vaginal mesh in the current medicolegal environment. Female Pelvic Med Reconstr Surg. 2015;21:241–243. [DOI] [PubMed] [Google Scholar]
- 92.Haber K, Hawkins E, Levie M, et al. Hysteroscopic morcellation: review of the Manufacturer and User Facility Device Experience (MAUDE) database. J Minim Invasive Gynecol. 2015;22:110–114. [DOI] [PubMed] [Google Scholar]
- 93.Yub Lee S, Won Youn S, Kyun Kim H, et al. Inadvertent detachment of a retrievable intracranial stent: review of Manufacturer and User Facility Device Experience. Neuroradiol J. 2015;28:172–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hubosky SG, Raval AJ, Bagley DH. Locked deflection during flexible ureteroscopy: incidence and elucidation of the mechanism of an underreported complication. J Endourol. 2015;29:907–912. [DOI] [PubMed] [Google Scholar]
- 95.Qureshi AI, Mian N, Siddiqi H, et al. Occurrence and management strategies for catheter entrapment with Onyx liquid embolization. J Vasc Interv Neurol. 2015;8:37–41. [PMC free article] [PubMed] [Google Scholar]
- 96.Shah AD, Hirsh DS, Langberg JJ. User-reported abrasion-related lead failure is more common with durata compared to other implantable cardiac defibrillator leads. Heart Rhythm. 2015;12:2376–2380. [DOI] [PubMed] [Google Scholar]
- 97.Sterling ME, Hartigan SM, Wein AJ, et al. A standardized surgical technique for removal of the Interstim tined lead. Can J Urol. 2016;23: 8471–8475. [PubMed] [Google Scholar]
- 98.Deso SE, Idakoji IA, Kuo WT. Evidence-based evaluation of inferior vena cava filter complications based on filter type. Semin Intervent Radiol. 2016;33:93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Omar A, Pendyala LK, Ormiston JA, et al. Review: stent fracture in the drug-eluting stent era. Cardiovasc Revasc Med. 2016;17:404–411. [DOI] [PubMed] [Google Scholar]
- 100.Koch CN, Mateo LS, Kayiaros S, et al. Spontaneous fractures of a modern modular uncemented femoral stem. HSS J. 2016;12:250–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pham JC, Williams TL, Sparnon EM, et al. Ventilator-related adverse events: a taxonomy and findings from 3 incident reporting systems. Respir Care. 2016;61:621–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Leiter V, White SK, Walters A. Adverse event reports associated with vaginal mesh: an interrupted time series analysis. Womens Health Issues. 2017;27:279–285. [DOI] [PubMed] [Google Scholar]
- 103.John RM, Kapur S, Ellenbogen KA, et al. Atrioesophageal fistula formation with cryoballoon ablation is most commonly related to the left inferior pulmonary vein. Heart Rhythm. 2017;14:184–189. [DOI] [PubMed] [Google Scholar]
- 104.Smith CD, Ganz RA, Lipham JC, et al. Lower esophageal sphincter augmentation for gastroesophageal reflux disease: the safety of a modern implant. J Laparoendosc Adv Surg Tech A. 2017;27:586–591. [DOI] [PubMed] [Google Scholar]
- 105.Shapiro AR. Nonadjunctive use of continuous glucose monitors for insulin dosing: is it safe? J Diabetes Sci Technol. 2017;11:833–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hill KD, Goldstein BH, Angtuaco MJ, et al. Post-market surveillance to detect adverse events associated with Melody® valve implantation. Cardiol Young. 2017;27:1090–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.He Y, Eguren D, Luu TP, et al. Risk management and regulations for lower limb medical exoskeletons: a review. Med Devices (Auckl). 2017;10:89–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Black-Maier E, Pokorney SD, Barnett AS, et al. Risk of atrioesophageal fistula formation with contact force-sensing catheters. Heart Rhythm. 2017;14:1328–1333. [DOI] [PubMed] [Google Scholar]
- 109.Li X, Alemzadeh H, Chen D, et al. Surgeon training in telerobotic surgery via a hardware-in-the-loop simulator. J Healthc Eng. 2017;2017:6702919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Shapiro AR The safety of nonadjunctive use of continuous glucose monitors for insulin dosing: still not resolved. J Diabetes Sci Technol. 2017;11:856–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rayess HM, Svider P, Hanba C, et al. Adverse events in facial implant surgery and associated malpractice litigation. JAMA Facial Plast Surg. 2018;20:244–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sandberg JM, Gray I, Pearlman A, et al. An evaluation of the Manufacturer and User Facility Device Experience database that inspired the United States Food and Drug Administration’s reclassification of transvaginal mesh. Investig Clin Urol. 2018;59:126–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Strong EB, Randall DR, Cates DJ, et al. Analysis of reported balloon malfunctions and proposed rescue strategy for malfunction during airway dilation. Otolaryngol Head Neck Surg. 2018;158:331–336. [DOI] [PubMed] [Google Scholar]
- 114.Galper BZ, Beery DE, Leighton G, et al. Comparison of adverse event and device problem rates for transcatheter aortic valve replacement and Mitraclip procedures as reported by the Transcatheter Valve Therapy Registry and the Food and Drug Administration postmarket surveillance data. Am Heart J. 2018;198:64–74. [DOI] [PubMed] [Google Scholar]
- 115.Nandra K, Ing R. Safety of orogastric tubes in foregut and bariatric surgery [published correction appears in Surg Endosc. 2019 Apr 12]. Surg Endosc. 2018;32:4068–4070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wang S, Manudhane A, Ezaldein HH, et al. A review of the FDA’s 510(k) approvals process for electromagnetic devices used in body contouring. J Dermatolog Treat. 2019;30:727–729. [DOI] [PubMed] [Google Scholar]
- 117.Abi-Rafeh J, Safran T, Al-Halabi B, et al. Death by implants: critical analysis of the FDA-MAUDE database on breast implant-related mortality. Plast Reconstr Surg Glob Open. 2019;7:e2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ahluwalia J, Avram MM, Ortiz AE. Lasers and energy-based devices marketed for vaginal rejuvenation: a cross-sectional analysis of the MAUDE database. Lasers Surg Med. 2019;51:671–677. [DOI] [PubMed] [Google Scholar]
- 119.Golovlev AV Hillegass MG. New onset tinnitus after high-frequency spinal cord stimulator implantation. Case Rep Anesthesiol. 2019; 2019:5039646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Krouwer JS. Reducing glucose meter adverse events by using reliability growth with the FDA MAUDE database. JDiabetes Sci Technol. 2019;13:959–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wang J, Liang H, Kang H, et al. Understanding health information technology induced medication safety events by two conceptual frameworks. Appl Clin Inform. 2019;10:158–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wang S, Manudhane A, Ezaldein HH, et al. United States Food and Drug Administration’s 510(k) pathway: drawing implications from the approvals of brachytherapy devices. Cureus. 2019;11:e4230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wakefield CJ, Eggerstedt M, Tajudeen BA, et al. Adverse events associated with absorbable implants for the nasal valve: a review of the Manufacturer and User Facility Device Experience database. Facial Plast Surg Aesthet Med. 2020. doi: 10.1089/fpsam.2020.0126. [DOI] [PubMed] [Google Scholar]
- 124.Abraham M, Gold J, Dweck J, et al. Classifying device-related complications associated with intrathecal baclofen pumps: a MAUDE study. World Neurosurg. 2020;139:e652–e657. [DOI] [PubMed] [Google Scholar]
- 125.Zeitler EP, Friedman DJ, Loring Z, et al. Complications involving the subcutaneous implantable cardioverter-defibrillator: lessons learned from MAUDE. Heart Rhythm. 2020;17:447–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Meier L, Wang EY, Tomes M, et al. Miscategorization of deaths in the US Food and Drug Administration adverse events database. JAMA Intern Med. 2020;180:147–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Manudhane AP, Wang S, Ezaldein HH, et al. Powered muscle stimulators: an investigation into newly FDA 510(k) approved devices marketed for muscle toning and esthetic benefit. J Dermatolog Treat. 2020;31:200–203. [DOI] [PubMed] [Google Scholar]
- 128.Clapp B, Klingsporn W, Lodeiro C, et al. Small bowel obstructions following the use of barbed suture: a review of the literature and analysis of the MAUDE database. Surg Endosc. 2020;34:1261–1269. [DOI] [PubMed] [Google Scholar]
- 129.Shah ED, Hosmer AE, Patel A, et al. Valuing innovative endoscopic techniques: endoscopic suturing to prevent stent migration for benign esophageal disease. Gastrointest Endosc. 2020;91:278–285. [DOI] [PubMed] [Google Scholar]
- 130.Ziapour B, Zaepfel C, Iafrati MD, et al. A systematic review of the quality of cardiovascular surgery studies that extracted data from the MAUDE database. J Vasc Surg. 2021;74:1708–1720.e5. [DOI] [PubMed] [Google Scholar]
- 131.Pier MM, Pasick LJ, Benito DA, et al. Adverse events associated with implantable Dopplers during microvascular surgery. J Reconstr Microsurg. 2021;37:365–371. [DOI] [PubMed] [Google Scholar]
- 132.Chugh Y, Khatri JJ, Shishehbor MH, et al. Adverse events with intravascular lithotripsy after peripheral and off-label coronary use: a report from the FDA MAUDE database. J Invasive Cardiol. 2021;33:E974–E977. [DOI] [PubMed] [Google Scholar]
- 133.Chen J, Akoh CC, Kadakia R, et al. Analysis of 408 total ankle arthroplasty adverse events reported to the US Food and Drug Administration from 2015 to 2018. Foot Ankle Spec. 2021;14:393–400. [DOI] [PubMed] [Google Scholar]
- 134.Ramai D, DeLuca M, Facciorusso A, et al. Analysis of reported adverse events with colonic stents: an FDA MAUDE database study. J Clin Gastroenterol. 2022;56:784–786. [DOI] [PubMed] [Google Scholar]
- 135.Klima M. Bent or broken: analysis of set screw fracture in the TFNa implant. J Orthop Traumatol. 2021;22:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Klima ML. Comparison of early fatigue failure of the TFNa and gamma 3 cephalomedullary nails in the United States from 2015 to 2019. J Orthop Trauma. 2021;35:e39–e44. [DOI] [PubMed] [Google Scholar]
- 137.Ward M, Ahmed M, Markosian C, et al. Complications associated with deep brain stimulation for Parkinson’s disease: a MAUDE study. Br J Neurosurg. 2021;35:625–628. [DOI] [PubMed] [Google Scholar]
- 138.Ramai D, DeLuca M, Enofe I, et al. Device failures associated with gastric pacemakers: a MAUDE database analysis. Dig Liver Dis. 2021;53: 1529–1530. [DOI] [PubMed] [Google Scholar]
- 139.Samuels JM, Overbey DM, Wikiel KJ, et al. Electromagnetic interference on cardiac pacemakers and implantable cardioverter defibrillators during endoscopy as reported to the US Federal Drug Administration. Surg Endosc. 2021;35:3796–3801. [DOI] [PubMed] [Google Scholar]
- 140.DeMarchi J, Schwiers M, Soberman M, et al. Evolution of a novel technology for gastroesophageal reflux disease: a safety perspective of magnetic sphincter augmentation. Dis Esophagus. 2021;34:doab036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Mascarenhas AK. Is fluoride varnish safe?: validating the safety of fluoride varnish. J Am Dent Assoc. 2021;152:364–368. [DOI] [PubMed] [Google Scholar]
- 142.Ramai D, Bhandari P, Facciorusso A, et al. Real-world experience of intragastric balloons for obesity: insights from the FDA Manufacturer and User Facility Device Experience (MAUDE) database. Obes Surg. 2021;31:3360–3364. [DOI] [PubMed] [Google Scholar]
- 143.Case BC, Yerasi C, Forrestal BJ, et al. Real-world experience of the MANTA closure device: insights from the FDA Manufacturer and User Facility Device Experience (MAUDE) database. Cardiovasc Revasc Med. 2021;27:63–66. [DOI] [PubMed] [Google Scholar]
- 144.Lalani C, Kunwar EM, Kinard M, et al. Reporting of death in US Food and Drug Administration medical device adverse event reports in categories other than death. JAMA Intern Med. 2021;181:1217–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Crumley C. Abdominal negative pressure wound therapy devices for management of the open abdomen: a technologic analysis. J Wound Ostomy Continence Nurs. 2022;49:124–127. [DOI] [PubMed] [Google Scholar]
- 146.Pier MM, Pasick LJ, Benito DA, et al. Adverse events associated with electromyogram endotracheal tubes in thyroid and parathyroid surgery. J Patient Saf. 2022;18:171–176. [DOI] [PubMed] [Google Scholar]
- 147.Tong JY, Pasick LJ, Benito DA, et al. Adverse events associated with ossicular prostheses: utility of a federal database. Otol Neurotol. 2022;43:e229–e234. [DOI] [PubMed] [Google Scholar]
- 148.Ramai D, Shapiro A, Barakat M, et al. Adverse events associated with transoral incisionless fundoplication (TIF) for chronic gastroesophageal reflux disease: a MAUDE database analysis. Surg Endosc. 2022;36:4956–4959. [DOI] [PubMed] [Google Scholar]
- 149.Mao J, Sedrakyan A, Sun T, et al. Assessing adverse event reports of hysteroscopic sterilization device removal using natural language processing. Pharmacoepidemiol Drug Saf. 2022;31:442–451. [DOI] [PubMed] [Google Scholar]
- 150.Megaly M, Zordok M, Mentias A, et al. Complications and failure modes of covered coronary stents: insights from the MAUDE database. Cardiovasc Revasc Med. 2022;35:157–160. [DOI] [PubMed] [Google Scholar]
- 151.Kaluski E, Ghosh P, Lone A. Complications and failure modes of polymer-jacketed guidewires; insights from the MAUDE database. Cardiovasc Revasc Med. 2022;36:136–137. [DOI] [PubMed] [Google Scholar]
- 152.Sedhom R, Abdelmaseeh P, Haroun M, et al. Complications of penumbra indigo aspiration device in pulmonary embolism: insights from MAUDE database. Cardiovasc Revasc Med. 2022;39:97–100. [DOI] [PubMed] [Google Scholar]
- 153.Artsen AM, Sassani JC, Moalli PA, et al. Complications reported to the Food and Drug Administration: a cross-sectional comparison of urogynecologic meshes. Female Pelvic Med Reconstr Surg. 2022;28:452–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kalenderian E, Lee JH, Obadan-Udoh EM, et al. Development of an inventory of dental harms: methods and rationale. J Patient Saf. 2022;18: 559–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kotamarti S, Michael Z, Silver D, et al. Device-related complications during renal cryoablation: insights from the Manufacturer and User Facility Device Experience (MAUDE) database. Urol Oncol. 2022;40:199.e9–199.e14. [DOI] [PubMed] [Google Scholar]
- 156.Topaz O Excimer laser-induced adverse coronary events: discerning the merits and shortcomings of the MAUDE database report. Cardiovasc Revasc Med. 2022;43:155–157. [DOI] [PubMed] [Google Scholar]
- 157.Lim Y, Wulkan A, Avram M. FDA MAUDE database reported adverse events on noninvasive body contouring, cellulite treatment, and muscle stimulation from 2015 to 2021. Lasers Surg Med. 2023;55:146–151. [DOI] [PubMed] [Google Scholar]
- 158.Crumley C Intra-abdominal pressure measurement devices: a technologic analysis. J Wound Ostomy Continence Nurs. 2022;49:220–225. [DOI] [PubMed] [Google Scholar]
- 159.Arora Y, D’Angelo L, Azarrafiy R, et al. Location of superior vena cava tears in transvenous lead extraction. Ann Thorac Surg. 2022;113:1165–1171. [DOI] [PubMed] [Google Scholar]
- 160.Cohen JL, Hicks J, Nogueira A, et al. Postmarket safety surveillance of delayed complications for recent FDA-approved hyaluronic acid dermal fillers. Dermatol Surg. 2022;48:220–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Xiong M, Chen C, Sereda Y, et al. Retrospective analysis of the MAUDE database on dermal filler complications from 2014–2020. J Am Acad Dermatol. 2022;87:1158–1160. [DOI] [PubMed] [Google Scholar]
- 162.Clapp B, Schrodt A, Ahmad M, et al. Stapler malfunctions in bariatric surgery: an analysis of the MAUDE database. JSLS. 2022;26:e2021.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Crumley C Topical wound therapy products with ionic Silver: a technologic analysis. J Wound Ostomy Continence Nurs. 2022;49:308–313. [DOI] [PubMed] [Google Scholar]
- 164.Burkett L, Moalli P, Ackenbom M. What is being reported about vaginal “lasers”?: an examination of adverse events reported to the Food and Drug Administration on energy-based devices. Aesthet Surg J. 2022;42:689–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Hamburger MI, Lakhanpal S, Mooar PA, et al. Intra-articular hyaluronans: a review of product-specific safety profiles. Semin Arthritis Rheum. 2003;32:296–309. [DOI] [PubMed] [Google Scholar]
- 166.Nussbaum DA, Gailloud P, Murphy K. A review of complications associated with vertebroplasty and kyphoplasty as reported to the Food and Drug Administration medical device related web site. J Vasc Interv Radiol. 2004;15:1185–1192. [DOI] [PubMed] [Google Scholar]
- 167.Brown SL, Woo EK. Surgical stapler-associated fatalities and adverse events reported to the Food and Drug Administration. J Am Coll Surg. 2004;199:374–381. [DOI] [PubMed] [Google Scholar]
- 168.Tambyraja RR, Gutman MA, Megerian CA. Cochlear implant complications: utility of federal database in systematic analysis. Arch Otolaryngol Head Neck Surg. 2005;131:245–250. [DOI] [PubMed] [Google Scholar]
- 169.Hignett S, Griffiths P. Do split-side rails present an increased risk to patient safety? Qual Saf Health Care. 2005;14:113–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Robinson TN, Clarke JH, Schoen J, et al. Major mesh-related complications following hernia repair: events reported to the Food and Drug Administration. Surg Endosc. 2005;19:1556–1560. [DOI] [PubMed] [Google Scholar]
- 171.Brott BC, Anayiotos AS, Chapman GD, et al. Severe, diffuse coronary artery spasm after drug-eluting stent placement. J Invasive Cardiol. 2006;18:584–592. [PubMed] [Google Scholar]
- 172.Delaney JW, Li JS, Rhodes JF. Major complications associated with transcatheter atrial septal occluder implantation: a review of the medical literature and the Manufacturer and User Facility Device Experience (MAUDE) database. Congenit Heart Dis. 2007;2:256–264. [DOI] [PubMed] [Google Scholar]
- 173.Hsi RS, Saint-Elie DT, Zimmerman GJ, et al. Mechanisms of hemostatic failure during laparoscopic nephrectomy: review of Food and Drug Administration database. Urology. 2007;70:888–892. [DOI] [PubMed] [Google Scholar]
- 174.Stapelmann H, Türp JC. The NTI-tss device for the therapy of bruxism, temporomandibular disorders, and headache—where do we stand? A qualitative systematic review of the literature. BMC Oral Health. 2008;8:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Wang HE, Weaver MD, Abo BN, et al. Ambulance stretcher adverse events. Qual Saf Health Care. 2009;18:213–216. [DOI] [PubMed] [Google Scholar]
- 176.Hsi RS, Ojogho ON, Baldwin DD. Analysis of techniques to secure the renal hilum during laparoscopic donor nephrectomy: review of the FDA database. Urology. 2009;74:142–147. [DOI] [PubMed] [Google Scholar]
- 177.Sfyroeras GS, Koutsiaris A, Karathanos C, et al. Clinical relevance and treatment of carotid stent fractures. J Vasc Surg. 2010;51:1280–1285. [DOI] [PubMed] [Google Scholar]
- 178.Chotikawanich E, Korman E, Monga M. Complications of stone baskets: 14-year review of the Manufacturer and User Facility Device Experience database. J Urol. 2011;185:179–183. [DOI] [PubMed] [Google Scholar]
- 179.Davis ID, Cizman B, Mundt K, et al. Relationship between drain volume/fill volume ratio and clinical outcomes associated with overfill complaints in peritoneal dialysis patients. Perit Dial Int. 2011;31:148–153. [DOI] [PubMed] [Google Scholar]
- 180.Mamas MA, Williams PD. Longitudinal stent deformation: insights on mechanisms, treatments and outcomes from the Food and Drug Administration Manufacturer and User Facility Device Experience database. EuroIntervention. 2012;8:196–204. [DOI] [PubMed] [Google Scholar]
- 181.Al-Safi ZA, Shavell VI, Hobson DT, et al. Analysis of adverse events with Essure hysteroscopic sterilization reported to the Manufacturer and User Facility Device Experience database. J Minim Invasive Gynecol. 2013;20:825–829. [DOI] [PubMed] [Google Scholar]
- 182.Johnson DT, Durack JC, Fidelman N, et al. Distribution of reported StarClose SE vascular closure device complications in the Manufacturer and User Facility Device Experience database. J Vasc Interv Radiol. 2013;24:1051–1056. [DOI] [PubMed] [Google Scholar]
- 183.Friedman DC, Lendvay TS, Hannaford B. Instrument failures for the da Vinci surgical system: a Food and Drug Administration MAUDE database study. Surg Endosc. 2013;27:1503–1508. [DOI] [PubMed] [Google Scholar]
- 184.Kwazneski D 2nd, Six C, Stahlfeld K. The unacknowledged incidence of laparoscopic stapler malfunction. Surg Endosc. 2013;27:86–89. [DOI] [PubMed] [Google Scholar]
- 185.Andreoli JM, Lewandowski RJ, Vogelzang RL, et al. Comparison of complication rates associated with permanent and retrievable inferior vena cava filters: a review of the MAUDE database. J Vasc Interv Radiol. 2014;25:1181–1185. [DOI] [PubMed] [Google Scholar]
- 186.Zelickson Z, Schram S, Zelickson B. Complications in cosmetic laser surgery: a review of 494 Food and Drug Administration Manufacturer and User Facility Device Experience reports. Dermatol Surg. 2014;40:378–382. [DOI] [PubMed] [Google Scholar]
- 187.Woerdeman PA, Cochrane DD. Disruption of silicone valve housing in a Codman Hakim Precision valve with integrated Siphonguard. J Neurosurg Pediatr. 2014;13:532–535. [DOI] [PubMed] [Google Scholar]
- 188.Metheny NA, Meert KL. Effectiveness of an electromagnetic feeding tube placement device in detecting inadvertent respiratory placement. Am J Crit Care. 2014;23:240–248. [DOI] [PubMed] [Google Scholar]
- 189.Munro MG, Nichols JE, Levy B, et al. Hysteroscopic sterilization: 10-year retrospective analysis of worldwide pregnancy reports. J Minim Invasive Gynecol. 2014;21:245–251. [DOI] [PubMed] [Google Scholar]
- 190.Johnson SM, Itoga N, Garnett GM, et al. Increased risk of cardiovascular perforation during ECMO with a bicaval, wire-reinforced cannula. J Pediatr Surg. 2014;49:46–50. [DOI] [PubMed] [Google Scholar]
- 191.Manoucheri E, Fuchs-Weizman N, Cohen SL, et al. MAUDE: analysis of robotic-assisted gynecologic surgery. J Minim Invasive Gynecol. 2014;21:592–595. [DOI] [PubMed] [Google Scholar]
- 192.Pokorney SD, Greenfield RA, Atwater BD, et al. Novel mechanism of premature battery failure due to lithium cluster formation in implantable cardioverter-defibrillators. Heart Rhythm. 2014;11:2190–2195. [DOI] [PubMed] [Google Scholar]
- 193.Wassell RW, Verhees L, Lawrence K, et al. Over-the-counter (OTC) bruxism splints available on the Internet. Br Dent J. 2014;216:E24. [DOI] [PubMed] [Google Scholar]
- 194.Mamas MA, Foin N, Abunassar C, et al. Stent fracture: insights on mechanisms, treatments, and outcomes from the food and drug administration Manufacturer and User Facility Device Experience database. Catheter Cardiovasc Interv 2014;83:E251–E259. [DOI] [PubMed] [Google Scholar]
- 195.Thennukonda RA, Natarajan BR. Adverse events associated with ultrasonic scalers: a Manufacturer and User Facility Device Experience database analysis. Indian J Dent Res. 2015;26:598–602. [DOI] [PubMed] [Google Scholar]
- 196.Naumann RW, Brown J. Complications of electromechanical Morcellation reported in the Manufacturer and User Facility Device Experience (MAUDE) database. J Minim Invasive Gynecol. 2015;22:1018–1021. [DOI] [PubMed] [Google Scholar]
- 197.Tremaine AM, Avram MM. FDA MAUDE data on complications with lasers, light sources, and energy-based devices. Lasers Surg Med. 2015;47:133–140. [DOI] [PubMed] [Google Scholar]
- 198.Chatterjee S, Herrmann HC, Wilensky RL, et al. Safety and procedural success of left atrial appendage exclusion with the lariat device: a systematic review of published reports and analytic review of the FDA MAUDE database. JAMA Intern Med. 2015;175:1104–1109. [DOI] [PubMed] [Google Scholar]
- 199.Overbey DM, Townsend NT, Chapman BC, et al. Surgical Energy-Based Device Injuries and Fatalities Reported to the Food and Drug Administration. J Am Coll Surg. 2015;221:197–205.e1. [DOI] [PubMed] [Google Scholar]
- 200.Alemzadeh H, Raman J, Leveson N, et al. Adverse events in robotic surgery: a retrospective study of 14 years of FDA data. PloS One. 2016;11:e0151470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Lopez J, Soni A, Calva D, et al. Iatrogenic surgical microscope skin burns: a systematic review of the literature and case report. Burns. 2016;42:e74–e80. [DOI] [PubMed] [Google Scholar]
- 202.Malgor RD, Gasparis AP, Labropoulos N. Morbidity and mortality after thermal venous ablations. Int Angiol. 2016;35:57–61. [PubMed] [Google Scholar]
- 203.Chen MM, Holsinger FC. Morbidity and mortality associated with robotic head and neck surgery: an inquiry of the Food and Drug Administration Manufacturer and User Facility Device Experience database. JAMA Otolaryngol Head Neck Surg. 2016;142:405–406. [DOI] [PubMed] [Google Scholar]
- 204.Kohani M, Pecht M. New Minimum Relative Humidity Requirements Are Expected to Lead to More Medical Device Failures [published correction appears in J Med Syst 2016 Apr;40(4):86]. J Med Syst. 2016;40:58. [DOI] [PubMed] [Google Scholar]
- 205.Everett KD, Conway C, Desany GJ, et al. Structural mechanics predictions relating to clinical coronary stent fracture in a 5 year period in FDA MAUDE database. Ann Biomed Eng. 2016;44:391–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Tanimoto R, Cleary RC, Bagley DH, et al. Ureteral avulsion associated with Ureteroscopy: insights from the MAUDE database. J Endourol. 2016;30:257–261. [DOI] [PubMed] [Google Scholar]
- 207.Li Y, Wang X, Bian R, et al. Zhongguo Yi Liao Qi Xie Za Zhi. 2017;41:48–50. [PubMed] [Google Scholar]
- 208.Allareddy V, Nalliah R, Lee MK, et al. Adverse clinical events reported during Invisalign treatment: analysis of the MAUDE database. Am J Orthod Dentofacial Orthop. 2017;152:706–710. [DOI] [PubMed] [Google Scholar]
- 209.Bielefeldt K. Adverse events of gastric electrical stimulators recorded in the manufacturer and user device experience (MAUDE) registry. Auton Neurosci. 2017;202:40–44. [DOI] [PubMed] [Google Scholar]
- 210.Bourgault AM, Aguirre L, Ibrahim J. Cortrak-assisted feeding tube insertion: a comprehensive review of adverse events in the MAUDE database. Am J Crit Care. 2017;26:149–156. [DOI] [PubMed] [Google Scholar]
- 211.Gupta P, Schomburg J, Krishna S, et al. Development of a classification scheme for examining adverse events associated with medical devices, specifically the DaVinci surgical system as reported in the FDA MAUDE database. J Endourol. 2017;31:27–31. [DOI] [PubMed] [Google Scholar]
- 212.Patel NH, Schulman AA, Bloom JB, et al. Device-related adverse events during percutaneous Nephrolithotomy: review of the Manufacturer and User Facility Device Experience database. J Endourol. 2017;31:1007–1011. [DOI] [PubMed] [Google Scholar]
- 213.Lin Y, Melby DP, Krishnan B, et al. Frequency of pacemaker malfunction associated with monopolar electrosurgery during pulse generator replacement or upgrade surgery. J Interv Card Electrophysiol. 2017;49:205–209. [DOI] [PubMed] [Google Scholar]
- 214.Srinivasa DR, Miranda RN, Kaura A, et al. Global adverse event reports of breast implant-associated ALCL: an international review of 40 government authority databases. Plast Reconstr Surg. 2017;139:1029–1039. [DOI] [PubMed] [Google Scholar]
- 215.Kang H, Wang F, Zhou S, et al. Identifying and synchronizing health information Technology (HIT) Events from FDA medical device reports. Stud Health Technol Inform. 2017;245:1048–1052. [PubMed] [Google Scholar]
- 216.Coelho DH, Tampio AJ. The utility of the MAUDE database for Osseointegrated auditory implants. Ann Otol Rhinol Laryngol. 2017;126:61–66. [DOI] [PubMed] [Google Scholar]
- 217.Brull SJ, Prielipp RC. Vascular air embolism: a silent hazard to patient safety. J Crit Care. 2017;42:255–263. [DOI] [PubMed] [Google Scholar]
- 218.Doran J, Ward M, Ward B, et al. Investigating complications associated with occipital nerve stimulation: a MAUDE study. Neuromodulation. 2018;21:296–301. [DOI] [PubMed] [Google Scholar]
- 219.Shida T, Umezu M, Iwasaki K. Investigation of adverse events associated with an off-label use of arterial stents and CE-marked iliac vein stents in the iliac vein: insights into developing a better iliac vein stent. J Artif Organs. 2018;21:254–260. [DOI] [PubMed] [Google Scholar]
- 220.Jazayeri MA, Vuddanda V, Turagam MK, et al. Safety profiles of percutaneous left atrial appendage closure devices: an analysis of the Food and Drug Administration Manufacturer and User Facility Device Experience (MAUDE) database from 2009 to 2016. J Cardiovasc Electrophysiol. 2018;29:5–13. [DOI] [PubMed] [Google Scholar]
- 221.Alicuben ET, Bell RCW, Jobe BA, et al. Worldwide experience with Erosion of the magnetic sphincter augmentation device. J Gastrointest Surg. 2018;22:1442–1447. [DOI] [PubMed] [Google Scholar]
- 222.Khalid N, Rogers T, Shlofmitz E, et al. Adverse events and modes of failure related to Impella RP: insights from the Manufacturer and User Facility Device Experience (MAUDE) database. Cardiovasc Revasc Med. 2019;20:503–506. [DOI] [PubMed] [Google Scholar]
- 223.Khalid N, Javed H, Rogers T, et al. Adverse events and modes of failure related to the FilterWire EZ embolic protection system: lessons learned from an analytic review of the FDA MAUDE database. Catheter Cardiovasc Interv. 2019;94:157–164. [DOI] [PubMed] [Google Scholar]
- 224.Khalid N, Rogers T, Shlofmitz E, et al. Adverse events and modes of failure related to the Impella percutaneous left ventricular assist devices: a retrospective analysis of the MAUDE database. EuroIntervention. 2019;15:44–46. [DOI] [PubMed] [Google Scholar]
- 225.Chen Y, Shah AA, Shlofmitz E, et al. Adverse events associated with the use of guide extension catheters during percutaneous coronary intervention: reports from the Manufacturer and User Facility Device Experience (MAUDE) database. Cardiovasc Revasc Med. 2019;20:409–412. [DOI] [PubMed] [Google Scholar]
- 226.Armstrong AA, Kroener L, Brower M, et al. Analysis of reported adverse events with uterine artery embolization for leiomyomas. J Minim Invasive Gynecol. 2019;26:667–670.e1. [DOI] [PubMed] [Google Scholar]
- 227.Agarwal A, Kelkar A, Garg Agarwal A, et al. Device-related complications associated with Magec rod usage for distraction-based correction of scoliosis. Spine Surg Relat Res. 2019;4:148–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Hauser RG, Sengupta J, Schloss EJ, et al. Internal insulation breaches in an implantable cardioverter-defibrillator lead with redundant conductors. Heart Rhythm. 2019;16:1215–1222. [DOI] [PubMed] [Google Scholar]
- 229.van Eijk AM, Zulaika G, Lenchner M, et al. Menstrual cup use, leakage, acceptability, safety, and availability: a systematic review and meta-analysis. Lancet Public Health. 2019;4:e376–e393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Diaz CL, Guo X, Whitman IR, et al. Reported mortality with rotating sheaths vs. laser sheaths for transvenous lead extraction. Europace. 2019;21:1703–1709. [DOI] [PubMed] [Google Scholar]
- 231.Souders C, Nik-Ahd F, Zhao H, et al. Robotic sacrocolpopexy: adverse events reported to the FDA over the last decade. Int Urogynecol J. 2019;30:1919–1923. [DOI] [PubMed] [Google Scholar]
- 232.Nik-Ahd F, Souders CP, Houman J, et al. Robotic urologic surgery: trends in Food and Drug Administration-reported adverse events over the last decade. J Endourol. 2019;33:649–654. [DOI] [PubMed] [Google Scholar]
- 233.Masoomi R, Lancaster E, Robinson A, et al. Safety of EndoAnchors in real-world use: a report from the Manufacturer and User Facility Device Experience database. Vascular. 2019;27:495–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Shlofmitz E, Garcia-Garcia HM, Rogers T, et al. Techniques to optimize the use of optical coherence tomography: insights from the Manufacturer and User Facility Device Experience (MAUDE) database. Cardiovasc Revasc Med. 2019;20:507–512. [DOI] [PubMed] [Google Scholar]
- 235.Kiely DJ, Oppenheimer LW, Dornan JC. Unrecognized maternal heart rate artefact in cases of perinatal mortality reported to the United States Food and Drug Administration from 2009 to 2019: a critical patient safety issue. BMC Pregnancy Childbirth. 2019;19:501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Halepas S, Lee KC, Higham ZL, et al. A 20-year analysis of adverse events and litigation with light-based skin resurfacing procedures. J Oral Maxillofac Surg. 2020;78:619–628. [DOI] [PubMed] [Google Scholar]
- 237.Hur K, Ge M, Kim J, et al. Adverse events associated with balloon sinuplasty: a MAUDE database analysis. Otolaryngol Head Neck Surg. 2020;162:137–141. [DOI] [PubMed] [Google Scholar]
- 238.Bestourous DE, Pasick LJ, Benito DA, et al. Adverse events associated with the Inspire implantable hypoglossal nerve stimulator: a MAUDE database review. Am J Otolaryngol. 2020;41:102616. [DOI] [PubMed] [Google Scholar]
- 239.Khalid N, Javed H, Ahmad SA, et al. Analysis of the Food and Drug Administration Manufacturer and User Facility Device Experience database for patient- and circuit-related adverse events involving extracorporeal membrane oxygenation. Cardiovasc Revasc Med. 2020;21:230–234. [DOI] [PubMed] [Google Scholar]
- 240.Ortiz AE, Ahluwalia J, Song SS, et al. Analysis of U.S. Food and Drug Administration data on soft-tissue filler complications. Dermatol Surg. 2020;46:958–961. [DOI] [PubMed] [Google Scholar]
- 241.Teames R, Joyce A, Scranton R, et al. Characterization of device-related malfunction, injury, and death associated with using elastomeric pumps for delivery of local anesthetics in the US Food and Drug Administration MAUDE database. Drug Healthc Patient Saf. 2020;12:293–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Tong JY, Pasick LJ, Benito DA, et al. Complications associated with tracheoesophageal voice prostheses from 2010 to 2020: a MAUDE study. Am J Otolaryngol. 2020;41:102652. [DOI] [PubMed] [Google Scholar]
- 243.Povolotskiy R, Abraham ME, Leverant AB, et al. Complications of palatal pillar implants: an analysis of the MAUDE database and literature review. Am J Otolaryngol. 2020;41:102303. [DOI] [PubMed] [Google Scholar]
- 244.Kang H, Gong Y. Creating a database for health IT events via a hybrid deep learning model. J Biomed Inform. 2020;110:103556. [DOI] [PubMed] [Google Scholar]
- 245.Thomas WC, Parvataneni HK, Vlasak RG, et al. Early polyethylene failure in a modern total hip prosthesis: a note of caution. J Arthroplasty. 2020;35:1297–1302. [DOI] [PubMed] [Google Scholar]
- 246.Hauser RG, Sengupta J, Casey S, et al. High shocking and pacing impedances due to defibrillation lead calcification. J Interv Card Electrophysiol. 2020;58:253–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Sengupta J, Storey K, Casey S, et al. Outcomes before and after the recall of a heart failure pacemaker. JAMA Intern Med. 2020;180:198–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Sassani JC, Artsen AM, Moalli PA, et al. Temporal trends of urogynecologic mesh reports to the U.S. Food and Drug Administration. Obstet Gynecol. 2020;135:1084–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Guo JZ, Souders C, McClelland L, et al. Vaginal laser treatment of genitourinary syndrome of menopause: does the evidence support the FDA safety communication? Menopause. 2020;27:1177–1184. [DOI] [PubMed] [Google Scholar]
- 250.Virk S, Phillips F, Khan S, et al. A cross-sectional analysis of 1347 complications for cervical disc replacements from medical device reports maintained by the United States Food and Drug Administration. Spine J. 2021;21:265–272. [DOI] [PubMed] [Google Scholar]
- 251.Assam JH, DeHaan MC, Bakken S, et al. Adverse event reporting in head and neck transoral robotic surgery: a MAUDE database study. J Robot Surg. 2021;15:899–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Tong JY, Pasick LJ, Benito DA, et al. Adverse events associated with laser use in the upper airway. Otolaryngol Head Neck Surg. 2021;164:911–917. [DOI] [PubMed] [Google Scholar]
- 253.Zhao H, Souders CP, Kuhlmann PK, et al. Adverse events associated with synthetic male slings: an analysis of the Food and Drug Administration Manufacturer and User Facility Device Experience database. Int Neurourol J. 2021;25:172–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Akoh CC, Chen J, Kadakia R, et al. Adverse events involving hallux metatarsophalangeal joint implants: analysis of the United States Food and Drug Administration data from 2010 to 2018. Foot Ankle Surg. 2021;27:381–388. [DOI] [PubMed] [Google Scholar]
- 255.Bansal A, Gupta S, Jain V, et al. Adverse events related to excimer laser coronary atherectomy: analysis of the FDA MAUDE database. Cardiovasc Revasc Med. 2021;27:88–89. [DOI] [PubMed] [Google Scholar]
- 256.Mahmoud K, Metikala S, O’Connor KM, et al. Adverse events related to total ankle replacement devices: an analysis of reports to the United States Food and Drug Administration. Int Orthop. 2021;45:2307–2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Philipson DJ, Cohen DJ, Fonarow GC, et al. Analysis of adverse events related to Impella usage (from the Manufacturer and User Facility Device Experience and National Inpatient Sample Databases). Am J Cardiol. 2021;140:91–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Hacherl CC, Patel NA, Jones K, et al. Characterizing adverse events of cranioplasty implants after craniectomy: a retrospective review of the federal Manufacturer and User Facility Device Experience database. Cureus. 2021;13:e16795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Bennett J, MacGuire J, Novakovic E, et al. Characterizing complications of deep brain stimulation devices for the treatment of parkinsonian symptoms without tremor: a federal MAUDE database analysis [published correction appears in Cureus. 2021 Aug 17;13(8):c46]. Cureus. 2021;13:e15539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Lee E, Tong JY, Pasick LJ, et al. Complications associated with PlasmaBlade TnA during tonsillectomy and adenoidectomy from 2010 to 2020: a MAUDE study. Am J Otolaryngol. 2021;42:102826. [DOI] [PubMed] [Google Scholar]
- 261.Bennett J, MacGuire J, Novakovic E, et al. Correction: characterizing complications of deep brain stimulation devices for the treatment of parkinsonian symptoms without tremor: a federal MAUDE database analysis. Cureus. 2021;13:c46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Rahimpour S, Kiyani M, Hodges SE, et al. Deep brain stimulation and electromagnetic interference. Clin Neurol Neurosurg. 2021;203:106577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Gopal N, Long B, Phillips J, et al. Endovascular stapler complications during minimally invasive nephrectomy: an updated review of the FDA MAUDE database from 2009–2019. Urology. 2021;153:181–184. [DOI] [PubMed] [Google Scholar]
- 264.Londoño Castillo J, Ramai D, Crinò SF, et al. Experience of the moray micro forceps biopsy for pancreatic cystic lesions: lessons and insights from the MAUDE database. Expert Rev Gastroenterol Hepatol. 2021;15:1345–1347. [DOI] [PubMed] [Google Scholar]
- 265.Khalid N, Pandey Y, Khalid U, et al. Modes of failure with fractional flow reserve guidewires: insights from the Manufacturer and User Facility Device Experience database. World J Cardiol. 2021;13:223–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Benham DA, Calvo RY, Carr MJ, et al. Revealing the scope of surgical device malfunctions: analysis of the “hidden” Food and Drug Administration device database. Am J Surg. 2021;221:1121–1126. [DOI] [PubMed] [Google Scholar]
- 267.Lee KC, Chintalapudi N, Halepas S, et al. The healthcare burden and associated adverse events from total alloplastic temporomandibular joint replacement: a national United States perspective. Int J Oral Maxillofac Surg. 2021;50:236–241. [DOI] [PubMed] [Google Scholar]
- 268.Morcos R, Megaly M, Desai A, et al. The transseptal puncture experience: safety insights from FDA MAUDE database. Catheter Cardiovasc Interv. 2021;98:E855–E861. [DOI] [PubMed] [Google Scholar]
- 269.Kucharski K, Adler DG. Comparison of technical failures and patient-related adverse events associated with 3 widely used mechanical lithotripters for ERCP: insights from the U.S. Food and Drug Administration Manufacturer and User Facility Device Experience database. Gastrointest Endosc. 2022;96:796–800. [DOI] [PubMed] [Google Scholar]
- 270.Koutsogiannis P, Khan S, Phillips F, et al. A cross-sectional analysis of 284 complications for lumbar disc replacements from medical device reports maintained by the United States Food and Drug Administration. Spine J. 2022;22:278–285. [DOI] [PubMed] [Google Scholar]
- 271.Bestourous DE, Davidson L, Reilly BK. A review of reported adverse events in MRI-safe and MRI-conditional Cochlear implants. Otol Neurotol. 2022;43:427. [DOI] [PubMed] [Google Scholar]
- 272.Narwani V Torabi SJ, Kasle DA, et al. Adverse events associated with corticosteroid-eluting sinus stents: a MAUDE database analysis. Otolaryngol Head Neck Surg. 2022;166:179–182. [DOI] [PubMed] [Google Scholar]
- 273.Koenig LR, Duong AT, Yuan M, et al. Adverse events associated with femtosecond laser-assisted cataract surgery reported to the MAUDE database. J Cataract Refract Surg. 2022;48:168–172. [DOI] [PubMed] [Google Scholar]
- 274.Rahl MD, Weistroffer J, Dall BE. Analysis of complications in sacroiliac joint fusions using FDA 510(k) cleared devices. Clin Spine Surg. 2022;35:E363–E367. [DOI] [PubMed] [Google Scholar]
- 275.Badger CD, Singh RA, Terhaar SJ, et al. Breaking it down: review and management of sialendoscopy device malfunctions. Am J Otolaryngol. 2022;43:103400. [DOI] [PubMed] [Google Scholar]
- 276.Juhász M, Sharma AN, Cohen JL. Characterizing ocular adverse events after facial dermal filler injection-reviewing the MAUDE database. Dermatol Surg. 2022;48:695–696. [DOI] [PubMed] [Google Scholar]
- 277.Dubrouskaya K, Hagenstein L, Ramai D, et al. Clinical adverse events and device failures for the Barrx™ radiofrequency ablation catheter system: a MAUDE database analysis. Ann Gastroenterol. 2022;35:345–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Hagenstein LD, Dubrouskaya K, Ramai D, et al. Clinical adverse events and device failures reported for the captivator and duette endoscopic mucosal resection (EMR) systems: A MAUDE database analysis. J Clin Gastroenterol. 2023;57:490–493. [DOI] [PubMed] [Google Scholar]
- 279.Megaly M, Morcos R, Khalil C, et al. Complications and failure modes of coronary embolic protection devices: insights from the MAUDE database. Catheter Cardiovasc Interv. 2022;99:405–410. [DOI] [PubMed] [Google Scholar]
- 280.Megaly M, Morcos R, Kucharik M, et al. Complications and failure modes of polymer-jacketed guidewires; insights from the MAUDE database. Cardiovasc Revasc Med. 2022;36:132–135. [DOI] [PubMed] [Google Scholar]
- 281.Lee E, Elzomor A, Zwemer C, et al. Complications associated with Dermabond® during head and neck surgery: MAUDE and literature review. Am J Otolaryngol. 2022;43:103330. [DOI] [PubMed] [Google Scholar]
- 282.Carey J, Gabbireddy S, Mammen L, et al. FDA MAUDE database analysis of titanium middle ear prosthesis. J Otol. 2022;17:18–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Correa J, Aribo C, Stuparich M, et al. Malfunction events in the US FDA MAUDE database: how does robotic gynecologic surgery compare with other specialties? J Minim Invasive Gynecol. 2022;29:300–307.e1. [DOI] [PubMed] [Google Scholar]
- 284.Dallas K, Souders CP, Caron A, et al. Manufacturer and user facility device experience reporting of events related to transvaginal mesh: understanding the data. Female Pelvic Med Reconstr Surg. 2022;28:332–335. [DOI] [PubMed] [Google Scholar]
- 285.Aminsharifi A, Kotamarti S, Silver D, et al. Major complications and adverse events related to the injection of the SpaceOAR hydrogel system before radiotherapy for prostate cancer: review of the Manufacturer and User Facility Device Experience database. J Endourol. 2019;33:868–871. [DOI] [PubMed] [Google Scholar]
- 286.Somerson JS, Matsen FA 3rd. Timely recognition of total elbow and radial head arthroplasty adverse events: an analysis of reports to the US Food and Drug Administration. J Shoulder Elbow Surg. 2019;28:510–519. [DOI] [PubMed] [Google Scholar]
- 287.Khalid N, Javed H, Shlofmitz E, et al. Adverse events and modes of failure related to rotational atherectomy system: the utility of the MAUDE database. Cardiovasc Revasc Med. 2021;27:57–62. [DOI] [PubMed] [Google Scholar]
- 288.Case BC, Kumar S, Yerasi C, et al. Real-world experience of suture-based closure devices: insights from the FDA Manufacturer and User Facility Device Experience. Catheter Cardiovasc Interv. 2021;98:572–577. [DOI] [PubMed] [Google Scholar]
- 289.Whitford M, Mitchell SJ, Marzloff GE, et al. Wheelchair mobility-related injuries due to inadvertent lower extremity displacement on footplates: analysis of the FDA MAUDE database from 2014 to 2018. J Patient Saf. 2021;17:e1785–e1792. [DOI] [PubMed] [Google Scholar]
- 290.D’Souza RS, Olatoye OO, Butler CS, et al. Adverse events associated with 10-kHz dorsal column spinal cord stimulation: a 5-year analysis of the Manufacturer and User Facility Device Experience (MAUDE) database. Clin J Pain. 2022;38:320–327. [DOI] [PubMed] [Google Scholar]
- 291.Ramai D, Facciorusso A, DeLuca M, et al. Adverse events associated with AXIOS stents: insights from the Manufacturer and User Facility Device Experience database. Endosc Ultrasound. 2022;11:231–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Pagani NR, Menendez ME, Moverman MA, et al. Adverse events associated with robotic-assisted joint arthroplasty: an analysis of the US Food and Drug Administration MAUDE database. J Arthroplasty. 2022;37:1526–1533. [DOI] [PubMed] [Google Scholar]
- 293.Heaton J, Rezkalla K, Fullmer J, et al. Adverse events of subcutaneous loop recorders: insights from the MAUDE database. Pacing Clin Electrophysiol. 2022;45:1306–1309. [DOI] [PubMed] [Google Scholar]
- 294.Giles TX, Bennett J, Stone CE, et al. Characterizing complications of intracranial responsive neurostimulation devices for epilepsy through a retrospective analysis of the federal MAUDE database. Neuromodulation. 2022;25:263–270. [DOI] [PubMed] [Google Scholar]
- 295.Shah VN, Pasick LJ, Benito DA, et al. Complications associated with PROPEL mometasone furoate bioabsorbable drug-eluting sinus stents from 2012 to 2020. Am J Rhinol Allergy. 2022;36:185–190. [DOI] [PubMed] [Google Scholar]
- 296.Megaly M, Sedhom R, Abdelmaseeh P, et al. Complications of the MANTA closure device: insights from MAUDE database. Cardiovasc Revasc Med. 2022;34:75–79. [DOI] [PubMed] [Google Scholar]
- 297.Rhodes SC. Ultrasonic device complications in endodontics: an analysis of adverse events from the Food and Drug Administration Manufacturer and User Facility Device Experience. J Patient Saf. 2022;18:269–275. [DOI] [PubMed] [Google Scholar]
- 298.Faulds ER, Wyne KL, Buschur EO, et al. Insulin pump malfunction during hospitalization: two case reports. Diabetes Technol Ther. 2016;18:399–403. [DOI] [PubMed] [Google Scholar]
- 299.Khan AR, Tripathi A, Farid TA, et al. Stent thrombosis with bioabsorbable polymer drug-eluting stents: insights from the Food and Drug Administration database. Coron Artery Dis. 2017;28:564–569. [DOI] [PubMed] [Google Scholar]
- 300.Metheny NA, Meert KL. Update on effectiveness of an electromagnetic feeding tube-placement device in detecting respiratory placements. Am J Crit Care. 2017;26:157–161. [DOI] [PubMed] [Google Scholar]
- 301.Rayess HM, Svider PF, Hanba C, et al. A cross-sectional analysis of adverse events and litigation for injectable fillers. JAMA Facial Plast Surg. 2018;20:207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Somerson JS, Hsu JE, Neradilek MB, et al. Analysis of 4063 complications of shoulder arthroplasty reported to the US Food and Drug Administration from 2012 to 2016. J Shoulder Elbow Surg. 2018;27:1978–1986. [DOI] [PubMed] [Google Scholar]
- 303.Patel NH, Uppaluri N, Iorga M, et al. Device malfunctions and complications associated with benign prostatic hyperplasia surgery: review of the Manufacturer and User Facility Device Experience database. J Endourol. 2019;33:448–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Isom N, Masoomi R, Thors A, et al. Guidewire fracture during orbital atherectomy for peripheral artery disease: insights from the Manufacturer and User Facility Device Experience database. Catheter Cardiovasc Interv. 2019;93:330–334. [DOI] [PubMed] [Google Scholar]
- 305.Sivanesan E, Bicket MC, Cohen SP. Retrospective analysis of complications associated with dorsal root ganglion stimulation for pain relief in the FDA MAUDE database. Reg Anesth Pain Med. 2019;44:100–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Mansukhani NA, Haleem MS, Eskandari MK. Thoracic endovascular aortic repair adverse events reported in the Food and Drug Administration Manufacturer and User Facility Device Experience database. Med Devices (Auckl). 2019;12:461–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Krouwer JS. Why the details of glucose meter evaluations matters. J Diabetes Sci Technol. 2019;13:559–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Beauvais D, Ferneini EM. Complications and litigation associated with injectable facial fillers: a cross-sectional study [published correction appears in J Oral Maxillofac Surg. 2021 May;79(5):1180]. J Oral Maxillofac Surg. 2020;78:133–140. [DOI] [PubMed] [Google Scholar]
- 309.Case BC, Forrestal BJ, Yerasi C, et al. Real-world experience of the sentinel cerebral protection device: insights from the FDA Manufacturer and User Facility Device Experience (MAUDE) database. Cardiovasc Revasc Med. 2020;21:235–238. [DOI] [PubMed] [Google Scholar]
- 310.Mahabir CA, DeFilippis EM, Aggarwal S, et al. The first 4 years of postmarketing safety surveillance related to the MitraClip device: a United States Food and Drug Administration MAUDE experience. J Invasive Cardiol. 2020;32:E130–E132. [DOI] [PubMed] [Google Scholar]
- 311.Wallace SL, Sokol ER, Enemchukwu EA. Vaginal energy-based devices: characterization of adverse events based on the last decade of MAUDE safety reports. Menopause. 2020;28:135–141. [DOI] [PubMed] [Google Scholar]
- 312.Jiang R, Kasle DA, Alzahrani F, et al. A Manufacturer and User Facility Device Experience analysis of upper aerodigestive endoscopy contamination: is flexible laryngoscopy different? Laryngoscope. 2021;131:598–605. [DOI] [PubMed] [Google Scholar]
- 313.Crowder HR, Bestourous DE, Reilly BK. Adverse events associated with Bonebridge and Osia bone conduction implant devices. Am J Otolaryngol. 2021;42:102968. [DOI] [PubMed] [Google Scholar]
- 314.Singh AK, Kasle DA, Torabi SJ, et al. Adverse events associated with ClariFix posterior nasal nerve cryoablation: a MAUDE database analysis. Otolaryngol Head Neck Surg. 2021;165:597–601. [DOI] [PubMed] [Google Scholar]
- 315.Bellamkonda N, Shiba T, Mendelsohn AH. Adverse events in hypoglossal nerve stimulator implantation: 5-year analysis of the FDA MAUDE database. Otolaryngol Head Neck Surg. 2021;164:443–447. [DOI] [PubMed] [Google Scholar]
- 316.Heaton JN, Singh S, Li M, et al. Adverse events with HeartMate-3 left ventricular assist device: results from the Manufacturer and User Facility Device Experience (MAUDE) database. Indian Heart J. 2021;73:765–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Crumley C. Negative pressure wound therapy devices with instillation/irrigation: a technologic analysis. J Wound Ostomy Continence Nurs. 2021;48:199–202. [DOI] [PubMed] [Google Scholar]
- 318.Weiss JK, Santucci NM, Sajadi KP, et al. Post-surgical complications after bladder outlet reducing surgery: an analysis of the FDA Manufacturer and User Facility Device Experience (MAUDE) database. Urology. 2021;156:211–215. [DOI] [PubMed] [Google Scholar]
- 319.Giffen Z, Ezzone A, Ekwenna O. Robotic stapler use: is it safe?-FDA database analysis across multiple surgical specialties. PloS One. 2021;16:e0253548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Reyes Orozco F, Ulloa R, Lin M, et al. Adverse events associated with image-guided sinus navigation in endoscopic sinus surgery: a MAUDE database analysis. Otolaryngol Head Neck Surg. 2023;168:501–505. [DOI] [PubMed] [Google Scholar]
- 321.Contractor T, Bhardwaj R, Mandapati R, et al. Adverse events associated with the AtriClip device for left atrial appendage occlusion: a Food and Drug Administration MAUDE database study. Heart Rhythm. 2022;19:1204–1205. [DOI] [PubMed] [Google Scholar]
- 322.Contractor T, Bhardwaj R, Mandapati R, et al. Adverse events associated with the bridge occlusion balloon for lead extraction: a MAUDE database study. Heart Rhythm. 2023;20:142–143. [DOI] [PubMed] [Google Scholar]
- 323.Contractor T, Bhardwaj R, Mandapati R, et al. Adverse events associated with the C304 his sheath used for conduction system pacing: a MAUDE database analysis. Am J Cardiol. 2022;178:174–176. [DOI] [PubMed] [Google Scholar]
- 324.Dodoo SN, Okoh AK, Oseni A, et al. Adverse events following transcatheter edge-to-edge repair (TEER) using MitraClip: lessons learned from the Manufacturer and User Facility Device Experience (MAUDE) registry. Cardiovasc Revasc Med. 2022;39:101–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Sheth AR, Al Yafeai Z, Dominic P. Adverse events related to AtriCure EPi-sense coagulation device-analysis of the FDA MAUDE database. J Cardiovasc Electrophysiol. 2022;33:151–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Metikala S, Mahmoud K, O’Connor KM, et al. Adverse events related to cartiva hemiarthroplasty of first metatarsal: an analysis of reports to the United States Food and Drug Administration. Foot Ankle Spec. 2022;15:113–118. [DOI] [PubMed] [Google Scholar]
- 327.Ofosu A, Ramai D, Mozell D, et al. Analysis of reported adverse events related to single-use duodenoscopes and duodenoscopes with detachable endcaps. Gastrointest Endosc. 2022;96:67–72. [DOI] [PubMed] [Google Scholar]
- 328.Kaplan-Marans E, Martinez M, Wood A, et al. Aquablation, prostatic urethral lift, and transurethral water vapor therapy: a comparison of device-related adverse events in a national registry. J Endourol. 2022;36:231–235. [DOI] [PubMed] [Google Scholar]
- 329.Brauer PR, Bryson PC, Wu SS, et al. Cancer risk associated with continuous positive airway pressure: a national study. Laryngoscope. 2022;132:2270–2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Megaly M, Sedhom R, Zordok M, et al. Complications and failure modes of stingray LP balloon: insights from the MAUDE database. Cardiovasc Revasc Med. 2022;35:187–188. [DOI] [PubMed] [Google Scholar]
- 331.Sedhom R, Megaly M, Abdelmaseeh P, et al. Complications and failure modes of the penumbra indigo CAT RX aspiration system in percutaneous coronary intervention: insights from the MAUDE database. Cardiovasc Revasc Med. 2022;37:147–148. [DOI] [PubMed] [Google Scholar]
- 332.Hakimi AA, Goshtasbi K, Kuan EC. Complications associated with nasopharyngeal COVID-19 testing: an analysis of the MAUDE database and literature review. Am J Rhinol Allergy. 2022;36:281–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Low SW, Swanson KL, Lee JZ, et al. Complications of endobronchial valve placement for bronchoscopic lung volume reduction: insights from the Food and Drug Administration Manufacturer and User Facility Device Experience (MAUDE). J Bronchology Interv Pulmonol. 2022;29:206–212. [DOI] [PubMed] [Google Scholar]
- 334.Wulkan AJ, Vazirnia A, Avram MM. Complications with noninvasive fat and cellulite reduction devices: a cross-sectional analysis of the US Food and Drug Administration Manufacturer and User Facility Device Experience database. Dermatol Surg. 2022;48:758–763. [DOI] [PubMed] [Google Scholar]
- 335.Case BC, Kumar S, Medranda GA, et al. Contemporary post-marketing adverse events and modes of failure related to VASCADE vascular closure system: the utility of the MAUDE database. Catheter Cardiovasc Interv. 2022;99:822–826. [DOI] [PubMed] [Google Scholar]
- 336.Hassanin SW, Kshirsagar RS, Eide JG, et al. Image-guided surgical device failures in functional endoscopic sinus surgery: a MAUDE analysis. Laryngoscope. 2023;133:1310–1314. [DOI] [PubMed] [Google Scholar]
- 337.Kewcharoen J, Shah K, Bhardwaj R, et al. Post-FDA approval “real-world” safety profile of different steerable sheaths during catheter ablation: a Food and Drug Administration MAUDE database study. Heart Rhythm. 2022;19:856–857. [DOI] [PubMed] [Google Scholar]
- 338.Garg J, Shah K, Pinkhas D, et al. Postapproval safety profile of Watchman FLX left atrial appendage occlusion device: analysis from the MAUDE database. Heart Rhythm. 2022;19:332–333. [DOI] [PubMed] [Google Scholar]
- 339.Alhusain R, Dayco J, Awadelkarim A, et al. Turnpike catheter failure, causes and mechanisms: insights from the MAUDE database. Ann Med Surg (Lond). 2022;78:103923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Heinemann L, Fleming GA, Petrie JR, et al. Insulin pump risks and benefits: a clinical appraisal of pump safety standards, adverse event reporting and research needs. A joint statement of the European Association for the Study of Diabetes and the American Diabetes Association Diabetes Technology Working Group. Diabetologia. 2015;58:862–870. [DOI] [PubMed] [Google Scholar]
- 341.Hauser RG, Gornick CC, Abdelhadi RH, et al. Major adverse clinical events associated with implantation of a leadless intracardiac pacemaker. Heart Rhythm. 2021;18:1132–1139. [DOI] [PubMed] [Google Scholar]
- 342.Pierce H, Roberts B, Scherr D, et al. Patient injuries and malfunctions associated with robotic prostatectomy: review of the Manufacturer and User Facility Device Experience database. J Robot Surg. 2021;15:179–185. [DOI] [PubMed] [Google Scholar]
- 343.Kass-Hout TA, Xu Z, Mohebbi M, et al. OpenFDA: an innovative platform providing access to a wealth of FDA’s publicly available data. J Am Med Inform Assoc. 2016;23:596–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.openFDA. Device Adverse Event API Endpoint. Available at: https://open.fda.gov/apis/device/event/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.


