Abstract
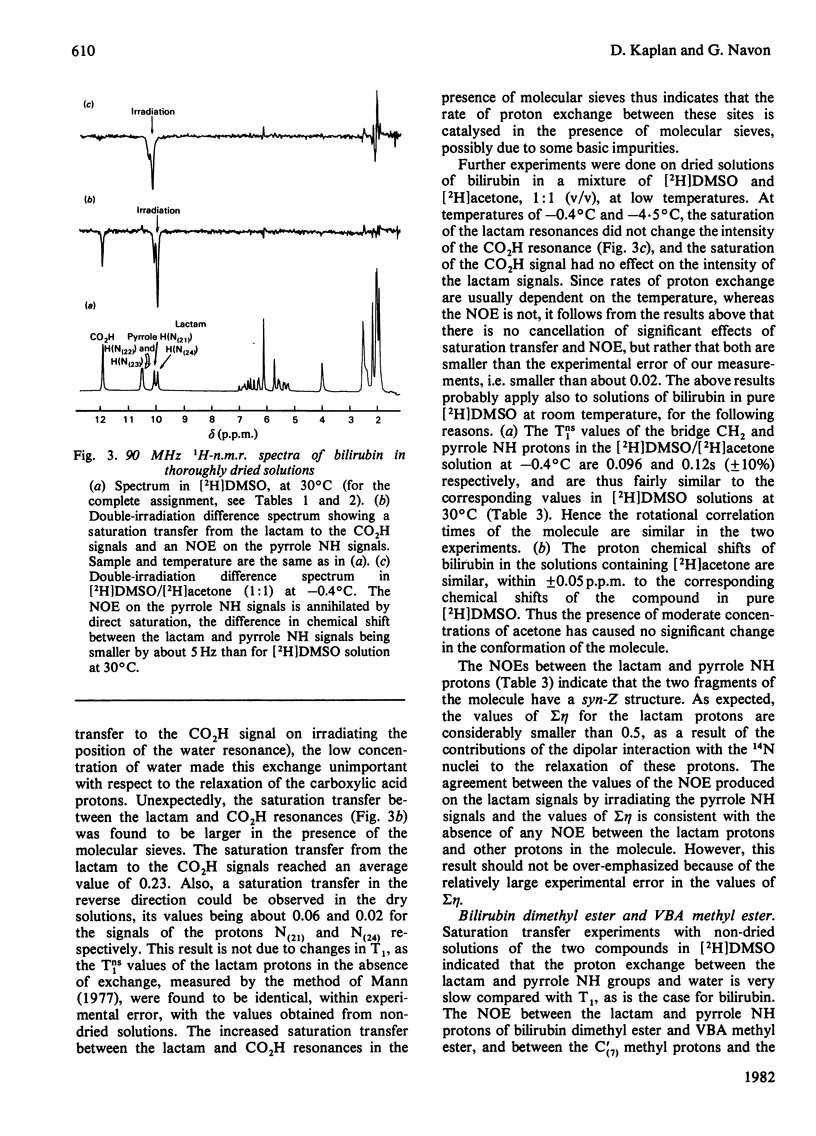
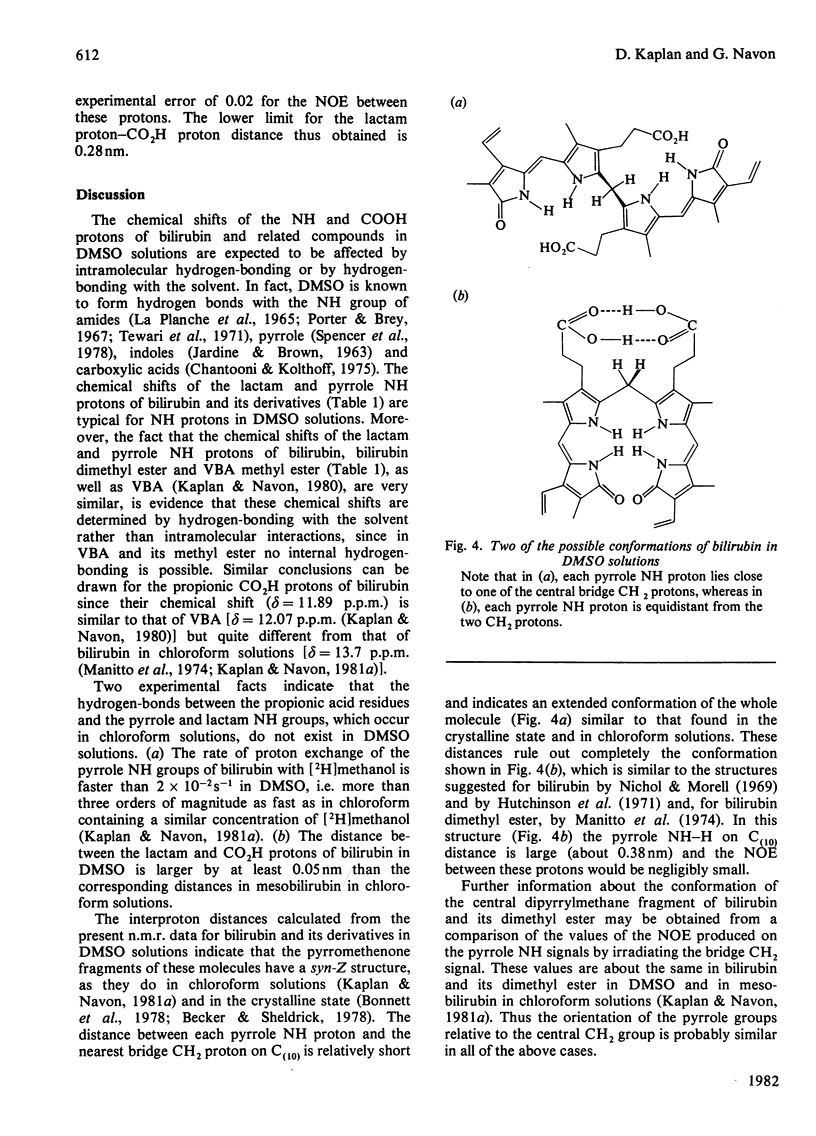
The conformation of bilirubin and its dimethyl ester in dimethyl sulphoxide (DMSO) was investigated by n.m.r. spectroscopy. The chemical shifts of the pyrrole NH and Lactam protons of bilirubin and its dimethyl ester in DMSO indicate a strong interaction with the solvent. Inter-proton distances were calculated from nuclear Overhauser effects (NOE), selective and non-selective relaxation times (T1) and rotational correlation times taken from 13C relaxation times. The interproton distances indicate that the conformation of the skeleton of bilirubin and its dimethyl ester in DMSO is similar to that of bilirubin and mesobilirubin in the crystalline state and in chloroform solutions, except for a possible slight twist of the pyrrolenone rings about the methine bonds, which may be a consequence of solvation of the NH groups by DMSO. Unlike in chloroform solutions, no direct hydrogen-bonding occurs between the carboxylic acid and the lactam groups of bilirubin in DMSO, as shown by the absence of an NOE between these groups. The fast exchange of the pyrrole NH protons with 2H shows that no hydrogen-bonding occurs between these protons and the propionic residues, in line with their solvation by DMSO. From the above results, and from the slowness of the internal motion of the propionic residues of bilirubin and its dimethyl ester, it is concluded that these residues are tied to the skeleton via bound solvent molecules.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonnett R., Davies J. E., Hursthouse M. B., Sheldrick G. M. The structure of bilirubin. Proc R Soc Lond B Biol Sci. 1978 Jun 23;202(1147):249–268. doi: 10.1098/rspb.1978.0066. [DOI] [PubMed] [Google Scholar]
- Brodersen R. Bilirubin transport in the newborn infant, reviewed with relation to kernicterus. J Pediatr. 1980 Mar;96(3 Pt 1):349–356. doi: 10.1016/s0022-3476(80)80671-8. [DOI] [PubMed] [Google Scholar]
- Chedekel M., Bovey F. A., Brewster A. I., Petryka Z. J., Weimer M., Watson C. J., Moscowitz A., Lightner D. A. On the existence of a mono-vinyl d-urobilin. Proc Natl Acad Sci U S A. 1974 May;71(5):1599–1601. doi: 10.1073/pnas.71.5.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta R. K., Redfield A. G. NMR double resonance study of azidoferricytochrome C. Biochem Biophys Res Commun. 1970 Oct 23;41(2):273–281. doi: 10.1016/0006-291x(70)90499-7. [DOI] [PubMed] [Google Scholar]
- Hutchinson D. W., Johnson B., Knell A. J. Tautomerism and hydrogen bonding in bilirubin. Biochem J. 1971 Jul;123(3):483–484. doi: 10.1042/bj1230483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuenzle C. C. Bilirubin conjugates of human bile. Nuclear-magnetic-resonance, infrared and optical spectra of model compounds. Biochem J. 1970 Sep;119(3):395–409. doi: 10.1042/bj1190395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navon G. The nuclear magnetic resonance spectrum of bilirubin. Birth Defects Orig Artic Ser. 1976;12(2):141–147. [PubMed] [Google Scholar]
- Nichol A. W., Morell D. B. Tautomerism and hydrogen bonding in bilirubin and biliverdin. Biochim Biophys Acta. 1969 May 6;177(3):599–609. doi: 10.1016/0304-4165(69)90325-0. [DOI] [PubMed] [Google Scholar]
- Plieninger H., el-Barkawi F., Ehl K., Kohler R., McDonagh A. F. Neue Synthese und 14 C-Markierung von Bilirubin-IXalpha. Justus Liebigs Ann Chem. 1972 Apr;758:195–201. doi: 10.1002/jlac.19727580122. [DOI] [PubMed] [Google Scholar]
- Porter D. M., Brey W. S., Jr A nuclear magnetic resonance study of hydrogen bonding in the succinimide-dimethyl sulfoxide system. J Phys Chem. 1967 Nov;71(12):3779–3783. doi: 10.1021/j100871a010. [DOI] [PubMed] [Google Scholar]


