Abstract
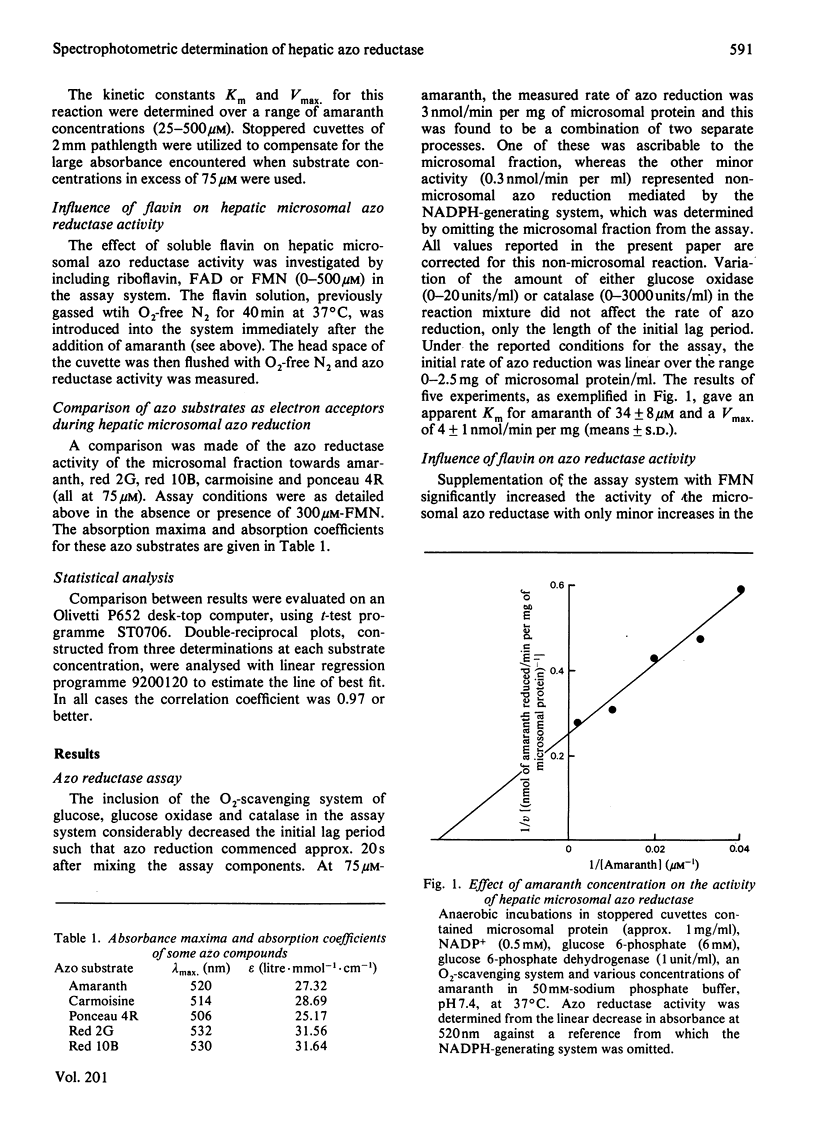
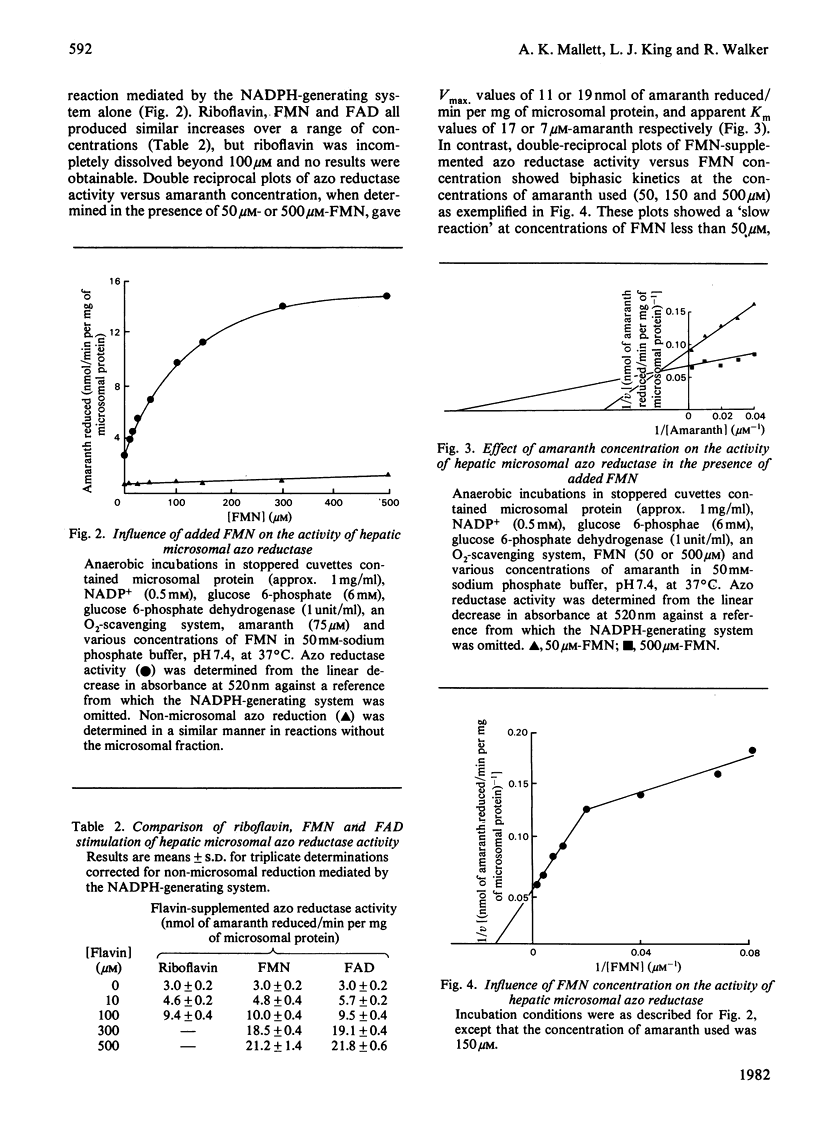
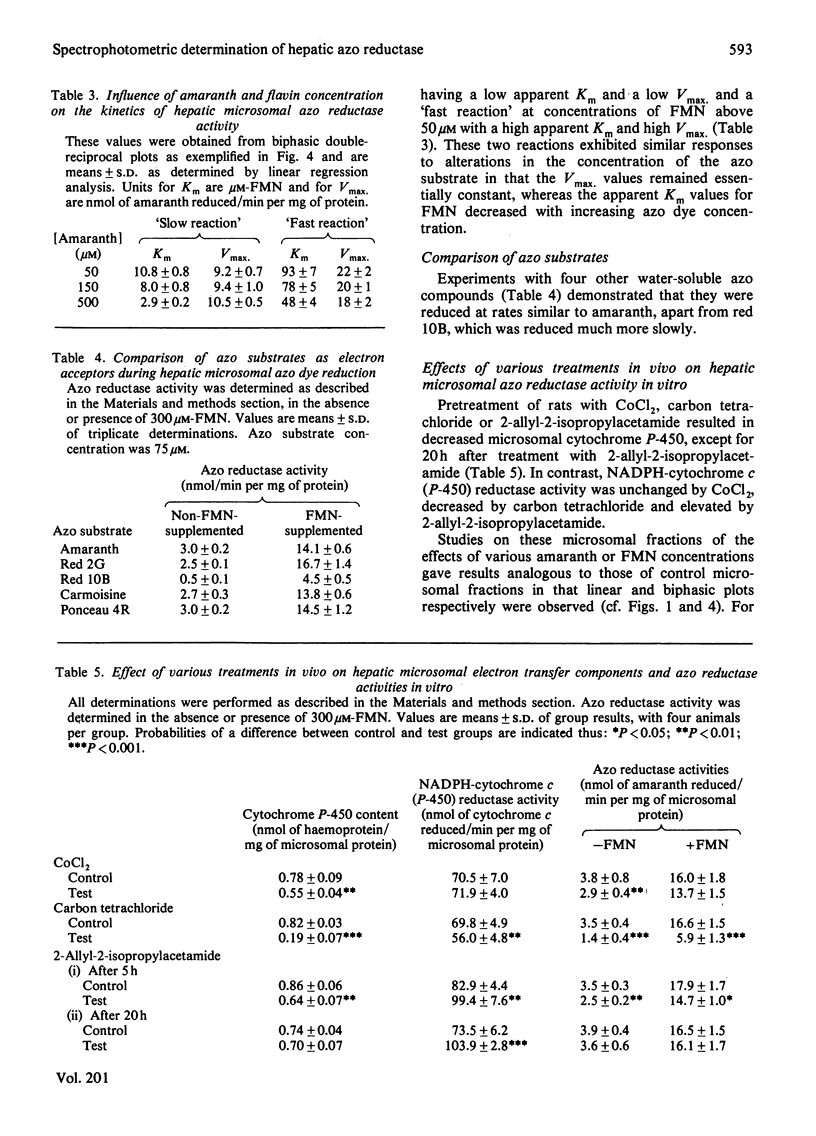
1. A continuous spectrophotometric determination of rat hepatic microsomal anaerobic azo reductase activity has been developed. 2. The addition of soluble flavins (riboflavin, FMN or FAD) greatly increased this NADPH-dependent activity towards a number of azo substrates. 3. Investigations with amaranth as substrate gave an apparent Km of 34 microM and Vmax. of 4 nmol/min per mg of microsomal protein. The inclusion of a fixed concentration of FMN increased Vmax. and greatly decreased Km, the magnitude of these changes reflecting the concentration of flavin present. 4. Investigations using a fixed amaranth concentration over a range of flavin concentrations gave biphasic double-reciprocal plots with two apparent Km and Vmax. values. 5. Pretreatment of animals with cobaltous chloride, 2-allyl-2-isopropylacetamide, carbon tetrachloride, phenobarbitone and 3-methylcholanthrene altered azo reductase activity in parallel with changes in cytochrome P-450 content. 6. The significance of these results is discussed in terms of the electron-transfer components present in the hepatic microsomal fraction.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alvares A. P., Mannering G. J. Two-substrate kinetics of drug-metabolizing enzyme systems of hepatic microsomes. Mol Pharmacol. 1970 May;6(3):206–212. [PubMed] [Google Scholar]
- FOUTS J. R., KAMM J. J., BRODIE B. B. Enzymatic reduction of prontosil and other azo dyes. J Pharmacol Exp Ther. 1957 Jul;120(3):291–300. [PubMed] [Google Scholar]
- Fujita S., Peisach J. Liver microsomal cytochromes P-450 and azoreductase activity. J Biol Chem. 1978 Jul 10;253(13):4512–4513. [PubMed] [Google Scholar]
- Gingell R., Walker R. Mechanisms of azo reduction by Streptococcus faecalis. II. The role of soluble flavins. Xenobiotica. 1971 May;1(3):231–239. doi: 10.3109/00498257109033172. [DOI] [PubMed] [Google Scholar]
- Hernandez P. H., Gillette J. R., Mazel P. Studies on the mechanism of action of mammalian hepatic azoreductase. I. Azoreductase activity of reduced nicotinamide adenine dinucleotide phosphate-cytochrome c reductase. Biochem Pharmacol. 1967 Oct;16(10):1859–1875. doi: 10.1016/0006-2952(67)90297-3. [DOI] [PubMed] [Google Scholar]
- Hernandez P. H., Mazel P., Gillette J. R. Studies on the mechanism of action of mammalian hepatic azoreductase. II. The effects of phenobarbital and 3-methylcholanthrene on carbon monoxide sensitive and insensitive azoreductase activities. Biochem Pharmacol. 1967 Oct;16(10):1877–1888. doi: 10.1016/0006-2952(67)90298-5. [DOI] [PubMed] [Google Scholar]
- MUELLER G. C., MILLER J. A. The reductive cleavage of 4-dimethylaminoazobenzene by rat liver: reactivation of carbon dioxide-treated homogenates by riboflavin-adenine dinucleotide. J Biol Chem. 1950 Jul;185(1):145–154. [PubMed] [Google Scholar]
- Mallett A. K., King L. J., Walker R. A continuous assay for hepatic microsomal azo reductase [proceedings]. Biochem Soc Trans. 1977;5(5):1522–1524. doi: 10.1042/bst0051522. [DOI] [PubMed] [Google Scholar]
- Mason R. P., Peterson F. J., Holtzman J. L. Inhibition of azoreductase by oxygen. The role of the azo anion free radical metabolite in the reduction of oxygen to superoxide. Mol Pharmacol. 1978 Jul;14(4):665–671. [PubMed] [Google Scholar]
- OMURA T., SATO R. THE CARBON MONOXIDE-BINDING PIGMENT OF LIVER MICROSOMES. I. EVIDENCE FOR ITS HEMOPROTEIN NATURE. J Biol Chem. 1964 Jul;239:2370–2378. [PubMed] [Google Scholar]
- Shargel L., Mazel P. Influence of 2,4-dichloro-6-phenoxyethyl-amine (DPEA) and -diethylaminoethyl diphenylpropylacetate (SKF-525A) on hepatic microsomal azoreductase activity from phenobarbital or 3-methylcholanthrene induced rats. Biochem Pharmacol. 1972 Jan;21(1):69–75. doi: 10.1016/0006-2952(72)90251-1. [DOI] [PubMed] [Google Scholar]
- WILLIAMS C. H., Jr, KAMIN H. Microsomal triphosphopyridine nucleotide-cytochrome c reductase of liver. J Biol Chem. 1962 Feb;237:587–595. [PubMed] [Google Scholar]
- Walker R., Gingell R., Murrells D. F. Mechanisms of azo reduction by Streptococcus faecalis. I. Optimization of assay conditions. Xenobiotica. 1971 May;1(3):221–229. doi: 10.3109/00498257109033171. [DOI] [PubMed] [Google Scholar]
- Walker R. The metabolism of azo compounds: a review of the literature. Food Cosmet Toxicol. 1970 Dec;8(6):659–676. doi: 10.1016/s0015-6264(70)80455-2. [DOI] [PubMed] [Google Scholar]
- Welton A. F., Aust S. D. Multiplicity of cytochrome P450 hemoproteins in rat liver microsomes. Biochem Biophys Res Commun. 1974 Feb 27;56(4):898–906. doi: 10.1016/s0006-291x(74)80273-1. [DOI] [PubMed] [Google Scholar]
- Williams J. R., Jr, Grantham P. H., Yamamoto R. S., Weisburger J. H. Effect of dietary riboflavin on azo dye reductase in liver and in bacteria of cecal contents of rats. Biochem Pharmacol. 1970 Aug;19(8):2523–2525. doi: 10.1016/0006-2952(70)90281-9. [DOI] [PubMed] [Google Scholar]


